Abstract
Transcriptional control of hypothalamic thyrotropin-releasing hormone (TRH) integrates central regulation of the hypothalamo-hypophyseal-thyroid axis and hence thyroid hormone (triiodothyronine (T3)) homeostasis. The two β thyroid hormone receptors, TRβ1 and TRβ2, contribute to T3 feedback on TRH, with TRβ1 having a more important role in the activation of TRH transcription. How TRβ1 fulfils its role in activating TRH gene transcription is unknown. By using a yeast two-hybrid screening of a mouse hypothalamic complementary DNA library, we identified a novel partner for TRβ1, hepatitis virus B X-associated protein 2 (XAP2), a protein first identified as a co-chaperone protein. TR–XAP2 interactions were TR isoform specific, being observed only with TRβ1, and were enhanced by T3 both in yeast and mammalian cells. Furthermore, small inhibitory RNA-mediated knockdown of XAP2 in vitro affected the stability of TRβ1. In vivo, siXAP2 abrogated specifically TRβ1-mediated (but not TRβ2) activation of hypothalamic TRH transcription. This study provides the first in vivo demonstration of a regulatory, physiological role for XAP2.
Keywords: XAP2, hypothalamus, TRH, transcription, TRβ1 specificity
Introduction
Hypothalamic thyrotropin-releasing hormone (TRH) is a central regulator of the hypothalamo-hypophyseal-thyroid axis, with the final output being the thyroid hormones (TH) tetraiodothyronine (T4) and triiodothyronine (T3), T3 being the biologically active form. TH homeostasis is vital both during development and maturity, and is maintained by negative feedback exerted by T3 on TRH and thyroid-stimulating hormone (TSH) production in the hypothalamus and pituitary, respectively.
As for other target genes, T3 modulates TRH transcription through its nuclear TH receptors (TRs). Among the TR subtypes, TRβ1 and TRβ2 (as opposed to TRα subtypes) are the key regulators of TRH. Both TRβ1 and TRβ2 are equally implicated in T3-dependent repression of TRH (Guissouma et al, 1998, 2002; Abel et al, 2001), whereas TRβ1 has a more marked role in activation (Dupre et al, 2004).
We addressed the mechanisms of TRβ1 activation of TRH by seeking TRβ1-specific partners. We used a yeast two-hybrid assay based on a library of adult male mice paraventricular nucleus (PVN) complementary DNA. One of the TRβ1 partners identified was the hepatitis virus B X-associated protein 2 (XAP2), also known as ARA9 or AIP (Carver & Bradfield, 1997; Ma & Whitlock, 1997; Meyer et al, 1998). XAP2 is known to modulate the transcriptional activity of the dioxin receptor (AhR) in vitro (Ma & Whitlock, 1997), associating with hsp90 and regulating the intracellular localization of AhR (Kazlauskas et al, 2001). However, no data have shown a physiological role for XAP2 or linked it to TRs.
Our experiments show that TRβ1 interacts specifically with XAP2 and that XAP2 exerts a functional role in modulating TRH transcription in vivo. By using a small inhibitory RNA (siRNA) knockdown approach, we show that XAP2 is necessary for T3-independent activation of TRH transcription mediated by TRβ1. Thus, our results show for the first time a functional and receptor isoform-specific in vivo role for XAP2.
Results and Discussion
XAP2 shows TRβ1-specific functional interactions
To identify new TRβ1 partners, we used a yeast two-hybrid system. Full-length mouse TRβ1, fused to the GAL4 DNA-binding domain in pGBKT7, was used to screen a mouse PVN cDNA library fused to the GAL4 activation domain; the library was screened with and without T3 (1 μM). XAP2 was isolated in the presence of T3. TRβ1 and XAP2 interactions in yeast were T3 dependent and TR isoform specific (Fig 1A). No other TR isoform tested (TRβ2 and TRα1) interacted with XAP2 with or without T3, but all TRs tested interacted with a known partner, RXRβ (Fig 1A).
Figure 1.
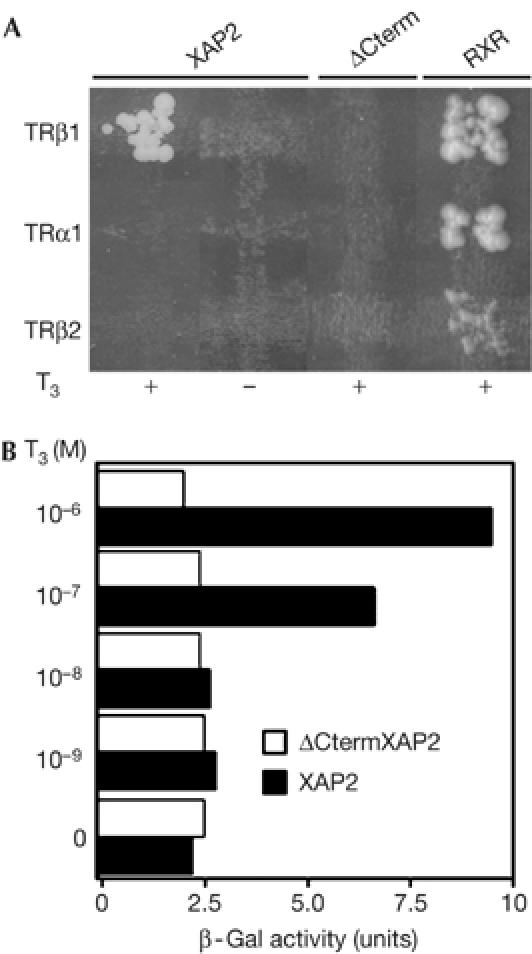
Functional interactions between hepatitis virus B X-associated protein 2 and thyroid hormone receptor in yeast. (A) XAP2: two-hybrid experiments in yeast show T3-dependent specific interactions of XAP2 with TRβ1 but not with TRα1 or TRβ2. ΔCterm: when the carboxy-terminal region of XAP2 is deleted, the interaction between TRβ1 and XAP2 is lost. RXR: positive control of interaction with each TR. (B) XAP2 and TRβ1 interactions in yeast depend on T3 concentration. Plasmids expressing different Gal4 fusion proteins were used in mating. Diploids were grown with increasing doses of T3 in selection medium. β-Galactosidase (β-Gal) activity was measured by densitometry. Each bar represents the average of two different transformants. T3, triiodothyronine; TR, thyroid hormone receptor; TRα1, TRβ1, TRβ2, TR subtypes; XAP2, hepatitis virus B X-associated protein 2.
To further analyse TRβ1 and XAP2 interactions, we deleted three conserved domains in the carboxy-terminal end of XAP2, the so-called tetratricopeptide repeats (TPR; ΔCtermXAP2 construct). These domains show high homologies with those of the steroid hormone receptor-interacting immunophilin FKBP52 (Carver & Bradfield, 1997; Ma & Whitlock, 1997; Meyer et al, 1998) implicated in protein–protein interactions (Lamb et al, 1995). These deletions caused loss of XAP2–TRβ1 interactions in yeast (Fig 1A). To further validate this finding, we tested the T3 dose dependency of the TRβ1–XAP2 or the TRβ1–ΔCtermXAP2 interaction in yeast using the β-galactosidase assay. Fig 1B shows that the strength of the TRβ1–XAP2 interaction is T3 dose dependent. Interaction between full-length XAP2 and TRβ1 was reduced by half as the T3 concentration decreased from 10−6 to 10−7 M and no interaction was seen at 10−8 M T3.
The TR–XAP2 interactions were tested in mammalian cell lines. The TRβ1 specificity was confirmed, but there was no interaction with TRα (Fig 2A, lanes 6,7). However, in contrast to the yeast data, XAP2 still interacted with TRβ1 in the absence of T3, but less than with T3. A similar discrepancy between ligand-dependent interactions in yeast and ligand-independent interactions in vitro in mammalian cells was described by Carver & Bradfield (1997) for XAP2 and AhR interactions.
Figure 2.
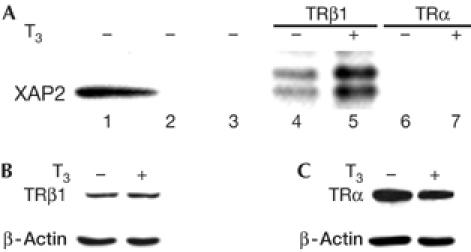
Hepatitis virus B X-associated protein 2–TRβ1 interactions in mammalian cells. (A) XAP2 interacts with TRβ1 (but not with TRα) in mammalian cells. P19 cells were incubated in the absence or presence of 1 μM of T3 for 2 h. Cells were then collected and whole-cell extracts (WCEs; lane 1) prepared. Extracts were used for immunoprecipitation using IgG (lane 2) or no antibody (lane 3) as controls, and TRβ1 (lanes 4,5) and TRα antibodies (lanes 6,7). The presence of XAP2 was examined by western blot analysis using XAP2 antibody. (B, C): Both TRβ1 (B) and TRα (C) are present in the P19 WCE. Samples (100 μg) of the WCE of the P19 cells used for the above experiment were fractionated on a 10% SDS–polyacrylamide gel electrophoresis gel. The levels of TRβ1 and TRα were detected by western blot analysis using TRβ1 and TRα antibodies, showing that both receptors were expressed in these cells. β-Actin was used as loading control. T3, triiodothyronine; TRα, TRβ1, thyroid hormone receptor subtypes; XAP2, hepatitis virus B X-associated protein 2.
XAP2 colocalizes with TRH and TRβ1 in the PVN
We next determined whether XAP2 was expressed in the PVN and whether it colocalized with TRH and TRβ1, as colocalization of TRH and TRβ1 had already been documented by Lechan et al (1994). Using in situ hybridization (supplementary information and supplementary Fig S1A online), we found that TRH and XAP2 transcripts were expressed in the same regions of the PVN, and XAP2 seemed to be more widely expressed than TRH. To assess the expression of XAP2 protein, we used immunocytochemistry with antibodies against XAP2 and TRβ1 on immediately successive PVN sections. XAP2 and TRβ1 were expressed in the same neurons (supplementary Fig S1B online). Thus, the localization of XAP2 is compatible with a functional interaction with TRβ1 in the hypothalamus, and with its role in regulating TRH transcription.
Extrapolating from the results reported on interactions of XAP2 and AhR (Kazlauskas et al, 2000), showing that XAP2 modified the subcellular localization of AhR, we next investigated whether TRβ1 localization was modified in the presence of XAP2. We used a green fluorescent protein (GFP)–TRβ fusion construct transfected into HeLa cells and followed GFP distribution by epifluorescence. The presence or absence of XAP2 did not modify the principally nuclear localization of TRβ1 (supplementary Fig S2 online). Moreover, addition of T3 did not affect the distribution of interactions, with or without XAP2 (supplementary Fig S2 online).
siXAP2 abrogates TRH activation in vivo
The central question that remained was whether XAP2 influences the effects of TRβ1 on activation of TRH transcription. To address this question, we used siRNA against XAP2 (siXAP2-1) and first tested their capacity to abrogate XAP2 protein production in HC11 cells in vitro. As shown in Fig 3A, siRNA against XAP2 diminished XAP2 protein expression after 24 h and even more at 48 h. The scrambled sequence (siCT) did not affect XAP2 protein production. To test whether XAP2 was implicated in the stability of TRβ1, we knocked down XAP2 protein and followed TRβ1 protein levels. XAP2 knockdown was associated with lowered TRβ1 protein levels (Fig 3B), especially in the presence of T3 (Fig 3B, right panel).
Figure 3.
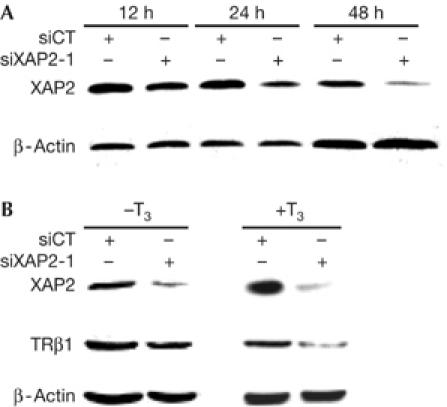
In vitro hepatitis virus B X-associated protein 2 small inhibitory RNA experiments. (A) Inhibition of XAP2 by siRNA: HC11 cells were transiently transfected with siRNA against mouse XAP2 (siXAP2-1). The cells were then collected after 12, 24 or 48 h and whole-cell extracts were prepared and fractionated on a 10% SDS–polyacrylamide gel electrophoresis gel. Levels of protein expression were examined by western blot analysis using XAP2 or β-actin antibodies. (B) The stability of TRβ1 is affected by the absence of XAP2: P19 cells were transiently transfected with scrambled siRNA (siCT) or siRNA against mouse XAP2 (siXAP2-1) and grown for 48 h. The cells were then incubated (+T3) or not (−T3) with 1 μM of T3 for 2 h. WCEs were prepared and fractionated on a 10% SDS–polyacrylamide gel electrophoresis gel. The stability of TRβ1 was detected by western blot analysis using TRβ1 antibody. XAP2 and β-actin antibodies were used as controls. siRNA, small inhibitory RNA; T3, triiodothyronine; TRβ1, thyroid hormone receptor subtype; XAP2, hepatitis virus B X-associated protein 2.
Next, we examined the effects of XAP2 knockdown in vivo at the site of TRH production, the hypothalamus, using a TRH-specific transcriptional assay and a recently developed, novel in vivo siRNA delivery technique (Hassani et al, 2005). siRNAs against XAP2 were complexed with JetSI lipids and injected into the hypothalamus of newborn mice along with a TRH reporter gene (TRH-luciferase (TRH-Luc); Guissouma et al, 1998; Dupre et al, 2004) and a TRβ1 expression vector. In this experimental paradigm, TRH-Luc expression is activated in the absence of T3, and repressed by more than 50% in the presence of T3 (Fig 4B, left-hand columns). When co-injecting with TRH-Luc and TRβ1 into the hypothalamus of hypothyroid newborn mice, siXAP2 (20 and 200 nM) abolished the T3-independent TRH-Luc activation (Fig 4A, middle columns). This effect was sequence specific, as no significant change in TRH transcription was seen with the control siRNA (Fig 4A, siCT). However, the effect at 20 nM was not statistically different from the effect seen at 200 nM, suggesting that the maximal level of inhibition was already attained with 20 nM. However, at 2 nM, no significant effect on TRH transcription was observed (data not shown). This inhibition of TRH activation was confirmed by the use of two different siRNAs (siXAP2-1 and siXAP2-2; Fig 4B). Given that siRNA against XAP2 abolished activation of TRH-Luc transcription in the short term (18 h post-transfection), we explored whether XAP2 knockdown affected TH production through modified endogenous TRH signalling. We found that, in the longer term (48 h post-transfection), siXAP2 increased total T4 levels slightly, but significantly (supplementary Fig S3 online), probably reflecting a rebound after the initial inhibition. This result confirms that XAP2 contributes to endogenous TRH control and that interference with XAP2 expression affects hypothalamo-hypophyseal-thyroid axis output.
Figure 4.
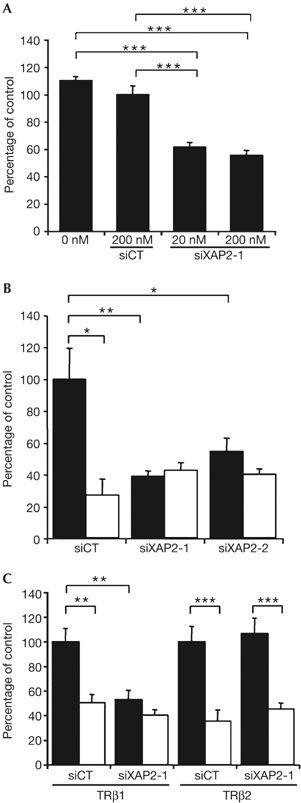
In the mouse hypothalamus, hepatitis virus B X-associated protein 2 small inhibitory RNA affects only TRβ1-mediated activation of thyrotropin-releasing hormone transcription. (A) Dose-dependent effect of XAP2 siRNA on basal TRH transcription. TRH-Luc transcription was followed in hypothyroid 2-day-old mice, 20 h after hypothalamic injection of 1 μg TRH-Luc, 100 ng TRβ1 expression vector and 0, 20 or 200 nM of siXAP2-1 or 200 nM of scrambled siRNA (siCT) complexed with JetSI. Values are means±s.e.m., n⩾30; ***P<0.001. (B) Two different XAP2 siRNAs affect the activation but not the T3-dependent repression of TRH transcription. TRH-Luc transcription was measured in hypothyroid 2-day-old mice, 20 h after hypothalamic injection of 1 μg reporter construct, 100 ng of TRβ1 expression vector and 200 nM of siXAP2-1, siXAP2-2 or scrambled siRNA (siCT) complexed with JetSI. After transfection, mice were treated with T3 (2.5 μg/g of body weight, filled bars) or saline (open bars). Values are means±s.e.m., n⩾6; ns: not significant; *P<0.05; **P<0.01. (C) XAP2 siRNA affects activation of TRH transcription mediated by TRβ1 but not by TRβ2. TRH-Luc transcription was measured in hypothyroid 2-day-old mice, 20 h after hypothalamic injection of 1 μg reporter construct, 100 ng of either TRβ1 or TRβ2 expression vectors and 200 nM of siXAP2-1 or scrambled siRNA (siCT) complexed with JetSI. After transfection, mice were treated with T3 (2.5 μg/g of body weight, filled bars) or saline (open bars). Values are means±s.e.m., n⩾14; ns: not significant; **P<0.01; ***P<0.001. Each experiment was repeated at least three times. siRNA, small inhibitory RNA; T3, triiodothyronine; TRβ1, TRβ2, thyroid hormone receptor subtypes; TRH, thyrotropin-releasing hormone; XAP2, hepatitis virus B X-associated protein 2.
The main result from these knockdown experiments is that although siRNA against XAP2 abrogated T3-independent activation of TRH transcription, it had no effect on TRH repression by T3 (compare means of white bars in Fig 4B). Thus, XAP2 is necessary for T3-independent TRH activation but not for T3-dependent repression. Interestingly, TRβ1 has a more pronounced role than TRβ2 in T3-independent activation of TRH (Dupre et al, 2004). We thus tested whether XAP2 knockdown differentially affected TRβ1 and TRβ2 effects on TRH transcription. As shown in Fig 4C, siXAP2 effects were specific to TRβ1-dependent regulation of TRH and had no effect if TRβ2 was overexpressed. Thus, the in vivo finding that siRNA against XAP2 specifically abrogated the TRβ1-dependent TRH activation provides a strong physiologic correlate for the TRβ1-specific XAP2 interaction seen in the different in vitro situations. To further confirm the role of TRβ1–XAP2 in T3-independent activation of TRH transcription, we looked for the presence of TRβ1 on the site 4 thyroid hormone response element (Satoh et al, 1996) of the TRH promoter. As expected, chromatin immunoprecipitation experiments on hypothalamus from hypothyroid newborn mice showed the presence of TRβ1 on the TRH promoter (representing about 3% of the input DNA; see supplementary Fig S4 online).
In conclusion, this report shows for the first time a central, regulatory role for XAP2, with XAP2 contributing specifically to TRβ1 activation of hypothalamic TRH transcription in a physiologically relevant context.
Methods
Plasmids TRH-Luc and pSG5-rTRβ1 were as described by Guissouma et al (2002), pSG5-rTRβ2 as described by Dupre et al (2004) and pSG5-hXAP2 as described by Kazlauskas et al (2000).
Two-hybrid experiments Plasmid construction. A Gal4 DBD-mTRβ1 (full-length) expression vector was constructed by inserting an EcoR1–Pst1 fragment encoding full-length mTRβ1 into the pGBKT7 vector (Clontech, Palo Alto, CA, USA). This fragment was generated by PCR using the following primers to create, respectively, the EcoR1 and Pst1 sites (in bold): 5′-CCGGAATTCATGACTCCTAACAGTATGACA-3′ and 5′-AACTGCAGTTAGTCCTCAAATACTTCTAA-3′. To obtain Gal4 DBD-mTRβ2 and Gal4 DBD-mTRα1 constructs, full-length mTRβ2 and mTRα1 were cloned by the same method using the following primers to create EcoR1 and BamH1 sites (in bold), respectively: for mTRβ2, 5′-CCGGAATTCATGAACTACTGTATGCCAGAG-3′ and 5′-CGGGATCCTTAGTCCTCAAATACTTCTAA-3′; for mTRα1, 5′-CCGGAATTCATGGAACAGAAGCCAAGCAAG-3′ and 5′-CGGGATCCTTAGACTTCCTGATCCTC-3′. The pGADT7-ΔCtermXAP2 plasmid was made by digesting pGADT7-XAP2 plasmid with AgeI and XhoI, thus deleting the region corresponding to the C terminus of XAP2, from base 420 to base 1,032, including the three TPR motifs and the GIFSH motif (Carver et al, 1998). The pGADT7-RXR plasmid was isolated in the two-hybrid screen.
Library construction, screening and analysis The MATCHMAKER Library Construction and Screening Kit (Clontech) was used to construct a cDNA library using PVN of adult male mice. Briefly, after generating double-stranded cDNA, haploid Mata yeast strain (AH109) was co-transformed with double-stranded cDNA and pGADT7-Rec vector, whereas the pGBKT7-TRβ1 vector served to transform haploid Matα yeast strain (Y187). Two-hybrid screening was carried out by mating the two transformed haploid yeast strains, according to the manufacturer's protocol. Diploids expressing the interacting proteins were identified by nutritional selection using three reporters (ADE2, HIS3 and MEL1) ±1 μM T3. After analysis, positive clones were rescued in Escherichia coli, sequenced and compared with GenBank.
Strength of interaction analysis by β-galactosidase assay A total of about 2 × 108 cells were grown in synthetic medium lacking leucine and tryptophan, in the presence of T3 (0, 10−9, 10−8, 10−7 and 10−6 M), and then incubated at 30°C for 3–4 h until cells reached the mid-log phase. The A600 was measured. An aliquot of each sample was centrifuged and resuspended in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl and 1 mM MgSO4). Cells were permeabilized by two snap-freezing cycles in liquid nitrogen. β-Galactosidase activity was assayed according to Lee et al (1995).
Cell cultures HeLa cells were propagated as described by Kazlauskas et al (2001). HC11 cells were grown according to Olsen et al (2002). Before transfection, the HC11 culture medium was changed to filtered RPMI without phenol red, supplemented with 5% charcoal-treated fetal bovine serum (FBS), L-glutamine (2 mM), gentamycin (1%), insulin (5 μg/ml) and epidermal growth factor (10 ng/ml). P19 cells were grown on Nunc plates coated with 0.1% gelatin in Dulbecco's minimum essential medium supplemented with 10% FBS, L-glutamine (2 mM) and 1% non-essential amino acids at 37°C and 9% CO2.
Cell extracts and immunoblot assay Immunoprecipitation and western blots were carried out according to Kazlauskas et al (2001). HC11 and P19 cells were grown in T3-depleted medium ±T3 (1 μM). Cells were collected and whole-cell extracts were prepared. For immunoprecipitation, 250 μg of cellular proteins were incubated with anti-TRβ1 or anti-TRα antibodies (Affinity BioReagents, Ozyme, St Quentin en Yvelines, France). Anti-XAP2 antibody (1:1,000 dilution; Novus Biologica, Littleton, CO, USA) was used for western blot (1:1,000 dilution). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse immunoglobulins (Dako Cytomation, Glostrup, Denmark).
siRNA experiments Generation of siRNAs. siRNAs against mouse XAP2 were designed using the HiPerformance Design Algorithm licensed from Novartis AG (Qiagen, Courtaboeuf, France). Duplexes targeted against mXAP2 nucleotides 489–509 were synthesized (siXAP2-1, 5′-CACGTAGTCCTGTATCCTCTA-3′). A second siRNA (SI00058562, siXAP2-2) was from Qiagen. Either a scrambled sequence (5′-CGTCACGAGTACTCCGAAGTT-3′) or an siRNA against an irrelevant GFP sequence served as the control (siCT).
In vitro siRNA transfection HC11 and p19 cells were transfected with a final concentration of 20 nM of siRNA (siXAP2-1 or siCT) using Lipofectamin Plus or Lipofectamin 2000, according to the manufacturer's instructions (Invitrogen, Cergy, Pontoise, France). The transfection medium was changed after 4 h (see the Cell cultures section). Cells were collected and used for western blotting using mouse anti-XAP2 antibody (1:1,000 dilution), mouse anti-TRβ1 antibody (1:1,000 dilution; Affinity BioReagents) or mouse β-actin antibody (1:5,000; Sigma, St Quentin Fallavier, France). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse immunoglobulins.
In vivo siRNA transfection–in vivo gene transfer Animal studies were conducted according to the principles and procedures of Guidelines for Care and Use of Experimental Animals. In vivo siRNA experiments were performed, as described previously (Hassani et al, 2005), using JetSI™ (Polyplus Transfection, France). XAP2 or control siRNA (0, 20 or 200 nM) was mixed with TRH-Luc (0.25 μg/μl; Plasmid Factory, Germany) and pSG5-TRβ1 or pSG5-TRβ2 (25 ng/μl). Complexes (2 μl) were injected bilaterally into the hypothalamus of hypothyroid 1-day-old mice, brains were dissected and luciferase assays were carried out 20 h after transfection (Guissouma et al, 1998).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
supplementary Figure 1
supplementary Figure 2
supplementary Figure 3
supplementary Figure 4
Acknowledgments
We thank G. Bolger and M. Plateroti for antibodies and Z. Hassani for help with siRNA experiments. The excellent technical assistance of the animal care personnel is gratefully acknowledged. This work was supported by EU grants Neurocoregulators, Pioneer and Cascade.
References
- Abel ED, Ahima RS, Boers ME, Elmquist JK, Wondisford FE (2001) Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest 107: 1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LA, Bradfield CA (1997) Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem 272: 11452–11456 [DOI] [PubMed] [Google Scholar]
- Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA (1998) Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem 273: 33580–33587 [DOI] [PubMed] [Google Scholar]
- Dupre SM, Guissouma H, Flamant F, Seugnet I, Scanlan TS, Baxter JD, Samarut J, Demeneix BA, Becker N (2004) Both thyroid hormone receptor (TR)β1 and TRβ2 isoforms contribute to the regulation of hypothalamic thyrotropin-releasing hormone. Endocrinology 145: 2337–2345 [DOI] [PubMed] [Google Scholar]
- Guissouma H, Ghorbel MT, Seugnet I, Ouatas T, Demeneix BA (1998) Physiological regulation of hypothalamic TRH transcription in vivo is T3 receptor isoform specific. FASEB J 12: 1755–1764 [DOI] [PubMed] [Google Scholar]
- Guissouma H, Dupre SM, Becker N, Jeannin E, Seugnet I, Desvergne B, Demeneix BA (2002) Feedback on hypothalamic TRH transcription is dependent on thyroid hormone receptor N terminus. Mol Endocrinol 16: 1652–1666 [DOI] [PubMed] [Google Scholar]
- Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, Behr JP, Demeneix BA (2005) Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med 7: 198–207 [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Poellinger L, Pongratz I (2000) The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem 275: 41317–41324 [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Sundstrom S, Poellinger L, Pongratz I (2001) The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol 21: 2594–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P (1995) Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci 20: 257–259 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Qi Y, Jackson IM, Mahdavi V (1994) Identification of thyroid hormone receptor isoforms in thyrotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Endocrinology 135: 92–100 [DOI] [PubMed] [Google Scholar]
- Lee JW, Choi HS, Gyuris J, Brent R, Moore DD (1995) Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol 9: 243–254 [DOI] [PubMed] [Google Scholar]
- Ma Q, Whitlock JP Jr (1997) A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem 272: 8878–8884 [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH (1998) Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol 18: 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen H, Hedengran Faulds MA, Saharinen P, Silvennoinen O, Haldosen LA (2002) Effects of hyperactive Janus kinase 2 signaling in mammary epithelial cells. Biochem Biophys Res Commun 296: 139–144 [DOI] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Iwasaki T, Mori M (1996) Negative regulation of the gene for the preprothyrotropin-releasing hormone from the mouse by thyroid hormone requires additional factors in conjunction with thyroid hormone receptors. J Biol Chem 271: 27919–27926 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information
supplementary Figure 1
supplementary Figure 2
supplementary Figure 3
supplementary Figure 4


