Abstract
Botulinum toxins are metalloproteases that act inside nerve terminals and block neurotransmitter release through their cleavage of components of the exocytosis machinery. These toxins are used to treat human diseases that are characterized by hyperfunction of cholinergic terminals. Recently, evidence has accumulated that gangliosides and synaptic vesicle proteins cooperate to mediate toxin binding to the presynaptic terminal. The differential distribution of synaptic vesicle protein receptors, gangliosides and toxin substrates in distinct neuronal populations opens up the possibility of using different serotypes of botulinum toxins for the treatment of central nervous system diseases caused by altered activity of selected neuronal populations.
Keywords: botulinum toxins, gangliosides, SV2, synaptic vesicles, synaptotagmin
Introduction
Several anaerobic bacteria of the genus Clostridium produce botulinum neurotoxins (BoNTs), which enter nerve terminals and cause a prolonged blockade of neuroexocytosis. This results in an impairment of muscle contraction and autonomic nerve functions; however, the effect is fully reversible provided the patient overcomes the acute phase of respiratory failure. Seven serotypes of BoNT (A–G) have been identified and are the most potent toxins known, with median lethal dose (LD50) values for mice ranging from 0.5 to 5 ng/kg depending on the serotype (Schiavo et al, 2000). These di-chain toxins are formed by a heavy (H) and a light (L) chain, linked by a single disulphide bond (Schiavo et al, 2000). BoNTs are adsorbed in the gastrointestinal tract, and, after entering the general circulation, bind to the presynaptic membrane of cholinergic nerve terminals and prevent the release of their neurotransmitter acetylcholine (Simpson, 2000). Intoxication proceeds through a four-step mechanism comprising binding, internalization, membrane translocation and proteolytic action (Schiavo et al, 2000). To accomplish this multistep process, BoNTs have a modular organization, with the carboxy-terminal domain of the H chain (HC) responsible for neurospecific binding, and the amino-terminal 50 kDa domain of the H chain (HN) involved in membrane translocation of the L chain. The L chain is a metalloprotease specific for components of the exocytosis machinery: BoNT/B, BoNT/D, BoNT/F and BoNT/G cleave synaptobrevin/vesicle-associated membrane protein (VAMP), which is an integral protein of the synaptic vesicle (SV) membrane; BoNT/C, BoNT/A and BoNT/E cleave the synaptosomal-associated protein of 25 kDa (SNAP-25), which is a protein located on the cytosolic face of the presynaptic membrane; and BoNT/C also cleaves the plasma membrane protein syntaxin (Schiavo et al, 2000). The combined properties of neurospecificity, potency and reversibility account for the use of BoNT/A in the treatment of various human diseases caused by the hyperfunction of cholinergic terminals, such as blepharospasm, spasticity, dystonia, hypersalivation and hyperhidrosis (Montecucco & Molgo, 2005). The number of human syndromes that are known to benefit from BoNT treatment is increasing, and a promising new area is the treatment of diseases involving the central nervous system (CNS). Indeed, when centrally administered, BoNTs can also block the release of other neurotransmitters, including glutamate, glycine, noradrenaline, dopamine, serotonin and neuropeptides (Schiavo et al, 2000). It is therefore important to elucidate the mechanisms that mediate their binding and internalization in neurons. Here, we review recent studies indicating that, to enter neurons, BoNTs parasitize the physiological process of SV recycling. Indeed, BoNTs bind to the inner surface of SVs during their exposure to the external medium, and are internalized by vesicle endocytosis. This mechanism of penetration probably favours internalization in hyperactive nerve terminals, which are treated with BoNTs to reduce acetylcholine release in human dystonias.
Presynaptic binding and entry of BoNTs
Gangliosides are sialylated glycosphingolipids that are involved in the development, function and maintenance of the nervous system. Complex gangliosides are particularly enriched at the presynaptic membrane (Hansson et al, 1977). Polysialogangliosides are thought to be involved in BoNT binding as a result of the following observations: BoNTs bind to polysialogangliosides, particularly to GD1b, GT1b and GQ1b (Schiavo et al, 2000), and the HC fragments of BoNT/A and B bind to GT1b through the conserved peptide motif H…SXWY…G (Rummel et al, 2004b); pre-incubation of BoNTs with polysialogangliosides partly prevents the poisoning of the neuromuscular junction; pre-treatment of cultured cells with polysialogangliosides increases their sensitivity to BoNT/A, whereas depletion of gangliosides in neuroblastoma cells impairs BoNT/A internalization; treatment of membranes with neuraminidase, which removes sialic-acid residues, decreases BoNT binding; and knockout mice defective in the production of polysialogangliosides show reduced sensitivity to BoNT/A and BoNT/B (Bullens et al, 2002; Kitamura et al, 2005; Schiavo et al, 2000; Yowler et al, 2002). More recently, polysialogangliosides GD1b and GT1b and phosphatidylethanolamine were reported to be the functional receptors of BoNT/C and BoNT/D, respectively (Tsukamoto et al, 2005).
However, polysialogangliosides alone do not account for the neurospecificity of binding of all BoNT serotypes. Indeed, SV proteins also participate in BoNT binding (Table 1). BoNT/B and BoNT/G interact with the luminal domain of the SV proteins synaptotagmin I (Syt-I) and Syt-II. BoNT/B has a higher affinity for Syt-II (Nishiki et al, 1996), whereas BoNT/G interacts preferentially with Syt-I (Dong et al, 2003; Nishiki et al, 1996; Rummel et al, 2004a). The following experimental evidence suggests that this binding is physiologically relevant: Syt-II-transfected Chinese hamster ovary cells bind BoNT/B after treatment with polysialogangliosides (Nishiki et al, 1994, 1996); entry of BoNT/B (but not BoNT/A or BoNT/E) into PC12 cells is dependent on Syt-I and Syt-II (Dong et al, 2003); mice are partly protected from BoNT/B toxicity by fragments corresponding to the luminal domain of Syt-II (Dong et al, 2003); BoNT/B and BoNT/G interact with Syt-I and Syt-II in pull-down assays; and, finally, peptides derived from the Syt-I and Syt-II luminal domains are able to partly inhibit neurotoxicity at the phrenic neuromuscular junction (Rummel et al, 2004a).
Table 1.
Receptors for BoNT/A, BoNT/B and BoNT/G
| Botulinum neurotoxin serotypes | Receptor | Kd/potency | Lipid requirement | Receptor expression at the neuro-muscular junction | Receptor expression in hippocampus/cortex excitatory neurons | Receptor expression in hippocampus/cortex inhibitory neurons |
|---|---|---|---|---|---|---|
| BoNT/B | Syt-I (Nishiki et al, 1996; Dong et al, 2003) | 2.3 nM (Nishiki et al, 1996) | Yes (Nishiki et al, 1996; Dong et al, 2003) | No (Li et al, 1994; Marqueze et al, 1995) | Yes (Geppert et al, 1991) | Yes (Geppert et al, 1991) |
| BoNT/B | Syt-II (Nishiki et al, 1996; Dong et al, 2003) | 0.23 nM (Nishiki et al, 1996) | Yes (Nishiki et al, 1996; Dong et al, 2003) | Yes (Li et al, 1994, Marqueze et al, 1995) | Yes (subpopulation; Geppert et al, 1991; Marqueze et al, 1995) | To be defined |
| BoNT/G | Syt-I (Rummel et al, 2004a) | Syt-I peptide 66% inhibition (Rummel et al, 2004a) | No (Rummel et al, 2004a) | See above | See above | See above |
| BoNT/G | Syt-II (Rummel et al, 2004a) | Syt-II peptide 37.9% inhibition (Rummel et al, 2004a) | No (Rummel et al, 2004a) | See above | See above | See above |
| BoNT/A | SV2C (Dong et al, 2006; Mahrhold et al, 2006) | Binding activity SV2C>SV2A>SV2B (Dong et al, 2006) | Yes, all isoforms (Dong et al, 2006) | Yes (Dong et al, 2006) | No Bajjalieh et al, 1994; Janz & Sudhof, 1999; Dong et al, 2006) | Yes (subpopulation only; C.V., C.G., M.M., unpublished data) |
| BoNT/A | SV2A (Dong et al, 2006) | Binding activity SV2C>SV2A>SV2B (Dong et al, 2006) | No (Mahrhold et al, 2006) | Yes (Dong et al, 2006) | Yes (Bajjalieh et al, 1994; Janz & Sudhof, 1999) | Yes (Bajjalieh et al, 1994; Janz & Sudhof, 1999) |
| BoNT/A | SV2B (Dong et al, 2006) | Binding activity SV2C>SV2A>SV2B (Dong et al, 2006) | No (Mahrhold et al, 2006) | Yes (Dong et al, 2006) | Yes (Bajjalieh et al, 1994; Janz & Sudhof, 1999) | No (Bajjalieh et al, 1994; Janz & Sudhof, 1999; C.V., C.G., M.M., unpublished data) |
BoNT, Botulinum neurotoxin; Kd, dissociation constant; SV, synaptic vesicle protein; Syt-I and -II, synaptotagmin I and II.
More recently, the specific interaction of BoNT/A with the luminal domain of SV2 has been reported (Dong et al, 2006; Mahrhold et al, 2006). SV2 is highly glycosylated and has 12 putative transmembrane regions (Fig 1), similar to transporters with cytosol-exposed amino and carboxy termini (Bajjalieh et al, 1992). The function of SV2 is not clearly defined, although it has been shown to be involved in vesicle recruitment to the plasma membrane in endocrine cells (Iezzi et al, 2005). SV2 isoforms bind to Syt-I (Schivell et al, 2005), providing an explanation for the spurious isolation of Syt-I as a BoNT/A-binding protein (Li & Singh, 1998). Vertebrates contain three distinct SV2 genes encoding homologous proteins called SV2A, SV2B and SV2C (Bajjalieh et al, 1992, 1994), which are also expressed by endocrine cells (Buckley & Kelly 1985; Iezzi et al, 2005; Portela-Gomes et al, 2000).
Figure 1.
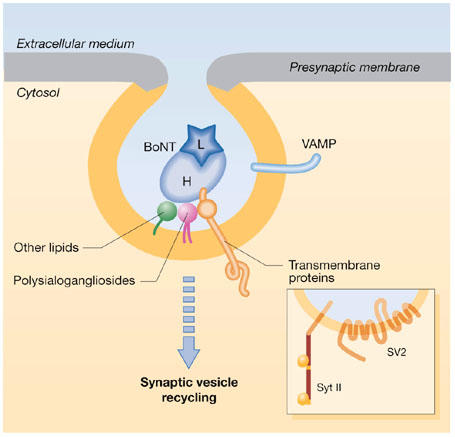
Binding of botulinum neurotoxins to the luminal surface of synaptic vesicles exposed to medium on neuroexocytosis. The illustration depicts the combined role of polysialogangliosides (purple) and a synaptic vesicle (SV) protein (SV2 or synaptotagmin; orange) in mediating botulinum neurotoxin (BoNT)-neurospecific binding and entry into neurons after the retrieval of the vesicle. It suggests additional low-affinity interactions with other molecules (lipids and/or proteins) of the SV membrane (green; modified from Montecucco et al, 2004). Multiple interactions with molecules of the SV membrane would allow almost irreversible neuron binding, which would then become completely irreversible on vesicle fission from the presynaptic membrane. The yellow and grey areas denote the different compositions of the SV and presynaptic membranes, respectively. H, heavy chain of BoNT; L, light chain of BoNT; VAMP, vesicle-associated membrane protein.
Consistent with the BoNT/A–SV2 interaction detected by pull-down assays, fragments of the SV2 domain that interact with BoNT/A inhibit the binding of toxin to neurons (Dong et al, 2006). Furthermore, in hippocampal neurons deficient in SV2A and SV2B, which are the main isoforms expressed in the hippocampus (see below and Fig 2), BoNT/A binding was abolished but was restored by the expression of SV2A, SV2B or SV2C. Finally, mice that lack SV2B have a reduced sensitivity to BoNT/A (Dong et al, 2006). Interestingly, BoNT/A has a much stronger interaction with the luminal L4 loop of SV2C than with the corresponding loops of SV2A and SV2B (Dong et al, 2006), although the latter proteins contribute to BoNT/A binding to hippocampal neurons in culture and to the neuromuscular junction (Dong et al, 2006). Possibly owing to the reduced binding of BoNT/A to SV2A and SV2B compared with SV2C, Mahrhold and colleagues found a specific interaction of BoNT/A with SV2C, but not with SV2A and SV2B (Mahrhold et al, 2006). A peptide comprising the intravesicular domain between transmembrane domains 7 and 8 specifically reduced the neurotoxicity of BoNT/A to phrenic nerve preparations, supporting the physiological relevance of this interaction (Mahrhold et al, 2006). Although it has been reported that either lipids or proteins alone are sufficient to account for BoNT-neurospecific binding (for a discussion, see Montecucco, 1986), these findings can be rationalized in terms of a double-receptor model in which BoNTs interact with both polysialogangliosides and SV proteins (Fig 1, Table 1; Montecucco, 1986). Indeed, the existence of protein–lipid microdomains has been shown in neurons (Tsui-Pierchala et al, 2002).
Figure 2.

Localization of Syt-I and Syt-II, and SV2A, SV2B and SV2C messenger RNA and protein. (A–D) In situ hybridization for synaptotagmin I (Syt-I) (A,C) and Syt-II (B,D) in rat brain (A,B) and spinal cord (C,D; modified from Berton et al, 1997). Note that Syt-II messenger RNA is restricted to the cerebellum (B) and is concentrated in the ventral horns of the spinal cord (D). (E–H) Immunoperoxidase staining of adult rat hippocampal sections for synaptic vesicle protein 2A (SV2A), SV2B and SV2C. SV2C immunoreactivity is largely undetectable (G), whereas SV2B (F) is excluded from inhibitory terminals, as identified by antibodies to glutamate decarboxylase 65 (GAD65; H). (I) Rat hemidiaphragms stained for α-bungarotoxin (α-Bgtx), which binds to postsynaptic nicotinic receptors, and for SV2C. Note the bright labelling for SV2C at the motor neuron terminals (see also Dong et al, 2006). (J) Western blot image of cortex and nerve muscle preparation (nmp) homogenates for SV2A and SV2C. Note the higher expression of SV2A compared with SV2C in rat cortex. The opposite situation is detectable in nerve muscle preparations.
BoNT activity in the central nervous system
Given that BoNTs do not cross the blood–brain barrier, their action is normally limited to peripheral nerve terminals. However, when injected directly into the brain, BoNTs can intoxicate neurons of the central nervous tissue. Indeed, SV proteins, ganglioside receptors and BoNT substrates are expressed in the CNS, although they have specific patterns of distribution (see above). The finding that BoNTs bind to protein receptor isoforms with different affinities opens up the possibility that the sensitivity of distinct terminals to BoNTs might depend on the isoforms expressed, and possibly on their glycosylation pattern. Motor neuron terminals express the protein receptor isoforms that bind more strongly to BoNTs, such as Syt-II (Juzans et al, 1996; Li et al, 1994; Marqueze et al, 1995) and SV2C (Fig 2; Janz & Sudhof, 1999). Conversely, high-affinity isoforms are poorly expressed in CNS neurons and terminals, in which Syt-I, SV2A and SV2B are the prevalent isoforms (Fig 2; Geppert et al, 1991; Marqueze et al, 1995). SV2C has been reported in only a small subset of neurons in the basal forebrain and brainstem, and was undetectable in the hippocampus (Janz & Sudhof, 1999) except for a subpopulation of inhibitory neurons (C.V., C.G. & M.M., unpublished data). The preferential expression of the high-affinity receptors at the neuromuscular junction might underlie the high efficiency of BoNT/A and BoNT/B intoxication at motor neuron terminals. Interestingly, BoNT/G, which binds preferentially to Syt-I, was shown to be far less efficient than BoNT/B in phrenic nerve intoxication, although high concentrations of recombinant BoNT/G were used in the study (Rummel et al, 2004a). Varying sensitivity to BoNTs might also result from specific patterns of polysialogangliosides and other lipids, expressed by selected neurons. It is therefore possible that high-affinity receptors for BoNTs might be localized specifically to those neurons that represent the in vivo cellular target of each BoNT serotype. The predominant expression of the low-affinity protein receptors (Syt-I, SV2A and SV2B) in the brain might explain the findings that BoNTs are less potent in hippocampal neurons than the CNS-acting tetanus neurotoxin, which penetrates through SV recycling (Matteoli et al, 1996; Schiavo et al, 2000; Verderio et al, 1999; C.V., C.G. & M.M., unpublished data).
The mechanisms of toxin penetration in brain neurons deserve thorough investigation, owing to the promising beneficial effect of BoNTs in CNS pathologies and in the treatment of pain (Montecucco & Molgo, 2005). Direct microinjection of BoNTs into the rat CNS can be used to inhibit neurotransmission reversibly and to control inflammatory pain centrally (Luvisetto et al, 2003, 2006). Another promising application of BoNTs is in the treatment of drug-resistant epilepsies. Indeed, the delivery of BoNT/E to the rat hippocampus markedly reduces both focal and generalized kainic-acid-induced seizures, supporting its possible applications as an anti-ictal and anti-epileptogenic agent (Costantin et al, 2005). Although a synaptic protein receptor for BoNT/E has not yet been identified, the possibility of an activity-dependent internalization in the CNS could provide the basis for a preferential penetration of the toxin in the hyperactive neurons of the epileptic foci. These neurons would indeed expose more receptors because of their high rate of SV exo/endocytosis.
Further aspects to be considered in relation to possible differential sensitivity of distinct nerve terminals to BoNTs are the expression and accessibility of toxin substrates (Table 2). For example, it is known that γ-aminobutyric acid (GABA)ergic terminals are less sensitive to BoNT/A and BoNT/E than glutamatergic terminals (Ashton & Dolly, 1988; Bigalke et al, 1981; Verderio et al, 2004). The molecular basis for this differential sensitivity is still undefined. However, the finding that mature hippocampal inhibitory terminals express undetectable levels of SNAP-25 (Verderio et al, 2004), together with the observation that inhibitory synapses express BoNT/A receptors that allow toxin penetration (C.V., C.G. & M.M., unpublished data) suggest that the relative resistance of GABAergic terminals to BoNT/A and BoNT/E could result from one of two scenarios: a BoNT/A-resistant SNAP, such as SNAP-23, SNAP-47 or SNAP-29 (Galli et al, 1998; Holt et al, 2006), might be involved in GABA exocytosis; or low amounts of SNAP-25 at inhibitory synapses might be segregated in such a way as to be inaccessible to the BoNT L chain. Irrespective of the molecular mechanism involved, the preferential action of BoNT/A and BoNT/E at excitatory terminals could be the basis for the use of these serotypes to treat pathologies that result from imbalances between excitatory and inhibitory terminals (Costantin et al, 2005). A similar concept could be extended to other BoNT substrates. For example, synaptobrevin/VAMP1, which, at least in the rat, is resistant to BoNT/B, has a selective distribution in the brain and is restricted to nuclei involved in modulating somatomotor functions (Raptis et al, 2005; Trimble et al, 1990). The distribution of different syntaxin isoforms in the brain still needs to be thoroughly investigated, particularly in relation to their sensitivity to BoNT/C. Once a more detailed picture of the distribution of toxin receptors and substrates is achieved, it will be possible to use different BoNTs for the therapy of neurological disorders caused by the unbalanced activity of selected neuronal populations.
Table 2.
The distribution of synaptosomal-associated protein isoforms at the neuromuscular junction and in different neuronal populations in the central nervous system
| SNAP isoform expression at the neuromuscular junction | SNAP isoform expression in the hippocampus/cortex excitatory neurons | SNAP isoform expression in the hippocampus/cortex inhibitory neurons |
|---|---|---|
| SNAP-25 (Washbourne et al, 2002) | SNAP-25 (mainly Vglut-1; F Conti, L Bragina, G Fattorini, S Giovedi, F Benfenati, C Candiracci, unpublished data) | SNAP-25 (largely undetectable; Verderio et al, 2004; Frassoni et al, 2005; F Conti, L Bragina, G Fattorini, S Giovedi, F Benfenati, C Candiracci, unpublished data, but see Tafoya et al, 2006) |
| SNAP-23 (to be defined) | SNAP-23 (mainly Vglut-2; F Conti, L Bragina, G Fattorini, S Giovedi, F Benfenati, C Candiracci, unpublished data) | SNAP-23 (subpopulation only; F Conti, L Bragina, G Fattorini, S Giovedi, F Benfenati, C Candiracci, unpublished data) |
| SNAP-47 (to be defined) | SNAP-47 (Holt et al, 2006) | SNAP-47 (to be defined) |
| SNAP-29 (to be defined) | SNAP-29 (Pan et al, 2005) | SNAP-29 (to be defined) |
SNAP, synaptosomal-associated protein.
Acknowledgments
We thank M. Francolini and F. Inverardi (Milano) for technical help, and M. Caleo and Y. Bozzi (Pisa) for critical reading of the manuscript. This study was supported by Telethon GGP04196, the European Community project EUSynapse, Ministero della Istruzione, Università e Ricerca FIRB–RBNE01RHZM and FIRB–RBNE01RHZMFIR. We gratefully acknowledge T. Sudhof for the gift of polyclonal antibodies to SV2C.
References
- Ashton AC, Dolly JO (1988) Characterization of the inhibitory action of botulinum neurotoxin type A on the release of several transmitters from rat cerebrocortical synaptosomes. J Neurochem 50: 1808–1816 [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH (1992) SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257: 1271–1273 [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH (1994) Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 14: 5223–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton F, Iborra C, Boudier JA, Seagar MJ, Marqueze B (1997) Developmental regulation of synaptotagmin I, II, III, and IV mRNAs in the rat CNS. J Neurosci 17: 1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke H, Ahnert-Hilger G, Habermann E (1981) Tetanus toxin and botulinum A toxin inhibit acetylcholine release from but not calcium uptake into brain tissue. Naunyn Schmiedebergs Arch Pharmacol 316: 143–148 [DOI] [PubMed] [Google Scholar]
- Buckley K, Kelly RB (1985) Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 100: 1284–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullens RW, O'Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Plomp JJ, Willison HJ (2002) Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci 22: 6876–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L et al. (2005) Antiepileptic effects of botulinum neurotoxin E. J Neurosci 25: 1943–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER (2003) Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol 162: 1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER (2006) SV2 is the protein receptor for botulinum neurotoxin A. Science 312: 592–596 [DOI] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D (1998) A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell 9: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Archer BT, Sudhof TC (1991) Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem 266: 13548–13552 [PubMed] [Google Scholar]
- Hansson HA, Holmgren J, Svennerholm L (1977) Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci USA 74: 3782–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, Jahn R (2006) Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem 281: 17076–17083 [DOI] [PubMed] [Google Scholar]
- Iezzi M, Theander S, Janz R, Loze C, Wollheim CB (2005) SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci 118: 5647–5660 [DOI] [PubMed] [Google Scholar]
- Janz R, Sudhof TC (1999) SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94: 1279–1290 [DOI] [PubMed] [Google Scholar]
- Juzans P, Molgo J, Faille L, Angaut-Petit D (1996) Synaptotagmin II immunoreactivity in normal and botulinum type-A treated mouse motor nerve terminals. Pflugers Arch 431: R283–R284 [DOI] [PubMed] [Google Scholar]
- Kitamura M, Igimi S, Furukawa K (2005) Different response of the knockout mice lacking β-series gangliosides against botulinum and tetanus toxins. Biochim Biophys Acta 1741: 1–3 [DOI] [PubMed] [Google Scholar]
- Li JY, Jahn R, Dahlstrom A (1994) Synaptotagmin I is present mainly in autonomic and sensory neurons of the rat peripheral nervous system. Neuroscience 63: 837–850 [DOI] [PubMed] [Google Scholar]
- Li L, Singh BR (1998) Isolation of synaptotagmin as a receptor for types A and E botulinum neurotoxin and analysis of their comparative binding using a new microtiter plate assay. J Nat Toxins 7: 215–226 [PubMed] [Google Scholar]
- Luvisetto S, Rossetto O, Montecucco C, Pavone F (2003) Toxicity of botulinum neurotoxins in central nervous system of mice. Toxicon 41: 475–481 [DOI] [PubMed] [Google Scholar]
- Luvisetto S, Marinelli S, Lucchetti F, Marchi F, Cobianchi S, Rossetto O, Montecucco C, Pavone F (2006) Botulinum neurotoxins and formalin-induced pain: central vs. peripheral effects in mice. Brain Res 1082: 124–131 [DOI] [PubMed] [Google Scholar]
- Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T (2006) The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett 580: 2011–2014 [DOI] [PubMed] [Google Scholar]
- Marqueze B, Boudier JA, Mizuta M, Inagaki N, Seino S, Seagar M (1995) Cellular localization of synaptotagmin I, II, and III mRNAs in the central nervous system and pituitary and adrenal glands of the rat. J Neurosci 15: 4906–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Verderio C, Rossetto O, Iezzi N, Coco S, Schiavo G, Montecucco C (1996) Synaptic vesicle endocytosis mediates the entry of tetanus neurotoxin into hippocampal neurons. Proc Natl Acad Sci USA 93: 13310–13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C (1986) How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci 11: 314–317 [Google Scholar]
- Montecucco C, Molgo J (2005) Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol 5: 274–279 [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rossetto O, Schiavo G (2004) Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol 12: 442–446 [DOI] [PubMed] [Google Scholar]
- Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S (1994) Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem 269: 10498–10503 [PubMed] [Google Scholar]
- Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sekiguchi M, Takahashi M, Kozaki S (1996) Binding of botulinum type B neurotoxin to Chinese hamster ovary cells transfected with rat synaptotagmin II cDNA. Neurosci Lett 208: 105–108 [DOI] [PubMed] [Google Scholar]
- Pan PY, Cai Q, Lin L, Lu PH, Duan S, Sheng ZH (2005) SNAP-29-mediated modulation of synaptic transmission in cultured hippocampal neurons. J Biol Chem 280: 25769–25779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela-Gomes GM, Lukinius A, Grimelius L (2000) Synaptic vesicle protein 2. A new neuroendocrine cell marker. Am J Pathol 157: 1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis A, Torrejon-Escribano B, Gomez de Aranda I, Blasi J (2005) Distribution of synaptobrevin/VAMP 1 and 2 in rat brain. J Chem Neuroanat 30: 201–211 [DOI] [PubMed] [Google Scholar]
- Rummel A, Karnath T, Henke T, Bigalke H, Binz T (2004) Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J Biol Chem 279: 30865–30870 [DOI] [PubMed] [Google Scholar]
- Rummel A, Mahrhold S, Bigalke H, Binz T (2004) The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol Microbiol 51: 631–643 [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C (2000) Neurotoxins affecting neuroexocytosis. Physiol Rev 80: 717–766 [DOI] [PubMed] [Google Scholar]
- Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM (2005) SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci 29: 56–64 [DOI] [PubMed] [Google Scholar]
- Simpson LL (2000) Identification of the characteristics that underlie botulinum toxin potency: implications for designing novel drugs. Biochimie 82: 943–953 [DOI] [PubMed] [Google Scholar]
- Tafoya LCR, Mameli M, Miyashita T, Guzowski JF, Valenzuela F, Wilson MC (2006) Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci 26: 7826–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble WS, Gray TS, Elferink LA, Wilson MC, Scheller RH (1990) Distinct patterns of expression of two VAMP genes within the rat brain. J Neurosci 10: 1380–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM (2002) Lipid rafts in neuronal signaling and function. Trends Neurosci 25: 412–417 [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Kohda T, Mukamoto M, Takeuchi K, Ihara H, Saito M, Kozaki S (2005) Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J Biol Chem 280: 35164–35171 [DOI] [PubMed] [Google Scholar]
- Verderio C, Coco S, Rossetto O, Montecucco C, Matteoli M (1999) Internalization and proteolytic action of botulinum toxins in CNS neurons and astrocytes. J Neurochem 73: 372–379 [DOI] [PubMed] [Google Scholar]
- Verderio C et al. (2004) SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41: 599–610 [DOI] [PubMed] [Google Scholar]
- Washbourne P et al. (2002) Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: 19–2611753414 [Google Scholar]
- Yowler BC, Kensinger RD, Schengrund CL (2002) Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem 277: 32815–32819 [DOI] [PubMed] [Google Scholar]


