Abstract
Background
We highlight a chronic inflammatory disease we call 'hyper-IgG4 disease', which has many synonyms depending on the organ involved, the country of origin and the year of the report. It is characterized histologically by a lymphoplasmacytic inflammation with IgG4-positive cells and exuberant fibrosis, which leaves dense fibrosis on resolution. A typical example is idiopathic retroperitoneal fibrosis, but the initial report in 2001 was of sclerosing pancreatitis.
Methods
We report an index case with fever and severe systemic disease. We have also reviewed the histology of 11 further patients with idiopathic retroperitoneal fibrosis for evidence of IgG4-expressing plasma cells, and examined a wide range of other inflammatory conditions and fibrotic diseases as organ-specific controls. We have reviewed the published literature for disease associations with idiopathic, systemic fibrosing conditions and the synonyms: pseudotumour, myofibroblastic tumour, plasma cell granuloma, systemic fibrosis, xanthofibrogranulomatosis, and multifocal fibrosclerosis.
Results
Histology from all 12 patients showed, to varying degrees, fibrosis, intense inflammatory cell infiltration with lymphocytes, plasma cells, scattered neutrophils, and sometimes eosinophilic aggregates, with venulitis and obliterative arteritis. The majority of lymphocytes were T cells that expressed CD8 and CD4, with scattered B-cell-rich small lymphoid follicles. In all cases, there was a significant increase in IgG4-positive plasma cells compared with controls. In two cases, biopsies before and after steroid treatment were available, and only scattered plasma cells were seen after treatment, none of them expressing IgG4. Review of the literature shows that although pathology commonly appears confined to one organ, patients can have systemic symptoms and fever. In the active period, there is an acute phase response with a high serum concentration of IgG, and during this phase, there is a rapid clinical response to glucocorticoid steroid treatment.
Conclusion
We believe that hyper-IgG4 disease is an important condition to recognise, as the diagnosis can be readily verified and the outcome with treatment is very good.
Background
While investigating a patient who had an uncharacterised multisystem disease with evidence of a severe acute-phase response, we found that similar rare cases had been described, usually as single reports [1], under an alarming list of synonyms [2] (Table 1). These conditions are all characterised by chronic inflammation leading to dense fibrosis, and retroperitoneal fibrosis (RPF) is a typical example. The principal synonyms that we found for these fibrosing conditions are: pseudotumour, myofibroblastic tumour, plasma cell granuloma, systemic fibrosis, xanthofibrogranulomatosis, and multifocal fibrosclerosis (Table 1). As can be seen in Tables 1 and 2, these conditions, such as RPF, Reidel's thyroiditis and sclerosing pancreatitis, are usually localised to one or two organs but can present with systemic multisystem disease.
Table 1.
Systemic fibrosis synonyms
| Pseudotumour, inflammatory pseudotumour, fibrous pseudotumour | Systemic[83-85] | Lung[86-89], liver[87,90-93], breast[94], pancreas[83,95-99], orbit[100], RPF[98-101], mesentery[102] |
| Inflammatory myofibroblastic tumour, myofibroblastoma | Systemic | Pancreas[96], lung[46,103,104], brain[105], breast[106] |
| Plasma cell granuloma | Systemic | lung[107,108], brain[107] |
| Systemic fibrosis, generalized form of Ormond's disease, systemic idiopathic fibrosis, idiopathic systemic sclerosing disease | Systemic[1,109-111] | PRF[109,111-113], orbit[112], lung[114,115] |
| Xanthofibrogranulomatosis, xanthogranuloma | Systemic[1,2,116,117] | RPF[101,118-121], brain[118,122], orbit[123] |
| Multifocal fibrosclerosis, multifocal idiopathic fibrosclerosis | Systemic[84,85,124-128] | RPF[124,125,128-133], orbit[124,129-131,134] thyroid[127,128,132,133], pancreas[9] |
RPF, retroperitoneal fibrosis.
Table 2.
Conditions associated with systemic fibrosis
| Name | Synonyms | |
| RPF | Ormond's disease | Systemic[109,111,113,135], lung[32,32,53,115,136,136,137], liver[138], breast, pancreas[5,139-141], |
| Retro-orbital tumour | Fibrous pseudotumour of the orbit, Graves' orbitopathy | RPF[124,129-131,142-145] |
| Riedel's thyroiditis | RPF[132,133,146-148] MFF[127,128,147,149,150] | |
| Chronic sclerosing sialadenitis[151] | Kuttner's tumour | |
| Panniculitis | Weber-Christian syndrome steatonecrosis, necrosing panniculitis | Systemic[110], RPF[135,152-155] Biliary cirrhosis[156], pancreas[157] |
| Sclerosing pancreatitis[158] | Primary inflammatory pancreatitis (P)[159], lymphoplasmacytic sclerosing P[7,160,161], autoimmune P[162], sclerosing pancreaticocholangitis[163], pancreatic pseudo-tumour[95] | RPF[5,97,139,161,164,165], cholangitis[95,160,164,166,167], systemic[83,168-171] gastric ulcer[172], lung[173,174] panniculitis[157], mesentery[175] |
| Sclerosing cholangitis | MFF[128], pancreas[92,166] | |
| Bronchiolitis obliterans with organizing pneumonia | Cryptogenic organizing pneumonia, pulmonary hyalinizing granuloma. | Systemic[50,176], pancreas[173,174], liver[177], RPF[115,136,137], renal[46,136], mediastinum[178,179] |
| Benign pleural mesothelioma[180,181], calcifying pseudotumour[182,183] | Asbestos-related? | RPF[11,26-28,32,32] peritoneum[56,184,185] pleura[54,55,186] |
RPF, retroperitoneal fibrosis; MFF, multifocal fibrosclerosis
In another patient, we observed the rare behaviour of idiopathic RPF behaving like a tumour, in which the inflammatory mass was invading both the kidney and liver and presented as a progressive cholangitis. This case was one of a series of patients seen in our gastroenterological department, who had sclerosing pancreatitis and chronic inflammation associated with immunoglobulin (Ig)G4-expressing plasma cells [3,4]. In biopsies from both these cases, we found typical fibrosis with lymphoplasmacytic inflammation and IgG4-bearing plasma cells [5].
Sclerosing (autoimmune) pancreatitis is a unique form of pancreatitis, characterized by hypergammaglobulinaemia and a lymphoplasmacytic inflammation of the pancreas that responds to glucocorticoid treatment. It has several synonyms (Table 2). A Japanese group investigating this condition [6] found that the sera of their patients had a polyclonal band in the rapidly migrating fraction of gammaglobulins, which was caused by a high concentration of the IgG4 gammaglobulin fraction. They also reported that serum concentrations of IgG4 were significantly and specifically raised in patients with sclerosing pancreatitis, and were closely associated with disease activity [6].
The same Japanese group found that of the 22 patients with sclerosing pancreatitis they studied, three also had concomitant hydronephrosis caused by a periureteric mass, diagnosed as RPF (Ormond's disease). Histological examination of the periureteric tissue showed abundant infiltration of IgG4-bearing plasma cells. Treatment with corticosteroids lowered serum concentrations of IgG4, and the authors proposed that IgG4 might have a pathological role in a systemic fibrosing process that includes pancreatic and retroperitoneal lesions [5].
Kamisawa et al have also published several reports emphasising the systemic nature of this process. Patients with autoimmune pancreatitis may also have involvement of lymph nodes and salivary glands as well as local tissues [7,8], in a pattern suggestive of multifocal fibrosclerosis [9,10].
We describe our index case, and report on the histological features of 11 other patients seen at our hospital with idiopathic RPF, for whom we have now been able to review the histology and examine sections for evidence of IgG4-expressing plasma cells. We also list the synonyms and disease associations that we believe are all part of the same disease process.
Methods
Histological examination
We report on the histological features of 16 biopsy specimens from 12 patients seen at the Middlesex hospital with a primary diagnosis of RPF, for whom we have now been able to review the histology and examine sections for evidence of IgG4-expressing plasma cells. Only archival paraffin wax-embedded material was used for this study. All the biopsies were taken for diagnostic reasons with the informed consent of patients.
All the samples were routinely fixed in formalin and embedded in paraffin wax. Dewaxed sections (μm) were stained with haematoxylin and eosin. Immunohistochemical analysis was performed using antibodies (all Dako UK Ltd, Cambridgeshire, UK) against CD3, CD4, CD8, CD20, and CD138, and the kappa and lambda light chains of immunoglobulins, by the standard streptavidin-biotin-peroxidase method. For IgG4, a mouse human IgG4 monoclonal antibody was used (1:100 dilution, MC011; The Binding Site, Birmingham, UK). Negative controls were created by substituting the primary antibody with similarly diluted non-immunized mouse serum.
We examined a wide variety of other inflammatory conditions and fibrotic diseases as organ-specific controls, which included liver (autoimmune hepatitis, chronic hepatitis C), pancreas (chronic and alcoholic pancreatitis), salivary gland (chronic sialadenitis), gallbladder (chronic cholecystitis), kidney (tubulointerstitial nephritis), duodenum (chronic duodenitis), colon (non-specific colitis), and other fibrotic processes (Dupuytren's, keloid scars). Because an association between RPF and asbestos exposure has been reported [11], we also included cases of malignant mesotheliomas. In addition, specimens from cases of "benign pleural plaques" were stained as controls.
Positive controls comprised tissue obtained from pancreas, salivary gland and liver from patients seen in our gastroenterological department who had sclerosing pancreatitis and chronic inflammation associated with IgG4-expressing plasma cells, details of which have been reported previously [3,4].
The degree of inflammation was assessed as severe [>100 inflammatory cells per high power field (), moderate (30–100 cells/HPF) and mild (30–10 cells/HPF). The presence of lymphoid-follicle formation, eosinophilic infiltration, venulitis and obliterative arteritis (on elastic van Gieson-stained sections) was also recorded. The number of IgG4-positive cells was estimated by counting 10 HPF, and scoring as follows: 0 (no IgG4 plasma cells/HPF), 1 (≤ 20 cells/HPF), 2 (20–50 cells/HPF) or 3 (≥ 50 cells/HPF) [12]. Fibrosis was assessed as - (no fibrosis), + (mild), ++ (moderate) or +++ (severe).
Literature review
We reviewed the published literature for disease associations with idiopathic, systemic fibrosing conditions using the following synonyms: pseudotumour, myofibroblastic tumour, plasma cell granuloma, systemic fibrosis, xanthofibrogranulomatosis and multifocal fibrosclerosis. We also looked for reports associating these conditions with organ-specific conditions, particularly sclerosing pancreatitis (see Table 2 for other synonyms), RPF, Reidel's thyroiditis, panniculitis, Weber-Christian syndrome and retro-orbital tumour, and with a range of autoimmune diseases (Table 3).
Table 3.
Rare associations with IgG4-related conditions (Case reports)
| Head and neck | |
| Submandibular gland fibrosis | Pancreas[95], cholangitis[187,188], pseudotumour[189] |
| Parotid involvement | pancreas[171] |
| Sjögren's syndrome | Pancreas[171,190], RPF[164], BOOP[191] |
| Maculopathy | MFF[126] |
| Uveitis | RPF[192] |
| Conjunctiva | Pseudotumour[86] |
| Hashimoto's disease, Graves' disease | RPF[193] |
| Cardiovascular | |
| Constrictive pericarditis | RPF[194] |
| Heart valves | RPF[20,22] |
| Mediastinal fibrosis | RPF[194-196] |
| Chronic periaortitis/aneurysm | RPF[197-205] |
| Vasculitis | RPF[125,206,207] |
| Occlusive phlebitis | MFF[149] |
| Intermittent claudication | RPF[208] |
| Rheumatology | |
| Rheumatoid arthritis | BOOP[176], RPF[209] |
| Polymyalgia rheumatica | BOOP[210] |
| Ankylosing spondylitis | RPF[211] |
| Periarticular fibrosis | RPF[212] |
| Skeletal hyperostosis | RPF[213] |
| Dermatomyositis | Pseudotumour[88], BOOP[214] |
| Polymyositis | BOOP[191,215] |
| Scleroderma | RPF[216] |
| Other autoimmune conditions | |
| Systemic lupus erythematosus | RPF[217] |
| Idiopathic thrombocytopenic purpura | RPF[218,219] |
| Haemolytic anaemia | Lung[220], cholangitis[221] |
| Central nervous system | |
| Brain | PCG[107], xanthogranuloma[118,222] |
| Sella | MFF[124,126] |
| Spinal cord compression | RPF[223] |
| Gastrointestinal | |
| Primary biliary cirrhosis | RPF[224], BOOP[176] |
| Mesentery | RPF[112,152,175,225], pseudotumour[102,153,155] |
| Bladder/pelvic mass | RPF[49,208,226,227] |
| Duodenal obstruction | RPF[228,229] |
| Portal hypertension | RPF[230] |
| Others | |
| Familial multifocal fibrosclerosis Familial RPF | MFF[43] RPF[42] |
| Proliferative crescentic glomerulonephritis, membranous glomerulonephritis | RPF[231] RPF[232] |
| Interstitial nephritis | Pancreas[233] |
| Testicular involvement | RPF[126,234] |
MFF, multifocal fibrosclerosis; RPF, retroperitoneal fibrosis; BOOP, bronchiolitis obliterans with organizing pneumonia; PCG, plasma-cell granuloma.
Many of these reports are case reports. We have not attempted to list every report but rather to draw up a comprehensive list of all possible associations with at least one citation.
Results
Index case
A 39-year-old Moroccan steel fitter had lived in the UK for 31 years. He presented in May 2003 with abdominal pain, weight loss, sweating, generalized lymphadenopathy, and severe anaemia and thrombocytosis. Investigations showed haemoglobin of 6.7 g/dL (normal range 13.0–17.0 g/dL), platelets 1031 × 109/L (150–400 × 109/L), erythrocyte sedimentation rate (ESR) 120 mm/h (0–15 mm/h), IgG 21.4 g/L (8.0–18.0 g/L), IgA 2.9 g/L (0.9–4.5 g/L), IgM 0.6 g/L (0.6–2.8 g/L) and C-reactive protein (CRP) 289 mg/L (normal 0–5 mg/L). Biochemistry was normal. Chest X-ray showed apical pleural thickening. An intravenous urogram (IVU) showed left hydronephrosis; an ultrasound scan (USS) confirmed this, and showed dilatation of the bile and pancreatic ducts and the gallbladder. Computed tomography (CT) showed multifocal upper zone and patchy nodular airspace shadowing, with right posterior pleural thickening and subcarinal lymphadenopathy. Biopsies of pleural, ileal and colonic tissue, bone marrow, and left inguinal lymph node were all either normal or showed reactive change. Cultures of blood, urine and sputum were all negative. The patient was also negative for human immunodeficiency virus types 1 and 2, hepatitis B and C, human T-lymphotropic virus, toxoplasmosis and treponemal disease. Mantoux test was weakly positive, and although bronchoalveolar lavage was smear-negative, there was one positive culture for a mycobacterium after 3 weeks.
The patient was commenced on quadruple therapy for tuberculosis (TB) in July 2003, for an organism that was strongly isoniazid-resistant. The patient presented again in November with fatigue, weight loss and fever, which was thought to be medication-related. He was given prednisolone 20 mg for 6 weeks of, taken off all TB therapy, and after 2 months was feeling so well that he was told his TB had been cured.
He re-presented 3 months later (March 2004) with a 6-week history of night sweats, low-grade fever, lethargy, weight loss, left pleuritic chest pain and axillary lymphadenopathy. Blood tests again showed microcytic anaemia, and the same acute phase response. Antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies, mitochondrial antibodies and smooth-muscle antibodies were all negative. CT showed many of the previous changes but with a right hydronephrosis that was confirmed on IVU (left hydronephrosis was no longer present). A repeat bone-marrow biopsy, inguinal lymph node and transjugular liver biopsies, endoscopy, colonoscopy, peritoneal fluid aspiration and bone scan were all either normal or non-specific. The patient was treated with quintuple anti-TB therapy in order to cover his original mycobacterium infection as well as an infection with Mycobacterium fortuitum that had been cultured from sputum on this occasion. He was also treated with steroids, initially 60 mg of prednisolone, tapered down to 20 mg over 3 months.
The patient was readmitted 6 months later with generalized abdominal pain and vomiting. He had stopped his steroids 11 days earlier because of side effects. Investigations showed only a marked inflammatory response. CT scan revealed no hydronephrosis, but now showed dilated, thick-walled small bowel in the left iliac fossa. During an exploratory laparotomy (October 2004), an inflamed omental mass adherent to the small-bowel mesentery was removed. Histology was reported as showing only some fibrosis with a mild chronic inflammatory cell infiltrate of lymphocytes and plasma cells. The patient was recommenced on steroids and quadruple anti-TB therapy.
He remained well for some months, but then presented (March 2005) with a 6-week history of pain in the right upper quadrant of the abdomen. His blood tests showed the same inflammatory response and a USS showed prominent intrahepatic ducts only. The histology of the omental mass was reviewed and showed a large number of IgG4-positve plasma cells throughout the fibroinflammatory reaction (Table 4). The patient's serum IgG concentration was 21.4 g/l and IgG4 2.4 (normal range 0–1.3 g/l). A diagnosis of hyper-IgG4 disease was made, all microbial therapy was stopped, and the patient was treated with prednisolone 20 mg/day with immediate improvement in his illness. He remains on azathioprine and 5 mg prednisolone.
Table 4.
Histopathology
| Patient no., sex, age | Organ | Fibrosis* | Lymphoplasmacytic infiltrate† | Lymphoid follicles | Venulitis | Eosinophilic component | IgG4-positive plasma cells‡ |
| (1) Male, 52 | Kidney | +++ | +++ | Yes | No | No | 3 |
| Liver | ++ | + | No | No | Yes | 0 | |
| (2) Male, 73 | Retroperitoneum | +++ | +++ | Yes | Yes | Yes | 3 |
| (3) Male, 39 | Omentum | + | ++ | Yes | No | No | 3 |
| (4) Male, 53 | Retroperitoneum | +++ | +++ | Yes | Yes | Yes | 3 |
| (5) Male, 45 | Periureter | +++ | +++ | Yes | Yes | No | 2 |
| (6) Female, 58 | Retroperitoneum | +++ | +++ | No | Yes | No | 3 |
| (7) Female, 55 | Para-aortic tissue | +++ | +++ | Yes | Yes | No | 3 |
| (8) Male, 47 | Periureter tissue | +++ | + | Yes | Yes | No | 2 |
| (9) Male, 48 | Retroperitoneum | +++ | ++ | No | No | No | 2 |
| (10) Male, 73 | Retroperitoneum | +++ | +++ | Yes | Yes | Yes | 1 |
| (11) Female, 56 | Retroperitoneum | +++ | ++ | Yes | No | No | 1 |
| (12) Male, 53 | Liver | ++ | + | No | No | No | 1 |
| Colon | ++ | + | No | No | No | 0 |
*Degree of interstitial fibrosis: +, mild; ++, moderate; +++, severe fibrosis.
†Degree of inflammation: +++ (severe), >100 inflammatory cells/HPF; ++ (moderate), 30–100 cells/HPF; + (mild), 10–30 cells/HPF
‡IgG4-positive plasma cells: 0, no IgG4 plasma cells/high power field (HPF); 1, ≤ 20 cells/HPF), 2, 20–50 cells/HPF; 3, ≥ 50 cells/HPF (criteria of Aoki et al [12]).
Pathology of IgG4 disease
We reviewed the histology and examined sections for evidence of IgG4-expressing plasma cells of 16 biopsies from 12 patients with a clinical diagnosis of idiopathic RPF seen at our hospital in the past 10 years. Biopsies comprised nine samples of retroperitoneal tissue, two of liver, and one each from kidney, colon and omentum. In addition, there were two further samples of retroperitoneal tissue taken from two patients after steroid therapy.
The results are shown in Table 4. All patients showed, to varying degrees, fibrosis, intense inflammatory cell infiltration with lymphocytes, plasma cells, scattered neutrophils and in some cases eosinophilic aggregates. The majority of lymphocytes expressed CD8 and CD4 positivity (T-cell markers), with B cells present to a lesser degree and with scattered B-cell-rich small lymphoid follicles. In addition, we saw vasculitis affecting small veins, and evidence of obliterative arteritis (Figures 1, 2, 3). In all cases, there was a significant increase in IgG4-positive plasma cells compared with controls (Figure 4). Even when few plasma cells were seen (patients 10–12) the majority expressed IgG4 (Figure 5). In two cases, biopsies before and after steroid treatment were available; only scattered plasma cells were seen after treatment, none of which expressed IgG4 (Fig 3). In all of our patients, the kappa and lambda light chains showed a polytypic pattern. We used PCR to look for evidence of oligoclonal infiltrates for IgH in one case (patient 1), as has been reported from Japan [13], but found none. None of the wide range of control sections showed significantly increased numbers of IgG4-positive cells.
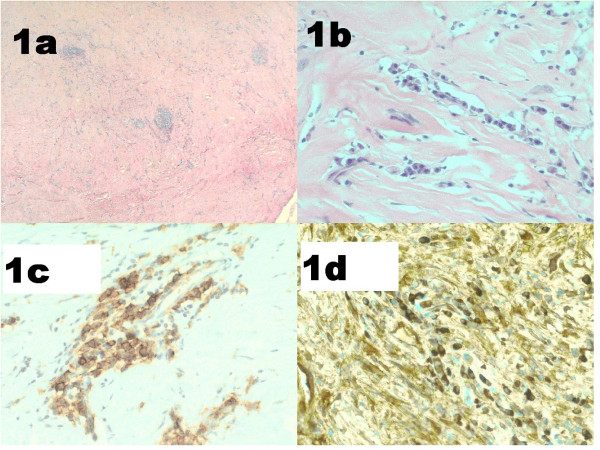
Figure 1.
Mesenteric mass. Patient 3 (index case): (a, b) fibrosis, lymphoid aggregates and plasma cells (haematoxylin and eosin (a) × 100, (b) ×400); (c) CD138-positive plasma cells; (d) IgG4-positive plasma cells.
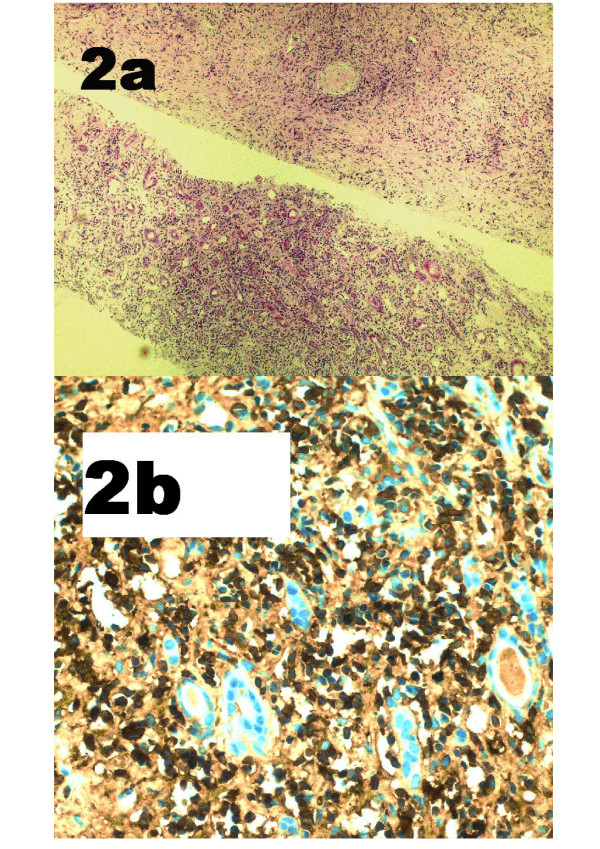
Figure 2.
Renal biopsy. Patient 1, showing renal involvement. (a) Cores of renal tissue with a conspicuous component of inter- and intra-tubular inflammatory cells (haematoxylin and eosin ×100); (b) IgG4-positive plasma cells.
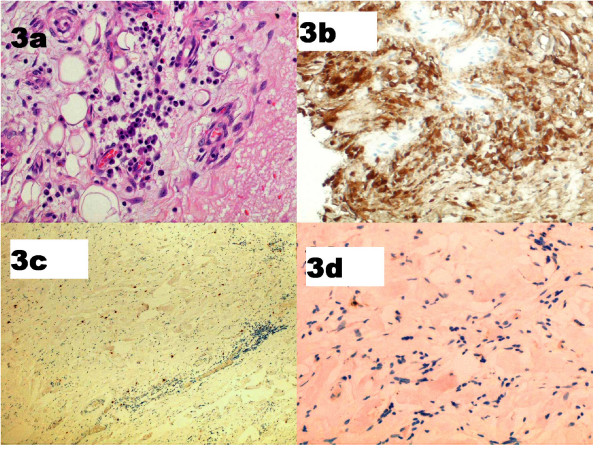
Figure 3.
Retroperitoneal tissue. Patient 7. Pre-glucocorticoid treatment: (a) fibrosis and plasma cells (haematoxylin and eosin ×400); (b) IgG4-positive plasma cells. Post-glucocorticoid treatment: (c) scattered CD138-positive plasma cells; (d) IgG4 staining reveals no positive cells.
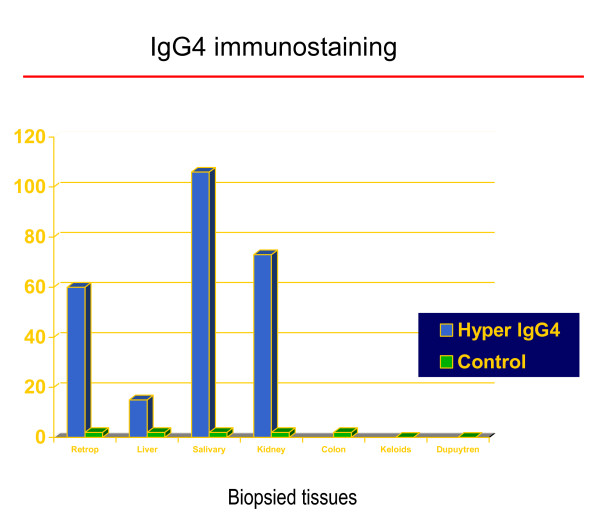
Figure 4.
Histopathology: IgG4 immunostaining (cases versus controls). Vertical axis (0–120) shows number of IgG4-positive plasma cells/high power field (see Methods for details). Histological data from salivary gland is positive control, (from previously reported study [3,4]).
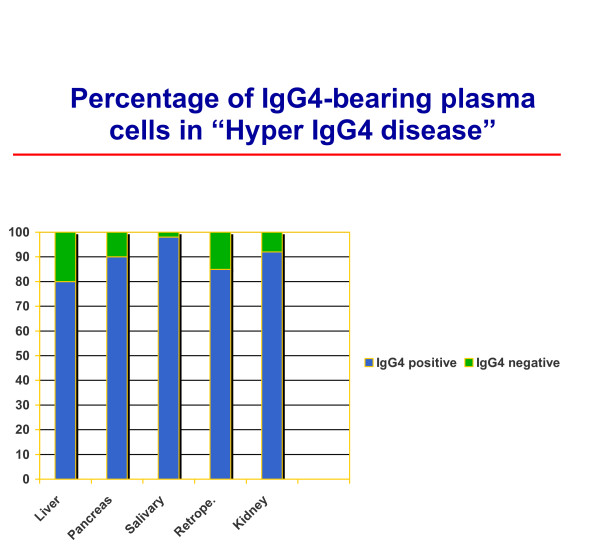
Figure 5.
Percentage of IgG4-bearing plasma cells.
We also examined biopsy specimens from liver (two patients), large bowel (one) and renal tissue (one). In all of them, we found mild fibrosis and a small increase in lymphoplasmacytic inflammatory cell infiltrate, although IgG4-positive plasma cells were not seen in one liver biopsy, nor in the colon biopsy (Table 4).
In summary, raised numbers of IgG4-positive plasma cells are seen in both peritoneal and non-peritoneal tissues in patients with hyper-IgG4 disease. The degree of IgG4-positive staining did not necessarily correlate with elevated serum IgG. Our clinical impression was that the number of IgG4-positive plasma cells was associated with disease activity.
Discussion
RPF is probably the most common example of the conditions associated with raised serum IgG4 and an IgG4-positive plasma cell infiltrate, which we call hyper-IgG4 diseases. The other most frequently recognised associations are sclerosing pancreatitis, Reidel's thyroiditis, retro-orbital tumour, panniculitis, and sclerosing cholangitis (see Table 2 for synonyms).
It is known that RPF can be associated with a similar fibrotic process in other organs, particularly adjacent tissues such as the mediastinum and pleura. Patients with RPF can have systemic symptoms and be febrile. We used RPF and our knowledge of its aetiology and pathogenesis to review the potential mechanisms of IgG4 disease.
Retroperitoneal disease
Idiopathic RPF generally presents in a non-specific manner with malaise, fatigue, fever and weight loss [11,14-16]. Pain in the back (lumbosacral) region, flank or lower abdomen is most common. The pathognomonic feature of RPF is a thick retroperitoneal fibrotic mass covering the abdominal aorta and compressing the ureters. The process of fibrosis can result in obstruction of the ureters and renal failure, or signs and symptoms may be related to the encasement or entrapment of other structures by the inflammatory mass, such as hydrocoeles or unilateral leg oedema. With renal involvement, severe hypertension is common.
The disease presents typically between the ages of 50 and 70 years, and men are affected 2–3 times more commonly than women [14]. The inflammatory nature of idiopathic RPF is demonstrated by elevated ESR and CRP, reduced haemoglobin and elevated gammaglobulins at presentation. An autoimmune nature is suggested by the finding of ANA in 10–20% of cases, an occasional association with different autoimmune diseases and good response to glucocorticosteroids. In one series of RPF associated with periaortitis, 10 of 16 patients were ANA-positive [17].
Although there are no randomised studies of treatment for RPF, patients who present during the acute inflammatory phase respond quickly to glucocorticosteroids. Patients with late presentation and dense fibrosis will not respond to medical treatment and surgery is often necessary[11].
Causes and associations
RPF has many causes, although in about 70% of cases the cause is unknown (idiopathic). The rest (secondary) develop as a result of malignant disease, radiation therapy, abdominal surgery, pancreatitis, retroperitoneal haemorrhage, urine extravasation, and infections. Carcinoid syndrome can cause systemic fibrosis [18], and RPF has been described without metastatic tumour [19].
Drugs
RPF has also been associated with the use of several drugs, but most commonly the ergotamine derivates, especially methysergide [20] and, more recently, those used as dopamine agonists to treat Parkinson's disease, such as pergolide and cabergoline [21].
From an aetiological perspective, drugs associated with RPF are important as they also cause fibrosis in various other sites. Systemic fibrosis is a prominent feature of methysergide use, with involvement of heart and lungs, particularly heart valves [20,22]. A similar distribution of pathology has been reported with the dopamine agonists pergolide and cabergoline [23-25].
Of pathogenetic importance is the observation that withdrawal of these offending drugs usually results in a rapid improvement in symptoms, reduction in inflammatory markers and regression of pathology [20]. Drug-induced cases are not associated with ANA [16].
Asbestos
Recently, asbestos exposure has been proposed as a causal factor for both pleural and retroperitoneal fibrosis [11,26-28]. Asbestos fibres also cause interstitial lung fibrosis (asbestosis), pleural fibrosis, pleural plaques, lung cancer, and pleural and peritoneal mesothelioma [29]. Patients exposed to asbestos, and particularly those with asbestosis, have an increased incidence of ANA and other autoantibodies, and raised levels of IgG and IgA. There is also a correlation between the degree of immunological abnormality and the likelihood of progression [30,31].
A case-control study in Finland of 43 patients with RPF (86% of eligible cases) found that the age-standardised incidence of RPF was 0.10 per 100000 person-years [11]. For every patient, five population-based matched controls were selected. There was a strong association with asbestos exposure. The odds ratio (OR) was 5.5 for <10 fibre-years of asbestos exposure and 8.8 for ≤ 10 fibre-years. Other risk factors were previous use of ergot derivates (OR = 9.92), abdominal aortic aneurysm (OR = 6.73) and smoking for >20 pack-years (OR = 4.73) [11].
We also saw pleural calcification in three of our patients with RPF, in whom it was not present at the presentation of the illness. One of those cases is included in our archival review (patient 5, Table 4). This raises the question of whether some of the pleural pathology associated with IgG4 disease can heal with calcification as well as fibrosis [32].
Aortitis
The relationship of aortic aneurysms with an inflamed and thickened aortic wall, extensive periaortal inflammation and RPF is well known [33]. As the inflammatory aneurysms of the abdominal aorta differ from RPF only in the diameter of the inflamed aorta, it has been suggested that both syndromes represent only variations of the same disease, which has been named 'chronic periaortitis' [34]. RPF has been suggested as the most severe form of chronic periaortitis [35,36]. Vasculitis of the vasa vasorum was thought to be a factor in the aneurysmal dilatation [34,37].
Several observations suggest an immunological pathogenesis for this periaortitis. As well as the periaortal tissue, the media and adventitia of the aorta are infiltrated by polyclonal B lymphocytes, activated CD4-positive T lymphocytes, and plasma cells [35]. In 85% of patients, the necrotic media contains deposits of IgG antibodies in close proximity to extracellular ceroid, and the serum often contains antibodies against ceroid and oxidised low-density lipoprotein [38].
Although it was initially proposed that the aortitis was caused by an autoimmune response to the components of atherosclerotic plaques [38] with antibodies to atheroma, more recent opinion has suggested that in view of the accompanying systemic symptoms and acute phase response, the aortitis is a primary autoimmune disease [17,36,39,40].
Autoimmune and other rare associations
While there is no one predominant association, a large range of autoimmune diseases has been described in association with RPF (Table 3), and 10–20% of cases will have ANA or other autoantibodies.
Paediatric
RPF in the paediatric population is rare with, only 23 cases reported in the English-language literature by 2003 [41]. A family has been reported in which two children had idiopathic retroperitoneal fibrosis, and they and their father had clinical and laboratory evidence of systemic immunological diseases [42].
Familial
Two brothers from a first-cousin consanguineous marriage were reported, who had different combinations of RPF, mediastinal fibrosis, sclerosing cholangitis, Riedel sclerosing thyroiditis, and pseudotumour of the orbit. One of the brothers had fibrotic contracture of the fingers [43]. Other reports are of RPF in three siblings with sickle-cell trait [44], and mediastinal and retroperitoneal fibrosis in two sisters with seronegative spondyloarthropathy [45].
RPF invading adjacent organs
Rarely, the inflammatory tissue seen in RPF can invade the renal parenchyma [46-48] and it can occasionally cause a tumour-like mass away from the retroperitoneum [49]. There are four isolated surgical case reports in the literature, with two being described as idiopathic RPF, one as systemic hyalinizing granuloma and the other as xanthofibrogranuloma. We have seen one case in which the IgG4-positive inflammatory mass (Figure 2) invaded the kidney and liver (patient 1, Table 4), and another in which it caused a large tumour like mass in the left iliac fossa (patient 2). Both cases responded promptly to steroids.
Summary
Although all our archival cases of idiopathic RPF have shown evidence of IgG4 involvement, it remains to be seen whether IgG4 plays any role in other types of RPF such as periaortitis, drug-induced and asbestos-related RPF. We certainly found it (in retroperitoneal tissue) in one of our patients who developed pleural calcification.
Lung disease
Part of the spectrum of disease in patients with hyper-IgG4 disease is parenchymal lung pathology, often described as either bronchiolitis obliterans with organizing pneumonia or cryptogenic organizing pneumonia [50]. This is seen typically as migratory pulmonary infiltrates, and is steroid-responsive. Nevertheless, there are at present no studies that have looked for evidence of IgG4 plasma cells in these conditions [51]. Other conditions associated with migratory pulmonary infiltrates that may be related, but not yet investigated, include pulmonary eosinophilia, hypersensitivity to drugs, parasitic infection, allergic bronchopulmonary aspergillosis and Churg-Strauss vasculitis [52]. Pleural thickening, with and without calcification, is well described [32,32,53]. There are also reports of calcifying pseudo-tumour of the pleura [54,55] and peritoneum [56].
Differential diagnosis of febrile illness
Fever of unknown origin (FUO) in an immunocompetent patient is defined as an illness of more than 3 weeks' duration with fever higher than 38.3°C (101°F) on several occasions, and with an uncertain diagnosis after 1 week of investigation [57]. Infection accounts for about one-third of cases (25–32%) of FUO, followed by non-infectious inflammatory disease (21–31%) and neoplasm (12–17%) [58-62]. Although many patients with FUO will be found to have bacterial infection, rarer diagnoses will be considered with time and extensive investigation. Nevertheless, at the end of all investigations, 18–30% of patients will have no diagnosis, although the majority of this idiopathic group will improve spontaneously [60]. We propose that hyper-IgG4 disease can be added to this list of systemic inflammatory diseases.
Pathogenesis
Conditions associated with elevated IgG4
IgG4 is the rarest of the IgG subclasses to be expressed, accounting for only 3–6% of total IgG in normal serum. It has a low affinity for target antigen. It is unique among the IgG subclasses, as it is unable to bind Clq complement and cannot activate the classic complement pathway [63]. IgG4 is a T-helper 2 (Th2)-dependent isotype. Interleukin (IL)-4 directs naive human B cells to switch to IgG4 and IgE production [64]; IL-10 and IL-13 are the other T-cell-derived cytokines that activate this differentiation.
High serum IgG4 concentrations are found in a limited number of other conditions, including atopic dermatitis [65], some parasitic diseases (helminthic diseases) [66], and pemphigus vulgaris (PV) and pemphigus foliaceus (PF)[67,68].
In allergic individuals, a feature of successful immunotherapy is the induction of allergen-specific IgG4 antibodies, even with the persistence of allergen-specific IgE [69,70]. High levels of the immunosuppressive cytokine IL-10 are found both in patients chronically infected with helminths and in those receiving allergen immunotherapy [71]. IL-10 also stimulates IgG4 production [72].
Th2 responses to allergens are suppressed by both CD4+ CD25+ T regulatory cells (Tregs) and IL-10-producing Tregs [73], and suppression by these subsets is decreased in allergic individuals. In animal models, Tregs can be induced by high- or low-dose inhaled antigen, and prior induction of Tregs prevents subsequent development of allergen sensitization and airway inflammation in inhaled challenge models. Allergen-injection immunotherapy may induce IL-10 Tregs, leading to both suppression of Th2 responses and a switch from IgE to IgG4 antibody production [73].
Helminths
Asymptomatic infections with helminth parasites are correlated with high levels of IgG4, and parasite-specific IgG4 antibodies can inhibit IgE-mediated degranulation of mast cells [66]. In most helminth infections, the Th2 response leads to rapid parasite expulsion or sequestration. During murine Schistosoma mansoni infection, however, parasites persist and the chronic Th2 response can induce severe pathological fibrosis in the gut and liver [74]. Evidence both from these mouse models and from human studies suggests that this progression to severe disease with intense fibrosis occurs in only a minority of cases, and is a consequence of the strongly profibrotic type 2 cytokine response mediated by IL-4 and IL-13 [74].
Pemphigus and pemphigoid
PV and PF are autoimmune skin diseases caused by autoantibodies against desmoglein 3 and desmoglein 1 respectively. These antibodies are predominantly of the IgG4 subclass [67,68]. Desmogleins are keratinocyte transmembrane proteins localized in the desmosome, and are members of the adhesion molecule family of cadherins. The interaction of antidesmoglein antibodies with their target antigens leads to loss of cell adhesion (acantholysis) and the formation of intraepithelial blisters of the skin and mucous membranes. The antibodies predominantly, but not exclusively, bind to the extracellular domains of the proteins and exert their pathogenic effect not through inhibition of the proteins' adhesive properties, but through an indirect pathway involving cellular signalling pathways, resulting in destabilization of desmoglein 1-based adhesive sites and desmosomes [75].
Bullous pemphigoid (BP) is an autoimmune blistering disease associated with autoantibodies against the hemidesmosomal glycoprotein BP180. The noncollagenous (NC)16A domain of BP180 has major antigenic sites recognized by BP sera. IgG4 and IgE are the major immunoglobulins that preferentially react with two distinct epitopes within this domain. Levels of these autoantibodies correlated with disease activity in BP [76].
Autoimmune pancreatitis
In sclerosing pancreatitis, evidence had been reported to suggest an immunological pathogenesis even before the description of a role for IgG4. In one study of 17 patients, ANA and antilactoferrin antibodies were detected in 76%, carbonic anhydrase II antibody in 59%, rheumatoid factor in 29% and anti-smooth-muscle antibody in 18% of patients. CD8+ and CD4+ cells were significantly increased in peripheral blood. CD4+ cells produced increased levels of interferon gamma compared with controls, but IL-4 was not increased [77]. However, in another Japanese study, which divided patients with sclerosing pancreatitis into a seronegative and seropositive group, found no differences in presentation, pathology or natural history between the groups [78].
HLA associations with IgG4-associated diseases
• PV was consistently found to be associated with DR4 and DR14 and, more precisely, with DRB1*0402 and DRB1*1401 subtypes [79]. Susceptibility to PF has been correlated with the presence of DR4, DR14 and DR1 alleles; however, in contrast to PV, no single DR4 or DR14 allele was shown to be associated with the disease.
• In an immunogenetic study of patients with autoimmune pancreatitis, a significant increase in DR4 was found. It was concluded that the DRB1*0405-DQB1*0401 haplotype was associated with autoimmune pancreatitis in the Japanese population [80].
• Aortoarteritis is a chronic inflammatory disease, mainly affecting the aorta and its major branches. In a Chinese Han population, the DRB1*04 and DRB1*07 alleles were significantly associated with aortoarteritis. Furthermore, there was no significant difference in the frequency of the DRB1*0405 subtype between the patient and control groups [81].
• In China, the HLA-DRB5*0101 allele and the IL-13 promoter A/A genotype were both found to be elevated in schistosomal hepatic fibrosis, although the two genes are located on different chromosomes. Subjects with both genotypes had odds ratios much higher (OR = 24.5) than the sum of the ratios for each individual genotype. The study strongly suggested that a pathogenic Th2 response directly influenced the prognosis of post-schistosomal liver fibrosis[82].
Conclusion
We believe that hyper-IgG4 disease is an important condition to recognise as the diagnosis can be verified by histology and simple blood tests, and the outcome with treatment during the acute phase is very good. A raised IgG concentration in the absence of hypergammaglobulinaemia is typical, but ideally IgG4 should also be measured. Even when pathology appears localised, as with RPF, constitutional symptoms can be present with evidence of an acute phase response. Rarely a systemic form of the disease will present with severe constitutional symptoms, and hyper-IgG4 disease can be added to list of unusual causes of FUO. Although the role and relevance of IgG4 is unknown, the dense fibrosis that accompanies healing would appear to be a typical example of Th2-mediated pathology.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
GH Neild was involved with clinical management of the patients and was responsible for the writing of the paper. Manuel Rodriguez-Justo reviewed and examined all the histological material and helped to write the paper. Catherine Wall and John O Connolly were both involved with the clinical management of the index case and several of the patients with RPF, and helped to write the paper.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We thank Ms P Munson for performing IgG4 immunostaining. We thank Drs Robin Woolfson and Helen Lachmann for reading the manuscript and making valuable contributions to its preparation. We would like to thank our colleagues Dr FD Thompson, Dr MA Mansell, Dr RG Woolfson and Dr BR Leaker for their help with the management of patients. Written consent was obtained from the patient for publication of the case history.
Contributor Information
Guy H Neild, Email: g.neild@ucl.ac.uk.
Manuel Rodriguez-Justo, Email: m.rodriguez-justo@ucl.ac.uk.
Catherine Wall, Email: Catherine.Wall@amnch.ie.
John O Connolly, Email: j.connolly@ucl.ac.uk.
References
- Streuli R, Egger C, Truniger B. Systemic fibrosis (generalized xanthofibrogranulomatosis) Nephrol Dial Transplant. 1997;12:608–609. doi: 10.1093/ndt/12.3.608. [DOI] [PubMed] [Google Scholar]
- Kastendieck H, Husselmann H. [Xanthofibrogranulomatosis. Classification, localization, morphology, pathogenesis] (author's translation) Virchows Arch A Pathol Anat Histol. 1978;380:237–259. doi: 10.1007/BF00430461. [DOI] [PubMed] [Google Scholar]
- Webster GJ, Wittman J, Seward E, Pereira SP, Hatfield AR. Autoimmune pancreatitis/choliopancreatopathy – evidence of multisystem involvement [abstract 048] Gut. 2006;54:a1–117. [Google Scholar]
- Deheragoda MG, Church NI, Rodriguez-Justo M, Seward E, Novelli MR, Pereira S, Hatfield ARW, Webster GJM. IgG4 immunostaining in autoimmune pancreatitis and in extra-pancreatic disease [abstract] Pancreatology. 2006;6:193. [Google Scholar]
- Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004;28:1114. doi: 10.1097/01.pas.0000126634.43301.45. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6:132–137. doi: 10.1159/000090033. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisawa T. IgG4-related sclerosing disease. Intern Med. 2006;45:125–126. doi: 10.2169/internalmedicine.45.0137. [DOI] [PubMed] [Google Scholar]
- Uibu T, Oksa P, Auvinen A, Honkanen E, Metsarinne K, Saha H, Uitti J, Roto P. Asbestos exposure as a risk factor for retroperitoneal fibrosis. Lancet. 2004;363:1422–1426. doi: 10.1016/S0140-6736(04)16100-X. [DOI] [PubMed] [Google Scholar]
- Aoki S, Nakazawa T, Ohara H, Sano H, Nakao H, Joh T, Murase T, Eimoto T, Itoh M. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients' sera. Histopathology. 2005;47:147–158. doi: 10.1111/j.1365-2559.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- Oshiro H, Ebihara Y, Serizawa H, Shimizu T, Teshima S, Kuroda M, Kudo M. Idiopathic retroperitoneal fibrosis associated with immunohematological abnormalities. Am J Med. 2005;118:782–786. doi: 10.1016/j.amjmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Baker LR, Mallinson WJ, Gregory MC, Menzies EA, Cattell WR, Whitfield HN, Hendry WF, Wickham JE, Joekes AM. Idiopathic retroperitoneal fibrosis. A retrospective analysis of 60 cases. Br J Urol. 1987;60:497–503. doi: 10.1111/j.1464-410x.1987.tb05028.x. [DOI] [PubMed] [Google Scholar]
- Yaqoob MM, Junaid I. The patient with urinary tract obstruction. In: Davison AM, Cameron JS, Grunfeld J-P, Ponticelli C, Ritz E, Winearls CG, et al, editor. Oxford Textbook of Clinical Nephrology. Oxford: Oxford University Press; 2005. pp. 2465–2470. [Google Scholar]
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002;60:231–242. [PubMed] [Google Scholar]
- Vaglio A, Corradi D, Manenti L, Ferretti S, Garini G, Buzio C. Evidence of autoimmunity in chronic periaortitis: a prospective study. Am J Med. 2003;114:454–462. doi: 10.1016/s0002-9343(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: an association with no explanation. Am J Gastroenterol. 2004;99:2466–2478. doi: 10.1111/j.1572-0241.2004.40507.x. [DOI] [PubMed] [Google Scholar]
- Chander S, Ergun EL, Chugani HT, Chugani DC, Juhasz C, Shields AF, Weaver DW. High 2-deoxy-2-[18F]fluoro-D-glucose accumulation in a case of retroperitoneal fibrosis following resection of carcinoid tumor. Mol Imaging Biol. 2002;4:363–368. doi: 10.1016/s1536-1632(02)00021-5. [DOI] [PubMed] [Google Scholar]
- Graham JR, Suby HI, LeCompte PR, Sadowsky NL. Fibrotic disorders associated with methysergide therapy for headache. N Engl J Med. 1966;274:359–368. doi: 10.1056/NEJM196602172740701. [DOI] [PubMed] [Google Scholar]
- Shaunak S, Wilkins A, Pilling JB, Dick DJ. Pericardial, retroperitoneal, and pleural fibrosis induced by pergolide. J Neurol Neurosurg Psychiatry. 1999;66:79–81. doi: 10.1136/jnnp.66.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JR. Cardiac and pulmonary fibrosis during methysergide therapy for headache. Am J Med Sci. 1967;254:1–12. doi: 10.1097/00000441-196707000-00001. [DOI] [PubMed] [Google Scholar]
- Horvath J, Fross RD, Kleiner-Fisman G, Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski H, Pache JC, Burkhard PR, Lang AE. Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov Disord. 2004;19:656–662. doi: 10.1002/mds.20201. [DOI] [PubMed] [Google Scholar]
- Bleumink GS, Molen-Eijgenraam M, Strijbos JH, Sanwikarja S, van Puijenbroek EP, Stricker BH. Pergolide-induced pleuropulmonary fibrosis. Clin Neuropharmacol. 2002;25:290–293. doi: 10.1097/00002826-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE. Valvular heart disease in patients taking pergolide. Mayo Clin Proc. 2002;77:1280–1286. doi: 10.4065/77.12.1280. [DOI] [PubMed] [Google Scholar]
- Maguire GP, Meggs LG, Addonizio J, Del Guercio LR. Association of asbestos exposure, retroperitoneal fibrosis, and acute renal failure. N Y State J Med. 1991;91:357–359. [PubMed] [Google Scholar]
- Boulard JC, Hanslik T, Doleris LM, Prinseau J, Baglin A. Asbestos and idiopathic retroperitoneal fibrosis. Lancet. 1995;345:1379. doi: 10.1016/s0140-6736(95)92584-8. [DOI] [PubMed] [Google Scholar]
- Sauni R, Oksa P, Jarvenpaa R, Parker JE, Roto P. Asbestos exposure: a potential cause of retroperitoneal fibrosis. Am J Ind Med. 1998;33:418–421. doi: 10.1002/(sici)1097-0274(199804)33:4<418::aid-ajim13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Manning CB, Vallyathan V, Mossman BT. Diseases caused by asbestos: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2:191–200. doi: 10.1016/s1567-5769(01)00172-2. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Suthar AM, Patel MM, Karnik AB, Dave SK, Kashyap SK, Venkaiah K. Humoral immunological profile of workers exposed to asbestos in asbestos mines. Indian J Med Res. 1993;98:274–277. [PubMed] [Google Scholar]
- Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ Health Perspect. 2005;113:25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Myou S, Ooka T, Fujimura M, Matsuda T. A case of pleural involvement followed by idiopathic retroperitoneal fibrosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:876–880. [PubMed] [Google Scholar]
- Walker DI, Bloor K, Williams G, Gillie I. Inflammatory aneurysms of the abdominal aorta. Br J Surg. 1972;59:609–614. doi: 10.1002/bjs.1800590807. [DOI] [PubMed] [Google Scholar]
- Mitchinson MJ. Chronic periaortitis and periarteritis. Histopathology. 1984;8:589–600. doi: 10.1111/j.1365-2559.1984.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Parums DV, Dunn DC, Dixon AK, Mitchinson MJ. Characterization of inflammatory cells in a patient with chronic periaortitis. Am J Cardiovasc Pathol. 1990;3:121–129. [PubMed] [Google Scholar]
- Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367:241–251. doi: 10.1016/S0140-6736(06)68035-5. [DOI] [PubMed] [Google Scholar]
- Lindell OI, Sariola HV, Lehtonen TA. The occurrence of vasculitis in perianeurysmal fibrosis. J Urol. 1987;138:727–729. doi: 10.1016/s0022-5347(17)43353-2. [DOI] [PubMed] [Google Scholar]
- Parums DV, Brown DL, Mitchinson MJ. Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaortitis. Arch Pathol Lab Med. 1990;114:383–387. [PubMed] [Google Scholar]
- Vaglio A, Buzio C. Chronic periaortitis: a spectrum of diseases. Curr Opin Rheumatol. 2005;17:34–40. doi: 10.1097/01.bor.0000145517.83972.40. [DOI] [PubMed] [Google Scholar]
- Moroni G, Del Papa N, Moronetti LM, Vitali C, Maglione W, Comina DP, Urgnani F, Sandri S, Ponticelli C, Cortelezzi A. Increased levels of circulating endothelial cells in chronic periaortitis as a marker of active disease. Kidney Int. 2005;68:562–568. doi: 10.1111/j.1523-1755.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- Miller OF, Smith LJ, Ferrara EX, McAleer IM, Kaplan GW. Presentation of idiopathic retroperitoneal fibrosis in the pediatric population. J Pediatr Surg. 2003;38:1685–1688. doi: 10.1016/s0022-3468(03)00590-6. [DOI] [PubMed] [Google Scholar]
- Doolin EJ, Goldstein H, Kessler B, Vinocur C, Marchildon MB. Familial retroperitoneal fibrosis. J Pediatr Surg. 1987;22:1092–1094. doi: 10.1016/s0022-3468(87)80715-7. [DOI] [PubMed] [Google Scholar]
- Comings DE, Skubi KB, Van Eyes J, Motulsky AG. Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel's thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967;66:884–892. doi: 10.7326/0003-4819-66-5-884. [DOI] [PubMed] [Google Scholar]
- Phills JA, Geggie P, Hidvegi RI, Oliva LA. Retroperitoneal fibrosis in three siblings with the sickle cell trait. Can Med Assoc J. 1973;108:1025–1029. [PMC free article] [PubMed] [Google Scholar]
- Goldbach P, Mohsenifar Z, Salick AI. Familial mediastinal fibrosis associated with seronegative spondylarthropathy. Arthritis Rheum. 1983;26:221–225. doi: 10.1002/art.1780260218. [DOI] [PubMed] [Google Scholar]
- Chalaoui J, Gregoire P, Sylvestre J, Lefebvre R, Amyot R. Pulmonary hyalinizing granuloma: a cause of pulmonary nodules. Radiology. 1984;152:23–26. doi: 10.1148/radiology.152.1.6203137. [DOI] [PubMed] [Google Scholar]
- Kell HH, Schumann HJ. Retroperitoneal xanthofibrogranuloma simulating kidney tumor. Zentralbl Chir. 1973;98:968–971. [PubMed] [Google Scholar]
- Kohler HP, Laeng RH, Egger C, Streuli R. Systemic fibrosis (generalized form of Ormond's disease). Report of a case which achieved complete remission with cyclophosphamide and corticosteroids. Schweiz Med Wochenschr. 1995;125:2131–2136. [PubMed] [Google Scholar]
- Takashima T, Onoda N, Ishikawa T, Koyama T, Inaba M, Nishizawa Y, Nakatani T, Wakasa K, Hirakawa K. Tumor-forming idiopathic retroperitoneal fibrosis: report of a case. Surg Today. 2004;34:374–378. doi: 10.1007/s00595-003-2695-z. [DOI] [PubMed] [Google Scholar]
- Duvic C, Desrame J, Leveque C, Nedelec G. Retroperitoneal fibrosis, sclerosing pancreatitis and bronchiolitis obliterans with organizing pneumonia. Nephrol Dial Transplant. 2004;19:2397–2399. doi: 10.1093/ndt/gfh050. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kawabe T, Komatsu Y, Matsubara S, Togawa O, Arizumi T, Yamamoto N, Nakai Y, Sasahira N, Tsujino T, Toda N, Isayama H, Tada M, Omata M. High-rate pulmonary involvement in autoimmune pancreatitis. Intern Med J. 2006;36:58–61. doi: 10.1111/j.1445-5994.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- King TE., Jr BOOP: an important cause of migratory pulmonary infiltrates? Eur Respir J. 1995;8:193–195. doi: 10.1183/09031936.95.08020193. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Furuichi S, Takahashi N, Koya Y, Akashiba T, Horie T. [A case of intrathoracic extension of retroperitoneal fibrosis] Nihon Kokyuki Gakkai Zasshi. 2001;39:748–752. [PubMed] [Google Scholar]
- Ammar A, El Hammami S, Horchani H, Sellami N, Kilani T. Calcifying fibrous pseudotumor of the pleura: a rare location. Ann Thorac Surg. 2003;76:2081–2082. doi: 10.1016/s0003-4975(03)00741-0. [DOI] [PubMed] [Google Scholar]
- Cavazza A, Gelli MC, Agostini L, Sgarbi G, De Marco L, Gardini G. Calcified pseudotumor of the pleura: description of a case. Pathologica. 2002;94:201–205. doi: 10.1007/s102420200032. [DOI] [PubMed] [Google Scholar]
- Kocova L, Michal M, Sulc M, Zamecnik M. Calcifying fibrous pseudotumour of visceral peritoneum. Histopathology. 1997;31:182–184. doi: 10.1046/j.1365-2559.1997.5860817.x. [DOI] [PubMed] [Google Scholar]
- Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- de Kleijn EM, Vandenbroucke JP, van der Meer JW. Fever of unknown origin (FUO). I A. prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine (Baltimore) 1997;76:392–400. doi: 10.1097/00005792-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Knockaert DC, Vanneste LJ, Bobbaers HJ. Fever of unknown origin in elderly patients. J Am Geriatr Soc. 1993;41:1187–1192. doi: 10.1111/j.1532-5415.1993.tb07301.x. [DOI] [PubMed] [Google Scholar]
- Mourad O, Palda V, Detsky AS. A comprehensive evidence-based approach to fever of unknown origin. Arch Intern Med. 2003;163:545–551. doi: 10.1001/archinte.163.5.545. [DOI] [PubMed] [Google Scholar]
- Zhiyong Z, Bingjun L, Xiaoju L, Xinjian F, Ping F, Wenya W. Fever of unknown origin: a report from China of 208 cases. Int J Clin Pract. 2003;57:592–596. [PubMed] [Google Scholar]
- Bleeker-Rovers CP, de Kleijn EM, Corstens FH, van der Meer JW, Oyen WJ. Clinical value of FDG PET in patients with fever of unknown origin and patients suspected of focal infection or inflammation. Eur J Nucl Med Mol Imaging. 2004;31:29–37. doi: 10.1007/s00259-003-1338-3. [DOI] [PubMed] [Google Scholar]
- Van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]
- Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal MR, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731–2737. [PubMed] [Google Scholar]
- Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–49. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, Klotz F, Ebner H, Kraft D, Scheiner O. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–1015. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]
- Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam TR, Reiman RM, Wynn TA. Exploiting worm and allergy models to understand Th2 cytokine biology. Curr Opin Allergy Clin Immunol. 2005;5:392–398. doi: 10.1097/01.all.0000182542.30100.6f. [DOI] [PubMed] [Google Scholar]
- Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42:577–583. [PubMed] [Google Scholar]
- Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kawaura Y, Satomura Y, Urabe T, Watanabe H, Motoo Y, Sawabu N. Duct-narrowing chronic pancreatitis without immunoserologic abnormality: comparison with duct-narrowing chronic pancreatitis with positive serological evidence and its clinical management. Dig Dis Sci. 2005;50:1414–1421. doi: 10.1007/s10620-005-2855-7. [DOI] [PubMed] [Google Scholar]
- Tron F, Gilbert D, Mouquet H, Joly P, Drouot L, Makni S, Masmoudi H, Charron D, Zitouni M, Loiseau P, Ben Ayed M. Genetic factors in pemphigus. J Autoimmun. 2005;24:319–328. doi: 10.1016/j.jaut.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, Hasebe O, Kiyosawa K. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- Dang A, Wang B, Zhang Y, Zhang P, Huang J, Liu G, Zheng D, Qiu C, Liu L. Association of the HLA-DRB1 gene with susceptibility to aortoarteritis in a Chinese Han population. Hypertens Res. 2002;25:631–634. doi: 10.1291/hypres.25.631. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Immunogenetic analysis of post-schistosomal liver fibrosis. Parasitol Int. 2004;53:193–196. doi: 10.1016/j.parint.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Voss SD, Kruskal JB, Kane RA. Chronic inflammatory pseudotumor arising in the hepatobiliary-pancreatic system: progressive multisystemic organ involvement in four patients. AJR Am J Roentgenol. 1999;173:1049–1054. doi: 10.2214/ajr.173.4.10511176. [DOI] [PubMed] [Google Scholar]
- van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Ann Intern Med. 2004;141:896–897. doi: 10.7326/0003-4819-141-11-200412070-00033. [DOI] [PubMed] [Google Scholar]
- Clark A, Zeman RK, Choyke PL, White EM, Burrell MI, Grant EG, Jaffe MH. Pancreatic pseudotumors associated with multifocal idiopathic fibrosclerosis. Gastrointest Radiol. 1988;13:30–32. doi: 10.1007/BF01889019. [DOI] [PubMed] [Google Scholar]
- Goto Y, Ohaki Y, Ibaraki N. A clinicopathologic case report of inflammatory pseudotumors involving the conjunctiva and lung. Jpn J Ophthalmol. 2004;48:573–577. doi: 10.1007/s10384-004-0117-4. [DOI] [PubMed] [Google Scholar]
- Hosler GA, Steinberg DM, Sheth S, Hamper UM, Erozan YS, Ali SZ. Inflammatory pseudotumor: a diagnostic dilemma in cytopathology. Diagn Cytopathol. 2004;31:267–270. doi: 10.1002/dc.20113. [DOI] [PubMed] [Google Scholar]
- Alam M, Morehead RS, Weinstein MH. Dermatomyositis as a presentation of pulmonary inflammatory pseudotumor (myofibroblastic tumor) Chest. 2000;117:1793–1795. doi: 10.1378/chest.117.6.1793. [DOI] [PubMed] [Google Scholar]
- Tuncozgur B, Ustunsoy H, Bakir K, Ucak R, Elbeyli L. Inflammatory pseudotumor of the lung. Thorac Cardiovasc Surg. 2000;48:112–113. doi: 10.1055/s-2000-9865. [DOI] [PubMed] [Google Scholar]
- Uetsuji S, Nakagawa A, Kwon AH, Komada H, Imamura A, Kamiyama Y. Inflammatory pseudotumor of the liver: report of a case and review of the literature. Surg Today. 1996;26:517–521. doi: 10.1007/BF00311559. [DOI] [PubMed] [Google Scholar]
- Sato Y, Harada K, Nakanuma Y. Hepatic inflammatory pseudotumour related to autoimmune pancreatitis. Histopathology. 2004;45:418–419. doi: 10.1111/j.1365-2559.2004.01909.x. [DOI] [PubMed] [Google Scholar]
- Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- Spivach A, Turoldo A, Pistan V, Colautti I, Zanconati F. Inflammatory pseudotumour of the liver: case report and review of the literature. Chir Ital. 2005;57:229–237. [PubMed] [Google Scholar]
- Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, Nakanuma Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005;29:275–278. doi: 10.1097/01.pas.0000147399.10639.f5. [DOI] [PubMed] [Google Scholar]
- Toosi MN, Heathcote J. Pancreatic pseudotumor with sclerosing pancreato-cholangitis: is this a systemic disease? Am J Gastroenterol. 2004;99:377–382. doi: 10.1111/j.1572-0241.2004.04075.x. [DOI] [PubMed] [Google Scholar]
- Esposito I, Bergmann F, Penzel R, di Mola FF, Shrikhande S, Buchler MW, Friess H, Otto HF. Oligoclonal T-cell populations in an inflammatory pseudotumor of the pancreas possibly related to autoimmune pancreatitis: an immunohistochemical and molecular analysis. Virchows Arch. 2004;444:119–126. doi: 10.1007/s00428-003-0949-1. [DOI] [PubMed] [Google Scholar]
- Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol. 1995;90:1155–1158. [PubMed] [Google Scholar]
- Zhao MF, Tian Y, Guo KJ, Ma ZG, Liao HH. Common bile duct obstruction due to fibrous pseudotumor of pancreas associated with retroperitoneal fibrosis: a case report. World J Gastroenterol. 2004;10:3078–3079. doi: 10.3748/wjg.v10.i20.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner W, Kocher T, Beglinger C, Harder F, Steinbrich W. Pseudotumor of the pancreatic head associated with idiopathic retroperitoneal fibrosis. Dig Surg. 2001;18:418–421. doi: 10.1159/000050184. [DOI] [PubMed] [Google Scholar]
- Van Hoe L, Oyen R, Gryspeerdt S, Baert AL, Bobbaers H, Baert L. Case report: pseudotumoral pelvic retroperitoneal fibrosis associated with orbital fibrosis. Br J Radiol. 1995;68:421–423. doi: 10.1259/0007-1285-68-808-421. [DOI] [PubMed] [Google Scholar]
- Kondo T, Onitsuka S, Kobayashi H, Ryoji O, Toma H. A case of retroperitoneal pseudotumor (xanthogranuloma) Hinyokika Kiyo. 1998;44:21–24. [PubMed] [Google Scholar]
- Ruiz-Cabello JM, Martinez Salmeron JF, Perez MJ, Salinas C, Rodrigo MM. Inflammatory mesenteric pseudotumor. Rev Esp Enferm Apar Dig. 1988;73:311–314. [PubMed] [Google Scholar]
- Sakurai H, Hasegawa T, Watanabe S, Suzuki K, Asamura H, Tsuchiya R. Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac Surg. 2004;25:155–159. doi: 10.1016/s1010-7940(03)00678-x. [DOI] [PubMed] [Google Scholar]
- Berman M, Georghiou GP, Schonfeld T, Feinmesser M, Horev G, Vidne BA, Saute M. Pulmonary inflammatory myofibroblastic tumor invading the left atrium. Ann Thorac Surg. 2003;76:601–603. doi: 10.1016/s0003-4975(02)05003-8. [DOI] [PubMed] [Google Scholar]
- Jeon YK, Chang KH, Suh YL, Jung HW, Park SH. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol. 2005;64:254–259. doi: 10.1093/jnen/64.3.254. [DOI] [PubMed] [Google Scholar]
- Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005;113:66–69. doi: 10.1111/j.1600-0463.2005.apm1130110.x. [DOI] [PubMed] [Google Scholar]
- Greiner C, Rickert CH, Mollmann FT, Rieger B, Semik M, Heindel W, Wassmann H. Plasma cell granuloma involving the brain and the lung. Acta Neurochir (Wien) 2003;145:1127–1131. doi: 10.1007/s00701-003-0109-z. [DOI] [PubMed] [Google Scholar]
- Urschel JD, Horan TA, Unruh HW. Plasma cell granuloma of the lung. J Thorac Cardiovasc Surg. 1992;104:870–875. [PubMed] [Google Scholar]
- Stewart TW, Jr, Friberg TR. Idiopathic retroperitoneal fibrosis with diffuse involvement: further evidence of systemic idiopathic fibrosis. South Med J. 1984;77:1185–1187. doi: 10.1097/00007611-198409000-00036. [DOI] [PubMed] [Google Scholar]
- Mitchinson MJ. Systemic idiopathic fibrosis and systemic Weber-Christian disease. J Clin Pathol. 1965;18:645–649. doi: 10.1136/jcp.18.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad N, Strongwater SL, Eypper S, Woda BA. Idiopathic retroperitoneal and mediastinal fibrosis mimicking connective tissue disease. Am J Med. 1987;82:363–366. doi: 10.1016/0002-9343(87)90089-1. [DOI] [PubMed] [Google Scholar]
- Binder SC, Deterling RA, Jr, Mahoney SA, Patterson JF, Wolfe HJ. Systemic idiopathic fibrosis. Report of a case of the concomitant occurrence of retractile mesenteritis and retroperitoneal fibrosis. Am J Surg. 1972;124:422–430. doi: 10.1016/0002-9610(72)90057-8. [DOI] [PubMed] [Google Scholar]
- Hershkowitz M, Fogari R, Chandra M. Idiopathic retroperitoneal fibrosis: implications for a systemic disorder. Clin Exp Rheumatol. 1983;1:157–160. [PubMed] [Google Scholar]
- Nakamura Y, Kohzaki S, Suyama N, Yamaguchi T, Kohno S, Hayashi K. Systemic idiopathic fibrosis with inflammatory pulmonary lesions. Br J Radiol. 1997;70:956–958. doi: 10.1259/bjr.70.837.9486075. [DOI] [PubMed] [Google Scholar]
- Kuramochi S, Kawai T, Yakumaru K, Mikata A, Torikata C, Kasuga Y, Fujiwara T. Multiple pulmonary hyalinizing granulomas associated with systemic idiopathic fibrosis. Acta Pathol Jpn. 1991;41:375–382. doi: 10.1111/j.1440-1827.1991.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Kastendieck H. Xanthofibrogranulomatosis – a tumor-like lesion of the intestinal adipose tissue with a tendency towards generalization. Verh Dtsch Ges Pathol. 1978;62:501. [PubMed] [Google Scholar]
- Miyachi S, Kobayashi T, Takahashi T, Saito K, Hashizume Y, Sugita K. An intracranial mass lesion in systemic xanthogranulomatosis: case report. Neurosurgery. 1990;27:822–826. doi: 10.1097/00006123-199011000-00025. [DOI] [PubMed] [Google Scholar]
- Grunow N, Goertchen R. Generalized retroperitoneal xanthofibrogranulomatosis with brain involvement. Pathologe. 1988;9:305–308. [PubMed] [Google Scholar]
- Verswijvel G, Stragier J, Verhelle J, Roskams T, Oyen RH. Bilateral xanthogranulomatous perinephritis without renal parenchymal involvement. Eur Radiol. 2000;10:900–902. doi: 10.1007/s003300051032. [DOI] [PubMed] [Google Scholar]
- Arvis G, de Boisgisson P, Schrameck E. Retroperitoneal xanthogranuloma. Apropos of 4 cases. J Urol Nephrol (Paris) 1975;81:550–557. [PubMed] [Google Scholar]
- Forster R, Ruschoff J, Kleinsorge F. Retroperitoneal xanthofibrogranulomatosis. Rofo. 1989;151:688–691. doi: 10.1055/s-2008-1047268. [DOI] [PubMed] [Google Scholar]
- Dauch WA, Rossberg C, Luttges J. Xanthofibrogranulomatosis with meningocortical involvement. Zentralbl Allg Pathol. 1986;132:33–36. [PubMed] [Google Scholar]
- Malhotra R, Porter RG, Selva D. Adult orbital xanthogranuloma with periosteal infiltration. Br J Ophthalmol. 2003;87:120–121. doi: 10.1136/bjo.87.1.120-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der PR, Nieuwenhuis MG, Mourits MP. Multifocal fibrosclerosis presenting as Grave's orbitopathy. Bilateral exophthalmos associated with retroperitoneal and sellar fibrosis. Graefes Arch Clin Exp Ophthalmol. 1999;237:256–258. doi: 10.1007/s004170050227. [DOI] [PubMed] [Google Scholar]
- Schnitzler L, Verret JL, Caron C, Tuchais E, Soret JY, Salaun Y. [Multifocal fibrosclerosis (retroperitoneal, mediastinal, mesenteric and pelvic involvement) associated with vasculitis and revealed by a livedo: clinical, histopathological and ultrastructural study in a case] (author's translation) Ann Dermatol Venereol. 1978;105:943–956. [PubMed] [Google Scholar]
- Brazier DJ, Sanders MD. Multifocal fibrosclerosis associated with suprasellar and macular lesions. Br J Ophthalmol. 1983;67:292–296. doi: 10.1136/bjo.67.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutuncu NB, Erbas T, Bayraktar M, Gedik O. Multifocal idiopathic fibrosclerosis manifesting with Riedel's thyroiditis. Endocr Pract. 2000;6:447–449. [PubMed] [Google Scholar]
- Laitt RD, Hubscher SG, Buckels JA, Darby S, Elias E. Sclerosing cholangitis associated with multifocal fibrosis: a case report. Gut. 1992;33:1430–1432. doi: 10.1136/gut.33.10.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassero A, Cracco C, Terrone C, Rossetti SR. Coexistence of orbital and retroperitoneal involvement in multifocal fibrosclerosis: case report. Arch Ital Urol Androl. 1998;70:11–14. [PubMed] [Google Scholar]
- Richards AB, Shalka HW, Roberts FJ, Flint A. Pseudotumor of the orbit and retroperitoneal fibrosis. A form of multifocal fibrosclerosis. Arch Ophthalmol. 1980;98:1617–1620. doi: 10.1001/archopht.1980.01020040469015. [DOI] [PubMed] [Google Scholar]
- Levine MR, Kaye L, Mair S, Bates J. Multifocal fibrosclerosis. Report of a case of bilateral idiopathic sclerosing pseudotumor and retroperitoneal fibrosis. Arch Ophthalmol. 1993;111:841–843. doi: 10.1001/archopht.1993.01090060129037. [DOI] [PubMed] [Google Scholar]
- Meijer S, Hoitsma HF, Scholtmeijer R. Idiopathic retroperitoneal fibrosis in multifocal fibrosclerosis. Eur Urol. 1976;2:258–260. doi: 10.1159/000472022. [DOI] [PubMed] [Google Scholar]
- Nielson HK. Multifocal idiopathic fibrosclerosis. Two cases with simultaneous occurrence of retroperitoneal fibrosis and Riedel's thyroiditis. Acta Med Scand. 1980;208:119–123. [PubMed] [Google Scholar]
- Aylward GW, Sullivan TJ, Garner A, Moseley I, Wright JE. Orbital involvement in multifocal fibrosclerosis. Br J Ophthalmol. 1995;79:246–249. doi: 10.1136/bjo.79.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Arnaud M, Bertin P, Treves R, Gotteron RD. Idiopathic retroperitoneal fibrosis with systemic manifestations. J Rheumatol. 1994;21:360–362. [PubMed] [Google Scholar]
- Hashimoto S, Fujii W, Takahashi T, Shiroshita K, Sakurai T, Ueda T, Kawata T. Pulmonary hyalinizing granuloma with hydronephrosis. Intern Med. 2002;41:463–466. doi: 10.2169/internalmedicine.41.463. [DOI] [PubMed] [Google Scholar]
- Dent RG, Godden DJ, Stovin PG, Stark JE. Pulmonary hyalinising granuloma in association with retroperitoneal fibrosis. Thorax. 1983;38:955–956. doi: 10.1136/thx.38.12.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejaco C, Ferenci P, Schober E, Kaserer K, Fugger R, Novacek G, Gangl A. Stenosis of the common bile duct due to Ormond's disease: case report and review of the literature. J Hepatol. 1999;31:156–159. doi: 10.1016/s0168-8278(99)80176-7. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Matsukawa M, Ohkawa M. Autoimmune pancreatitis associated with retroperitoneal fibrosis. JOP. 2005;6:260–263. [PubMed] [Google Scholar]
- Pereira-Lima JC, Kromer MU, Adamek HE, Riemann JF. Cholestatic jaundice due to Ormond's disease (primary retroperitoneal fibrosis) Hepatogastroenterology. 1996;43:992–994. [PubMed] [Google Scholar]
- Cappell MS. Obstructive jaundice due to retroperitoneal fibrosis involving the head of the pancreas. J Clin Gastroenterol. 1994;18:53–56. doi: 10.1097/00004836-199401000-00013. [DOI] [PubMed] [Google Scholar]
- Fruh D, Jaeger W, Kafer O. Orbital involvement in retroperitoneal fibrosis (morbus Ormond) Mod Probl Ophthalmol. 1975;14:651–656. [PubMed] [Google Scholar]
- Lenhard V, Bommer J, Andrassy K, Krampien B, Orth H, Ritz E. Retro-orbital fibrosis in Ormond's disease (author's transl) Dtsch Med Wochenschr. 1974;99:2289–2286. doi: 10.1055/s-0028-1108127. [DOI] [PubMed] [Google Scholar]
- Schonder AA, Clift RC, Brophy JW, Dane LW. Bilateral recurrent orbital inflammation associated with retroperitoneal fibrosclerosis. Br J Ophthalmol. 1985;69:783–787. doi: 10.1136/bjo.69.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reittner P, Riepl T, Goritschnig T, Preidler KW, Koele W, Szolar DH. Bilateral orbital pseudotumour due to Ormond's disease: MR imaging and CT findings. Neuroradiology. 2002;44:272–274. doi: 10.1007/s002340100697. [DOI] [PubMed] [Google Scholar]
- Kruit WH, den Ottolander GJ. Riedel's thyroiditis in a patient with retroperitoneal fibrosis. Neth J Med. 1991;39:17–19. [PubMed] [Google Scholar]
- Miro I, Bakir R, Chanu B, Brocheriou C, Brenot F, Rouffy J. Riedel's thyroiditis and retroperitoneal fibrosis. Apropos of a case of multiple fibrosing disease. Ann Med Interne (Paris) 1984;135:212–216. [PubMed] [Google Scholar]
- Rao CR, Ferguson GC, Kyle VN. Retroperitoneal fibrosis associated with Riedel's struma. Can Med Assoc J. 1973;108:1019–1021. [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Hausman R. Occlusive phlebitis in multifocal fibrosclerosis. Am J Clin Pathol. 1976;65:274–283. doi: 10.1093/ajcp/65.3.274. [DOI] [PubMed] [Google Scholar]
- Wich M, Steegmuller KW, Junginger T, Teifke A. Riedel's thyroiditis and idiopathic multifocal fibrosclerosis. A case report. Chirurg. 1991;62:211–213. [PubMed] [Google Scholar]
- Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, Watanabe K, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Tsuneyama K, Saito K, Haratake J, Takagawa K, Nakanuma Y. Abundant IgG4-Positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Kuttner's Tumor) Am J Surg Pathol. 2005;29:783–791. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- Leger L, Roberti A, Deloche dN, Lemaigre G. Mesenteric panniculitis, a variety of retroperitoneal fibrosclerosis. Presse Med. 1967;75:75–79. [PubMed] [Google Scholar]
- Godquin B, Saout R, Loupias P, Garipuy A, Blanchard J. Inflammatory and necrotic pseudotumor of the mesentery. Classification of mesenteric panniculitis. J Chir (Paris) 1972;104:167–175. [PubMed] [Google Scholar]
- Paraf A, Menager C, Texier J. Mesenteric panniculitis, retroperitoneal lipodistrophy and pancreatic diseases. Apropos of a case. Presse Med. 1968;76:2263–2265. [PubMed] [Google Scholar]
- Decker GA, Gaylis H. Mesenteric panniculitis simulating an abdominal aortic aneurysm. S Afr Med J. 1972;46:750. [PubMed] [Google Scholar]
- Herr W, Lohse AW, Spahn TW, Dienes HP, Trautmann F, Meyer zum Buschenfelde KH, Marker-Hermann E. Nodular nonsuppurative panniculitis in association with primary biliary cirrhosis and Hashimoto's thyroiditis. Z Rheumatol. 1996;55:122–126. [PubMed] [Google Scholar]
- Proenca NG, Frucchi H. Weber-Christian febrile recurrent nodular nonsuppurative panniculitis and panniculitis caused by pancreopathy: differential diagnosis and enzymatic mechanisms. Rev Paul Med. 1978;91:82–83. [PubMed] [Google Scholar]
- Motoo Y, Minamoto T, Watanabe H, Sakai J, Okai T, Sawabu N. Sclerosing pancreatitis showing rapidly progressive changes with recurrent mass formation. Int J Pancreatol. 1997;21:85–90. doi: 10.1007/BF02785924. [DOI] [PubMed] [Google Scholar]
- Sarles H, Sarles J, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas – an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Ito T, Nakano I, Koyanagi S, Miyahara T, Migita Y, Ogoshi K, Sakai H, Matsunaga S, Yasuda O, Sumii T, Nawata H. Autoimmune pancreatitis as a new clinical entity. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42:1458–1468. doi: 10.1023/a:1018862626221. [DOI] [PubMed] [Google Scholar]
- Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreato-cholangitis responsive to steroid therapy. Lancet. 1999;354:43–44. doi: 10.1016/s0140-6736(99)00603-0. [DOI] [PubMed] [Google Scholar]
- Fukui T, Okazaki K, Yoshizawa H, Ohashi S, Tamaki H, Kawasaki K, Matsuura M, Asada M, Nakase H, Nakashima Y, Nishio A, Chiba T. A case of autoimmune pancreatitis associated with sclerosing cholangitis, retroperitoneal fibrosis and Sjogren's syndrome. Pancreatology. 2005;5:86–91. doi: 10.1159/000084494. [DOI] [PubMed] [Google Scholar]
- Fukukura Y, Fujiyoshi F, Nakamura F, Hamada H, Nakajo M. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR Am J Roentgenol. 2003;181:993–995. doi: 10.2214/ajr.181.4.1810993. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Kondo S, Ambo Y, Hirano S, Ohmi M, Okushiba S, Morikawa T, Shimizu M, Katoh H. Primary sclerosing cholangitis associated with autoimmune pancreatitis. Hepatogastroenterology. 2002;49:1221–1224. [PubMed] [Google Scholar]
- Nishino T, Toki F, Oyama H, Oi I, Kobayashi M, Takasaki K, Shiratori K. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005;30:76–82. [PubMed] [Google Scholar]
- Kamisawa T. IgG4-positive plasma cells specifically infiltrate various organs in autoimmune pancreatitis. Pancreas. 2004;29:167–168. doi: 10.1097/00006676-200408000-00014. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- Uchida K, Okazaki K, Konishi Y, Ohana M, Takakuwa H, Hajiro K, Chiba T. Clinical analysis of autoimmune-related pancreatitis. Am J Gastroenterol. 2000;95:2788–2794. doi: 10.1111/j.1572-0241.2000.03187.x. [DOI] [PubMed] [Google Scholar]
- Pickartz T, Pickartz H, Lochs H, Ockenga J. Overlap syndrome of autoimmune pancreatitis and cholangitis associated with secondary Sjogren's syndrome. Eur J Gastroenterol Hepatol. 2004;16:1295–1299. doi: 10.1097/00042737-200412000-00010. [DOI] [PubMed] [Google Scholar]
- Shinji A, Sano K, Hamano H, Unno H, Fukushima M, Nakamura N, Akamatsu T, Kawa S, Kiyosawa K. Autoimmune pancreatitis is closely associated with gastric ulcer presenting with abundant IgG4-bearing plasma cell infiltration. Gastrointest Endosc. 2004;59:506–511. doi: 10.1016/s0016-5107(03)02874-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Ko M, Seko S, Nishida O, Inoue F, Kobayashi H, Saiga T, Okamoto M, Fukuse T. Interstitial pneumonia associated with autoimmune pancreatitis. Gut. 2004;53:770–771. [PMC free article] [PubMed] [Google Scholar]
- Arai K, Kawamura O, Naruse I, Tsunekawa K, Hayashi A, Yonezu M, Oya N, Takagi H, Mori M, Kon Y. A case of chronic pancreatitis with diffuse irregular narrowing of the pancreatic duct complicated by Sjogren's syndrome and interstitial pneumonia. Nippon Shokakibyo Gakkai Zasshi. 2001;98:847–852. [PubMed] [Google Scholar]
- Phillips RH, Carr RA, Preston R, Pereira SP, Wilkinson ML, O'Donnell PJ, Thompson RP. Sclerosing mesenteritis involving the pancreas: two cases of a rare cause of abdominal mass mimicking malignancy. Eur J Gastroenterol Hepatol. 1999;11:1323–1329. [PubMed] [Google Scholar]
- Harada M, Hashimoto O, Kumemura H, Taniguchi E, Shiratsuchi M, Harada R, Sakamoto M, Yoshida H, Fukuda T, Sakisaka S, Sata M. Bronchiolitis obliterans organizing pneumonia in a patient with primary biliary cirrhosis and rheumatoid arthritis treated with prednisolone. Hepatol Res. 2002;23:301. doi: 10.1016/s1386-6346(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sakamoto N, Okada M, Tamechika Y, Ishibashi H. [Interstitial pneumonia observed during acute exacerbation of autoimmune hepatitis] Nippon Shokakibyo Gakkai Zasshi. 2000;97:719–722. [PubMed] [Google Scholar]
- Yousem SA, Hochholzer L. Pulmonary hyalinizing granuloma. Am J Clin Pathol. 1987;87:1–6. doi: 10.1093/ajcp/87.1.1. [DOI] [PubMed] [Google Scholar]
- Gossot D, Fromont G, Galetta D, Debrosse D, Grunenwald D. Pulmonary hyalinising granuloma with mediastinal fibrosis: a rare cause of dysphagia. Ann Chir. 2003;128:622–625. doi: 10.1016/j.anchir.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Burrig KF, Kastendieck H. Ultrastructural observations on the histogenesis of localized fibrous tumours of the pleura (benign mesothelioma) Virchows Arch A Pathol Anat Histopathol. 1984;403:413–424. doi: 10.1007/BF00737290. [DOI] [PubMed] [Google Scholar]
- Burrig KF, Kastendieck H, Husselmann H. Localized fibrous pleural tumor (benign mesothelioma). Clinico-pathological study of 24 cases for classification, morphogenesis and prognosis. Pathologe. 1983;4:120–129. [PubMed] [Google Scholar]
- Lee HY, Chuah KL, Tan PH. Test and teach. Number fifty-three. Diagnosis: Calcifying fibrous pseudotumour. Pathology. 2003;35:166–169. [PubMed] [Google Scholar]
- Dargent JL, Delplace J, Roufosse C, Laget JP, Lespagnard L. Development of a calcifying fibrous pseudotumour within a lesion of Castleman disease, hyaline-vascular subtype. J Clin Pathol. 1999;52:547–549. doi: 10.1136/jcp.52.7.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik M, Boudova L, Sulc M, Michal M. Location of calcifying fibrous pseudotumour: peritoneal predominance. Histopathology. 2005;46:346. doi: 10.1111/j.1365-2559.2004.01996.x. [DOI] [PubMed] [Google Scholar]
- Weynand B, Draguet AP, Bernard P, Marbaix E, Galant C. Calcifying fibrous pseudotumour: first case report in the peritoneum with immunostaining for CD34. Histopathology. 1999;34:86–87. doi: 10.1046/j.1365-2559.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- Peachell M, Mayo J, Kalloger S, Flint J, English J. Calcifying fibrous pseudotumour of the lung. Thorax. 2003;58:1018–1019. doi: 10.1136/thorax.58.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneyama K, Saito K, Ruebner BH, Konishi I, Nakanuma Y, Gershwin ME. Immunological similarities between primary sclerosing cholangitis and chronic sclerosing sialadenitis: report of the overlapping of these two autoimmune diseases. Dig Dis Sci. 2000;45:366–372. doi: 10.1023/a:1005429130150. [DOI] [PubMed] [Google Scholar]
- Sjogren I, Wengle B, Korsgren M. Primary sclerosing cholangitis associated with fibrosis of the submandibular glands and the pancreas. Acta Med Scand. 1979;205:139–141. doi: 10.1111/j.0954-6820.1979.tb06019.x. [DOI] [PubMed] [Google Scholar]
- Monteil RA, Saint-Paul MC, Hofman P, Jehl-Pietri C, Michiels JF, Raspaldo H. Oral inflammatory pseudotumour: immunohistochemical investigation of a case involving the submandibular gland and review of the literature. Oral Oncol. 1997;33:215–219. doi: 10.1016/s0964-1955(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Luttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Kloppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- Imasaki T, Yoshii A, Tanaka S, Ogura T, Ishikawa A, Takahashi T. Polymyositis and Sjogren's syndrome associated with bronchiolitis obliterans organizing pneumonia. Intern Med. 1996;35:231–235. doi: 10.2169/internalmedicine.35.231. [DOI] [PubMed] [Google Scholar]
- Doi M, Uji Y. A case of uveitis associated with idiopathic retroperitoneal fibrosis. Am J Ophthalmol. 1994;117:358–362. doi: 10.1016/s0002-9394(14)73146-0. [DOI] [PubMed] [Google Scholar]
- Armigliato M, Paolini R, Bianchini E, Monesi G, Zamboni S, D'Andrea E. Hashimoto's thyroiditis and Graves' disease associated with retroperitoneal fibrosis. Thyroid. 2002;12:829–831. doi: 10.1089/105072502760339415. [DOI] [PubMed] [Google Scholar]
- Hanley PC, Shub C, Lie JT. Constrictive pericarditis associated with combined idiopathic retroperitoneal and mediastinal fibrosis. Mayo Clin Proc. 1984;59:300–304. doi: 10.1016/s0025-6196(12)61424-4. [DOI] [PubMed] [Google Scholar]
- van Bommel EF, Bouvy ND, Liem E, Boeve ER, Weimar W. Coexistent idiopathic retroperitoneal and mediastinal fibrosis presenting with portal hypertension. Neth J Med. 1994;44:174–177. doi: 10.1016/0300-2977(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Morgan AD, Loughridge LW, Calne RY. Combined mediastinal and retroperitoneal fibrosis. Lancet. 1966;1:67–70. doi: 10.1016/s0140-6736(66)92360-9. [DOI] [PubMed] [Google Scholar]
- Mitchinson MJ, Wight DG, Arno J, Milstein BB. Chronic coronary periarteritis in two patients with chronic periaortitis. J Clin Pathol. 1984;37:32–36. doi: 10.1136/jcp.37.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DS, Larson TS. Idiopathic retroperitoneal fibrosis. J Am Soc Nephrol. 1993;3:1748–1752. doi: 10.1681/ASN.V3111748. [DOI] [PubMed] [Google Scholar]
- Debrand-Passard A, Wilhelm H. Ormond's disease or aortic aneurysm? Case reports. Int Urol Nephrol. 1996;28:295–304. doi: 10.1007/BF02550489. [DOI] [PubMed] [Google Scholar]
- Vaideeswar P, Shenoy AS, Sivaraman A, Deshpande JR, Pandit AA. Idiopathic retroperitoneal fibrosis: a report of 3 cases. J Postgrad Med. 1993;39:95–98. [PubMed] [Google Scholar]
- Remedios D, Coppen M, Bradbeer J, Theodossi A. Chronic periaortitis presenting as common bile duct obstruction. Gut. 1991;32:713–714. doi: 10.1136/gut.32.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdrojewski Z. Retroperitoneal fibrosis and chronic peri-aortitis – new hypothesis. Pol Merkuriusz Lek. 1998;4:50–53. [PubMed] [Google Scholar]
- Turo AJ, Torres LJ, Peiro MF, Cabrera Cabrera JA. Obstructive uropathy caused by retroperitoneal fibrosis secondary to an aneurysm of the abdominal aorta. Actas Urol Esp. 2000;24:764–766. doi: 10.1016/s0210-4806(00)72542-3. [DOI] [PubMed] [Google Scholar]
- Breems DA, Haye H, van der MJ. The role of advanced atherosclerosis in idiopathic retroperitoneal fibrosis. Analysis of nine cases. Neth J Med. 2000;56:38–44. doi: 10.1016/s0300-2977(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Serra RM, Engle JE, Jones RE, Schoolwerth AC. Perianeurysmal retroperitoneal fibrosis. An unusual cause of renal failure. Am J Med. 1980;68:149–153. doi: 10.1016/0002-9343(80)90187-4. [DOI] [PubMed] [Google Scholar]
- Hautekeete ML, Babany G, Marcellin P, Gayno S, Palazzo E, Erlinger S, Benhamou JP. Retroperitoneal fibrosis after surgery for aortic aneurysm in a patient with periarteritis nodosa: successful treatment with corticosteroids. J Intern Med. 1990;228:533–536. doi: 10.1111/j.1365-2796.1990.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Servais A, Launay-Vacher V, Deray G. Retroperitoneal fibrosis due to Wegener's granulomatosis: a misdiagnosis as tuberculosis. Am J Med. 2002;113:164–166. doi: 10.1016/s0002-9343(02)01170-1. [DOI] [PubMed] [Google Scholar]
- Regan F, Tran T, Crofton ME. Case report: idiopathic retroperitoneal fibrosis: an unusual cause of an ovarian mass. Br J Radiol. 1993;66:558–560. doi: 10.1259/0007-1285-66-786-558. [DOI] [PubMed] [Google Scholar]
- Tsai TC, Chang PY, Chen BF, Huang FY, Shih SL. Retroperitoneal fibrosis and juvenile rheumatoid arthritis. Pediatr Nephrol. 1996;10:208–209. doi: 10.1007/BF00862082. [DOI] [PubMed] [Google Scholar]
- Stey C, Truninger K, Marti D, Vogt P, Medici TC. Bronchiolitis obliterans organizing pneumonia associated with polymyalgia rheumatica. Eur Respir J. 1999;13:926–929. doi: 10.1034/j.1399-3003.1999.13d37.x. [DOI] [PubMed] [Google Scholar]
- Bezza A, Maghraoui AE, Ghadouane M, Tabache F, Abouzahir A, Abbar M, Ghafir D, Ohayon V, Archane MI. Idiopathic retroperitoneal fibrosis and ankylosing spondylitis. A new case report. Joint Bone Spine. 2002;69:502–505. doi: 10.1016/s1297-319x(02)00438-4. [DOI] [PubMed] [Google Scholar]
- Ewald EA, Gikas PW, Castor CW. Periarticular fibrosis associated with idiopathic retroperitoneal fibrosis. J Rheumatol. 1988;15:1443–1446. [PubMed] [Google Scholar]
- Sozay S, Bayramoglu M, Karatas M, Ozker R. Diffuse idiopathic skeletal hyperostosis in a patient with idiopathic retroperitoneal fibrosis: a case report. Rheumatol Int. 2002;22:249–252. doi: 10.1007/s00296-002-0252-5. [DOI] [PubMed] [Google Scholar]
- Knoell KA, Hook M, Grice DP, Hendrix JD., Jr Dermatomyositis associated with bronchiolitis obliterans organizing pneumonia (BOOP) J Am Acad Dermatol. 1999;40:328–330. doi: 10.1016/s0190-9622(99)70478-0. [DOI] [PubMed] [Google Scholar]
- Fata F, Rathore R, Schiff C, Herzlich BC. Bronchiolitis obliterans organizing pneumonia as the first manifestation of polymyositis. South Med J. 1997;90:227–230. doi: 10.1097/00007611-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Mansell MA, Watts RW. Retroperitoneal fibrosis and scleroderma. Postgrad Med J. 1980;56:730–733. doi: 10.1136/pgmj.56.660.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Takahira S, Sugahara S, Nakamoto H, Suzuki H. Retroperitoneal fibrosis and systemic lupus erythematosus. Nephrol Dial Transplant. 1999;14:1300–1302. doi: 10.1093/ndt/14.5.1300. [DOI] [PubMed] [Google Scholar]
- Wallach PM, Flannery MT, Adelman HM, PowSang J, Saba H, Altus P, Espinoza LR. Retroperitoneal fibrosis accompanying immune thrombocytopenia. Am J Hematol. 1991;37:204–205. doi: 10.1002/ajh.2830370315. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Funatomi H, Katagiri A, Katayose K, Kitamura K, Seki T, Yamamura F, Aoyagi Y, Nishida H, Mitamura K. A case of autoimmune pancreatitis complicated with immune thrombocytopenia during maintenance therapy with prednisolone. Dig Dis Sci. 2003;48:1968–1971. doi: 10.1023/a:1026170304531. [DOI] [PubMed] [Google Scholar]
- Iwami S, Ryu T, Kasai S, Tokuda H, Saitoh T. Idiopathic interstitial pneumonia with autoimmune hemolytic anemia. Nippon Naika Gakkai Zasshi. 2003;92:1079–1081. doi: 10.2169/naika.92.1079. [DOI] [PubMed] [Google Scholar]
- Pineau BC, Pattee LP, McGuire S, Sekar A, Scully LJ. Unusual presentation of primary sclerosing cholangitis. Can J Gastroenterol. 1997;11:45–48. doi: 10.1155/1997/948191. [DOI] [PubMed] [Google Scholar]
- Briffod M, Berlie J, Clavel B, Hebert H. Retroperitoneal malignant xanthogranuloma (author's transl) Sem Hop. 1982;58:113–119. [PubMed] [Google Scholar]
- de Sa J, Pimentel J, Carvalho M, Evangelista P, Martins P. Spinal cord compression secondary to idiopathic retroperitoneal fibrosis. Neurosurgery. 1990;26:678–681. doi: 10.1097/00006123-199004000-00020. [DOI] [PubMed] [Google Scholar]
- Sevenet F, Capron-Chivrac D, Delcenserie R, Lelarge C, Delamarre J, Capron JP. Idiopathic retroperitoneal fibrosis and primary biliary cirrhosis. A new association? Arch Intern Med. 1985;145:2124–2125. [PubMed] [Google Scholar]
- Rozynek M. Mesenteric lipodystrophy as a variant of Ormond's disease. Patol Pol. 1975;26:525–528. [PubMed] [Google Scholar]
- Verit A, Yeni E, Unal D, Kafali H, Ozturk A, Ozardali I. Idiopathic retroperitoneal fibrosis mimicking a pelvic tumor: a case of pericystitis plastica. Yonsei Med J. 2003;44:548–550. doi: 10.3349/ymj.2003.44.3.548. [DOI] [PubMed] [Google Scholar]
- Haciyanli M, Erkan N, Elverdi B, Avci G, Fuzun M, Dicle O. Retroperitoneal fibrosis mimicking a rectal tumor: report of a case. Dis Colon Rectum. 1998;41:664–666. doi: 10.1007/BF02235280. [DOI] [PubMed] [Google Scholar]
- Yamada H, Komatsu R, Nagae H, Fujioka Y, Fujita M. Idiopathic retroperitoneal fibrosis with duodenal obstruction successfully treated with corticosteroids. Intern Med. 1998;37:592–598. doi: 10.2169/internalmedicine.37.592. [DOI] [PubMed] [Google Scholar]
- Jun BM, Lee EY, Yoon YJ, Kim EK, Ahn MS, Lee CK, Cho YS, Yoo B, Moon HB. Retroperitoneal fibrosis with duodenal stenosis. J Korean Med Sci. 2001;16:371–374. doi: 10.3346/jkms.2001.16.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini S, Reiner M, Bernasconi S, Pedrinis E. Retroperitoneal fibrosis and portal hypertension. Schweiz Med Wochenschr. 1993;123:26–28. [PubMed] [Google Scholar]
- Zabetakis PM, Novich RK, Matarese RA, Michelis MF. Idiopathic retroperitoneal fibrosis: a systemic connective tissue disease? J Urol. 1979;122:100–102. doi: 10.1016/s0022-5347(17)56273-4. [DOI] [PubMed] [Google Scholar]
- Moroni G, Farricciotti A, Cappelletti M, Ponticelli C. Retroperitoneal fibrosis and membranous nephropathy. Improvement of both diseases after treatment with steroids and immunosuppressive agents. Nephrol Dial Transplant. 1999;14:1303–1305. doi: 10.1093/ndt/14.5.1303. [DOI] [PubMed] [Google Scholar]
- Uchiyama-Tanaka Y, Mori Y, Kimura T, Sonomura K, Umemura S, Kishimoto N, Nose A, Tokoro T, Kijima Y, Yamahara H, Nagata T, Masaki H, Umeda Y, Okazaki K, Iwasaka T. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis. 2004;43:e18–e25. doi: 10.1053/j.ajkd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Nistal M, Paniagua R, Torres A, Hidalgo L, Regadera J. Idiopathic peritesticular fibrosis associated with retroperitoneal fibrosis. Eur Urol. 1986;12:64–68. doi: 10.1159/000472580. [DOI] [PubMed] [Google Scholar]