Abstract
Caspofungin is a potent antifungal inhibiting glucan synthesis in Candida species. However, caspofungin is not 100% curative in candidiasis. Therefore, we evaluated combinations of fluconazole with caspofungin for murine candidemia. We could not show any benefit of combined therapy over individual antifungal drugs.
Caspofungin, an echinocandin antifungal drug, inhibits synthesis of the fungal cell wall by blocking beta 1,3-d-glucan synthesis in the cell wall (2). Triazoles, such as fluconazole, act more slowly to impair synthesis of ergosterol in fungal cell membranes (1). Both echinocandins and triazoles are effective clinically against candidemia when given alone. However, response rates are in the range of 60 to 75%, leaving some room for improvement (5, 7). There has been some concern that these drugs may be additive or synergistic when used together. However, “additive” and “synergistic” are terms that are usually limited to in vitro studies. For antifungal drugs, there are few clear in vivo correlates. Most animal studies have involved drug combinations for experimental aspergillosis or candidiasis. Results have been mixed (3, 4; A. Cacciapuoti, M. Gurnani, J. Halpern, F. Gheyas, and R. Hare, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 415, 2002, and J. C. Luque, K. V. Clemons, and D. A. Steens, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 397, 2001). Because of inconsistent results and the few in vivo data available for candidiasis, we evaluated combined echinocandin and triazole therapy in murine disseminated candidiasis.
Candida albicans #R-1590, a clinical isolate, was obtained from The Fungus Testing Laboratory at The University of Texas Health Science Center at San Antonio. In vitro MIC determinations were performed with the National Committee for Clinical Laboratory Standards M27-A method (6). The MICs of fluconazole at 24 and 48 h were 0.25 and 0.25 μg/ml and of caspofungin were ≤0.125 and ≤ 0.125 μg/ml. Unmanipulated male outbred ICR or BALB/c mice were infected intravenously with 4 × 103 to 2 × 106 CFU/mouse in a 0.2-ml vol. Fluconazole was administered orally twice a day (BID) by gavage in a 0.2-ml vol at doses ranging from 0.06 mg/kg of body weight to 5 mg/kg. Caspofungin was administered in a 0.2-ml vol at doses ranging from 0.0005 to 5 mg/kg daily. Various combinations were also administered.
Efficacy was measured as reduction of kidney tissue burden of Candida. Drug therapy was administered days 1 to 7 postchallenge. On day 8 (or the day of death if earlier), the kidneys were homogenized and serial dilutions were cultured. The Mann-Whitney test was used for comparisons of tissue burdens. Because comparisons of combination groups were made with untreated controls and with each single drug arm, we adjusted the requirement for P ≤ 0.02 for significance.
In a preliminary study exploring doses of fluconazole, we found that controls had a median log count of 5.89 log CFU/kidney and that fluconazole at 0.08 mg/kg/dose reduced the counts to 5.0 log CFU/kidney, 1.25 mg/kg reduced the count to 3.63 log CFU/kidney, and 5 mg/kg reduced the count to 2.67 log CFU/kidney. All fluconazole regimens were effective at P < 0.02. Controls for a second study evaluating caspofungin showed a median of 4.78 log CFU/kidney. Caspofungin from 0.006 mg/kg to 0.1 mg/kg reduced the median kidney burden to <log 1.3 CFU/kidney. P was <0.02 for all caspofungin doses compared with controls.
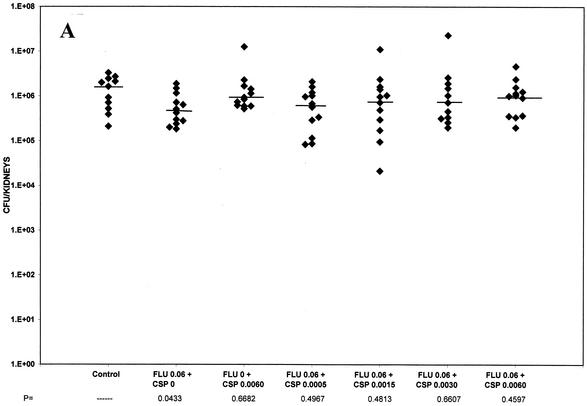
Figure 1 shows the effects of fluconazole therapy alone at a very low dose of 0.06 mg/kg BID (Fig. 1A), an intermediate dose of 1 mg/kg (Fig. 1B), and a high dose of 5 mg/kg BID (Fig. 1C). Fluconazole was used alone at 0.06 mg/kg BID and was combined with caspofungin at doses ranging from 0.0005 through 0.0060 mg/kg (Fig. 1A). Caspofungin was also used alone at 0.0060 mg/kg. These doses were set just below the margin of effective therapy and were selected to show any profound additive effect which a combination might show over that of each drug alone. None of the treatment regimens significantly reduced kidney counts below those of controls at these doses.
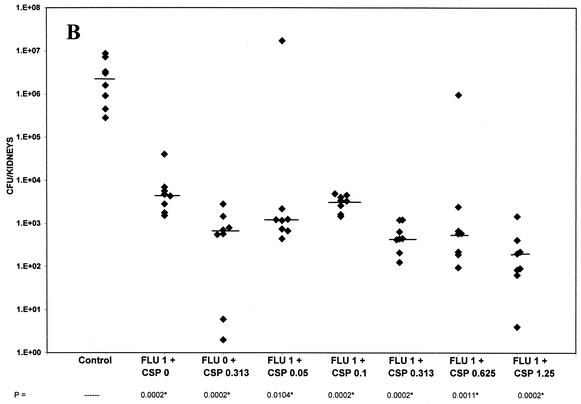
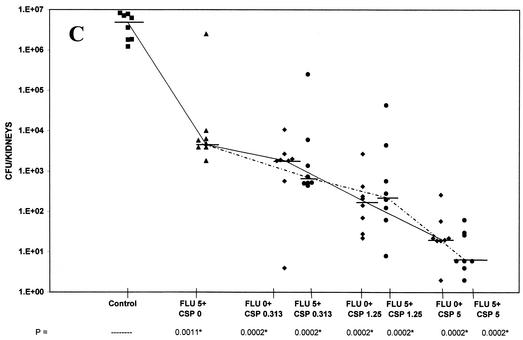
FIG. 1.
(A) Tissue burden of kidneys of groups of 11 to 12 mice infected intravenously with 3.1 × 105 CFU of C. albicans/mouse and treated from days 1 to 7 with fluconazole at 0.06 mg/kg BID, caspofungin alone at 0.0060 mg/kg, and combinations of fluconazole with caspofungin at 0.0005, 0.00015, 0.0030, or 0.0060 mg/kg. (B) Tissue burden of kidneys of groups of eight mice infected intravenously with 4.8 × 105 CFU of C. albicans/mouse and treated from days 1 to 7 with fluconazole at 1 mg/kg BID, caspofungin alone at 0.313 mg/kg, and combinations of fluconazole with caspofungin at 0.05, 0.1, 0.313, 0.625, or 1.25 mg/kg. (C) Tissue burden of kidneys of groups of eight mice infected intravenously with 1.2 × 106 CFU of C. albicans/mouse and treated from days 1 to 7 with fluconazole at 5 mg/kg BID, caspofungin alone at 0.313, 1.25, and 5 mg/kg, and combinations of fluconazole with caspofungin at 0.313, 1.25, or 5 mg/kg. The P values marked with asterisks at the bottom of panels B and C represent P ≤ 0.02 for significance compared to those for controls.
As shown in Fig. 1B, fluconazole was given at 1 mg/kg BID and caspofungin was given at 0.313 mg/kg alone. These doses were chosen because treatment with each individual drug reduced kidney counts and because it should be possible to show either additive or antagonistic effects at intermediate doses. As expected, all treatment groups were significantly superior to controls in reducing kidney burden counts (P ≤ 0.02). Caspofungin at 0.313 mg/kg was superior to fluconazole at 1 mg/kg (P = 0.0006). A combination of fluconazole (1 mg/kg BID) and caspofungin (0.1 mg/kg) was not superior to fluconazole alone (P > 0.02). All other combinations, including that of fluconazole at 1 mg/kg BID and caspofungin at 0.313 mg/kg (P = 0.0002) and that of fluconazole at 1 mg/kg BID and caspofungin at 1.25 mg/kg (P = 0.0002), were superior to fluconazole alone. However, none of the combinations were superior to caspofungin alone at 0.313 mg/kg. Therefore, this study showed indifference of fluconazole and caspofungin at this intermediate range of doses.
In the tissue burden study (Fig. 1C), fluconazole was given at a high 5-mg/kg BID dose both alone and in combination with caspofungin. The high dose of fluconazole should also have allowed demonstration of either additive or antagonistic effects of combination therapy. Caspofungin was given alone at doses of 0.313 to 5 mg/kg and at these same doses in combination with fluconazole. All groups were significantly superior to controls in reducing kidney burden counts (P ≤ 0.02). Caspofungin alone at all three doses and in combination with fluconazole was superior to fluconazole alone (P ≤ 0.02). However, just as described above and shown in Fig. 1B, none of the combination groups were superior to caspofungin alone at the same doses used in the combinations.
Although we explored a broad range of doses, the present studies were limited by what we did not examine. Treatments with the two drugs were initiated concurrently at 1 day after infection. Thus, there was no examination of sequencing of drugs or any study of the effects of delayed treatment or host immune suppression. We studied a single fluconazole-susceptible C. albicans isolate. Mice clear antifungals at different rates than humans, and while we administered fluconazole twice daily to compensate for this, we did not explore a variety of dose intervals. Nevertheless, these studies should give some pause to those who—in an uncontrolled manner—leap from preclinical studies to routine clinical practice. Combining fluconazole and caspofungin may be advantageous. Our studies do not discourage a clinical trial in that we found no antagonism. The major disadvantage of a clinical trial appears to us to be an issue of cost, but echinocandins are also costly. The data from present studies suggest that we should consider a comparative clinical trial before accepting a presumed benefit of combined fluconazole and caspofungin therapy for candidemia.
Acknowledgments
This work was supported by the NIH (contract N01-AI-85351).
REFERENCES
- 1.Como, J. A., and W. E. Dismukes. 1994. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med. 330:263-272. [DOI] [PubMed] [Google Scholar]
- 2.Hector, R. F. 1993. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 6:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob. Agents Chemother. 44:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis, R. E., R. A. Prince, J. Chi, and D. P. Kontoyiannis. 2002. Itraconazole preexposure attenuates the efficacy of subsequent amphotericin B therapy in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora-Duarte, J., R. Betts, C. Rotstein, A. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing for yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, and C. D. Webb. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]