Abstract
To elucidate Campylobacter jejuni resistance to antibiotics in Germany, MICs of ciprofloxacin, moxifloxacin, erythromycin, clindamycin, and tetracycline were determined (using agar dilution) for 144 clinical isolates. The data indicate a considerable ciprofloxacin resistance (45.1%) without a clonal relationship of the strains and a greater in vitro activity of moxifloxacin, erythromycin, and clindamycin.
Fluoroquinolone resistance of Campylobacter spp. appears to be selected by treatment of patients with these drugs (1). However, the development of fluoroquinolone resistance coincided not only with the extended usage of these drugs in humans but also with the introduction of fluoroquinolones into the raising of livestock (29). Enrofloxacin is closely related to ciprofloxacin and widely used in poultry to treat infections with Escherichia coli. Soon after its licensing in The Netherlands, the first resistant Campylobacter isolates were detected in treated animals and in humans (7). Experimentally, supplementation of drinking water with enrofloxacin rapidly led to the induction of enrofloxacin and ciprofloxacin resistance of Campylobacter spp. in broilers (14, 15). Fluoroquinolones have been licensed in Germany for use in cattle and swine since 1989 and in poultry since 1990. Although Campylobacter infections currently are found twice as frequently as infections with Salmonella spp. in some parts of Germany (20), no data on MICs of antimicrobials for Campylobacter jejuni isolates are available. Therefore, one aim of the present study was to determine the ciprofloxacin resistance in C. jejuni isolates in Germany.
Moxifloxacin is a newer 8-methoxyquinolone with excellent bioavailability and tissue distribution. Compared to ciprofloxacin, moxifloxacin exerts stronger activity against most gram-positive organisms and mycobacteria and some nonfermenting bacteria, anaerobes, and chlamydia as well as mycoplasma, while the MICs of moxifloxacin for Enterobacteriaceae and Pseudomonas aeruginosa are usually higher (3, 4, 8, 21, 31). Moxifloxacin shows improved activity against topoisomerase IV, which is encoded by the parC and parE genes. Accordingly, a second aim of the present study was to determine the MICs of moxifloxacin and the standard alternative (i.e., erythromycin) as well as of other drugs potentially used in the treatment of C. jejuni infections.
A total of 144 C. jejuni isolates were collected from patients between January 1998 and April 2001 (before antimicrobial treatment was started in cases in which patients were to receive treatment). Isolates were differentiated using routine laboratory techniques (13). All isolates were stored at −20°C until tests of susceptibility and pulsed-field gel electrolysis (PFGE) analysis were conducted. Since no National Committee for Clinical Laboratory Standards (NCCLS)-approved method had been published at the time of the study, an agar dilution method following guideline M7-A5 of the NCCLS was applied and standardized using C. jejuni ATCC 33560 as control. MICs of ciprofloxacin and moxifloxacin (both from Bayer, Leverkusen, Germany), erythromycin and tetracycline (both from Sigma, Deisenhofen, Germany), and clindamycin (Pharmacia & Upjohn, Erlangen, Germany) were determined by an agar dilution method as follows: using a multipoint inoculator, 10 μl of medium containing 106 CFU was applied onto Mueller-Hinton agar (Oxoid, Wesel, Germany) supplemented with 5% horse blood, and the plates were incubated at 36°C for 40 to 42 h in an atmosphere of reduced oxygen (Campy-Gen; Oxoid). A method using a similar bacterial inoculum and comparable culture conditions for susceptibility testing of C. jejuni isolates was recently reported by Ge et al. (11). Since NCCLS does not recommend specific breakpoints for tests of Campylobacter spp., in this study ciprofloxacin MICs of ≥4 μg/ml (as recommended for Enterobacteriaceae) and erythromycin, clindamycin, and tetracycline MICs of ≥8 μg/ml (as recommended for staphylococci and enterococci) were considered to indicate resistance. No breakpoint has been defined for moxifloxacin so far. A total of 14 tests of C. jejuni ATCC 33560 resulted in concentration values of 0.25 to 0.5 μg/ml for ciprofloxacin, 1 to 4 μg/ml for erythromycin, and 0.5 to 1 μg/ml for tetracycline. These data are comparable to the tentative agar dilution quality control ranges approved by the NCCLS veterinary antimicrobial susceptibility testing subcommittee (11). Further concentration values resulting from tests of C. jejuni ATCC 33560 were 0.125 to 0.25 μg/ml for moxifloxacin and 0.125 to 2 μg/ml for clindamycin.
To rule out a possible expansion of a limited number of resistant clones, PFGE analysis was performed, which had previously been shown to be the most discriminatory typing method compared to fatty acid profile typing, serotyping, and biotyping (25). C. jejuni ATCC 33560 was used as control. Bacterial suspensions were prepared in phosphate-buffered saline, diluted with TEN buffer (100 mM Tris [pH 7.5], 100 mM EDTA, 150 mM NaCl, distilled H2O), and mixed with agarose (Bio-Rad, Richmond, Calif.) up to a final concentration of 1% agarose. DNA was extracted from agarose-bacterium blocks by incubation with lysozyme (5 h, 37°C) and proteinase K (overnight, 50°C), and whole chromosomal DNA in agarose was digested with the restriction endonuclease SmaI (Boehringer Mannheim, Mannheim, Germany). The restriction fragments were separated in a CHEF DRII apparatus (Bio-Rad) as described by Pfaller et al. (16). A lambda ladder (Bio-Rad) was used as a molecular weight marker. Electrophoresis was performed for 23 h at 6 V/cm with pulse times of 5 to 40 s at 14°C. Gels were stained with ethidium bromide, illuminated under UV light, and photographed. Pictures of the PFGE gels were scanned, and the banding patterns were analyzed by GelCompare software (Applied Math, Kortrijk, Belgium). Cluster analysis was performed using an unweighted pair group method with arithmetic average (correlation, Dice coefficient [maximum tolerance for each band, 2.0%; minimum surface, 0.0%]). Isolates with >95% similarity were considered a single clone.
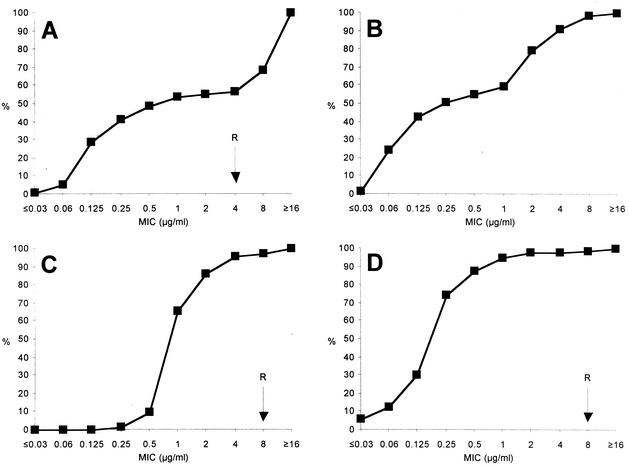
In examinations of the susceptibility of the 144 C. jejuni isolates from human stool samples, 45.1% exhibited resistance against ciprofloxacin (MICs ≥ 4 μg/ml) (Fig. 1A). Interestingly, a similar proportion (45.8%) of ciprofloxacin-resistant isolates was found in 24 C. jejuni isolates that were collected from carcasses from livestock (12 poultry, 7 calves, and 5 pigs) at 4 different slaughterhouses between November 1998 and July 1999 (data not shown). A total of 111 of those 144 C. jejuni isolates were typed by PFGE and represented 79 different clones. While 59 clones were found only once, 20 clones were isolated between two and six times (Table 1). These data demonstrate that the ciprofloxacin-resistant C. jejuni isolates did not represent the expansion of a few resistant clones.
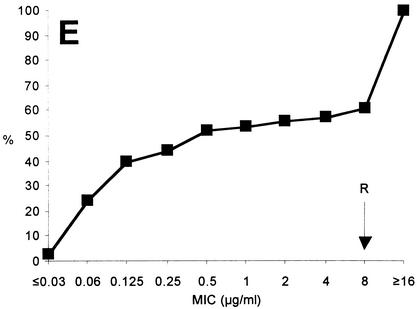
FIG. 1.
Cumulative MICs of ciprofloxacin (A), moxifloxacin (B), erythromycin (C), clindamycin (D), and tetracycline (E) for 144 C. jejuni isolates from human stool samples. R, breakpoint for resistance (not yet defined for moxifloxacin); %, percentage of isolates tested.
TABLE 1.
PFGE results for 111 C. jejuni isolatesa
| No. of isolates/clone | No. of clones |
|---|---|
| 1 | 59 |
| 2 | 14 |
| 3 | 3 |
| 4 | 1 |
| 5 | 1 |
| 6 | 1 |
Clones represent isolates with similar (>95%) banding patterns.
Comparing the MICs of ciprofloxacin and moxifloxacin, the MIC at which 50% of the isolates tested were inhibited (MIC50) and MIC90 were higher for ciprofloxacin (0.25 and ≥ 16 μg/ml, respectively) than for moxifloxacin (0.125 μg/ml and 4 μg/ml, respectively) (Fig. 1A and B). Therefore, moxifloxacin appears to exhibit greater in vitro activity against ciprofloxacin-resistant C. jejuni isolates. A clearly different pattern was observed with respect to resistance to erythromycin, to which only 4.2% of the clinical C. jejuni isolates were resistant (erythromycin MICs ≥ 8 μg/ml; Fig. 1C). Of note, 1 of the 65 ciprofloxacin-resistant C. jejuni isolates (1.5%) was also resistant to erythromycin. Out of 13 clinical isolates from children of age 16 or younger (in cases in which these drugs are contraindicated), 5 (38.5%) were ciprofloxacin resistant while all 13 isolates were susceptible to erythromycin. This finding and similar data reported by others (5, 19, 26) further emphasize the importance of livestock as a source for infections with such isolates. In fact, a recent study revealed that more than 40% of German broiler carcasses were contaminated with Campylobacter (2). However, although the infectious dose of C. jejuni appears to be low compared to that of other enteric pathogens, these results do not necessarily mean that there were sufficient numbers of C. jejuni on all of the carcasses to cause disease. Clindamycin and tetracycline could be additional alternative antibiotics in the treatment of C. jejuni infections. Here, clindamycin (Fig. 1D) and tetracycline (Fig. 1E) exhibited MICs ≥ 8 μg/ml for 2.0% and 43.1% of the isolates, respectively.
Increasing rates of fluoroquinolone resistance have been reported for Campylobacter spp. from England and Wales (10.5%) (28), Spain (82%) (17), Finland (11%) (18), Greece (30.6%) (5), The Netherlands (29%) (26), Canada (12.7%) (9), and the United States (10.2%) (24). Although a highly significant correlation could be found between traveling to Spain or Thailand and enteritis caused by fluoroquinolone-resistant Campylobacter strains (as determined by analysis of isolates from English and Swedish travelers to those countries) (10, 23), Smith et al. (24) recently demonstrated that travel and quinolone use account for only a small percentage of Campylobacter infections with fluoroquinolone-resistant isolates in the United States. They related the risk of domestically acquiring such isolates to the licensing of fluoroquinolones for use in poultry in 1995. The mechanisms of fluoroquinolone resistance in C. jejuni have previously been identified as mutations in the gyrA and parC genes (12, 30), and similar mutations can be postulated for the isolates examined in the present study. Moxifloxacin MICs lower than those of ciprofloxacin against C. jejuni have previously been shown for three isolates with defined mutations in the gyrA locus (27). Similarly, moxifloxacin possessed greater activity against ciprofloxacin-resistant Staphylococcus aureus isolates (with mutations in grlA, grlB, gyrA, and gyrB) and ciprofloxacin-resistant Streptococcus pneumoniae isolates (6, 22).
In conclusion, high rates of ciprofloxacin resistance of Campylobacter can also be found in Germany. Similarly to other countries, these are most likely correlated to the use of these drugs in animal husbandry. Considering the high incidence of diarrhea caused by Campylobacter in Germany, the status of ciprofloxacin as a first-choice drug in the treatment of acute gastroenteritis in cases in which the identity of the causative agent has not been established is questionable. Since MICs of moxifloxacin are lower than those of ciprofloxacin for C. jejuni, clinical trials would be desirable for evaluations of whether this translates into a therapeutical benefit. Erythromycin should be the first choice for treatment of patients in Germany with infections caused by C. jejuni of unknown susceptibility.
Acknowledgments
We thank Jutta Imlau for expert technical assistance.
The study was supported by Bayer Pharmaceuticals.
REFERENCES
- 1.Adler-Mosca, H., J. Luthy-Hottenstein, G. Martinetti Lucchini, A. Burnens, and M. Altwegg. 1991. Development of resistance to quinolones in five patients with campylobacteriosis treated with norfloxacin or ciprofloxacin. Eur. J. Clin. Microbiol. Infect. Dis. 10:953-957. [DOI] [PubMed] [Google Scholar]
- 2.Atanassova, V., and C. Ring. 1999. Prevalence of Campylobacter spp. in poultry and poultry meat in Germany. Int. J. Food Microbiol. 51:187-190. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind, A. 1997. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J. Antimicrob. Chemother. 40:639-651. [DOI] [PubMed] [Google Scholar]
- 4.Bebear, C. M., H. Renaudin, A. Boudjadja, and C. Bebear. 1998. In vitro activity of BAY 12-8039, a new fluoroquinolone against mycoplasmas. Antimicrob. Agents Chemother. 42:703-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatzipanagiotou, S., E. Papavasileiou, A. Lakumenta, A. Makri, C. Nicolaou, K. Chantzis, S. Manganas, and N. I. Legakis. 2002. Antimicrobial susceptibility patterns of Campylobacter jejuni strains isolated from hospitalized children in Athens, Greece. J. Antimicrob. Chemother. 49:803-805. [DOI] [PubMed] [Google Scholar]
- 6.Coyle, E. A., G. W. Kaatz, and M. J. Rybak. 2001. Activities of newer fluoroquinolones against ciprofloxacin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1654-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endtz, H. P., G. J. Ruijs, B. van Klingeren, W. H. Jansen, T. van der Reyden, and R. P. Mouton. 1991. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J. Antimicrob. Chemother. 27:199-208. [DOI] [PubMed] [Google Scholar]
- 8.Fass, R. J. 1997. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone. Antimicrob. Agents Chemother. 41:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaunt, P. N., and L. J. Piddock. 1996. Ciprofloxacin resistant Campylobacter spp. in humans: an epidemiological and laboratory study. J. Antimicrob. Chemother. 37:747-757. [DOI] [PubMed] [Google Scholar]
- 11.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, P. F. McDermott, and J. Meng. 2002. Comparison of the Etest and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 12.Gibreel, A., E. Sjögren, B. Kaijser, B. Wretlind, and O. Sköld. 1998. Rapid emergence of high-level resistance to quinolones in Campylobacter jejuni associated with mutational changes in gyrA and parC. Antimicrob. Agents Chemother. 42:3276-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasmick, A. 1992. Processing and interpretation of bacterial fecal cultures. In H. D. Isenberg (ed.) Clinical microbiology procedures handbook, vol. 1, p. 1.10.1-1.10.25. American Society for Microbiology, Washington, D.C.
- 14.Jacobs-Reitsma, W. F., C. A. Kan, and N. M. Bolder. 1994. The induction of quinolone resistance in Campylobacter bacteria in broilers by quinolone treatment. Lett. Appl. Microbiol. 19:228-231. [Google Scholar]
- 15.McDermott, P. F., S. M. Bodeis, L. L. English, D. G. White, R. D. Walker, S. Zhao, S. Simjee, and D. D. Wagner. 2002. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Dis. 185:837-840. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., R. J. Hollis, and H. S. Sader. 1992. PFGE analysis of chromosomal restriction fragments. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, vol. 2, p. 10.5.c.1-10.5.c.12. American Society for Microbiology, Washington, D.C.
- 17.Prats, G., B. Mirelis, T. Llovet, C. Munoz, E. Miró, and F. Navarro. 2000. Antibiotic resistance trends in enteropathogenic bacteria isolated in 1985-1987 and 1995-1998 in Barcelona. Antimicrob. Agents Chemother. 44:1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rautelin, H., O.-V. Renkonen, and T. U. Kosunen. 1991. Emergence of fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli in subjects from Finland. Antimicrob. Agents Chemother. 35:2065-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reina, J., M. J. Ros, and A. Serra. 1994. Susceptibilities to 10 antimicrobial agents of 1,220 Campylobacter strains isolated from 1987 to 1993 from feces of pediatric patients. Antimicrob. Agents Chemother. 38:2917-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert-Koch-Institut. 2002. Epidemiologisches Bulletin 3/2002. Aktuelle Statistik der meldepflichtigen Infektionskrankheiten. Stand 31:12.2001. Robert-Koch-Institut, Berlin, Germany.
- 21.Rodriguez, J. C., M. Ruiz, A. Climent, and G. Royo. 2001. In vitro activity of four fluoroquinolones against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 17:229-231. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz, F. J., K. Kohrer, S. Scheuring, J. Verhoef, A. Fluit, H. P. Heinz, and M. E. Jones. 1999. The stability of grlA, grlB, gyrA, gyrB and norA mutations and MIC values of five fluoroquinolones in three different clonal populations of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 5:287-290. [DOI] [PubMed] [Google Scholar]
- 23.Sjogren, E., G. B. Lindblom, and B. Kaijser. 1997. Norfloxacin resistance in Campylobacter jejuni and Campylobacter coli isolates from Swedish patients. J. Antimicrob. Chemother. 40:257-261. [DOI] [PubMed] [Google Scholar]
- 24.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, and the Investigation Team. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 25.Steele, M., B. McNab, L. Fruhner, S. DeGrandis, D. Woodward, and J. A. Odumeru. 1998. Epidemiological typing of Campylobacter isolates from meat processing plants by pulsed-field gel electrophoresis, fatty acid profile typing, serotyping, and biotyping. Appl. Environ. Microbiol. 64:2346-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talsma, E., W. G. Goettsch, H. L. Nieste, P. M. Schrijnemakers, and M. J. Sprenger. 1999. Resistance in Campylobacter species: increased resistance to fluoroquinolones and seasonal variation. Clin. Infect. Dis. 29:845-848. [DOI] [PubMed] [Google Scholar]
- 27.Tankovic, J., R. Bachoual, S. Ouabdesselam, A. Boudjadja, and C. J. Soussy. 1999. In-vitro activity of moxifloxacin against fluoroquinolone-resistant strains of aerobic gram-negative bacilli and Enterococcus faecalis. J. Antimicrob. Chemother. 43(Suppl. B):19-23. [DOI] [PubMed] [Google Scholar]
- 28.Thwaites, R. T., and J. A. Frost. 1999. Drug resistance in Campylobacter jejuni, C. coli, and C. lari isolated from humans in northwest England and Wales, 1997. J. Clin. Pathol. 52:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bogaard, A. E., and E. E. Stobberingh. 1999. Antibiotic usage in animals: impact on bacterial resistance and public health. Drugs 58:589-607. [DOI] [PubMed] [Google Scholar]
- 30.Wang, Y., W. M. Huang, and D. E. Taylor. 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 37:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodcock, J. M., J. M. Andrews, F. J. Boswell, N. P. Brenwald, and R. Wise. 1997. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob. Agents Chemother. 41:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]