Abstract
Garenoxacin (T-3811ME, BMS-284756) is a novel des-F(6) quinolone that has been shown to be effective in vitro against a wide range of clinically important pathogens, including gram-positive and gram-negative aerobes and anaerobes. This study was conducted to evaluate the safety and tolerability of multiple oral doses (100 to 1,200 mg/day) of garenoxacin in healthy subjects and to determine its multiple-dose pharmacokinetics. Forty healthy male and female subjects (18 to 45 years of age) were enrolled in this randomized, double-blind, placebo-controlled, sequential, multiple- and ascending-dose study. Each subject received a once-daily oral dose of garenoxacin (100, 200, 400, 800, or 1,200 mg) or a placebo for 14 days. Blood and urine samples were collected for measurements of garenoxacin by validated liquid chromatography with dual mass spectrometry, and plasma garenoxacin concentration-time data were analyzed by noncompartmental methods. The effects of garenoxacin on Helicobacter pylori, psychometric test performance, and electrocardiograms were assessed, as was drug safety. Over the 14 days of dosing, geometric mean peak concentrations of garenoxacin in plasma (Cmax) at the 100- and 1,200-mg doses were within the ranges of 1.2 to 1.6 and 16.3 to 24 μg/ml, respectively. The corresponding values for the geometric mean area under the concentration-time curve over the dosing interval (AUCτ) for garenoxacin in plasma at the 100- and 1,200-mg doses were within the ranges of 11.5 to 15.7 and 180 to 307 μg · h/ml, respectively. Increases in systemic exposure to garenoxacin in terms of AUC and Cmax were approximately dose proportional over the 100- to 400-mg dose range but demonstrated increases that were somewhat greater than the dose increments at the 800- and 1,200-mg doses. Median values for the time to achieve Cmax were in the range of 1.13 to 2.50 h for all doses. The mean elimination half-life for garenoxacin in plasma appeared to be independent of dose and ranged from 13.3 to 17.8 h (day 14). Approximately 30 to 50% of an administered garenoxacin dose was excreted unchanged in the urine. At doses of 100 to 400 mg, steady-state concentrations of garenoxacin in plasma appeared to be attained by the fourth dose. Multiple oral doses of garenoxacin were well tolerated and did not demonstrate clinically significant effects on QTc or psychometric test results. Garenoxacin administered alone for 14 days at doses of ≥400 mg demonstrated activity against H. pylori. These results suggest that multiple once-daily oral doses of garenoxacin of up to 1,200 mg are safe and well tolerated and that the pharmacokinetics of garenoxacin support once-daily administration.
The 6-fluoroquinolones are synthetic antimicrobials that have a unique mechanism of action (binding to DNA gyrase), a broad spectrum of antibacterial activity, good absorption from the gastrointestinal tract, and generally favorable pharmacokinetic properties. Antimicrobials in this class have proven to be very effective for a large number of indications, including infections of the respiratory tract, the skin and skin structure, and the urinary tract and infections that are sexually transmitted. Fluoroquinolones have also been shown to be safe for the treatment of a wide variety of patients with such infections (6).
The older drugs in this class have good activity against a wide range of pathogens, including Pseudomonas aeruginosa and Staphylococcus aureus (methicillin susceptible) as well as other staphylococci, but they are limited by lower activity against clinically important gram-positive organisms such as streptococcal and enterococcal species. In addition, older drugs in this class have relatively poor activity against anaerobes and atypical pathogens such as Mycoplasma spp. (4, 6).
More recently developed 6-fluoroquinolones (e.g., sparfloxacin, trovafloxacin, clinafloxacin, and grepafloxacin) have broader antimicrobial spectra (1, 6, 11, 13, 19), but their use has been limited by significant toxicities, including cardiotoxicity (sparfloxacin and grepafloxacin), hepatotoxicity (trovafloxacin), and phototoxicity (sparfloxacin and clinafloxacin) (2, 5, 6, 21).
Garenoxacin (T-3811ME, BMS-284756) is a novel des-F(6) quinolone that has been shown to be effective in vitro against a wide range of clinically important pathogens, including gram-positive and gram-negative aerobes and anaerobes (9, 10, 12, 20, 22, 23). The ex vivo serum protein binding capability of garenoxacin has been determined to be 75% and is independent of time and garenoxacin concentration (0.3 to 6.5 μg/ml) (A. Bello, D. Hollenbaugh, D. Gajjar, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-45, 2001). The present study was designed to evaluate the safety and tolerability of multiple daily oral doses (100 to 1,200 mg/day) of garenoxacin in healthy adult subjects and to determine the multiple-dose pharmacokinetics following this route of administration.
MATERIALS AND METHODS
Study design.
A randomized, double-blind, placebo-controlled, sequential- and multiple-dose, dose escalation design was used to evaluate the pharmacokinetics of garenoxacin in 40 healthy adult subjects.
Subjects.
This study was performed in accordance with the good clinical practice guidelines of the Food and Drug Administration, and all subjects were required to give informed, written consent (approved by the institutional review board) prior to the initiation of any study-specific procedures. The study subjects included both males and females, 18 to 45 years of age, with a body mass index in the range of 18 to 30 kg/m2, who were in good health as determined by medical history, electrocardiogram (ECG), physical examination, and clinical laboratory studies carried out within 14 days prior to the start of the study. No concomitant medications or therapies, prescription or over-the-counter, were permitted during study participation unless they were prescribed by the investigator for treatment of specific adverse events. The subjects were not permitted to consume alcohol or alcohol-containing beverages during the study. The subjects were required to remain in the clinical facility for 3 days before dosing, during dosing, and for at least 72 h after administration of the last dose of the study drug. The subjects were assigned to one of five sequential-dosing groups (n = 8 each). Within each dosing group, the subjects were randomized in a 3:1 ratio to receive garenoxacin or a placebo.
Dosing procedures.
Each subject was given a once-daily oral dose of either 100, 200, 400, 800, or 1,200 mg of garenoxacin in capsule form (100 or 200 mg) or a placebo for 14 days. The subjects were required to fast (nothing to eat or drink except water) for at least 8 h prior to each dose and until 4 h after administration of the study drug. After a dose level was found to be safe and well tolerated in at least six of eight subjects in a dosing group, the succeeding panel of eight subjects received the next higher dose level. Throughout the study, each subject maintained his or her dosing assignment, and no intrasubject dose escalation was permitted.
Sample collection and assay.
Blood samples (5 to 8 ml) and urine samples (5 ml) for measurement of garenoxacin concentrations were obtained up to 24 h postdosing on days 1 and 7 and up to 72 h postdosing on day 14. Blood collection times for the plasma assay were 15, 30, and 45 min and 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, and 24 h after dosing on days 1, 7, and 14. Additional blood samples were collected at 36, 48, and 72 h after the last dose on day 14. Trough blood samples were also collected immediately before dosing on days 3, 4, 5, 6, 12, and 13. The blood samples were collected into vials containing EDTA as an anticoagulant and were centrifuged to obtain plasma. Total urine output was collected over the intervals of 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing on days 1, 7, and 14 (urine volume was recorded prior to the separation of the analytical sample). Plasma and urine samples were stored at −70°C prior to subsequent analysis.
The concentrations of garenoxacin in plasma and urine samples were determined by validated liquid chromatography with tandem mass spectrometry assays that utilized an internal standard. The limits of quantification based on garenoxacin standard curves ranged from 0.01 to 10 μg/ml for plasma and from 0.5 to 100 μg/ml for urine. The intra- and interday coefficients of variation were no greater than 5.2%.
Calculation of pharmacokinetic parameters.
Concentration-time data for garenoxacin in plasma were analyzed by using noncompartmental methods. The peak plasma drug concentration (Cmax), time to reach Cmax (Tmax), and trough concentration (Cmin) were recorded directly from experimental observations. The slope of the terminal phase of the plasma drug concentration profile (K) was determined from ≥3 data points by a log-linear regression analysis. The absolute value of K was used to estimate the apparent terminal half-life (t1/2) by the equation t1/2 = ln 2/K. The area under the plasma drug concentration-time curve over the dosing interval (AUCτ) was calculated by trapezoidal and log-trapezoidal summation for dosing interval τ (24 h). In all cases, the concentrations of garenoxacin in plasma were quantifiable for at least 24 h after a single-dose administration and AUC values were extrapolated to include estimates beyond the last quantifiable determination to infinity (AUC∞). The apparent total body clearance (CLT/F) was calculated by dividing the administered dose by either AUC∞ (day 1) or AUCτ (day 7 or 14), as appropriate. The mean residence time (MRT) on day 1 was calculated by dividing the area under the first moment of the plasma garenoxacin concentration-time curve extrapolated to infinity (AUMC∞) by AUC∞. MRT values on days 7 and 14 were determined by dividing AUMCτ plus [τ × (plasma drug concentration at time τ/K)] by AUCτ.
The amount of garenoxacin excreted in the urine during each collection interval was estimated by multiplying the concentration of the intact drug in each urine sample by the volume of urine obtained during the respective collection period. The total urinary recovery of the unaltered drug (percent UR) was calculated as the cumulative amount excreted over 24 h for days 1, 7, and 14 and was expressed as a percentage of the administered dose. The renal clearance (CLR) of garenoxacin was calculated as follows: CLR = UR0-T/AUC0-T, where UR0-T and AUC0-T were, respectively, the urinary recovery and area under the plasma garenoxacin concentration-time curve from time of dosing (time zero) to time T, where T is 24 h.
Pharmacodynamic assessments. (i) Assessment of intestinal flora.
The effect of garenoxacin on fecal flora was evaluated in this study, and the results have been reported elsewhere (16).
(ii) Assessment of H. pylori.
The presence of H. pylori was determined by a [14C]urea breath test performed on days −2, 17, and 45. Breath samples (sufficient to fill the test balloon) were collected 10 min after the subjects ingested capsules containing [14C]urea, dissolved in a collection fluid, and analyzed for CO2 by liquid scintillation counting. Results of <50 or >199 dpm were interpreted as negative or positive, respectively, for H. pylori, and intermediate values were judged as indeterminate. Positive indications of H. pylori were confirmed by H. pylori immunoglobulin G (IgG) antibody immunoassays of blood samples taken by a direct vein puncture or via an indwelling catheter.
(iii) Psychometric tests.
Psychometric tests were performed to evaluate the potential effects of garenoxacin on the central nervous system. The psychometric tests of short-term memory, reaction time, and sustained attention or arousal included digit symbol substitution, simple reaction time, and continuous performance. These assessments were performed predosing (baseline), at 2 h postdosing on days 1, 7, and 14, and at 72 h after the day 14 dosing. Variables indicative of accuracy of response, latency, sustained attention, and/or completion time were statistically summarized during treatment with garenoxacin and compared with baseline performance.
Safety assessments.
The evaluation of drug safety included a review of treatment-emergent clinical adverse events, clinical laboratory findings, vital signs, and ECG results. Treatment-emergent clinical adverse events were defined as illnesses, signs, or symptoms, independent of causality, that appeared or worsened from the initial dosing to discharge. Laboratory adverse events were defined as results deemed clinically significant by the investigator.
Physical examinations were performed at screening, within 48 h prior to the initial dosing, and on discharge from the facility (day 17). Vital signs were also obtained at screening, 2 days prior to dosing, and on days 17 and 45. In addition, blood pressure and pulse rate, taken after the subject had been seated for at least 5 min, were recorded on day 1 before dosing and at 1, 2, 4, 6, 12, and 24 h after dosing. Twelve-lead ECGs were recorded at screening, 2 days prior to dosing, and at 1, 2, 4, 6, 12, and 24 h after dosing on days 1, 7, and 14. Each value for QT was recorded directly from the ECG tracing and corrected for heart rate (QTc) by using Bazett's formula (3) as follows:
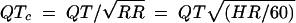 |
where QT is the QT interval in milliseconds, QTc is the QT interval corrected for heart rate, RR is the RR interval in milliseconds, and HR is the heart rate in beats per minute.
Blood and urine samples were obtained for clinical laboratory tests at screening, 2 days prior to the initial dosing, and on days 4, 7, 11, 14, 17, 21, 28, and 45. Laboratory blood analysis included hematology assessments (hemoglobin, hematocrit, complete blood cell count with differential, and platelet count) and serum chemistry.
Urine samples were analyzed for specific gravity, glucose, protein, urobilinogen, pH, ketones, blood, leukocytes, nitrites, and microscopic examination of sediment. On days 2 and 11, fresh urine samples were microscopically examined for signs of drug-related crystalluria. Additional screening assessments included tests for the hepatitis C virus and the human immunodeficiency virus and of urine for drugs of abuse.
Statistical methods.
Subject demographics, vital signs, clinical laboratory data, and physical examinations were statistically summarized and/or tabulated in frequency distributions for each dosage panel. Summary statistics for the pharmacodynamic measures, the numbers of microorganisms in fecal samples, the H. pylori IgG antibody test, the [14C]urea breath test, and the three psychometric assessments were calculated and graphically analyzed for time and/or dose dependency.
The distributions of the pharmacokinetic variables Cmax, Tmax, AUC∞, AUCτ, and t1/2 were summarized by dose group and by time relative to dosing. For each treatment panel, ECG intervals were statistically summarized, as were differences from baseline in mean QTc and PR intervals, and these were also plotted as functions of time relative to dosing. Differences in QTc versus AUCτ or Cmax for the period of 0 to 6 h following dosing were plotted and analyzed by linear regression.
RESULTS
Subject demographics and baseline characteristics.
Forty subjects were serially enrolled in five study panels (six to receive garenoxacin and two to receive placebo in each panel). Overall, 39 (97.5%) subjects completed treatment and 38 (95.0%) completed both treatment and posttreatment follow-up. One subject randomized to receive placebo was lost to follow-up, and a second volunteer in the garenoxacin 400-mg panel discontinued participation on day 10 due to adverse events (abdominal pain, diarrhea, fatigue, lethargy, somnolence, headache, sore throat, vomiting, and discoloration of the tongue). The demographic characteristics of the subjects were similar across the treatment groups. The subjects' ages ranged from 18 to 45 years, with an average of 34 years; their weights ranged from 54.8 to 97.0 kg, with an average of 79.1 kg; their heights ranged from 160 to 192 cm, with an average of 176.4 cm; and their body mass indexes ranged from 19.0 to 29.5 kg of body weight/m2, with an average of 25.4 kg/m2.
Pharmacokinetics.
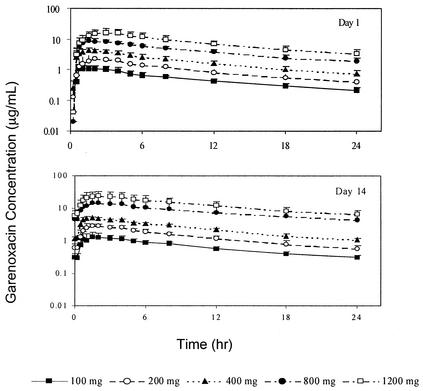
The concentrations of garenoxacin in plasma increased with increasing doses following once-daily administration of 100, 200, 400, 800, or 1,200 mg for 14 days (Fig. 1; day 7 data not shown). For garenoxacin doses in the ratio of 1:2:4:8:12, geometric mean values for garenoxacin Cmax increased to the ratios of 1:2.0:3.8:7.9:13.6 on day 1, 1:1.9:3.5:8.4:14.3 on day 7, and 1:1.9:3.5:9.0:15.0 on day 14. The corresponding geometric mean AUCτ values increased to the ratios of 1:2.0:3.9:8.8:15.6 on day 1, 1:2.0:3.8:10.1:17.4 on day 7, and 1:2.1:3.7:11.5:19.6 on day 14, and geometric mean AUC∞ values increased to the ratio of 1:2.0:3.7:8.6:15.2 on day 1. AUC∞ values on day 1 were similar to the corresponding AUCτ values on days 7 and 14 for the 100- to 400-mg dose groups, suggesting that steady state had been achieved. For the 800- and 1,200-mg dose groups, values for AUCτ on days 7 and 14 were approximately 20 to 40% higher than for the corresponding day 1 AUC∞ values. Overall, these findings suggested that within the 100- to 400-mg dose range, increases in garenoxacin Cmax and AUC were approximately proportionate to dose and were time independent (Table 1). At doses of 800 and 1,200 mg, increases in the systemic exposure to garenoxacin appeared to be somewhat greater than the dose increment and to demonstrate some degree of time dependence with repeated once-daily administration.
FIG. 1.
Mean plasma garenoxacin concentration-versus-time profiles for garenoxacin following oral administration of 100, 200, 400, 800, or 1,200 mg on days 1 and 14.
TABLE 1.
Summary of statistics for garenoxacin pharmacokinetic parameters tabulated by dose group and day
| Parameter and garenoxacin dose (mg) | Result fora:
|
||
|---|---|---|---|
| Day 1 | Day 7 | Day 14 | |
| Cmax, μg/ml [geometric mean (% CV)] | |||
| 100 | 1.2 (23.2) | 1.5 (23.1) | 1.6 (37.0) |
| 200 | 2.4 (15.2) | 2.9 (15.9) | 3.0 (9.2) |
| 400 | 4.6 (14.8) | 5.2 (15.8) | 5.6 (17.6) |
| 800 | 9.5 (12.8) | 12.6 (32.2) | 14.4 (40.4) |
| 1,200 | 16.3 (31.8) | 21.5 (37.9) | 24.0 (34.8) |
| AUC∞, μg/ml [geometric mean (% CV)] | |||
| 100 | 15.1 (24.4) | N/Ad | N/A |
| 200 | 29.6 (20.4) | N/A | N/A |
| 400 | 55.2 (18.5) | N/A | N/A |
| 800 | 130.3 (16.5) | N/A | N/A |
| 1,200 | 230.0 (33.1) | N/A | N/A |
| AUGτ,b μg/ml [geometric mean (% CV)] | |||
| 100 | 11.5 (21.3) | 15.6 (18.6) | 15.7 (20.5) |
| 200 | 23.3 (19.0) | 31.0 (17.6) | 32.7 (17.3) |
| 400 | 45.2 (15.6) | 58.5 (23.1) | 58.6 (19.9) |
| 800 | 100.7 (10.7) | 157.9 (44.5) | 180.8 (48.8) |
| 1,200 | 179.8 (30.0) | 271.0 (36.5) | 307.3 (31.2) |
| t1/2, h [mean (SD)] | |||
| 100 | N/A | N/A | 17.8 (3.3) |
| 200 | N/A | N/A | 15.5 (4.4) |
| 400 | N/A | N/A | 13.3 (2.2) |
| 800 | N/A | N/A | 15.5 (4.0) |
| 1,200 | N/A | N/A | 15.4 (3.4) |
| Tmax, h [median (minimum to maximum)] | |||
| 100 | 1.25 (0.75-2.00) | 1.13 (0.75-4.00) | 1.13 (0.50-4.00) |
| 200 | 1.75 (1.00-2.00) | 1.50 (1.00-3.00) | 1.75 (1.00-2.00) |
| 400 | 1.75 (0.75-2.00) | 1.25 (0.50-1.50) | 1.50 (0.75-2.00) |
| 800 | 1.75 (1.00-4.00) | 1.50 (0.75-3.00) | 2.50 (1.50-4.00) |
| 1,200 | 2.50 (2.00-4.00) | 2.50 (1.50-4.00) | 2.00 (1.50-4.00) |
| MRT, h [mean (SD)] | |||
| 100 | 16.9 (3.0) | 18.8 (5.1) | 19.4 (2.8) |
| 200 | 15.6 (2.4) | 16.0 (5.1) | 18.2 (3.5) |
| 400 | 13.9 (1.1) | 15.6 (1.7) | 17.1 (1.8) |
| 800 | 16.0 (2.7) | 20.0 (5.7) | 21.2 (5.5) |
| 1,200 | 16.0 (2.9) | 17.3 (2.2) | 20.4 (2.7) |
| % UR [mean (SD)] | |||
| 100 | 37.3 (8.3) | 50.7 (9.0) | 52.0 (19.7)c |
| 200 | 28.0 (6.4) | 44.4 (6.0) | 44.3 (13.1) |
| 400 | 23.2 (7.7) | 37.6 (9.8) | 43.6 (3.2) |
| 800 | 21.1 (3.5)c | 36.6 (9.1) | 33.1 (6.0) |
| 1,200 | 25.4 (2.8) | 36.5 (4.1) | 40.9 (3.6) |
| CLR, ml/min [mean (SD)] | |||
| 100 | 56.1 (19.9) | 55.2 (15.2) | 55.7 (11.7) |
| 200 | 40.6 (11.5) | 48.4 (11.5) | 46.9 (18.5) |
| 400 | 34.9 (13.2) | 44.6 (17.1) | 50.4 (11.0) |
| 800 | 27.0 (5.6) | 33.3 (17.8) | 25.6 (10.0) |
| 1,200 | 29.4 (10.1) | 28.0 (8.5) | 27.5 (8.2) |
n = 6 for all groups but the 400-mg dose group.
AUCτ is 24 h.
n = 5.
N/A, not applicable.
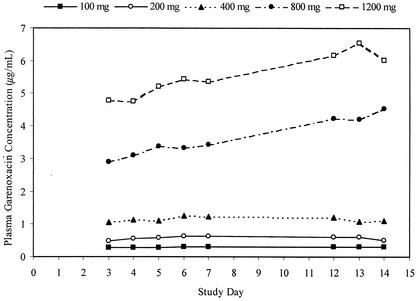
Following oral administration of garenoxacin, concentrations of the drug in plasma increased rapidly at each dose level and demonstrated median Tmax values of between 1.13 and 2.50 h (for all dose groups and on all study days). Tmax values appeared to increase with increasing doses but remained relatively constant within individual dose groups and across study days. Mean t1/2 and MRT values were similar for all doses and ranged from 13.3 to 17.8 h and 13.9 to 21.2 h, respectively. These parameters appeared to be dose independent but demonstrated an apparent increase between days 7 and 14 at all doses. Consistent with the longer t1/2 of garenoxacin relative to those of some other quinolones, a modest accumulation in systemic exposure to the drug was noted with daily oral doses of 100, 200, and 400 mg. Subjects in these dose groups generally attained steady state by day 4. Trough concentrations of garenoxacin continued to rise over the 14-day dosing period for subjects administered doses of 800 and 1,200 mg (Fig. 2), indicating that steady state was not achieved in these high-dose groups over the 14 days of treatment.
FIG. 2.
Mean trough plasma garenoxacin concentration versus study day following oral administration of 100, 200, 400, 800, or 1,200 mg.
The mean values for CLT/F at doses from 100 to 400 mg (103.5 to 122.3 ml/min) did not differ markedly with dose and demonstrated a small but consistent decrease with repeated administration. For subjects treated with garenoxacin doses of 800 and 1,200 mg, the mean values for CLT/F (67.8 to 103.3 ml/min) decreased with increasing doses and dosing days. The CLR of garenoxacin ranged from 27.0 to 56.1 ml/min across all dose groups. The CLR also decreased with the dose escalation but did not vary markedly between days 1 and 14. Approximately 20 to 50% of an administered garenoxacin dose was excreted unchanged in the urine; the mean percent UR decreased with increasing doses on each study day and was lower overall on day 1 (37.3% for the 100-mg dose and 25.4% for the 1,200-mg dose) than on day 14 (52.0 and 40.9%, respectively).
Pharmacodynamics. (i) H. pylori.
The administration of garenoxacin reduced H. pylori activity, as determined by the [14C]urea breath test and confirmed by an H. pylori IgG antibody test. The change from baseline was greatest at garenoxacin doses of ≥400 mg and was comparable to that of the placebo at doses of 100 and 200 mg. Three days after the termination of dosing (day 17), one of two previously H. pylori-positive subjects randomized to receive 400 mg of garenoxacin was negative for H. pylori activity, as were one of four and one of two subjects in the 800- and 1,200-mg dose groups, respectively. Reductions in H. pylori activity were maintained in both higher dose groups to day 45 (Table 2).
TABLE 2.
Cross tabulation of baseline and follow-up positive PYtest ([14C]urea breath test) results following 14-day oral administration of garenoxacin
| Study medication and dose (mg) | No. of patients at day −2 | No. (%) of test-positive subjects who were:
|
|||
|---|---|---|---|---|---|
| Positive at follow-up
|
Negative at follow-up
|
||||
| Day 17 | Day 45 | Day 17 | Day 45 | ||
| Placebo | 7 | 7 (100) | 6 (100)a | 0 (0) | 0 (0) |
| Garenoxacin | |||||
| 100 | 2 | 2 (100) | 2 (100) | 0 (0) | 0 (0) |
| 200 | 3 | 3 (100) | 3 (100) | 0 (0) | 0 (0) |
| 400 | 2 | 1 (50) | 2 (100) | 1 (50) | 0 (0) |
| 800 | 4 | 3 (75) | 3 (75) | 1 (25) | 1 (25) |
| 1,200 | 2 | 1 (50) | 1 (50) | 1 (50) | 1 (50) |
One subject was missing but was considered positive for the percent calculation.
(ii) Psychometric tests.
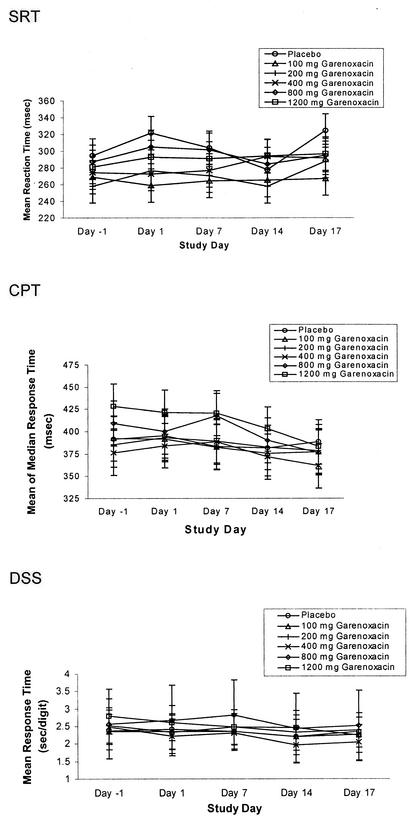
The administration of garenoxacin did not appear to have an effect on the performance of the subjects on any of the psychometric tests on any study day (Fig. 3).
FIG. 3.
Mean responses for simple reaction time (SRT), continuous performance time (CPT), and digit symbol substitution (DSS) versus time by treatment group following multiple-dose oral administration of garenoxacin.
Safety. (i) Adverse events.
There were no serious adverse events. One subject receiving 400 mg of garenoxacin/day discontinued participation on day 10, reporting epigastric pain and fatigue, both of which had been characteristic of this individual's pretreatment history. Treatment-emergent clinical adverse events were reported by, or observed in, 24 of 40 (60%) subjects, including 20 of 30 (67%) subjects who received garenoxacin and 4 of 10 (40%) randomized to receive placebo. A total of 61 adverse events were reported by the 24 subjects: 54 by those dosed with garenoxacin and 7 by those receiving placebo. With a single exception, all adverse events were mild or moderate in intensity and all were resolved without treatment. The most common adverse events in the subjects who received garenoxacin were headache (n = 7; 23%), pharyngitis (n = 5; 17%), dizziness (n = 4; 13%), and white exudate (n = 4; 13%). There did not appear to be any relationship between the garenoxacin dose and either the type or the frequency of adverse events.
(ii) Clinical laboratory findings.
No subject discontinued participation due to abnormal clinical laboratory values. For both hematology and serum chemistry assessments, clinical laboratory changes from baseline were minor and none was considered clinically significant. No subject who received either garenoxacin or placebo had an alanine aminotransferase elevation of >2.5 times the upper limit of normal.
(iii) Vital signs, physical examination findings, and ECGs.
No subject discontinued participation due to abnormalities in vital signs or ECGs. Moreover, there was no evidence that garenoxacin had any relevant effect on either vital signs or physical examination findings.
ECGs were further analyzed for changes in QTc intervals. No clinically meaningful changes were apparent in the ECG results, particularly in the QTc interval. There was no male subject with a QTc interval of >430 ms or female subject with a QTc interval of >450 ms, and there was no subject with a QTc change from baseline of >60 ms in any of the dose groups.
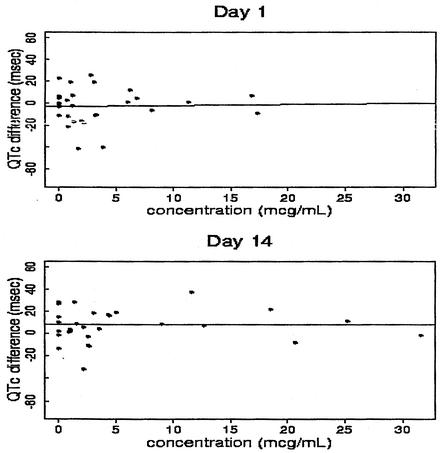
Visual examination of the mean plots for QTc suggests that there were neither substantial differences between the placebo and active treatment groups nor any apparent dose-response relationships across time points for those ECG measurements. The observed overall means for the derived QTc endpoints (average QTc, maximum QTc, and QTc at Tmax) and for their changes from baseline QTc for all active doses combined were lower than the corresponding means for the placebo on all study days. Further, the relationships between the derived QTc endpoints (average QTc, maximum QTc, QTc at Tmax, and the corresponding changes from baseline) and Cmax and the average plasma garenoxacin concentration were examined. Overall, the majority of the linear regression analysis produced 95% confidence intervals for the slopes that included zero, suggesting that there was no apparent concentration-dependent increase in QTc intervals with the oral administration of garenoxacin. The results of QTc analysis discussed above suggest that there was no clinically significant effect of garenoxacin on the ECG in general or on the QTc interval specifically. A representative plot for the changes from baseline QTc to maximum QTc versus Cavg (0-6 h) for all of the subjects by study day is presented in Fig. 4.
FIG. 4.
Differences in maximum QTc versus Cavg (0 to 6) for all subjects by study day following multiple-dose oral administration of garenoxacin.
DISCUSSION
The results of this study indicate that garenoxacin is rapidly absorbed after oral administration, demonstrates linear and dose-proportional pharmacokinetics up to oral doses of 400 mg/day, and has an elimination t1/2 that permits once-daily dosing. The results of pharmacodynamic assessments indicate that garenoxacin has no clinically significant effects on ECGs (including QTc prolongation) or evaluations of the central nervous system function. In addition, garenoxacin administered alone for 14 days at higher doses may be active against H. pylori. Finally, garenoxacin was well tolerated by healthy adult subjects at oral doses of up to 1,200 mg/day for up to 14 days.
The pharmacokinetic profile for garenoxacin is generally similar to that of many of the recently developed quinolones. These drugs (e.g., levofloxacin, sparfloxacin, gatifloxacin, and moxifloxacin) have elimination t1/2s that permit once-daily dosing and demonstrate pharmacokinetic profiles that are generally linear and dose proportional over the clinical-dose range (6, 15, 24). The nonlinear relationship between dose and drug exposure observed for garenoxacin at the 800- and 1,200-mg/day dose groups has also been noted for high doses of grepafloxacin and has been attributed to both saturation of metabolism and distribution into a peripheral compartment (24). Interestingly, values for the garenoxacin CLR decreased with increasing dose and were greater than the glomerular filtration rate (approximately 120 ml/min) when expressed as the CLR of unbound garenoxacin (data not shown) at doses of up to 400 mg. However, values for CLR of unbound garenoxacin at the 800- and 1,200-mg doses were similar to those for the glomerular filtration rate. These findings appear to suggest that the excretion of garenoxacin by the kidneys occurs via a combination of glomerular filtration and net tubular secretion and that the secretory process demonstrates saturation at doses of 800 mg or greater. The deviation from linearity noted in systemic exposure to garenoxacin is unlikely to be clinically significant, as it was noted at doses outside the anticipated dosing range.
One additional characteristic of the pharmacokinetic profile of garenoxacin is worthy of mention. The renal elimination of this des-F(6) quinolone accounted for between 30 and 50% of the garenoxacin doses evaluated in this study. This finding suggests that the elimination of garenoxacin is relatively balanced between renal and nonrenal pathways. This finding contrasts with those for many of the other quinolones that are eliminated almost exclusively by either hepatic metabolism (grepafloxacin) (8) or urinary excretion (levofloxacin) (15). Results from work with other agents that are eliminated by multiple pathways (such as bisoprolol by the liver and kidney) (18) suggest that the overall elimination of garenoxacin may not be substantially altered in patients with either renal or hepatic insufficiency and could thus potentially simplify dosing in these patient groups.
While quinolones have been highly useful for the treatment of a wide range of bacterial infections, the potential cardiotoxicity of some of the newer agents in this class has raised general concerns about their safety (6, 14). Administration of certain quinolones has been shown to prolong the QT interval, an event which may be associated with the onset of torsade de pointes and potentially life-threatening ventricular arrhythmias (7, 17). The results from the present study indicate that the use of garenoxacin is not likely to be limited by concerns regarding QTc prolongation. None of the subjects who received garenoxacin experienced a change in QTc of >60 ms, and none of the observed changes in QTc intervals was considered to be clinically significant. The results of the QTc analysis suggested that garenoxacin does not produce any dose-related and/or concentration-related effects on the QTc interval. Moreover, there was no apparent relationship between the garenoxacin dose or Cmax and either the average QTc interval or the change from baseline for this parameter.
In summary, the results of this study indicate that the pharmacokinetics of garenoxacin are dose proportional over the anticipated therapeutic dose range and that there is only modest accumulation of the drug over 14 days of oral dosing. Garenoxacin steady state was attained after 4 days, and the >13-h t1/2 demonstrated for this des-F(6) quinolone is supportive of once-daily dosing regimens. The multiple-dose administration of garenoxacin is well tolerated in healthy subjects, with no clinically significant effects on QTc interval or psychometric test results. Overall, these results indicate that multiple daily oral doses of garenoxacin of up to 1,200 mg administered for up to 14 days are safe and well tolerated.
REFERENCES
- 1.Applebaum, P. C. 1999. Quinolone activity against anaerobes. Drugs 58(Suppl. 2):60-64. [DOI] [PubMed] [Google Scholar]
- 2.Ball, P., L. Mandell, Y. Niki, and G. Tillotson. 1999. Comparative tolerability of the newer floroquinolone antibacterials. Drug Saf. 21:407-421. [DOI] [PubMed] [Google Scholar]
- 3.Bazett, H. C. 1920. An analysis of the time relations of electrocardiograms. Heart 7:353-370. [Google Scholar]
- 4.Bebear, C. M., J. Renaudin, A. Charron, H. Renaudin, B. de Barbeyrac, T. Schaeverbeke, and C. Bebear. 1999. Mutations in the gyrA, parC, and parE genes associated with fluoroquinolone resistance in clinical isolates of Mycoplasma hominis. Antimicrob. Agents Chemother. 43:954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertino, J., Jr., and D. Fish. 2000. The safety profile of the fluoroquinolones. Clin. Ther. 22:798-817. [DOI] [PubMed] [Google Scholar]
- 6.Blondeau, J. M. 1999. Expanded activity and utility of the new fluoroquinolones: a review. Clin. Ther. 21:3-40. [DOI] [PubMed] [Google Scholar]
- 7.Chiba, K., A. Sugiyama, Y. Satoh, H. Shiina, and K. Hashimoto. 2000. Proarrhythmic effects of fluoroquinolone antibacterial agents: in vivo effects as physiologic substrate for torsades. Toxicol. Appl. Pharmacol. 169:8-16. [DOI] [PubMed] [Google Scholar]
- 8.Efthymiopoulos, C., S. L. Bramer, and A. Maroli. 1997. Pharmacokinetics of grepafloxacin after oral administration of single and repeat doses in healthy young males. Clin. Pharmacokinet. 33(Suppl. 1):1-8. [DOI] [PubMed] [Google Scholar]
- 9.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huczko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoellman, D. B., L. M. Kelly, M. R. Jacobs, and P. C. Applebaum. 2001. Comparative antianaerobic activity of BMS 284756. Antimicrob. Agents Chemother. 45:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, R. N., and M. A. Pfaller. 2000. In vitro activity of newer fluoroquinolones for respiratory tract infections and emerging patterns of antimicrobial resistance: data from the SENTRY Antimicrobial Surveillance Program. Clin. Infect. Dis. 31(Suppl. 2):S516-S523. [DOI] [PubMed]
- 12.Jones, R. N., M. A. Pfaller, and M. Stilwell. 2001. Activity and spectrum of BMS 284756, a new des-F (6) quinolone, tested against strains of ciprofloxacin-resistant Gram-positive cocci. Diagn. Microbiol. Infect. Dis. 39:133-135. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, M. T., A. C. Gales, H. S. Sader, M. A. Pfaller, R. N. Jones, and the SENTRY Participants Group (Latin America). 2000. Frequency of occurrence and antimicrobial susceptibility patterns for pathogens isolated from Latin American patients with a diagnosis of pneumonia: results from the SENTRY antimicrobial surveillance program (1998). Diagn. Microbiol. Infect. Dis. 37:63-74. [DOI] [PubMed] [Google Scholar]
- 14.Lipsky, B. A., and C. A. Baker. 1999. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin. Infect. Dis. 28:352-364. [DOI] [PubMed] [Google Scholar]
- 15.Lubasch, A., I. Keller, K. Borner, P. Koeppe, and H. Lode. 2000. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob. Agents Chemother. 44:2600-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nord, C. E., D. A. Gajjar, and D. M. Grasela. 2002. Ecological impact of the des-F(6)-quinolone, BMS-284756, on the normal intestinal microflora. Clin. Microbiol. Infect. 8:229-239. [DOI] [PubMed] [Google Scholar]
- 17.Patmore, L., S. Fraser, D. Mair, and A. Templeton. 2000. Effects of sparfloxacin, grepafloxacin, moxifloxacin, and ciprofloxacin on cardiac action potential duration. Eur. J. Pharmacol. 406:449-452. [DOI] [PubMed] [Google Scholar]
- 18.Payton, C. D., J. G. Fox, N. F. Pauleau, J. M. Boulton-Jones, C. Loannides, A. Johnston, and P. Thomas. 1987. The single dose pharmacokinetics of bisoprolol (10 mg) in renal insufficiency: the clinical significance of balance clearance. Eur. Heart J. 8(Suppl. M):15-22. [DOI] [PubMed] [Google Scholar]
- 19.Sader, H. S., R. N. Jones, A. C. Gales, P. Winokur, K. C. Kugler, M. A. Pfaller, G. V. Doern, and the SENTRY Latin American Study Group. 1998. Antimicrobial susceptibility patterns for pathogens isolated from patients in Latin American medical centers with a diagnosis of pneumonia: analysis of results from the SENTRY Antimicrobial Surveillance Program (1997). Diagn. Microbiol. Infect. Dis. 32:289-301. [DOI] [PubMed] [Google Scholar]
- 20.SENTRY Participants Group (Latin America), A. Gales, H. Sader, and R. N. Jones. 2001. Activities of BMS 284756 (T-3811) against Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae isolates from SENTRY Antimicrobial Surveillance Program medical centers in Latin America (1999). Antimicrob. Agents Chemother. 45:1463-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahlmann, R., and H. Lode. 1999. Toxicity of quinolones. Drugs 58(Suppl. 2):37-42. [DOI] [PubMed] [Google Scholar]
- 22.Takahata, M., J. Mitsuyama, Y. Yamashiro, M. Yonezawa, H. Araki, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 1999. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob. Agents Chemother. 43:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahata, M., M. Shimakura, R. Hori, K. Kizawa, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 2001. In vitro and in vivo efficacies of T-3811ME (BMS-284756) against Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 45:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnidge, J. 1999. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 58(Suppl. 2):29-36. [DOI] [PubMed] [Google Scholar]