Abstract
Mussels (Mytilus edulis) are economically important in their role as an aquaculture species and also with regard to marine biofouling. They attach tenaciously to a wide variety of submerged surfaces by virtue of collagenous attachment threads termed ‘byssi’. The aim of this study was to characterize the spreading of the byssal attachment plaque, which mediates attachment to the surface, on a range of surfaces in response to changes in wettability. To achieve this, well characterized self-assembled monolayers of ω-terminated alkanethiolates on gold were used, allowing correlation of byssal plaque spreading with a single surface characteristic—wettability. The present results were inconsistent with those from previous studies, in that there was a positive correlation between plaque size and surface wettability; a trend which is not explained by conventional wetting theory for a three-phase system. A recent extension to wetting theory with regard to hydrophilic proteins is discussed and the results of settlement assays are used to attempt reconciliation of these results with those of similar previous studies and, also, with recent data presented for the spreading of Ulva linza spore adhesive.
Keywords: Mytilus edulis, byssus, wettability, self-assembled monolayers
1. Introduction
Mussels of the genera Mytilus, Dreissena and Perna cause a serious and persistent fouling problem affecting, e.g. aquaculture nets, off-shore rigs and industrial coolant outflows (Kingsbury 1981; Forteath et al. 1984; Edyvean et al. 1985; Southgate & Myers 1985; Relini & Monranari 1999; Nishida et al. 2003). Their large size and accessibility of the attachment apparatus make mussels highly suitable model organisms for adhesion (Crisp et al. 1984; Waite & Qin 2001) and antifouling studies (da Gama et al. 2004).
Mytilus edulis is a wide-spread, temperate water species that inhabits rocky intertidal coastlines around northern Europe and north-eastern USA. Although they have the appearance of sessile organisms, the juveniles of this species are highly motile by virtue of their muscular foot. The foot has a sensory role (Mahéo 1970), but functions primarily to attach mussels to surfaces. The method of attachment by multiple, collagenous, attachment threads, or byssi (Waite et al. 1998) is unique to its class (Yonge 1962). The byssi attach with great tenacity to almost any submerged surface (Young & Crisp 1981) and ensure that the organisms can remain anchored on wave-beaten shores.
The byssal thread is a complex structure that can be divided into three main areas: (i) the root that is embedded in the base of the muscular foot; (ii) the threads that are produced along a ventral groove that runs the length of the foot; and (iii) the attachment disc, or plaque, as it will be referred to here, which mediates adhesion to the substratum. The byssus is composed primarily of five proteins (termed Mefp 1–5) as well as collagen. The relatively small protein, Mefp-3, is of particular interest to this study as Mefp-3 is believed to contribute to adhesion of the byssal plaque to the substratum (Yu et al. 1999) and has also been referred to as an adhesive primer (Warner & Waite 1999). The basis for these hypotheses lies in the high concentration (20 mol%) of the amino acid 3,4-dihydroxyphenyl-L-alanine (DOPA; Warner & Waite 1999; Floriolli et al. 2000; Waite & Qin 2001) in Mefp-3, as well as the observation that multiple forms (at least 10 electrophoretic variants) of the protein may be expressed by individual mussels in response to different surface compositions. It is suggested that protein expression may be determined by the physical properties of the attachment surface (Vreeland et al. 1998; Warner & Waite 1999), i.e. the adhesive could be matched to the surface for maximal adhesive force, although this has not been shown conclusively.
Despite their status as model organisms for adhesion studies, direct measures of mussel adhesion are surprisingly rare. There is also insufficient information to construct a clear picture of the preference of mussels for different substrata (Hansen et al. 1994; Yamamoto et al. 1997; Nishida et al. 2003). Young & Crisp (1981) investigated the deposition and spreading of the byssal plaque of M. edulis on surfaces differing in free energy and polarity, namely slate, glass, paraffin wax and PTFE (polytetrafluoroethylene). Their results demonstrated that the byssal plaque spread significantly further and tenacity was lower on the low-energy surfaces (PTFE and paraffin wax, which were also non-polar). They concluded that a simple balance of thermodynamic forces governs the deposition and spreading of the plaque and a combination of Young–Dupré equations (see §4) was invoked to describe the competition between the adhesive and the liquid medium (seawater) for the test surface. In reality, however, their test surfaces differed in many other properties, including rugosity and bulk/elastic modulus. Therefore, their findings must be viewed as equivocal.
Recently, a similar study was carried out on the spreading of the secreted adhesive of spores of the green alga Ulva linza. This used surfaces of different wettability in the form of self-assembled monolayers (SAMS) of CH3- or OH-terminated alkanethiolates on gold, or mixtures of the two (Callow et al. 2005). The use of SAMs as model surfaces with which to study the adhesion processes of marine organisms (e.g. Ista et al. 1996, 2004; Callow et al. 2000a; Finlay et al. 2002) is advantageous compared with heterogeneous substrates like slate, glass, etc. since they are chemically defined, uniform in surface topography and modulus, and in the case of CH3- and OH-terminations, uncharged at the pH of seawater.
The results of Callow et al. (2005) were not consistent with those reported for mussel adhesive spreading by Young & Crisp (1981) since spreading of the spore adhesive was greatest on hydrophilic surfaces and least on hydrophobic surfaces. They were also not consistent with standard thermodynamic wetting theory based on the Young-Dupré equations for a 3-phase system (surface, fluid adhesive, liquid water) since standard theory would predict that a fluid adhesive should more easily ‘wet’, i.e. spread more, on a less wettable surface. In a novel extension of thermodynamic wetting theory, it was shown that this apparent contradiction can be explained on the basis that a very polar adhesive protein could indeed effectively compete with water to wet a hydrophilic surface.
In view of the results of Callow et al. (2005) it was decided to re-evaluate the findings of Young & Crisp (1981) on the spreading of the adhesive plaques of blue mussel byssi, using as substrates a range of SAMs differing in wettability. Assays were also performed to identify any preference mussels may have for specific surfaces.
2. Material and methods
2.1 Treatment of mussels
Juvenile mussels were collected from the shore at Seaton Sluice (NZ 335 765 GB Grid ref.), Northeast England two weeks prior to experimentation. They were maintained at 10 °C under constant light in aquaria containing 50 l aerated natural seawater. The seawater was changed weekly after the mussels had been fed Tetraselmis chui (500 ml, culture strength ca 105 cells ml−1).
Prior to experimentation mussels of the desired size (15±1 mm), chosen for their more extensive exploratory behaviour in comparison with fully grown adults, were separated from the culture and allowed to explore for 5 h in a shallow tray containing natural seawater. Only those mussels showing exploratory behaviour during this interval were selected for testing.
2.2 Production of SAM surfaces
SAM surfaces were produced by the method of Bain et al. (1989), having been used in similar studies previously (Ista et al. 1996; 2004; Callow et al. 2000a). Briefly, the SAMs were produced on standard glass microscope slides coated with thin layers of chromium and gold using a metal evaporator evacuated to 10−6 torr. Resulting surfaces were immersed in 1 mM ethanolic solutions of dodecanethiol, mercapto-undecanol, or mixtures of the two to produce surfaces with either CH3 or OH groups or combinations thereof at the surface. The surfaces ranged in wettability as defined using advancing water contact angles [θAW] from θAW 15° (hydrophilic) to 107° (hydrophobic).
SAMs were sealed in Coplin jars containing de-oxygenated nanopure water and delivered to Newcastle overnight. SAMs were used on the day of delivery following a preparation procedure which comprised a rinse in ethanol followed by nanopure water. The slides were then dried under nitrogen before use.
2.3 Experimental set-up
Two batches of SAMs were used during this investigation. Batch 1 comprised five different SAMs with θAW values of 103°, 80°, 63°, 30° and 17° with six replicates of each. Batch 2 comprised two different SAMs with θAW values of 15° and 107° and 36 replicates of each. Batch 1 was used for a deposition study and spreading measurements, while batch 2 was used to replicate spreading measurements and for a ‘choice’ experiment.
2.3.1 Byssal deposition
Polypropylene containers were leached with distilled water for two weeks prior to use. One valve of each mussel was stuck to polystyrene rods using cyanoacrylate adhesive. This immobilized the mussels during the assay, while still allowing free movement of the foot over the test surfaces (figure 1). Polystyrene rods were, in turn, attached to the base of the tubs using high modulus clear silicone sealant (B&Q plc. UK.). The containers were filled with 1 l of natural seawater and incubated for 24 h at 10 °C under constant dim light and static water conditions.
Figure 1.
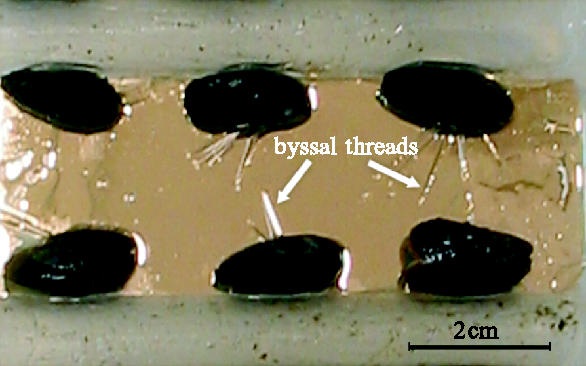
Juvenile Mytilus immobilized for deposition experiments such that they deposit byssi primarily onto the test surfaces. Gold surface is an ω-substituted alkane thiolate SAM.
For the first batch of SAMs, 36 mussels were used for each treatment (six mussels per slide) with all five treatments (θAW 103°, 80°, 63°, 30° and 17°) tested simultaneously. The number of byssi deposited by all mussels was counted every hour for the first 8 h of the experiment and then again after 24 h. At the end of the 24 h period the byssi deposited by individual mussels were marked by scratching the gold surface of the SAMs and then cut with fine scissors. SAM-coated slides bearing byssal plaques were removed and fixed with 3% glutaraldehyde in 0.45 μm filtered seawater, for 1 h at 5 °C. They were then stored prior to measurement (over a 5 day period) at 5 °C in 0.45 μm filtered seawater. No change in plaque size was observed over this period and measurements were conducted in a random order to negate any possible effect of storage.
2.3.2 Choice assay
Most of the second batch of SAMs was used in a choice assay. Thirty mussels were allowed to ‘choose’ between OH-terminated (θAW 15°) and CH3-terminated (θAW 107°) SAMs in a chamber designed to restrict deposition to one of these two surfaces. This was achieved by coating all other surfaces with nylon plankton mesh (150 μm pore size); a surface inhibitory to mussel settlement. The assay was conducted over 8 h.
The assay was considered to be unbiased. As a single SAM can only be used once, the chamber could not be calibrated and the surfaces were assumed to be equally likely to be selected, with no significant influence of surface treatment. This allowed comparison of the resultant binomial data with a 50% cumulative frequency distribution.
2.4 Data analysis
Images of the byssi were captured onto an IBM-compatible PC using an analogue CCTV camera attached to an Olympus BH-2 compound microscope. The areas of the byssi were calculated in Matrox Inspector software for Windows. Mean areas were taken for each mussel to reduce the skewing factor introduced for those mussels that habitually produced more byssi. A mean spreading area per surface could then be calculated to allow inter-comparison of surfaces.
Due to the variability in byssal plaque shape, size and irregularities highlighted by transmitted light, it was necessary to manually define the edges of the adhesive plaque. A macro designed for this purpose may have been less time-consuming; however, it would have been less accurate. The use of the natural autofluorescence of the structures did not yield any increase in accuracy of measurements and in fact did not demonstrate the full extent of spreading. For these reasons, the measurements were taken from captured images of byssal adhesive plaques (greater than 500 in total) in their native state (figure 2).
Figure 2.
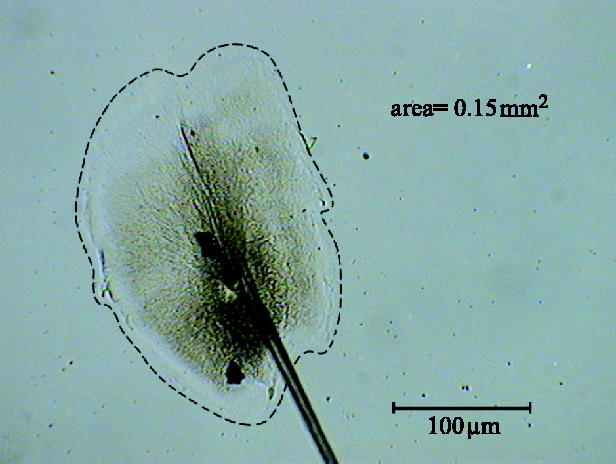
An image of byssal plaque, similar to those used for measurement.
3. Results
3.1 Attachment of mussels
In general, mussels attached more rapidly to high wettability surfaces than to lower wettability surfaces. Attachment was defined as the deposition of one or more byssi on a surface and the time that this initial attachment occurred was recorded for each mussel. After a comparatively slow initial rate of attachment to the 103° surface, mussels attached at a high rate. In comparison, the mussels on the 17° surface attached in higher numbers immediately. Nevertheless, although attachment rates were measured throughout the investigation, no significant trend of increased attachment rate with increased wettability was detected (data not shown).
3.2 Number of byssi produced and rate of deposition on SAMs
The mean number of byssi produced, per individual, on each of the 5 surfaces was determined at all time intervals. The values for 24 h are shown in figure 3. A strong correlation coefficient and significant regression (F=20.3, p=0.02) suggested that byssal production increased significantly with decreasing wettability. The rate of byssus deposition on each surface is presented in figure 4. For clarity only two regressions, 17° and 103°, representing byssus deposition at the extremes of the wettability spectrum, are shown. The 24 h data points were removed from the regression, but in all cases were similar to the 8 h reading where deposition was maximal.
Figure 3.
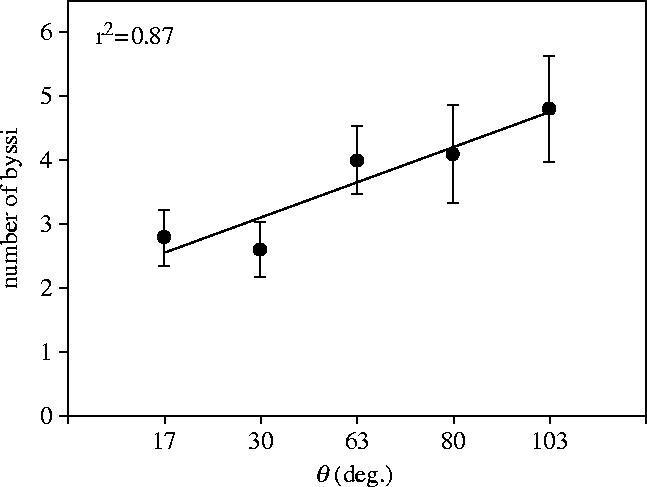
The mean number of byssi deposited per individual plotted against internal θAW on all test surfaces after 24 h.
Figure 4.
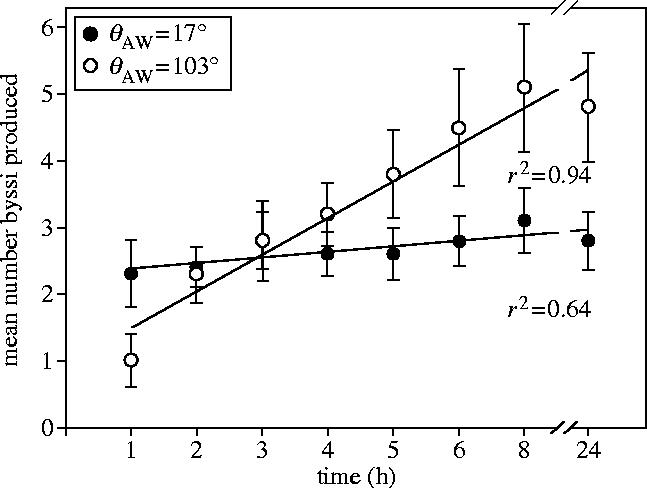
Regressions of the rate of deposition of byssi on 100% OH (hydrophilic) terminated (17° θAW) and 100% CH3 (hydrophobic) terminated (103° θAW) SAMs over 8 h.
There was a significant linear increase of byssal deposition with time on the 103° SAM (F=113.28, p=<0.001), but not on the 17° SAM (F=2.94, p=0.174). On the 17° SAM, most byssi were produced within the first hour, whereas byssi were continually deposited until 8 h on the 103° SAM.
3.3 Comparison of byssus plaque areas on different surfaces
The measurement of byssal areas on each of the surfaces after 24 h revealed a significant (F=99.39, p=0.002) positive linear relationship between plaque area and increasing wettability (figure 5). This trend was validated by a repeat experiment that also showed a significant difference (F=10.9, p=0.002) between plaque areas deposited on the θAW 15° and 107° SAMs. Mean plaque areas ranged from 0.16±s.e. 0.0077 mm2 for the 17° SAMs to 0.14±0.006 mm2 for the 103° SAMs.
Figure 5.
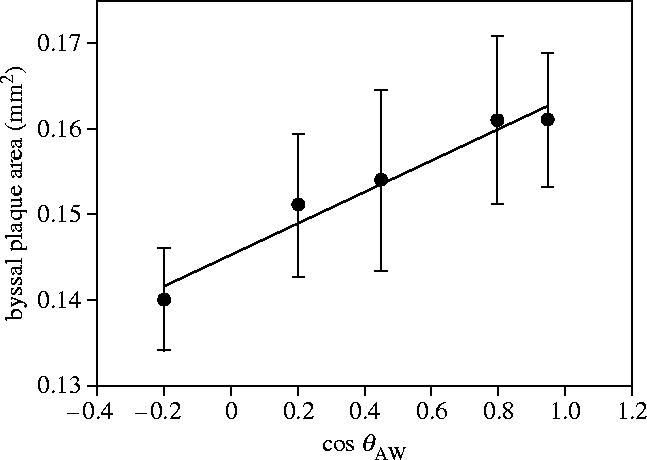
Mean byssal plaque area plotted against the cosine of internal θAW. As wettability increases, θAW measured internally decreases and cos θAW becomes larger.
3.4 Choice assay
Out of 30 mussels tested, 24 selected a surface by 8 h. Eighteen of these deposited the majority of their byssi onto the 15° SAM in preference to the 107° SAM. The probability of this occurring by chance alone, according to the 50% cumulative frequency plot, is less than 0.001 and can therefore be attributed to the introduction of a significant bias by the surfaces. The 15° SAM can thus be considered significantly more attractive to settling mussels than the 107° SAM.
4. Discussion
The results of this study were not consistent with previous findings (Young & Crisp 1981) since a positive correlation was observed between adhesive plaque spreading and wettability. The most obvious explanation for the different results is that while the present study used SAMs, Young & Crisp (1981) used highly heterogeneous materials for their test surfaces (slate, glass, PTFE and paraffin wax). These would have differed in wettability, but also in chemistry, topography, polarity and modulus and it is therefore possible that their results were influenced by one or more of these properties, other than, or in combination with, wettability. Surface roughness in particular is known to affect adhesion of marine invertebrates (Berntsson et al. 2000a,b, 2004). Further, the number of measurements taken here (greater than 500 and replication) in comparison to nine individuals used by Young & Crisp (1981) allows confidence to be placed in the present results.
Given the discrepancies between studies, the applicability of conventional wetting should be examined. From the Dupré equation
where θ is the adhesive contact angle, AS describes the adhesive/solid interface, SL the solid/liquid interface and LA the liquid/liquid interface, Crisp et al. (1984) derived the equivalent of
where WSA and WSL are the changes in adhesive energy, or work of adhesion, of the solid/adhesive and solid/liquid interfaces, respectively. γLA remains constant. (θ, here, is an external contact angle that is used to describe the spreading of the adhesive on a surface, under water. This should not be confused with the internal contact angle formed by a water droplet on a surface, which is used as a measure of surface wettability; typically as its cosine. To avoid confusion the latter is referred to as θAW throughout this paper). This demonstrates that as WSL increases from low to high wettability, the external contact angle θ will decrease, leading to restricted spreading of the adhesive on highly wettable surfaces. This trend has also been demonstrated empirically using inert probe liquids (Clint & Wicks 2001). The fact that mussel plaques did not behave in this way in the present study suggests that the adhesive has a higher surface tension than the ambient medium—seawater.
In Callow et al. (2005), attention was drawn to the fact that proteins are highly complex molecules containing both polar and non-polar domains. There is potential then for them to show quite different spreading characteristics compared to inert probe liquids. Mefp-3, for example, is extensively hydroxylated and can, therefore, engage readily in hydrogen bonding (Waite & Qin 2001). Further, catechol, a DOPA side chain, is able to form strong hydrogen bonds with hydrophilic polymers (Wiegemann 2005). This hydrophilic character, however, would lead logically to the conclusion that de-wetting of the adhesive for successful contact with the surface would be difficult. Thus, in order for adhesion to be successful, the work of adhesion between the adhesive and the surface (WSA) must also be greater than the energy needed to dehydrate the liquid adhesive (WLA). Callow et al. (2005) suggested that for a highly polar proteinaceous adhesive, WSA can be sufficiently large to allow further spreading of adhesive on high-energy surfaces than low-energy surfaces. The highly hydrophilic character of mussel byssal proteins may, therefore, be crucially important in their ability to spread well on high-energy surfaces.
Briefly, the conclusion of the Callow et al. (2005) study was that two different components of surface wettability affected the spreading of Ulva adhesive. Namely, dispersion forces in relation to the CH3-terminated regions of the SAMs and hydrogen bonding in relation to the OH regions.
It is well established that high-energy surfaces allow the formation of stronger adhesive bonds than low-energy surfaces (Baier et al. 1968; Lindner 1992) and marine organisms, especially fouling species, may have evolved mechanisms of attachment that yield strong adhesion to low-energy surfaces when necessary; or, conversely, methods of de-wetting high-energy surfaces in order to facilitate attachment. In the case of mussels, this adaptation could be an adhesive with properties that efficiently displace water from high-energy surfaces and/or the mussel may perceive the surface properties (Mahéo 1970) and alter the composition of the adhesive to effect maximal adhesion (Vreeland et al. 1998; Warner & Waite 1999).
Although further study is clearly needed to help explain the observed trend of increasing byssal plaque size with increasing surface wettability, a detailed examination of the results here is illuminating. Mussels on the hydrophobic (θAW=∼103°) SAMs attached differently to those on the more hydrophilic surfaces: initially, deposition was slow, with the rate increasing after the first hour. Mussels ultimately produced fewer byssi on the 17° surface than on the 103° surface (figure 3), with most attachments occurring in the first hour (figure 4) on the former. This is also contrary to the findings of Young & Crisp (1981). Mussels deposited byssi at a comparatively slow rate for the first hour on the 103° SAM but then continued to deposit byssi for up to 8 h with no appreciable increase thereafter. Nishida et al. (2003), likewise found that the mussel, Perna viridis, deposited more byssi onto nylon, rubber, silicone and PTFE (low-energy surfaces) than onto glass (high-energy). However, no statistical analyses were presented.
Although the data can be interpreted in several ways, one plausible sequence of events when a mussel is presented with a high-energy surface is as follows:
high-energy surface is detected;
identified as a good attachment site and the mussel attaches quickly;
byssi are deposited immediately;
byssal plaque spreads well, resulting in high tenacity attachment;
few byssi are required, so byssal production stops, conferring an energetic advantage (energy that would be used to produce byssi can be used for other purposes) to the mussel.
The opposite might apply for low-energy surfaces. This scheme of events is favoured because it relies upon a physical process mediated by either the SAM surface, or an adhesive that overcomes the natural tendency of water to coat high-energy surfaces, making adhesive wetting difficult. Some marine organisms adhere preferentially to low-energy surfaces when the liquid medium is of high surface tension, e.g. water (Absolom et al. 1983; van Loosdrecht et al. 1987; Otto et al. 1999; Callow et al. 2000a; Finlay et al. 2002), so it is possible that the mussel can detect the electrostatic properties of the surface and spread its adhesive accordingly. If mussels prefer to attach to high-energy surfaces, a selection that might have reflected an increased strength of adhesion, then this hypothesis could be tested experimentally. However, if this adaptive compensation occurred in response to a physical restriction on byssal plaque spreading, similar spreading of the adhesive on all surfaces would be anticipated. This is not the case here (figure 5).
In theory at least, the Ulva case is made easier to explain with the knowledge that there can be no deliberate spreading of the adhesive, therefore excluding a behavioural explanation. Spores contain a finite quantity of pre-formed adhesive, which is expressed externally and spreads passively (Callow et al. 2000b).
This work represents the first step towards investigating the adhesion of M. edulis systematically and moves beyond the usual application of this organism as an attachment bioassay, towards gaining a fuller understanding of why these surface preferences exist in M. edulis, as well as other fouling organisms. Greater knowledge of how these adhesives work would seem to be fundamental to the development of effective, minimally adhesive marine coatings. Indeed, from this study alone it is made clear that although we may know certain surfaces to be deleterious to adhesion, our explanations for this characteristic may be far from complete.
Following directly from this work, an important future step is to test the strengths of adhesion of byssi deposited onto SAMs, and to extend the test protocol further to studies of other marine fouling organisms.
Acknowledgments
N.A. was supported by a NERC studentship and funded, in part, by the US Office of Naval Research grants awarded to A.S.C., J.A.C. and M.E.C. (N00014-02-1-0311) and to G.P.L. (N0014-02-1-0377). The authors would also like to thank M. K. Chaudhury for his constructive comment and analysis throughout the drafting of this paper.
References
- Absolom D.R, Lamberti F.V, Policova Z, Zingg W, van Oss C, Neumann W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier R.E, Shafrin E.G, Zisman W.A. Adhesion: mechanisms that assist or impede it. Science. 1968;162:1360–1368. doi: 10.1126/science.162.3860.1360. [DOI] [PubMed] [Google Scholar]
- Bain C.D, Troughton E.B, Tao Y.T, Evall J, Whitesides G.M, Nuzzo R.G. Formation of monolayer films by spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989;111:323–335. [Google Scholar]
- Berntsson K.M, Andreasson H, Jonsson P.R, Larsson L, Ring K, Petronis S, Gatenholm P. Reduction of barnacle recruitment on micro-textured surfaces: analysis of effective topographic characteristics and evaluation of skin friction. Biofouling. 2000;16:245–261. [Google Scholar]
- Berntsson K.M, Jonsson P.R, Lejhall M, Gatenholm P. Analysis of behavioural rejection of micro-textured surfaces and implications for recruitment by the barnacle Balanus improvisus. J. Exp. Mar. Biol. Ecol. 2000;251:59–83. doi: 10.1016/s0022-0981(00)00210-0. doi:10.1016/S0022-0981(00)00210-0 [DOI] [PubMed] [Google Scholar]
- Berntsson K.M, Jonsson P.R, Larsson A.I, Holdt S. Rejection of unsuitable substrata as a potential driver of aggregated settlement in the barnacle Balanus improvisus. Mar. Ecol. Prog. Ser. 2004;275:199–210. [Google Scholar]
- Callow M.E, Callow J.A, Ista L.K, Coleman S.E, Nolasco A.C, López G.P. Use of self-assembled monolayers of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 2000a;66:3249–3254. doi: 10.1128/aem.66.8.3249-3254.2000. doi:10.1128/AEM.66.8.3249-3254.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow J.A, Crawford S.A, Higgins M.J, Mulvaney P, Wetherbee R. The application of atomic force microscopy to topographical studies and force measurements on the secreted adhesive of the green alga Enteromorpha. Planta. 2000b;211:641–647. doi: 10.1007/s004250000337. doi:10.1007/s004250000337 [DOI] [PubMed] [Google Scholar]
- Callow J.A, Callow M.E, Ista L.K, Lopez G, Chadhury M.K. The influence of surface energy on the wetting behaviour of the spore adhesive of the marine alga Ulva linza (synonym Enteromorpha linza) J. R. Soc. Interface. 2005;2:319–325. doi: 10.1098/rsif.2005.0041. doi:10.1098/rsif.2005.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clint J.H, Wicks A.C. Adhesion under water: surface energy considerations. Int. J. Adhes. Adhesives. 2001;21:267–273. doi:10.1016/S0143-7496(00)00029-4 [Google Scholar]
- Crisp D.J, Walker G, Young G.A, Yule A.B. Adhesion and substrate choice in mussels and barnacles. J. Col. Int. Sci. 1984;104:40–50. doi:10.1016/0021-9797(85)90007-4 [Google Scholar]
- da Gama B.A.P, Pereira R.C, Soares A.R, Teixeira V.L, Yoneshigue-Valentin Y. Is the mussel test a good indicator of antifouling activity? A comparison between laboratory and field assays. Biofouling. 2003;19:161–169. doi: 10.1080/0892701031000089534. doi:10.1080/0892701031000089534 [DOI] [PubMed] [Google Scholar]
- Edyvean E.G, Terry J.I.A, Picken G.B. Marine fouling and its effects on offshore structures in the North Sea—a review. Int. Biodeter. 1985;21:227–284. [Google Scholar]
- Finlay J.A, Callow M.E, Ista L.K, López G.P, Callow J.A. The influence of surface wettability on the adhesion strength of settled spores of the green alga Enteromorpha and the diatom Amphora. Integr. Comp. Biol. 2002;42:1116–1122. doi: 10.1093/icb/42.6.1116. [DOI] [PubMed] [Google Scholar]
- Floriolli R.Y, von Langen J, Waite J.H. Marine surfaces and the expression of specific byssal adhesive protein variants in Mytilus. Mar. Biotechnol. 2000;2:352–363. doi: 10.1007/s101269900032. [DOI] [PubMed] [Google Scholar]
- Forteath G.N.R, Picken G.B, Ralph R. Patterns of macrofouling on steel platforms in the central and northern North Sea. In: Lewis J.R, Mercer A.D, editors. Corrosion and marine growth on offshore structures. Ellis Horwood Limited; Chichester: 1984. pp. 10–22. [Google Scholar]
- Hansen D.C, Luther G.W, III, Waite J.H. The adsorption of the adhesive protein of the blue mussel Mytilus edulis L. onto type 304L stainless steel. J. Col. Int. Sci. 1994;168:206–216. doi:10.1006/jcis.1994.1410 [Google Scholar]
- Ista L.K, Fan H, Baca O, López G.P. Attachment of bacteria to model solid surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microbiol. Lett. 1996;142:59–63. doi: 10.1111/j.1574-6968.1996.tb08408.x. doi:10.1016/0378-1097(96)00243-1 [DOI] [PubMed] [Google Scholar]
- Ista L.K, Callow M.E, Finlay J.A, Coleman S.E, Nolasco A.C, Simons R.H, Callow J.A, Lopez G.P. Effect of substratum chemistry and surface energy on attachment of marine bacteria and algal spores. Appl. Environ. Microbiol. 2004;70:4151–4157. doi: 10.1128/AEM.70.7.4151-4157.2004. doi:10.1128/AEM.70.7.4151-4157.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury R.W.S.M. Proc. of the Society for Underwater Technology Conference. Marine fouling of offshore structures. vol. 1. World Surface Coatings Abstracts, Paint Research Associates; London: 1981. Marine fouling of North Sea installations; pp. 1–22. [Google Scholar]
- Lindner E. A low surface energy approach in the control of marine biofouling. Biofouling. 1992;6:192–205. [Google Scholar]
- Mahéo R. Étude de la pose et de l'activité de secretion du byssus de Mytilus edulis L. Cah. Biol. Mar. 1970;11:475–483. [Google Scholar]
- Nishida A, Ohkawa K, Ueda I, Yamamoto H. Green mussel Perna viridis L.: attachment behaviour and preparation of antifouling surfaces. Biomol. Eng. 2003;20:381–387. doi: 10.1016/s1389-0344(03)00057-1. doi:10.1016/S1389-0344(03)00057-1 [DOI] [PubMed] [Google Scholar]
- Otto K, Elwing H, Hermansson M. The role of type 1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic sufaces. Coll. Surf. B Bioint. 1999;15:99–111. doi:10.1016/S0927-7765(99)00050-8 [Google Scholar]
- Relini G, Monranari M. Proc. 10th Int. Cong. on Marine Corrosion and Fouling, Melbourne, 7–12 February 1999 (Additional Papers), DSTO-GD-0287. DSTO Aeronautical and Marine Research Laboratory; Fishermans Bend, VIC: 1999. Macrofouling role of mussels in Italian seas: a short review; pp. 17–32. [Google Scholar]
- Southgate T, Myers A.A. Mussel fouling on the Celtic Sea Kinsale Field gas platform. Estuar. Coast. Shelf. Sci. 1985;20:651–659. doi:10.1016/0272-7714(85)90023-X [Google Scholar]
- van Loosdrecht M.C.M, Lyklema J, Norde W, Schraa G, Zehnder A. J.B. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland V, Waite J.H, Epstein L. Polyphenols and oxidases in substratum adhesion by marine algae and mussels. J. Phycol. 1998;34:1–8. doi:10.1046/j.1529-8817.1998.340001.x [Google Scholar]
- Waite J.H, Qin X. Phosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry. 2001;40:2887–2893. doi: 10.1021/bi002718x. doi:10.1021/bi002718x [DOI] [PubMed] [Google Scholar]
- Waite J.H, Qin X, Coyne K.J. The peculiar collagens of mussel byssus: a minireview. Mar. Biol. 1998;17:93–106. doi: 10.1016/s0945-053x(98)90023-3. [DOI] [PubMed] [Google Scholar]
- Warner S.C, Waite J.H. Expression of multiple forms of an adhesive plaque protein in an individual mussel, Mytilus edulis. Mar. Biol. 1999;134:729–734. doi:10.1007/s002270050589 [Google Scholar]
- Wiegemann M. Adhesion in blue mussels (Mytilus edulis) and barnacles (genus Balanus): mechanisms and technical applications. Aquat. Sci. 2005;67(2):166–176. [Google Scholar]
- Yamamoto H, Ogawa T, Nishida A. Studies on quinone cross-linking adhesion mechanism and preparation of antifouling surfaces toward the blue mussel. J. Mar. Biotechnol. 1997;5:133–136. [Google Scholar]
- Yonge C.M. On the primitive significance of the byssus in the bivalvia and its effects in evolution. J. Mar. Biol. Assoc. UK. 1962;42:113. [Google Scholar]
- Young G.A, Crisp D.J. Marine animals and adhesion. In: Allen K.W, editor. Adhesion. Applied Science Publishers Ltd; UK: 1981. pp. 19–39. [Google Scholar]
- Yu M.E, Hwang J.Y, Deming T.J. Role of L-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999;121:5825–5826. doi:10.1021/ja990469y [Google Scholar]


