Abstract
An analytical method for the determination of voriconazole (UK-109,496; Pfizer) in plasma was developed and validated. The method utilizes solid-phase extraction technology and high-performance liquid chromatography. The lower limit of quantitation is 0.2 μg/ml, and the range of linearity tested was 0.2 to 10 μg/ml.
Voriconazole (VRC; UK-109,496 [C16H14N5OF3]; Pfizer Pharmaceuticals) is a novel broad-spectrum triazole antifungal that is used in the treatment of a wide range of opportunistic fungal infections, including aspergillosis (4). VRC is marketed in formulations for administration both orally (tablet) and intravenously. Previously described assays for VRC include a bioassay procedure and two different high-performance liquid chromatography (HPLC) methods. The former lacks the required sensitivity, and the latter HPLC methods either lacked the necessary sensitivity (4) or were lengthy and technically difficult (6). This assay includes the use of an internal standard (UK-115,794) and sample preparation by solid-phase extraction (SPE). Validation guidelines published by Shah et al. (5) were used to determine the method's accuracy, precision, reproducibility, and specificity.
Pfizer Research and Development, Sandwich, United Kingdom, provided VRC and internal-standard powders. The HPLC system (Beckman Coulter, Fullerton, Calif.) consisted of a 168 diode array detector, a 126 solvent pump, a 508 autosampler, an IBM NT-based computer work station, and 32 Karat software.
Stock and working VRC standards (1,000, 100, and 10 μg/ml, respectively) and stock and working internal standards (1,000 and 100 μg/ml, respectively) were all made in methanol (MeOH) and stored at −20°C. Calibration standards (0.2, 0.5, 1, 2, 4, 6, 8, and 10 μg/ml) were prepared in pooled plasma from the VRC working standards on the day of the analysis.
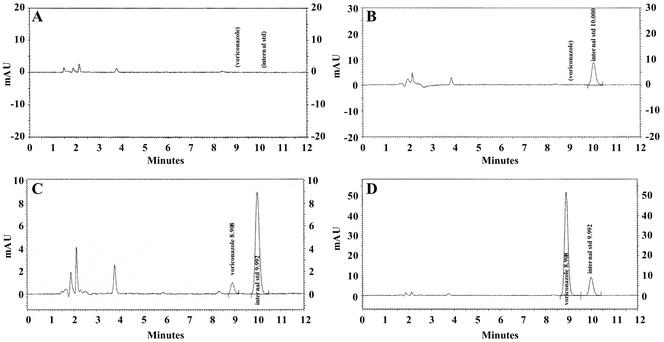
Plasma controls (0.2, 0.5, 4, and 8 μg/ml) were made in batches and frozen for analyses over a period of time from an independent weighing of VRC powder. Five hundred microliters of each blank, standard, or control was pipetted into an appropriately labeled tube. Ten microliters of a 100-μg/ml internal standard was added to each tube except the “blank-blank” tube. All samples were buffered with 700 μl of 0.2 M borate buffer (pH 9.0). Samples were extracted by SPE with C18, 100 mg, 1-ml Bond Elut columns (Varian, Inc., Harbor City, Calif.). The columns were conditioned with separate washings in the following order: 1 ml of MeOH, 1 ml of H2O, and 1 ml of 0.2 M borate buffer (pH 9.0). The buffered plasma samples were added to each respective column. After the columns completely drained, they were washed with separate and independent washings of the following reagents: 1 ml of 0.2 M borate buffer, followed by 1 ml of MeOH-H2O (50:50, vol/vol). Inside the vacuum manifold glass chamber, microcentrifuge tubes were positioned for collection of each eluted sample from its respective SPE column. One milliliter of the eluent, MeOH-glacial acetic acid (99:1, vol/vol), was added to each column. The collected eluate was dried under a stream of nitrogen at ambient temperature with a Turbo-Vap LV evaporator (ZYMARK, Hopkinton, Mass.) and reconstituted in a 200-μl mobile phase. The mobile phase consisted of 0.01 M TEMED (N,N,N′,N′-tetramethylethylenediamine) phosphate buffer (adjusted to pH 7.4 with phosphoric acid) added to acetonitrile (55:45, vol/vol). TEMED was obtained from Sigma, St. Louis, Mo. Twenty microliters of each reconstituted sample was injected under the following HPLC conditions: reverse-phase Luna 5-μm C18 column (250 by 4.6 mm), preceded by a universal SecurityGuard cartridge (Phenomenex, Torrance, Calif.), a detector wavelength of 254 nm, and a mobile-phase flow rate of 1 ml/min. Retention times were approximately 9.0 and 10.0 min for VRC and the internal standard, respectively. Representative chromatograms are shown in Fig. 1.
FIG. 1.
Representative chromatograms of VRC and the internal standard (std) (9- and 10-min retention times, respectively) in human plasma. Panels: A, blank pooled human plasma; B, pooled human plasma spiked with the internal standard at 1.0 μg/ml; C, pooled human plasma spiked with VRC at 0.2 μg/ml (LOQ) and the internal standard at 1.0 μg/ml; D, pooled human plasma spiked with VRC at 10 μg/ml and the internal standard at 1.0 μg/ml. mAU, milli-absorbance units.
Essential parameters in validating an analytical procedure, such as accuracy (percent bias), precision (expressed as the coefficient of variation [%CV]), sensitivity, response function (linearity), and specificity, were evaluated to determine the robustness of the method (2, 3, 5).
For this method, the lower limit of quantitation (LOQ) was determined to be 0.2 μg/ml. This method was evaluated in excess of the recommended time frame of 20 days (1). The interday %CVs for concentrations of 0.2, 0.5, 4, and 8 μg/ml were 16, 11.4, 7.8, and 9.2%, respectively (n = 24 for each concentration). Intra- and interday precision and accuracy data for quality control validation samples are presented in Tables 1 and 2. Percent recoveries of VRC at concentrations of 0.2, 0.5, 4.0, and 8 μg/ml were 93.5, 89.3, 90.9, and 100.4%, respectively. The internal standard recovery percentage at the concentration used was 100.5%.
TABLE 1.
Intraday precision and accuracy data for quality control samples
| Nominal control concn and mean concna (μg/ml) | Precisionb (% CV) | Accuracyc (%) |
|---|---|---|
| 0.2 (LOQ) | ||
| 0.165 | 15.7 | 82.6 |
| 0.215 | 12.9 | 107.3 |
| 0.166 | 10.7 | 83.0 |
| 0.181 | 8.1 | 90.5 |
| 0.5 | ||
| 0.465 | 4.6 | 93.0 |
| 0.415 | 8.2 | 83.1 |
| 0.545 | 2.5 | 108.9 |
| 0.471 | 7.7 | 94.1 |
| 4.0 | ||
| 3.579 | 1.5 | 89.3 |
| 3.536 | 7.1 | 88.4 |
| 4.014 | 2.6 | 100.4 |
| 4.021 | 6.5 | 100.5 |
| 8.0 | ||
| 7.395 | 3.03 | 92.4 |
| 7.411 | 3.90 | 92.5 |
| 8.250 | 2.41 | 103.1 |
| 9.052 | 3.35 | 113.1 |
Mean concentration of six replicates for four different run days (intraday n = 6).
Intraday %CV (n = 6).
Determined with the formula (mean assayed concentration n = 6/nominal concentration) × 100.
TABLE 2.
Interday precision and accuracy data for quality control samplesa
| Concn (μg/ml) | Overall mean concn (μg/ml) ± SD | Overall precision (%CV) | Overall inaccuracy (% bias) | Mean accuracy (%) |
|---|---|---|---|---|
| 0.2 | 0.182 ± 0.029 | 16.0 | −9.2 | 90.9 |
| 0.5 | 0.474 ± 0.054 | 11.4 | −5.2 | 94.8 |
| 4.0 | 3.787 ± 0.295 | 7.8 | −5.3 | 94.6 |
| 8.0 | 8.027 ± 0.740 | 9.2 | 0.3 | 100.3 |
The overall value is the mean value for assayed controls at each concentration (n = 24). Overall inaccuracy (percent bias) = (overall mean − nominal concentration/nominal concentration). Mean accuracy = overall mean/nominal concentration × 100.
The slope-intercept linear model: y = mx + b was chosen to express the linearity, and the best-fit line was generated without forcing through zero. Results were analyzed with the Beckman 32 Karat software and data system. For this study, the minimum goodness of fit (r2) for six standard curves was 0.996.
The specificity of the method was evaluated by analyzing plasma samples from six normal volunteers in which no interference was noted. Plasma samples from persons receiving itraconazole, fluconazole, amphotericin B, and/or nystatin and a blank plasma sample spiked with 10 μg of caspofungin per ml were also analyzed to document a lack of interference by those antifungal agents with the assay. Notably, this study did not test all of the other possible multiple-drug cocktails that patients may receive; however, no interference by the aforementioned antifungal agents was observed.
To resolve potential stability concerns, plasma controls at 0.5, 4, and 8 μg/ml were left at room temperature for 7 days to determine changes in results from day 1 to day 7. The percent changes in the three controls ranged from 2.8 to 10%. Although there does not appear to be a significant change in samples left at room temperature for up to 7 days, long-term storage at −20°C is recommended. Studies that determined the stability of VRC in plasma samples subjected to freeze-thaw cycles were conducted by Stopher and Gage (6). In those studies, VRC in pooled plasma was stable through two freeze-thaw cycles and concentrations of VRC remained stable in samples frozen at −25°C for 14 months (6).
In conclusion, the analytical method developed to quantitate VRC in plasma has been successfully validated on the basis of principles established to determine accuracy, precision, linearity, sensitivity, and specificity. The VRC assay using SPE and HPLC met the criteria established by regulatory agencies that provide guiding principles for the validation of an analytical method.
Acknowledgments
This work was supported by Pfizer, Inc., New York, N.Y.
REFERENCES
- 1.NCCLS. 1999. Evaluation of precision performance of clinical chemistry devices: approved guidelines. Approved guidelines document EP5-A, no. 2. NCCLS, Wayne, Pa.
- 2.NCCLS. 2001. Evaluation of the linearity of quantitative analytical method; proposed guidelines—second edition. EP6-P2, vol. 21, no. 20. NCCLS, Wayne, Pa.
- 3.NCCLS. 1999. Statistical quality control for quantitative measurements: principles and definition: approved guidelines document C24-A2, vol. 19, no. 5, second ed. NCCLS, Wayne, Pa.
- 4.Perea, S., G. Pennick, A. Modak, A. Fothergill, D. Sutton, D. Sheehan, and M. Rinaldi. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah, V., K. Midha, S. Dighe, I. McGilveray, J. Skelly, A. Yacobi, T. Layloff, C. Viswanathan, C. Cook, R. McDowall, K. Pittman, and S. Spector. 1992. Analytical methods validation: bioavailability, bioequivalence, and pharmacokinetic studies. J. Pharm. Sci. 81:309-312. [DOI] [PubMed] [Google Scholar]
- 6.Stopher, D., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size exclusion column. J. Chromatogr. B 691:441-448. [DOI] [PubMed] [Google Scholar]