Abstract
At least three organs (skin, peripheral nerves and the conjunctiva) have been induced to regenerate partially in adults following application of porous, degradable scaffolds with highly specific structure (templates). Templates blocked contraction and scar formation by inducing a reduction in the density of contractile fibroblasts (probably myofibroblasts) and by preventing these cells to organize themselves appropriately in the wound. In contrast, during early foetal healing, myofibroblasts were absent and wounds did not close by contraction but rather by spontaneous regeneration. The adult regenerative process has so far led to imperfect recovery of the physiological anatomy of skin (skin appendages were missing), while early foetal healing has led to apparently complete restoration. Furthermore, the mechanism of the adult regenerative process involves thwarting of myofibroblast function while, during early foetal healing, differentiation of myofibroblasts has not yet occurred. The data suggest that induced organ regeneration in the adult is the result of partial reversion to early foetal healing. If so, the adult may conceal a foetal response that may be subject to activation following application of highly active scaffolds or of other substances or cells.
Keywords: skin regeneration, peripheral nerve regeneration, contraction blocking, scaffolds, regeneration templates
1. Introduction
Limb regeneration in certain amphibians is a spectacular feat by which an amputated limb grows back to its original form and recovers its normal function. It is the prototypical paradigm of regeneration. Very few adult amphibians, and none of the adult mammals, can replicate this feat. Instead, the vast majority of mammals, including adult humans, respond to severe injury by a spontaneous repair process. Repair closes the wound by contraction and synthesis of scar tissue without recovery of the uninjured tissues. In contrast, regeneration closes the wound by synthesis of the missing organ at the original site, yielding a regenerate. Regeneration restores the normal structure and function of the organ; repair does not.
Induced regeneration is the synthesis of non-regenerative tissues in a severely injured adult organ that leads to, at least partial, recovery of physiological structure and function. In clinical terms, it amounts to partial or total replacement of an organ that has stopped functioning due to severe trauma or chronic disease. When an organ can be induced to regenerate, even partially, the patient can be spared most of the problems accompanying alternative procedures that are commonly used. For example, in organ transplantation, the immune response of the host must be suppressed, typically at substantial cost of quality of life or even longevity, for the host; in autografting, the donor site contributes significant morbidity, including severe scarring. These problems are obviated by induced regeneration.
In clear contrast to the adult, the mammalian foetus heals its wounds spontaneously by regeneration, provided that the injury has been inflicted at a sufficiently early stage of gestation, typically before the third trimester. At that stage of development, healing processes undergo an early foetal to late foetal (often called ‘adult’) transition; the outcome of the transition is a conversion of healing by regeneration to healing by contraction and scar formation. Although not identical to the early foetal response, healing by induced regeneration in an adult is clearly distinct from the spontaneous adult healing response. The data pose the question whether the developmental transition in healing response that occurs during gestation is reversed, at least partially, during induced regeneration. Indeed, the combined evidence leads to the hypothesis that the foetal response is a sort of ‘default setting’ for healing processes in the organism and that it is partially reactivated when the adult setting is appropriately blocked.
In this article we first review the phenomenology of organ healing in the mammal. The evidence for induced regeneration is then summarized, followed by a discussion of mechanism. The two foetal healing modes, early and late foetal healing, are then discussed and compared to induced regeneration in adults.
2. Phenomenological principles of spontaneous healing processes
2.1 The tissue triad
A useful classification of tissue types in an organ focuses on three tissue types that are present in organs: epithelia, basement membrane and supporting tissue or stroma (e.g. Burkitt et al. 1993). Persistent, independent observations of adult healing following injury have shown that, in a very large number of organs, excised epithelial tissues and its associated basement membrane regenerate spontaneously following excision while the excised stroma does not (figure 1; for a detailed review of the literature for several organs see Yannas 2001). Stroma regeneration in an adult occurs, therefore, only if induced by the investigator and, for this reason, it appears to be the central problem in studies of organ regeneration.
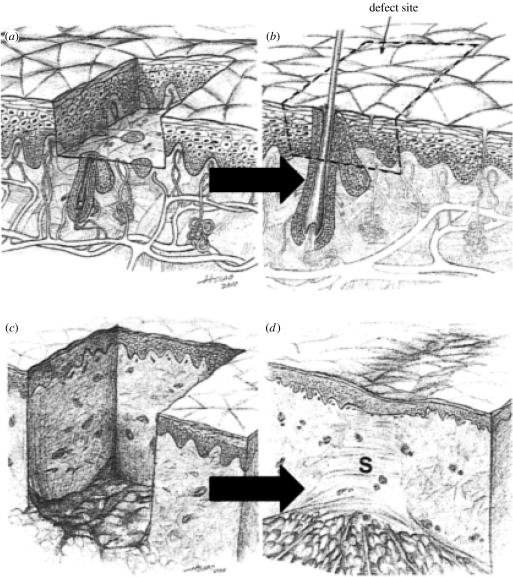
Figure 1.
Following controlled injury (a) the epidermis regenerates spontaneously (b). A much deeper injury leads to excision of the dermis (c), which does not regenerate (d); instead, the severe wound closes by contraction and scar formation (S).
2.2 The defect closure rule
Even though specification of the products of the healing process as scar and physiological tissue appears, at first glance, to describe wound closure exhaustively, this description omits a critically important quantity: the extent to which wound closure has occurred due to contraction, i.e. the centripetal movement of wound edges. (We define ‘contraction’ as the quantitative contribution to wound closure, measured at or near the time of closure, of centripetal translation and deformation of perilesional skin; the term ‘contracture’ has been often used to describe qualitatively the long-term clinical outcome, such as limb and joint deformation, of the wound contraction process.) Quantitative study of the phenomenology of healing reveals the relative importance of the outcome contributed by each process. In order to arrive at a quantitative description of the outcome of the healing process, it is necessary to include data from anatomically well-defined defects and to define the initial state (injury) and final state (wound closure) of the healing process (Yannas 2001).
The contribution to the healing outcome of each of these three processes, contraction, scar formation and regeneration in a given healing response can be represented most simply by explicitly reporting the fraction of the initial wound area that was eventually closed by each process. Even though these three processes occur simultaneously during healing, their relative contribution to the outcome of healing (wound closure) can be measured separately. Thus, while the contribution of contraction to wound closure comes from translation and contraction of perilesional skin in the direction towards the centre of the wound, scar formation and regeneration contribute to wound closure by synthesis of new tissue. This reasoning leads to the defect closure rule which states that wounds close exclusively by the three processes described above, which means that the fractional contribution of all processes add up to 1 (or the percentage contribution adds up to 100)
| (2.1) |
Here, C, S and R represent the percentage of initial wound area closed by each process.
Quantitative methods for separate measurement of each of the above quantities have been described in detail elsewhere (Yannas 2001).
Representative values of C, S and R from several investigations have been tabulated in table 1. In certain species, such as rodents, which have a mobile integument, contraction accounts for almost all of wound closure; in the human, where the skin is tethered to subcutaneous tissues, contraction accounts for a little more than a third of the closure process. For example, the result of spontaneous healing of a full-thickness skin wound in the dorsal region of the rabbit can be described in the final state by C=96±1%, S=4±1% and R=0 (estimated from data by Kennedy & Cliff 1979). For simplicity of presentation, the configuration of the final state (closed wound) is enclosed in brackets while the percentage symbol and error limits are omitted, leading to the representation [96, 4, 0] for the above example of rabbit data.
Table 1.
Spontaneous healing of an organ defect. Representative data on configuration of the final state (additional data in Yannas 2001).
| wounds prepared by complete excision of stroma (defects) | configuration of final state (C, % contraction; S, % scar; R, % regeneration) |
|---|---|
| general case of organ defect healing | [C, S, R] |
| ideal early foetal healing of dermis-free defect (perfect regeneration model) | [0, 0, 100] |
| spontaneous healing of dermis-free skin defect in rabbit dorsum (Kennedy & Cliff 1979). Values are representative of several adult rodents and lagomorphs | [96, 4, 0] |
| spontaneous healing of dermis-free skin defect in the adult human (Ramirez et al. 1969) | [37, 63, 0] |
| spontaneous healing of transected adult rat sciatic nerve (Yannas 2001) | [96, 4, 0] |
| spontaneous healing of stroma-free defect in adult conjunctiva (rabbit) (Yannas 2001) | [45, 55, 0] |
Early mammalian foetal healing occurs with negligible contraction and no scar formation, leading to
| (2.2) |
while healing in the late mammalian foetal models and in adult mammals occurs by contraction and scar formation, with no incidence of regeneration during late foetal and adult healing
| (2.3) |
During the process of induced regeneration in adults, either C or S or both must be reduced appropriately to accommodate a non-zero value of R.
3. Spontaneous wound healing in skin is compared to healing in other organs
In this section, we continue to focus on the macroscopic outcome of the spontaneous healing process, rather than on its detailed molecular/cellular mechanism. Rules derived about macroscopic outcomes will then be used to survey healing processes in different organs.
3.1 Skin wounds
The large bulk of quantitative data on wound healing in the literature have come from studies with wounded skin which can be studied much more conveniently than other organs.
Spontaneous closure of skin wounds in adults occurs by contraction and scar formation. In rodents, where the integument is mobile, contraction is by far the main engine of closure of skin wounds, while scar formation has been shown to be quantitatively much less important. In humans, where the integument is tethered more securely onto subcutaneous tissues, the two processes contribute approximately equally to wound closure (see table 1 for representative data).
Scar formation in skin wounds appears to be secondary to contraction during healing. Scar formation was nearly abolished when healing processes in skin wounds were manipulated with scaffolds that partially blocked contraction (Yannas 1981; Yannas et al. 1981, 1982, 1984, 1989). Quantitative measurement of the orientation of collagen fibres in dermal scars by laser light scattering showed that the fibres were persistently oriented in the plane of the wound and along the direction of the major contraction vector of the wound rather than being quasi-randomly oriented, as in physiological dermis (Ferdman & Yannas 1993). Other findings have demonstrated a relationship between fibroblast axis orientation and orientation of collagen fibres synthesized during healing (Trelstad & Birk 1984). The combined data have suggested that scar formation amounts to synthesis of oriented stroma by fibroblasts that have themselves been previously oriented in the presence of the mechanical field (primarily a plane stress field in skin wounds) that is generated by the contraction process. Such a mechanism suggests that scar formation should disappear following interference with this mechanical field, as observed when scaffolds that block contraction, even to a relatively minor extent, have been used. Although additional data are required to elucidate these processes further, the available evidence is consistent with the tentative view that contraction, not scar formation, is the main engine for closure of skin wounds (Yannas 2001).
3.2 Wounds in organs other than skin
Contraction has been observed on several occasions in almost all organs other than skin but has very rarely been studied systematically (for review see Yannas 2001). It is widely recognized that wound contraction results in contractures, strictures and stenosis of various organs (e.g. Peacock & Van Winkle 1976; Rudolph et al. 1992). Thus, although contraction has been observed in diverse organs, with very few exceptions, quantitative data on organs other than skin are practically absent from the literature. Lack of quantitative reports of contraction has contributed to widespread misunderstanding of the relative importance of this critical healing process in regeneration of adult organs. Emphasis is placed on contraction in this article because it is a basic, although unheralded, tool that can be used to normalize the description and comparison of healing processes across ontogenesis as well as across phyla.
In summary, spontaneous healing in skin as well as in other organs results in wound closure, the result of a generous contribution from contraction of wound edges.
4. The adult mammal can be induced to heal severe wounds by partial regeneration
There is accumulating evidence that the healing process of an injured organ in the adult mammal can be modified to yield a partly or wholly regenerated organ. In almost all such processes the critical ‘reactant’ supplied by the investigators was a scaffold of one type or another, usually seeded with autologous epithelial cells (Yannas 2004). The most extensive data on induced regeneration are available with skin. Data with other organs have been presented in a recent volume (Yannas 2005a,b).
4.1 Regeneration of skin
Detailed examples of induced skin regeneration have been described elsewhere (Yannas et al. 1981, 1982, 1984, 1989; Murphy et al. 1990; Ferdman & Yannas 1993; Butler et al. 1998, 1999; Compton et al. 1998). The data describe the structural and functional similarities and differences among normal skin, scar and regenerated skin in the adult guinea pig and the swine following grafting of dermis-free defects with the keratinocyte-seeded dermis regeneration template (DRT), a scaffold characterized by unusual regenerative activity. DRT is a macromolecular network synthesized as a highly porous analogue of the extracellular matrix. Among other characteristics, regenerated skin is mechanically competent, fully vascularized and sensitive to touch as well as heat or cold. The regenerated dermal–epidermal junction, with its extensive formations of rete ridges and capillary loops (figure 2), leave no doubt that de novo regenerated skin organ is clearly not scar; however, regenerated skin differs from physiological skin in the absence of skin appendages (hair follicles, sweat glands, etc.).
Figure 2.
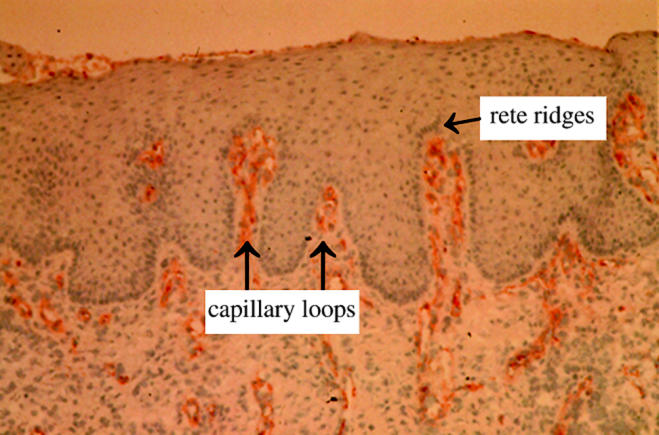
The dermal–epidermal junction of regenerated skin is viewed following immunostaining for Factor VIII in order to visualize the capillary loops inside the epidermal folds (rete ridges) (Compton et al. 1998). Neither rete ridges nor capillary loops form in scar.
Seeding with keratinocytes leads to simultaneous regeneration of a dermis and an epidermis, while grafting of the cell-free template leads to sequential regeneration of dermis and epidermis. The simultaneous process leads to a clinically desirable result within about two to three weeks but is complicated by the need to prepare the seeded template in the clinical setting. The period required for regeneration can be shortened by culture of keratinocytes prior to seeding (Butler et al. 1999). The sequential process is obviously simpler to implement; following grafting, the template is spontaneously epithelialized from the wound margin or a thin autoepidermal graft is applied on the newly synthesized dermis. However, closure by spontaneous epithelialization is much slower and, especially with large wounds in which the epidermis spontaneously regenerates across large distances, it is often unacceptably slow to be clinically useful.
Although seeding of DRT with autologous keratinocytes, harvested from the reference organ, was required to accelerate the kinetics of organ regeneration, seeding was not required to affect the outcome itself (regeneration versus repair). Neither was seeding with fibroblasts required. Furthermore, studies of skin wounds under the same experimental conditions as above showed that treatment of the wounds with a large variety of growth factors (Greenhalgh et al. 1990; Puolakkainen et al. 1995) or epidermal cell suspensions or epidermal cell sheets (Billingham & Reynolds 1952; Carver et al. 1993), or with a number of scaffolds based on synthetic polymers (Cooper et al. 1991; Hansbrough et al. 1993), failed to induce dermis regeneration. These and related observations (for review see Yannas 2001) motivate study of the mechanism, discussed below, by which DRT induces stroma regeneration.
In spite of the lack of skin appendages, the cell-free scaffold that induces regeneration of dermis (DRT) has been approved by the US Food and Drug Administration (FDA) for use with massively burned patients and with patients undergoing plastic and reconstructive surgery of the skin, as described in early clinical studies (Burke et al. 1981; Heimbach et al. 1988; Stern et al. 1990). Recently, the cell-free DRT scaffold (Integra) was found to be a very effective treatment, by induction of skin regeneration, for deep skin ulcers in patients (Gottlieb & Furman 2004).
4.2 Regeneration of adult organs other than skin
In addition to skin, confirmed observations of at least partial regeneration, or significant progress in the study of regeneration, have been also reported with the following adult organs: conjunctiva (Hatton & Rubin 2005), bone (Mistry & Mikos 2005), heart valves (Rabkin-Aikawa et al. 2005), articular cartilage (Kinner et al. 2005), urological organs (Atala 2005), the spinal cord (Verma & Fawcett 2005) and peripheral nerves (Zhang & Yannas 2005). The reader is referred to the relevant publications for further details.
5. Antagonistic relation between contraction and regeneration
The evidence suggests that contraction blocks induced regeneration. Data supporting this view will be reviewed briefly below; an extensive discussion has appeared elsewhere (Yannas 2001, 2005c).
5.1 Emerging dominance of contraction with loss of regenerative activity during the early foetal to late foetal transition in mammals
A developmental transition, occurring during late mammalian gestation, leads from healing primarily by regeneration to healing by repair. During this transition closure of the injured site by regeneration is largely replaced by closure based on contraction and scar formation. Detailed observations are summarized below in the section that focuses on the transition from early foetal to late foetal healing.
5.2 Gradual replacement of regeneration by contraction during amphibian development
Unlike studies with mammalian foetal models that are hindered by experimental difficulties, healing of a skin defect in an amphibian (anuran) model (North American bullfrog) can be studied in great detail during development, since the organism is accessible to observation without complicated experimental manipulation. During tadpole development, contraction eventually becomes clearly dominant at the expense of regeneration. A small component of scar formation is first observed after metamorphosis of the tadpole to the adult frog; at this adult stage, regeneration has been abolished and contraction accounts for almost all of closure of the defect (Yannas et al. 1996), consistent with the generalized description of the repair process in adult mammals that possess a mobile integument (see above).
5.3 Regeneration is induced in adult mammals following blocking of contraction by use of templates
The skin was the first organ to be induced to regenerate. Grafting of the unseeded DRT, a highly porous graft copolymer of type I collagen and chondroitin 6-sulphate, initially unseeded with cells, onto full-thickness skin wounds in the guinea pig, blocked wound contraction by over 25 days, a dramatic delay (Yannas 1981; Yannas et al. 1989). When the cell-seeded scaffold was grafted, contraction was not simply delayed but clearly arrested; instead, the wound closed by simultaneous regeneration of a dermis and an epidermis over almost the entire initial wound area (Yannas et al. 1981, 1982, 1989).
In a study with rabbits (Hsu et al. 2000), the conjunctiva was excised through the full depth of the conjunctival stroma (equivalent in depth to a full-thickness skin wound). Tenon's capsule was also excised. The deep wound was grafted with DRT. It was observed that ungrafted wounds closed by contraction and formation of scar tissue, the latter comprising an aligned array of dense collagen populated with occasional fibroblasts. Grafting of cell-free DRT resulted in regeneration of the conjunctival stroma, followed by epithelialization of the stroma (Hsu et al. 2000).
In studies of the peripheral nervous system, a very common animal model is the fully transected rat (or mouse) sciatic nerve (figure 6). The two stumps, separated by a gap of controlled length, are typically inserted inside a tube fabricated from an experimental material (Lundborg et al. 1982). In early studies, it was repeatedly observed that the cross section area of the distal nerve stump eventually was reduced by as much as 50–60% (Holmes & Young 1942; Weiss 1944; Weiss & Taylor 1944; Sunderland 1990; table 1). A current view of failure to regenerate a transected peripheral nerve is one in which the two stumps close by contraction and formation of neural scar (Yannas 2001).
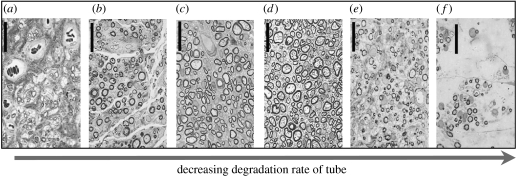
Figure 6.
Effects of decreasing degradation rate of the scaffold/tube on the quality of peripheral nerve regeneration. Quality of regeneration was maximal at half-life of three weeks (d) and decreased on either side of the maximum. Axon morphology observed in cross-sections obtained at the midpoint of the regenerated nerve trunk. The number of axons with diameter larger than 6 μm ((a) fibres; control conduction velocity) is maximum in (d). Degradation rate of scaffold decreased gradually from left to right with the following values of the half-life for each panel (weeks): (a) <1.5; (b) <1.5; (c) 2; (d) 3; (e) >100; (f) >100. Scale bars, approximately 25 μm (Harley et al. 2004).
5.4 Scar formation is secondary to contraction
As discussed above, the rodent data support a view of contraction as the main engine of defect closure while scar formation is viewed as a derivative (secondary) process. In these animal models, contraction clearly dominates scar formation during skin wound closure. Insufficient data are available with humans, with whom contraction is somewhat less important than scar formation as closure mode, to suggest a similar relation between contraction and scar.
5.5 However, although impaired healing in adults is accompanied by loss of contraction, regeneration is not observed
Experimental study of several models of impaired healing of skin wounds has been based on use of pharmacological agents (e.g. steroids), controlled infection, mechanical splinting, or on animal models of genetically impaired healing, such as the diabetic, or obese mouse. In all of these models, contraction was blocked almost completely; yet, regeneration was not induced (see review in Yannas 2001). Data from these models clearly show that blocking of contraction is not necessarily accompanied by regeneration.
5.6 Summary of observed changes in C, S and R
In summary, we consider the change (Δ) in contribution of each closure mode, C, S and R, associated with changes in experimental conditions and conclude as follows:
| (5.1) |
The inequalities state that blocking of contraction (ΔC<0) is required for induced regeneration (ΔR>0) as well as for the concomitant abolition of scar (S→0). These conditions are, however, not sufficient, since regeneration was not induced when contraction was impaired in several models of impaired healing. Studies of the mechanism of induced regeneration will now be focused on various mechanistic pathways for contraction blocking.
6. Mechanistic pathways for induction of regeneration
Having highlighted the empirical evidence for an antagonistic relation between contraction and regeneration in adults, we now seek mechanistic pathways that account for such a relation. It has been pointed out in §5 that, although contraction blockade appears to be required, it certainly does not suffice for induction of regeneration. Although there may be a number of reasons, most of them not currently understood, why contraction blockade is insufficient for regeneration, one of these is the clear requirement for an additional step, a step during which the regenerate is being synthesized de novo. A search for mechanism must, therefore, account for the following two steps that appear to be both required and sufficient for induction of regeneration in adults:
6.1 The contractile fibroblast is the main cell type associated with contraction
A cell type that plays a key role during contraction will be briefly described below. The differentiated myofibroblast, referred to simply as myofibroblast (MFB) in this article, has been credited with generation of most of the contractile forces in skin wounds (Rudolph et al. 1992; Tomasek et al. 2002; Thannickal et al. 2003). Even though MFB may not be the only cell type that participates in contraction (Rudolph et al. 1992; Ehrlich et al. 1999), the proposed mechanistic pathways are based on the assumption that MFB are indeed the dominant cell type. The specific feature which provides the most useful operational distinction of MFB differentiation is expression of the α-smooth muscle actin phenotype (Tomasek et al. 2002).
There is considerable evidence that myofibroblast differentiation is regulated by at least one cytokine (the transforming growth factor-beta1, TGF-β1), the presence of mechanical tension and an extracellular matrix component (the ED-A splice variant of cellular fibronectin (Desmoulière et al. 2005).
6.2 Models of the macroscopic contractile force that closes wounds in skin and peripheral nerves
The macroscopic force to contract a skin wound spontaneously is estimated at about 0.1 N (Yannas 2005a–c). An individual dermal fibroblast in culture is capable of developing a force of order 1–10 nN (Brown et al. 1998; Freyman et al. 2001). The number of contractile fibroblasts required to develop the macroscopic force that suffices to close the wound is, therefore, at least 10−1N/10 nN =107 cells, suggesting a factor of this magnitude to scale up from cell to organ.
In a simple model of an anatomically well-defined skin wound, contraction results from a plane stress field that is generated by contractile cells with their contractile axes lying in the plane of the wound. The macroscopic force vector, Fc, is considered as the product of three contributions: the total number of MFB in the wound, N, the fraction of cells, ϕ, bound to the matrix and capable of applying traction and the average contractile force vector generated per MFB, expressed as the in-plane vector component of the force per cell, fi (Yannas 2005c)
| (6.1) |
A similar model accounts for closure of the wounded stumps in a transected peripheral nerve by neuroma formation. A myofibroblast capsule has been observed to surround the nerve stumps as well as the nerve regenerate emanating from the stumps (Chamberlain et al. 2000). The models for contractile forces developed during spontaneous healing of skin and nerve wounds differ primarily in terms of the geometry of the two organs (planar in skin, cylindrical in nerve).
6.3 Reduction of contractile force by scaffolds
Two major mechanisms appear to account well for reduction of the contractile force Fc by scaffolds. The first mechanism depends on reduction of the number of MFB, N, while the second depends on reduction of the sum of forces generated by MFB in the wound. Simple mechanical splinting makes no significant contribution to contraction blocking by these scaffolds (Yannas 2005c).
DRT and the template that induces peripheral nerve regeneration (nerve regeneration template, NRT) differ in the processes by which each reduces N; while DRT works by downregulating recruitment of MFB, the NRT scaffold additionally allows MFB to escape from the wound. Downregulation of N by DRT is supported by two observations: (i) MFB comprise only about 10% of the total number of fibroblasts in the presence of the template inside a standardized, severe skin wound, compared to about 50% in its absence (Murphy et al. 1990) and (ii) DRT is practically free of collagen banding (without loss of the triple helical structure of the collagen molecule), that is normally required for platelet aggregation in wounds; although platelets adhere on DRT, they have failed to aggregate (Sylvester et al. 1989). Since, platelet aggregation is an important early source of TGF-β1, itself known to be required for MFB differentiation (see above), the data suggest that DRT contributes to relative depletion of TGF-β1 from the wound (Yannas 1990). Another possible mechanism for MFB depletion is based on the observation that TGF-β1 and TGF-β2 bind avidly, though non-specifically, on the extensive specific surface of DRT (Ellis et al. 1997, unpublished observations); such binding may contribute further to relative unavailability of TGF-β1 and TGF-β2 in the wound fluid.
The NRT mechanism for reduction of N is based on three observations; (i) the MFB density surrounding regenerating nerves of high quality is very low while being very high around a poorly regenerating nerve (Chamberlain et al. 2000); (ii) myofibroblasts have been shown to migrate outward through the wall of cell-permeable tubes fabricated from NRT (Chamberlain et al. 2000); and (iii) cell-impermeable tubes in which nerve stumps have been inserted have consistently led to very poorly regenerated nerves while their cell-permeable controls have led to regenerates of high quality (Jenq & Coggeshall 1985a,b). The available data can be most simply explained by hypothesizing that a scaffold, such as NRT, that facilitates exit of MFB reduces N and eventually attenuates the macroscopic contractile force.
The second major mechanism for contraction blocking, i.e. reduction of the sum of forces generated by MFB, has been observed so far only with DRT. Once having migrated inside DRT and become bound on the extensive surface of the highly porous scaffold, the long axes of MFB lose their in-plane orientation, becoming almost randomly oriented (figure 3). Accordingly, the contribution of the entire cell assembly to the macroscopic force can be reduced to a collection of pairs of vectors that are oriented at opposite directions from each other. In such a random assembly of force vectors the sum of forces, Fc, must be near zero. Cells that remain outside the scaffold are oriented in the plane and are free to generate their full contractile force.
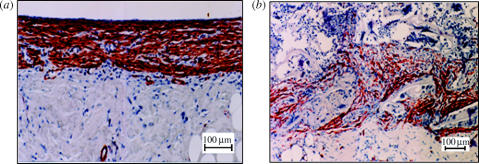
Figure 3.
The DRT scaffold that possesses high regenerative activity has disorganized the myofibroblast layer inside a deep skin wound. Myofibroblasts (brown stain) were observed inside a full-thickness skin wound in the guinea pig model 10 days after injury. (a) Contraction is proceeding vigorously in this untreated skin wound (negative control). A thick, continuous myofibroblast layer is present at the surface of the skin wound. (b) Contraction has been blocked in this skin wound that was treated with the DRT scaffold. Myofibroblasts are dispersed inside the scaffold 10 days after injury; cell–cell binding is practically absent and the axes of contractile cells are almost randomly arranged in the space of the wound. Stained with monoclonal antibody against α-smooth muscle actin (Troxel 1994). (Images reproduced courtesy of M.I.T.)
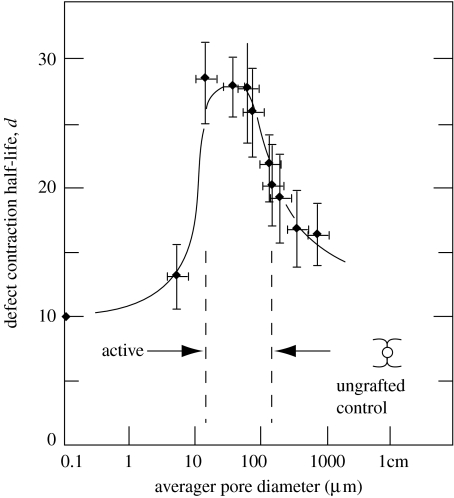
The contraction blocking activity of a scaffold clearly depends on its ability to bind most of the contractile cells in the wound; structural features that control cell–scaffold binding should therefore play major roles. For example, fibroblast–DRT binding requires participation of specific ligands, in particular those mediated by the β1 integrins that have been shown (Racine-Samson et al. 1997) to control myofibroblast–matrix binding during contraction. Ligand density is another critical feature of scaffold activity; a large concentration of ligands should lead to binding of large numbers of cells on the scaffold, resulting in loss of their ability to scale up contraction forces. At a very small pore size, cells are prevented from entering and binding inside the scaffold; at very large pore size the specific surface is very low, corresponding to low levels of the ligand density. Ligand density is therefore, expected to be minimal at the extremes of pore size. In a homologous series of scaffolds, where scaffold members possess increasingly larger pore size, one should therefore expect that contraction blocking activity should go through a maximum, as observed in the range 20–120 μm (Yannas et al. 1989; figure 4).
Figure 4.
Sharp differences in regenerative activity of collagen-based scaffolds that are matched closely in structure. The contraction half-life (inverse measure of contraction rate) of a skin wound model (guinea pig) is shown for several scaffolds with identical structure except pore diameter. The half-life reaches a maximum (corresponding to maximum regenerative activity) when the scaffold pore diameter is in the range 20–120 μm. Other scaffolds were inactive (Yannas et al. 1989).
6.4 Isomorphous and synchronous tissue synthesis
Once contraction has been blocked by a scaffold with the appropriate structure the stage has been set for synthesis of the organ. We recall that the critical synthetic step of an induced regeneration process is synthesis of the stroma: once the stroma has been regenerated, the epithelia and basement membrane of the organ can spontaneously be formed as well (Yannas 2004).
Fibroblasts deposit newly synthesized collagen fibres immediately outside their cell membrane, with the fibre axis oriented parallel to the major cell axis (Trelstad & Birk 1984). It follows that the three-dimensional architecture of fibroblasts at the time of collagen synthesis largely determines the architecture of the new stroma. A scaffold that binds fibroblasts avidly on its surface, as is true of DRT, controls their three-dimensional pattern, as well as their synthetic output and hence the architecture of the resulting stroma. We will refer to stroma synthesized under these conditions as being isomorphous (topographically similar) to the scaffold that controls fibroblast orientation.
The network of collagen fibres synthesized by these cells in DRT is a replica of the dermis, a quasi-random assembly of collagen fibres (figure 5a). Likewise, the porous architecture of a scaffold that has induced peripheral nerve regeneration after being implanted between the two stumps (rather than being used in the alternate experimental configuration of a tubular bridge, as described above) is a porous structure characterized by highly oriented pore channels (figure 5b). A similar architecture characterizes the endoneurial stroma in which nerve fibres are embedded with an orientation along the major axis of the nerve trunk.
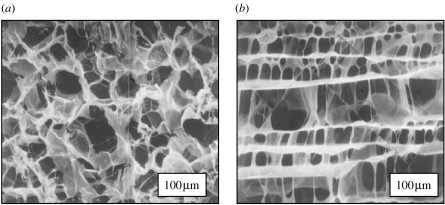
Figure 5.
(a) A collagen-based scaffold that has induced regeneration of the dermis in animals and humans. Composition: graft copolymer of type I collagen and chondroitin 6-sulphate. Scanning electron micrograph. Pore channel orientation is almost completely random. Average pore diameter, 80 μm. (b) Peripheral nerve was induced to regenerate across a 15 mm gap in the rat sciatic nerve using this scaffold as a bridge between the two stumps inside a silicone tube. Scanning electron micrograph. Pore channel orientation along major nerve axis. Average pore diameter, 20 μm. (Images reproduced courtesy of M.I.T.)
There is, however, one more requirement for the desired synthesis: The scaffold has to get out of the way of newly synthesized tissue; the exit has to be timely, otherwise the new tissue will not be formed in a sufficiently large space, leading to synthesis of stroma with incorrect topography. It has, in fact, been observed in skin wounds that when the half-life for scaffold degradation, td, was much longer than about 21 days, corresponding to the half-life for tissue synthesis during wound healing, th (very roughly equal to 21 days for the standardized full-thickness skin wound), contraction was blocked but scar formed between the intractable scaffold and the defect. A lower bound also existed since, when td was much lower than th, the scaffold did not block contraction. These findings have led to the rule of synchronous tissue synthesis
| (6.2) |
In words, synthesis of dermis in skin wounds (regeneration) was observed when the time constants for scaffold degradation and new tissue synthesis were approximately equal (Yannas & Burke 1980).
Further support for the requirement for an optimal scaffold duration has been also obtained from data on peripheral nerve regeneration. The data (figure 6) clearly indicate the existence of an optimal scaffold degradation rate of three weeks at which the quality of nerve regeneration is maximized.
In summary, induced regeneration comprises contraction blocking and synthesis of new stroma. Synthesis of stroma appears to require both synchronous and isomorphous replacement: the template is replaced at about the same rate that it is being degraded by newly synthesized tissue (stroma) with similar architecture. This discussion provides a mechanistic justification for the structural determinants of regeneration templates, as summarized in table 2.
Table 2.
Structural determinants of regenerative activity of two scaffolds based on ECM analogues.
| structural parameter of scaffold that is required for regenerative activity | skin regeneration (DRT; figure 5a) | nerve regeneration (filling of silicone chamber; figure 5b) | structural features of scaffold involved in contraction blocking |
|---|---|---|---|
| type I collagen/GAG, w/w | 98/2 | 98/2 | ligand identity required for binding of α2β1 integrin and other fibroblast integrins |
| residual collagen fibre banding (Yannas 1990) | ca 5% of native collagen | ca 5% of native collagen | platelet aggregation downregulated |
| average molecular weight between crosslinks, Mc (kDa) | 5–15 | 40–60 | scaffold maintains undegraded structure during contraction process |
| average pore diameter (μm) | 20–120 | 5–10 | maximum ligand density |
| pore channel orientation | random | axial | ligand orientation specific for stroma of organ |
Sources: skin regenerations—Yannas et al. (1989) ; nerve regeneration—Yannas (2001). GAG: glycosaminoglycan.
7. Two current views of the ontogenetic transition from scarless-to-scarring healing
Quantitative data on healing processes in mammalian foetuses have been relatively slow in being reported due to experimental difficulties involved in studies of wound healing in foetuses. Although scarless healing is often referred to as ‘foetal’ while scarring healing is referred to as ‘adult’, the transition in healing modes takes place in the foetal stage. In what follows, healing by regeneration will be designated ‘early foetal’ while healing by contraction and scar formation in a foetal model will be referred to as ‘late foetal’.
Two major viewpoints have been advanced to explain the phenomenon of loss in regenerative activity that is observed during the early foetal to late foetal transition in healing response. The first is focused on the detailed local pathways by which the organism responds to trauma. The second viewpoint takes a systemic view of organismic defences, noting that development of the immune system coincides with loss of regenerative activity and considering the former as a precursor for the latter.
Over the years at least three major differences in local healing responses involved in early foetal and late foetal skin wound healing have been established: healing in the early foetal gestation stages has been characterized by the relative lack of inflammatory response and lack of scarring, increased levels of hyaluronic acid (hyaluronan, HA) synthesis and lack of contraction. It is not clear whether all three of these foetal characteristics are lost simultaneously, i.e. at the same stage of the gestation period for a given organ of the species under study. The loss of each of these three features of scarless healing with increase in gestation age will be reviewed briefly below with emphasis on data from skin wound healing studies, where data are most numerous. The reader is referred to a recent review of foetal healing (Colwell et al. 2005a,b).
A persistent observation that has distinguished early foetal from late foetal healing has been lack of scarring, typically associated also with various measures of diminished inflammatory response. Investigators have reported various aspects of the cytokine profile before and after the foetal–adult transition. Several cytokines, including platelet derived growth factor (PDGF) (Whitby & Ferguson 1991; Haynes et al. 1994; Olutoye et al. 1996; Peled et al. 2001; Ferguson & O'Kane 2004), fibroblast growth factor (FGF) (Whitby & Ferguson 1991; Dang et al. 2003), endothelial growth factor (EGF) (Peled et al. 2001), vascular endothelial growth factor (VEGF) (Colwell et al. 2005a,b), various isoforms of these factors as well as occasional receptors have been studied in an effort to elicit information that would implicate one or more of them in the transition from scarring to scarless healing; however, the results have so far been largely equivocal.
Much more informative were studies of TGF-β and its three isoforms, TGF-β1, -β2 and -β3. Basing themselves on the early evidence (Roberts et al. 1986) that TGF-β upregulates collagen synthesis during wound healing, investigators hypothesized that this family of cytokines may play a major role in scar formation. Two basic approaches have been particularly useful: in early foetal wounds, TGF-β or its isoforms have been exogenously added while, in late foetal wounds, neutralizing antibodies for TGF-β or its isoforms have been added. Studies with models of foetal healing showed that addition of exogenous TGF transformed the early foetal into a late foetal response (Krummel et al. 1988) while, in another study, the size of the scar obtained in an early foetal model was proportional to the amount of TGF-β1 (Sullivan et al. 1995). In a later study, exogenous TGF-β1 induced early foetal wounds to upregulate expression of the gene for pro-collagen type 1 alpha 1, leading to fibrotic healing in the early foetal model (Lanning et al. 1999). The reverse phenomenon was also demonstrated in models of late foetal healing. Addition of neutralizing antibody to TGF-β 1, 2 in a late foetal model reduced fibrosis (Shah et al. 1994) while addition of neutralizing antibody to each of the three TGF-β isoforms in late foetal wounds showed isoform-specific differences: while addition of neutralizing antibodies to TGF-β1 and -β2 decreased scarring, exogenous addition of TGF-β3 (rather than the antibody) had a similar effect (Shah et al. 1995). Relatively rapid clearance of TGF-β1 during early foetal healing had been detected (Martin et al. 1993) and later studies with well-characterized models of the early foetal to late foetal transition revealed additional details of the ontogeny of expression of the three TGF-β isoforms. In one such study, expression of the three TGF-β ligands (isoforms) as well as their receptors were studied in early and late foetal animals; a sequence of model wounds, ranging from early gestation wounds that healed without scar to late gestation wounds healing with scar were studied. In the scarless (early foetal) model, expression of TGF-β1 was decreased and was rapidly cleared while expression of TGF-β3 was increased and prolonged. Furthermore, in the scarring (late foetal) model expression of TGF-β1 and -β2 was increased and prolonged while expression of TGF-β3 was decreased and delayed. Study of receptor expression gave similar results (Soo et al. 2003). The combined data suggest that the transition from scarless to scarring healing which happens during late gestation is under differential transcriptional control of these three TGF-β isoforms.
HA has been detected in early foetal wounds in far larger concentrations over a longer period during healing than in late foetal wounds. It was observed that the HA content was much higher in early foetal than in late foetal wounds (Krummel et al. 1987; DePalma et al. 1989; Siebert et al. 1990) and that it persisted much longer than in late foetal wounds (Longaker et al. 1991a,b), possibly reflecting the presence of higher levels of hyaluronidase in late foetal wounds (West et al. 1997). There is evidence that HA is replaced by sulphated glycosaminoglycans (GAGs) during the early foetal to late foetal transition (Freund et al. 1993). Addition of HA to early foetal wounds led to formation of much finer bundles of collagen fibres than in late foetal wounds (Iocono et al. 1998). It is speculated that the known inability of HA to precipitate collagen in the form of coarse fibres at neutral pH (Yannas et al. 1980) is consistent with the fine structure of collagen fibres observed during early foetal healing (Iocono et al. 1998); sulphation of GAGs during late foetal healing provides, according to this hypothesis, conditions for effective precipitation of collagen, leading to deposition of coarser bundles of collagen observed during this later stage and associated with scarring.
Large differences in degree of wound contraction between early foetal and late foetal healing models have been reported. Provided that healing was being studied at a sufficiently early stage of the gestation period, investigators observed lack of contraction of untreated, excisional skin wounds in the rabbit; instead, significant wound expansion was documented in three rabbit studies (Ledbetter et al. 1991; Lanning et al. 1999, 2000). At sufficiently late stages of the gestation period, or following appropriate manipulation, investigators reported healing with contraction in excisional skin wounds in the foetal rabbit (Lanning et al. 1999, 2000) and foetal lamb models (Longaker et al. 1991a,b; Horne et al. 1992; Stelnicki et al. 2000). Quantitative data obtained with an amphibian model showed that, prior to metamorphosis (tadpole stages), the contribution of contraction to skin wound closure continuously increased at the expense of regeneration while scar formation was negligible; after metamorphosis (frog), contraction dominated over scar formation while regeneration was undetectable (Yannas et al. 1996). The amphibian trend is consistent with the available mammalian data.
Since, there is considerable evidence that fibroblasts that have expressed the α-smooth muscle actin phenotype (myofibroblasts) are largely responsible for wound contraction in adult wounds (see discussion above) it is worth looking for a changing role for MFB during the early foetal to late foetal transition. Myofibroblasts were, in fact, identified in excisional skin wounds in the foetal lamb that closed by contraction (Longaker et al. 1991a,b) or by scar formation (Cass et al. 1997) but not in wounds that healed with no scar formation (Cass et al. 1997). Absent in wounds made at an early gestation stage, MFB were observed in increasing amounts at later gestation times in the foetal lamb model (Estes et al. 1994).
A much more systemic view of the early foetal to late foetal healing transition is based on the hypothesis that loss of the regenerative activity is closely associated with concomitant development of the immune system (Harty et al. 2003; Mescher & Neff 2005). This concept is consistent with recognition that the vertebrate immune system has evolved with two functions that are closely associated, both being related to the defence of the organism against trauma. Following severe trauma of the skin, for example, the resulting large wound simultaneously facilitates massive infection and rapid loss of bodily fluids, seriously threatening survival of the organism by either of these pathways. The adult organism resists infection with its immune system and closes open wounds by contraction and scar formation (see above). Neither of these two protective functions is fully developed in the early foetal stages. In this viewpoint, the emergence of the immune system spawns not only several defence mechanisms directed against a variety of antigens but also the inflammatory response to injury. Development of immunocompetence in the late foetal stages coincides, therefore, with development of the adult-like inflammatory response that blocks wound closure by regeneration and favours closure by contraction and scarring. Several phenotypes of the immune response that are related to the inflammatory response, e.g. growth factors and cytokines released by antigen-presenting cells and lymphocytes, have been reviewed (Mescher & Neff 2005).
The two views of the ontogenetic transition in healing response that eliminates regenerative activity appear to be complementary. The first view, based on the expression of phenotypes that characterize the classical inflammatory response, appears to be a local version of a systemic phenomenon. For example, the amplified and prolonged presence of TGF-β1 and -β2 that appears to control, at least in part, the loss of regenerative activity (see above) may be related to the increased density of T lymphocytes observed in foetal wounds treated with TGF-β, suggesting that the foetus mounts an attenuated T lymphocyte response compared to the adult (Adolph et al. 1993).
A great deal of work needs to be done to identify hypothetically common pathways by which the healing response and the immune response are expressed. One approach could be based on the observation that HoxB13, a member of the highly conserved family of Hox transcription factors, is downregulated during the early foetal stages, when healing occurs by regeneration, but not in later stages (Stelnicki et al. 1998; Mack et al. 2003; White et al. 2003). In a related approach, data from RNA differential display of samples from scarless wound healing in a foetal model have been used to identify novel genes differentially expressed during healing (Darden et al. 2000; Li et al. 2000; Soo et al. 2002). In one study, the data have suggested that downregulation of the CCT chaperonin in scarless wound healing may inhibit differentiation of myofibroblasts, the primary cell type involved in contraction (see above), by preventing folding of sufficient α-smooth muscle actin units to form the stress fibres characteristic of these cells (Darden et al. 2000).
8. Major differences between induced regeneration in adults and early foetal healing
Let us summarize briefly the major conclusions that were reached in the preceding sections. A study of the phenomenology of spontaneous healing in several organs led to the conclusion that a healing process can be viewed as the outcome from just three processes, contraction, scar formation and regeneration. Of the three processes, contraction (rather than scar formation) is the clearly dominant process for spontaneous wound closure in adult experimental models (typically in rodents; much less so in humans). Three organs, namely skin, the conjunctiva and peripheral nerves, have been induced to regenerate, at least partially, using grafts synthesized as porous scaffolds with highly specific structure (regeneration templates). Scaffolds with regenerative activity have blocked contraction effectively in each of the three organs. Blocking of contraction, orginating in the stroma, appears to be required (but does not suffice) to induce regeneration and to abolish scar. Mechanistic pathways to induced regeneration have focused on two mechanisms, both of which lead to, at least partial, blockade of contraction: In the first, the scaffold downregulates the number of contractile fibroblasts (myofibroblasts); in the second mechanism, myofibroblasts end up being bound on specific ligands on the extensive specific surface of a template, an event that reduces the contractile forces collectively generated by MFB in the wound.
Two lines of evidence suggest a more extensive comparison between induced regeneration in adults and early foetal healing. In both cases, healing results in some form of organ regeneration under conditions that are largely free of contraction. On the other hand, the early foetal model leads to apparently complete recovery while induced regeneration in adults has so far led to imperfect recovery of physiological organs.
The outcomes of the two healing processes are compared first. In two organs studied so far in some detail (skin and peripheral nerves), the outcomes of induced regeneration processes, at their current state of development, have been imperfectly physiological organs. One may ask whether these instances of induced regeneration in adults can be modelled as imperfect analogues of early foetal healing. An analogue of imperfect skin regeneration in a foetal model has been described: An intermediate stage of healing of the lips of the rhesus monkey foetus, labelled ‘transition wound’ by the authors, was characterized by regeneration of a normal reticular dermal collagen pattern, i.e. no scar formation, but also lacking skin appendages (Lorenz et al. 1993). The morphological description of skin that was imperfectly induced to regenerate following use of the DRT suggests that regenerated skin differed clearly from scar and resembled normal skin, in the structure of the dermis, basement membrane and the dermal–epidermal junction; however, it differed from physiological skin in its absence of appendages. Basing ourselves on the early observations with foetal healing (Lorenz et al. 1993), we tentatively describe the process of induced skin regeneration in the adult as the result of ‘transition healing’. This line of reasoning suggests that during induced skin regeneration using the DRT, the spontaneous adult healing processes (similar in outcome to those during late foetal healing) were blocked insufficiently by the template to induce their total, rather than partial, reversion to early foetal healing. Nevertheless, the partial reversion of adult healing to early foetal healing suggests a plasticity in the developmental process that has not been previously obvious. Somewhat speculatively it can even be hypothesized that the foetal response is a sort of ‘default setting’ for healing processes in the organism and that the foetal setting is partially reactivated when the adult setting is appropriately blocked.
Let us now consider the pathway followed during induced regeneration in the two modes of healing. As described above, during induced regeneration processes in skin that are controlled by a template, the contractile cells (myofibroblasts) have expressed the α-smooth muscle actin phenotype; they are, however, much fewer and their contractile axes are much less well oriented in the plane than in models of spontaneous adult healing by contraction and scar formation. The template works by reducing contractile cell density and by cancelling the scale-up of the contractile force of individual cells to the macroscopic level that suffices to close the wound. In contrast, in two independent studies of early foetal healing (no scarring), the α-smooth muscle actin phenotype was absent, appearing at a later stage of gestation (late foetal healing) when healing occurred with scarring (Estes et al. 1994; Cass et al. 1997). Clearly, induction of regeneration in the adult model by the template was not achieved by a pathway characteristic of regeneration during early foetal healing: rather than blocking expression of the contractile phenotype the template thwarted the function of contractile fibroblasts. Since the blocking action occurs so far downstream, the regenerative activity of the template reminds of the activity of many enzymes or pharmacological agents. In fact, the progressive loss in contraction-blocking activity with gradual increase in average pore size in the template (which corresponds, as described above, to gradual reduction in ligand density and in number of cells bound on the template) suggests that contraction-blocking can be ‘dosed’.
Finally, a question that is posed by the comparative data from studies of induced regeneration in adults and from studies of early foetal healing is the contribution of stem cells in each healing mode. Recent evidence has been accumulating that skin wounding recruits adult bone marrow cells to the site of injury (Badiavas et al. 2003; Fathke et al. 2004). In one study it was shown that multi-potent stem cells, present in a bone marrow preparation that had been topically applied to skin wounds, became differentiated into myofibroblasts (Yamaguchi et al. 2005). The evidence is still sparse and does not lead to firm conclusions. Nevertheless, stem cell recruitment following wounding, together with the synthesis of more active scaffolds, present new variables that should accelerate the effort to induce organ regeneration in adults.
References
- Adolph V.R, DiSanto S.K, Bleacher J.C, Dillon P.W, Krummel T.M. The potential role of the lymphocyte in fetal wound healing. J. Pediatr. Surg. 1993;28:1316–1320. doi: 10.1016/s0022-3468(05)80320-3. [DOI] [PubMed] [Google Scholar]
- Atala A. Regeneration of urologic tissues and organs. Adv. Biochem. Eng. Biotechnol. 2005;94:179–208. doi: 10.1007/b100004. [DOI] [PubMed] [Google Scholar]
- Badiavas E.V, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J. Cell. Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- Billingham R.E, Reynolds J. Transplantation studies on sheets of pure epidermal epithelium and epidermal cell suspensions. Br. J. Plast. Surg. 1952;5:25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- Brown R.A, Prajapati R, McGrouther D.A, Yannas I.V, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Burke J.F, Yannas I.V, Quinby W.C, Bondoc C.C, Jung W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981;194:413–428. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt H.G, Young B, Heath J.W, editors. Wheater's functional histology. 3rd edn. Churchill Livingstone; Edinburgh: 1993. [Google Scholar]
- Butler C.E, Orgill D.P, Yannas I.V, Compton C.C. Effect of keratinocyte seeding of collagen–glycosaminoglycan membranes on the regeneration of skin in a porcine model. Plast. Reconstr. Surg. 1998;101:1572–1579. doi: 10.1097/00006534-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Butler C.E, Yannas I.V, Compton C.C, Correia C.A, Orgill D.P. Comparison of cultured and uncultured keratinocytes seeded into a collagen–GAG matrix for skin replacements. Br. J. Plast. Surg. 1999;52:127–132. doi: 10.1054/bjps.1997.3047. [DOI] [PubMed] [Google Scholar]
- Carver N, Navsaria H.A, Green C.J, Leigh I.M. The effect of backing materials on keratinocyte autograft take. Br. J. Plast. Surg. 1993;46:228–234. doi: 10.1016/0007-1226(93)90173-9. [DOI] [PubMed] [Google Scholar]
- Cass D.L, Sylvester K.G, Yang E.Y, Crombleholme T.M, Adzick N.S. Myofibroblast persistence in fetal sheep wounds is associated with scar formation. J. Pediatr. Surg. 1997:1017–1021. doi: 10.1016/s0022-3468(97)90390-0. (discussion 1021–1022.) [DOI] [PubMed] [Google Scholar]
- Chamberlain L.J, Yannas I.V, Hsu H.-P, Spector M. Connective tissue response to tubular implants for peripheral nerve regeneration: the role of myofibroblasts. J. Comp. Neurol. 2000;417:415–430. doi: 10.1002/(sici)1096-9861(20000221)417:4<415::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Colwell A.S, Beanes S.R, Soo C, Dang C, Ting K, Longaker M.T, Atkinson J.B, Lorenz H.P. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast. Reconstr. Surg. 2005;115:204–212. [PubMed] [Google Scholar]
- Colwell A.S, Longaker M.T, Lorenz H.P. Mammalian fetal organ regeneration. Adv. Biochem. Eng. Biotechnol. 2005;93:83–100. doi: 10.1007/b99972. [DOI] [PubMed] [Google Scholar]
- Compton C.C, Butler C.E, Yannas I.V, Warland G, Orgill D.P. Organized skin structure is regenerated in vivo from collagen–GAG matrices seeded with autologous keratinocytes. J. Invest. Dermatol. 1998;110:908–916. doi: 10.1046/j.1523-1747.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- Cooper M.L, Hansbrough J.F, Spielvogel R.L, Cohen R, Bartel R.L, Naughton G. In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh. Biomaterials. 1991;12:243–248. doi: 10.1016/0142-9612(91)90207-q. [DOI] [PubMed] [Google Scholar]
- Dang C.M, Beanes S.R, Soo C, Ting K, Benhaim P, Hedrick M.H, Lorenz H.P. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plast. Reconstr. Surg. 2003;111:1969–1979. doi: 10.1097/01.PRS.0000054837.47432.E7. [DOI] [PubMed] [Google Scholar]
- Darden D.L, et al. RNA differential display of scarless wound healing in fetal rabbit indicates downregulation of a CCT chaperonin subunit and upregulation of a glycophorin-like gene transcript. J. Pediatr. Surg. 2000;35:406–419. doi: 10.1016/s0022-3468(00)90204-5. [DOI] [PubMed] [Google Scholar]
- DePalma R.L, Krummel T.M, Durham L.A, III, Michna B.A, Thomas B.L, Nelson J.M, Diegelmann R.F. Characterization and quantitation of wound matrix in the fetal rabbit. Matrix. 1989;9:224–231. doi: 10.1016/s0934-8832(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich H.P, Keefer K.A, Myers R.L, Passaniti A. Vanadate and the absence of myofibroblasts in wound contraction. Arch. Surg. 1999;134:494–501. doi: 10.1001/archsurg.134.5.494. [DOI] [PubMed] [Google Scholar]
- Estes J.M, Vande Berg J.S, Adzick N.S, MacGillivray T.E, Desmouliere A, Gabbiani G. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation. 1994;56:173–181. doi: 10.1046/j.1432-0436.1994.5630173.x. [DOI] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdman A.G, Yannas I.V. Scattering of light from histologic sections: a new method for the analysis of connective tissue. J. Invest. Dermatol. 1993;100:710–716. doi: 10.1111/1523-1747.ep12472364. [DOI] [PubMed] [Google Scholar]
- Ferguson M.W, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R Soc. B Biol. Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R.M, Siebert J.W, Cabrera R.C, Longaker M.T, Eidelman Y, Adzick N.S, Garg H.G. Serial quantitation of hyaluronan and sulfated glycosaminoglycans in fetal sheep skin. Biochem. Mol. Biol. Int. 1993;29:773–783. [PubMed] [Google Scholar]
- Freyman T.M, Yannas I.V, Yokoo R, Gibson L.J. Fibroblast contraction of a collagen–GAG matrix. Biomaterials. 2001;22:2883–2891. doi: 10.1016/s0142-9612(01)00034-5. [DOI] [PubMed] [Google Scholar]
- Gottlieb M.E, Furman J. Successful management and surgical closure of chronic and pathological wounds using Integra. J. Burns Surg. Wound Care. 2004;3:4–48. [Google Scholar]
- Greenhalgh D.G, Sprugel K.H, Murray M.J, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Hansbrough J.F, Morgan J.L, Greenleaf G.E, Bartel R. Composite grafts of human keratinocytes grown on a polyglactin mesh-cultured fibroblast dermal substitute function as a bilayer skin replacement in full-thickness wounds on athymic mice. J. Burn Care Rehabil. 1993;14:485–494. doi: 10.1097/00004630-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Harley B.A, Spilker M.H, Wu J.W, Asano K, Hsu H.P, Spector M, Yannas I.V. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153–165. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff A.W, King M.W, Mescher A.L. Regeneration or scarring: an immunologic perspective. Dev. Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Hatton M.P, Rubin P.A.D. Conjunctival regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:125–140. doi: 10.1007/b100002. [DOI] [PubMed] [Google Scholar]
- Haynes J.H, Johnson D.E, Mast B.A, Diegelmann R.F, Salzberg D.A, Cohen I.K, Krummel T.M. Platelet-derived growth factor induces fetal wound fibrosis. J. Pediatr. Surg. 1994;29:1405–1408. doi: 10.1016/0022-3468(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, Jordan M, McManus W, Solem L, et al. Artificial dermis for major burns. Ann. Surg. 1998;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W, Young J.Z. Nerve regeneration after immediate and delayed suture. J. Anat. (London) 1942;77:63–96. [PMC free article] [PubMed] [Google Scholar]
- Horne R.S, Hurley J.V, Crowe D.M, Ritz M, O'Brien B.M, Arnold L.I. Wound healing in foetal sheep: a histological and electron microscope study. Br. J. Plast. Surg. 1992;45:333–344. doi: 10.1016/0007-1226(92)90001-e. [DOI] [PubMed] [Google Scholar]
- Hsu W.C, Spilker M.H, Yannas I.V, Rubin P.A.D. Inhibition of conjunctival scarring and contraction by a porous collagen–GAG implant. Invest. Ophthalmol. Vis. Sci. 2000;41:2404–2411. [PubMed] [Google Scholar]
- Iocono J.A, Ehrlich H.P, Keefer K.A, Krummel T.M. Hyaluronan induces scarless repair in mouse limb organ culture. J. Pediatr. Surg. 1998;33:564–567. doi: 10.1016/s0022-3468(98)90317-7. [DOI] [PubMed] [Google Scholar]
- Jenq C.-B, Coggeshall R.E. Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res. 1985;326:29–40. doi: 10.1016/0006-8993(85)91381-2. [DOI] [PubMed] [Google Scholar]
- Jenq C.-B, Coggeshall R.E. Long-term patterns of axon regeneration in the sciatic nerve and its tributaries. Brain Res. 1985;345:34–44. doi: 10.1016/0006-8993(85)90833-9. [DOI] [PubMed] [Google Scholar]
- Kennedy D.F, Cliff W.J. A systematic study of wound contraction in mammalian skin. Pathology. 1979;11:207–222. doi: 10.3109/00313027909061947. [DOI] [PubMed] [Google Scholar]
- Kinner B, Capito R.M, Spector M. Regeneration of articular cartilage. Adv. Biochem. Eng. Biotechnol. 2005;94:91–123. doi: 10.1007/b100001. [DOI] [PubMed] [Google Scholar]
- Krummel T.M, Michna B.A, Thomas B.L, Sporn M.B, Nelson J.M, Salzberg A.M, Cohen I.K, Diegelmann R.F. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J. Pediatr. Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Krummel T.M, Nelson J.M, Diegelmann R.F, Lindblad W.J, Salzberg A.M, Greenfield L.J, Cohen I.K. Fetal response to injury in the rabbit. J. Pediatr. Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Lanning D.A, Nwomeh B.C, Montante S.J, Yager D.R, Diegelmann R.F, Haynes J.H. TGF-beta1 alters the healing of cutaneous fetal excisional wounds. J. Pediatr. Surg. 1999;34:695–700. doi: 10.1016/s0022-3468(99)90358-5. [DOI] [PubMed] [Google Scholar]
- Lanning D.A, Diegelmann R.F, Yager D.R, Wallace M.L, Bagwell C.E, Haynes J.H. Myofibroblast induction with transforming growth factor-beta1 and -beta3 in cutaneous fetal excisional wounds. J. Pediatr. Surg. 2000;35:183–187. doi: 10.1016/s0022-3468(00)90007-1. (discussion 187–188.) [DOI] [PubMed] [Google Scholar]
- Ledbetter M.S, Morykwas M.J, Ditesheim J.A, Vander Ark W.D, La Rosee J.R, Argenta L.C. The effects of partial and total amniotic fluid exclusion on excisional fetal rabbit wounds. Ann. Plast. Surg. 1991;27:139–145. doi: 10.1097/00000637-199108000-00008. [DOI] [PubMed] [Google Scholar]
- Li H.S, Hebda P.A, Kelly L.A, Ehrlich G.D, Whitcomb D.C, Dohar J.E. Up-regulation of prostaglandin EP4 receptor messenger RNA in fetal rabbit skin wound. Arch. Otolaryngol. Head Neck Surg. 2000;126:1337–1343. doi: 10.1001/archotol.126.11.1337. [DOI] [PubMed] [Google Scholar]
- Longaker M.T, Burd D.A, Gown A.M, Yen T.S, Jennings R.W, Duncan B.W, Harrison M.R, Adzick N.S. Midgestational excisional fetal lamb wounds contract in utero. J. Pediatr. Surg. 1991;26:942–947. doi: 10.1016/0022-3468(91)90841-g. (discussion 947–948.) [DOI] [PubMed] [Google Scholar]
- Longaker M.T, Chiu E.S, Adzick N.S, Stern M, Harrison M.R, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann. Surg. 1991;213:292–296. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz H.P, Whitby D.J, Longaker M.T, Adzick N.S. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Ann. Surg. 1993;217:391–396. doi: 10.1097/00000658-199304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G, Dahlin L.B, Danielsen N, Gelberman R.H, Longo F.M, Powell H.C, Varon S. Nerve regeneration in silicone model chambers: influence of gap length and of distal stump components. Exp. Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- Mack J.A, Abramson S.R, Ben Y, Coffin J.C, Rothrock J.K, Maytin E.V, Hascall V.C, Largman C, Stelnicki E.J. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 2003;17:1352–1354. doi: 10.1096/fj.02-0959fje. Epub May 20, 2003. [DOI] [PubMed] [Google Scholar]
- Martin P, Dickson M.C, Millan F.A, Akhurst R.J. Rapid induction and clearance of TGF beta 1 is an early response to wounding in the mouse embryo. Dev. Genet. 1993;14:225–238. doi: 10.1002/dvg.1020140309. [DOI] [PubMed] [Google Scholar]
- Mescher A.L, Neff A.W. Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mistry A.S, MIkos A.G. Tissue engineering for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- Murphy G.F, Orgill D.P, Yannas I.V. Partial dermal regeneration is induced by biodegradable collagen–glycosaminoglycan grafts. Lab. Invest. 1990;62:305–313. [PubMed] [Google Scholar]
- Olutoye O.O, Yager D.R, Cohen I.K, Diegelmann R.F. Lower cytokine release by fetal porcine platelets: a possible explanation for reduced inflammation after fetal wounding. J. Pediatr. Surg. 1996;31:91–95. doi: 10.1016/s0022-3468(96)90326-7. [DOI] [PubMed] [Google Scholar]
- Peacock E.E, Jr., Van Winkle W.Wound repair2nd edn1976Saunders; Philadelphia, PA [Google Scholar]
- Peled Z.M, Rhee S.J, Hsu M, Chang J, Krummel T.M, Longaker M.T. The ontogeny of scarless healing II: EGF and PDGF-B gene expression in fetal rat skin and fibroblasts as a function of gestational age. Ann. Plast. Surg. 2001;47:417–424. doi: 10.1097/00000637-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Puolakkainen P.A, Twardzik D.R, Ranchalis J.E, Pankey S.C, Reed M.J, Gombotz W.R. The enhancement in wound healing by transforming growth factor-b1(TGF-b1) depends on the topical delivery system. J. Surg. Res. 1995;58:321–329. doi: 10.1006/jsre.1995.1050. [DOI] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Mayer J.E, Jr., Schoen F.J. Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:141–178. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- Racine-Samson L, Rockey D.C, Bissell D.M. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J. Biol. Chem. 1997;272:30 911–30 917. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- Roberts A.B, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R, Van de Berg J, Ehrlich H.P. Wound contraction and scar contracture. In: Cohen I.K, Diegelmann R.F, Lindblad W.J, editors. Wound healing. Biochemical clinical aspects. Saunders; Philadelphia, PA: 1992. pp. 96–114. [Google Scholar]
- Shah M, Foreman D.M, Ferguson M.W. Neutralising antibody to TGF-b1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994;107:1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman D.M, Ferguson W.J. Neutralization of TGF-b1 and TGF-b2 or exogenous addition of TGF-b3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Siebert J.W, Burd A.R, McCarthy J.G, Weinzweig J, Ehrlich H.P. Fetal wound healing: a biochemical study of scarless healing. Plast. Reconstr. Surg. 1990;85:495–502. (discussion 503–504.) [PubMed] [Google Scholar]
- Soo C, Sayah D.N, Zhang X, Beanes S.R, Nishimura I, Dang C, Freymiller E, Ting K. The identification of novel wound-healing genes through differential display. Plast. Reconstr. Surg. 2002;110:787–797. doi: 10.1097/00006534-200209010-00011. (discussion 798–800.) [DOI] [PubMed] [Google Scholar]
- Soo C, et al. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am. J. Pathol. 2003;163:2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelnicki E.J, Arbeit J, Cass D.L, Saner C, Harrison M, Largman C. Modulation of the human homeobox genes PRX-2 and HOXB13 in scarless fetal wounds. J. Invest. Dermatol. 1998;111:57–63. doi: 10.1046/j.1523-1747.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Stelnicki E.J, Doolabh V, Lee S, Levis C, Baumann F.G, Longaker M.K, Mackinnon S. Nerve dependency in scarless fetal wound healing. Plast. Reconstr. Surg. 2000;105:140–147. doi: 10.1097/00006534-200001000-00024. [DOI] [PubMed] [Google Scholar]
- Stern R, McPherson M, Longaker M.T. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J. Burn Care Rehabil. 1990;11:7–13. doi: 10.1097/00004630-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Sullivan K.M, Lorenz H.P, Meuli M, Lin R.Y, Adzick N.S. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J. Pediatr. Surg. 1995;30:198–202. doi: 10.1016/0022-3468(95)90560-x. (discussion 202–203.) [DOI] [PubMed] [Google Scholar]
- Sunderland S. The anatomy and pathology of nerve injury. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- Sylvester M.F, Yannas I.V, Salzman E.W, Forbes M.J. Collagen banded fibril structure and the collagen–platelet reaction. Thromb. Res. 1989;55:135–148. doi: 10.1016/0049-3848(89)90463-5. [DOI] [PubMed] [Google Scholar]
- Thannickal V.J, Lee D.Y, White E.S, Cui Z, Larios J.M, Chacon R, Horowitz J.C, Day R.M, Thomas P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12 384–12 389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Tomasek J.J, Gabbiani G, Hinz B, Chaponnier C, Brown R.A. Myofibroblasts and mechanoregulation of connective tissue remodeling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Trelstad R.L, Birk D.E. Collagen fibril assembly at the surface of polarized cells. In: Trelstad R.L, editor. The role of extracellular matrix in development. Liss; New York: 1984. pp. 513–543. [Google Scholar]
- Troxel, K. 1994 Delay of skin wound contraction by porous collagen–GAG matrices. Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, MA.
- Verma P, Fawcett J. Spinal cord regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:43–66. doi: 10.1007/b99999. [DOI] [PubMed] [Google Scholar]
- Weiss P. The technology of nerve regeneration: a review. Sutureless tabulation and related methods of nerve repair. J. Neurosurg. 1944;1:400–450. [Google Scholar]
- Weiss P, Taylor A.C. Further experimental evidence against “neurotropism” in nerve regeneration. J. Exp. Zool. 1944;95:233–257. [Google Scholar]
- West D.C, Shaw D.M, Lorenz P, Adzick N.S, Longaker M.T. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int. J. Biochem. Cell Biol. 1997;29:201–210. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- Whitby D.J, Ferguson M.W. Immunohistochemical localization of growth factors in fetal wound healing. Dev. Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- White P, Thomas D.W, Fong S, Stelnicki E, Meijlink F, Largman C, Stephens P. Deletion of the homeobox gene PRX-2 affects fetal but not adult fibroblast wound healing responses. J. Invest. Dermatol. 2003;120:135–144. doi: 10.1046/j.1523-1747.2003.12015.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, et al. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br. J. Dermatol. 2005;152:616–622. doi: 10.1111/j.1365-2133.2005.06402.x. [DOI] [PubMed] [Google Scholar]
- Yannas I.V. Use of artificial skin in wound management. In: Dineen P, editor. The surgical wound. Lea & Febiger; Philadelphia, PA: 1981. pp. 171–190. [Google Scholar]
- Yannas I.V. Biologically active analogs of the extracellular matrix. Angew. Chem. 1990;29:20–35. (Engl. edn.) [Google Scholar]
- Yannas I.V. Springer; New York: 2001. Tissue and organ regeneration in adults. [Google Scholar]
- Yannas I.V. Synthesis of tissues and organs. Chembiochem. 2004;3:26–39. doi: 10.1002/cbic.200300682. [DOI] [PubMed] [Google Scholar]
- Yannas, I. V. (ed.) 2005aRegenerative medicine I. Theories, models and methods Adv. Biochem. Eng./Biotech., vol. 93 (series ed. T. Scheper). Heidelberg: Springer.
- Yannas, I. V. (ed.) 2005bRegenerative medicine II. Clinical and preclinical applications. Adv. Biochem. Eng./Biotech., vol. 94 (series ed. T. Scheper). Heidelberg: Springer.
- Yannas I.V. Facts and theories of induced organ regeneration. Adv. Biochem. Eng. Biotechnol. 2005;93:1–38. doi: 10.1007/b99965. [DOI] [PubMed] [Google Scholar]
- Yannas I.V, Burke J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- Yannas I.V, Burke J.F, Gordon P.L, Huang C, Rubenstein R.H. Design of an artificial skin. II. Control of chemical composition. J. Biomed. Mater. Res. 1980;14:107–132. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- Yannas I.V, Burke J.F, Warpehoski M, Stasikelis P, Skrabut E.M, Orgill D, Giard D.J. Prompt, long-term functional replacement of skin. Trans. Am. Soc. Artif. Intern. Organs. 1981;27:19–22. [PubMed] [Google Scholar]
- Yannas I.V, Burke J.F, Orgill D.P, Skrabut E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- Yannas, I. V., Orgill, D. P., Skrabut, E. M., & Burke, J. F. 1984 Skin regeneration with a bioreplaceable polymeric template. In Polymeric materials and artificial organs (ed. C. G. Gebelein), pp. 191–197. ACS Symposium Series, No. 256. Washington, DC: American Chemical Society.
- Yannas I.V, Lee E, Orgill D.P, Skrabut E.M, Murphy G.F. Synthesis and characterization of a model extracellular matrix which induces partial regeneration of adult mammalian skin. Proc. Natl Acad. Sci. USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannas I.V, Colt J, Wai Y.C. Wound contraction and scar synthesis during development of the amphibian Rana catesbeiana. Wound Repair Regen. 1996;4:31–41. doi: 10.1046/j.1524-475X.1996.40107.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yannas I.V. Peripheral nerve regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:67–89. doi: 10.1007/b100000. [DOI] [PubMed] [Google Scholar]