Abstract
Regardless of the underlying pathological mechanisms oxidative stress seems to be present in all forms of hypertension. Thus, we tested the hypothesis that chronic presence of high pressure itself elicits increased arterial O2.− production. Hypertension was induced in rats by abdominal aortic banding (Ab). Rats with Ab had elevated pressure in vessels proximal and normal pressure in vessels distal to the coarctation, yet both vascular beds were exposed to the same circulating factors. Compared to normotensive hind limb arteries (HLAs) hypertensive forelimb arteries (FLAs) exhibited 1) impaired dilations to acetylcholine and the nitric oxide donor S-nitroso-N-acetyl-d,l-penicillamine that were restored by administration of superoxide dismutase; 2) an increased production of O2.− (measured by lucigenin chemiluminescence and ethidium bromide fluorescence) that was inhibited or reduced by superoxide dismutase, the NAD(P)H oxidase inhibitors diphenyleneiodonium and apocynin, or the protein kinase C (PKC) inhibitors chelerythrine and staurosporine or by the angiotensin-converting enzyme (ACE) inhibitor captopril; and 3) increased ACE activity. In organ culture, exposure of isolated arteries of normotensive rats to high pressure (160 mmHg, for 24 hours) significantly increased O2.− production compared to that in arteries exposed to 80 mmHg. High pressure-induced O2.− generation was reduced by inhibitors of ACE and PKC. Incubation of cultured arteries with angiotensin II elicited significantly increased O2.− generation that was inhibited by chelerythrine. Thus, we propose that chronic presence of high pressure itself can elicit arterial oxidative stress, primarily by activating directly a PKC-dependent NAD(P)H oxidase pathway, but also, in part, via activation of the local renin-angiotensin system.
In several forms of hypertension increased arterial superoxide (O2.−) production has been shown to decrease the bioavailability of endothelium-derived vasodilator nitric oxide (NO), thereby contributing to the maintenance of elevated peripheral resistance.1–6 However, the stimuli and mechanisms underlying increased O2.− production in hypertension are not completely understood.
The vascular effects of hypertension are complex and are likely to be induced, at least in part, by increased levels of neurohumoral factors. Among them, angiotensin II has been suggested to increase O2.− generation in vascular cells.3,4,7 However, oxidative stress seems to be present in virtually all forms of hypertension4,5 (including low-renin hypertension,8,9 genetic hypertension, angiotensin II-induced hypertension,3,4 renovascular hypertension,2,10 pheochromocytoma-related hypertension11) despite the differences in plasma levels of circulating factors. Importantly, it has been reported that in angiotensin II-infused rats reduction of blood pressure with hydralazine or spironolactone (that are unlikely to affect angiotensin II levels) normalized aortic O2.− production.7,12
Thus, it is logical to hypothesize that high intraluminal pressure itself promotes vascular O2.− generation in hypertension. This idea is congruent with our recent findings that in vitro acute increases in pressure up-regulate arterial O2.− production by activating NAD(P)H oxidase.13 Previous studies also showed that short-term increases in pressure both in vivo and in vitro impair endothelial function,14–16 an effect that can be prevented by superoxide dismutase (SOD).17 To test the role of chronic exposure to high pressure in vitro in the up-regulation of arterial O2.− production, isolated arteries maintained in vessel culture were pressurized to normotensive or hypertensive levels. To test the differential role of chronic presence of high pressure in vivo, hypertension was induced in rats by abdominal aortic banding (Ab). The Ab model of hypertension provides the advantage that blood vessels proximal to the coarctation are exposed to high pressure, whereas in distal vascular beds pressure is close to normotensive levels. Because both vascular beds are exposed to the same circulating factors, the in vivo effects of chronic presence of high blood pressure on vascular O2.− production could be independently assessed.
Materials and Methods
Suprarenal Aortic Constriction Hypertension
In male Wistar-Hannover rats (n = 11) Ab hypertension was induced according to the protocol of Taconic Biotechnology Co. In brief, animals were anesthetized with intraperitoneal sodium pentobarbital and a dorsal midline incision was made. The abdominal aorta was exposed and a constriction (∼0.7 mm in diameter) was applied cranial to both renal arteries. Then, the abdomen and skin were closed and animals were allowed to recover. Successful Ab was indicated by an undetectable pulse pressure measured by the tail cuff method after the operation. Weight-matched sham-operated control animals (C, n = 11) were subjected to the same procedure, without Ab. Animals were monitored for 6 weeks after the operation. Systolic blood pressure was measured by the tail cuff method once a week. All subsequent experiments were performed 6 weeks after operation,18,19 when plasma angiotensin II levels are close to normal values in rats with suprarenal Ab.19 Animals were heparinized and anesthetized (intraperitoneal sodium pentobarbital), the left carotid and femoral artery were cannulated, and arterial pressures were simultaneously recorded. Then the animals were sacrificed and the hearts were excised and weighed.
Vessel Isolation and Functional Studies
Arteries of the forelimbs (FLAs) and the hind limbs (HLAs) of control and Ab rats were exposed (brachial and femoral arteries, respectively) and branches supplying the skeletal muscle were isolated for further studies. In isolated, pressurized FLA and HLA branches (diameter, ∼200 μm) dilations to acetylcholine and to the NO donor S-nitroso-N-acetyl-d,l-penicillamine in the absence and presence of SOD (200 U/ml) were measured, as described.17 Responses to the phosphodiesterase inhibitor papaverine (10−4 mol/L) were also obtained.
Measurement of Vascular Superoxide Levels
Vascular O2.− production was assessed by the lucigenin chemiluminescence method as previously described.20 In brief, the vessels were placed in scintillation vials containing HEPES-buffered (10 mmol/L, pH 7.4) physiological salt solution (PSS) and lucigenin (10 μmol/L) chemiluminescence was measured in a liquid scintillation counter (LS-6000IC; Beckman, Fullerton, CA) in the absence or presence of SOD (200 U/ml) or diphenyleneiodonium (DPI) [10−5 mol/L, an inhibitor of flavoprotein-containing oxidases, including NAD(P)H oxidases] or chelerythrine [10−6 mol/L, an inhibitor of protein kinase C (PKC) and PKC-induced NAD(P)H activity3] or in the absence of extracellular Ca2+. In separate experiments, O2.− production in FLAs of Ab rats was determined in the absence and presence (30 minutes incubation) of apocynin [3 × 10−4 mol/L, an inhibitor of superoxide production by NAD(P)H oxidases5,21] or Nω-nitro-l-arginine-methyl-ester (L-NAME) (3 × 10−4 mol/L, an inhibitor of NO synthesis20) or captopril [10−4 mol/L, an angiotensin-converting enzyme (ACE) inhibitor, 1 hour incubation] or staurosporine (10−6 mol/L, an inhibitor of PKC), or Tiron (10 mmol/L, a superoxide scavenger). Scintillation counts were obtained 15 to 20 minutes after addition of vessels (averaged) and background-corrected values were normalized to tissue weight.
Ethidium Bromide Fluorescence
Hydroethidine was used to localize superoxide production, as described.20 Samples exposed to hydroethidine in the presence of SOD, Tiron, or DPI served as control. In other experiments arterial segments from the same control rat were incubated in the absence or presence of phorbol myristate acetate (PMA) (10−6 mol/L), PMA plus SOD, or PMA plus DPI and then were incubated with hydroethidine.
Measurement of Vascular ACE Activity
ACE activity was measured in vascular homogenates in the absence or presence of the ACE inhibitors captopril or enalaprilat using the synthetic ACE-specific substrate hyppuryl histidyl leucine by a high pressure liquid chromatography (HPLC)-based assay according to the protocols of Meng and colleagues22 and Koiter and colleagues.23 In brief, vessels were pulverized in liquid nitrogen and homogenized (at 0°C for 1 minute) in 0.02 mol/L potassium phosphate buffer (pH 8.3). One hundred μL of homogenate containing 10 mg of vascular tissue23 was added to 500 μl of reaction mixture24 (300 mmol/L NaCl, 10−4 mol/L CoCl2, in 0.1 mol/L phosphate buffer, pH 8.3) in three test tubes, stored on ice. Samples were incubated with or without captopril (10−4 mol/L) or enalaprilat (10−4 mol/L) for 15 minutes. The reaction was started by adding hyppuryl histidyl leucine (final concentration, 5 mmol/L) to the reaction mixture and transferring the tubes to a 37°C water bath. After 15 minutes of incubation generation of the product (hippuric acid, HA) was stopped by adding 250 μL of HCl (1 mol/L) then the internal standard N-benzoyl-l-alanine (BA, 0.1 mg/sample) was added. To extract HA and BA 1 ml of ethyl acetate was added to the sample according to the method of Cushman and Cheung.24 After vortexing for 1 minute and 10 minutes of centrifugation (3000 × g), 0.8 ml of the ethyl acetate layer was transferred to a glass tube and evaporated at 60°C. The residue was dissolved in 150 μL mobile phase. Twenty-five μL of the sample was analyzed with the System Gold HPLC system (Beckman Coulter, www.beckman.com) equipped with C18 guard and 150 × 2.0-mm MiniBore Ultrasphere 5μC18 column (Beckman Coulter) and a UV detector set at 228 nm. The mobile phase consisted of sodium acetate buffer (10 mmol/L, pH 4.0) with 10% methanol and 7.5% acetonitrile (flow-rate, 1.0 ml/min). Peaks for HA and BA were identified by comparison with retention times of standard compounds. Standard solutions of HA and BA were prepared daily by dissolving HA and BA in the HPLC mobile phase and diluted serially to provide calibration standards. Under the conditions described above, a clear separation of HA and BA was achieved. Quantification was performed by the 32Karat software (Beckman Coulter) measuring peak areas of HA (elution time, 2.7 minutes) in relation to the internal standard BA (elution time, 3.5 minutes) at 228 nm. Measurements were performed in duplicate. The intra-assay coefficient of variation was 4%. Doubling of the incubation time doubled the generation of HA showing the linearity of the assay. ACE activity was expressed in units (U), defined as the formation of nmol HA per minute at 37°C per g vascular tissue wet weight. The site-specific ACE inhibitors captopril and enalaprilat eliminated the HA peak in the samples showing the specificity of the assay to ACE activity.
Detection of 3-Nitrotyrosine
To characterize ONOO− formation in vascular samples we used the modified method of Csiszar and colleagues20 and Ungvari and colleagues25 using a dot-blot system that allows for the determination of the total 3-nitrotyrosine content in all proteins without molecular size limitations. Immunolabeling of membranes was performed using a primary antibody against nitrated tyrosine residues of proteins (1:100; Cell Signaling Technology Co, Beverly, MA) and a goat anti-rabbit secondary antibody conjugated to biotin (Vector Laboratories, Burlingame, CA). 3-Nitrotyrosine was localized in arterial sections by immunohistochemistry as described.20,25 Sections were stained using an avidin-biotinylated enzyme complex (ABC Vectastain, Vector Laboratories, www.vectorlabs.com) and Vector-DAB (diaminobenzidine tetrahydrochloride) substrate, counterstained with hematoxylin.
Vessel Culture Studies
To investigate vascular O2.− generation under controlled conditions isolated FLAs of control rats were maintained in vessel culture. In brief, arteries were cannulated on both sides in a stainless steel vessel culture chamber (Danish Myo Technology, www.dmt.dk) under sterile conditions and continuously superfused with F12 medium (Life Technologies, Inc., Grand Island, NY) containing antibiotics (100 UI/L penicillin, 100 mg/L streptomycin, and 10 μg/L fungizone) and supplemented with 5% fetal calf serum (Boehringer-Mannheim, Indianapolis, IN) according to the modified technique of Bakker and colleagues26 and Bolz and colleagues,27,28 as previously described.29 Arteries were exposed to 80 mmHg or 160 mmHg (to mimic hypertensive conditions) for 24 hours in the absence or presence of enalaprilat (10−4 mol/L), captopril (10−4 mol/L), or chelerythrine (10−6 mol/L). In separate experiments, vessels were incubated with the PKC activator PMA (10−6 mol/L, for 30 minutes in oxygenated PSS, at 37°C) or with angiotensin II (10−8 to 10−6 mol/L, for 24 hours) in the absence or presence of chelerythrine, SOD, or DPI. Minimal intraluminal flow was maintained only to renew the culture medium within the intraluminal space and maintaining minimal shear stress (<0.5 dyn/cm2). At the end of the culture period vascular O2.− generation was determined.
Data Analysis
Lucigenin chemiluminescence data and densitometric ratios were normalized to the respective control mean values. Data are expressed as means ± SEM. Statistical analyses of data were performed by Student’s t-test or by two-way analysis of variance followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
Results
Ab rats had elevated mean arterial blood pressure proximal and normal blood pressure distal to the coarctation (Table 1), as previously reported.30
Table 1.
Hemodynamic Data and Heart Weight
| Parameter | Sham-operated | Ab rats |
|---|---|---|
| MAP, carotid artery (mmHg) | 92 ± 2 | 139 ± 9*† |
| MAP, femoral artery (mmHg) | 87 ± 3 | 69 ± 9 |
| SBP (tail cuff, mmHg) | 127 ± 1 | n.d. |
| HR (tail cuff, BPM) | 416 ± 10 | n.d. |
| BW (g) | 322 ± 9 | 333 ± 9 |
| HW/BW (mg/g) | 2.6 ± 0.1 | 3.8 ± 0.4* |
Hemodynamic data and heart weight in sham-operated control (n = 4 to 11) and aortic-banded (n = 4 to 11) rats. MAP, mean arterial pressure measured in anesthetized animals; SBP, systolic blood pressure measured in awake animals by the tail cuff method; HR, heart rate; BPM, beats per minute, n.d., nondeterminable; BW, body weight; HW, heart weight. Values are mean ± SEM.
P < .05 versus control.
P < .05 versus femoral artery.
Arterial Dilations
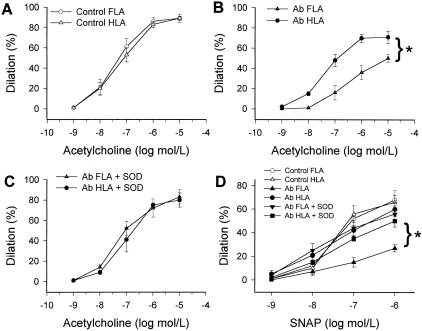
Dilations to ACh and S-nitroso-N-acetyl-d,l-penicillamine in Ab FLAs were significantly decreased as compared to responses of HLAs of the same rats (Figure 1). Previous studies also showed impaired ACh-induced relaxation in high pressure-exposed thoracic segments, but not normal pressure exposed abdominal segments, of the aorta of Ab rats.30 Administration of SOD did not affect significantly ACh- (not shown) or S-nitroso-N-acetyl-d,l-penicillamine-induced responses in FLAs and HLAs of control rats and in HLAs of Ab rats, whereas it restored responses of Ab FLAs (Figure 1, C and D). Arterial dilations to papaverine did not differ among the four groups of vessels (not shown).
Figure 1.
A–D: Dilations to acetylcholine and to S-nitroso-N-acetyl-d,l-penicillamine in isolated FLAs and HLAs of sham-operated control and Ab rats in the absence and presence of SOD (200 U/ml) (n = 4 to 7 rats). Data are mean ± SEM. *, P < 0.05.
Vascular Superoxide Production
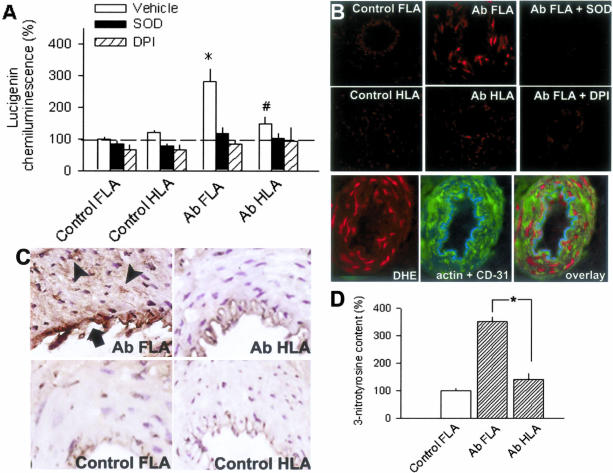
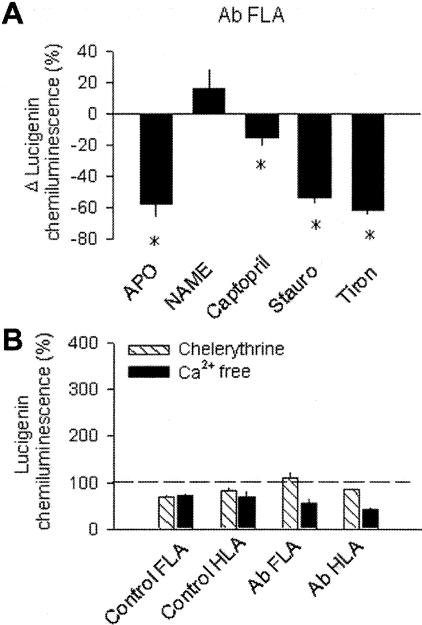
Under basal conditions, lucigenin chemiluminescence in FLAs was significantly higher than in HLAs of the same Ab rats or in FLAs and HLAs from control rats (C FLA, 23.4 × 103 counts/mg/minute; Figure 2A). Increased O2.− generation in Ab FLAs was inhibited by SOD or DPI eliminating the difference between the groups (Figure 2A). Increased O2.− generation by Ab FLAs was also blocked by apocynin, staurosporine, and Tiron, whereas it was not significantly affected by L-NAME (Figure 3A). Captopril also elicited a partial, but significant, reduction of O2.− generation in Ab FLAs (Figure 3A), whereas chelerythrine and removal of extracellular Ca2+ completely inhibited increased O2.− generation in Ab FLAs and eliminated the differences between the groups (Figure 3B). In Ab FLAs (n = 3) the relative number of ethidium bromide (EB)-positive nuclei was significantly increased both in the media and intima of Ab FLAs (Figure 2B). Incubation of vessels with DPI or SOD prevented EB staining (Figure 2B).
Figure 2.
A: Generation of O2− as determined by lucigenin chemiluminescence in isolated FLAs and HLAs of sham-operated control or Ab rats under control conditions and in the presence of SOD (200 U/ml) or DPI (10−5 mol/L). Data are normalized to the mean value of the control FLA group. *, P < 0.05 vs. control and #, P < 0.05 vs. FLA (n = 4 to 15 rats). B: Top: Representative fluorescent photomicrographs of control and Ab FLAs and HLAs labeled with the dye dihydroethidium, which produces a red fluorescence when oxidized to ethidium bromide (EB) by O2−. Sections of Ab FLA incubated with DPI or SOD are also shown. Bottom: Overlaying EB-stained fluorescent images of an Ab FLA with images of the same vessel section stained for α-smooth muscle actin (green) and the endothelium-specific marker CD-31 (blue) show that increased O2·− levels are present both in the smooth muscle and the endothelium of Ab FLA. Similar findings were observed in three separate experiments. C: Hypertensive Ab FLA showed more prevalent immunostaining for 3-nitrotyrosine (brown reaction product, DAB staining) that was localized both to the endothelium (arrow) and the media (arrowheads), than normotensive vessels (Ab HLA, control FLA and HLA). Hematoxylin counterstaining. D: Summary data for Western blot analysis of protein tyrosine nitration in normotensive and hypertensive vessels (n = 3 in each group). *, P < 0.05. Original magnifications: ×40 (B); ×20 (C).
Figure 3.
A: Changes in O2− generation in isolated FLAs and HLAs of sham-operated control or Ab rats in response to apocynin (Apo, 3 × 10−4 mol/L), L-NAME (3 × 10−4 mol/L), captopril (10−4 μmol/L), staurosporine (10−6 μmol/L), or Tiron (10 mmol/L). *, P < 0.05 (n = 4 to 7 rats). B: O2− generation in FLAs and HLAs of control and Ab rats in the presence of chelerythrine (Chel, 10−6 mol/L) or in the absence of Ca2+. Data are normalized to the mean value of the untreated control FLA group. *, P < 0.05 (n = 5 to 7).
Vascular 3-Nitrotyrosine Content
In FLAs of Ab rats there was a significantly increased 3-nitrotyrosine content (Figure 2D), as shown by Western blotting. Enhanced immunostaining for 3-nitrotyrosine in FLAs of Ab rats was localized both to the endothelium and media (Figure 2C). The media of Ab HLAs and arteries of control rats were relatively free from 3-nitrotyrosine immunoreactivity. In control experiments there was no evidence of nonspecific immunostaining.
Vascular ACE Activity
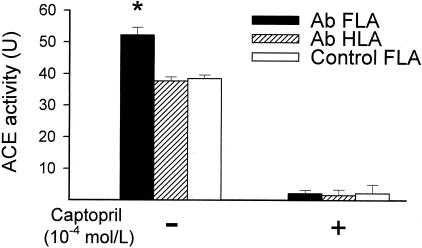
ACE activity was significantly greater in FLAs than in HLAs of Ab rats and could be inhibited by enalaprilat (not shown) and captopril (Figure 4).
Figure 4.
Vascular ACE activity, measured by a HPLC-based assay, in FLAs and HLAs of sham-operated control and Ab rats. Data are mean ± SEM. *, P < 0.05.
Vessel Culture Studies
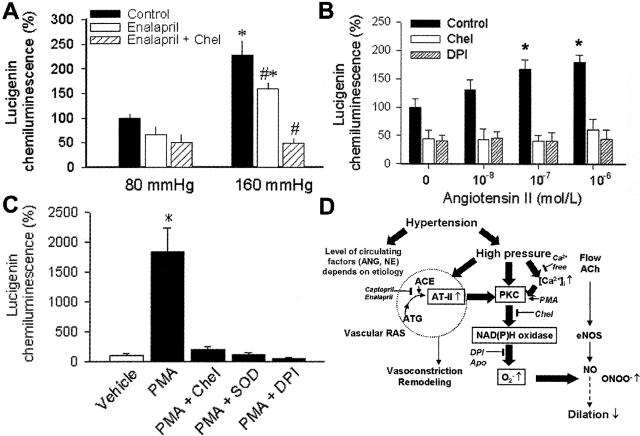
Lucigenin chemiluminescence measurements showed that chronic pressurization of cultured arteries to hypertensive levels (160 mmHg) elicited significant increases in vascular O2.− generation, as compared to that in vessels exposed to 80 mmHg (Figure 5A). Increased O2.− generation in high pressure-treated vessels was partly reduced by incubation with enalaprilat (Figure 5A), which still left a large portion of O2.− generation intact and did not eliminate the difference between the groups. Similar results were obtained with captopril as well (not shown). Additional administration of chelerythrine completely inhibited pressure-induced increases in O2.− production (Figure 5A). Incubation of cultured arteries with angiotensin II (Figure 5B) or pharmacological activation of PKC with PMA (Figure 5C) also elicited concentration-dependent increases in O2.− generation that were inhibited by chelerythrine, DPI, or SOD.
Figure 5.
A: O2− generation in FLAs of control rats pressurized to normotensive (80 mmHg) and hypertensive levels (160 mmHg) in organ culture (24 hours) in the presence and absence of enalaprilat (10−4 mol/L) or enalaprilat plus chelerythrine. *, P < 0.05 versus 80 mmHg; #, P < 0.05 versus untreated (n = 4 to 5 rats). B: O2− generation in cultured arteries with or without incubation with angiotensin II (24 hours) in the presence and absence of chelerythrine and DPI. *, P < 0.05 (n = 4 to 5 rats). C: O2− generation in cultured arteries of control rats under control conditions and in the presence of PMA (10−6 mol/L), or PMA plus chelerythrine, or PMA plus DPI, or PMA plus SOD. Data are normalized to the control mean value. *, P < 0.05 (n = 4 to 5 rats). D: Proposed scheme for high pressure-induced activation of Ca2+-PKC pathway promoting NAD(P)H oxidase-derived O2.− production and endothelial dysfunction. High pressure-induced up-regulation of vascular RAS increases tissue concentrations of angiotensin II contributing to increased oxidative stress, vascular remodeling, and proinflammatory alterations.
Discussion
The main findings of the present study are that chronic presence of high intraluminal pressure itself, both in vivo and in vitro enhance O2.− production in peripheral arteries. Using the Ab rat model of hypertension we have demonstrated that in the presence of the same circulating factors regional increases in blood pressure resulted in selective impairment of NO-mediated dilations that could be restored by SOD (Figure 1). In contrast, dilations (Figure 1) and O2.− generation (Figure 2A) in normotensive arteries of the same animals were similar to those in vessels from sham-operated control rats. Previous studies also showed impairment of NO-mediated relaxations in high pressure-exposed thoracic aorta and coronary arteries, but not in normal pressure-exposed abdominal aorta of Ab rats.19,31 We demonstrated, that hypertensive arteries selectively exhibit a SOD-sensitive enhanced O2.− production (Figure 2A). EB-staining showed that O2.− production was localized both in the endothelium and the smooth muscle of hypertensive vessels (Figure 2B). The presence of elevated O2.− levels in both vascular layers provides an explanation for the simultaneously impaired dilations to both endothelium-derived NO and NO released from exogenous NO donors (Figure 1) frequently observed in hypertensive humans and animals.4,19,32 In hypertensive FLAs (Figure 2, C and D) and aorta19 of Ab rats, but not in normal pressure-exposed HLAs there was an increased protein 3-nitrotyrosine content localized both to the endothelium and smooth muscle (Figure 2C), indicating that in both vascular layers there are increased O2.− levels present in vivo, which scavenge endothelium-derived NO by forming ONOO−. Because hypertensive and normotensive vascular beds in Ab animals are exposed to the same circulating factors, it is likely that increases in O2.− production in hypertensive arteries are predominantly because of the prevailing high intraluminal pressure.
An important role for high pressure in up-regulation of O2.− production is further supported by our findings that a developmental increase in blood pressure coincides with the increase in vascular O2.− production and appearance of endothelial dysfunction33 in spontaneously hypertensive rats (SHR). Also, in angiotensin II- and/or norepinephrine-infused rats reduction of blood pressure with hydralazine to control levels (which is unlikely to affect plasma levels of circulating factors) normalized the increased aortic O2.− production7 and decreased the elevated plasma levels of 8-epi-PGF2α, a marker of oxidative stress in vivo.34 Although previous studies proposed that in rabbits hydralazine decreased enzymatic production of vascular O2.− production and improved NO-induced aortic relaxations, these effects have not been correlated with hydralazine-induced changes in blood pressure.35 Taken together, in hypertensive conditions in vivo high intraluminal pressure itself can increase O2.− production, which may explain why an increased oxidative stress has been found in high pressure-exposed vessels in virtually all forms of hypertension.2,5,7
The primary source of O2.− in hypertensive vessels is likely the vascular NAD(P)H oxidase, because increased lucigenin chemiluminescence of hypertensive arteries was inhibited by DPI (Figure 2A) and by apocynin (Figure 3A). Previous studies also reported an increased NAD(P)H oxidase activity in most peripheral vascular beds of animals with various forms of hypertension,7 including genetic hypertension, angiotensin II-induced hypertension,3,4 renovascular hypertension,2 and low-renin hypertension.5
Because captopril reduced O2.− production in hypertensive arteries of Ab rats (Figure 3A), it is likely that increased vascular levels of angiotensin II, because of an up-regulated local renin-angiotensin system (RAS)36 contribute to the activation of NAD(P)H oxidase. Indeed, we found that in hypertensive FLAs (Figure 4) and thoracic aorta37 of Ab rats there is an increased ACE activity, which may be because of an increased expression of ACE,37 although other mechanisms cannot be excluded. Also, previous studies showed that chronic inhibition of ACE in Ab hypertensive guinea pigs significantly decreased NAD(P)H oxidase activity in vascular cells.38 In the present study vessels were exposed to high pressure only for 6 weeks, thus one can speculate that longer exposure of vessels, such as years or decades would have a more substantial effect on vascular RAS. Because inhibition of PKC (Figure 3B) substantially decreased O2.− production in the hypertensive vessels of Ab rats, it is likely that PKC plays an important role in chronic high pressure-induced activation of NAD(P)H oxidase in vivo. Correspondingly, previous studies also showed increased PKC activity39,40 in high pressure-exposed aorta of Ab rats. It is likely that PKC phosphorylates the regulatory p47phox subunit of NAD(P)H oxidase,13 which is thought to be essential to angiotensin II-induced oxidative stress.41
To further test the role of ACE and PKC in chronic high pressure-related O2.− production we pressurized isolated arteries of control rats to normal and hypertensive pressure levels in vessel culture. Compared to normotensive pressure level, chronic exposure of cultured arteries in vitro to high pressure resulted in an increased O2.− production, which was reduced, at least in part, by ACE inhibitors (Figure 5A). Thus, we propose that high intraluminal pressure-induced activation of local RAS in the vascular wall contributes to any hypertension-induced vascular oxidative stress. This view is further supported by previous studies by Bardy and colleagues42 showing an increased angiotensin II concentration in the culture medium of high pressure-exposed cultured arteries. It is likely that angiotensin II increases vascular NAD(P)H oxidase activity via activating PKC,3,43 because incubation of cultured arteries with exogenous angiotensin II also significantly increased arterial O2.− generation that could be prevented by inhibition of PKC (Figure 5B).
Importantly, however, a significant part of high pressure-induced oxidative stress appears to be independent of local and systemic RAS, because in the presence of ACE inhibitors O2.− production was still substantially greater in high pressure-exposed arteries than in arteries pressurized to normotensive level (Figure 5A). Importantly, this pressure-induced, ACE-independent O2.− generation was abolished by inhibition of PKC (Figure 5A). Previously we have demonstrated in isolated arteries that exposure to high pressure itself activates PKC13 and in the present study we showed that pharmacological activation of PKC2 increases vascular NAD(P)H oxidase activity (Figure 5C). These findings are in line with our recent observations that increases in pressure13 or stretching of arteries44 (that are unlikely to up-regulate ACE activity acutely) can elicit PKC-mediated phosphorylation and apocynin-sensitive translocation of the NAD(P)H oxidase regulatory subunit p47−phox44 increasing NAD(P)H oxidase activity with the consequent impairment of NO-mediated dilations of vessels of normotensive rats.17 One of the mechanisms by which high intraluminal pressure can activate PKC is an increased Ca2+ influx. Indeed, we have shown that high pressure in vitro increases [Ca2+]i in the vascular wall.13 Because in the absence of Ca2+, O2.− production was significantly decreased both in hypertensive arteries (Figure 3B) and high pressure-exposed isolated arteries,13 it is likely that increased Ca2+-induced PKC activity is an important stimulator of vascular NAD(P)H oxidase even in the absence of activation of local RAS.
On the basis of the present and previous findings we propose (Figure 5D) that in peripheral arteries the chronic presence of high intravascular pressure increases [Ca2+]i and activates PKC, increasing NAD(P)H oxidase-derived O2.− production. In addition, the high pressure-induced NAD(P)H oxidase activity in vivo is likely further modulated by the presence of pulsatility and an increased pulsatility,45,46 such as in systolic hypertension. Chronic presence of high pressure also activates the local renin-angiotensin system, thereby increasing angiotensin II bioavailability in the vascular wall, contributing further to the activation of PKC-NAD(P)H oxidase axis. The resulting oxidative stress reduces NO-mediated regulation of arterial resistance. The high pressure-induced up-regulation of vascular RAS, together with the increased arterial O2.− and ONOO− production could be the initial steps leading to remodeling47 and proinflammatory alterations of arterial wall.48 Our findings may explain the cardiovascular protective effects attributed to ACE inhibitors in cases of human essential hypertension that are associated with low plasma angiotensin II levels. Nevertheless, the primary role of high pressure itself in the development of vascular oxidative and nitrosative stress and proinflammatory microenvironment emphasize the importance of early and effective reduction of high blood pressure by behavioral changes, dietary means, and pharmacological treatments.
Footnotes
Address reprint requests to Akos Koller M.D., Ph.D., Department of Physiology, Basic Sciences Building., Room 617, New York Medical College, Valhalla, NY 10595. E-mail: koller@nymc.edu.
Supported by grants from the National Institutes of Health (PO-1-HL-43023, HL-46813, HL-59417), the American Heart Association (0430108N, 00500849T, 0020144T, and 0120166T), and Hungarian National Scientific Research Fund (T-033117, T-34779).
Z.U. and A.C. contributed equally to this study.
References
- Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, III, Panza JA. Selective defect in nitric oxide synthesis may explain the impaired endothelium-dependent vasodilation in patients with essential hypertension. Circulation. 1998;97:851–856. doi: 10.1161/01.cir.97.9.851. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:58e–65e. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Matsuura H, Hara K, Goto C, Oshima T, Chayama K. Excess norepinephrine impairs both endothelium-dependent and -independent vasodilation in patients with pheochromocytoma. Hypertension. 2002;39:513–518. doi: 10.1161/hy02t2.102820. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- De Bruyn VH, Nuno DW, Cappelli-Bigazzi M, Dole WP, Lamping KG. Effect of acute hypertension in the coronary circulation: role of mechanical factors and oxygen radicals. J Hypertens. 1994;12:163–172. [PubMed] [Google Scholar]
- Ghaleh B, Hittinger L, Kim SJ, Kudej RK, Iwase M, Uechi M, Berdeaux A, Bishop SP, Vatner SF. Selective large coronary endothelial dysfunction in conscious dogs with chronic coronary pressure overload. Am J Physiol. 1998;274:H539–H551. doi: 10.1152/ajpheart.1998.274.2.H539. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res. 1998;83:960–965. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- Lang D, Mosfer SI, Shakesby A, Donaldson F, Lewis MJ. Coronary microvascular endothelial cell redox state in left ventricular hypertrophy: the role of angiotensin II. Circ Res. 2000;86:463–469. doi: 10.1161/01.res.86.4.463. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30:934–941. doi: 10.1161/01.hyp.30.4.934. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Meng QC, Balcells E, Dell’Italia L, Durand J, Oparil S. Sensitive method for quantitation of angiotensin-converting enzyme (ACE) activity in tissue. Biochem Pharmacol. 1995;50:1445–1450. doi: 10.1016/0006-2952(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Koiter J, Navis G, de Jong PE, van Gilst WH, de Zeeuw D. Sample dilution: a methodological pitfall in the measurement of tissue but not serum ace-activity. J Pharmacol Toxicol Methods. 1998;39:45–49. doi: 10.1016/s1056-8719(97)00099-3. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Bagi Z, Koller A. Impaired nitric oxide-mediated flow-induced coronary dilation in hyperhomocysteinemia: morphological and functional evidence for increased peroxynitrite formation. Am J Pathol. 2002;161:145–153. doi: 10.1016/S0002-9440(10)64166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker ENTP, van der Meulen ET, Spaan JAE, VanBavel E. Organoid culture of cannulated rat resistance arteries: effect of serum factors on vasoactivity and remodeling. Am J Physiol. 2000;278:H1233–H1240. doi: 10.1152/ajpheart.2000.278.4.H1233. [DOI] [PubMed] [Google Scholar]
- Bolz SS, Pieperhoff S, De Wit C, Pohl U. Intact endothelial and smooth muscle function in small resistance arteries after 48 h in vessel culture. Am J Physiol. 2000;279:H1434–H1439. doi: 10.1152/ajpheart.2000.279.3.H1434. [DOI] [PubMed] [Google Scholar]
- Bolz S, Pieperhoff S, De Wit C, Pohl U. Chronic increases in transmural pressure reduce NO-mediated dilations in isolated resistance arteries of the hamster. Acta Physiol Scand. 2000;168:113–117. doi: 10.1046/j.1365-201x.2000.00633.x. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J, Vanheel B, Leusen I. Depressed endothelium-dependent relaxation in hypertension: relation to increased blood pressure and reversibility. Pflugers Arch. 1988;411:500–504. doi: 10.1007/BF00582370. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Pinto A, Mullane KM. Impaired endothelium-dependent relaxations in rabbits subjected to aortic coarctation hypertension. Hypertension. 1987;10:164–170. doi: 10.1161/01.hyp.10.2.164. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Pu Q, Park JB. Effect of amlodipine compared to atenolol on small arteries of previously untreated essential hypertensive patients. Am J Hypertens. 2002;15:105–110. doi: 10.1016/s0895-7061(01)02290-7. [DOI] [PubMed] [Google Scholar]
- Koller A, Huang A. Development of nitric oxide and prostaglandin mediation of shear stress-induced arteriolar dilation with aging and hypertension. Hypertension. 1999;34:1073–1079. doi: 10.1161/01.hyp.34.5.1073. [DOI] [PubMed] [Google Scholar]
- Aizawa T, Ishizaka N, Usui S, Ohashi N, Ohno M, Nagai R. Angiotensin II and catecholamines increase plasma levels of 8-epi-prostaglandin F(2alpha) with different pressor dependencies in rats. Hypertension. 2002;39:149–154. doi: 10.1161/hy1201.097301. [DOI] [PubMed] [Google Scholar]
- Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrion D, Benessiano J, Levy BI. In vitro modulation of a resistance artery diameter by the tissue renin-angiotensin system of a large donor artery. Circ Res. 1997;80:189–195. doi: 10.1161/01.res.80.2.189. [DOI] [PubMed] [Google Scholar]
- Goetz RM, Holtz J. Angiotensin-converting enzyme: induction by hypertension-induced vessel distension. Blood Press. 2000;9:40–46. doi: 10.1080/080370500439416. [DOI] [PubMed] [Google Scholar]
- Bell JP, Mosfer SI, Lang D, Donaldson F, Lewis MJ. Vitamin C and quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am J Physiol. 2001;281:H1704–H1710. doi: 10.1152/ajpheart.2001.281.4.H1704. [DOI] [PubMed] [Google Scholar]
- Pucci ML, Tong X, Miller KB, Guan H, Nasjletti A. Calcium- and protein kinase C-dependent basal tone in the aorta of hypertensive rats. Hypertension. 1995;25:752–757. doi: 10.1161/01.hyp.25.4.752. [DOI] [PubMed] [Google Scholar]
- Turla MB, Park SM, Webb RC. Vascular responsiveness to phorbol esters in coarctation-hypertensive rats. J Hypertens. 1990;8:191–196. doi: 10.1097/00004872-199002000-00015. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy N, Merval R, Benessiano J, Samuel JL, Tedgui A. Pressure and angiotensin II synergistically induce aortic fibronectin expression in organ culture model of rabbit aorta. Evidence for a pressure-induced tissue renin-angiotensin system. Circ Res. 1996;79:70–78. doi: 10.1161/01.res.79.1.70. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Waack BJ, Weno BL, Heistad DD. Increases in pulse pressure impair acetylcholine-induced vascular relaxation. Am J Physiol. 1995;268:H359–H363. doi: 10.1152/ajpheart.1995.268.1.H359. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Oemar BS, Yang Z, Luscher TF. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res. 1997;81:797–803. doi: 10.1161/01.res.81.5.797. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Molecular and cellular mechanisms regulating vascular function and structure—implications in the pathogenesis of hypertension. Can J Cardiol. 2000;16:1137–1146. [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]