Abstract
The early inflammatory response within organ allografts is initiated by ischemia/reperfusion (I/R) and promotes subsequent alloantigen-primed T cell recruitment into and rejection of the graft. Polymorphonuclear leukocyte (PMN)-mediated tissue damage is a primary component of the early inflammation in allograft rejection. We sought to compare and elucidate the mechanism of early PMN infiltration into cardiac isografts and allografts. Despite identical production of PMN attractant chemokines, PMN infiltration following reperfusion into syngeneic and allogeneic grafts was not equivalent. PMN infiltration into isografts peaked at 9 to 12 hours post-transplant and quickly resolved. In contrast, PMN infiltration into allografts continued to elevated levels, peaking at 24 hours post-reperfusion. This amplified PMN infiltration into allografts did not resolve until 72 hours post-reperfusion and was accompanied by marked parenchymal necrosis. This early innate inflammatory response was regulated by IFN-γ-producing CD8+ T cells present in the recipient before detectable alloantigen T cell priming. Co-culture with CD62Llow CD8+ T cells, but not CD62Lhigh CD8+ or CD62Llow CD4+ T cells, harvested from naïve animals induced allogeneic endothelial cells to express IFN-γ-dependent chemokines. These data demonstrate CD8+ T cell-mediated attack on the vascular endothelium of allografts within hours following organ reperfusion that amplifies innate immune-mediated intra-graft inflammation and necrosis.
Organ transplantation is a commonly used therapy for end-stage organ failure. With few exceptions, transplanted organs are allogeneic and preventing T cell-mediated rejection is a primary goal of post-transplant medical care. Acute rejection is initiated by the priming of alloreactive T cells and the recruitment of primed T cells and other leukocytes into the allograft.1,2 This model predicts that the allograft is not subject to specific adaptive immune detection and attack until alloreactive T cells are primed and directed to the graft site. Although the rejection rate of allogeneic organs has been significantly reduced by the utilization of potent immunosuppressive drugs, direct graft loss from acute rejection remains a significant clinical problem. In addition, clinical studies have shown that acute rejection episodes are a strong predisposing risk factor for the development of transplant-associated vasculopathy or chronic rejection, the leading cause of graft loss.3–5 Thus, elucidating mechanisms directing leukocyte trafficking to and interactions with the vascular endothelium of the allograft is critical for understanding rejection and for the development of strategies to prevent graft loss.
Surgical trauma and ischemia/reperfusion (I/R) injury are inherent consequences of the organ transplantation procedure. Immediately following reperfusion, pro-inflammatory cytokines, including TNF-α and IL-1, are produced by the graft endothelium.6,7 These cytokines up-regulate expression of major histocompatibility complex (MHC) and adhesion molecules on the surface of the vascular endothelium and induce the production of polymorphonuclear leukocyte (PMN) attractant chemokines, including KC/CXCL1 (the murine homologue of Gro-α) and macrophage-inflammatory protein (MIP)-2/CXCL2. Numerous studies have demonstrated the central role of PMN infiltration and activity in tissue damage and organ dysfunction following reperfusion of ischemic tissues.8,9 Antagonism of KC/CXCL1 and MIP-2/CXCL2 in rodent models of renal I/R injury attenuates PMN infiltration and prevents renal dysfunction and mortality.10 Similar findings have been reported in animal models of liver, lung, and hind limb I/R injury.11
In clinical transplantation, these inflammatory events negatively impact organ allografts. Longer ischemic times are associated with a stronger inflammatory response, delayed graft function, and increased risk of rejection.12 However, the role of early inflammatory events has not been carefully examined in an organ transplant model. Previous studies from this laboratory demonstrated a delay in T cell infiltration into cardiac allografts following antagonism of KC/CXCL1.13 Consistent with the correlation between time of ischemia and allograft function and survival, these studies indicate innate immune regulation of antigen-specific T cell interaction with allografts.
The production of KC/CXCL1 and MIP-2/CXCL2 within the graft is a potentially important step in recruiting PMNs and establishing inflammatory foci within the graft vasculature. Since these events occur quickly in response to the surgical trauma imposed during transplantation, the levels of early inflammation would be expected to be similar in isografts and allografts. The goal of this study was to test the levels of the inflammatory response to syngeneic and allogeneic heart grafts immediately following reperfusion and to elucidate mechanisms by which the response operates. Up-regulated expression of several cytokine genes during the early inflammatory response to allogeneic organ transplantation has been reported although mechanisms mediating this expression remain undefined.14,15 Herein we demonstrate an early CD8+ T cell-mediated immune response to heart allografts before detectable recipient alloreactive T cell priming in the lymphoid tissue draining the graft site. This IFN-γ-dependent immune response up-regulates the intensity of early innate immunity that mediates intra-graft inflammation and tissue necrosis following transplantation.
Materials and Methods
Mice
A/J (H-2a) and C57BL/6 (H-2b) mice were obtained through Dr. C. Reeder at the National Cancer Institute (Frederick, MD). C3H/HeJ (H-2k) and SJL/J (H-2s) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Adult males of 8 to 12 weeks of age were used throughout this study.
Heterotopic Cardiac Transplantation
Heterotopic cardiac transplants were performed using the method of Corry and co-workers.16 Briefly, donor hearts were harvested and placed in chilled Ringer’s solution (Baxter Healthcare Corporation, Deerfield, IL) while the recipient mice were prepared. The donor heart was anastomosed to the recipient abdominal aorta and inferior vena cava using microsurgical techniques. On completion of the anastomosis and organ reperfusion, the heart grafts resumed spontaneous contraction. The strength and quality of cardiac graft impulses were monitored each day by palpation of the abdomen. Rejection of cardiac grafts was considered complete by cessation of impulse and was confirmed visually for each graft by laparotomy.
Antibodies
Antibodies used for immunohistological analyses included GK1.5 (rat anti-mouse CD4) and 53–6.7 (rat anti-mouse CD8α) from BD Pharmingen (San Diego, CA). Rat anti-Ly-6G mAb (RB6–8C5) was purified from culture supernatant using Protein G-Sepharose. To deplete CD4+, CD8+, or natural killer (NK) cells mice received a cocktail of 100 μg of YTS191 and 100 μg of GK1.5, 100 μg of YTS169 and 100 μg of TIB105, or 100 μg of PK136, respectively, on 3 consecutive days before cardiac transplantation. Depletion of cell populations was confirmed by FACS analysis and immunohistology.
Immunohistology
A mid-ventricular portion of the cardiac graft was embedded in OCT compound (Sakura Finetek U.S.A., Torrance, CA), immediately frozen in liquid nitrogen following harvest and 8-μm thick sections were prepared as previously described. Slides were immersed in phosphate-buffered saline (PBS) for 10 minutes and 0.1% H2O2 in PBS for 5 minutes at room temperature (RT) to eliminate endogenous peroxidase activity. Slides were stained with 10 μg/ml GK1.5 (for CD4+ cells), 53–6.7 (for CD8+ cells), F4/80 (for macrophages), or RB6–8C5 (for PMNs) in PBS with 1% bovine serum albumin (BSA) for 1 hour at RT and then with biotinylated rabbit anti-rat IgG (DAKO Corporation, Carpinteria, CA) diluted 1:300 in PBS for 20 minutes at RT. The slides were developed with DAB for color change and counter-stained by immersion in hematoxylin for 3 minutes. Images were captured and analyzed with Image Pro Plus (Media Cybernetics, Silver Spring, MD). The number of positive cells was counted in a blinded fashion in 10 random fields per slide in two non-consecutive sections per heart and for three hearts per time point at ×200 magnification. The counts were then normalized to the area of tissue and expressed in mm2.
ELISA
Following harvest from recipients, cardiac allografts were immediately snap-frozen in liquid nitrogen, pulverized in liquid nitrogen, and dissolved in 500 μl of PBS with 0.01 mol/L EDTA and a proteinase inhibiting cocktail (10 μg/ml phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 100 μg/ml Pefabloc SC, and 100 μl/ml chymostatin). One ml of 1.5% Triton X-200 in PBS was added before agitation at 4°C for 30 minutes. The samples were centrifuged at 12,000 × g for 10 minutes, the supernatant collected and total protein concentration determined with the Coomassie Plus Protein Assay (Pierce Biotechnology, Rockford, IL). KC/CXCL1 and MIP-2/CXCL2 were quantified by sandwich ELISA using Quantikine M kits (R&D Systems, Minneapolis, MN) as per the manufacturer’s instructions.
Cell Culture and Leukocyte Enrichment
The C3H (H-2k)-derived SV-40 transformed murine endothelial cell line, 2F-2B, was purchased from American Type Culture Collection (ATCC, CRL-2168, Manassas, VA). Cells were cultured in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO), 2 mmol/L L-glutamine, 5 × 10−5 M 2-mercaptethanol, 10 mmol/L HEPES, and 20 μg/ml gentamicin. The endothelial cells were seeded in 6-well plates at 2 × 104 per well and grown for 3 days to 90% confluence. The endothelial cells were activated with 1 ng/ml TNF-α (R&D Systems, Minneapolis, MN) 12 hours before co-culture with T cell populations. Spleen and lymph node suspensions from naïve C57BL/6, naïve C3H, and C57BL/6 mice primed to C3H skin grafts were centrifuged through a Lympholyte-M (Cedarlane Laboratories, Ontario, Canada) gradient and interface cells were collected. Cells were incubated with reagents from the CD4+ or CD8+ T Cell Isolation kit, respectively, (Miltenyi Biotec, Auburn, CA) and eluted through magnetic columns as per the manufacturer’s instructions. To isolate naïve versus memory phenotype cells, the flow-through cells from the first selection were washed and incubated with CD62L Microbeads (Miltenyi Biotec) at 1:5 dilution of the recommended amount and eluted through magnetic separation columns. The positive and negative fractions were collected, counted and FACS analysis was performed to verify purity. In all experiments, the purity of cell populations was over 90% for CD4+ versus CD8+ and over 80% for CD62L high/low.
RNA Extraction, in Vitro Transcription, and Ribonuclease Protection Assay
Following co-culture, whole endothelial cell RNA was extracted using Trizol reagent (Life Technologies). The [32P]UTP-radiolabeled anti-sense murine monokine induced by IFN-γ (Mig) (cDNA generously provided by Dr. C. Tannenbaum at The Cleveland Clinic Foundation)and murine glyceraldehyde-3-phosphate dehydrogenase(GAPDH) (cDNA from Pharmingen) riboprobes for RNase protection assays (RPAs) were synthesized and purified using MAXIscript In Vitro Transcription kit (Ambion, Austin, TX) according to the manufacturer’s protocol. Endothelial cell expression of Mig and GAPDH was quantified by RPA using RiboQuant RPA kits (BD Pharmingen) according to the manufacturer’s protocol. The intensity of each signal was measured with ImageQuant (Molecular Dynamics) and standardized to the intensity of the GAPDH signal for each sample.
ELISPOT Assay
ELISPOT assays for IFN-γ were performed as previously described.17 Briefly, ELISPOT plates (Immunospot M200 plates; Cellular Technologies Ltd, Cleveland, OH) were coated with IFN-γ-specific mAb and blocked with sterile 1% BSA in PBS. Allograft recipient spleen cells (106/well) were plated in HL-1 media with mitomycin C-treated stimulator cells (300,00/well) for 24 hours. After washing with PBS and PBS 0.025% Tween 20, biotin-rat-anti-mouse IFN-γ detection antibody (XMG1.2 at 4 μg/ml, BD Pharmingen) was added and incubated overnight at 4°C. Alkaline phosphatase-conjugated anti-biotin antibody (Vector Laboratories, Burlingame, CA) was added for 1.5 hours at RT and the plates were developed with nitroblue tetrazolium chloride (NBT, Bio-Rad Laboratories, Hercules, CA) and 5-bromo-4-chloro-3-indolyl phosphate substrate (BCIP, Sigma Chemical Company, St. Louis, MO). The resulting spots were counted on an ImmunoSpot Series 2 Analyzer (Cellular Technologies Ltd).
Mixed Leukocyte Reaction Assay
Alloantigen priming of heart allograft recipients was also tested by mixed leukocyte reaction assays (MLR) as previously described.18 Responder T cell suspensions were cultured with spleen cells suspensions from syngeneic (C57BL/6), allograft donor (A/J), or third party allogeneic (SJL) stimulator cells. After 48 hours, cultures were pulsed with 0.25 μCi [3H] thymidine, incubated for 16 hours then harvested onto fiber filter mats. The amount of 3H incorporation was determined by liquid scintillation counting.
Results
Temporal Production of KC/CXCL1 and MIP-2/CXCL2 in Cardiac Grafts
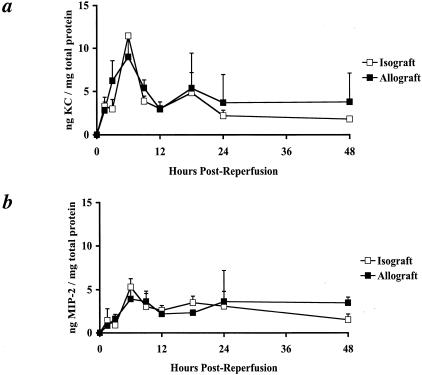
To test the temporal production of KC/CXCL1 and MIP-2/CXCL2 in heart transplants, heterotopically transplanted C57BL/6 (H-2b) cardiac isografts or A/J (H-2a) cardiac allografts were harvested from recipient C57BL/6 mice at multiple time points between 90 minutes and 48 hours following graft reperfusion. Heart graft protein homogenates were tested for levels of KC/CXCL1 and MIP-2/CXCL2 production. Identical levels of KC/CXCL1 and of MIP-2/CXCL2 protein were observed in isografts and allografts over the initial 48 hours post-transplant (Figure 1). First detectable at 90 minutes, production of both chemokines peaked at 6 to 9 hours post-reperfusion and returned to low levels by 18 to 24 hours post-reperfusion. KC/CXCL1 production was quantitatively higher than MIP-2/CXCL2 in both isografts and allografts at these early times. These results demonstrate the equivalent production of KC/CXCL1 and MIP-2/CXCL2 in cardiac isografts and allografts rapidly following reperfusion.
Figure 1.
Equivalent KC/CXCL1 and MIP-2/CXCL2 production in heterotopic cardiac isografts and allografts. Cardiac isografts and allografts were retrieved from recipients at the indicated time points following graft reperfusion. Tissue homogenates were prepared and tested by ELISA for KC/CXCL1 and MIP-2/CXCL2 content and normalized to total cardiac protein. a: KC/CXCL1 production peaks at 6 to 9 hours post-reperfusion in both cardiac isografts (□) and allografts (▪) and returns to low levels by 24 hours post-reperfusion. b: MIP-2/CXCL2 levels also peak at 6 to 9 hours post-reperfusion and return to low levels by 24 hours in both groups. All results are displayed as the mean and SD of at least two dilutions of three to four grafts in each group for each time point.
Temporal Pattern of PMN Infiltration
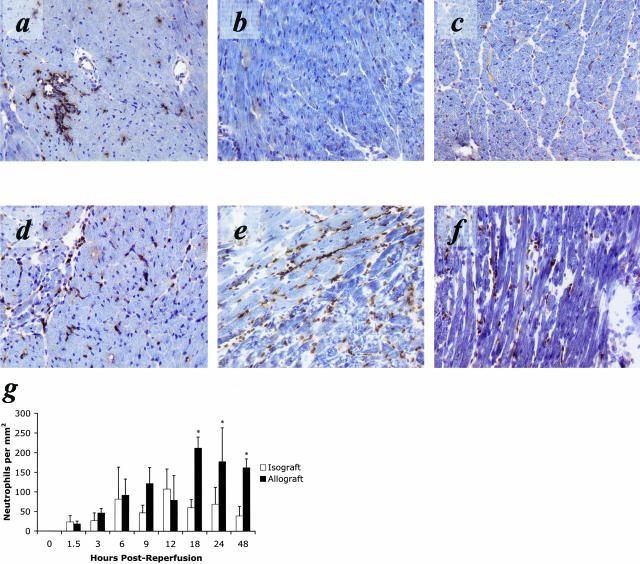
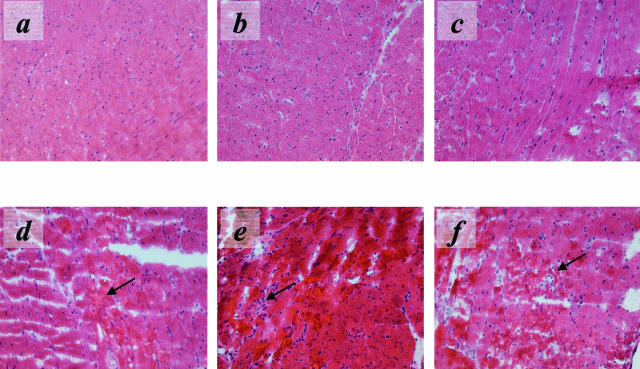
The equivalent production of KC/CXCL1 and MIP-2/CXCL2 in cardiac isografts and allografts predicted a similar magnitude and equivalent tempo of PMN infiltration into the grafts. Cardiac isografts and allografts were retrieved at several time points following reperfusion and mid-ventricular sections were stained with anti-Ly6G mAb (RB6–8C5) to detect PMN infiltration (Figure 2, a to g). Consistent with the production of KC/CXCL1 and MIP-2/CXCL2, PMNs were observed as early as 90 minutes post-reperfusion in both isografts and allografts (Figure 2g). PMN infiltration increased with equivalent kinetics in both groups until approximately 12 hours post-reperfusion. After this time the number of PMNs and inflammatory infiltrate observed in the isografts quickly subsided whereas the infiltrate into the allografts continued to increase. The PMN infiltrate into the allograft remained elevated at 48 hours post-reperfusion (Figure 2, a to f) and did not resolve until 72 hours post-reperfusion (data not shown). At 48 hours post-reperfusion, parenchymal necrosis in the allograft was more marked than in the isograft controls, consistent with the increased level and duration of PMN infiltration (Figure 3).
Figure 2.
PMN infiltration into cardiac allografts is increased in magnitude and duration compared to PMN infiltration observed in cardiac isografts controls. Immunohistology with anti-Ly6G (RB6–8C5) demonstrates an equivalent PMN infiltrate in cardiac isografts (a) and cardiac allografts (d) at 12 hours post-reperfusion. Sections taken at 24 hours post-reperfusion demonstrate the resolution of inflammation in isografts (b) but an enhanced PMN infiltrate into allografts (e). This PMN dominant inflammation is maintained at 48 hours in the allograft (f), while the isograft tissue (c) at this time appears normal. g: Quantification of immunohistology demonstrates that PMN infiltration into isografts (□) peaks around 12 hours post-reperfusion and returns to low levels by 24 hours post-reperfusion. However, PMN infiltration into allografts (▪) increases to twice that level between 12 hours and 24 hours post-reperfusion and remains elevated beyond 48 hours post-reperfusion (*, P < 0.01). All results are displayed as the mean and SD of at least 10 random myocardial fields from two non-consecutive sections from three to four grafts per group counted twice in a blinded fashion. The counts are normalized to tissue area and shown in mm2.
Figure 3.
Hematoxylin and eosin staining of sections of isografts and allografts retrieved at 48 post-reperfusion. Staining reveals more normal tissue in isograft sections (a, b, and c), whereas marked tissue damage and necrosis (indicated by arrows) are observed in allografts (d, e, and f).
PMN Infiltration Following Depletion of CD4+, CD8+, or NK Cells
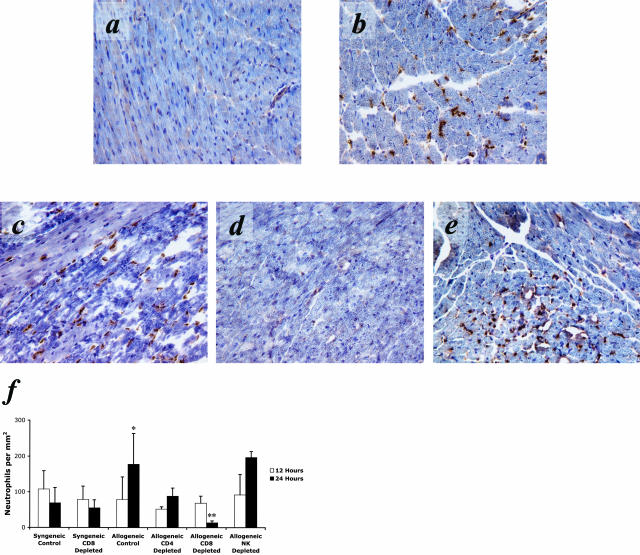
The striking increase in PMN infiltration into allografts at times after resolution in isografts indicated the ability of the allograft recipient to detect the presence of allogeneic tissue as early as 12 hours post-transplant. This suggested an adaptive immune mechanism mediating the quantitative difference in early inflammatory cell infiltration. To begin to test potential mechanisms, recipient CD4+, CD8+, or NK cells were depleted before cardiac transplantation. At 12 hours post-reperfusion, the PMN infiltrate into the cardiac isografts and allografts was similar to wild-type controls for all groups (Figure 4f). However, at 24 hours post-reperfusion depletion of CD8+ cells in allograft recipients (Figure 4d) resulted in significantly fewer PMNs within the cardiac allograft parenchyma. PMN infiltration at 24 hours into allografts in recipients depleted of CD4+ (Figure 4c) or NK (Figure 4e) cells was not significantly different from allograft controls (Figure 4b). A similar decrease in PMN infiltration was observed in allografts harvested from CD8 −/− recipients (data not shown). These data indicate that in the absence of CD8+ cells the magnitude and duration of the PMN infiltrate into cardiac allografts is comparable to levels observed in isografts.
Figure 4.
PMN infiltration at 24 hours post-reperfusion into cardiac isografts and allografts following depletion of CD4+, CD8+, or NK cells. Immunohistology using specific primary mAb for CD4+, CD8+, or NK cells demonstrated a greatly attenuated PMN infiltrate into allografts in recipients depleted of CD8+ cells (d) pre-transplant. Little to no effect is observed in allograft recipients depleted of CD4+ (c) or NK cells (e) pre-transplant. Immunohistology of an isograft (a) and an allograft (b) are shown for comparison. f: Quantification of PMN infiltration following depletion of specific leukocyte populations pre-transplant. Depletion of CD8+ cells in cardiac allograft recipients greatly attenuates PMN infiltration (**, P < 0.001). Depletion of CD4+ cells or NK cells in allograft recipients did not have a significant effect on PMN infiltration. Depletion of CD8+ cells in cardiac isograft recipients also did not have a significant effect on PMN infiltration. The PMN infiltration into cardiac allografts in significantly higher than cardiac isograft controls at 24 hours post-reperfusion (*, P < 0.01). All results are displayed as the mean and SD of at least 10 random myocardial fields from two non-consecutive sections from three to four grafts per group counted twice in a blinded fashion. The counts are normalized to tissue area and shown in mm2.
Role of IFN-γ on Early PMN Infiltration into Allografts
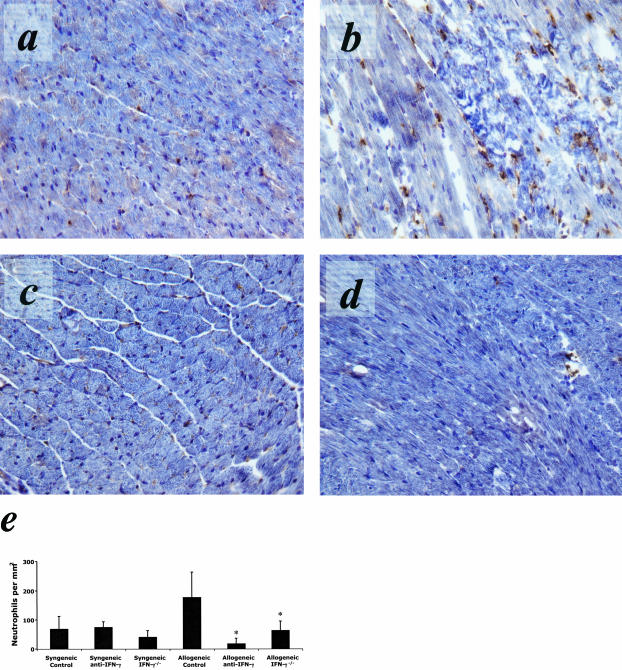
IFN-γ production is a key effector function expressed by CD8+ T cells to promote allograft rejection. To test the influence of IFN-γ on early PMN infiltration into allografts, recipients were treated with anti-IFN-γ mAb (XMG1.2) 8 hours before and 8 hours following transplantation. Cardiac allografts were harvested at 24 hours post-reperfusion and sections stained to assess PMN infiltration. Although treatment with anti-IFN-γ antibody did not alter PMN infiltration into isograft, the PMN infiltrate into cardiac allografts was greatly reduced at 24 hours post-reperfusion in recipients treated with IFN-γ neutralizing antibody compared to control-treated recipients (Figure 5c). Similar results were observed at 24 hours post-transplant in A/J heart allografts retrieved from B6.IFN-γ−/− recipients (Figure 5d).
Figure 5.
Immunohistology demonstrates attenuated PMN infiltration into cardiac allografts in IFN-γ-blocked or -deleted recipients at 24 hours post-reperfusion. PMN infiltration into cardiac allografts was significantly reduced in wild-type recipients receiving IFN-γ-blocking mAb (XMG1.2) (c) pre- and post-transplant. PMN infiltration was also reduced in IFN-γ−/− recipients (d). Isograft (a) and allograft (b) sections taken at 24 hours post-reperfusion are shown for comparison. e: Quantification of PMN infiltration into cardiac isografts and allografts following at 24 hours post-reperfusion when IFN-γ is blocked or absent. Blocking INF-γ with a mAb (XMG1.2) resulted in attenuated PMN infiltration into cardiac allografts comparable to wild-type isografts (*, P < 0.01). Recipients with a gene deletion of IFN-γ showed similar results. PMN infiltration in recipients treated with anti-IFN-γ (XMG1.2) or in IFN-γ−/− recipients of cardiac isografts was unchanged from control-treated isograft recipients. All results are displayed as the mean and SD of at least 10 random myocardial fields from two non-consecutive sections from three to four grafts per group counted twice in a blinded fashion. The counts are normalized to tissue area and shown in mm2.
Alloreactive T Cell Priming Is Detected No Earlier than Day 3 Post-Transplant
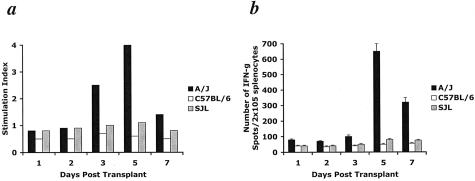
The results indicate a rapid CD8+ T cell-mediated response to graft alloantigens that sustains and up-regulates PMN infiltration into allografts 12 hours after graft reperfusion. Although unlikely, a potential mechanism regulating this innate immune response was the rapid priming of allogeneic-specific CD8+ T cells in response to the vascularized graft. Therefore, cardiac allograft recipient splenocytes were tested to determine the time post-transplant when alloantigen-specific T cell priming was first detectable. Three-day MLR assays demonstrated recipient splenocyte proliferation in response to A/J stimulator cells no earlier than day 3 following transplantation (Figure 6). Overnight IFN-γ ELISPOT assays demonstrated a specific increase in alloreactive T cells producing IFN-γ no earlier than day 5 post-transplant. Splenocyte reactivity to third party and syngeneic controls remained low throughout the rejection process in both assays verifying an alloantigen-specific response. Therefore, the earliest time that alloantigen T cell priming can be detected is substantially later than the enhanced PMN infiltration into allografts is observed.
Figure 6.
Alloantigen-primed T cells are not detectable in heart allograft recipients by MLR or ELISPOT assays until day 3 to 5 post-transplant. Allogeneic-specific T cell priming (A/J stimulators) is first detected at day 3 following transplant by 3-day MLR assay (a) and day 5 by overnight IFN-γ ELISPOT assay (b). In each case, self-reactivity (C57BL/6 stimulators) and reactivity to a third party (SJL stimulators) remained at background levels. In either case, T cell priming is detected long after the early inflammatory difference is observed.
Memory but Not Naïve CD8+ T Cells Respond to Allogeneic Endothelium
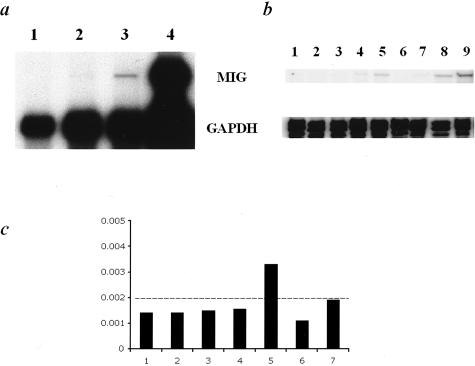
Since T cell priming was not detectable for several days after the amplified inflammatory response was observed, specific subpopulations of T cells from naïve mice were tested for reactivity to allogeneic endothelial cells. Based on a lower threshold for activation, T cells of a memory phenotype (eg, CD62Llow) would be expected to be quickly reactive to allogeneic endothelium. Therefore, purified populations of CD62Lhigh and CD62Llow CD4+ and CD8+ T cell from naïve C57BL/6 mice (H-2b) were tested for the ability to induce allogeneic (H-2k) endothelial cells to express monokine induced by IFN-γ (Mig/CXCL9). Mig/CXCL9 expression is entirely dependent on IFN-γ induction and therefore Mig/CXCL9 can be considered a specific marker of IFN-γ production.19 As a positive control, CD4+ and CD8+ T cells derived from a C57BL/6 mouse that had previously rejected a C3H (H-2k) skin graft induced high levels of Mig/CXCL9 expression following a 24 hour co-culture with the 2F-2B (H-2k) endothelial cell line (Figure 7). Importantly, memory phenotype CD8+ T cells (CD62Llow) isolated from naïve C57BL/6 mice (H-2b) also induced the endothelial cells to express Mig/CXCL9 whereas naïve phenotype CD8+ T cells (CD62Lhigh) isolated from the naïve mice did not. Additionally, CD4+ CD62Lhigh and CD62Llow T cells from naïve C57BL/6 mice were unable to stimulate the production of Mig/CXCL9. Finally, co-culture with either CD8+ CD62Lhigh or CD62Llow T cells from syngeneic C3H mice (H-2k) did not induce the endothelial cells to express Mig/CXCL9. This result indicates that CD8+ CD62Llow T cells isolated from naïve mice and without previous alloantigen priming produce IFN-γ during interaction with allogeneic endothelium and stimulate the endothelial cells to express the IFN-γ-dependent chemokine Mig/CXCL9.
Figure 7.
Mig expression by endothelial cells following co-culture with CD8+ CD62Llow T cells derived from naïve mice. RNA was purified from endothelial cell cultures and tested from for monokine induced by γ interferon (Mig/CXCL9) and GAPDH levels by ribonuclease protection assay (RPA). a: Experiment 1 shows that CD8+ CD62Llow T cells (lane 3) isolated from naïve mice are reactive to allogeneic endothelium and induce the expression of Mig/CXCL9 by the endothelial cells. CD8+ CD62Lhigh T cells isolated from the same mice (lane 2) are unable to induce the expression of Mig/CXCL9. Endothelial cells alone (lane 1) did not express Mig/CXCL9, whereas, as expected, specific alloantigen-primed T cells induced high levels of Mig/CXCL9 expression (lane 4). b: Experiment 2 demonstrates the same result for CD8+ CD62Lhigh and CD8+ CD62Llow T cells in lanes 4 and 5, respectively. In addition, this experiment demonstrates that neither syngeneic CD8+ CD62Lhigh (lane 6) and CD62Llow (lane 7) CD8+ nor allogeneic CD4+ CD62Lhigh (lane 2) and CD62Llow (lane 3) T cells induce the expression of Mig/CXCL9 above background levels. CD4+ and CD8+ T cells from mice that had previously rejected a C3H skin graft were used as positive controls (lanes 8 and 9, respectively) and endothelial cells without T cell co-culture as a negative control (lane 1). c: Phosphorscreen quantification demonstrates that the normalized intensity of allogeneic CD8+ CD62Llow T cells (lane 5) induces the expression of Mig/CXCL9 above background levels measured in endothelial cells alone (lane 1), endothelial cells co-cultured allogeneic CD4+ CD62Lhigh (lane 2) or CD62Llow (lane 3), with allogeneic CD8+ CD62Lhigh T cells (lane 4), with syngeneic CD8+ CD62Lhigh (lane 6) or CD62Llow T cells (lane 7). The signal intensity measured from co-culture with CD4+ and CD8+ T cells (lanes 8 and 9, respectively) from mice that had previously rejected a C3H skin graft were off the scale and therefore not plotted. The Mig/CXCL9 signal was normalized to the GAPDH signal performed concurrently as an internal control within each sample.
Discussion
Allograft rejection is initiated by inherent non-specific injury imposed on the graft in response to ischemia and reperfusion. Following reperfusion of an ischemic tissue, an intra-graft inflammatory state mediated by components of the innate immune system rapidly develops.20–23 Pro-inflammatory signals also activate donor dendritic cells resident within the graft to mature and emigrate to draining lymphoid tissue where these cells prime alloreactive recipient T cells through the direct presentation pathway.24,25 Following clonal proliferation and development to effector cells, the alloantigen-primed T cells traffic to the graft and are activated to express the effector functions mediating rejection.1,2,18,26
Although allograft rejection is primarily mediated by alloantigen-primed T cells, components of the innate immune system have strong regulatory effects on the initiation and activity of the adaptive immune response. Despite strong evidence for an important role in allograft rejection,13 many of these early events and their down-stream sequelae following transplantation have not been widely studied. We initially hypothesized that the early events at the graft vascular endothelium, principally those mediated by PMNs, play a central, but non-alloantigen-specific, role in initiating and amplifying the inflammatory response following transplantation. Consistent with this model we expected to observe inflammatory responses at levels of equal intensity in isografts and allografts. Instead, this study provides strong evidence for a novel adaptive immune response to the allograft that occurs within hours following organ reperfusion. Moreover, this early IFN-γ-dependent CD8+ T cell-mediated immune response is shown to regulate the intensity and duration of innate immunity within cardiac allografts.
The assumption that post-transplant intra-graft inflammation following reperfusion is solely a response to non-specific signals proved to be false. The PMN infiltrate into cardiac isografts and allografts followed the production of KC/CXCL1 and MIP-2/CXCL2 and was found to be equivalent for the initial 12 hours following reperfusion. Although KC/CXCL1 and MIP-2/CXCL2 are among the most potent murine PMN chemokines and therefore selected for testing, it remains possible that alternative PMN chemokines that also bind CXCR2 such as PF4/CXCL4, ENA-78/CXCL5, or NAP-2/CXCL7 may play a role in the differential infiltration observed in the allograft group. After the initial 12 hours the inflammation promptly resolved in the isograft group, but was elevated and maintained for an extended duration in the allograft group. Previous reports using RT-PCR indicated increased inflammatory cytokine expression in allografts versus isografts at 24 hours post-reperfusion of murine cardiac grafts.14 We attempted to repeat those experiments using RPA, however, little difference was noted in the expression levels of multiple cytokines in isografts and allografts from control and CD8+-depleted recipients. We attribute this result to the probable local and patchy nature of this immune attack. As noted in previous studies, intra-graft IFN-γ-dependent chemokines at day 3 post-transplant were not uniformly expressed throughout the graft endothelium, but rather were localized to focal regions of the vascular endothelium.18
In addition to a quantitative elevation in PMN infiltration, tissue damage and necrosis were markedly more apparent in the parenchyma of allografts at 48 hours post-transplant. A previous histological study noted an amplified infiltration of PMNs into rat renal allografts although mechanisms directing this were not defined.27 Of note, the primary antibody used in the immunohistochemistry of the current study to detect PMNs also reacts with the Ly-6G antigen found on most granulocytes, including eosinophils. However, ribonuclease protection assays do not detect intra-graft eotaxin/CCL11 mRNA expression at any time point following transplantation and H/E staining reveals that the overwhelming majority of infiltrating cells that stain positive with the RB6–8C5 antibody also possess a classic polymorphic nucleus suggesting a PMN. The observed tissue damage caused by the enhanced inflammation observed in cardiac allografts at 48 hours post-transplant is potentially permanent and may have considerable down-stream effects. For example, antigen leak from the graft and/or parenchymal fibrosis may establish a state of chronic inflammation that continually promotes the infiltration of T cells and other leukocytes into the graft.
To elucidate the mechanism of the enhanced PMN infiltrate into allografts, specific populations of leukocytes were depleted in wild-type mice before cardiac allograft transplantation. Depletion of NK or CD4+ T cells had little to no effect on the observed amplification of PMN infiltration. In contrast, depletion of CD8+ T cells before transplantation resulted in a significantly attenuated inflammatory response to allografts that was quantitatively and qualitatively equivalent to isografts. In addition, anti-IFN-γ mAb treatment or utilization of IFN-γ−/− recipients resulted in a similarly attenuated inflammatory response at these early times. The dendritic cell population that expresses CD8α and produces IFN-γ may also participate in this early immune response in vivo. However, the IFN-γ-dependent increase in PMN infiltration was observed in allo- but not iso-grafts suggesting the requirement for CD8+ T cells. In addition, co-culture experiments demonstrated the ability of allogeneic endothelial cells to stimulate highly purified CD8+ T cells to induce expression of IFN-γ-dependent genes. These results suggest that CD8+ T cell production of IFN-γ is sufficient to promote increased PMN infiltration into allograft and that CD8α+ DC production of IFN-γ in this situation would have to be mediated through this CD8+ T cell activity. Taken together, these results indicate that the amplified inflammatory response observed in allografts is mediated by a population of circulating CD8+ T cells that produce IFN-γ during interaction with the graft.
In conjunction with other studies, the current results indicate a complex role for IFN-γ in allograft rejection. We find that IFN-γ produced in allografts at these early times post-reperfusion amplifies the inflammatory response, particularly PMN infiltration, and parenchymal injury of cardiac allografts. It is worth noting a recent report by Finn and colleagues28 that demonstrated early expression of IFN-γ and other pro-inflammatory cytokine genes in cardiac allografts at 24 hours post-transplant. Moreover, this expression was not observed in recipients with a T cell-targeted defect in NF-κB signaling. These results provide further support for the proposal that the critical IFN-γ producing cell in allografts at early times post-transplant is the CD8+ T cell and not a CD8α+ dendritic cell. In contrast, previous studies from this and other laboratories have indicated a protective role for IFN-γ at later times after reperfusion of vascularized allografts.29,30 Indeed, a recent study from this laboratory found that IFN-γ-deficient animals mounted a massive PMN-mediated inflammatory response beginning at day 3 post-transplant that rejected cardiac allografts several days earlier than wild-type controls.31 Collectively, these studies demonstrate a complex and, at times, opposing role for IFN-γ in the immune response to allografts.32
To further elucidate this early immune attack against the allograft, specific T cell populations were isolated and co-cultured with allogeneic endothelial cells. CD62Llow CD8+ T cells (memory phenotype) from naïve animals responded to allogeneic vascular endothelium which induced the production of the IFN-γ-dependent chemokine, Mig/CXCL9. No other T cell subpopulation tested induced Mig/CXCL9 expression indicating the lack of IFN-γ production. These data suggest that pre-existing CD62Llow CD8+ T cells recognize and respond to allogeneic endothelium by producing IFN-γ without the need for alloantigen priming. The consequence of this response is the early inflammatory response observed in vascularized allografts characterized by intense PMN infiltration and tissue necrosis.
Animal models have demonstrated that memory T cells induced to skin allografts accelerate the rejection of a subsequent heart allograft.33,34 Furthermore, studies have shown wide cross-reactivity between altered-self or peptide primed T cell clones and allogeneic MHC molecules.35,36 The frequency of alloreactivity of T cell clones to one or more haplotypes from mice immunized to model proteins was shown to be greater than 60%, suggesting a great potential for cross reactivity between exogenous peptide/self MHC molecules and peptide/allogeneic MHC molecules.37 These studies support the clinical problem faced by sensitized patients who have received multiple transplants.38 The results in the current study suggest the presence of established memory CD8+ T cells that have not been primed to alloantigens and yet have alloreactivity. One potential source of such cells is CD8+ T cell-mediated responses to environmental antigens (eg, food and microbial antigens). Recent results have indicated that memory T cells generated in response to viral infections can mediate rejection of skin allografts.39 In further support of this postulate, recent studies by Valujskikh and colleagues40 showed that CD4+ T cells isolated from animals infected with Leishmania major had specific alloreactivity to H-2p, but not other haplotype, antigens and rejected H-2p skin grafts with second order kinetics. Whereas these previous studies used graft rejection to detect memory T cells with cross-reactivity to alloantigens, the current study suggests that their activity may be more insidious. The impact of this early response on acute and chronic allograft pathology is under investigation.
An important issue regarding the memory T cells involved in the regulation of early inflammation reported in the current study is the restriction to the CD8+, and not the CD4+, T cell compartment. Resting murine endothelium has been reported to lack MHC class II expression, providing an explanation for the little influence CD4+ T cells exhibited in promoting innate immune-mediated inflammation in the cardiac allografts.41 Human endothelium, however, does express detectable levels of MHC class II suggesting that both CD4+ and CD8+ T cells could regulate the early inflammatory response to vascularized human organs.42 Our preliminary studies indicate that the enhanced inflammation observed in allografts is resistant to commonly used immunosuppressive drugs. Recipient animals pre-treated with high dose cyclosporine A for 2 days before receiving a cardiac allograft, mounted an equally strong inflammatory response to cardiac allografts as vehicle-treated, control recipients (T. El-Sawy, unpublished observations). This suggests that the CD8+ T cells promoting this early inflammation via interaction with allogeneic endothelium are resistant to calcineurin inhibitors. Based on these results we propose that this population of memory phenotype, alloantigen reactive CD8+ T cells may play an important role in the development of transplant-associated vasculopathy. We hypothesize that chronic IFN-γ-mediated damage directed at the graft vasculature by this pre-existing T cell population, under the cover of immunosuppression, may promote the vascular disease observed in the clinical transplantation setting that continues to be the leading cause of graft loss.
In summary, the results presented in this study indicate that a transplanted allogeneic organ is detected and under attack by the recipient adaptive immune system within hours following reperfusion. Current dogma has assumed that inflammation is a non-specific result of the trauma of surgery, however, in reality it is a highly coordinated response by components of the innate and adaptive arms of the immune system. To protect clinical grafts from this early T cell-regulated inflammatory damage and potential down-stream vascular effects, therapeutic strategies aimed at interfering with the early interaction of recipient T cells and the graft endothelium should be investigated.
Footnotes
Address reprint requests to Tarek El-Sawy, Department of Immunology, NB3–30, The Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, Ohio 44195. E-mail: tarek@crwu.edu.
Supported by National Institutes of Health Grant AI 40459 and AI 51620 (to R.L.F.) and American Heart Association Grant 0215112B (to T.E.S.).
References
- Hall B. Cells mediating allograft rejection. Transplantation. 2001;51:1141–1151. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1993;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- Nisco SJ, Reitz BA. Developments in cardiac transplantation. Curr Opin Cardiol. 1994;9:237–246. doi: 10.1097/00001573-199403000-00014. [DOI] [PubMed] [Google Scholar]
- Hornick P, Smith J, Pomerance A, Mitchell A, Banner N, Rose M, Yacoub M. Influence of acute rejection episodes, HLA matching, and donor/recipient phenotype on the development of “early” transplant-associated coronary artery disease. Circulation. 1997;96 (Suppl 9):148–153. [PubMed] [Google Scholar]
- Matan AJ, Gillingham KJ, Payne WD, Najarian JS. The impact of an acute rejection episode on long-term renal allograft survival (t1/2). Transplantation. 1994;57:857–859. doi: 10.1097/00007890-199403270-00015. [DOI] [PubMed] [Google Scholar]
- Laskowski I, Pratschke J, Wilhelm MJ, Gasser M, Tilney NL. Molecular and cellular events associated with ischemia/reperfusion injury. Ann Transplant. 2000;5:29–35. [PubMed] [Google Scholar]
- Bergese SD, Huang EH, Pelletier RP, Widmer MB, Ferguson RM, Orosz CG. Regulation of endothelial VCAM-1 expression in murine cardiac grafts: expression of allograft endothelial VCAM-1 can be manipulated with antagonist of IFN-α or IL-4 and is not required for allograft rejection. Am J Path. 1995;147:166–175. [PMC free article] [PubMed] [Google Scholar]
- Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat: the role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Miura M, Fu X, Zhang Q, Remick DG, Fairchild RL. Neutralization of Gro-a and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines GC, Welborn MB, Moldawer LL, Huber TS, Harward TR, Seeger JM. Attenuation of skeletal muscle ischemia/reperfusion injury by inhibition of tumor necrosis factor. J Vasc Surg. 1999;29:370–376. doi: 10.1016/s0741-5214(99)70390-3. [DOI] [PubMed] [Google Scholar]
- Dragun D, Hoff U, Park JK, Qun Y, Schneider W, Luft FC, Haller H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173–1181. doi: 10.1046/j.1523-1755.2001.0600031173.x. [DOI] [PubMed] [Google Scholar]
- Morita K, Miura M, Paolone DR, Engeman TM, Kapoor A, Remick DG, Fairchild RL. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J Immunol. 2001;167:2979–2984. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- Dallman MJ, Larsen CP, Morris PJ. Cytokine gene transcription in vascularized organ grafts: analysis using semi-quantitative polymerase chain reaction. J Exp Med. 1991;174:493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H, Nadeau KC, Takada M, Kusaka M, Tilney NL. Sequential cellular and molecular kinetics in acutely rejecting renal allografts in rats. Transplantation. 1997;63:1101–1108. doi: 10.1097/00007890-199704270-00009. [DOI] [PubMed] [Google Scholar]
- Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice: the role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Gorbachev AV, Heeger PS, Fairchild RL. CD4+ and CD8+ T cell priming for contact hypersensitivity occurs independently of CD40-CD154 interactions. J Immunol. 2001;166:2323–2332. doi: 10.4049/jimmunol.166.4.2323. [DOI] [PubMed] [Google Scholar]
- Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, Fairchild RL. Monokine induced by IFN-γ is a dominant factor directing T cells into murine cardiac allograft during acute rejection. J Immunol. 2001;167:3494–3504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- Fuggle SV, Sanderson JB, Gray DW, Richardson A, Morris PJ. Variation in expression of endothelial adhesion molecules in pre-transplant and transplanted kidneys: correlation with intragraft events. Transplantation. 1993;55:117–123. doi: 10.1097/00007890-199301000-00022. [DOI] [PubMed] [Google Scholar]
- Nawroth P, Handley D, Matsueda G, De Waal R, Gerlach H, Blohm D, Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986;163:1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GRO-a, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct cells chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naïve T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1519. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragun D, Hoff U, Park JK, Qun Y, Schneider W, Luft FC, Haller H. Ischemia-reperfusion injury in renal transplantation is independent of the immunologic background. Kidney Int. 2000;58:2166–2177. doi: 10.1111/j.1523-1755.2000.00390.x. [DOI] [PubMed] [Google Scholar]
- Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-κB-dependent T cell activation abrogates acute allograft rejection. J Immunol. 2001;167:5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- Halloran PF, Miller LW, Urmson J, Ramassar V, Zhu LF, Kneteman NM, Solez K, Afrouzian M. IFN-γ alters the pathology of graft rejection: protection from early necrosis. J Immunol. 2001;166:7072–7081. doi: 10.4049/jimmunol.166.12.7072. [DOI] [PubMed] [Google Scholar]
- Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-γ is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell co-stimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-γ. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo LG, Halloran PF. Role of IFN-γ in allograft rejection. Crit Rev Immunol. 2002;22:317–349. [PubMed] [Google Scholar]
- Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Shen XD, Gao F, Coito AJ, Wasowska BA, Salama A, Schmitt I, Busuttil RW, Sayegh MH, Kupiec-Weglinski JW. The CD154-CD40 T cell co-stimulation pathway is required for host sensitization of CD8+ T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169:1270–1276. doi: 10.4049/jimmunol.169.3.1270. [DOI] [PubMed] [Google Scholar]
- Matis LA, Sorger SB, McElligot DL, Fink PJ, Hedrick SM. The molecular basis of alloreactivity in antigen-specific, major histocompatibility complex-restricted T cell clones. Cell. 1987;51:59–69. doi: 10.1016/0092-8674(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Killer cells reactive to altered-self antigens can also be alloreactive. Proc Natl Acad Sci USA. 1977;74:2094–2098. doi: 10.1073/pnas.74.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell JD, Chen C, Schwartz RH. High frequency and non-random distribution of alloreactivity in T cell clones selected for recognition of foreign antigen in association with self class II molecules. J Immunol. 1986;136:389–395. [PubMed] [Google Scholar]
- Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pre-transplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of post-transplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- Choo JK, Seebach JD, Nickeleit V, Shimizu A, Lei H, Sachs DH, Madsen JC. Species differences in the expression of major histocompatibility complex class II antigens on coronary artery endothelium: implications for cell-mediated xenoreactivity. Transplantation. 1997;64:1315–1322. doi: 10.1097/00007890-199711150-00014. [DOI] [PubMed] [Google Scholar]
- Rose ML, Coles MI, Griffin RJ, Pomerance A, Yacoub MH. Expression of class I and class II major histocompatibility antigens in normal and transplanted human heart. Transplantation. 1986;41:776–780. doi: 10.1097/00007890-198606000-00021. [DOI] [PubMed] [Google Scholar]