Abstract
Fibrosis is a hallmark of progressive organ diseases. Monocyte chemoattractant protein (MCP)-1, also termed as macrophage chemotactic and activating factor (MCAF/CCL2) and its receptor, CCR2 are presumed to contribute to progressive fibrosis. However, the therapeutic efficacy of MCP-1/CCR2 blockade in progressive fibrosis remains to be investigated. We hypothesized that blockade of CCR2 may lead to the improvement of fibrosis. To achieve this goal, we investigated renal interstitial fibrosis induced by a unilateral ureteral obstruction in CCR2 gene-targeted mice and mice treated with propagermanium or RS-504393, CCR2 inhibitors. Cell infiltrations, most of which were F4/80-positive, were reduced in CCR2 knockout mice. In addition, dual staining revealed that CCR2-positive cells were mainly F4/80-positive macrophages. Importantly, CCR2 blockade reduced renal interstitial fibrosis relative to wild-type mice. Concomitantly, renal transcripts and protein of MCP-1, transforming growth factor-β, and type I collagen were decreased in CCR2-null mice. Further, this CCR2-dependent loop for renal fibrosis was confirmed by treatment with CCR2 antagonists in a unilateral ureteral obstruction model. These findings suggest that the therapeutic strategy of blocking CCR2 may prove beneficial for progressive fibrosis via the decrease in infiltration and activation of macrophages in the diseased kidneys.
Fibrosis is characteristic in progressive diseases, resulting in organ failure. Monocyte chemoattractant protein (MCP)-1 (also termed as monocyte chemotactic and activating factor/CCL2) is presumed to be a key molecule in chemotaxis and activation of macrophages.1 MCP-1 has been implicated in a variety of renal diseases, including progressive renal damage such as chronic rejection of renal transplantation, lupus nephritis, IgA nephropathy, crescentic glomerulonephritis, and diabetic nephropathy in human and experimental models.2–9 CCR2, a cognate receptor of MCP-1 expressed mainly on monocytes, has been reported to be involved in human crescentic glomerulonephritis and experimental models of progressive renal disease.10,11 In addition to inducing tissue infiltration and activation of macrophages, MCP-1 expression and the consequent accumulation of CCR2-positive cells are considered to be closely related to pulmonary fibrosis.12 Thus, the strategy of blocking MCP-1/CCR2 interaction might be effective in preventing macrophage-induced tissue damage. Supporting this notion, neutralization of MCP-1 has been reported to reduce macrophage infiltration and progressive kidney damage.5,8,9,13
Newly developed antagonists against chemokine receptors are now available. For example, propagermanium is an organic germanium compound with a chemical structure of [(O1/2)3GeCH2CH2CO2H]n, which shows potent anti-inflammatory activity in various inflammatory experimental models,14,15 and has been used as a therapeutic agent in chronic hepatitis type B. A recent study revealed that propagermanium inhibited the MCP-1-induced migration of monocytes via glycosylphosphatidylinositol-anchored protein associated with CCR2.16 In addition, RS-504393 also has the capacity to inhibit MCP-1-induced chemotaxis and ischemia-reperfusion injury in kidneys, where MCP-1 plays a role.17 However, whether the blockade of CCR2 might be effective for the treatment of renal interstitial fibrosis has not been fully examined.
In this study, we hypothesized that the blockade of CCR2 might be therapeutically beneficial for progressive fibrosis. To achieve this goal, we evaluated renal interstitial fibrosis induced by unilateral ureteral obstruction (UUO), a well-known renal fibrosis model,18,19 in CCR2 gene-targeted mice and mice treated with propagermanium or RS-504393. We report here that the blockade of CCR2 represents a beneficial therapeutic approach for progressive fibrosis in kidney.
Materials and Methods
Animals
Mice deficient in the expression of CCR2 were generated by the process of gene targeting in murine embryonic stem cells20 and a breeding colony was maintained under specific pathogen-free conditions. Control male C57BL/6J and 129/Ola mice were purchased from Charles River, Japan Inc. (Atsugi, Kanagawa, Japan). The male CCR2-deficient and wild-type control animals were on an outbred C57BL/6 × 129/Ola genetic background (n > 8 generations) and were used at 8 weeks of age. All procedures used in the animal experiments complied with the standards set out in the Guidelines for the Care and Use of Laboratory Animals in Takara-machi Campus of Kanazawa University.
Unilateral Ureteral Obstruction Model
The general procedure of a UUO model is well described elsewhere.21 CCR2 gene-targeted and wild-type mice were anesthetized with diethyl ether and pentobarbital sodium. A flank incision was made and the left ureter ligated with 4-0 silk suture at two points. Sham operation was performed in a similar manner, except for left ureteral ligation. For pathological examination, both the obstructed and contralateral kidneys were harvested from UUO animals 4, 7, and 14 days after ureteral ligation (n = 6, 6, and 8 for each group at each time point).
Treatment with CCR2 Antagonists
To evaluate the therapeutic effects of MCP-1/CCR2 signaling, either propagermanium (3 or 8 mg/kg orally once a day) or RS-504393 (2 mg/kg orally twice a day) was mandatorily injected into their mouths to wild-type mice from 3 days before ureteral ligation until the day of sacrifice. In addition, to determine the viability for the usage of CCR2 antagonists for the treatment of renal fibrosis, propagermanium (8 mg/kg) was given daily, beginning 4 days after ureter ligation. For pathological examination, both the obstructed and contralateral kidneys were harvested from UUO animals 4, 7, and 14 days after ureteral ligation (n = 5 at each time point). Untreated age-matched male wild-type mice and CCR2-deficient mice were used as normal control (n = 6 for each group). Since propagermanium treatment was started from 3 days before ureteral ligation, mice treated with propagermanium for 3 days at day 0 were used as a negative control (n = 5).
Tissue Preparation
One portion of the renal tissue was fixed in 10% buffered formalin (pH 7.2), embedded in paraffin, cut at 4 μm, stained with hematoxylin and eosin, periodic acid-Schiff, or Mallory-azan and observed under a light microscope. Two independent observers with no previous knowledge of the experimental design evaluated each section. Mean interstitial fibrotic area, expressed as blue in Mallory-azan staining, was evaluated from the whole area of cortex and outer medulla in the individual complete sagittal kidney section and expressed as percentage/mm2 of the field using Mac Scope version 6.02 (Mitani Shoji Co., Ltd., Fukui, Japan).
Immunohistochemical Studies
The other portion of fresh renal tissue, embedded in O.C.T. compound and snap-frozen in n-hexane cooled with a mixture of dry ice and acetone, was cut at 6 μm on a cryostat (Tissue-Tek Systems; Miles, Naperville, IL). The presence of F4/80-positive macrophages or CD3-positive T cells was detected immunohistochemically using rat anti-mouse F4/80 monoclonal antibody (clone A3–1; BMA Biomedicals AG, Augst, Switzerland) or rat anti-mouse CD3 monoclonal antibody (R&D Systems, Minneapolis, MN). The number of interstitial infiltrated F4/80-positive macrophages or CD3-positive T cells was counted in the whole area of the outer medulla, where cell migration was maximal, and expressed as the mean number ± SE (SEM)/mm2. The presence of MCP-1 and transforming growth factor (TGF)-β1 was demonstrated immunohistochemically on formalin-fixed, paraffin-embedded renal tissue specimens using the indirect avidin-biotinylated peroxidase complex method with rabbit anti-murine MCP-1 polyclonal antibodies and rabbit anti-murine TGF-β1 polyclonal antibodies (clone sc-146; Santa Cruz Biotechnology, Santa Cruz, CA). The antigen was retrieved with Target Retrieval Solution (DAKO Co., Glostrup, Denmark). To evaluate the specificity of these antibodies, we stained tissue specimens with normal rabbit IgG or antibodies for MCP-1 and TGF-β absorbed with the excess amount of each molecule or a blocking peptide. The presence of type I collagen was also demonstrated immunohistochemically on paraffin-embedded renal tissue with rabbit anti-murine type I collagen polyclonal antibodies (Chemicon Int., Inc., Temecula, CA). The positive area of MCP-1, TGF-β1, and type I collagen was evaluated from the whole area of cortex and outer medulla in the individual complete sagittal kidney section and expressed as a percentage/mm2 using Mac Scope version 6.02.
Dual Staining
To determine the phenotypes of CCR2-positive cells, dual-labeled immunofluorescence immunohistochemistry was performed. Briefly, sections were first incubated with goat anti-mouse CCR2 antibodies (clone sc-6228; Santa Cruz Biotechnology) overnight. After rinsing in PBS, the rat anti-mouse F4/80 or rabbit anti-murine TGF-β1 polyclonal antibodies were added and the sections incubated overnight. CCR2 was visualized by incubating sections for 120 minutes with fluorescein isothiocyanate-conjugated donkey anti-goat IgG antibodies (1:200; Jackson Immunoresearch Laboratory, Inc., West Grove, PA). After rinsing, sections were incubated for 120 minutes with Cy3-conjugated donkey anti-rat IgG antibodies (1:200; Jackson Immunoresearch Laboratory, Inc.) to visualize F4/80 or with Cy3-conjugated goat anti-rabbit IgG antibodies (1:200; Jackson Immunoresearch Laboratory, Inc.) to visualize TGF-β1. Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) was used for image handling and the three-color channels were handled separately.
Detection of MCP-1 and TGF-β Transcripts in Diseased Kidneys by Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To determine transcripts of MCP-1 and TGF-β, total RNA was extracted from the whole kidneys. cDNA was reverse-transcribed from 1 μg of total RNA, combined from five mice in each group (1 μg of RNA per a mouse), by using a SuperScript II RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the following parameters: 10 minutes at 25°C, 30 minutes at 48°C, and 5 minutes at 95°C. For all PCR experiments, the LightCycler (Roche Diagnostics, Basel, Switzerland) was used. In quantitative RT-PCR for murine MCP-1 (primers: forward, 5′-ACTGAAGCCAGCTCTCTCTTCCTC-3′ and reverse, 5′-TTCCTTCTTGGGGTCAGCACAGAC-3′),22 1 μg of cDNA prepared above described and performed by SYBR Green I format with LightCycler Fast Start DNA Master SYBR Green I (Roche Diagnostics). Transcripts of murine TGF-β and GAPDH were determined using primers and hybridization probe kit (TGF-β, Roche Diagnostics; GAPDH, Nihon Gene Research Lab’s, Inc., Sendai, Japan). The reactions for MCP-1 were incubated at 95°C for 10 minutes followed by 40 cycles of 15 seconds at 95°C, 10 seconds at 55°C, and 20 seconds at 72°C. The reactions for TGF-β were incubated at 95°C for 10 minutes followed by 40 cycles of 10 seconds at 95°C, 15 seconds at 62°C, and 11 seconds at 72°C. mRNA expression of MCP-1 and TGF-β in each sample was finally described after correction with GAPDH expression. No PCR product was detected in the real-time PCR procedure without reverse transcription, indicating that the contamination of genomic DNA was negligible. Gels of the PCR products after quantification of MCP-1, TGF-β, or GAPDH by real-time RT-PCR showed a single band (270, 293, and 230 bp, respectively) with the expected size (data not shown).
Detection of Type I Collagen Transcripts in Diseased Kidneys by RT-PCR
To determine transcripts of type I collagen, total RNA was also extracted from the whole kidneys from all mice in each group 4, 7, and 14 days after ureteral ligation to perform RT-PCR. cDNA was reverse-transcripted from 5 μg of total RNA from each mouse by using a RT-PCR kit (Takara Shuzo Co., Ltd., Tokyo, Japan). The cDNA product was amplified by PCR. Primers for type I collagen [5′-TCGTGACCGTGACCTTGCG-3′ (sense); 5′-GGATGAGTCGGCAGACACGGA-3′ (anti-sense)]23 were used to detect type I collagen transcripts. The housekeeping gene GAPDH was used for PCR controls. Ten μl of PCR products were run on 2.0% agarose gel and stained with ethidium bromide, then gene-specific bands were visualized under ultraviolet light. The quantities of target cDNA were analyzed by NIH image. Scanner analysis of photographs of the DNA-stained agarose gels was evaluated by the band intensity comparison of GAPDH expression versus type I collagen expression in computer image analysis.
Statistical Analysis
The mean and SEM were calculated on all of the parameters determined in this study. Statistical analyses were performed using Wilcoxon rank-sum test, Kruskal-Wallis test, and analysis of variance test. P < 0.05 was accepted as statistically significant.
Results
Renal Lesions Were Reduced in CCR2 Knockout Mice
Histopathological examination was performed using periodic acid-Schiff-stained renal tissues. Severe cell infiltration, tubular atrophy, and interstitial fibrosis were observed 14 days after ureteral ligation in the outer medulla of wild-type mice (Figure 1a). In contrast, the lesions in the outer medulla were significantly reduced in CCR2 gene-targeted mice (Figure 1b). However, renal damage was still evident as compared with sham-operated mice (Figure 1d). Thus, the loss of CCR2 correlated with reduced renal lesions.
Figure 1.
Inhibition of CCR2 reduced renal pathology. a: Severe cell infiltration, tubular atrophy, and interstitial fibrosis were observed 14 days after ureteral ligation in the outer medulla of wild-type mice. In contrast, such lesions in the outer medulla were significantly reduced in CCR2 gene-targeted mice (b) and propagermanium-treated mice at the dose of 3 mg/kg (c). d: A sham-operated mouse kidney.
Reduced Renal Interstitial Fibrosis and Type I Collagen Expression in CCR2 Knockout Mice
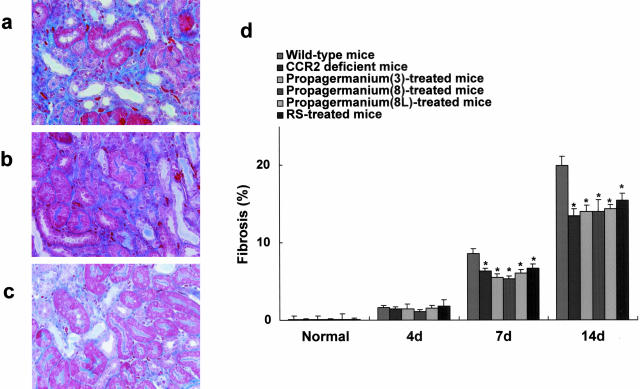
To determine the impact of MCP-1/CCR2 interactions on progressive renal interstitial injury, interstitial fibrotic areas expressed as blue in Mallory-azan staining were examined by computer analysis (Figure 2; a to c). Ureteral ligation caused progressive renal interstitial fibrosis in wild-type mice (Figure 2d). In contrast, mean interstitial fibrosis, expressed as percentage/mm2, was reduced in CCR2 gene-targeted mice as compared with wild-type mice (Figure 2d). However, the CCR2 deficiency did not reduce interstitial fibrotic areas to the levels shown in sham-operated mice or normal mice (Figure 2d).
Figure 2.
CCR2 deletion reduced interstitial fibrosis. a and d: Progressive interstitial lesions exhibited interstitial fibrosis in wild-type mice on day 14. In contrast, the mean interstitial fibrosis, expressed as percentage involvement of the field, was reduced in CCR2 gene-targeted mice (b, d) and mice treated with propagermanium (c, d) or RS-504393 (d) as compared with wild-type mice. Values are the mean ± SEM. PG(3) mice treated with propagermanium at the dose of 3 mg/kg; PG(8) mice treated with propagermanium (8 mg/kg); PG(8L), mice treated with propagermanium (8 mg/kg) from 4 days after ureter ligation; RS, RS-504393. *, P < 0.05 as compared with wild-type mice. Original magnifications, ×200.
Moreover, the type I collagen-positive area was extensive in wild-type mice 14 days after ureteral ligation, but was reduced by CCR2 deficiency (Figure 3, a and b). Furthermore, ureteral ligation enhanced type I collagen mRNA expression in wild-type mice. The up-regulated mRNA expression of type I collagen in diseased kidneys was reduced by CCR2 deficiency, whereas mRNA expression of type I collagen was faintly detected in normal kidneys (Figure 3c). Thus, CCR2 deficiency appears to play a role in the pathogenesis of renal interstitial fibrosis by resulting in a reduction in type I collagen synthesis.
Figure 3.
CCR2 deletion reduced type I collagen expression. a: Type I collagen was detected by immunohistochemical analysis. b: Type I collagen expression was reduced in the interstitium in CCR2 gene-targeted mice and propagermanium-treated mice compared with that of wild-type mice. c: The up-regulated mRNA expression of type I collagen in diseased kidneys was reduced by CCR2 blockade, whereas mRNA expression of type I collagen was faintly detected in normal kidneys. Values are the mean ± SEM. PG(3) mice treated with propagermanium at the dose of 3 mg/kg. *, P < 0.05 as compared with wild-type mice. Original magnification, ×400.
Reduced Expression of Renal MCP-1 by CCR2 Deficiency
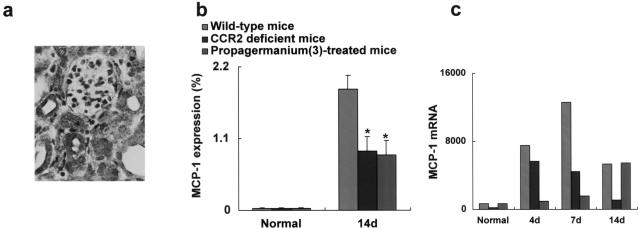
To clarify the impact of MCP-1/CCR2 system on MCP-1 expression in the diseased kidneys, MCP-1 protein was evaluated immunohistochemically at day 14. MCP-1 protein was faintly detected in normal kidneys and sham-operated kidneys (data not shown). On the contrary, MCP-1 protein was readily detected in tubular epithelial cells and interstitially infiltrated cells in diseased kidneys of wild-type mice (Figure 4a). The percentage of MCP-1-positive area in interstitium was reduced in CCR2 gene-targeted mice relative to that in wild-type mice (Figure 4b).
Figure 4.
Reduced expression of renal MCP-1 by CCR2 deletion. a: MCP-1 protein was detected mainly in tubular epithelial cells and infiltrates at day 14. b: MCP-1 protein expression was reduced in the interstitium in CCR2 gene-targeted mice and propagermanium-treated mice (3 mg/kg) compared with that of wild-type mice. Values are the mean ± SEM. *, P < 0.05 as compared with wild-type mice. c: Transcripts of MCP-1 were faintly detected from normal kidneys by real-time RT-PCR. In contrast, transcripts of MCP-1 in diseased kidneys were up-regulated in wild-type mice, which were reduced by CCR2 blockade. Original magnification, ×400.
Transcripts of MCP-1 were faintly detected in normal and sham-operated kidneys (data not shown) by real-time RT-PCR. In contrast, transcripts of MCP-1 in diseased kidneys in wild-type mice were up-regulated, whereas the levels of these transcripts were reduced in CCR2 knockout mice (Figure 4c). Therefore, CCR2 deficiency, in turn, led to a decrease in MCP-1 expression in the renal interstitium.
Renal TGF-β Expression Was Reduced in CCR2 Knockout Mice
The presence of TGF-β1 protein, a potent fibrogenic factor, was evaluated immunohistochemically in diseased kidneys. Overexpression of TGF-β1 protein was detected mainly in tubular epithelial cells in wild-type mice (Figure 5, a and e) as compared with that in normal (Figure 5e) and sham-operated mice (data not shown). The area of TGF-β1-positive cells was reduced in CCR2 gene-targeted mice (Figure 5, b and e). TGF-β1 immunoreactivity was not detected in sections incubated with the absorbed antibodies with excess amount of a blocking peptide (Figure 5d) or control rabbit IgG (data not shown), which suggests that this staining was specific for TGF-β1.
Figure 5.
Renal TGF-β expression was reduced by the deletion of CCR2. a: Up-regulation of TGF-β1 protein was detected mainly in tubular epithelial cells in diseased kidneys in wild-type mice at day 14. In contrast, TGF-β1 protein was reduced of interstitium in CCR2 gene-targeted mice (b) and propagermanium-treated mice (3 mg/kg) (c). TGF-β1 immunoreactivity was not detected in sections incubated with the blocking peptide (d). e: The percentage of TGF-β1-positive area in one field is shown. f: Transcripts of TGF-β were faintly detected in normal kidneys by real-time RT-PCR. In contrast, transcripts of TGF-β in diseased kidneys were up-regulated in wild-type mice. The levels of these transcripts were reduced by CCR2 blockade. In wild-type mice, CCR2-positive cells were visualized with fluorescein isothiocyanate (g) and TGF-β1-positive cells with Cy3 (i). h: Some of the infiltrating CCR2-positive cells were also positive for TGF-β1 in injured kidneys at day 14. Values are the mean ± SEM. *, P < 0.05 as compared with wild-type mice. Original magnifications, ×400.
Transcripts of TGF-β were faintly detected in normal and sham-operated kidneys by real-time RT-PCR. In contrast, transcripts of TGF-β in diseased kidneys in wild-type mice were up-regulated. Furthermore, CCR2 deficiency reduced the up-regulation of renal TGF-β transcripts (Figure 5f). Thus, the loss of CCR2 down-regulated the expression of TGF-β, which might, in turn, contribute to the decrease in fibrogenesis in diseased kidneys. Further, to determine the TGF-β1 expression in CCR2-positive cells, dual staining was performed. In wild-type mice, CCR2-positive (Figure 5g) and TGF-β1-positive (Figure 5i) cells were detected and most of the infiltrating TGF-β1-positive cells were also positive for CCR2 in injured kidneys (Figure 5h) at day 14.
Interstitial F4/80-Positive Macrophages, Most of Which Were Positive for CCR2, Were Reduced in CCR2 Knockout Mice
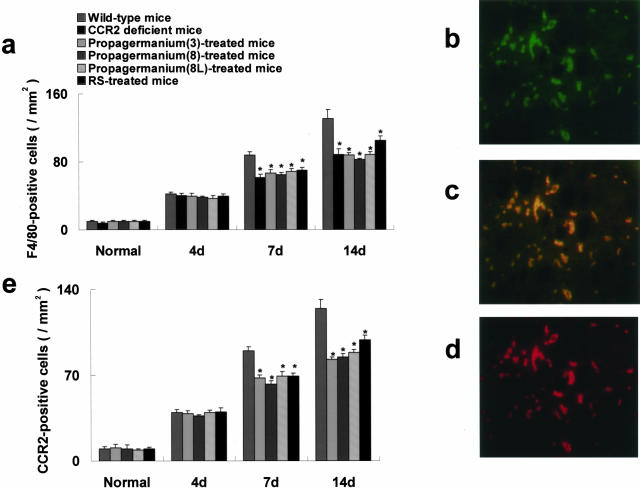
F4/80-positive macrophages infiltrated mainly in the outer medulla in a murine UUO model. The number of interstitially infiltrated F4/80-positive macrophages was reduced in CCR2 gene-targeted mice 7 and 14 days after ureteral ligation compared with that of wild-type mice (Figure 6a). In contrast, the cell number in the glomeruli did not differ at any time point after ureteral ligation (data not shown). In addition, CCR2-positive cells, which increased during the experimental periods, were decreased in number in CCR2 knockout mice (Figure 6, b and e). To determine the presence of CCR2 on F4/80-positive cells, dual labeling of immunofluorescence immunohistochemistry was performed. In wild-type mice, CCR2-positive (Figure 6b) and F4/80-positive (Figure 6d) cells were detected in diseased kidneys 14 days after ureteral ligation. Most infiltrating F4/80-positive cells were also positive for CCR2 in injured kidneys (Figure 6c). Therefore, deficiency of CCR2, predominantly expressed on macrophages, correlated directly with macrophage infiltration in diseased kidneys.
Figure 6.
Interstitial F4/80-positive macrophages, most of which were positive for CCR2, were reduced. a: The number of F4/80-positive cells was reduced in CCR2 gene-targeted mice and mice treated with propagermanium or RS-504393 7 and 14 days after ureteral ligation compared with that of wild-type mice. F4/80-positive cells were visualized with Cy3 and CCR2-positive cells with fluorescein isothiocyanate. In wild-type mice, CCR2 (b)- and F4/80 (d)-positive cells were detected in the outer medulla 14 days after ureteral ligation. c: Most infiltrated F4/80-positive cells were also positive for CCR2 in injured kidneys. e: The number of CCR2-positive cells was similarly reduced in mice treated with propagermanium or RS-504393 7 and 14 days after ureteral ligation compared with that of wild-type mice. Values are the mean ± SEM. PG(3), mice treated with propagermanium at the dose of 3 mg/kg; PG(8), mice treated with propagermanium (8 mg/kg); PG(8L), mice treated with propagermanium (8 mg/kg) 4 days after ureter ligation; RS, RS-504393. *, P < 0.05 as compared with wild-type mice. Original magnifications, ×400.
Blockade of CCR2 by CCR2 Antagonists Ameliorated Renal Interstitial Fibrosis
Although deficiency of CCR2 showed the anti-fibrogenic effects in the renal interstitium in CCR2 knockout mice, the genetic deficiency of CCR2 might be fundamentally different from the inhibition of MCP-1/CCR2 interactions in diseased kidneys. Therefore, to determine the impact of blockade of CCR2, treatment with propagermanium or RS-504393 was examined in a UUO model. Similar to the results obtained from CCR2-deficient mice, the treatment with propagermanium or RS-504393 significantly reduced renal pathology, especially the extensive interstitial fibrosis mediated by decrease in type I collagen synthesis (Figure 1, b and c; Figure 2; Figure 3). TGF-β1-positive area was reduced in mice treated with propagermanium (Figure 5, c and e). Moreover, CCR2 blockade also reduced F4/80-positive cells, which were mostly positive for CCR2, by possibly reducing the macrophage-derived expression of MCP-1 in diseased kidneys (Figure 4; Figure 6, a and e). As a result, the reduction in the number of F4/80-positive macrophages did not differ in CCR2 gene-targeted mice from those in propagermanium- or RS-504393-treated mice. In particular, propagermanium treatment (3 mg/kg) led to the anti-fibrotic effect associated with decreased macrophage infiltration similar to that in mice treated with propagermanium at the dose of 8 mg/kg. It was also noted that the administration of propagermanium from day 4 also reduced renal fibrosis (Figure 2d), which was associated with the decrease in number of infiltrated macrophages (Figure 6a). Thus, it should be noted that blockade of CCR2 via CCR2 antagonists similarly ameliorated progressive fibrosis in diseased kidneys.
Impacts of CCR2 Blockade on T-Cell Infiltration in Diseased Kidneys
CD3-positive T cells were not reduced by CCR2 blockade. The infiltration of CD3-positve T cells increased in wild-type mice 14 days after ureter ligation (8.6 ± 2.5/mm2) as compared with that in normal control mice (0.9 ± 0.6/mm2). The number of T cells in interstitium in CCR2-deficient mice (7.7 ± 2.0/mm2) or propagermanium-treated mice (7.2 ± 1.9/mm2) did not differ from that of wild-type mice.
Discussion
In this report, to explore the impact of MCP-1/CCR2 interactions as the therapeutic targets in progressive fibrosis in kidney, we inhibited CCR2 signaling in a murine UUO model in mice genetically deficient in CCR2 and mice treated with CCR2 antagonists. We now report that inhibition of CCR2 reduced the degree of macrophage infiltration and the extent of fibrosis in a murine UUO model. We also noted that inhibition of CCR2 reduced the expression of TGF-β and type I collagen as well as MCP-1. The results, obtained from CCR2-deficient mice, were similar to those in mice treated with CCR2 antagonists. Further, reduced renal fibrosis associated with decrease in macrophage infiltration was noted in mice treated with a CCR2 antagonist given after ureter ligation. Thus, we conclude that CCR2 signaling via MCP-1 is an appealing therapeutic target for combating progressive fibrosis in kidney.
Our findings are consistent with previous reports that CCR2 signaling via MCP-1 plays a key role in human and experimental renal damage, especially in the interstitium.4,6,7,10 It is worth noting that MCP-1 deficiency contributed to interstitial injury, but not glomerular lesions in a murine experimental model of glomerulonephritis induced by anti-glomerular basement membrane antibodies.24 In addition to renal diseases, the blockade of CCR2 demonstrated the critical role of MCP-1/CCR2 interaction in macrophage-mediated pathogenesis in various diseases, such as bronchiolitis obliterans syndrome,25 acetaminophen-induced hepatotoxicity,26 experimental autoimmune encephalomyelitis,27 and atherosclerosis.28 Further, accumulating evidence indicates the existence of a regulatory loop between MCP-1 and TGF-β in isolated glomeruli and the diseased kidneys in Thy-1 nephritis.29,30 In this study, dual staining revealed that most of the infiltrated TGF-β1-positive cells were also positive for CCR2. These findings, combined with our present study, suggest that diminished CCR2 signaling may down-regulate TGF-β expression, thereby attenuating progressive fibrosis in the diseased kidney. Thus, taken together, CCR2 signaling via MCP-1 is instrumental in promulgating progressive fibrosis and the blockade of this signaling is therapeutically promising.
We investigated the inhibitory effects of CCR2 on renal fibrosis using CCR2 antagonists, propagermanium and RS-504393. We previously reported that propagermanium prevented MCP-1-induced chemotaxis but not interleukin-8-induced, RANTES-induced, or MIP-1α-induced chemotaxis of monocytes. Propagermanium also inhibited MCP-3-induced migration of monocytes.16 In addition, in our unpublished data, propagermanium did not inhibit eotaxin-induced chemotaxis of eosinophils or TARC-induced chemotaxis of CCR4-transfected THP-1 cells. Therefore, these results examined suggest that the inhibitory effect of propagermanium on monocyte migration would be CCR2-dependent. The molecular mechanism of the action of propagermanium has been revealed. Propagermanium targets glycosylphosphatidylinositol-anchored proteins that are closely associated with CCR2 and interferes with the MCP-1-induced monocyte chemotaxis.16 Propagermanium improved massive liver damage induced by Corynebacterium parvum followed by lipopolysaccharide administration in mice.15 We also reported that propagermanium significantly reduced tumor necrosis factor-α production from interferon-γ-pretreated mice.14 Further, RS-504393 inhibited MCP-1-induced chemotaxis in a dose-dependent manner, whereas it did not inhibit MIP-1α-induced chemotaxis.31 RS-504393 reduced acute tubular necrosis and cell infiltration in ischemia reperfusion injury in kidneys.17 Our results strongly suggest that these anti-inflammatory effects may contribute to the reduction in renal injury in this particular model. Our results also provide the first evidence of the preventive effect of CCR2 antagonists on progressive renal injury characterized by fibrosis associated with inflammatory cell infiltration. Therefore, clinical trials using CCR2 antagonists to prevent progressive organ fibrosis including renal diseases would be encouraged.
The expression of MCP-1 was significantly reduced via MCP-1/CCR2 blockade. This finding may be explained by some speculations as follows: 1) this reduction of MCP-1 may be proportional to the decline in macrophage infiltration in the diseased kidneys. Human CD14-positive monocytes could up-regulate MCP-1 expression dependent on MCP-1 stimulation, which is inhibited by the CCR2 blockade (manuscript in preparation). 2) The interaction between macrophages and renal epithelial cells would be important. Activated macrophages by CCR2 signaling produce proinflammatory cytokines and chemokines including MCP-1, which in turn stimulate renal resident cells such as renal tubular epithelial cells or endothelial cells to produce cytokines and chemokines. Supporting this notion, it was reported that MCP-1 activates AP-1 and nuclear factor-κB in tubular epithelial cells,32 which are responsible for the production of interleukin-6 and ICAM-1. Moreover, AP-1 and nuclear factor-κB are critical for production of MCP-1 in tubular epithelial cells.33 These data suggest that MCP-1/CCR2-dependent activation of nuclear factor-κB and AP-1 might amplify the local inflammation of the diseased kidneys including MCP-1 production itself. Therefore, MCP-1/CCR2 inhibition may directly or indirectly lead to the reduced MCP-1 expression in diseased kidneys.
Progressive fibrosis is a common pathological finding in various organs, resulting in organ failure. In addition to renal fibrosis, the involvement of MCP-1/CCR2 interactions has been reported in the pathogenesis of progressive fibrosis in lungs12,25 and lung granuloma models.23,34 Moreover, recent studies also suggest the critical role of MCP-1/CCR2 interactions and the benefits of its therapeutic inhibition in atherosclerosis and vascular restenotic changes (neointimal hyperplasia).35,36 More recently, Moore and colleagues37 have shown that alveolar epithelial cells from CCR2-deficient mice suppress fibroblast proliferation more than alveolar epithelial cells from CCR2 intact mice. These findings could provide a key to the protection from pulmonary fibrosis in CCR2-deficient mice. Therefore, targeting MCP-1/CCR2 interaction provides a novel way to control progressive fibrosis and vascular change beyond macrophage chemotaxis and activation. Further, in this present study, propagermanium and RS-504393 were proven to have the similar anti-CCR2 inhibitory effect to that obtained from CCR2-deficient mice. Thus, these findings imply the potential clinical application of CCR2 antagonists such as propagermanium and RS-504393 for progressive fibrosis, such as renal interstitial fibrosis.
In summary, our results suggest that the MCP-1/CCR2 is pivotal in the causation of renal interstitial damage and that the blockade of CCR2 is an effective therapeutic strategy for progressive fibrosis in the diseased kidneys.
Acknowledgments
We thank Dr. Joost J. Oppenheim (National Cancer Institute-Frederick Cancer Research and Development Center-FCRDC) for his critical review of the manuscript and Dr. Toshikazu Kondo (Wakayama Medical University, Wakayama, Japan) for his advice in a dual staining.
Footnotes
Address reprint requests Dr. Takashi Wada, Division of Blood Purification and Department of Gastroenterology and Nephrology, Graduate School of Medical Science, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-8641, Japan. E-mail: twada@medf.m.kanazawa-u.ac.jp.
Supported in part by a grant-in-aids from the Ministry of Health, Labor, and Welfare of Japan; and the Ministry of Education, Science, Sports, and Culture in Japan (no. 14571019 to T.W.).
References
- Matsushima K, Larrsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, Cartron JP, Colin Y, Schlöndorff D, Alpers CE. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am J Kidney Dis. 2001;37:518–531. [PubMed] [Google Scholar]
- Wada T, Yokoyama H, Mukaida N, Furuichi K, Segawa S, Hisada Y, Ohta S, Takasawa K, Kobayashi K, Matsushima K. Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int. 1996;49:761–767. doi: 10.1038/ki.1996.105. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Wada T, Furuichi K, Segawa C, Shimizu M, Kobayashi K, Su S, Mukaida N, Matsushima K. Urinary levels of chemokines (MCAF/MCP-1, IL-8) reflect distinct disease activities and phases of human IgA nephropathy. J Leukoc Biol. 1998;63:493–499. doi: 10.1002/jlb.63.4.493. [DOI] [PubMed] [Google Scholar]
- Wada T, Yokoyama H, Furuichi K, Kobayashi K, Harada K, Naruto M, Su S, Akiyama M, Mukaida N, Matsushima K. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1). FASEB J. 1996;10:1418–1425. [PubMed] [Google Scholar]
- Wada T, Furuichi K, Shimizu M, Sakai N, Kida H, Kobayashi K, Mukaida N, Ohmoto N, Matsushima K, Yokoyama H. MIP-1 alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int. 1999;56:995–1003. doi: 10.1046/j.1523-1755.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Wada T, Furuichi K, Sakai N, Iwata Y, Kida H, Kobayashi K, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- Wada T, Yokoyama H, Matsushima K, Kobayashi K. Chemokines in renal diseases. Int Immunopharmacol. 2001;4:637–645. doi: 10.1016/s1567-5769(01)00004-2. [DOI] [PubMed] [Google Scholar]
- Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, Tomosugi N, Matsushima K, Egashira K, Yokoyama H. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15:940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- Segerer S, Cui Y, Hudkins KL, Goodpaster T, Eitner F, Mack M, Schlöndorff D, Alpers CE. Expression of the chemokine monocyte chemoattractant protein-1 and its receptor chemokine receptor 2 in human crescentic glomerulonephritis. J Am Soc Nephrol. 2000;11:2231–2242. doi: 10.1681/ASN.V11122231. [DOI] [PubMed] [Google Scholar]
- Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlöndorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;15:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- Fujinaka H, Yamamoto T, Takeya M, Feng L, Kawasaki K, Yaoita E, Kondo D, Wilson CB, Uchiyama M, Kihara I. Suppression of anti-glomerular basement membrane nephritis by administration of anti-monocyte chemoattractant protein-1 antibody in WKY rats. J Am Soc Nephrol. 1997;8:1174–1178. doi: 10.1681/ASN.V871174. [DOI] [PubMed] [Google Scholar]
- Ishiwata Y, Yokochi S, Hashimoto F, Ninomiya F, Suzuki T. Protection against concanavalin A-induced murine liver injury by the organic germanium compound, propagermanium. Scand J Immunol. 1998;48:605–614. doi: 10.1046/j.1365-3083.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- Yokochi S, Ishiwata Y, Hashimoto H, Ninomiya F, Suzuki T. Hepatoprotective effect of propagermanium on Corynebacterium parvum and lipopolysaccharide-induced liver injury in mice. Scand J Immunol. 1998;48:183–191. doi: 10.1046/j.1365-3083.1998.00356.x. [DOI] [PubMed] [Google Scholar]
- Yokochi S, Hashimoto H, Ishiwata Y, Shimokawa H, Haino M, Terashima Y, Matushima K. An anti-inflammatory drug, propagermanium, may target GPI-anchored proteins associated with an MCP-1 receptor, CCR2. J Interferon Cytokine Res. 2001;21:389–398. doi: 10.1089/107999001750277862. [DOI] [PubMed] [Google Scholar]
- Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziiel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14:2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- Wright F. Effects of urinary tract obstruction on glomerular filtration rate and renal blood flow. Semin Nephrol. 1982;2:5–16. [Google Scholar]
- Klahr S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983;23:414–426. doi: 10.1038/ki.1983.36. [DOI] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlöndorff D. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Gibbs L, Flower RJ, Das AM, Perretti M. Investigation of the functional role played by the chemokine monocyte chemoattractant protein-1 in interleukin-1-induced murine peritonitis. Br J Pharmacol. 1998;125:319–326. doi: 10.1038/sj.bjp.0702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL. Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol. 1998;153:1861–1872. doi: 10.1016/S0002-9440(10)65700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor 2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TC, Kuziel WA, Osahar TA, Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- Wolf G, Jocks T, Zahner G, Panzer U, Stahl RA. Existence of regulatory loop between MCP-1 and TGF-beta in glomerular immune injury. Am J Physiol Renal Physiol. 2002;283:F1075–F1084. doi: 10.1152/ajprenal.00349.2001. [DOI] [PubMed] [Google Scholar]
- Schneider A, Panzer U, Zahner G, Wenzel U, Wolf G, Thaiss F, Helmchen U, Stahl RA. Monocyte chemoattractant protein-1 mediates collagen deposition in experimental glomerulonephritis by transforming growth factor-beta. Kidney Int. 1999;56:135–144. doi: 10.1046/j.1523-1755.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Diehl F, Ebi B, Bhakta S, Polsky I, McCarley D, Mulkins M, Weatherhead GS, Lapierre JM, Danwardt J, Morgan D, Jr, Wilhelm R, Jarnagin K. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- Viedt C, Dechend R, Fei J, Hansch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells. J Am Soc Nephrol. 2002;13:1534–1547. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-κB dependent. Kidney Int. 2000;57:2011–2022. doi: 10.1046/j.1523-1755.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163:2193–2201. [PubMed] [Google Scholar]
- Inoue S, Egashira K, Ni W, Kitamoto S, Usui M, Ohtani K, Ishibashi M, Hiasa K, Nishida K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy limits progression and destabilization of established atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2002;106:2700–2706. doi: 10.1161/01.cir.0000038140.80105.ad. [DOI] [PubMed] [Google Scholar]
- Usui M, Egashira K, Ohtani K, Kataoka C, Ishibashi M, Hiasa K, Katoh M, Zhao Q, Kitamoto S, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy inhibits restenotic changes (neointimal hyperplasia) after balloon injury in rats and monkeys. FASEB J. 2002;16:1838–1840. doi: 10.1096/fj.02-0094fje. [DOI] [PubMed] [Google Scholar]
- Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, III, Toews GB. Aveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol. 2003;284:L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]