Abstract
Collagenous Alzheimer amyloid plaque component (CLAC) is a unique non-Aβ amyloid component of senile plaques (SP) derived from a transmembrane collagen termed CLAC-precursor. Here we characterize the chronological and spatial relationship of CLAC with other features of SP amyloid in the brains of patients with Alzheimer’s disease (AD), Down syndrome (DS), and of PSAPP transgenic mice. In AD and DS cerebral cortex, CLAC invariably colocalized with Aβ42 but often lacked Aβ40- or thioflavin S (thioS)-reactivities. Immunoelectron microscopy of CLAC-positive SP showed labeling of fibrils that are more loosely dispersed compared to typical amyloid fibrils in CLAC-negative SP. In DS cerebral cortex, diffuse plaques in young patients were negative for CLAC, whereas a subset of SP became CLAC-positive in patients aged 35 to 50 years, before the appearance of Aβ40. In DS cases over 50 years of age, Aβ40-positive SP dramatically increased, whereas CLAC burden remained at a constant level. In PSAPP transgenic mice, CLAC was positive in the diffuse Aβ deposits surrounding huge-cored plaques. Thus, CLAC and Aβ40 or thioS exhibit mostly separate distribution patterns in SP, suggesting that CLAC is a relatively early component of SP in human brains that may have inhibitory effects against the maturation of SP into β-sheet-rich amyloid deposits.
Alzheimer’s disease (AD) is characterized pathologically by a massive accumulation of amyloid deposits comprised of amyloid β peptides (Aβ) as senile plaques (SP) or cerebral amyloid angiopathy (CAA).1 While genetic, pathological, and biochemical studies have provided firm evidence supporting the causative significance of Aβ deposition in AD,1 a number of non-Aβ proteinacious components have been detected in SP amyloid, some of which have been shown to affect Aβ deposition in vivo in the brains of transgenic or knockout mice. For example, the ablation of murine Apolipoprotein E gene (APO E) attenuated the accumulation of Congophilic β-amyloid, whereas transgenic supplementation of human Apolipoprotein E protein (Apo E), especially E4 isoform, restored and promoted β-sheet-rich amyloid deposits;2,3 overexpression of α1-antichymotrypsin accelerated the accumulation of β-amyloid,4 whereas ablation of Apolipoprotein J attenuated it.5 Thus, identification of Aβ-associated proteins in SP amyloid and characterization of their pathological functions is important in the elucidation of the pathobiology of β-amyloid formation and AD.
We have searched for novel components of SP amyloid by raising monoclonal antibodies (mAbs) against crude amyloid fractions extracted from AD brains, and identified a novel protein that we named CLAC (collagenous Alzheimer amyloid plaque component).6 CLAC is derived from the ectodomain of a novel membrane-bound, neuron-specific collagen that we termed CLAC-precursor (CLAC-P) or collagen type XXV, through shedding by furin.6 Recent work suggests that CLAC is identical to the AMY antigen7 that had previously been identified in SP.8,9 In vitro studies show that recombinant CLAC specifically binds aggregated Aβ, but not its soluble form.6 Pathologically, CLAC-immunoreactivity (IR) was detected in a subset of SP in AD brains, especially in primitive plaques or in the periphery of typical plaques, whereas amyloid cores, CAA, or diffuse plaques lacked CLAC-IR.6 This selectivity in the distribution of CLAC deposition in a specific subfraction of amyloid deposits is unique, and not observed with any other non-Aβ SP component proteins, ie, Apo E, complement component C1q or heparan sulfate proteoglycan (10–12, Sakakura T, Kowa H, Iwatsubo T, unpublished observations). Other well-known features of SP amyloid exhibiting selective distributions are the heterogenous C termini of Aβ, Aβ40, and Aβ42. Aβ exhibits two major C-terminal variants by the heterogeneity in positions of γ-secretase cleavage of β-amyloid precursor protein (βAPP): Aβ42 with a longer C terminus is a relatively minor secreted species13 but has a higher propensity to aggregate14 and deposits initially and widely in SP.15,16 In contrast, Aβ40, a major secreted species,13 accumulates later robustly in a subset of SP as well as in CAA. However, the temporal and spatial relationships between deposition of CLAC, Aβ40, and Aβ42, each of which shows unique deposition patterns, as well as the pathological significance of CLAC deposition in β-amyloid formation, remains elusive. In this study, we have examined the relationship between deposition of CLAC and different Aβ species in the brains of patients with AD or Down syndrome (DS), as well as those of transgenic (TG) mice developing β-amyloid plaques, using multiple labeling and morphometric evaluation. We find that CLAC-positive SP and Aβ40/thioflavin S (thioS)-positive SP show mostly separate distribution patterns, suggesting a possible role of CLAC binding to prevent further maturation of β-sheet-rich, dense amyloid deposits.
Materials and Methods
Cases
Blocks from frontal neocortex (Brodmann area 8/9) were obtained at autopsy from 74 patients with AD (male: 37 cases, female: 37 cases; age 44 to 92 years, 71.5 ± 10.0 (mean ± SE)), as well as from 26 patients with DS (age 31 to 71 years, 52.7 ± 10.9). All AD patients had pathologically confirmed AD based on the consensus criteria of the National Institute of Aging, and were at Braak stages 5 and 6. Tissues from 28 AD patients were fixed in 10% formalin for 18 to 24 hours, then maintained in phosphate-buffered saline (PBS) at 4°C. Tissue blocks from the rest of the AD cases, as well as from all DS cases, were fixed in 10% buffered formalin for 2 to 4 weeks, then embedded in paraffin wax, cut in serial sections of 6-μm thickness and immunostained as below. Brains of TG mice that doubly express human K670N/M671L mutant βAPP gene and M146L mutant PS1 (PSAPP mice17) at ages 3, 6, 9, 12, and 19 months (total, 9 animals) were fixed by immersion in 70% ethanol/150 mmol/L NaCl for 2 weeks, then embedded in paraffin as previously described.18
Antibodies, Tissue Processing, and Immunohistochemistry
For CLAC immunostaining in human brains, a mouse mAb 9D2, that was originally developed against a crude amyloid fraction of AD brains and specifically recognizes CLAC, whose epitope is located at the pyroglutamated N terminus of CLAC,6 was used. Rabbit affinity-purified antibodies against synthetic peptides corresponding to the three non-collagenous (NC) domains of CLAC-P (anti-NC2–2, anti-NC3, and anti-NC4), as well as to the pyroglutamated N terminus of CLAC (anti-pyroGlu113) also were used.6 The immunostaining patterns with antibodies to the NC domains of CLAC-P were essentially similar to that obtained with 9D2.6 Detection of deposits of murine CLAC in the brains of TG mice was performed with anti-NC2–2.6 Mouse mAbs BC05 and BA27, which specifically react with the C termini of Aβ42 and Aβ40, respectively, have been described;15,16 sections were pretreated with 99% formic acid, followed by incubation with 0.1% trypsin at 37°C before immunostaining for BC05 (BA27 immunostaining was performed solely with formic acid pretreatment). 9D2 optimally detects CLAC-positive SP in 50-μm thick unembedded vibratome sections fixed in 10% formalim for 24 hours without pretreatment.6 Therefore, for CLAC immunostaining of formalin-fixed, paraffin-embedded sections, antigen retrieval of deparaffinized sections by microwave treatment (550W, 10 minutes) in citrate buffer (pH 6.0) followed by proteinase K treatment (100 μg/ml, 10 minutes), a methodology developed for immunostaining of AMY antigen,9 was effective and routinely performed. Immunoperoxidase staining using avidin-biotin complex using diaminobenzidine was performed as described.15,16 Fluorescence labeling of amyloid by thioS was performed as described:19,20 briefly, sections were incubated with 1% thioS in distilled water for 10 minutes, followed by brief wash in 50% ethanol, and then a final wash in tap water. For double or triple fluorescence labeling, sections were incubated by mixture of primary antibodies, followed by incubation with secondary antibodies against mouse or rabbit IgG tagged with Alexa fluorophores and observed with Olympus fluoview confocal microscope as described.21 Fluorescence signals obtained by Alexa 488, 594, and 647 were displayed in pseudocolors of green, red, and blue, respectively.
Morphometry
Deposition of CLAC or Aβ in peroxidase-labeled sections was evaluated by quantifying the total percentage of cortical surface area covered by CLAC- or Aβ-IRs (percent CLAC or amyloid burden) as described.16,18 Images were captured by HC-2500 digital image recording system (Fujix, Tokyo, Japan) mounted on a BX51 microscope (Olympus, Tokyo, Japan) in five randomly selected cortical areas encompassing the entire depth of cortex (0.57 mm2 each; corresponding to 1280 × 1000 pixels), and the percentage of positive areas was calculated by MacSCOPE image analysis software (Mitani Company, Tokyo, Japan). To evaluate the correlation between patient age and CLAC or amyloid burden in DS cases, regression plots were drawn on patient groups younger or older than 50 years of age, using simple regression method on Statview, version 5.0 (SAS Institute Inc., Cary, NC). CLAC/amyloid burden in double fluorescence specimens was analyzed in a representative cortical area (0.13 mm2) in eight representative AD cases. The percentages of single- or double-positive areas for given probes were calculated by MacSCOPE, as above.
Immunoelectron Microscopy
Double immunolabeling for electron microscopic observation of CLAC/Aβ42 or CLAC/Aβ40 in AD cortices was performed as follows: 50-μm thick, floating sections fixed in 10% formalin for 24 hours were incubated with mAb 9D2 overnight. After washing, sections were incubated with anti-mouse IgG antibody tagged with 1-nm gold particles (Nanoprobe) for 24 hours. After washing in 10 mmol/L phosphate buffer, sections were post-fixed in 2.5% glutaraldehyde for 1 hour, and transferred to 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid(HEPES) buffer (pH 5.8). After washing in distilled water (DW), silver intensification was performed using HQ-silver kit (Nanoprobes) according to manufacturer’s instructions. After stopping the silver intensification by washing in DW, the sections were post-fixed in 2% osmium tetroxide for 1 hour, dehydrated, and embedded in epoxy resin. Subsequent post-embedding immunolabeling for Aβ40/42 was performed as described by Yamaguchi et al22 with some modifications. Briefly, ultra-thin sections were cut at 80 nm and treated with 3% H2O2 for 10 minutes followed by 1% sodium periodate for 10 minutes on a nickel grid. After blocking in 3% bovine serum albumin in 100 mM phosphate buffer (pH 7.4) for 30 minutes, sections were incubated with BC05 or BA27, followed by reaction with anti-mouse IgG antibodies tagged with 10-nm gold particles. The sections were double-stained by uranium-acetate and lead-citrate, and viewed in electron microscope (1200EXII, JEOL).
Results
CLAC-IR in SP of AD Neocortex Overlaps with Aβ42 but Minimally with Aβ40 or ThioS Fluorescence
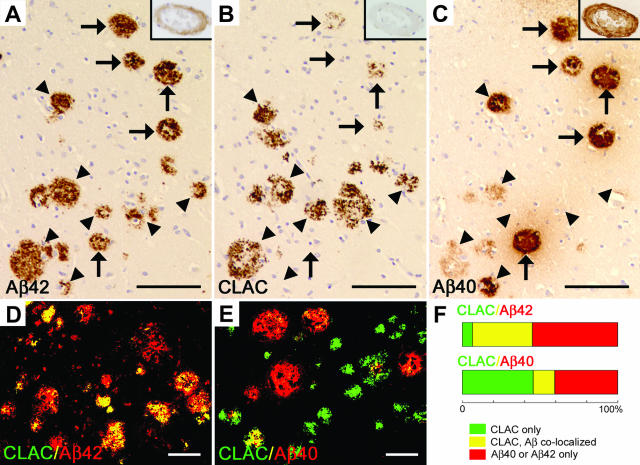
We have previously found that a subset of SP in the cerebral neocortex in AD, especially the primitive or neuritic types, is immunolabeled by anti-CLAC mAb 9D2 in a thick bundle- or coarse granule-like pattern.6 Notably, CLAC-IRs were always accompanied by Aβ-IRs. This observation prompted us to further examine the relationship between CLAC-IR and those of the major two Aβ C-terminal species, Aβ42 and Aβ40, which exhibit distinct distributions in AD brain.15,16 Comparison of three serial sections of frontal neocortex from sporadic AD cases immunolabeled for Aβ42, CLAC, and Aβ40, respectively, showed that most of the CLAC-positive SP was simultaneously positive for Aβ42 (Figure 1, A and B, arrowheads). In contrast, CLAC was often negative in Aβ40-positive amyloid deposits that are often uniformly and densely immunostained for Aβ40, including the cores of typical SP and blood vessels affected by cerebral amyloid angiopathy (Figure 1, B and C, arrows). Accordingly, the distribution pattern of Aβ42 overlaps and encompasses that of CLAC, whereas that of Aβ40 shows only a limited extent of overlap with that of CLAC. We then directly addressed the co-localization of CLAC and Aβ42 or Aβ40 by double immunofluorescence labeling combined with morphometric analysis. Approximately ∼43% of Aβ42-positive areas were CLAC-positive, and ∼93% of the CLAC-positive areas (CLAC burden) were simultaneously Aβ42-positive, only ∼7.2% remaining Aβ42-negative (Figure 1, D and F). In sharp contrast, only ∼26% of Aβ40-positive areas were CLAC-positive and ∼32% of CLAC-positive areas were Aβ40-positive (Figure 1, E and F). We further analyzed the relationship between CLAC and thioS-positive Aβ deposits, which are regarded to represent highly β-sheeted amyloid structures, by multiple fluorescence labeling. The number of thioS-positive SP in frontal neocortex was variable among AD cases. Numerous thioS-positive SP were observed (ie, detectable in every visual field of ∼3.4 mm2, occupying ∼5% of total areas) in ∼40% of the cases. Among these, only ∼9% of the thioS-positive area was CLAC-positive, and ∼7% of the CLAC-positive SP area was thioS-positive, showing minimal and even less extent of overlap compared to Aβ42/CLAC or Aβ40/CLAC (Figure 2, A to C). Comparison of Aβ40 and thioS reactivities, on serial mirror sections, showed that thioS-positive areas (Figure 2A, green) were always Aβ40-positive (Figure 2B, red), and accounted for ∼50% of the Aβ40-positive areas within the entire depth of cerebral cortex. Occasional SP showed co-localization of Aβ40 and CLAC, in which thioS was invariably negative (Figure 2, A and B, a SP in the lower left corner).
Figure 1.
Immunohistochemistry of Aβ42, CLAC and Aβ40 in the frontal neocortex of AD brain. A-C: Six-μm thick serial sections were immunostained with mAbs BC05 (Aβ42, A), 9D2 (CLAC, B), or BA27 (Aβ40, C). Arrowheads show Aβ42/CLAC-positive SP that are negative or only weakly positive for Aβ40, and arrows indicate Aβ40-positive SP that are negative or only weakly positive for CLAC. Insets show immunostaining of CAA in the subarchnoid space. Bar, 100 μm. D–E: Double immunofluorescence labeling of 50-μm thick floating sections for CLAC (anti-pyroGlu113, green) and Aβ42 (BC05, red) (D) or Aβ40 (BA27, red) (E), viewed by confocal microscopy. Bar, 50 μm (F). Relative ratios of percentage areas of SP that are CLAC-positive, Aβ42 or 40-negative (green), CLAC- and Aβ42 or 40-positive (yellow) or CLAC-negative, Aβ42 or 40-positive (red), in sections stained as in D (for Aβ42) or E (for Aβ40). Average levels in eight AD cases fixed in 10% formalin for 24 hours are shown. Note that the total SP areas (100%) correspond to those positive for CLAC plus Aβ42 in the upper bar, and CLAC plus Aβ40 in the lower bar, respectively.
Figure 2.
A–B: Six-μm thick, mirror-sectioned serial sections from frontal neocortex of AD brain doubly stained for CLAC (blue, anti-pyroGlu113) and thioS (green) (A) or for CLAC (blue, anti-pyroGlu113) and Aβ40 (red, BA27) (B). Note that most of the thioS-positive areas in A are overlapped and included within the Aβ40-positive areas in B, and occasional CLAC/Aβ40-positive SP (visualized in purple in B; eg, a SP in the lower left) are thioS-negative in A. Bar, 50 μm. C: Relative ratios of CLAC-positive, thioS-negative (blue), CLAC- and thioS-positive (light blue) and CLAC-negative, thioS-positive (green) SP areas in sections stained as in A (mean value in eight AD cases in which numerous thioS-positive SP were observed) are shown. The total areas (100%) correspond to those positive for CLAC plus thio-S.
Ultrastructural Characteristics of CLAC-Positive and -Negative Aβ Deposits in AD Brains
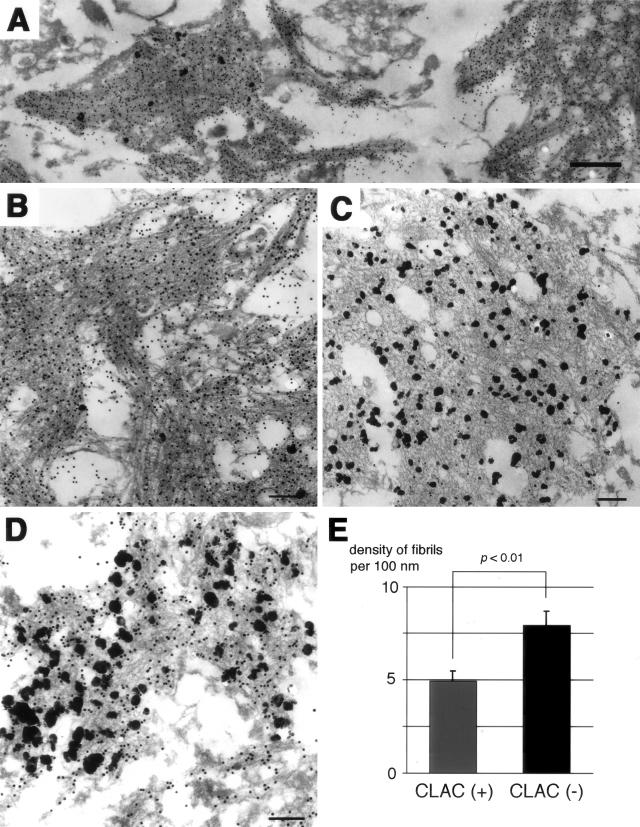
The distinct distribution patterns of CLAC- or Aβ40/thioS-positive SP led us to examine the ultrastructural characteristics of Aβ deposits that are positive or negative for CLAC. To rigorously compare the fine structure of CLAC-positive or negative structures in an identical manner, we used double immunoelectron microscopy, visualizing CLAC-IR by pre-embedding, 1-nm immunogold labeling/silver intensification, and Aβ40- or Aβ42-IRs by post-embedding with 10-nm gold particles, and compared their ultrastructures within an identical ultra-thin section. Because thioS-labeling cannot be applied to EM, we examined Aβ40-positive deposits at the core of dense SP, that are predicted to be thioS-positive at a light microscopic level, as representative areas of highly β-sheeted deposits. The latter type of deposit showed the typical morphology of amyloid fibrils, ie, dense bundles composed of relatively straight and smooth-surfaced filaments of ∼10 nm in diameter, that were intensely decorated by an anti-Aβ40 antibody (Figure 3, A and B). In contrast, CLAC-positive materials were apparently composed of fibrils, although they sometimes formed a mesh-like structure, in which individual fibrils were loosely packed, less electron dense and smaller in diameter, and distributed in rather random directions (Figure 3C). These CLAC-positive fibrils were strongly positive for Aβ42 (Figure 3D). We quantified the density of amyloid fibrils and found that CLAC-positive fibrils were more loosely distributed compared to CLAC-negative ones (4.9 ± 0.50 versus 7.9 ± 0.72 per 100-nm width in amyloid bundles) (Figure 3E).
Figure 3.
Double immunoelectron microscopic analysis of amyloid deposits in the frontal neocortices of AD brains. A–C, Aβ40 (post-embedding, BA27, 10 nm gold) and CLAC (pre-embedding, 9D2, 1 nm immunogold/silver intensification). All electron micrograms in A–C were taken at different areas on an identical ultra-thin section. D: Aβ42 (post-embedding, BC05, 10 nm gold) and CLAC (pre-embedding, 9D2, 1 nm immunogold/silver intensification). Bar: A, 500 nm; B–D, 200 nm. E: Densities of amyloid fibrils per 100 nm width within CLAC-positive and -negative amyloid bundles in B and C measured at 30 points (mean ± SE).
Chronological Relationships between CLAC and Aβ Deposition in Down Syndrome Brains
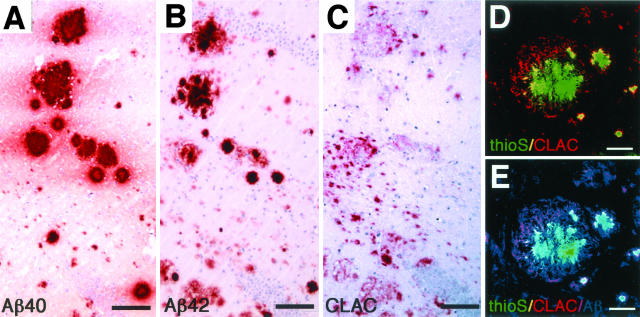
The pathological findings observed in autopsied AD brains were considered to represent an end-stage of AD pathology, lacking information regarding the temporal sequence of events in the development of AD pathology. To gain insight into the chronological and spatial relationships between CLAC deposition and those of Aβ40 and Aβ42, we studied the frontal neocortex of patients with Down syndrome (DS), dying at ages ranging from 31 to 71 years, by immunohistochemistry for Aβ42, Aβ40, and CLAC. Purely diffuse plaques, which predominate in the neocortex in young individuals with DS and which are known to be exclusively positive for Aβ42 (Figure 4A) but negative for Aβ40 (Figure 4G) (see also16), were CLAC-negative (Figure 4D). However, a subset of Aβ42-positive SP (Figure 4B), especially those of a primitive type, observed in DS individuals dying between ages of 35 and 50 years became CLAC-positive (Figure 4E), and the CLAC burden (percentage of CLAC-positive areas) gradually increased, showing a modest correlation with age (r2 = 0.395; blue dotted line in <50-year group) and amounting to ∼30 to 100% of the Aβ42-positive areas (Figure 4K); Aβ40 was only occasionally present (Figure 4H), especially in CLAC-negative SP. Accordingly, Aβ42-positive, CLAC-negative diffuse plaques and Aβ42/CLAC-positive primitive plaques accounted for most of the SP at this stage. After 50 years of age, the Aβ42 burden remained at a relatively constant level (Figure 4C), whereas those of Aβ40-positive and thioS-positive SP dramatically increased (Figure 4I), showing an age-related increase in Aβ40 levels (r2 = 0.614). In contrast, the CLAC burden remained at a relatively constant level (∼3% of total area) (Figure 4F and K). The overlap between CLAC-positive and Aβ40-positive areas was minimal in the neocortex of older (>50 years) individuals with DS, similar to that seen in the neocortex in patients with AD (Figure 4J).
Figure 4.
Immunohistochemistry of frontal neocortices from patients with Down syndrome for Aβ42 (BC05, A–C), CLAC (9D2, D–F), and Aβ40 (BA27, G–H). Three serial sections immunostained for Aβ42, CLAC, and Aβ40 from patients dying at the ages of 31 years (A, D, G), 44 years (B, E, H), and 58 years (C, F, I) are shown. J: Double immunofluorescence labeling of SP for CLAC (anti-pyroGlu113, green) and Aβ40 (BA27, red) in the frontal neocortex of a 58-year-old DS patient. Bar, 100 μm. K: Morphometry of the percentage of SP areas positive for Aβ42 (gray bar), CLAC (blue square) and Aβ40 (red diamond). The abscissa represents the age of the patients and the ordinate shows the percentage of immunoreactive areas. The dotted lines represent regression plots for Aβ42 (gray), Aβ40 (red), and CLAC (blue) in patients younger or older than 50 years of age, respectively.
Deposition of CLAC in the Brains of TG Mice Overexpressing Mutant PS and APP Genes
To examine if β-amyloid plaques that appear in the brains of TG mice overexpressing FAD mutant APP and PS genes are associated with endogenous CLAC, and if the separate distributions of CLAC- and Aβ40/thioS-positive areas are also present in TG brains, we immunostained the cerebrum and hippocampus of PSAPP TG mice. The huge core-like amyloid plaques, that are Aβ40-positive (as described previously18), and predominate at 3 to 6 months of age, were CLAC-negative (data not shown). After 12 months of age, in addition to the Aβ40-predominant huge-cored plaques (Figure 5A), small-sized diffuse deposits, positive for Aβ42 but not for Aβ40, appeared in the periphery of the core-like huge plaques or in the neuropil of the surrounding areas, most prominently in the molecular layer of the dentate gyrus of hippocampus (Figure 5B); these were immunoreactive for CLAC (Figure 5, C to E). Thus, the predilection for CLAC to deposit in Aβ40/thioS-negative Aβ plaques was also seen in the brains of TG mice in which Aβ deposition is highly accelerated.
Figure 5.
Immunohistochemisty of the hilar region of dentate gyrus of the hippocampus in PSAPP TG mouse (19-month-old). Six-μm thick serial sections were immunostained for Aβ40 (BA27, A), Aβ42 (BC05, B), and CLAC (anti-NC2–2, C), and then counterstained with hematoxylin. Bar, 100 μm. D–E: Triple immunofluorescence labeling of cored plaques and surrounding diffuse deposits for thioS, CLAC, and Aβ. ThioS-positive core (green) and CLAC-positive deposits in the surrounding areas (red) are shown (D). E: Superimposition of immunoreactivity for human Aβ (blue, BAN50) shows that areas positive for CLAC (purple) or thioS (light green) are Aβ-positive. Bar, 50 μm.
Discussion
In this present study, we have examined Aβ plaques in the cerebral cortices of humans with AD and DS, and in the brains of PSAPP TG mice, by immunohistochemistry for CLAC, a novel neuron-specific collagen and a non-Aβ amyloid plaque protein, as well as for the two major C-terminal variants of Aβ, ie, Aβ40 and Aβ42. We have shown the following: CLAC exhibits a mostly separate distribution from that of Aβ40, and especially of the thioS-positive amyloid; CLAC is associated with Aβ42-positive deposits comprised of a loosely packed, fibrillar form of Aβ, but not with pure diffuse plaques; CLAC deposition precedes that of Aβ40 and shows a mutually exclusive distribution to each other in DS; and that CLAC appears later than the Aβ40-rich, huge-cored plaques in the brains of PSAPP TG mice, but again exhibits a distinct distribution from that of Aβ40/thioS. These findings illustrate the presence of two different populations of Aβ deposits in brains, suggesting that binding of CLAC to amyloid may play a role in the determination of the two distinct maturation pathways of β-amyloid deposits.
It is well recognized that Aβ42 has a higher propensity to aggregate in vitro14 and accumulates earlier than Aβ40 in human brains.15,16 However, it has remained unknown why a significant proportion of Aβ42-positive SP remain negative for Aβ40 and/or thioS, despite the in vitro results that once a “seed” for β-amyloid is initially formed, a rapid incorporation of Aβ (either Aβ40 or Aβ42) follows.14 Our observation that CLAC- and Aβ42-positive SP show a mostly separate distribution from that of Aβ40/thioS-positive SP suggests that binding of CLAC may play a mechanistic role in the evolution of SP, possibly by inhibiting incorporation of Aβ40, abundantly secreted from neurons,23 thereby precluding the formation of dense, typical amyloid deposits that are positively labeled by amyloid-sensitive dyes like thioS.19 The reason why CLAC preferentially binds to a specific subset of SP, especially Aβ42-positive, Aβ40-negative primitive type SP, but not to more typical thioS-positive amyloid or to early diffuse plaques composed of amorphous aggregates of Aβ,24 is unknown. Our immunoelectron microscopic observations that CLAC- and Aβ42-positive deposits in AD brains exhibited the morphology of fibrils, although they were less typical compared to the Aβ40/thioS-positive ones, suggest that CLAC preferentially binds to a fibrillar form of Aβ that has some structural characteristics in its β-sheet structure, which does not allow the binding of thioS. Alternatively, it is possible that once CLAC is bound to Aβ fibrils, the binding site(s) for thioS is occupied, rendering the fibrils thioS-negative. In either case, CLAC binding may block the binding site on Aβ fibrils for further incorporation of Aβ1–40 and inhibit growth of amyloid fibrils. Indeed, our preliminary in vitro experiments suggest that co-incubation of recombinant CLAC with synthetic Aβ peptides inhibits the formation of Aβ fibrils (Nishimura A, Hashimoto T, Iwatsubo T, unpublished observation), supporting the assumption that binding of CLAC plays a “protective” role against deposition of Aβ.
Our observation on a series of DS individuals dying at various ages further substantiates this hypothesis. Based on the findings in young DS persons, as well as in non-demented aged individuals, it has been widely believed that one of the major forms of early Aβ deposition in human brains are diffuse plaques that are composed of amorphous, non-fibrillar aggregates of Aβ, especially of Aβ42 species.16 Further accumulation/removal of Aβ may gradually remodel the plaques into primitive or classical types comprised of a more fibrillar form of Aβ. The pure diffuse plaques in young DS brains were negative for CLAC, whereas SP readily became CLAC-positive as they acquired the morphology of primitive SP during the period between 30 to 50 years of age. Aβ40/thioS-positive SP subsequently appeared, forming a distinct population from CLAC-positive SP. Such Aβ40-positive SP dramatically increased after 50 years of age, whereas the level of CLAC-positive, Aβ40-negative SP remained constant and these SP did not appear to develop into Aβ40/thioS-positive SP. A most plausible interpretation of this chronological progression would be that after 50 years of age, some factor in the extracellular space of DS brains is altered in such a way as to lower the threshold for the incorporation of Aβ1–40 onto the amyloid seeds, without allowing CLAC to bind and intervene in the rapid formation of Aβ40-rich amyloid. In sharp contrast, Aβ40-predominant giant-cored plaques appeared early in the brains of PSAPP TG mice, with Aβ42- and CLAC-positive small, diffuse deposits only appearing at a later stage. This may be because the level of overproduction of Aβ (1–40 as well as 1–42) in the brains of TG mice is so high as to allow the rapid formation of core-like seeds, as well as the incorporation of Aβ1–40 into amyloid plaques at an early stage, and that the diffuse deposits that grow slower in areas “resistant” to rapid amyloid deposition “attract” endogenous murine CLAC. In this regard, it is interesting to note that amyloid plaques in PDAPP TG mice were negative for immunostaining with the AMY117 antibody, although the immunostaining patterns in AD and DS brains (eg, precedence of Aβ42 deposition as diffuse plaques to the appearance of AMY-IR, absence in vascular amyloid deposits) were very similar.9 The reason for this discrepancy is unknown at present. However, our preliminary results in different lines of APP TG mice (including PDAPP mice; unpublished observations) suggested that a small amount of endogenous murine CLAC deposits in amyloid plaques irrespective of the type of APP transgenic mice. Although it has been clearly shown that AMY antigen is identical to CLAC,7 the sensitivity and specificity of AMY antibodies may be somewhat different from those of our CLAC antibodies.
It will be highly informative to produce TG mice overexpressing human CLAC-P and to cross these with others expressing human βAPP, to determine the precise mechanistic role of CLAC in the formation of amyloid plaques in vivo. If the appearance of Aβ40/thioS-positive plaques is delayed and CLAC/Aβ42-positive “primitive” plaques similar to those seen in human AD and DS brains are increased in the double TG mice, the in vivo “protective” effect of CLAC binding against formation of dense, classical amyloid plaques will be confirmed. This may then pave the way toward therapeutics for AD through the modulation of CLAC.
Acknowledgments
We thank Haruyasu Yamaguchi for valuable suggestions on immunoelectron microscopic analysis, Takaomi C. Saido for anti-Aβ(pyroGlu3), John Q. Trojanowski, Virginia M.-Y. Lee, Jan Naslund, Yoshihide Osada, and Akiko Nishimura for helpful discussions, and Takeda Chemical Industries for the monoclonal antibodies to the C terminus of Aβ.
Footnotes
Address reprint requests to Takeshi Iwatsubo, M.D., Department of Neuropathology and Neuroscience, Graduate School of Pharmaceutical Sciences, University of Tokyo 7–3-1 Bunkyoku Hongo Tokyo 113-0033, Japan. E-mail: iwatsubo@mol.f.u-tokyo.ac.jp.
Supported by Grants-in-Aid from the Ministry of Education, Science, Culture and Sports for the 21st Century Center of Excellence Programme and by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research, Japan.
References
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid β-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol (Berl) 2000;100:451–458. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- Nilsson LN, Bales KR, DiCarlo G, Gordon MN, Morgan D, Paul SM, Potter H. α-1-antichymotrypsin promotes β-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, O’Dell MA, Parsadanian M, Taylor JW, Harmony JA, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DMA, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg L, Zhukareva V, Bogdanovic N, Hashimoto T, Winblad B, Iwatsubo T, Lee VM-Y, Trojanowski JQ, Naslund J. Molecular identification of AMY, and Alzheimer disease amyloid-associated protein. J Neuropathol Exp Neurol. 2003;62:1108–1117. doi: 10.1093/jnen/62.11.1108. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Lee VM, Forman M, Chiu TS, Trojanowski JQ. Monoclonal antibodies to a 100-kd protein reveal abundant Aβ-negative plaques throughout gray matter of Alzheimer’s disease brains. Am J Pathol. 1997;151:69–80. [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Grenfell TJ, Selkoe DJ. The AMY antigen co-occurs with Aβ and follows its deposition in the amyloid plaques of Alzheimer’s disease and Down’s syndrome. Am J Pathol. 1999;155:29–37. doi: 10.1016/s0002-9440(10)65095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Hyman BT, Greenberg SM, Rebeck GW. Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Aβ aggregation. J Neuropathol Exp Neurol. 2001;60:342–349. doi: 10.1093/jnen/60.4.342. [DOI] [PubMed] [Google Scholar]
- Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ, Lemere CA. Temporal accrual of complement proteins in amyloid plaques in Down’s syndrome with Alzheimer’s disease. Am J Pathol. 2000;156:489–499. doi: 10.1016/S0002-9440(10)64753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, Otte-Holler I, David G, Maat-Schieman ML, van den Heuvel LP, Wesseling P, de Waal RM, Verbeek MM. Heparan sulfate proteoglycan expression in cerebrovascular amyloid β deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol (Berl) 2001;102:604–614. doi: 10.1007/s004010100414. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ-monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Mann DMA, Odaka A, Suzuki N, Ihara Y. Amyloid β protein (Aβ) deposition: aβ42(43) precedes Aβ40 in Down syndrome. Ann Neurol. 1995;37:294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DMA, Hyman BT, Iwatsubo T. Age-related amyloid β deposition in transgenic mice overexpressing both presenilin 1 and amyloid β precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer’s disease: a double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132:86–101. [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Robinson KA, Lee VM, Trojanowski JQ. Chemical and immunological heterogeneity of fibrillar amyloid in plaques of Alzheimer’s disease and Down’s syndrome brains revealed by confocal microscopy. Am J Pathol. 1995;147:503–515. [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Maat-Schieman ML, van Duinen SG, Prins FA, Neeskens P, Natte R, Roos RA. Amyloid β protein (Aβ) starts to deposit as plasma membrane-bound form in diffuse plaques of brains from hereditary cerebral hemorrhage with amyloidosis-Dutch type, Alzheimer disease, and non-demented aged subjects. J Neuropathol Exp Neurol. 2000;59:723–732. doi: 10.1093/jnen/59.8.723. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Tomita T, Matsunaga H, Ishibashi Y, Saido TC, Iwatsubo T. Primary cultures of neuronal and non-neuronal rat brain cells secrete similar proportions of amyloid β peptides ending at Aβ40 and Aβ42. Neuroreport. 1999;10:2965–2969. doi: 10.1097/00001756-199909290-00017. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Nakazato Y, Shoji M, Takatama M, Hirai S. Ultrastructure of diffuse plaques in senile dementia of the Alzheimer type : comparison with primitive plaques. Acta Neuropathol (Berl) 1991;82:13–20. doi: 10.1007/BF00310918. [DOI] [PubMed] [Google Scholar]