Abstract
The accumulation of macrophage foam cells in atherosclerotic lesions is associated with both initiation and progression of this disease. Scavenger receptors CD36 and SRA are the primary receptors responsible for conversion of macrophages into foam cells. Integrin αVβ3 plays a role in the differentiation of several cell types, but its involvement in the transition of macrophages into foam cells and the potential role of this receptor in atherosclerosis have not been examined. Using an in vitro model of single surface receptor activation by binding with an immobilized monoclonal antibody specific to αVβ3 integrin we show that ligation of αVβ3 integrin prevents differentiation of blood monocytes and macrophages into the foam cell phenotype via coordinate down-regulation of CD36 and SRA. This effect of αVβ3 integrin ligation can be reproduced by contact with endothelial cells, whereas the inhibition of αVβ3 receptor ligation restores the uptake of oxidized low-density lipoprotein. Moreover, we found that αVβ3 integrin is readily detected in situ on macrophages in early and advanced atherosclerotic lesions and that in vitro exposure to oxidized low-density lipoprotein up-regulates αVβ3 integrin expression. We hypothesize that αVβ3 integrin regulates macrophage functional maturation into foam cells in a persistent manner, and therefore, by targeting αVβ3 receptor it could potentially be possible to regulate progression of atherosclerosis in humans.
Animal studies, as well as cell culture experiments and studies of human tissue samples, support the view that the initiation and progression of atherosclerosis represents a response of monocytes and macrophages to the accumulation and modification of lipoproteins in the arterial intima.1–7 The recruitment of blood monocytes into the arterial wall, followed by their differentiation into tissue macrophages, is a key process in atherosclerosis.6–10 During this differentiation, macrophages acquire the ability to take up oxidized low-density lipoprotein (oxLDL) by scavenger receptor pathways. This unrestricted uptake, which is not limited by intracellular cholesterol levels, eventually leads to the formation of lipid-filled foam cells, the hallmark of atherosclerosis.4,6,10–13 Of the many cell-surface proteins described as scavenger receptors, the class A type I and II macrophage scavenger receptors (SRA) and CD36 are thought to be the major receptors involved in foam cell formation, mediating the influx of lipids into the macrophages12,14–20 and play a key role in atherosclerosis by regulating fundamental macrophage functions. Identification and characterization of novel genes regulating the scavenger receptor-dependent macrophage functions and the transition of macrophages into foam cells, particularly genes that persistently control scavenger receptor activity, could be crucial in deciphering the mechanisms of atherosclerosis. However, to date, little is known regarding regulation of scavenger receptors by specific receptor-mediated signaling pathways and ligands.
The αVβ3 integrin (CD51/CD61) is a ubiquitous receptor that is expressed on a variety of cell types and interacts with ligands present in extracellular matrix or expressed on the cell surface. As a consequence, this integrin plays a role in diverse biological processes.21–25 Consistent with its expression profile in vivo, αVβ3 integrin is thought to play a key role in the initiation or progression of several human diseases, including osteoporosis, rheumatoid arthritis, cancer, and ocular diseases, as well as restenosis of arteries after angioplasty.24,26,27 As a result, αVβ3 antagonists may be expected to provide an approach for the treatment of these conditions, and some are currently in clinical trials.26,27 However, αVβ3 integrin has received little attention as a potential contributor to atherosclerosis, and possible effects of αVβ3 integrin antagonists on macrophage functions have not been taken into consideration. Monocyte and macrophage functions are known to be profoundly influenced by adherence.28,29 We have previously shown that adherence of monocyte-derived macrophages (MDMs) to endothelial cells (ECs) regulates proliferation of the MDMs in response to the cytokine M-CSF.30 We therefore asked whether adhesion molecules also regulate the process of differentiation of monocytes into foam cells.
We now demonstrate that αVβ3 integrin is consistently detected on the macrophages in early and advanced human atherosclerotic lesions, and that its expression is up-regulated by atherogenic stimuli (oxLDL, M-CSF) in vitro. Ligation of αVβ3 receptor on human blood monocytes and differentiated MDMs results in a sustained phenotypic change characterized by persistent down-regulation of multiple scavenger receptors and resultant lipid accumulation. We postulate that the signaling pathways initiated by αVβ3 integrin ligation play an important role in progression of atherosclerosis by controlling macrophage CD36- and SRA-dependent responses in the vessel wall.
Materials and Methods
Antibodies and Reagents
Monoclonal antibodies (mAbs) to αVβ3 (LM609) and αVβ5 (P1F6) integrins were from Chemicon International (Temecula, CA), primary mAbs against macrophages (HAM56 or CD68) were from DAKO Corp. (Carpinteria, CA), anti-human smooth muscle cell (SMC) mAb (HHF35) was from Enzo Life Sciences, Inc. Isotype control IgG and fluorescein isothiocyanate (FITC)-conjugated IgG1 were from Pharmingen (San Diego, CA). A cocktail of FITC-conjugated mAbs to CD11b, CD11c (Caltag Laboratories, Burlingame, CA), CD15 (Pharmingen) was used for MDM identification. FITC-conjugated mAb to human CD36 (FA6–152) was from Immunotech (Marseille, France). Human recombinant M-CSF (1.9 × 10 U/ml by bone marrow assay) was a gift from Genetics Institute, Inc. (Cambridge, MA). Cyclic RGD was a gift from Merck KGaA (Darmstadt, Germany). Human lipoproteins were purchased from Intracel (Rockville, MD) or Biomedical Technologies (Stoughton, MA). LY294002 and SB203580 were purchased from Biomol Research Laboratories (Plymouth, PA), PD98059 from New England Biolabs (Beverly, MA), and wortmannin from Calbiochem (San Diego, CA).
Leukocyte Isolation and in Vitro Model of αVβ3 Ligation
Human monocytes were obtained from 20 healthy donors of either sex (24 to 38 years old) by leukocytopheresis followed by counterflow centrifugation as described30 under a protocol approved by our Institutional Review Board. These cells (>95% monocytes by morphology and cell surface markers) were either used immediately or cryopreserved in liquid nitrogen. Fresh and frozen monocytes behaved indistinguishably in all assays. Peripheral blood monocytes and MDMs were used. To obtain MDMs, monocytes were differentiated in vitro for 5 to 9 days in the presence of M-CSF (200 U/ml) as described.30 M-CSF is important in maintaining long-term survival of MDMs,31,32 has been detected in atherosclerotic lesions33 and has been found to enhance macrophage scavenger receptor functions and αVβ3 expression on MDMs in vitro.34,35 For αVβ3 ligation, MDMs were harvested on ice and reseeded on mAbs immobilized on plastic dishes. Antibodies were immobilized on substrate by incubating dishes with 10 μg/ml of mAbs in phosphate-buffered saline (PBS) for 18 hours at 4°C. Before seeding, dishes were blocked for 40 minutes with 20% fetal bovine serum in M199. When the effects of αVβ3 ligation on monocyte differentiation were of interest, monocytes were seeded directly on immobilized mAbs and were cultured in the same conditions for 1 to 7 days before analysis.
Analysis of Modified LDL Uptake, Intracellular Lipid Accumulation, and CD36 Expression by Flow Cytometry
For lipoprotein uptake measurement, monocytes cultured on immobilized mAbs or reseeded MDMs were incubated with DiI-labeled oxLDL (DiI-oxLDL) (5 μg/ml) or DiI-labeled acetylated LDL (DiI-acLDL) (5 μg/ml) for 3 hours at 37°C, harvested on ice, fixed with 1% formaldehyde, and analyzed by flow cytometry. To examine lipid accumulation in cholesterol storage vacuoles induced by lipoproteins, cells were incubated with oxLDL (50 μg/ml) or ac-LDL (100 μg/ml) for 48 to 78 hours and harvested cells were stained with the lipophilic probe Nile Red36 before analysis by flow cytometry. To examine CD36 surface expression (or αVβ3 integrin expression) cells were harvested mechanically and stained in suspension with FITC-conjugated primary mAbs or FITC-conjugated isotype-matched IgG1 for 40 minutes at 4°C in 0.2% bovine serum albumin/PBS before analysis by flow cytometry. Where CD36 expression and oxLDL uptake by the same cells were of interest, cells were first incubated with DiI-oxLDL and then stained with anti-CD36 mAb as described above.
Involvement of PI3-Kinase/Akt Signaling Pathway
To elucidate which signaling pathways may be involved in the regulation of CD36 expression and oxLDL uptake, MDMs were harvested, preincubated for 20 minutes at 37°C in suspension with the PI3-kinase inhibitors LY294002 (10 μmol/L) or wortmannin (100 nmol/L), p38SAPK inhibitor (SB203580, 20 μmol/L), ERK-1,2 inhibitor (PD98059, 20 μmol/L), or control vehicle (dimethyl sulfoxide), and then MDMs were reseeded on immobilized mAbs in the presence of inhibitors for 6 hours. MDMs were then incubated with DiI-oxLDL for 3 more hours, and DiI-oxLDL uptake was analyzed by flow cytometry. For CD36 detection, MDMs reseeded on anti-αVβ3 integrin mAb or control IgG1 were analyzed by flow cytometry using FITC-labeled mAb to CD36 or FITC-conjugated isotype control antibody.
EC and Monocyte Co-Culture Model
Human umbilical vein ECs were isolated and co-cultured with monocytes as previously described.30 To examine the effect of adhesion of MDMs to ECs on oxLDL-inducible foam cell formation, monocytes were co-cultured with ECs for 5 days, then EC monolayers were mechanically wounded to allow floating MDMs generated by proliferation in co-culture to adhere to denuded areas. Then co-cultures were exposed to oxLDL (50 μg/ml) for 48 to 72 hours, fixed with buffered formaldehyde, and then stained for lipids with Oil Red O and counterstained with hematoxylin. To examine oxLDL uptake by MDMs both adherent to ECs and floating, as well as the effect of αVβ3 inhibitor cRGD on DiI-oxLDL uptake by these cells, monocytes were co-cultured with ECs for 5 to 7 days (which induced their proliferation and generated significant numbers of MDMs both floating and adherent to ECs30). Co-cultures exposed to cRGD (10 μg/ml) for 24 hours or control co-cultures without cRGD treatment were incubated with DiI-oxLDL (5 μg/ml) for 3 hours. Floating and loosely adherent MDMs were harvested from ECs by gentle pipetting; the remaining cells were then harvested by trypsinization. Cells were stained with a cocktail of FITC-labeled mAbs to the MDM lineage markers CD11b, CD11c, CD15, or FITC-labeled isotype-matched control mAb for 40 minutes at 4°C in 0.2% bovine serum albumin/PBS, washed with PBS, fixed in formaldehyde, and analyzed by flow cytometry for DiI-oxLDL uptake by two separate MDM populations.
Immunohistochemistry
Hearts and aortas (thoracic and abdominal) of patients who died suddenly of coronary causes were obtained as described previously.37 Atherosclerotic plaques were classified using a modification of the American Heart Association Scheme.38 Serial cryostat sections (6 μm) from advanced lesions of human coronary arteries identified as fibroatheromas (lesions with relativity thick fibrous cap and necrotic lipid core) or early lipid lesions (fatty streak) from aortas were randomly selected from seven and three patients, respectively, and used for immunohistochemical studies according to a standard protocol for cryostat tissue sections. The labeling of primary antibodies was achieved using a biotinylated link antibody and positive staining was visualized using the 3-amino-9-ethylcarbazole substrate-chromogen system; the sections were counterstained with Gill’s hematoxylin. To demonstrate expression of αVβ3 integrin on macrophages, co-localization studies were performed. Anti-αVβ3 mAb was labeled with a biotinylated link secondary antibody and visualized with the fluorescent marker Alexa Fluor 488 (Molecular Probes, Eugene, OR) conjugated to streptavidin. After staining for αVβ3 integrin, sections were rinsed in PBS and incubated with anti-macrophage mAb CD68 tagged with Alexa Fluor 568 using a Zeon labeling kit according to the manufacturer’s instructions (Molecular Probes). Sections were counterstained with 4,6-diamidino-2-phenylindole and examined using a Leica TCS SP2 spectral confocal imaging system (Leica, Heidelberg, Germany).
Quantitative Real-Time Polymerase Chain Reaction
MDMs differentiated for 5 days in culture were reseeded on immobilized anti-αVβ3 integrin mAb or immobilized control IgG1 mAb for 24 hours, and total RNA was isolated using Qiagen RNeasy mini kit according to the manufacturer’s instructions. For quantitative real-time reverse transcriptase-polymerase chain reaction analysis of CD36 and SRA expression, total RNA was reverse-transcribed and amplified by LightCycler System (Roche Molecular Biochemicals) using primers for CD36, forward primer 5′-GAG AAC TGT TAT GGG GCT AT-3′ and reverse primer 5′-TTC AAC TGG AGA GGC AAA GG-3; and for SRA, 5′-CCA GGG ACA TGG GAA TGC AA-3′ and 5′-CCA GTG GGA CCT CGA TCT CC-3′. β-Actin-specific primers were used as control. Specific RNA levels were quantified using the manufacturer’s software and compared to standard curves of control preparations of MDMs RNA.
Reproducibility of Data
Experiments were repeated with monocytes isolated from at least five different donors with consistent results.
Results
Expression of αVβ3 Integrin on Macrophages in Human Atherosclerotic Lesions
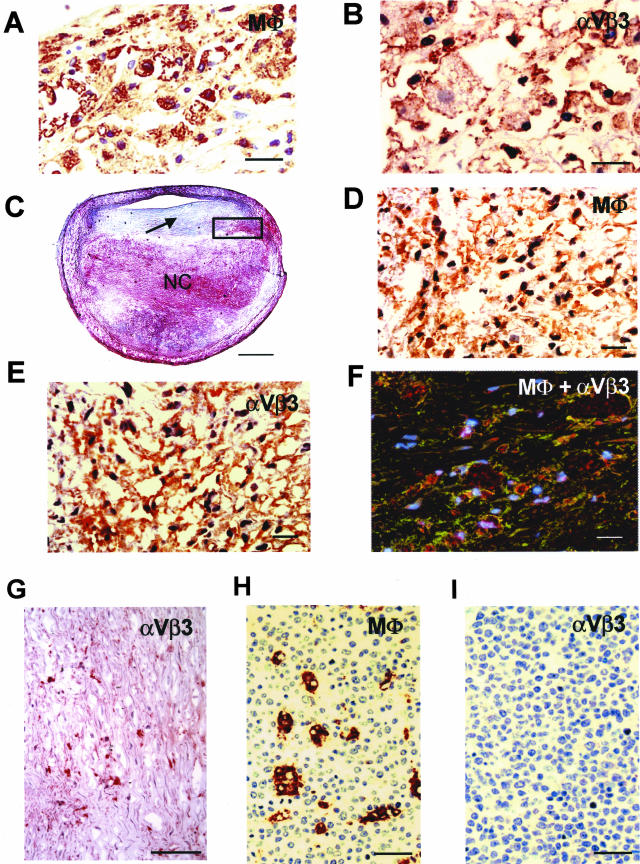
We initially investigated αVβ3 integrin expression on macrophages present in human early fatty streak lesions and advanced plaques. We found that resident macrophages, including typical macrophage foam cells, accumulated in the subendothelial compartment of early lipid (fatty streak) lesions demonstrate high levels of αVβ3 expression (Figure 1, A and B). In human coronary lesions (fibroatheromas), strongly positive cells that expressed αVβ3 integrin were consistently found in the perimeter of the necrotic core and shoulder of advanced plaques in areas rich in CD68-positive macrophages (Figure 1; C, D, and E). Using two-color staining for αVβ3 integrin and the macrophage lineage marker CD68, those cells were identified as macrophages (Figure 1F). We also found that some of the SMCs within the plaque showed positive staining for αVβ3 integrin (Figure 1G), but the vast majority of SMCs in the lesions examined was αVβ3 integrin-negative, whereas SMCs of the medial wall demonstrated mild to moderate reactivity to αVβ3 integrin staining (data not shown). Immunostaining for αVβ3 integrin was also noted in ECs localized to the lumen and in occasional microvessels of the plaque (data not shown). Importantly, αVβ3 integrin expression is not a constitutive feature of all tissue macrophages. In contrast with macrophages in atherosclerotic lesions, macrophages located in uninvolved tissues such as tonsils (Figure 1H, HAM56-positive cells) do not express αVβ3 integrin (Figure 1I). Thus, to the best of our knowledge, here we demonstrate for the first time that αVβ3 integrin is expressed on activated macrophages in both fatty streak and more advanced fibroatheromas, suggesting that this receptor could be involved in lesion development and progression. For this reason, and because lesion SMCs showed little αVβ3 integrin expression, subsequent studies were focused on macrophage αVβ3 integrin.
Figure 1.
Expression of αVβ3 integrin on macrophages in a human atherosclerotic lesion. A: Accumulation of HAM-56-positive macrophages in early lipid lesion. B: Macrophages in the same lesion show strong positive staining for αVβ3 integrin expression (brownish-red reaction product). C: Low-power view of a human atherosclerotic plaque (fibroatheroma) from mid left anterior descending coronary artery. There is a large necrotic core (NC) and relatively thick fibrous cap (arrow). D: High-power view of the black box represented in C demonstrating numerous CD68-positive macrophages in the perimeter of the necrotic core. E: Positive staining for αVβ3 integrin expression on macrophages in a similar area as in D. F: Image from confocal microscopy showing co-localization of macrophages (red) and αVβ3 integrin (green) in the perimeter of the necrotic core. Regions of overlap appear yellow. The nuclei (blue) are stained with 4,6-diamidino-2-phenylindole. G: Immunostaining for αVβ3 integrin expression on SMCs within the fibrous cap of this lesion shows a relatively low number of αVβ3-positive SMCs. H: HAM-56-positive macrophages in tonsil. I: Absence of staining for αVβ3 integrin in macrophage in tonsil. Scale bars: 20 μm (A, B, D–F), 1 mm (C), 100 μm (G), 29 μm (H and I).
Effect of M-CSF and oxLDL on αVβ3 Expression on MDMs in Vitro
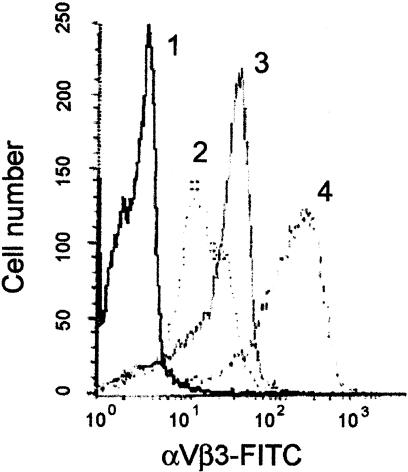
We next determined whether αVβ3 integrin expression on MDMs is regulated in vitro by M-CSF and oxLDL, which are known to be present in lesions in vivo.2–4,10,31,32 We found that freshly isolated monocytes express a clearly detectable level of αVβ3 integrin (Figure 2, line 2). When monocytes were differentiated in the presence of M-CSF for 5 to 7 days they demonstrate a significant increase in surface αVβ3 expression (Figure 2, compare lines 2 and 3). Treatment of these MDMs with oxLDL (20 μg/ml) results in rapid (6 to 8 hours) and sustained (up to 72 hours) up-regulation of even this high level of αVβ3 expression (Figure 2, line 4). Thus, when we mimic conditions existing in atherosclerotic lesions using our cell culture model, MDMs demonstrate significant increases of αVβ3 integrin expression, which we speculate correlates with our findings in vivo, suggesting that exposure of lesion macrophages to atherogenic oxLDL could lead to observed αVβ3 expression on lesion macrophages.
Figure 2.
M-CSF and oxLDL synergistically up-regulate αVβ3 integrin surface expression on MDMs. Differentiation of freshly isolated blood monocytes in the presence of M-CSF for 6 days results in increases in αVβ3 expression on MDMs (compare lines 2 and 3). Treatment of these MDMs with oxLDL (20 μg/ml) for 24 hours significantly up-regulates αVβ3 integrin expression (line 4). Monocytes and harvested MDMs were stained for αVβ3 integrin surface expression with FITC-labeled anti-αVβ3 integrin mAb or FITC-labeled isotype-matched control IgG1 (line 1) and analyzed by flow cytometry (refer to Materials and Methods).
Effect of αVβ3 Ligation on Scavenger Receptor Activity and Foam Cell Formation
Ligation of αVβ3 Integrin on Blood Monocytes
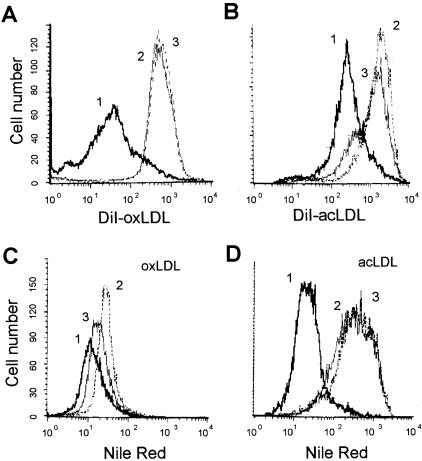
We next examined the effects of αVβ3 ligation on blood monocyte phenotype when they were differentiated in vitro on immobilized antibodies to αVβ3 integrin. As functional measures for scavenger receptor activity, we analyzed the effect of αVβ3 ligation on uptake of DiI-oxLDL and DiI-acLDL, and on lipid accumulation induced by exposure to these modified LDLs. Under these conditions, cells differentiated on αVβ3 integrin mAb had persistently (from day 3 up to day 7) reduced DiI-oxLDL and DiI-acLDL uptake (Figure 3, A and B) and lipid accumulation induced by modified LDL (Figure 3, C and D) as compared with control (immobilized isotype-matched IgG1) antibody, or antibody to the closely related αVβ5 integrin. Because adhesion to all three antibodies was comparable, the effect could not be attributed to adhesion alone, suggesting that these effects are specific for αVβ3 integrin. Thus, ligation of αVβ3 integrin on blood monocytes results in their differentiation into a sustained phenotype characterized by decreased uptake of modified LDL and resultant lipid accumulation. From these data (Figure 2 and Figure 3), we hypothesize that a feedback mechanism leading from oxLDL to αVβ3 expression and down-regulation of scavenger receptor activity is important in foam cell formation.
Figure 3.
Differentiation of blood monocytes on immobilized anti-αVβ3 integrin mAb results in down-regulation of DiI-oxLDL and DiI-acLDL uptake and resultant lipid accumulation induced by modified LDL. Blood monocytes were seeded directly on immobilized anti-αVβ3 integrin mAb (thick line), anti-αVβ5 integrin mAb (thin line), or control isotype-matched IgG1 (dotted line) and were cultured for 7 days. On day 7 MDMs were incubated with 5 μg/ml of DiI-oxLDL (A) or 5 μg/ml of DiI-acLDL (B) for 3 hours. To induce lipid accumulation in cholesterol storage vacuoles, MDMs were exposed to 50 μg/ml of oxLDL (C) or 100 μg/ml of acLDL (D) for 48 hours (from day 5 to day 7). At the end of the treatment cells were harvested, stained for intracellular lipids with Nile Red (C and D only), and analyzed by flow cytometry.
Ligation of αVβ3 Integrin on Differentiated MDMs
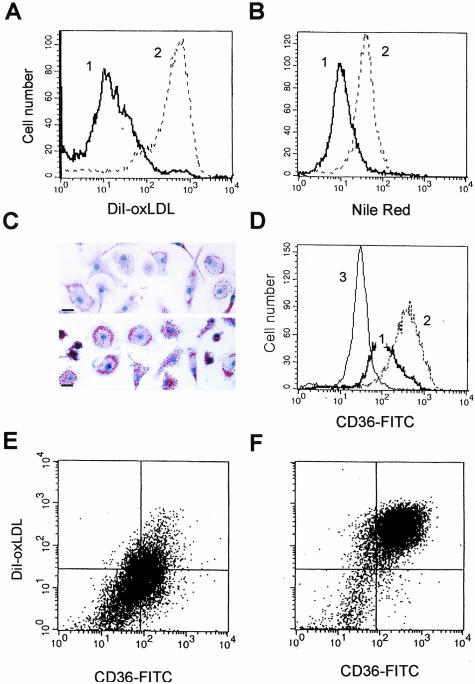
Scavenger receptor activity on terminally differentiated macrophages is considered not to be down-regulated by physiological stimuli in persistent manner. We therefore next examined whether ligation of αVβ3 integrin could regulate CD36 and SRA activity on macrophages after they had differentiated in vitro and acquired a phenotype characterized by constitutive and unregulated high levels of oxLDL and acLDL uptake. More than 95% of these MDMs can be transformed into lipid-laden foam cells by exposure to modified LDL,12,13,39,40 so this primary human monocyte-derived macrophage system is a useful and well-characterized in vitro model of atherogenic foam cell formation. Monocytes were differentiated in culture for 5 to 9 days, harvested, and reseeded on anti-integrin antibodies or on control antibody immobilized on substrate. We found that MDMs reseeded on αVβ3 integrin antibodies took up 5- to 10-fold less DiI-oxLDL than MDMs reseeded on control IgG1 (Figure 4A) or on antibodies to αVβ5 integrin (data not shown). Lipid accumulation was correspondingly reduced in cells exposed to αVβ3 integrin ligation. When MDMs reseeded on αVβ3 integrin antibodies were incubated with oxLDL (50 μg/ml) for 48 to 72 hours, they demonstrated a greater than fourfold reduction in intracellular cholesterol accumulation (Figure 4B). Lipid accumulation, as viewed by Oil Red O staining, was also visibly reduced by αVβ3 ligation (Figure 4C). The reduction in DiI-oxLDL uptake appeared receptor-mediated. We found that ligation of αVβ3 integrin on MDMs resulted in more than fourfold down-regulation of surface expression of CD36 as measured by flow cytometry (Figure 4D). Two-color staining with FITC-labeled antibodies to CD36 and DiI-oxLDL of MDMs reseeded on αVβ3 integrin mAb (Figure 4E) or control IgG1 (Figure 4F) further supported this hypothesis, demonstrating that decreased CD36 expression correlated with a significant reduction of DiI-oxLDL uptake by the same cells. Using quantitative real-time reverse transcriptase-polymerase chain reaction, we found that ligation of αVβ3 integrin on MDMs caused a twofold to ninefold reduction in the amount of CD36 mRNA (n = 6), and a threefold to fivefold fold reduction of SRA mRNA (n = 5), suggesting that down-regulation of CD36 and SRA protein expression by αVβ3 integrin ligation occurred at the transcriptional level. The effect of αVβ3 integrin ligation on DiI-oxLDL uptake and CD36 expression was persistent and observed as early as 4 to 6 hours after reseeding, and continued for up to 4 days (observation time). Ligation of αVβ3 integrin on differentiated MDMs, as well as on blood monocytes, had an identical effect on both CD36 and SRA activity, consistent with coordinate regulation of CD36 and SRA expression by αVβ3 integrin. Although donor variability was evident, αVβ3 integrin ligation on MDMs consistently induced significant down-regulation of scavenger receptor activity and prevented foam cell formation in MDMs from all 20 donors tested. Thus, αVβ3 integrin ligation down-regulates expression of multiple scavenger receptors, even in fully differentiated macrophages with high pre-existing expression of these receptors.
Figure 4.
Ligation of αVβ3 integrin on differentiated MDMs decreases DiI-oxLDL uptake and prevents oxLDL-induced foam cell formation via a mechanism requiring down-regulation of CD36 expression. MDMs (5 to 9 days of culture in vitro) were harvested and reseeded on immobilized antibodies to αVβ3 integrin (thick line) or control isotype-matched IgG1 (dotted line), and analyzed by flow cytometry 24 hours after reseeding. A: DiI-oxLDL uptake by MDMs after 3 hours of incubation with 5 μg/ml of labeled oxLDL. B: Lipid accumulation in cholesterol storage vacuoles induced by incubation with 50 μg/ml of oxLDL for 48 hours revealed by Nile Red staining. C: Reduction of foam cell formation in vitro induced by incubation with oxLDL (50 μg/ml) for 48 hours of MDMs reseeded on immobilized anti-αVβ3 mAb. Top: MDMs reseeded on immobilized anti-αVβ3 mAb. Bottom: MDM reseeded on immobilized control IgG1. Oil Red O lipid staining (red), hematoxylin nuclear counterstain (blue). D: Down-regulation of CD36 surface expression on MDMs induced by αVβ3 ligation revealed by staining with FITC-labeled mAb to CD36 and after analysis by flow cytometry (thin line shows isotype-matched control). E and F: CD36 surface expression (x axis) and DiI-oxLDL uptake (y axis) by MDMs reseeded on αVβ3 integrin antibodies (E) or control IgG1 (F). Scale bars, 20 μm.
Role of PI3-Kinase/Akt Signaling Pathway
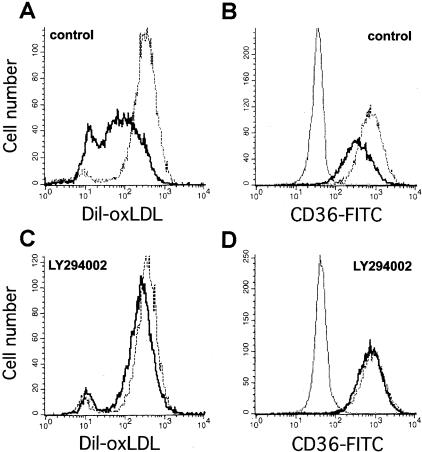
Signal transduction via the phosphatidylinositol 3-kinase (PI3-kinase)/Akt pathway is known to be important in monocyte survival, proliferation, and differentiation.32,41 Data obtained from ECs and other cell types suggested that the extracellular signal-regulated protein kinases (ERK-1,2) and p38 stress-activated protein kinases (p38SAPK) may also mediate cellular responses to αVβ3 integrin activation.41,42 We found that specific inhibitors of the PI3-kinase/Akt pathway LY294002 (10 μmol/L) or wortmannin (100 nmol/L) abolished the reduction of DiI-oxLDL uptake (Figure 5, A and C) and down-regulation of CD36 expression (Figure 5, B and D) induced by ligation of αVβ3 integrin. Inhibitors of p38SAPK (SB203580; 20 μmol/L) or ERK-1,2 (PD98059; 20 μmol/L) did not affect CD36 expression or DiI-oxLDL uptake (data not shown). The effect of these inhibitors on DiI-acLDL uptake by MDMs was identical, consistent with coordinate regulation of CD36 and SRA expression by αVβ3 integrin. These data identified the PI3-kinase/Akt signaling pathway initiated by αVβ3 integrin ligation on MDMs as a principal pathway for regulation of scavenger receptor expression and resultant foam cell formation.
Figure 5.
Inhibition of PI3-kinase/Akt signaling pathway abolishes the down-regulation of DiI-oxLDL uptake and CD36 expression induced by αVβ3 integrin ligation on MDMs. MDMs were differentiated in vitro for 6 days, harvested, incubated in suspension with LY294002 (10 μmol/L) or control vehicle (dimethyl sulfoxide) for 20 minutes, and reseeded on immobilized antibodies to αVβ3 integrin (thick line) or control IgG1 (dotted line) for 6 hours in the presence of inhibitor. MDMs were incubated with DiI-oxLDL (5 μg/ml) for 3 more hours, then DiI-oxLDL uptake was analyzed by flow cytometry. For CD36 detection, MDMs reseeded on anti-αVβ3 or control mAbs were analyzed by flow cytometry using FITC-labeled mAb to CD36 or FITC-conjugated isotype control antibody (thin line). A: DiI-oxLDL uptake. B: CD36 expression by MDMs treated with vehicle control. C and D: Treatment of MDMs with LY294002 prevents effects of αVβ3 integrin ligation on DiI-oxLDL uptake (C) and CD36 expression (D).
Effect of Adhesion of MDMs to ECs on Scavenger Receptor Activity and Foam Cell Formation
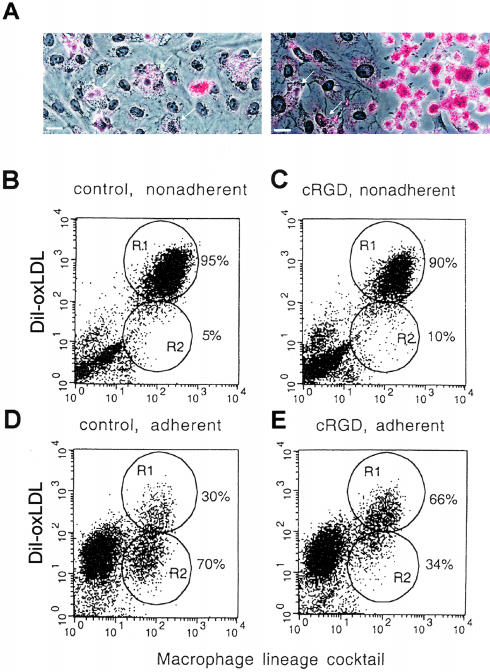
Finally, we wished to determine whether more physiological adhesion of monocytes to ECs had the same effect on lipid uptake as immobilized antibodies. Oil Red O staining for lipids revealed that MDMs adherent to an intact EC monolayer had low levels of lipid accumulation (Figure 6A, left). In contrast, MDMs adherent to plastic in mechanically denuded areas of the same culture demonstrated high-lipid accumulation and typical macrophage foam cell morphology (Figure 6A, right). These results were confirmed by flow cytometry, which showed that nonadherent and loosely adherent MDMs demonstrated high levels of DiI-oxLDL uptake (Figure 6B, R1), whereas the majority of MDMs that were firmly adherent to ECs showed significantly less uptake of DiI-oxLDL (Figure 6D, R2). To test the hypothesis that this effect on lipid uptake was specifically because of ligation of αVβ3 integrin on MDMs by ECs, we used a specific inhibitor of αVβ3 integrin, cyclic RGD peptide (cRGD), which blocks αVβ3 binding.26,27 We found that the population of adherent MDMs with low lipid uptake in co-cultures of MDMs and ECs that were treated with cRGD (10 μg/ml) for 24 hours was significantly reduced from 70% (Figure 6D, R2) to 34% (Figure 6E, R2). Exposure to cRGD had no effect on nonadherent MDMs, which displayed a uniformly high lipid uptake (Figure 6, B and C). Thus, although we cannot totally exclude the possibility that some of the effect of cRGD might be indirectly mediated (eg, via an effect on ECs), these findings support the hypothesis that contact with ECs down-regulates lipid uptake by mechanisms involving αVβ3 integrin, and that activation of multiple adhesion molecules on MDMs by contact with ECs does not abolish the effect of αVβ3 integrin ligation on scavenger receptor activity.
Figure 6.
Adhesion of MDMs to ECs prevents foam cell formation and down-regulates DiI-oxLDL uptake via mechanisms requiring αVβ3 integrin. A: Monocytes were co-cultured with ECs for 5 days, then EC monolayers were partially denuded with a rubber scraper to allow floating MDMs from co-culture to adhere to the plastic in the denuded area. Foam cell formation was induced by incubation of co-cultures with oxLDL (50 μg/ml) for 48 hours. MDMs adherent to ECs (left, arrows) show low lipid accumulation compared to those adherent to denuded area (right). Oil Red O lipid staining (red), hematoxylin counterstain (blue). B–E: Effect of the αVβ3 inhibitor cRGD on DiI-oxLDL uptake by MDMs adherent or nonadherent to ECs. Monocytes were co-cultured with ECs for 5 days, then cRGD (10 μg/ml) was added for 24 hours. Co-cultures were incubated with DiI-oxLDL (5 μg/ml) for 3 hours, then adherent and nonadherent MDMs were harvested (refer to Materials and Methods) and analyzed separately by flow cytometry for MDMs lineage markers (x axis), and uptake of DiI-oxLDL (y axis). B: Nonadherent MDMs from control co-cultures. C: Nonadherent MDMs from co-cultures treated with cRGD. D: Adherent MDMs from control co-cultures. E: Adherent MDMs from co-cultures treated with cRGD. Gated regions represented populations of MDMs with high (R1, mean fluorescence = 330) and low (R2, mean fluorescence = 34) DiI-oxLDL uptake. Scale bars, 20 μm.
Scavenging of Modified LDL by MDMs Generated via Proliferation in Co-Culture with ECs
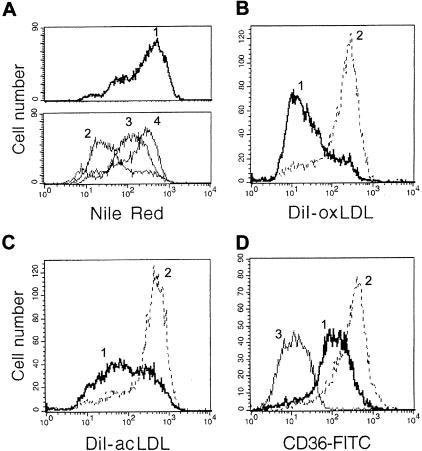
We have previously demonstrated that monocytes co-cultured with ECs undergo enhanced and prolonged proliferation.30 In that study we found that firm adhesion of monocytes to ECs was necessary for this proliferative burst. We next examined the ability of MDMs generated in co-culture to scavenge modified LDL. MDMs were co-cultured with ECs for 5 days. All floating and loosely adherent cells were then removed, and the accumulation of lipids by floating MDMs that arose from the adherent population via proliferation was measured. The loss of contact with ECs by MDMs resulted in a progressive increase in lipid accumulation induced by both oxLDL and acLDL (Figure 7A). However, when these MDMs were reseeded on anti-αVβ3 integrin immobilized mAb, and αVβ3 was thus ligated, MDMs showed both significant decreases in uptake of modified LDL (Figure 7, B and C) and reduced CD36 expression (Figure 7D). Taken together, these results suggest that when ligation of αVβ3 integrin is interrupted (floating MDMs), MDMs differentiate into a phenotype with persistent high CD36 and SRA activity, and thus are able to scavenge atherogenic LDL. However, activation of αVβ3 receptor on these macrophages by its ligand down-regulates scavenger receptor activity and resultant lipid accumulation.
Figure 7.
Scavenging of modified LDL by MDMs generated in co-culture via proliferation may be reversed by αVβ3 ligation. A: Monocytes were co-cultured with ECs for 5 days, then all nonadherent MDMs were removed, and cells were followed as they progressively proliferated, became nonadherent, and lost contact with ECs. The nonadherent population harvested at 24 hours (line 2), 48 hours (line 3), and 72 hours (line 4) after the initial wash shows progressive increases in lipid accumulation induced by incubation with acLDL (100 μg/ml) for 24 hours before harvest. Lipid uptake was analyzed by flow cytometry after staining with Nile Red. In the top panel, line 1 shows lipid accumulation by control MDMs differentiated on plastic for 8 days. B–D: Ligation of αVβ3 integrin on MDMs generated in co-culture down-regulates uptake of modified LDL and CD36 expression. Floating MDMs from co-culture (same MDMs as shown on A, line 4) were harvested and reseeded on immobilized mAb to αVβ3 integrin (thick line) or control IgG1 (dotted line) and analyzed for DiI-oxLDL uptake (B), DiI-acLDL uptake (C), and CD36 expression (D, thin line shows FITC-conjugated IgG1 control) by flow cytometry (see Materials and Methods).
Discussion
Expression of αVβ3 Integrin on Lesion Macrophages in Situ and Its Regulation by oxLDL in Vitro
To hypothesize that αVβ3 receptor plays a role in the pathogenesis of atherosclerosis, it should be demonstrated that this receptor is expressed in vivo in pathological conditions and that its expression can be regulated by pathogenic stimuli, but it is not significantly expressed in normal tissues. Here we have demonstrated that αVβ3 integrin is highly expressed on the majority of macrophages present in both human aortic fatty streak lesions and advanced fibroatheromas plaques located in the coronary arteries. However, αVβ3 expression is not a constitutive feature of all tissue macrophages, as shown by its absence on macrophages in tonsil. In a previous study in advanced coronary artery lesions obtained from patients undergoing heart transplantation,43 it was found that the number of lesion macrophages expressing αVβ3 integrin was insignificant, whereas SMCs in the lesion showed positive staining for αVβ3 integrin. We also have demonstrated αVβ3 integrin expression on SMCs present in fibrous caps and in the medial wall, thus our data are comparable with the cited one. The apparent discrepancy in αVβ3 integrin expression on macrophages may be simply because of the high content of activated macrophages in the lesion areas analyzed in our study (lipid-rich core and shoulder of advanced plaques, but not in the fibrous cap). Alternatively, it is possible that αVβ3 integrin expression on macrophages depends on their activation by risk factors or other as yet unknown stimuli. Indeed, our data show that αVβ3 integrin is significantly expressed on MDMs differentiated in the presence of M-CSF, and that exposure of these cells to oxLDL results in sustained up-regulation of even this high level of αVβ3 integrin expression. Nevertheless, local targeting of macrophages in the zones of active plaque development may have therapeutic value for this disease. Expression of αVβ3 integrin on other cell types involved in atherosclerosis (SMCs, ECs) has been shown, but these studies do not link αVβ3 integrin expression on cells present in the lesion to the regulation of their functions by αVβ3 integrin. Lack of these data may explain contradictory results from animal models. We hypothesize that the conditions existing in atherosclerotic plaques, but not in unaffected tissue, can induce local increases of αVβ3 expression on macrophages in the lesion. Activation of αVβ3 integrin on these cells by its ligands will result in down-regulation of scavenger receptor activity, decrease of oxLDL uptake, and prevention of foam cell formation. Interruption of the signaling pathway initiated by αVβ3 integrin ligation, for example by αVβ3 antagonists, adhesion to ECs or, as it was recently shown in a mice model of atherosclerosis44 by deficiency of β3 integrin, will restore scavenger receptor activity and resultant foam cell formation. Thus, the balance between αVβ3 integrin expression and activation, exposure to atherogenic stimuli, and scavenger receptor activity is important in lesion development.
Ligation of αVβ3 Integrin on Blood Monocytes and Macrophages Prevents Their Differentiation into Foam Cell Phenotype in Vitro
Progression of the early fatty streak lesion to the more complex, clinically significant atherosclerotic plaque is characterized by activation of scavenger receptors on macrophages and subsequent unregulated accumulation of lipids, resulting in foam cell formation, and by macrophage-related inflammatory responses.10,45 Our studies have demonstrated a previously unrecognized function of αVβ3 integrin on human peripheral blood monocytes and differentiated macrophages in regulating foam cell formation. Specifically, these findings identify persistent down-regulation of at least two key scavenger receptors (CD36 and SRA) induced by αVβ3 integrin ligation as the mechanism that prevents the transition of macrophages into foam cells. Additionally, these findings demonstrate that a signaling pathway initiated by αVβ3 integrin ligation can also reverse CD36 and SRA expression and the uptake of oxLDL by differentiated macrophages, which show maximal scavenger receptor activity before ligation (Figure 4). To the best of our knowledge, this is the first demonstration that the phenotype of human MDMs traditionally thought to be atherogenic (which leads to foam cell formation and altered inflammatory responses) can be reversed by αVβ3 receptor ligation, resulting in persistent down-regulation of multiple scavenger receptors.
Ligation of αVβ3 Integrin on Blood Monocytes and Macrophages Results in Sustained Phenotypic Changes
Previous in vitro studies have demonstrated that multiple soluble mediators and cytokines such as tumor necrosis factor-α, interferon-γ, M-CSF, bacterial lipopolysaccharide, as well as CD40/CD40 ligand and others may transiently down-regulate or activate scavenger receptors.34,39,40,44,46–48 However, the transition of macrophages into foam cells requires persistent activation of scavenger receptors, and thus the transient down-regulation of CD36 and SRA expression reported in these previous studies would be unlikely to prevent foam cell formation when macrophages are chronically exposed to atherogenic LDL. In contrast, we found that αVβ3 ligation on both blood monocytes and differentiated macrophages resulted in sustained down-regulation of scavenger receptor activity and lipid accumulation. Thus, the αVβ3 signaling pathway emerges as a key regulator of macrophage transition into the foam cell phenotype, a process strongly associated with lesion progression.
Role of Contact with ECs
Adhesion of monocytes to endothelium, necessary for their recruitment into the arterial wall, is known to profoundly influence monocyte and macrophage functions and the expression of multiple receptors. It is possible that this complicated physiological process can modify the effect of αVβ3 integrin ligation by regulating its expression or by other as yet unknown mechanisms. Our findings support the hypothesis that contact with endothelium prevents foam cell formation by mechanisms involving αVβ3 integrin, and that crosstalk between multiple adhesion molecules activated by contact with ECs does not abolish the effect of αVβ3 ligation on scavenger receptor activity.
Potential Role of αVβ3 Integrin Inhibition in Atherosclerosis
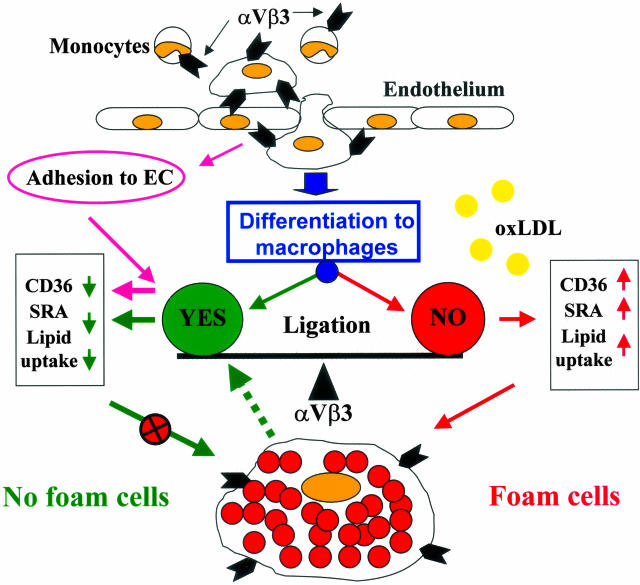
The pathophysiological role of activation or inhibition of scavenger receptors and terminal differentiation of macrophages into the foam cell phenotype is still controversial. It is not clear whether foam cells accelerate the progression of lesions by activating proatherogenic responses or, alternatively, whether they are beneficial scavengers of modified LDL. We have previously postulated that, at least in early lesions, lipid accumulation by macrophages, and their subsequent exit from the lesion is anti-atherogenic and may contribute to lesion regression or reduced progression.30 However, if risk factors are present constitutively, they will lead to continued accumulation of foam cells in the lesion, resulting in increases of lesion size, deposition of intra- and extracellular lipids, cell death and formation of necrotic core, as well as enhanced macrophage-related inflammatory responses leading to progression and complication of the lesion. Contradictory data from animal models of atherosclerosis have been reported to suggest both that blocking of αVβ3 integrin in vivo may be protective and that the lack of β3 integrin hastens progression of atherosclerosis. Taken together, our results establish an important regulatory role for αVβ3 integrin signaling in atherosclerosis for both proatherogenic or anti-atherogenic scenarios and raise the possibility that pharmacological targeting of αVβ3 integrin may be of therapeutic value in human vascular diseases. Figure 8 illustrates our hypothetical model of the regulation of foam cell formation by αVβ3 integrin ligation and rationale for potential therapeutic targeting of αVβ3 receptor.
Figure 8.
Hypothetical model of the regulation of foam cell formation by αVβ3 integrin ligation. Adhesion of blood monocytes to the endothelium results in αVβ3 ligation and leads to the down-regulation of CD36 and SRA expression and prevention of macrophage transition into foam cells. When these cells migrate deeper into the lesion, they lose contact with ECs and may lose αVβ3 integrin activation although it is still expressed. As a result, these macrophages are differentiated into foam cells. Alternatively, as suggested by our results, if αVβ3 integrin is persistently activated via its ligation, the monocytes may differentiate to a macrophage phenotype characterized by down-regulation of scavenger receptors, and thus they do not form foam cells as long as the αVβ3 receptor is activated. Moreover, our data suggest that if other ligands are present in a particular microenvironment that can activate αVβ3-dependent signaling pathways, the foam cell phenotype may be reversed by down-regulation of scavenger receptor activity (dotted line). The prediction from our hypothetical model is that when αVβ3 integrin is persistently ligated/activated, it will prevent macrophage differentiation into the foam cell phenotype. Conversely, blocking of the αVβ3 receptor may lead to up-regulation of scavenger receptor expression and increased foam cell formation.
Acknowledgments
We thank F. Wittke, J. Waller, and R. Eisenstein for technical assistance; M. Keskintepe for assistance with flow cytometry; M. Curuk for help with reverse transcriptase-polymerase chain reaction; S. Peiper, H. Farber, and D. Stern for discussions; K. Smith for immunohistochemistry; and N. Freeman for help with manuscript preparation.
Footnotes
Address reprint requests to Dr. Alexander S. Antonov, Department of Pathology, BF 231, Medical College of Georgia, 1120 15th St., Augusta, GA 30912. E-mail: aantonov@mail.mcg.edu.
Supported by grants from National Institutes of Health (RO1 57930 to R.G.G. and RO1 HL 71148-01 to F.D.K.) and the Department of Pathology, Medical College of Georgia (to A.S.A.).
References
- Schaffner T, Taylor K, Bartucci EJ, Fischer-Dzoga K, Beeson JH, Glagov S, Wissler RW. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980;100:57–80. [PMC free article] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modification of low density lipoproteins that increase its atherogeneity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Atherosclerosis: scavenging for receptors. Nature. 1990;343:508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis. I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis. II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Hiltunen MO, Turunen M, Herttuala SY, Kodama T, Gordon S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36 and atherosclerosis. Curr Opin Lipidol. 2000;11:483–491. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- Geng Y, Kodama T, Hansson GK. Differential expression of scavenger receptor isoforms during monocyte-macrophage differentiation and foam cell formation. Arterioscler Thromb. 1994;14:798–806. doi: 10.1161/01.atv.14.5.798. [DOI] [PubMed] [Google Scholar]
- Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- Kodama T, Reddy P, Kishimoto PC, Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci USA. 1988;85:9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayakawa I, Kanamori H, Aburatani H, Takaku F, Suzuki H, Kobari Y, Miyai T, Takahashi K, Cohen EH, Wydro R, Housman DE, Kodama T. Human macrophage scavenger receptors: primary structure, expression and localization in atherosclerotic lesion. Proc Natl Acad Sci USA. 1990;87:9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Nakata A, Nakagawa Y, Nishida M, Nozaki S, Miyagawa J, Nakagawa T, Tamura R, Matsumoto K, Kameda-Takemura K, Yamashita S, Matsuzawa Y. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T. A role for the macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor, CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. The role of alpha v integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzova TV, Rabbani R, D’Souza SE, Plow EF. Role of integrin alpha (v) beta 3 in vascular biology. Thomb Haemost. 1998;80:726–734. [PubMed] [Google Scholar]
- van Zanten GH, de Graaf S, Slootweg PJ, Heijnen HF, Connolly TM, de Groot PG, Sixma JJ. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93:615–632. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker GC. Inhibitors of integrins. Curr Opin Pharmacol. 2002;2:394–402. doi: 10.1016/s1471-4892(02)00175-3. [DOI] [PubMed] [Google Scholar]
- Miller WH, Keenan RM, Willette RN, Lark MW. Identification and in vivo efficacy of small-molecule antagonists of integrin alpha v beta 3 (the vitronectin receptor). Drug Discov Today. 2000;5:397–408. doi: 10.1016/s1359-6446(00)01545-2. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signaling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov AS, Munn DH, Kolodgie FD, Virmani R, Gerrity RG. Aortic endothelial cells regulate proliferation of human monocytes in vitro via a mechanism synergistic with macrophage colony-stimulating factor. Convergence at the cyclin E/p27 (Kip1) regulatory checkpoint. J Clin Invest. 1997;99:2867–2876. doi: 10.1172/JCI119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Yla-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300. [PMC free article] [PubMed] [Google Scholar]
- Yesner LM, Huh HY, Pearce SF, Silverstein RL. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]
- DeNichilo MO, Burns GF. Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate αv integrin expression on cultured human macrophages. Proc Natl Acad Sci USA. 1993;90:2517–2521. doi: 10.1073/pnas.90.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P, Mayer EP, Fowler SD. Nile Red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death. A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Tromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Fogelman AM, Haberland ME, Seager J, Hokom M, Edwards PA. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981;22:1131–1141. [PubMed] [Google Scholar]
- Geng YJ, Hansson GK. Interferon-γ inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992;89:1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38SAP kinase, and NF-kB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Strömblad S, Cheresh DA. Integrin αvβ3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM. αVβ3 integrin expression in normal and atherosclerotic artery. Circ Res. 1995;77:1129–1135. doi: 10.1161/01.res.77.6.1129. [DOI] [PubMed] [Google Scholar]
- Weng S, Zemany L, Standley KN, Novack DV, La Regina M, Bernal-Mizrachi C, Coleman T, Semenkovich CF. Beta3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci USA. 2003;100:6730–6735. doi: 10.1073/pnas.1137612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- van Lenten BJ, Fogelman AM. Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-α. J Immunol. 1992;148:112–116. [PubMed] [Google Scholar]
- Han J, Hajjar DP, Tauros JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ. J Biol Chem. 2000;275:1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- Hrboticky N, Draude G, Hapfelmeier G, Lorenz R, Weber PC. Lovastatin decreases the receptor-mediated degradation of acetylated and oxidized LDLs in human blood monocytes during the early stage of differentiation into macrophages. Arterioscler Thromb Vasc Biol. 1999;19:1267–1275. doi: 10.1161/01.atv.19.5.1267. [DOI] [PubMed] [Google Scholar]