Abstract
The study of lymphatic endothelial cells and lymphangiogenesis has, in the past, been hampered by the lack of lymphatic endothelial-specific markers. The recent discovery of several such markers has permitted the isolation of lymphatic endothelial cells (LECs) from human skin. However, cell numbers are limited and purity is variable with the different isolation procedures. To overcome these problems, we have transfected human dermal microvascular endothelial cells (HDMVECs) with a retrovirus containing the coding region of human telomerase reverse transcriptase (hTERT), and have produced a cell line, hTERT-HDLEC, with an extended lifespan. hTERT-HDLEC exhibit a typical cobblestone morphology when grown in culture, are contact-inhibited, and express endothelial cell-specific markers. hTERT-HDLEC also express the recognized lymphatic markers, Prox-1, LYVE-1 and podoplanin, as well as integrin α9, but do not express CD34. They also form tube-like structures in three-dimensional collagen gels when stimulated with vascular endothelial growth factors -A and -C. Based on these currently recognized criteria, these cells are LEC. Surprisingly, we also found that the widely studied HMEC-1 cell line expresses recognized lymphatic markers; however, these cells are also CD34-positive. In summary, the ectopic expression of hTERT increases the life span of LECs and does not affect their capacity to form tube-like structures in a collagen matrix. The production and characterization of hTERT-HDLEC will facilitate the study of the properties of lymphatic endothelium in vitro.
The blood and lymphatic vascular systems are anatomically and histologically closely related but distinct, and do not anastomose except at the level of the main lymphatic ducts. The blood vascular system supplies nutrients and oxygen to the body while the lymphatic vasculature is crucial for immune cell traffic and the uptake of extracellular fluid. The formation of new blood vessels from pre-existing blood vessels (angiogenesis) occurs in physiological (eg, postnatal growth, inflammation, and tissue repair) and pathological (eg, tumor recruitment of blood vessels) settings. Lymphangiogenesis is the formation of new lymphatic vessels from pre-existing lymphatic capillaries, and occurs in similar settings. Blood endothelial cells (BECs) and lymphatic endothelial cells (LECs) are quiescent cells that line the lumina of blood and lymphatic vessels.
The lymphatic system has traditionally been overshadowed by the greater emphasis placed on the blood vascular system. This has been due in part to the absence of suitable markers that distinguish lymphatic from blood vascular endothelium. In the past few years, this limitation has been overcome. Lymphatic markers include LYVE-1, a lymphatic endothelial receptor for the extracellular matrix/lymphatic fluid glycosaminoglycan hyaluronan;1 Prox-1, a homeobox gene product involved in regulating early lymphatic development;2 podoplanin, a glomerular podocyte membrane mucoprotein;3 and vascular endothelial growth factor receptor-3 (VEGFR-3), a transmembrane tyrosine kinase receptor for vascular endothelial growth factors-C (VEGF-C) and VEGF-D.4,5 Recent reports indicate that the expression of the integrin α9 subunit is restricted to lymphatic endothelium.6
Use of these markers has permitted the isolation of relatively pure populations of BECs and LECs using fluorescence-activated cell sorting (FACS)7 or immunomagnetic beads.8,9 Initial characterization of these populations highlighted major differences between the two cell types. However, differences have also been observed in molecular expression and cellular function within the same cell type, depending on the isolation procedure used. An additional limitation remains the limited number of cells that can be obtained from a single isolation, and primary cultures of BECs and LECs enter senescence after 8 to 10 passages. Cell purity can also be a problem as contaminating cell types rapidly overgrow endothelial cell (ECs). Primary isolates, while most accurately representing the “physiological state”, have the inherent problems of tissue availability and batch-to-batch variation, which can affect reproducibility of results.
Various approaches have been used to overcome EC senescence. These include ectopic expression of viral oncogenes10–13 and spontaneous transformation.10–13 However, the immortalized cell lines thus generated lose important EC functions and ultimately classical EC markers. An alternative way to overcome or delay senescence is by ectopic expression of human telomerase reverse transcriptase (hTERT). This approach has been successful for immortalizing fibroblasts and retinal pigment epithelial cells14–16 and also for human dermal microvascular endothelial cells (HDMVEC),17,18 and can be achieved without converting the cells to a transformed phenotype.
In the work presented herein, we have transfected commercially available primary HDMVEC with hTERT and compared these cells to a well-characterized immortalized cell line, HMEC-1.10,19 HMEC-1 was generated by stably transfecting dermal microvascular endothelial cells (also isolated from human skin) with the coding region of the simian virus SV40 large antigen. We have found that our hTERT-HDLEC retain typical EC morphology and marker expression beyond 40 passages, express currently recognized LEC markers but not CD34, and retain the capacity to form capillary-like tubes in three-dimensional collagen gels. Interestingly, we also demonstrate that HMEC-1, which is used as a blood vascular-derived endothelial cell line, expresses many lymphatic markers and also CD34. Overall, a comparison of the panel of markers expressed by and functional characteristics of hTERT-HDLEC with the current LEC and BEC profiles, suggests that these cells are LEC.
Materials and Methods
Reagents
Recombinant human VEGF-A (165aa isoform) was purchased from PeproTech Inc. (Rocky Hill, NJ). Recombinant human FGF-2 was provided by Dr. P. Sarmientos (Farmitalia Carlo Erba, Milan, Italy), VEGF-CΔNΔC (designated VEGF-C from hereon) was provided by Dr. M. Skobe (Cancer Center, Mount Sinai Medical Center, New York), and mutant VEGF-C15620 was provided by Dr. K. Alitalo (Biomedicum, Helsinki, Finland). Type I collagen was extracted from rat tail tendons as described previously.21
Cell Lines and Cell Culture
Primary HDMVEC were purchased from Clonetics (neonatal pooled, Cambrex Bio Science Inc, Walkersville, MD, USA) or PromoCell (PromoCell, Heidelberg, Germany) and cultured in EGM-2MV and ECGM-MV2 media, respectively. hTERT-transfected HDMVEC were cultured in EGM-2MV medium. HMEC-1 were kindly provided by Drs. T.J. Lawley and E.W. Ades (Centers for Disease Control, Atlanta, GA) and were grown in EBM131 supplemented with 1 μg/ml hydrocortisone, 10 ng/ml hEGF, and 10% donor calf serum (DCS). SkHep-1 were grown in DMEM (glucose 450 × g/L, Life Technologies Inc., Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS).
Antibodies
Mouse mAbs anti-VE-cadherin,22 -CD34 (QBEND-10), -VEGFR-2 (p1C11),23 and -VEGFR-3 (p3C5)24 were gifts from Dr. J. Gamble (Hanson Centre for Cancer Research, Adelaide, Australia), Dr. M. Greaves (Leukemia Research Fund Center, Chester Beatty Laboratories, London, UK), and Drs. D. Hicklin and B. Pytowski (ImClone Systems Inc., New York), respectively. Rat anti-CD4425 and isotype control MEL14 were gifts from Dr. M. Aurrand-Lions (Department of Pathology, University Medical Center, Geneva, Switzerland). Biotinylated mAb anti-CD31 (Ancell, Bayport, MA, USA) was revealed by PE-conjugated streptavidin (Becton Dickinson, NJ, USA).
Polyclonal rabbit anti-podoplanin and -LYVE-1 were provided by Dr. D. Kerjaschki (Institute of Pathology, University of Vienna, Austria) and Dr. D.G. Jackson (MRC Human Immunology Unit, Institute of Molecular Medicine, Oxford, UK), respectively. Polyclonal rabbit anti-Prox-1 was provided by Dr. J. Wilting (Children’s Hospital, University of Goettingen, Germany) and Reliatech (Braunschweig, Germany). Irrelevant mouse IgG1k (anti-Keyhole Limpet Hemocyanin (KLH) antibody) and normal rabbit immunoglobulins were purchased from Pharmingen and Santa Cruz, respectively. Rabbit polyclonal anti-human podoplanin antibody (no. 201853) was generated by Covalab (Lyon, France) by immunizing with peptides CDVVTPGTSEDRYKSG (RN16CG), CESTVHAQEQSPSATA (RN16CA), andCEGASTGQPEDDTETT (RN16CT), and was partially purified against immobilized peptides.
Cloning of Human Telomerase and Transfection of HDMVEC with hTERT
hTert was recloned from a human immortalized lymphocyte cell line by RT-PCR (details of the cloning can be obtained on demand). The full-length cDNA was verified by sequencing. It was then cloned into pBABEhygro vectors.26 A stock of high-titer retrovirus was prepared by transfecting the Phoenix-Ampho packaging cell line as described.27 Neonatal pooled HDMVEC (Clonetics) at passage 5 were plated the day before infection at 104 cells/cm2 in 6-well plates. Virus stock at 106 CFU/ml was diluted threefold in ECGM-MV2 medium and was added to the cells together with polybrene that was added to a final concentration of 8 μg/ml. The plate was centrifuged at 32°C for 45 minutes at 10,000 × g to increase infection efficiency and thereafter was incubated overnight in a CO2 incubator at 37°C. Two days after infection, selection was initiated with 100 μg/ml hygromycin.
RT-PCR
Total RNA was extracted from human cell lines and tissues using Trizol (Life Technologies, Gaithersburg, MD, USA). Total RNA (2 μg) was reverse-transcribed (RT) using random hexanucleotides (Boehringer Mannheim, Mannheim, Germany) and Superscript II reverse transcriptase (Life Technologies). One twentieth of the RT products were amplified using Expand High Fidelity PCR System (Roche Molecular Biochemicals, Mannheim Germany) or with TaqDNA Polymerase (Invitrogen, Carlsbad, CA, USA). Amplification of the acidic ribosomal phosphoprotein P0 was used to control for RNA integrity and reverse-transcription efficiency.28 Where indicated, RT was omitted. Equal volumes of PCR products were analyzed on 2% agarose gels. Bands representing the partial cDNAs of podoplanin and LYVE-1 were excised from the gel, subcloned into pGEMT-Easy vector (Promega, Madison, WI), and sequenced on both strands.
Immunocytochemistry
ECs were cultured on gelatin-coated dishes. Confluent cells were fixed with 4% paraformaldehyde and post-fixed with cold methanol. ECs were incubated for 30 minutes in blocking buffer (1% bovine serum albumin (BSA) in phosphate- buffered saline (PBS)) before exposure to 5 μg/ml rabbit anti-Prox-1 or normal rabbit IgGs overnight at 4°C. Staining was revealed with Alexa 488-labeled secondary antibody (Molecular Probes, Leiden, The Netherlands). Nuclei were counterstained with 0.5 μg/ml 4′-6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO, USA).
FACS Analysis
Confluent monolayers were dissociated by gently pipetting in the presence of Cell Dissociation Solution (Sigma). Cell pellets were resuspended in PBS containing 0.2% BSA to a final cell number of 1 to 2 × 105 cells/sample, and incubated for 45 minutes on ice with 2 μg/ml anti-PECAM-1, 2 μg/ml CD34, 5 μg/ml CD44, or hybridoma supernatant (diluted 1:2) containing anti-VE-cadherin. Rabbit antisera were diluted as follows: 1:500 for anti-podoplanin (D.K.), 1:100 for anti-podoplanin 201853, and 1:100 for anti-LYVE-1. Primary antibody binding was revealed with an FITC-labeled goat anti-rabbit antibody (Biosys, Compiegne, France) or with streptavidin-phycoerythrin (PE) conjugate (Pharmingen, San Diego, CA, USA). Negative controls included omission of the first antibody for anti-PECAM-1 and anti-VE-cadherin, pre-immune sera for rabbit antisera, and substitution of primary antibodies by appropriate irrelevant antibodies. Cells were analyzed by flow cytometry using a FACScan instrument and CellQuest software, and overlays were executed with either CellQuest or Winmdi software.
RNase Protection Assay
RNase protection assays were performed as previously described.29 Total cellular RNA was extracted using Trizol reagent (Invitrogen). [32P]-dUTP cRNA probes were generated using cDNAs for human VEGFR-1, VEGFR-2, and VEGFR-330 and human acidic ribosomal phosphoprotein P0, the latter serving as an internal control.31 Autoradiograms were scanned with a Laser ScanJet IIex Instrument (Hewlett Packard, Palo Alto, CA) and bands were quantitated using ImageQuant 3.3 software (Molecular Dynamics, Sunnyvale, CA).
Proliferation Assays
hTERT-HDLEC were seeded into 12-well plates at 104 cells per well and grown in VEGF-A- and FGF-2-depleted EGM-2MV medium (incomplete medium) for 24 hours; this was done to avoid interference by cytokines contained in the medium. The cells were left untreated or were treated with FGF-2 (10 ng/ml), or with VEGF-A (100 ng/ml), VEGF-C (100 ng/ml), or VEGF-C156 (100 and 500 ng/ml) alone or in combination with FGF-2 (10 ng/ml). Medium and cytokines were renewed every 2 days. After 6 days, the cells were harvested using trypsin and suspended in 1 ml, and were then counted for 1 minute using a FACScan instrument and CellQuest software. Results represent the mean of four independent experiments (passages 17–24) ± SEM per condition. Mean values were compared using Student’s unpaired t-test, and a significant value was taken as P < 0.05.
In Vitro Angiogenesis Assays
The ability of HMEC-1 and hTERT-HDLEC to form capillary-like structures in vitro was assessed in three-dimensional collagen gel assays.9,21,32 Cells were either allowed to form monolayers on top of collagen gels to assess their invasive capacity, or seeded as single cells in suspension within collagen gels, or “sandwiched” between two layers of collagen. Cells were seeded onto collagen gels in 16-mm wells at 1 × 105 cells/well for the invasion assay, at 0.5 × 106 cells/ml in the suspension assay and at a concentration of 3.4 × 104 cells/cm2 in the sandwich assay. HMEC-1 and hTERT-HDLEC were cultured in EBM 131 or EGM-2MV medium (complete or incomplete), respectively. For cell suspension or sandwich assays, cells were treated after collagen polymerization, while for cells seeded onto collagen gels, treatment was begun only after the cells had reached confluence (approximately 1 week). Cells were treated with 10 ng/ml FGF-2, and 100 ng/ml VEGF-A, 100 ng/ml VEGF-C, 100 or 500 ng/ml VEGF-C156 alone or in combination with 10 ng/ml FGF-2. Media and cytokines were renewed every 2 to 3 days. After 7 days, cells were photographed under phase contrast microscopy using a Nikon Diaphot TMD inverted photomicroscope (Nikon, Tokyo, Japan). Tube formation in the invasion assay was quantitated as described,33 and results are expressed as mean additive sprout length ± SEM (in μm) from three fields per experiment for at least three experiments per condition. Mean values were compared using Student’s unpaired t-test, and a significant value was taken as P < 0.05.
Semi-Thin and Thin Sections
Collagen gel cultures were fixed in situ overnight with 2.5% glutaraldehyde in 100 mmol/L sodium cacodylate buffer (pH 7.4). After rinsing in the same buffer, the gels were cut into 2 × 2-mm fragments and post-fixed in 1% osmium tetroxide in Veronal acetate buffer for 60 minutes, stained en bloc with 2.5% uranyl acetate in 50% ethanol, dehydrated in graded ethanols, and embedded in Epon 812 in flat molds. Semi-thin (2 μm) and thin (40 nm) sections were cut with an LKB ultramicrotome (LKB Instruments, Gaithersburg, MD) and were stained with 1% methylene blue and photographed using a transmission light microscope (Carl Zeiss, Orberkochen, Germany).32 Thin sections were stained with uranyl acetate and lead citrate and examined in a Philips CM10 electron microscope (Philips, Eindhoven, The Netherlands).
Zymography and Reverse Zymography
Matrix metalloproteinase (MMP) activity was analyzed using gelatin zymography.34 Confluent monolayers of hTERT-HDLEC or HMEC-1 were washed with PBS and the cells incubated in their corresponding serum- and cytokine-free media. After a 15 hour incubation at 37°C, conditioned media were collected, supplemented with 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF) and 15 mmol/L N-(2-hydroxyethyl)piperazine-N′-(2 ethanesulfonic acid) (HEPES), centrifuged at 340 × g for 5 minutes, and the resulting supernatants were stored at −80°C until use. Supernatants (30 μl) were electrophoresed in 10% SDS-PAGE gels co-polymerized with 1 mg/ml gelatin. After soaking in 2.5% Triton X-100 for 30 minutes, the gels were incubated in reaction buffer (50 mmol/L Tris-HCl, pH 8) containing 150 mmol/L NaCl, 10 mmol/L CaCl2, and 0.02% NaN3) at 37°C for 16 hours and stained with ethanol:acetic acid:water (30:10:60) containing 0.25% Coomassie Blue R250 for 4 hours. Gelatinolytic activity was detected as clear bands against a background of uniform staining. Conditioned media from MCF-7 and U937 cell lines, which secrete MMP-2 and MMP-9, respectively, were used as positive controls. For casein zymography and reverse zymography, the cells were treated as mentioned above, and cell extracts or supernatants were prepared and analyzed as previously described.29,35,36
Array Analysis
Targeted cDNA arrays designed to analyze adhesion molecule and protease expression (GEArray HGEA9913090 and HGEA 9914030) were purchased from SuperArray Bioscience Corporation (Frederick, MD). Probe synthesis and hybridization from total cellular RNA from HMEC-1 (passage 16) or hTERT-HDLEC (passage 21) were performed as a service by Artus-Biotech (Hamburg, Germany) according to the array manufacturer’s instructions. Hybridization was quantitated using a Typhoon 9210 phosphoimager (Molecular Dynamics) and data were analyzed with ImageQuant 3.3 (Molecular Dynamics) software. Briefly, the lowest hybridization signal (considered as background) was subtracted from the average hybridization for each gene (duplicate spots for each gene), and values were normalized with respect to GAPDH. Any gene with a hybridization signal greater than two times that of pUC18 was deemed to be expressed. Results are summarized as supplementary data at http://ajp.amjpathol.org.
Results
hTERT-HDLEC Have a Markedly Extended Lifespan
HDMVEC were transduced with a plasmid containing hTERT cDNA. The cells were obtained from Clonetics (reference number CC-2516), and were pooled by the suppliers from at least three neonatal foreskins. Cells from each donor were isolated and grown separately before being pooled. Foreskins were enzymatically processed and endothelial cells were isolated using mesh strainers to catch clusters of endothelial cells together with differential split techniques (information obtained from the suppliers).
Similar to previous reports, we have found that human microvascular endothelial cells transduced with telomerase exhibit a longer life-span. The cells retained the characteristic endothelial cell cobblestone morphology and displayed contact-inhibited growth (Figure 1A). hTERT-transduced cells were analyzed for expression of the transduced hTERT gene by RT-PCR. Infected cells were found to express the hTERT cDNA (Figure 1B) and, as shown by TRAP assay (Figure 1C), also displayed detectable hTERT enzymatic activity on an articifial template. Cell growth was robust until about 40 passages, following which a subpopulation exhibited a flattened morphology typical of senescent cells (data not shown). Under the same culture conditions, the primary parental endothelial cells entered senescence after 8 to 10 passages. Thus, ectopic expression of hTERT in endothelial cells extended their replicative life span at least four times, technically defining these cells as immortalized.37 There was no evidence for a transformed phenotype in vitro, since the cells retained their typical EC contact-inhibited phenotype and did not form colonies in soft agar (data not shown). All attempts to obtain independent populations by clonal expansion failed, as indicated by the fact that from the 20 colonies initially cloned using cloning rings, only three grew up to a maximum of five passages before exhibiting cell senescence.
Figure 1.
Endothelial life span is increased by hTERT. A: Passage 27 hTERT-HDLEC were photographed under phase contrast microscopy. The cells retain their typical cobblestone morphology and are contact inhibited. Bar, 100 μm. B: RT-PCR assay. Samples were run in a 1.5% agarose gel. The expected 350-bp band is indicated. Marker (1-kb ladder, Invitrogen), primary HDMVEC (negative control, lane 1), H20 (lane 2), RPE-hTERT (positive control, lane 3), hTERT-HDLEC, passage 9 (lane 4). C: Cell extracts were prepared and the TRAP assay was performed using the Intergen TRAPeze kit. Samples were run in an 8% acrylamide gel. Marker kit control, Mdx12 hTERT fibroblasts (positive control, lane 1), primary HDMVEC, passage 6 (lane 2), hTERT-HDLEC passage 9 (lane 3), H2O (lane 4).
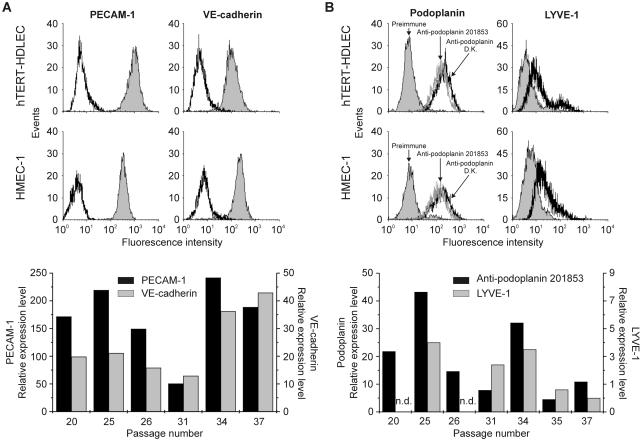
Expression of EC markers was assessed by FACS. CD31 (PECAM-1), and VE-cadherin were expressed up to the passages analyzed (passage 37). FACS profiles with both markers revealed the presence of a single population, and the relative expression of the two markers was similar at the different passages analyzed (Figure 2A). HMEC-1 cells were used as a positive control. Interestingly, CD34 was detected in both cell lines by RT-PCR while only HMEC-1 expressed detectable levels of the protein by FACS (data not shown). Similarly, von Willebrand factor was virtually undetectable in hTERT-HDLEC when compared to primary HDMVEC and human umbilical vein endothelial cells. We also found that both hTERT-HDLEC and HMEC-1 express CD44 as assessed by RT-PCR and FACS (data not shown).
Figure 2.
Expression of endothelial and lymphatic markers. A: Typical profiles obtained with the endothelial markers PECAM-1 and VE-cadherin on either hTERT-HDLEC (passage 26) or HMEC-1 (passage 18) (top). hTERT-HDLEC at different passages were stained with PECAM-1 (black bars, left axis) and VE-cadherin (gray bars, right axis) (bottom). The mean fluorescence intensities were normalized to the fluorescence with the secondary antibody alone (to which a value of 1 was assigned). B: Typical profiles obtained with the lymphatic markers podoplanin and LYVE-1 on either hTERT-HDLEC or HMEC-1. hTERT-HDLEC at different passages were stained with podoplanin (black bars, left axis) and LYVE-1 (gray bars, right axis), and the mean fluorescence intensities were normalized to fluorescence with pre-immune sera (to which a value of 1 was assigned). n.d., not determined.
hTERT-HDLEC Characterization
Neonatal HDMVEC purchased from Clonetics were pooled, by the supplier, from at least three donors; cells from each donor were isolated and grown separately before being pooled. It was thus likely that they contained both BEC and LEC populations. Transfection of neonatal pooled HDMVEC with hTERT could have immortalized either or both populations. Our first objective was to determine whether these cells express LEC markers. To this end, we generated a polyclonal antibody against peptides contained in the extracellular domain of human podoplanin (antibody characterization is shown as supplementary data at http://ajp.amjpathol.org). Both hTERT-HDLEC and HMEC-1 displayed similar FACS profiles, in that both cell lines contained only one population, which stained positively with three different antibodies (anti-podoplanin 201853, anti-LYVE-1, and anti-podoplanin (from D.K.)) to two reliable LEC markers (Figure 2B). Despite variations, hTERT-HDLEC expressed podoplanin up to at least passage 37 while LYVE-1 expression was lost between passages 35 and 37 where the mean fluorescence intensity reached a value of 1 (the value which was assigned to the mean fluorescence intensity of pre-immune serum) (Figure 2B).
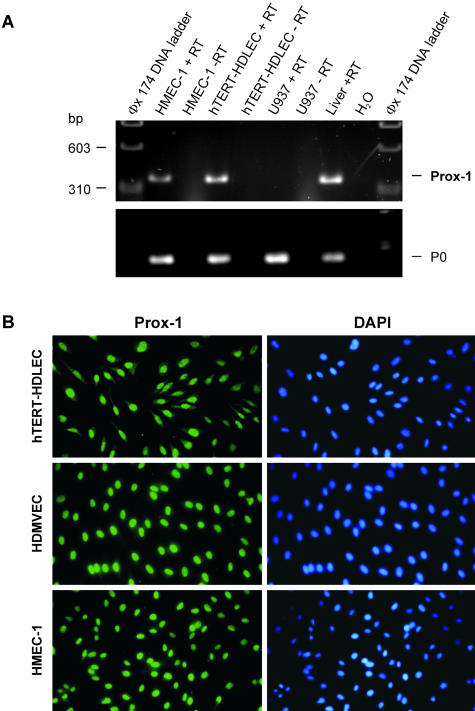
We next assessed the expression of the transcription factor Prox-1, one of the most reliable lymphatic markers described to date.38 RT-PCR using the human Prox-1-specific primers indicated in Table 1 amplified a band of the predicted size (333 bp) in both HMEC-1 and hTERT-HDLEC (Figure 3A). Immunocytochemistry was used to confirm Prox-1 expression at the protein level, and was repeated at least three times with HMEC-1 (passages 17–21) or with hTERT-HDMVEC (passages 21–37). Over 95% of nuclei stained positively for Prox-1 when compared to DAPI counterstaining (Figure 3B). (In fact, virtually 100% of the cells were positively stained; nuclei that gave a low signal were considered as negative.)
Table 1.
Sequences of PCR Primers, Length of PCR Product, Optimal Annealing Temperature and Number of Amplification Cycles
| Molecule | Sense (5′-3′) | Antisense (5′-3′) | Template (bases) | Annealing temperature (°C) | Number of cycles |
|---|---|---|---|---|---|
| Integrin α1 | GCTACTGCTTCTTCTGGAGATGTG | TGAATTGTGCTGCCGAGATGAAC | 345 | 55 | 30 |
| Integrin α2 | AGTGTGCTGTGTTCAGTTGATGTG | TGTGCGGATAGTGCCCTGATG | 318 | 60 | 35 |
| Integrin α5 | ACCTGCTACCTCTCCACAGATAAC | TCACCAACAGCCACAGAGTATCC | 327 | 55 | 30 |
| Integrin α6 | AGTCTCGTTCCTGTTCCTGCTAAC | CATCTGCCTTGCTGGTTCATGTAG | 341 | 55 | 30 |
| Integrin α9 | ACTATGAAGCCGACCACATCCTAC | ATAAACCTTGCCGATGCCTTTGTC | 386 | 60 | 40 |
| Integrin β1 | CAATGAAGGGCGTGTTGGTAGAC | TACAGACACCACACTCGCAGATG | 383 | 55 | 30 |
| hVEGF-C | TCCGGACTCGACCTCTCGGAC | CCCCACATCTATACACACCTCC | 324 | 60 | 40 |
| hLYVE-1 | GGCAAGGACCAAGTTGACACAG | TGGAGCAGGAGGAGTAGTAGTAGG | 376 | 55 | 35 |
| hPodoplanin | AGCCAGAAGATGACACTGAGACTAC | ACCACAACGATGATTCCACCAATG | 357 | 55 | 35 |
| hProx-1 | CCACCTGAGCCACCACCCTTG | GCTTGACGTGCGTACTTCTCCATC | 333 | 60 | 35 |
| hCD34 | AAGCCTAGCCTGTCACCTGGAAATG | GGCAAGGAGCAGGGAGCATACC | 300 | 60 | 35 |
| hCD44 | TGGGTTCATAGAAGGGCATGTGGTG | ATTCTGTCTGTGCTGTCGGTGATCC | 420 | 60 | 35 |
| hMMP-2 | GCGACAAGAAGTATGGCTTC | TGCCAAGGTCAATGTCAGGA | 390 | 62 | 40 |
| hMMP-9 | CGCAGACATCGTCATCCAGT | GATGCCATTCACGTCGTCCTTATG | 829 | 60 | 40 |
| hMMP-14 | CAACACTGCCTACGAGAGGA | GTTCTACCTTCAGCTTCTGG | 380 | 60 | 40 |
| hTERT | CTCTCCCCCTTGAACCTCCTCTTTC | AGGACACCTGGCGGAAGGAG | 350 | 55 | 35 |
Figure 3.
Expression of Prox-1. A: The pair of primers indicated in Table 1 was used to amplify partial cDNAs encoding for Prox-1, and equal volumes of the PCR products were analyzed on 2% agarose gels. cDNA fragments of ± 330 bp were amplified from hTERT-HDLEC, HMEC-1 and human liver (used as a positive control) but not from U937 cells. This fragment corresponds in size to the predicted Prox-1 amplification product. Amplification of the acidic ribosomal phosphoprotein P0 was used as a control for RNA integrity and reverse-transcriptase efficiency. Where indicated, RT was omitted to exclude genomic contamination of the signal. B: Nuclear localization of Prox-1 (left) was detected by immunocytochemistry in hTERT-HDLEC (top), neonatal pooled HDMVEC (Clonetics, middle) and HMEC-1 (bottom). Nuclei were counterstained with DAPI (right). One representative staining out of at least three independent experiments is shown.
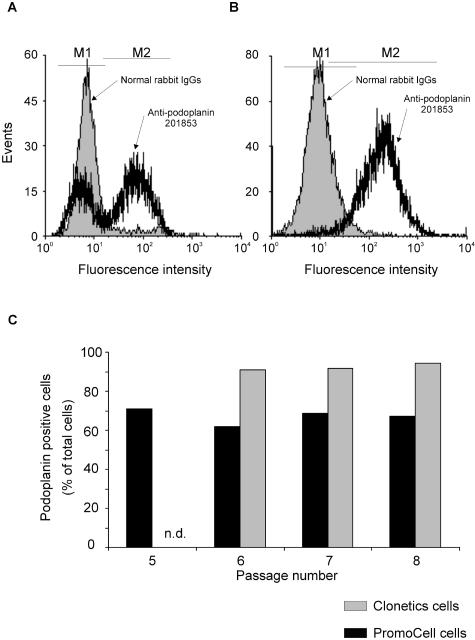
Both EC lines express reliable lymphatic markers, and thus could be defined as LECs when compared to current LEC and BEC molecular profiles. We wished to know however whether HDMVEC purchased from Clonetics acquire the capacity to express these markers in culture or are derived from a mixed population of BEC and LEC with BEC being lost during cell culture. To answer this question, we cultured primary endothelial cells purchased from Clonetics or PromoCell in their respective media and performed FACS analysis to determine the pattern of podoplanin expression at different passages. With regard to cells from PromoCell, 60 to 70% were podoplanin-positive, suggesting that these cells contain a mixed population of BECs and LECs, which is in accordance with previous reports.38–40
This ratio did not change with subsequent passaging suggesting that, at least in this passage range, the BEC did not acquire the capacity to express podoplanin. In addition, since the ratio of BEC:LEC did not change, we can conclude that BECs and LECs retain their molecular identities/characteristics in culture, and that neither population is lost or is overgrown by the other. Since the HDMVEC purchased from PromoCell are isolated from a single donor, we performed the experiment on three different batches. Similar results were obtained with each batch, and one representative experiment is shown in Figure 4. Experiments performed on neonatal-pooled ECs from Clonetics showed that over 98% of the cells expressed podoplanin from passage 6 (Figure 4). Since the cells are grown in different media, it is possible that one medium might favor LEC growth and therefore increase the LEC to BEC ratio. To address this question, we performed the same experiments but switched the media: PromoCell cells were cultured in Clonetics medium and vice versa. This gave similar results as described above when cells were cultured in their own media (data not shown). This suggests that once the cells are established in culture (ie, when they are received from the supplier), the medium does not have a significant effect on the growth of one population versus the other. Furthermore, experiments performed in our lab in which a heterogeneous population of cells derived from neonatal foreskins were grown for two weeks, gave rise to a 99% or 60% pure CD31+/podoplanin+ population when grown in EGM-2MV (Clonetics) or ECGM-MV2 (PromoCell) media, respectively (data not shown). Furthermore, and of major significance for our study, Clonetics cells are initially LEC-enriched. This is likely to explain why virtually all of the cells in our hTERT-HDLEC line express characteristic LEC markers.
Figure 4.
PromoCell versus Clonetics: podoplanin expression. Human dermal microvascular endothelial cells purchased from PromoCell or Clonetics were cultured in their respective media. Podoplanin expression was determined by FACS analysis. The cells were incubated with control IgGs or with affinity-purified anti-podoplanin 201853, and labeling was revealed with a FITC-conjugated secondary antibody. A: Cells from PromoCell. B: Cells from Clonetics. C: The experiment was performed at different passages and the percentage of podoplanin-positive cells for each passage is indicated. n.d., not determined.
Cytokine-Induced hTERT-HDLEC Proliferation
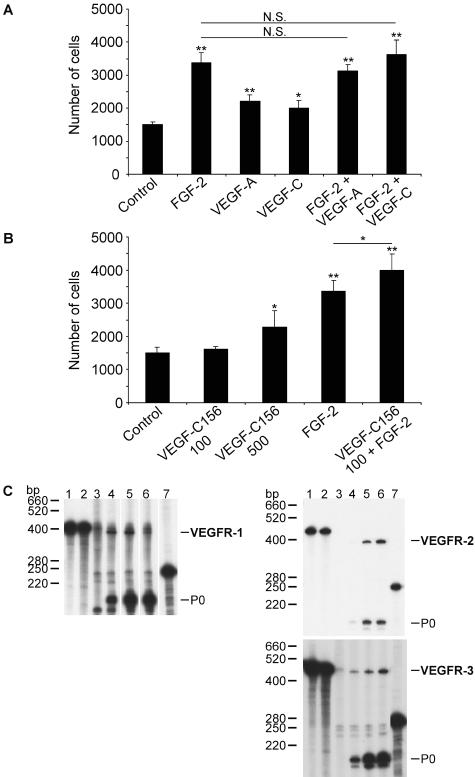
A characteristic of endothelial cell lines is their ability to respond mitogenically to angiogenic or lymphangiogenic cytokines. Thus, we treated hTERT-HDLEC with different cytokines in FGF-2- and VEGF-A-depleted EGM-2MV medium. FGF-2, VEGF-A, and VEGF-C significantly increased cell proliferation. VEGF-A and VEGF-C alone induced a similar response and did not synergize with FGF-2 (Figure 5A). Interestingly, VEGF-C156 induced a mild, but significant, effect at 500 ng/ml39 and at 100 ng/ml VEGF-C156 only potentiated FGF-2 response (Figure 5B). These results suggested hTERT-HDLEC express VEGF receptors and were confirmed by the detection of VEGFR-1 (Flt-1), VEGFR-2 (Flk-1), and VEGFR-3 (Flt-4) transcripts by RNase protection assay (Figure 5C).
Figure 5.
Cytokine-induced hTERT-HDLEC proliferation. hTERT-HDLEC were seeded into 12-well plates at 104 cells per well and starved for 24 hours in EGM-2MV medium, from which VEGF-A and FGF-2 had been omitted. A: The cells were left untreated or were treated with FGF-2 (10 ng/ml), VEGF-A (100 ng/ml), or VEGF-C (100 ng/ml) alone or in combination with FGF-2 (10 ng/ml). B: The cells were treated with FGF-2, VEGF-C156 (100 ng/ml or 500 ng/ml), or VEGF-C156 (100 ng/ml) in combination with FGF-2 (10 ng/ml). Medium and growth factors were renewed every 2 days. After 6 days, the cells were harvested and counted. Values are the mean from four independent experiments ± SEM. Mean values were compared using Student’s unpaired t-test (*, P < 0.05; **, P < 0.005). C: RNase protection analysis for VEGFR-1, -2, and -3 mRNA in HMEC-1 and hTERT-HDLEC. Purified 32P-labeled cRNA probes (lane 1) were hybridized to hybridization mix (lane 2), yeast tRNA (lane 3), 10 μg of total RNA from human liver (lane 4), HMEC-1 (lane 5) or hTERT-HDLEC (lane 6). As an internal control, an equal amount of the P0 cRNA probe was included in all samples or loaded onto the gel (lane 7). One of two experiments with similar results is shown.
In Vitro Tubulogenesis Assays
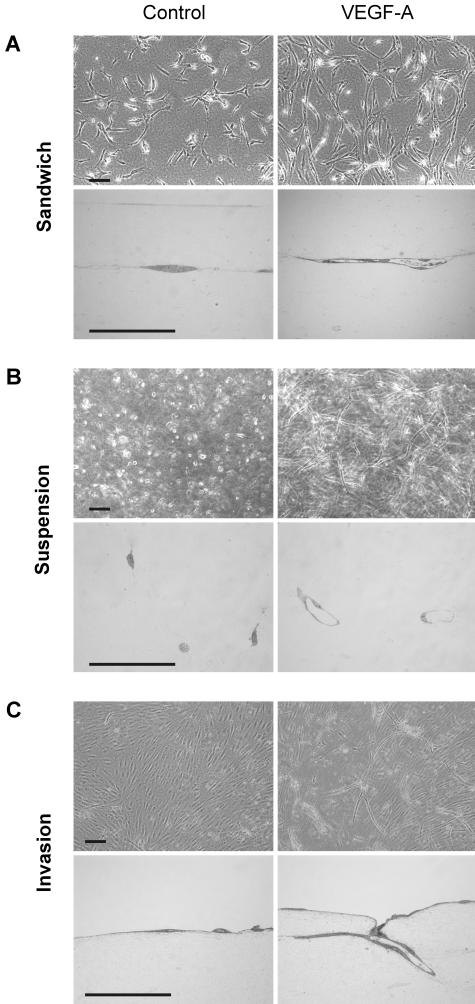
In agreement with previous reports,17,18 cytokine treatment induced HMEC-1 and hTERT-HDLEC lines to form tube-like structures. With regard to hTERT-HDLEC, the same results were obtained when the cells were treated in complete or FGF-2- and VEGF-A-depleted EGM-2MV. Thus, the concentration of FGF-2 and VEGF-A contained in commercial medium appears to be insufficient to induce major morphological modifications. Cytokine stimulation of both cell lines when “sandwiched” between two three-dimensional collagen layers (Figure 6A) or in suspension as single isolated cells in a collagen gel (Figure 6B), promoted cell survival and increased the formation of tube-like structures. Tube formation was confirmed by semi-thin and thin section. Electron microscopic analysis revealed that hTERT-HDLECs delimited a patent lumen containing cell debris (Figure 7A) and formed tight junctions at sites of intercellular contact (Figure 7B). Furthermore, long overlapping interdigitating junctions were observed (Figure 7C) as well as focal discontinuities in the basal lamina underlying the abluminal plasma membrane (Figure 7D). These are two characteristic morphological features of lymphatics.41 Results obtained with the different cytokines are summarized in Table 2. When grown on the surface of three-dimensional collagen gels, limited superficial spontaneous invasion by isolated HMEC-1 was observed, which was slightly increased by cytokine treatment. Similarly, minimal superficial spontaneous invasion of isolated hTERT-HDLEC was observed. In contrast, cytokine-stimulated hTERT-HDLEC reproducibly and extensively invaded the underlying collagen gels, within which they formed clearly distinguishable tube-like structures (Figure 6C, Figure 7A, and Table 2). VEGF-A induced a maximal response after 3 days and tube regression began after 7 days. FGF-2 and VEGF-C increased invasion and also induced tube formation, albeit to a lesser degree than VEGF-A. VEGF-A and -C both synergized with FGF-2 (Figure 8A). In contrast, VEGF-C156 never induced invasion or tube formation in any of the three collagen models, even after 2 weeks of culture at 100 ng/ml and 1 week at 500 ng/ml. However, as seen with proliferation assays, VEGF-C156 at a concentration of 100 ng/ml slightly but significantly potentiated the effect of FGF-2 (Figure 8C).
Figure 6.
Tube formation by hTERT-HDLEC and HMEC-1 in collagen gels. Cells were either sandwiched between two collagen gels (A), seeded in suspension as isolated single cells in a collagen gel (B), or seeded on the surface a collagen gel (C). Cells were left untreated (left) or treated with the indicated cytokines (right). After 5 to 7 days of culture, cells were photographed, and semi-thin sections (bottom) were analyzed to assess whether tube formation had occurred. Bar, 100 μm. Endothelial cell responses to cytokines are summarized in Table 2.
Figure 7.
Ultrastructural analysis of endothelial tubes. A–C: hTERT-HDLECs were sandwiched between two collagen layers and grown for 7 days in the presence of 100 ng/ml VEGF-A. A: Cells delimit a patent lumen containing cell debris. Bar, 2 μm. B: Higher magnification of the area outlined in A. A tight junction (arrows) is recognizable as a focal pentalaminar fusion of the plasma membranes of two adjacent endothelial cells. Bar, 0.1 μm. C: Higher magnification of the area outlined in A. A long overlapping interdigitated junction, typically found in lymphatics, is clearly visible. Bar, 0.5 μm. D: hTERT-HDLECs were suspended in a three-dimensional collagen gel and grown for 7 days in the presence of 100 ng/ml VEGF-A. The abluminal plasma membrane of an endothelial cell delimiting a tube-like structure is underlined by a basal lamina showing focal discontinuities. Bar, 0.5 μm.
Table 2.
Effect of Angiogenic and/or Lymphangiogenic Cytokines on HMEC-1 and hTERT-HDLEC in Three-Dimensional Collagen Gel Tubulogenesis Assays
| hTERT-HDLEC (treatment ng/ml) | Sandwich | Suspension | Invasion |
|---|---|---|---|
| Control | + | + | − |
| FGF-2 (10) | ++ | ++ | + |
| VEGF-A (100) | +++ | +++ | +++ |
| VEGF-C (100) | ++ | ++ | + |
| VEGF-C156 (100 or 500) | + | + | − |
| FGF + VEGF-A (10 + 100) | n.d. | ++++ | ++++ |
| FGF + VEGF-C (10 + 100) | n.d. | n.d. | +++ |
| FGF + VEGF-C156 (10 + 100) | n.d. | n.d. | + |
| HMEC-1 | Sandwich | Suspension | Invasion |
|---|---|---|---|
| Control | − | − | − |
| FGF-2 (10) | n.d. | n.d. | − |
| VEGF-A (100) | + | + | − |
| VEGF-C (100) | + | + | − |
| VEGF-C156 (100 or 500) | n.d. | n.d. | n.d. |
| FGF + VEGF-A (10 + 100) | n.d. | n.d. | n.d. |
| FGF + VEGF-C (10 + 100) | n.d. | n.d. | n.d. |
| FGF + VEGF-C156 (10 + 100) | n.d. | n.d. | n.d. |
| PMA (10) | n.d. | n.d. | − |
+, tube formation; −, absence of tube formation; n.d., not determined.
Figure 8.
Quantitation of cytokine-induced hTERT-HDLEC invasion of collagen gels. Confluent monolayers of hTERT-HDLEC (between passage 18 and 25) on three-dimensional collagen gels were left untreated or were treated (A) with FGF-2 (10 ng/ml), VEGF-A (100 ng/ml), VEGF-C (100 ng/ml) alone or in combination with FGF-2 (10 ng/ml) or (B) with FGF-2, VEGF-C156 (100 ng/ml or 500 ng/ml), or VEGF-C156 (100 ng/ml) in combination with FGF-2 (10 ng/ml). Cells were cultured for 7 days; media and growth factors were renewed every 2 days. Data are shown as mean values, at least three experiments per condition (at least three fields per condition per experiment) ± SEM. Mean values were compared using Student’s unpaired t-test (*, P < 0.5; **, P < 0.005). C: hTERT-HDLEC were left untreated or were treated with VEGF-C (100 ng/ml) alone or together with 20 μg/ml p1C11 (anti-VEGFR-2), 20 μg/ml p3C5 (anti-VEGFR-3) or both antibodies in combination; anti-KLH 20 μg/ml was used as an isotype control. Data are shown as mean percentage of total cell invasion with respect to untreated cells (100%), ± SEM.
Since VEGF-C activates both VEGFR-2 and -3, and VEGF-C156 has no effect by itself, VEGF-C-driven invasion is likely to be due to activation of VEGFR-2. To investigate this hypothesis, we stimulated hTERT-HDLEC with VEGF-C alone or in combination with neutralizing antibodies to VEGFR-2 (p1C11) or VEGFR-3 (p3C5); an isotype-matched IgG was used as a control (Figure 8C). VEGFR-2 blockade reduced invasion to untreated levels, whereas blockade of VEGFR-3 alone had no effect. These results demonstrate that VEGF-C drives hTERT-HDLEC invasion via the activation of VEGFR-2. Interestingly, similar invasion profiles were observed with cells at passages 36–38 (data not shown), albeit to a lesser degree than in Figure 8A and Figure 8B, demonstrating that the cells still respond to angiogenic or lymphangiogenic cytokines even at high passage numbers.
Molecular Characterization of hTERT-HDLEC
During invasion and tube formation, endothelial cells degrade their basement membrane, and proliferate and migrate into the surrounding collagen-rich matrix. This complex process requires the coordinated activities of many different molecules, including VEGFRs, integrins, and proteinases. RNase protection assays confirmed expression of all three VEGFRs in both HMEC-1 and hTERT-HDLEC (Figure 5C). VEGFR-2 expression on the surface of the two lines was confirmed by FACS analysis (data not shown). Metalloproteinase and integrin expression were analyzed using SuperArray filters (see supplementary data at http://ajp.amjpathol.org) and several molecules implicated in the invasive process were subsequently validated by RT-PCR. hTERT-HDLEC express mRNA for integrin subunits responsible for interactions with type-I collagen, namely α1β1 and α2β1, the fibronectin receptor α5β1 and the laminin receptor α6β1 (Figure 9A). Interestingly, we detected expression of the transcript coding for the α9 integrin subunit which has previously been reported to be restricted to lymphatic endothelium.6 Furthermore, we could not detect expression of VEGF-C, which previous reports have indicated is restricted to BECs. hTERT-HDLEC also expressed αvβ3 and αvβ5 integrins, as assessed by FACS analysis using LM609 and P1F6 antibodies (data not shown). These integrins have previously been reported to mediate angiogenesis.42,43
Figure 9.
Molecular characterization of h-TERT-HDMVEC. A: hTERT-HDLEC integrin and MMP expression were assessed by RT-PCR. The total cellular RNA, isolated from confluent hTERT-HDLEC monolayers (at passage 26) cultured in EGM-2MV Bullet kit medium, was reverse-transcribed and amplified by PCR. The specific conditions and primers pairs are indicated in Table 1. Equal amounts of PCR products were analyzed on a 2% agarose gel. Marker (123-bp DNA ladder), hTERT-HDLEC + RT (lane 1), hTERT-HDLEC − RT (lane 2), human liver + RT (lane 3), H2O (lane 4). The expression of αvβ3 and αvβ5 integrin complexes was confirmed by FACS analysis (data not shown). B and C: Zymographic analysis of HMEC-1 and hTERT-HDLEC. Cells were cultured in serum-free media lacking FGF-2 or VEGF-A. Confluent monolayers in 35-mm tissue culture dishes were left untreated (control) or exposed to 100 ng/ml VEGF-A or 100 ng/ml VEGF-C for 15 hours. Supernatants were analyzed by gelatin (B) or casein (C) zymography. uPA activity was confirmed by its inhibition with amiloride (data not shown).
We next assessed whether hTERT-HDLEC express proteolytic enzymes implicated in angiogenesis/lymphangiogenesis.44,45 With regard to MMP expression, we detected mRNA for MMP-2, MMP-9, and MMP-14 by RT-PCR (Figure 9,A). While MMP-2 activity was also detected in conditioned media by gelatin zymography, MMP-9 was not detected and may be expressed at levels below the detection limit (Figure 9B). Zymography and reverse zymography demonstrated hTERT-HDLEC express urokinase and tissue-type plasminogen-activators (uPA and tPA) as well as PA inhibitor-1 (PAI-1) (Figure 9C). The identity of uPA was confirmed by its inhibition by amiloride (data not shown). As previously described,45 VEGF-A, and to a lesser extent VEGF-C, up-regulate both uPA and tPA activities with no effect on PAI-1. In contrast, only VEGF-A induced a small increase in tPA activity in HMEC-1 with no measurable effect on uPA (one of three experiments with similar results is shown).
Discussion
The major objectives of this study were to generate endothelial cell lines with an extended lifespan by ectopic expression of hTERT in HDMVEC and to study their molecular profile and their ability to respond to angiogenic and/or lymphangiogenic cytokines. We report the generation of hTERT-immortalized human dermal lymphatic endothelial cells (hTERT-HDLEC). The cells have been grown to 44 passages (at least a fourfold increase over parental cells) and maintain a phenotype that is similar to the primary cells from which they were derived. Senescence was observed at higher passage numbers, which may explain our inability to clone these cells.
Detailed phenotypic and molecular analysis of hTERT-HDLEC revealed several interesting features. First, they retain typical EC cobblestone morphology, and in agreement with other reports,18 hTERT expression did not induce EC instability or a transformed phenotype. Also, hTERT-HDLEC exhibit contact inhibition, ie, cell density-induced proliferation arrest, and do not form colonies when cultured in soft agar. Second, the cells stably express typical EC markers including PECAM-1 and VE-cadherin. However, they do not express CD34 protein, which is in accord with the currently accepted notion that CD34 is strongly expressed by BEC but not by LEC.
In fact, several reports have demonstrated that CD34 is specifically expressed by blood vascular but not by lymphatic endothelium in neonatal foreskins8,9 and rodent skin.2 However, a recent report7 showed co-expression of CD34 and podoplanin in a subpopulation of endothelial cells in adult human skin. These discrepancies might be related to the age of the donor and/or the donor site (neonatal foreskins versus breast reduction or abdominoplasty). Third, hTERT-HDLEC form tube-like structures in collagen gels when stimulated with the angiogenic and/or lymphangiogenic cytokines FGF-2, VEGF-A, and VEGF-C. Fourth, hTERT-HDLEC express many molecules that have been shown to mediate angiogenesis and/or lymphangiogenesis42,43 such as VEGFRs, integrins, MMPs, and members of the PA family of proteases. Finally, both hTERT-HDLEC and the well-characterized HMEC-1 line express all of currently recognized lymphatic markers analyzed (ie, Prox-1, podoplanin, LYVE-1, VEGFR-3, and integrin α9). The lack of CD34 expression in hTERT-HDLEC supports our conclusion that they are a pure population of lymphatic endothelial cells. In contrast, the fact that HMEC-1 express lymphatic markers as well as CD34 and PAL-E10 suggests that these cells may be hybrid BEC-LEC. Whether this difference between hTERT-HDLEC and HMEC-1 is due to the different immortalization techniques (hTERT versus SV40) is not known.
Expression of CD44 by hTERT-HDLEC may appear to contradict their definition as lymphatic, since it appears that in vivo, expression is restricted to blood vessels. However, recent microarray reports have indicated that cultured LECs express CD448,9 (confirmed by real-time PCR in the latter report). Griffioen et al46 and have suggested that since CD44 is not expressed by HUVECs in vivo, expression in vitro is artifactual. In keeping with this idea is the observation that CD44 expression is increased with passage number and by FGF-2 or VEGF-A in HUVECs and microvascular endothelial cells isolated from the dermis. These observations might be relevant to hTERT-HDLEC.
hTERT was chosen for immortalization since spontaneous transformation or ectopic viral oncogene expression10–13 had previously been shown to generate cells which lose many EC markers and functional characteristics. Furthermore, recent reports have demonstrated successful immortalization of human microvascular EC using hTERT. These cells formed tube-like structures either in three-dimensional collagen or fibrin gels or on matrigel,18 and behaved as early passage primary EC in vitro. Interestingly, hTERT-immortalized human microvascular endothelial cells have been reported to form functional capillaries in vivo.47 However, blood vascular versus lymphatic phenotypic characterization was not assessed in these cells.
Comparative experiments were carried out on endothelial cells obtained from PromoCell and Clonetics in their respective media, and also by switching media. Once the cells are established in culture (ie, on receipt from the manufacturer), we have observed that BEC do not acquire the capacity to express lymphatic markers with increasing passage. The media do not drastically alter the growth rate of one population with respect to the other, as the proportion of both populations remains constant with increasing passage number (PromoCell cells) independent of the culture medium. Our hTERT-HDLEC are a pure population of LEC because they were derived from EC that were already a relatively pure LEC population. This fact, taken together with our own (unpublished) observations on primary EC isolation, suggests that some of the currently used isolation procedures give rise to a greater proportion of LEC than BEC (also reported by Kriehuber et al7), and that the choice of medium may be a critical determinant in early passages (below passage 5).
Both HMEC-1 and hTERT-HDLEC exhibited interesting functional properties. Both responded to angiogenic cytokines, such as FGF-2 and VEGF-A, when sandwiched between two collagen gels or grown in suspension in a three-dimensional collagen matrix. However, we were unable to induce HMEC-1 invasion or tube formation when these cells were seeded on the surface of a collagen gel, irrespective of the angiogenic stimulus (which also included phorbol myristate acetate (PMA)). In contrast, hTERT-HDLEC reproducibly formed measurable tube-like structures when seeded on the top of collagen gels. The robust induction of hTERT-HDLEC invasion by VEGF-A and the synergistic effect of FGF-2 with VEGF-A and VEGF-C is in line with other reports performed on bovine primary EC.33,48 hTERT-HDLEC formed tube-like structures in the sandwich assay or when grown in suspension in collagen gels when stimulated with the lymphangiogenic cytokine VEGF-C. Our data with VEGFR-2-neutralizing antibodies and with VEGF-C156 suggest that the effect of VEGF-C on invasion was mediated by VEGFR-2. VEGFR-3 activation induced proliferation but not tube formation in the three different models. However, specific stimulation of VEGFR-3 potentiated FGF-2-induced proliferation and invasion. Taken together, these findings suggest that VEGFR-3 activation is necessary for proliferation, and also modulates signal transduction pathways induced by other cytokines. More specifically, VEGFR-3 signaling may be linked to the FGF-2 pathway (also reported by Kubo et al49).
Using recently identified markers of lymphatic endothelium, we have characterized two immortalized human microvascular endothelial cell lines namely hTERT-HDLEC (described herein) and HMEC-1.10 From their molecular expression profiles we conclude that hTERT-HDLEC are derived from lymphatic endothelium and represent an immortalized LEC line. HMEC-1 may represent a hybrid BEC-LEC line. hTERT-HDLEC will be useful for the further investigation of lymphatic endothelial physiology and pathology, including lymphangiogenesis.
Supplementary Material
Acknowledgments
We thank Corinne Di Sanza, Mireille Quayzin, Esther Sutter, Evelyne Meier, and Veronique Hoddeand for their excellent technical assistance.
Footnotes
Address reprint requests to Prof. Michael S. Pepper, Department of Morphology, University Medical Center, 1, rue Michel Servet, 1211 Geneva 4, Switzerland. E-mail: michael.pepper@medecine.unige.ch.
Supported by Swiss National Science Foundation grant number 3100–064037.00 (to M.S.P.).
References
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Jackson DG, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci USA. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- Dubois NA, Kolpack LC, Wang R, Azizkhan RG, Bautch VL. Isolation and characterization of an established endothelial cell line from transgenic mouse hemangiomas. Exp Cell Res. 1991;196:302–313. doi: 10.1016/0014-4827(91)90265-v. [DOI] [PubMed] [Google Scholar]
- Candal FJ, Rafii S, Parker JT, Ades EW, Ferris B, Nachman RL, Kellar KL. BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc Res. 1996;52:221–234. doi: 10.1006/mvre.1996.0060. [DOI] [PubMed] [Google Scholar]
- Fontijn R, Hop C, Brinkman HJ, Slater R, Westerveld A, van Mourik JA, Pannekoek H. Maintenance of vascular endothelial cell-specific properties after immortalization with an amphotrophic replication-deficient retrovirus containing human papilloma virus 16 E6/E7 DNA. Exp Cell Res. 1995;216:199–207. doi: 10.1006/excr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- Gagnon E, Cattaruzzi P, Griffith M, Muzakare L, LeFlao K, Faure R, Beliveau R, Hussain SN, Koutsilieris M, Doillon CJ. Human vascular endothelial cells with extended life spans: in vitro cell response, protein expression, and angiogenesis. Angiogenesis. 2002;5:21–33. doi: 10.1023/a:1021573013503. [DOI] [PubMed] [Google Scholar]
- Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- Xu Y, Swerlick RA, Sepp N, Bosse D, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1). J Invest Dermatol. 1994;102:833–837. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]
- Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. 1998;273:6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Litwin M, Clark K, Noack L, Furze J, Berndt M, Albelda S, Vadas M, Gamble J. Novel cytokine-independent induction of endothelial adhesion molecules regulated by platelet/endothelial cell adhesion molecule (CD31). J Cell Biol. 1997;139:219–228. doi: 10.1083/jcb.139.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]
- Persaud K, Tille JC, Liu M, Zhu Z, Jimenez X, Peirera D, Miao HQ, Witte L, Pepper MS, Pytowski B. Involvement of the VEGF receptor-3 in capillary morphogenesis demonstrated with a human anti-human VEGFR-3 antibody that antagonizes receptor activation by VEGF-C. J Cell Sci. 2004;117:2745–2756. doi: 10.1242/jcs.01138. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high-titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83:852–859. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Sappino AP, Stocklin R, Montesano R, Orci L, Vassalli JD. Up-regulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993;122:673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandriota SJ, Pepper MS. Lymphangiogenesis and biological activity of vascular endothelial growth factor-C. J Soc Biol. 1999;193:159–163. [PubMed] [Google Scholar]
- Pepper MS, Mandriota SJ. Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp Cell Res. 1998;241:414–425. doi: 10.1006/excr.1998.4072. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Sonzogni L, Vergani V, Hosseini G, Ceruti R, Ghilardi C, Bastone A, Toschi E, Borsotti P, Scanziani E, Giavazzi R, Pepper MS, Stetler-Stevenson WG, Bani MR. Post-transcriptional stimulation of endothelial cell matrix metalloproteinases 2 and 1 by endothelioma cells. Exp Cell Res. 2000;258:384–394. doi: 10.1006/excr.2000.4936. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-β 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111:743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Rosnoblet C, Di Sanza C, Kruithof EK. Synergistic induction of t-PA by vascular endothelial growth factor and basic fibroblast growth factor and localization of t-PA to Weibel-Palade bodies in bovine microvascular endothelial cells. Thromb Haemost. 2001;86:702–709. [PubMed] [Google Scholar]
- Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial re-programming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veikkola T, Lohela M, Ikenberg K, Makinen T, Korff T, Saaristo A, Petrova T, Jeltsch M, Augustin HG, Alitalo K. Intrinsic versus microenvironmental regulation of lymphatic endothelial cell phenotype and function. EMBO J. 2003;17:2006–2013. doi: 10.1096/fj.03-0179com. [DOI] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival, and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak LV, Jones M. Lymphatic endothelium isolation, characterization, and long-term culture. Anat Rec. 1993;236:641–652. doi: 10.1002/ar.1092360408. [DOI] [PubMed] [Google Scholar]
- Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. alphav β 3 and alphav β 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. Role of α v integrins during angiogenesis. Cancer J. 2000;6(Suppl 3):S245–S249. [PubMed] [Google Scholar]
- Pepper MS. Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86:346–355. [PubMed] [Google Scholar]
- Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Coenen MJ, Damen CA, Hellwig SM, van Weering DH, Vooys W, Blijham GH, Groenewegen G. CD44 is involved in tumor angiogenesis; an activation antigen on human endothelial cells. Blood. 1997;90:1150–1159. [PubMed] [Google Scholar]
- Yang J, Nagavarapu U, Relloma K, Sjaastad MD, Moss WC, Passaniti A, Herron GS. Telomerized human microvasculature is functional in vivo. Nature Biotechnol. 2001;19:219–224. doi: 10.1038/85655. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol. 1998;177:439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.