Abstract
Angiogenesis inhibitors are receiving increased attention as cancer therapeutics, but little is known of the cellular effects of these inhibitors on tumor vessels. We sought to determine whether two agents, AG013736 and VEGF-Trap, that inhibit vascular endothelial growth factor (VEGF) signaling, merely stop angiogenesis or cause regression of existing tumor vessels. Here, we report that treatment with these inhibitors caused robust and early changes in endothelial cells, pericytes, and basement membrane of vessels in spontaneous islet-cell tumors of RIP-Tag2 transgenic mice and in subcutaneously implanted Lewis lung carcinomas. Strikingly, within 24 hours, endothelial fenestrations in RIP-Tag2 tumors disappeared, vascular sprouting was suppressed, and patency and blood flow ceased in some vessels. By 7 days, vascular density decreased more than 70%, and VEGFR-2 and VEGFR-3 expression was reduced in surviving endothelial cells. Vessels in Lewis lung tumors, which lacked endothelial fenestrations, showed less regression. In both tumors, pericytes did not degenerate to the same extent as endothelial cells, and those on surviving tumor vessels acquired a more normal phenotype. Vascular basement membrane persisted after endothelial cells degenerated, providing a ghost-like record of pretreatment vessel number and location and a potential scaffold for vessel regrowth. The potent anti-vascular action observed is evidence that VEGF signaling inhibitors do more than stop angiogenesis. Early loss of endothelial fenestrations in RIP-Tag2 tumors is a clue that vessel phenotype may be predictive of exceptional sensitivity to these inhibitors.
Inhibitors of angiogenesis are now making their way through clinical trials.1–3 Some results with inhibitors of vascular endothelial growth factor (VEGF) are promising,4,5 but challenges are faced in selecting the right patients, determining effective doses, and evaluating responses. Patient selection is made difficult by lack of understanding of which tumors have drug-sensitive blood vessels, and limited information is available on the cellular changes tumor vessels undergo in response to angiogenesis inhibitors. Conventional measurements of microvascular density, one of the most common microscopic methods used to quantify angiogenesis in tumors,6 is not always an accurate measure of efficacy because tumor mass may decrease in parallel with the number of blood vessels.1 Other standard endpoints, such as tumor burden, provide little insight into whether drugs act on blood vessels or tumor cells and may not show whether tumor growth is stabilized by angiogenesis inhibition. Thus, new ways are needed for evaluating vascular effects of angiogenesis inhibitors.
Blood vessels in tumors have multiple abnormalities. Tumor vessels express unique proteins7 and have bizarre morphological features, including loss of arteriole-capillary-venule hierarchy, tortuosity, variable diameter, defective endothelial monolayer, and leakiness.8,9 Even pericytes (mural cells) of tumor vessels are abnormal, as evidenced by altered gene expression and loss of intimate contact with endothelial cells.10,11 Abnormalities of the basement membrane of tumor vessels are also present and reflect the disturbances of endothelial cells and pericytes.12 We reasoned that a better understanding of these abnormalities and how they respond to treatment could give insight into the cellular effects of angiogenesis inhibitors.
One approach to blocking angiogenesis involves inhibition of VEGF.4,13 VEGF and its receptors, VEGFR-1 (flt-1) and VEGFR-2 (flk-1/KDR), play key roles in the formation and growth of normal blood vessels and in tumor angiogenesis.14 There is compelling evidence that VEGF is a survival factor for some tumor vessels and that the growth of some tumors is dependent on VEGF-induced angiogenesis. The strength of the evidence stems in part from the use of multiple different approaches to inhibit VEGF signaling, including neutralizing antibodies against VEGF15 or VEGFR-2,16 anti-sense VEGF cDNA,17 conditional expression of the VEGF gene,18 soluble VEGF receptors,19,20 chimeric proteins consisting of the extracellular domain of VEGFR-1 and VEGFR-2 joined to the Fc portion of IgG,21 adenoviral expression of soluble VEGF receptors22 or dominant-negative VEGFR-2,23 and small molecules that inhibit VEGF receptor tyrosine kinase phosphorylation.24–27
Inhibition of VEGF signaling not only blocks angiogenesis in tumors but can also change or destroy tumor vessels.28–31 VEGF/VEGFR inhibition can decrease the diameter, tortuosity, and permeability of tumor vessels28 and even transform surviving tumor vessels into a more normal phenotype.32,33 Considering the important role of VEGF and its receptors in regulating vascular function, we sought to characterize the changes in tumor vessels produced by agents that block the action of this growth factor.
In the present studies, we examined the cellular effects of two inhibitors of VEGF signaling, VEGF-Trap and AG013736, on blood vessels in spontaneous pancreatic islet tumors in RIP-Tag2 transgenic mice34 and implanted Lewis lung carcinoma (LLC) in syngeneic mice. VEGF-Trap is a decoy construct of VEGFR-1 and VEGFR-2 that inhibits VEGF signaling by selectively binding the ligand and has potent anti-angiogenic activity in preclinical tumor models.21,31 AG013736 is a small molecule inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, and related tyrosine kinase receptors that has potent anti-angiogenic and anti-tumor effects in mice.27,35 We focused on changes occurring during the first week of treatment of established tumors to identify primary vascular effects of the inhibitors that precede or accompany the reduction in tumor growth documented by others.21,27,31,33,35,36 Fluorescence, confocal, and electron microscopic approaches applied to tissues fixed in situ by vascular perfusion provided cellular and molecular readouts for assessing patency and blood flow of individual vessels, endothelial sprouts and fenestrations, VEGFR-2 and VEGFR-3 immunoreactivity, pericyte morphology, and changes in the vascular basement membrane. The orderly microvasculature of the mouse trachea was used to validate some of the methods in a simpler system.37 Cellular changes observed as soon as 24 hours after the onset of treatment reflected drug activity and suggested novel features that may help to identify types of blood vessels that are sensitive to inhibition of VEGF signaling.
Materials and Methods
VEGF/VEGFR Inhibitors
VEGF-Trap (also called VEGF-TrapR1R2), which consists of the second immunoglobulin (Ig) domain of VEGFR-1 and third Ig domain of VEGFR-2 fused to the constant region (Fc) of human IgG1,21 was supplied by Regeneron Pharmaceuticals, Inc., Tarrytown, NY. AG013736, which is a potent small molecule inhibitor of VEGF/platelet-derived growth factor (PDGF) receptor tyrosine kinases (IC50 = 1.2 nmol/L for VEGFR-1, 0.25 nmol/L for VEGFR-2, 0.29 nmol/L for VEGFR-3, 2.5 nmol/L for PDGFR-β, 2.0 for cKit, and 218 nmol/L for FGFR-1),35 was supplied by Pfizer Global Research and Development, San Diego, CA.
Animals and Treatment
Tumor-bearing RIP-Tag2 transgenic mice34 (C57BL/6 background, 10 to 12 weeks of age) were injected with VEGF-Trap (25 mg/kg in a volume of 5 μl/g i.p.) or its vehicle (5 μl/g; Chinese hamster ovary cell-derived human Fc domain in 40 mmol/L phosphate) on day 0 and studied on day 1 or 2 or were injected on days 0, 3, and 6 and studied on day 7. Alternatively, RIP-Tag2 mice were injected with AG013736 (25 mg/kg in a volume of 5 μl/g i.p.) or its vehicle (5 μl/g; 3 parts PEG 400 to 7 parts acidified H2O, pH 2 to 3) twice daily for 1, 2, or 7 days or once daily for 7 or 21 days. In addition, wild-type C57BL/6 mice, 10 weeks of age, with a 1-mm3 piece of LLC tumor implanted under the dorsal skin for 4 to 6 days, were treated with AG013736 (25 mg/kg i.p.) or vehicle twice daily for 7 days. These intervention treatment regimens38 examined the effects of the agents on established RIP-Tag2 tumors and LLC tumors. Normal wild-type mice (FVB/n background, 8 weeks of age) were treated with AG013736 (25 mg/kg i.p.) twice daily for <10 days, VEGF-Trap for 7 days, or vehicle for developing the model using the tracheal microvasculature.
Lectin Injection and Fixation by Vascular Perfusion
At the end of the treatment period, mice were anesthetized with ketamine (100 mg/kg i.m.) plus xylazine (10 mg/kg i.m.). In mice used for immunohistochemistry, blood flow and patency of individual tumor vessels were assessed by injection of 100 μg of fluorescein isothiocyanate-labeled Lycopersicon esculentum lectin in 0.9% NaCl (100 μl into femoral vein; Vector Laboratories, Burlingame, CA).8,11 Two minutes later the chest was opened rapidly, and the vasculature was perfused for 2 minutes at a pressure of 120 mmHg with fixative [1% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, Sigma, St. Louis, MO] from an 18-gauge cannula inserted into the aorta via an incision in the left ventricle. Blood and fixative exited through an opening in the right atrium. Tissues were removed, immersed in fixative for 1 hour at 4°C, and then processed for immunohistochemistry. In mice used for transmission electron microscopy (TEM), tissues were fixed by vascular perfusion of fixative containing 3% glutaraldehyde in 75 mmol/L cacodylate buffer, pH 7.1, 4% polyvinylpyrrolidone, 0.05% CaCl2, and 1% sucrose.8,11 The fixative for scanning electron microscopy (SEM) contained 2% glutaraldehyde in 100 mmol/L phosphate buffer. Fixative was perfused at 120 mmHg for 2 minutes and then 100 mmHg for 3 minutes. Tissues were removed, immersed in fixative for at least 18 hours (4°C), and then processed for TEM or SEM.
Electron Microscopy
For TEM, RIP-Tag2 tumors, LLC tumors, pancreas, thyroid, and tongue were fixed by vascular perfusion and then were trimmed to a maximal dimension of 5 mm, embedded in 7% agarose, and 0.25- to 1-mm slices were cut with a tissue chopper or razor blade. Specimens measuring ∼1 to 3 mm on a side were cut from the slices, rinsed with 100 mmol/L cacodylate buffer, fixed with OsO4 (1% in 100 mmol/L cacodylate buffer at 4°C, 2 hours), rinsed with water, en bloc stained with uranyl acetate (2% aqueous for 48 hours at 38°C), dehydrated with acetone, and embedded in epoxy resin.8,11 Sections 0.5 μm in thickness were stained with toluidine blue for light microscopy, and sections 60 to 80 nm in thickness were stained with lead citrate and examined with a Zeiss EM-10C electron microscope. For SEM, RIP-Tag2 tumors and thyroid glands were fixed by vascular perfusion, trimmed to a maximal dimension of 7 mm, and treated with 30% potassium hydroxide in distilled water for 8 minutes at 60°C to remove the extracellular matrix around blood vessels and tumor cells.39 The specimens were stained with a solution of 2% tannic acid and 1% OsO4, dehydrated with a graded series of ethanol, transferred to isoamyl acetate, and critical point-dried in liquid CO2. Some tumors were cracked with fine forceps under a dissection microscope to expose blood vessels within the specimen. Dried specimens were put on aluminum stubs, coated with OsO4 in an Osmium Plasma Coater (Vacuum Device Corp., Japan), and examined with a Hitachi S-4300N scanning electron microscope.
Immunohistochemistry
Endothelial cells of tumor vessels were evaluated by immunohistochemistry using six primary antibodies: rat monoclonal anti-CD31 (PECAM-1, clone MEC 13.3, 1:1000; Pharmingen, San Diego, CA), hamster monoclonal anti-CD31 (clone 2H8, 1:1000; Chemicon, Temecula, CA), rabbit polyclonal anti-VEGFR-2 (VEGF receptor-2, antibody T014, 1:2000; gift from Rolf Brekken and Philip Thorpe, University of Texas Southwestern Medical Center), goat polyclonal anti-VEGFR-3 (VEGF receptor-3, 1:1000; R&D Systems, Minneapolis, MN), rat monoclonal anti-CD105 (endoglin, clone MJ7/18, 1:500; Pharmingen), and rat monoclonal anti-α5 integrin [CD49e, clone 5H10-27 (MFR5), 1:400; Pharmingen). Pericytes were examined with two primary antibodies: Cy3-conjugated mouse monoclonal anti-α-smooth muscle actin (SMA) (clone 1A4, 1:1000; Sigma Chemical Co., St. Louis, MO) and rat monoclonal anti-PDGFR-β (PDGF receptor-β, clone APB5, 1:2000; gift from Akiyoshi Uemura, Kyoto University, Japan). Vascular basement membrane was examined with rabbit polyclonal anti-type IV collagen antibody (1:10,000; Cosmo Bio Co., Tokyo, Japan).12 Secondary antibodies were goat anti-rat IgG labeled with fluorescein isothiocyanate or Cy3 for rat CD31, CD105, α5 integrin, and PDGFR-β antibodies; goat anti-hamster IgG labeled with fluorescein isothiocyanate for hamster CD31 antibody; donkey anti-rabbit IgG labeled with Cy3 for rabbit anti-VEGFR-2 antibody; donkey anti-goat IgG labeled with Cy3 for goat anti-VEGFR-3 antibody; and goat anti-rabbit IgG labeled with Cy3 or Cy5 for rabbit anti-type IV collagen antibody (1:400; Jackson ImmunoResearch, West Grove, PA).
After fixation by vascular perfusion, tracheas were removed, incised along the ventral midline, and processed as whole mounts to visualize the three-dimensional structure of the microvasculature.37 Other organs and tumors were removed, rinsed several times with PBS, infiltrated with 30% sucrose, and frozen in OCT compound. Cryostat sections were cut 80 μm in thickness for all studies except for measurement of immunofluorescence intensity in which they were cut at 20 μm. Sections were dried on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) for 1 to 2 hours (20-μm sections) or 12 to 15 hours (80-μm sections). Specimens were permeabilized with PBS containing 0.3% Triton X-100 (Lab Chem Inc., Pittsburgh, PA) and incubated in 5% normal serum (Jackson ImmunoResearch) in PBS+ [PBS containing 0.3% Triton X-100, 0.2% bovine serum albumin (Sigma), and 0.01% thimerosal (Sigma)] for 1 hour at room temperature to block nonspecific antibody binding. Sections were incubated with primary antibodies diluted in 5% normal serum in PBS+ for 2 hours (20-μm sections) or 12 to 15 hours (80-μm sections and trachea) at room temperature (20- and 80-μm sections) or 4°C (trachea). Most primary antibodies were used in combination with anti-CD31. Anti-CD31 was used alone to examine the distribution of lectin staining. Control specimens for immunofluorescence intensity analysis were incubated in 5% normal serum instead of primary antibody for the same period. After rinsing with PBS containing 0.3% Triton X-100, specimens were incubated for 1 hour (20-μm sections) or 4 to 6 hours (for 80-μm sections and trachea) at room temperature with fluorophore-conjugated (fluorescein isothiocyanate, Cy3 or Cy5) secondary antibodies diluted in 5% normal serum in PBS+. Specimens were rinsed with PBS containing 0.3% Triton X-100, fixed in 4% paraformaldehyde (PFA), rinsed again with PBS, and mounted in Vectashield (Vector Laboratories).
Fluorescence Imaging
Specimens were examined with a Zeiss Axiophot fluorescence microscope equipped with single, dual, and triple fluorescence filters and a low-light, externally cooled, three-chip charge-coupled device camera (480 × 640 pixel RGB-color images, CoolCam; SciMeasure Analytical Systems, Atlanta, GA) and with a Zeiss LSM 510 confocal microscope with argon, helium-neon, and UV lasers (512 × 512 or 1024 × 1024 pixel RGB-color images). In each mouse, four or five images were obtained from sections cut approximately through the midpoint of RIP-Tag2 tumors, ranging from 0.5 to 2 mm in diameter, or LLCs, measuring ∼5 mm in diameter. Images were obtained from central and peripheral regions of tumors too large to be included in one image.
Fluorescence Intensity Measurements
Fluorescence intensity of blood vessels in 20-μm-thick cryostat sections of RIP-Tag2 and LLC tumors from mice treated with AG013736, VEGF-Trap, or vehicle for 7 days was measured on digital images (×20 objective, ×1 Optovar, tissue region 480 μm by 640 μm) of specimens stained for VEGFR-2, VEGFR-3, α5-integrin, CD31, CD105, or PDGFR-β immunoreactivity. Measurements of mean vessel brightness involved four steps. 1) On the fluorescence microscope, the background fluorescence (nonspecific fluorescence of the tissue) of each image was set to a barely detectable level by adjusting the gain of the charge-coupled device camera, and then the image was captured. The brightness of blood vessel fluorescence was ignored in this step. 2) RGB images were converted to 8-bit gray scale images (fluorescence intensity range: 0 < 255) using ImageJ software (http://rsb.info.nih.gov/ij). 3) The intensity of the background fluorescence was measured on images of tissues (RIP-Tag2 and LLC tumors and normal pancreatic islets) stained with Cy3-labeled secondary antibody without a primary antibody. Analysis of these images showed that >97% of pixels had a fluorescence intensity of <15. A fluorescence intensity of 15 was thus established as the threshold for distinguishing pixels of the vasculature from those of the background. 4) The fluorescence intensity of the vasculature represented the average brightness of all vessel-related pixels. This value was calculated from all pixels with fluorescence intensities ≥15 as follows: the sum of the number of pixels at each fluorescence intensity times that fluorescence intensity was divided by the total number of pixels with brightness ≥15. The mean value was calculated for four images of tissue stained with a particular antibody from each mouse (n = 4 to 8 mice per group).
The distribution of VEGFR-2 intensity values of individual tumor vessels was measured to determine whether the treatment-related decrease in mean vessel brightness was because of selective pruning of vessels with the highest VEGFR-2 expression. For this purpose, the peak VEGFR-2 fluorescence intensity of 10 sequentially selected vessels was measured using the Plot Profile function of ImageJ on each of the digital images just described for RIP-Tag2 tumors with or without AG013736 treatment for 7 days (four images per mouse, n = 4 mice per group).
Tumor Vascularity
An index of area density (proportion of sectional area) was measured in fluorescence microscopic digital images to quantify tumor vessel blood flow (lectin staining of perfused blood vessels), endothelial cells (CD31 immunoreactivity), pericytes (α-SMA immunoreactivity), and vascular basement membrane (type IV collagen immunoreactivity) in 80-μm-thick cryostat sections of RIP-Tag2 and LLC tumors with or without treatment. Blood vessel fluorescence was analyzed in images (×10 objective, ×1 Optovar, tissue region 960 × 1280 μm) captured from sections of four to five tumors (RIP-Tag2) or four regions of tumor (LLC) in each mouse. Based on fluorescence intensities ranging from 0 to 255, blood vessels were distinguished from background by empirically determining threshold values that included only blood vessels in specimens from vehicle-treated mice (intensity value 40 to 55 for RIP-Tag2 tumors and 35 for LLC tumors). The threshold was constant for all measurements of a given marker in each experiment. The area density of blood vessels stained with lectin, CD31, α-SMA, or type IV collagen was calculated as the proportion of pixels having a fluorescence intensity value equal to or greater than the corresponding threshold. An average value was calculated for all tumors or regions of tumor in each mouse (n = 4 mice per group). The values were not true area densities because of the section thickness. Indeed, they overestimated the true area density because measurements were made on two-dimensional projections of three-dimensional (80-μm thick) specimens. However, by including more tissue, thicker specimens expanded the range of possible values and the sensitivity of the measurements. All specimens were handled identically. Curve fitting using a second order polynomial equation with data from 1-μm- to 32-μm-thick projections made from 1-μm confocal optical sections obtained from 80-μm physical sections showed that an area density index of 57.7%, as measured above, corresponded to a true area density of 15.9%. Thus, blood vessels occupied ∼16% of untreated RIP-Tag2 tumors.
Vascularity of Thyroid and Tongue
Blood vessel profiles were quantified in 0.5-μm-thick, toluidine blue-stained epoxy sections of thyroid and tongue with or without treatment on real-time color bright-field video images by using a Zeiss Axiophot microscope with a three-chip charge-coupled device color video camera (model DXC 755; Sony, Tokyo, Japan) interfaced to a real-time color video digitizing card (DVA-4000; Video-Logic, Cambridge, MA) in a Compaq computer (Houston, TX). Measurements were made with a digitizing tablet (Digipad, model 1111A; GTCO, Rockville, MD) and image analysis software developed in our laboratory. Vascular density in sections of thyroid viewed with a ×20 objective (×1 Optovar) was expressed as number of capillary profiles per millimeter of follicle perimeter (10 thyroid follicles per mouse; n = 3 mice per group). Blood vessel profiles in sections of tongue viewed with a ×40 objective (×1 Optovar) were counted in regions measuring 22,337 μm2, and vascular density was expressed as number of vessel profiles per 104 μm2 of lingual muscle in each mouse (n = 3 mice per group).
Number of Endothelial Fenestrations
Fenestrations in endothelial cells of blood vessels in RIP-Tag2 and LLC tumors, thyroid, and tongue smooth muscle with or without treatment were counted on TEM images (total magnification ×17,640, 10 blood vessel profiles per mouse, n = 3 to 4 mice per group). Fenestrations were identified as 60- to 90-nm diaphragm-covered openings in endothelial cells.40 Vessel perimeters were measured with a digitizing tablet on TEM images at ×4480 or ×5600. Values are expressed as mean number of endothelial fenestrations per 100 μm of vessel perimeter.
Estimate of Tumor Vessel Diameter
The effect on vessel diameter of 7-day treatment with AG013736 was measured on printed fluorescent images of RIP-Tag2 tumors stained for VEGFR-2 immunoreactivity (×20 objective, ×1 Optovar, tissue region 480 μm by 640 μm). Three horizontal lines were drawn across each image. The diameter of 10 blood vessels that intersected these lines was measured with the digitizing tablet (four images per mouse, n = 4 mice per group).
Estimate of Tumor Size
The effect of treatment on tumor size was assessed by treating 10-week-old RIP-Tag2 mice with AG013736 or vehicle as above for 21 days (n = 4 mice per group). After fixation by vascular perfusion with 1% PFA, the pancreas was frozen, and 80-μm-thick cryostat sections were stained for type IV collagen immunoreactivity. Digital fluorescence microscopic images of all tumors visible in any of four sections cut at different levels of each pancreas were captured (×5 objective, ×1 Optovar, tissue region 1920 μm by 2560 μm), and then the sectional area of each tumor (8 to 20 tumors per mouse) was measured with ImageJ. Tumors too large to fit into a single image were recorded as multiple images and the data combined. The volume of each tumor was calculated from the sectional area with the assumption the tumors were spherical.
Statistics
The significance of differences between groups was assessed using analysis of variance followed by the Bonferroni-Dunn or Fisher’s test for multiple comparisons. Values are expressed as means ± SE. P values <0.05 were considered significant except where lower values were indicated in Bonferroni-Dunn tests.
Results
Treatment with VEGF-Trap or AG013736 caused multiple changes in the blood vessels of spontaneous RIP-Tag2 tumors and implanted LLC tumors. Changes in endothelial cells of tumor vessels were evident as soon as 24 hours after the onset of treatment, demonstrating the rapid onset of vascular actions of these drugs (Table 1). Both agents had anti-vascular effects in the tumors, with more than 50% of the vessels undergoing regression within 7 days (Table 1).
Table 1.
Effect of VEGF/VEGFR Inhibitors on Endothelial Fenestrations and Vascular Density
| Control | AG013736
|
VEGF-Trap
|
|||
|---|---|---|---|---|---|
| 1 day | 7 days | 1 day | 7 days | ||
| RIP-Tag2 tumor | |||||
| Endothelial fenestrations | 68.0 ± 21.3 | 5.0 ± 3.0* (−93%) | 1.1 ± 0.5* (−98%) | 9.1 ± 3.7* (−87%) | 13.7 ± 5.3* (−80%) |
| Vascular density | 55.7 ± 1.7 | 33.7 ± 2.1* (−39%) | 11.7 ± 0.6* (−79%) | 37.6 ± 2.0* (−32%) | 15.4 ± 1.2* (−72%) |
| Lewis lung carcinoma | |||||
| Endothelial fenestrations | 0 | – | 0 | – | – |
| Vascular density | 23.3 ± 2.3 | – | 11.3 ± 1.2* (−52%) | – | – |
| Thyroid gland | |||||
| Endothelial fenestrations | 52.6 ± 3.3 | – | 6.2 ± 2.7* (−88%) | – | 34.4 ± 3.0 (−35%) |
| Vascular density | 42.5 ± 3.2 | – | 19.2 ± 1.6* (−55%) | – | 45.4 ± 2.0 (+7%) |
| Tongue muscle | |||||
| Endothelial fenestrations | 0 | – | 0 | – | – |
| Vascular density | 24.9 ± 1.9 | – | 26.9 ± 0.3 (+8%) | – | – |
Comparison of endothelial fenestrations and vascular densities in mice with or without treatment. Fenestrations (number per 100 μm of vessel perimeter) were quantified on TEM images of 10 blood vessel profiles per mouse. Vascular densities in tumors (area density index expressed as percent of tumor area) were measured on 80-μm-thick sections stained for CD31 immunoreactivity by calculating proportion of pixels with fluorescence intensity greater than threshold (see Materials and Methods). Vascular densities in thyroid (number of capillaries/mm perimeter of follicles) and in tongue (number of vessels per 104 μm2 of muscle) were measured on toluidine blue-stained epoxy sections 0.5 μm in thickness. Percent change in relation to corresponding control value shown in parentheses.
Significantly different from control value (P < 0.05). Values are mean ± SE; n = 3 to 4 mice per group.
–, Not examined.
Loss of Vessel Patency and Blood Flow before Endothelial Cell Regression
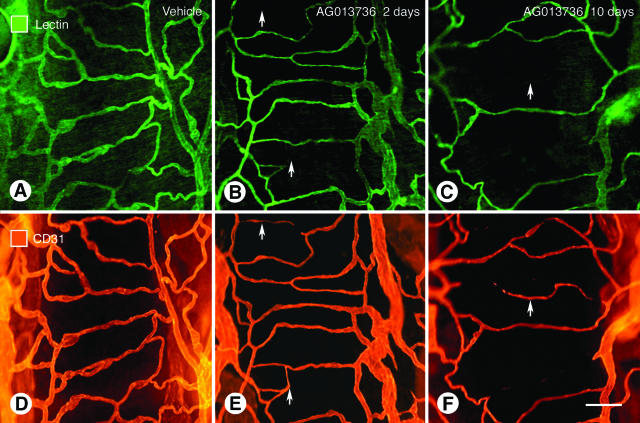
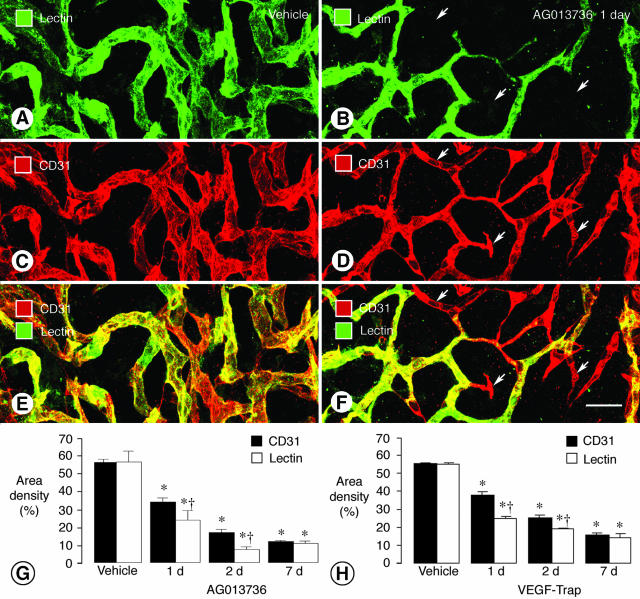
L. esculentum lectin injected into the bloodstream binds rapidly and uniformly to the luminal surface of the vasculature, thus marking blood vessels that are patent and perfused.8,11 In the simple vascular network of the trachea, lectin staining uniformly co-localized with CD31 immunoreactivity (Figure 1, A and D). After 2 days of treatment with AG013736, some CD31-positive vessels lacked lectin staining (Figure 1, B and E), and after a week or more reductions in both markers were evident, indicative of vessel loss (Figure 1, C and F). Similarly, in untreated RIP-Tag2 tumors most blood vessels had uniform lectin staining and CD31 immunoreactivity (Figure 2; A, C, and E), showing the vessels were patent, perfused, and lined by endothelial cells. However, after 1 day of treatment with AG013736, some CD31-positive vessels in RIP-Tag2 tumors lacked lectin staining (Figure 2; B, D, and F). The discrepancy between CD31 immunoreactivity and lectin staining involved 30% of the tumor vasculature at 1 day and 57% at 2 days but was minimal at 7 days (Figure 2G). Similar changes were found with VEGF-Trap (Figure 2H). The amount of lectin staining and CD31 immunoreactivity decreased by more than 70% during the first week, indicative of the extensive vessel loss (Figure 2, G and H). At that point, the amounts of lectin staining and CD31 immunoreactivity were again matched, showing that the nonperfused vessels had degenerated, and vessels that survived treatment were patent and perfused.
Figure 1.
Loss of vessel patency in simple vascular network of trachea after inhibition of VEGF signaling. Fluorescence micrographs showing vasculature of mouse tracheas stained by intravenous injection of lectin (A–C) and same vessels stained for CD31 immunoreactivity (D and E). Three conditions are compared: A and D, normal state; B and E, after 2 days of treatment with AG013736; and C and F, after 10 days of treatment with AG013736. After 2 days of treatment the lectin stains fewer vessels than CD31 because vessel patency is lost and blood flow stops before the CD31 disappears as endothelial cells degenerate. At 10 days, fewer vessels are evident by either method. Arrows mark regions where the lectin staining and CD31 immunoreactivity do not match. Scale bar, 30 μm.
Figure 2.
Reduction in tumor vessel patency and blood flow after inhibition of VEGF signaling. Confocal microscopic images of tumor vessels in RIP-Tag2 mice treated with vehicle (A, C, E) or AG013736 (B, D, F) for 1 day and injected with lectin before fixation by vascular perfusion. Lectin staining (A, green) of blood vessels in vehicle-treated tumor closely matches CD31 immunoreactivity (C, red). However, after treatment with AG013736, multiple vessels (arrows) lack lectin staining (B, green) but have CD31 immunoreactivity (D, red), indicating loss of vessel patency. Vessels without flow appear red in merged images (E, F). Bar graphs show area density (%) of lectin staining and CD31 immunoreactivity in vessels of RIP-Tag2 tumors after treatment for 1, 2, or 7 days with AG013736 (G) or VEGF-Trap (H) or their respective vehicle. After 1 or 2 days of treatment by either agent, the area density of lectin staining is significantly less than the amount of CD31 immunoreactivity. By 7 days, this discrepancy no longer exists, suggesting that the surviving vessels have blood flow. *, Different from vehicle (P < 0.05). †, Different from CD31 (P < 0.05). Scale bar, 25 μm.
Reduction of Endothelial Sprouts and Fenestrations
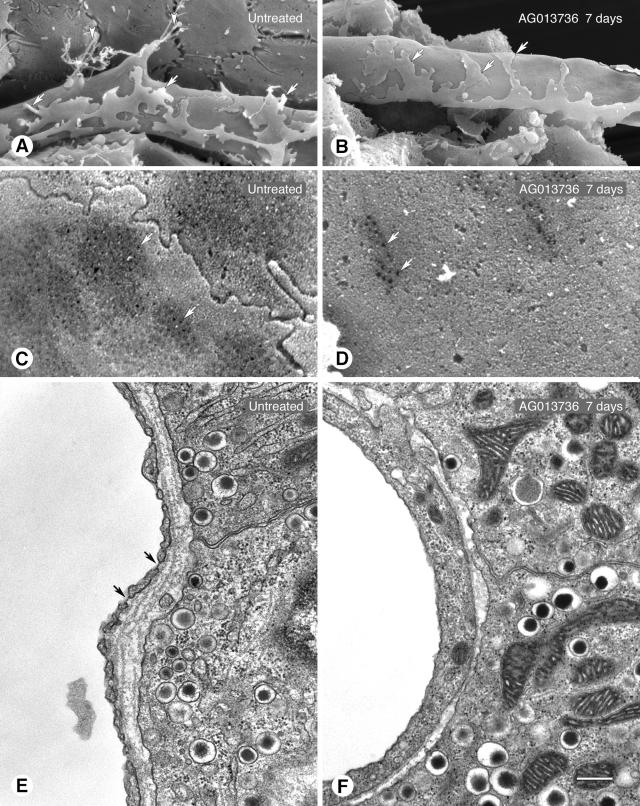
Endothelial sprouts, appearing as cell protrusions tipped by filopodia, were abundant on blood vessels in untreated RIP-Tag2 tumors (Figure 3A) but were rare on tumor vessels after 7 days of treatment with AG013736 (Figure 3B). Most blood vessels of tumors in RIP-Tag2 mice have abundant endothelial fenestrations, as do capillaries of pancreatic islets from which the tumorsarise.8,41 Because VEGF is known to induce fenestrations in endothelial cells40 and targeted deletion of VEGF in pancreatic islets results in nonfenestrated vessels,41 we asked whether inhibition of VEGFR signaling would reduce the number of fenestrations in tumor vessels. Surprisingly, the reduction was rapid and almost complete in RIP-Tag2 tumor vessels (Figure 3; C to F). Treatment with VEGF-Trap for only 24 hours reduced the number of endothelial fenestrations by 87% (Table 1). Treatment with AG013736 reduced fenestrations by 93% after 1 day and by 98% after 7 days (Figure 3, D and F, and Table 1).
Figure 3.
Reduction in endothelial sprouts and fenestrations in RIP-Tag2 tumors after inhibition of VEGF signaling. A and B: SEM of outer surface of tumor vessels showing endothelial sprouts (arrowheads) without treatment (A) but not after treatment with AG013736 for 7 days (B). Processes of pericytes are also visible (arrows), those without treatment being more loosely associated with the endothelium than after treatment. C and D: Higher magnification SEM showing much more abundant endothelial fenestrations (arrows) on abluminal surface of untreated tumor vessel (C) than after treatment (D, AG013736, 7 days). E and F: TEM showing abundance of endothelial fenestrations (arrows) in untreated tumor vessel (E) compared to none after treatment (F, AG013736, 1 day). In sections of tumor vessels examined by TEM, 63% and 83% had no fenestrations in the 1- and 7-day AG013736 groups, respectively, but only 7% had none without treatment. Scale bar: 5 μm (A, B); 1 μm (C, D); 0.5 μm (E, F).
Next we asked whether the presence of endothelial fenestrations was an indicator of vessel sensitivity to the anti-vascular effects of AG013736 and VEGF-Trap. Two approaches were used. First, we examined LLC tumor vessels, which had no endothelial fenestrations (Table 1). The number of blood vessels in LLC tumors was reduced by 52% after 7 days of treatment with AG013736 (Table 1). Although still an appreciable reduction in tumor vascularity, the change in LLC tumors was significantly less than the 79% reduction found in RIP-Tag2 tumors (Table 1). Second, we examined the response of normal blood vessels with or without endothelial fenestrations to VEGF-Trap or AG013736. In blood vessels of the thyroid, which are heavily fenestrated, VEGF-Trap reduced the number of fenestrations by 35% and produced no reduction in vascularity (Table 1). However, treatment with AG013736 for 7 days reduced the number of fenestrations by 88% and caused a 55% reduction in vascularity (Table 1). These changes were not accompanied by weight loss or other obvious evidence of impaired health (data not shown). By contrast, the vasculature of tongue musculature, which had no endothelial fenestrations, showed no reduction in vessel number after treatment withAG013736 (Table 1). Together, the findings suggest that some blood vessels with endothelial fenestrations are dependent on VEGF signaling for survival, and the magnitude of reduction in fenestrations may predict the amount of blood vessel destruction produced by VEGF/VEGFR inhibitors.
Decreased Endothelial Cell VEGFR-2 and VEGFR-3 Immunofluorescence
Because inhibition of VEGF signaling can decrease VEGFR-2 expression in certain types of blood vessels,31,37,42 we asked whether VEGF/VEGFR blockade decreased receptor expression in our tumor models. We reasoned that the intensity of endothelial cell immunofluorescence in digital images could provide a measure of protein expression in blood vessels, if the brightness of vessel-related pixels was distinguished from the number of vessel-related pixels.
Fluorescence intensity was analyzed in microscopic images of blood vessels in immunohistochemically stained sections of RIP-Tag2 tumors, LLC tumors, and normal pancreatic islets after treatment for 7 days with AG013736, VEGF-Trap, or their respective vehicles. Blood vessels in these tissues were immunoreactive for VEGFR-2, VEGFR-3, α5 integrin, CD31, and CD105 in endothelial cells and PDGFR-β in pericytes.
Treatment of RIP-Tag2 mice with AG013736 for 7 days had two effects on tumor vessel immunofluorescence. First, the number of vessel-related pixels decreased with all endothelial cell markers, because of the 79% decrease in number of tumor vessels. Second, the brightness of fluorescence of certain markers decreased in the surviving vessels. VEGFR-2 immunofluorescence showed the largest change in brightness. Fluorescence microscopic images and surface plots of vessel fluorescence intensity showed conspicuous reductions in VEGFR-2 immunoreactivity in addition to the large decrease in vessel number (Figure 4; A to C). Tumors stained for CD105 immunoreactivity showed the same reduction in vessel number but no appreciable change in vessel brightness (Figure 4; D to F). Measurements confirmed that the reduction in brightness of VEGFR-2 immunofluorescence (−49%) was significant but that of CD105 immunofluorescence was not (−10%, Figure 4G).
Figure 4.
Reduction in brightness of receptor immunofluorescence and decrease in vessel number after inhibition of VEGF signaling. Fluorescence micrographs comparing high VEGFR-2 immunoreactivity and high vessel density in vehicle-treated RIP-Tag2 tumor (A) with low VEGFR-2 immunoreactivity and low vessel density after AG013736 treatment for 7 days (B). Reduced brightness of VEGFR-2 immunoreactivity is illustrated by lower peaks in the surface plot of fluorescence intensity (C). Reduced vessel number is indicated by fewer peaks (C). By contrast, after staining for CD105 immunoreactivity, vessel brightness is not reduced by the treatment, but vessel number is clearly reduced (D–F). Consistent with molecule-specific changes in expression levels indicated by brightness of fluorescence, intensity measurements show significant reductions in VEGFR-2, VEGFR-3, and α5-integrin immunoreactivities of RIP-Tag2 tumor vessels after treatment with AG013736 for 7 days but little or no reduction in CD105, CD31, or PDGFR-β (G, values for vehicle normalized to 100%). VEGF-Trap for 7 days also reduced brightness of VEGFR-2 immunofluorescence in RIP-Tag2 tumor vessels, but the change was smaller (H, values for vehicle normalized to 100%). AG013736 had a smaller effect (−16%) on brightness of VEGFR-2 immunofluorescence in LLCs than found in RIP-Tag2 tumors, both in fluorescence micrographs (I, J) and surface plot (K). *, Different from corresponding vehicle (P < 0.05). †, Different from VEGF-Trap (P < 0.05). Scale bar, 80 μm.
To determine whether the decrease in VEGFR-2 immunofluorescence resulted from selective pruning of vessels with the highest receptor expression, thereby leaving vessels with low VEGFR-2 expression, we compared the range of fluorescence intensities of vessel populationsin RIP-Tag2 tumors with or without treatment withAG013736. Because the treatment caused a 79% reduction in vascularity, leaving 21% of the vessels, we compared the dimmest 21% of vessels in untreated tumors to the mean brightness of the surviving vessels. Measurements of individual vessels showed that the fluorescence intensity of the 21% of vessels with least VEGFR-2 expression (intensity = 125 ± 3) in untreated tumors was significantly greater than the 21% of vessels still present after treatment (intensity = 83 ± 4, n = 4 per group, P < 0.001). This finding suggests that the treatment not only reduced vessel number but also reduced vessel VEGFR-2 expression in RIP-Tag2 tumors. Further, the decrease in VEGFR-2 fluorescence appears to reflect a change in epitope density on individual vessels rather than reduced vessel caliber because there was no significant change in vessel size (mean diameter 9.7 μm after vehicle compared to 11.6 μm) after treatment with AG013736 for 7 days.
Other measurements showed that reductions in brightness of VEGFR-3 (−29%) and α5-integrin (−21%) immunofluorescence were also significant (Figure 4G). However, changes in brightness of CD31 (−14%) and PDGFR-β (+5%) were not significant (Figure 4G). VEGF-Trap had a smaller effect in this assay, reducing brightness of VEGFR-2 immunofluorescence in RIP-Tag2 tumors by 18% (Figure 4H). Treatment of LLC tumors with AG013736 for 7 days caused a 52% decrease in vascularity but a much smaller decrease in brightness of VEGFR-2 immunofluorescence (−16%) than was found in RIP-Tag2 tumors (Figure 4; I to K).
Overall, the largest decrease in brightness of VEGFR-2 immunofluorescence in tumor vessels coincided with the largest reduction in vascularity (Table 1), suggesting that diminished immunofluorescence was indicative of robust action on tumor vessels. To the extent that brightness of fluorescence reflects amount of expression, vessels with the highest initial VEGFR-2 expression were most likely to be destroyed or transformed into vessels with lower VEGFR-2 expression.
Normalization of Pericytes on Tumor Vessels
Pericytes identified by α-SMA immunoreactivity are loosely associated with endothelial cells in RIP-Tag2 and LLC tumors.11 Bizarre pericyte-endothelial cell relationships were particularly conspicuous in LLC tumors (Figure 5A). Similar abnormalities were present but less extreme in RIP-Tag2 tumors (Figure 5B).
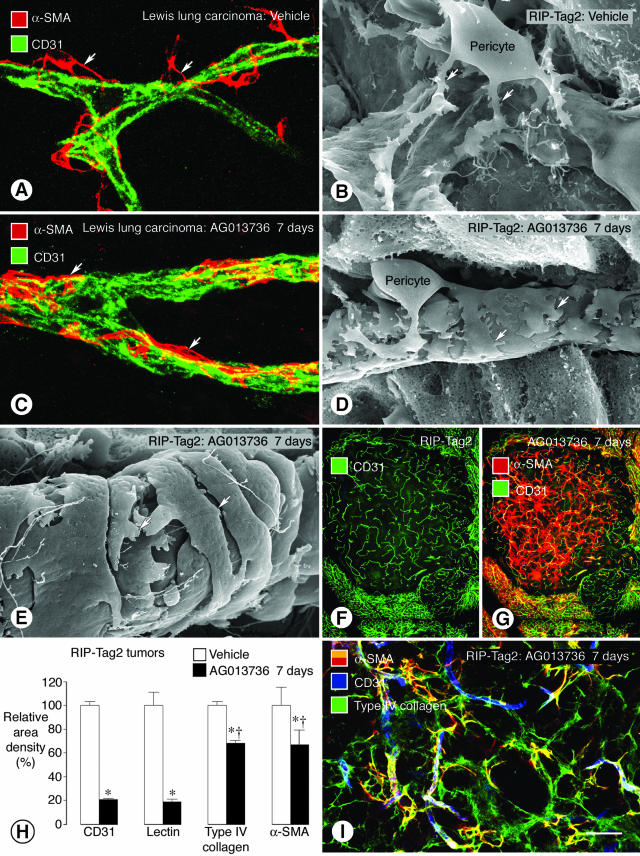
Figure 5.
Tightening of pericytes on tumor vessels after inhibition of VEGF signaling. Confocal microscopic and SEM images show changes in pericyte-endothelial cell relationships (arrows) in tumor vessels. In vessels of LLC, α-SMA immunoreactive pericytes (A, red) are loosely attached to CD31-positive endothelial cells (green). A similar loose association of pericytes to endothelial cells is also evident by SEM in RIP-Tag2 tumor vessels (B). By comparison, after treatment with AG013736 for 7 days, α-SMA-positive pericytes are tighter on endothelial cells imaged by immunofluorescence (C) or SEM (D). Pericytes on some treated vessels acquire a smooth muscle-like phenotype (E). RIP-Tag2 tumor vessels still present after AG013736 for 7 days (F) are accompanied by a disproportionately large number of α-SMA-positive cells, many of which are not associated with blood vessels (G). After treatment with AG013736, reductions in α-SMA and type IV collagen immunoreactivities are about the same but are much less than corresponding reductions in CD31 and lectin (H, values for vehicle normalized to 100%). After the ∼80% reduction in RIP-Tag2 tumor vessels treated with AG013736 for 7 days, most α-SMA-positive cells are still covered by type IV collagen basement membrane (I). *, Different from corresponding vehicle (P < 0.05). †, Different from CD31. Scale bar: 20 μm (A, C); 5 μm (B, D); 500 μm (F, G); 50 μm (I).
α-SMA-positive pericytes underwent multiple changes after treatment with VEGF-Trap (data not shown) or AG013736 for 7 days (Figure 5, C and D). One population of pericytes became closely associated with surviving vessels in LLC tumors (Figure 5C) and RIP-Tag2 tumors (Figure 5D). These pericytes were more tightly apposed to endothelial cells than in untreated tumors (compare Figure 5, B and D). Unlike most pericytes in untreated tumors, some were oriented circumferentially around vessels (Figure 5E), resembling smooth muscle cells on arterioles. Another population of α-SMA-positive cells had no apparent association with blood vessels (Figure 5; F and G). Many of these cells did not co-localize with the surviving CD31-positive blood vessels (Figure 5, F and G). As evidence of the greater impact of treatment on endothelial cells than pericytes, the reductions in CD31 immunoreactivity and lectin staining were more than double the reduction in α-SMA immunoreactivity (Figure 5H). Treatment with AG013736 for 7 days reduced staining of α-SMA-positive pericytes (−33%) to the same extent as type IV collagen in basement membrane (−32%) (Figure 5H). Despite their lack of vessel association, virtually all α-SMA-positive cells co-localized with type IV collagen (Figure 5I). Cells with PDGFR-β immunoreactivity had similar properties (data not shown).
Persistence of Vascular Basement Membrane after Blood Vessel Regression
The basement membrane of blood vessels that regress naturally during development may persist long after endothelial cells disappear.43,44 We asked whether the same occurs in tumors after endothelial cell degeneration is triggered by angiogenesis inhibitors. Using CD31 and type IV collagen immunoreactivities as markers,12 we compared the fates of endothelial cells and vascular basement membrane during blood vessel regression. Initially, the idea was tested in a simple system by comparing the distribution of the two markers in the simple, well-organized microvasculature of the mouse trachea. Under baseline conditions, type IV collagen staining consistently co-localized with CD31 staining of tracheal blood vessels (Figure 6A). However, after treatment with AG013736 for 7 days, distinct strands of type IV collagen unaccompanied by CD31 staining indicated the presence of empty sleeves of basement membrane (Figure 6B). These strands were in locations normally occupied by ∼20% of capillaries. Strands were similarly numerous in tracheas treated with VEGF-Trap. Absence of lectin staining showed that the strands were not blood vessels without endothelial cells (data not shown).
Figure 6.
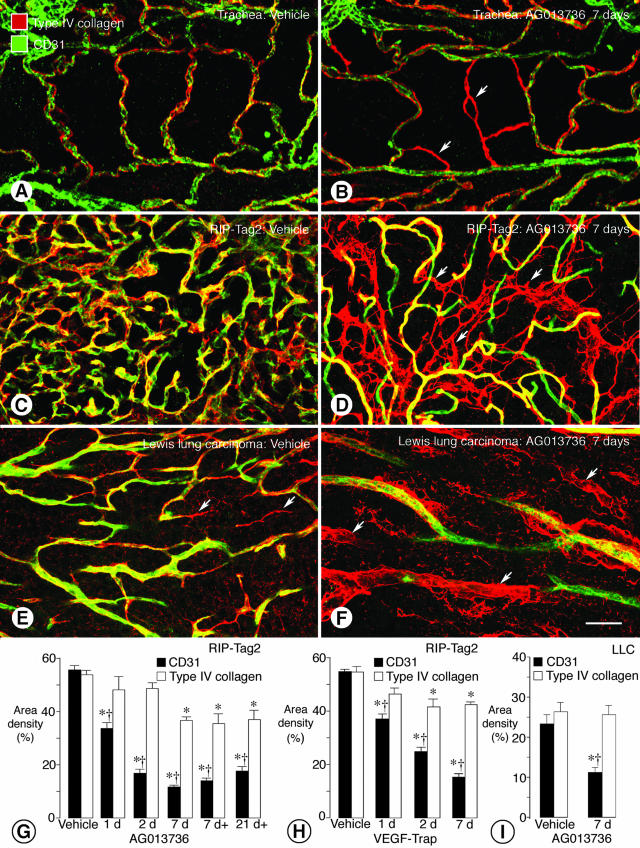
Persistence of vascular basement membrane after endothelial cells degenerate. Confocal micrographs show co-localization of CD31 immunoreactivity (green) and type IV collagen immunoreactivity (red) on simple vasculature of normal adult mouse trachea (A). After treatment with AG013736 for 7 days, sleeves of type IV collagen immunoreactivity devoid of CD31 immunoreactive endothelial cells are present among blood vessels of the tracheal mucosa (B, arrows). Similarly, in vehicle-treated RIP-Tag2 tumors, CD31 and type IV collagen immunoreactivities co-localize on most vessels (C), but after treatment with AG013736 for 7 days, empty sleeves of type IV collagen immunoreactivity are abundant (D, arrows). In LLC, some type IV collagen sleeves are present without treatment (E, arrows) but are much more numerous after treatment (F, arrows). Measurements show that area densities of CD31 and type IV collagen immunoreactivities were about the same in vehicle-treated RIP-Tag2 tumors, but after treatment with AG013736 for 1 to 21 days (d, twice daily; d+, once daily), CD31 immunoreactivity decreased much more than type IV collagen (G). This difference was detectable even at 1 day. A similar discrepancy was found after treatment with VEGF-Trap (H). In LLC, type IV collagen immunoreactivity remained essentially unchanged after treatment with AG013736 for 7 days despite the 52% reduction in CD31 staining (I). *, Different from corresponding vehicle (P < 0.05). †, Different from type IV collagen. Scale bar: 30 μm (A, B); 50 μm (C–F).
As in the trachea, most CD31-positive blood vessels in untreated RIP-Tag2 tumors had type IV collagen immunoreactivity (Figure 6C), and the area densities of the two was essentially the same (56% versus 54%). By comparison, after treatment with AG013736 for 7 days, the conspicuous loss of CD31-positive vessels was not accompanied by a corresponding reduction in type IV collagen (Figure 6D). Numerous strands of type IV collagen, unaccompanied by CD31 immunoreactivity, interconnected the surviving blood vessels (Figure 6D). In LLC tumors, some strands of type IV collagen were present in the absence of treatment (Figure 6E), but they were much more abundant after treatment with AG013736 (Figure 6F). Strands of type IV collagen that did not co-localize with CD31 resembled empty sleeves of basement membrane (Figure 6F).
After treatment of RIP-Tag2 tumors with AG013736 for 1, 2, or 7 days, CD31-positive staining decreased by 39%, 70%, and 79%, respectively, but type IV collagen staining decreased by only 11%, 10%, and 32% (Figure 6G). Once or twice daily treatment with AG013736 for 7 days had about the same effect on the tumor vasculature. Even after 21 days of once-daily treatment, there was still a large discrepancy between the reduction in CD31 (−68%) and the reduction in type IV collagen (−31%). The reduction in CD31-positive vessel density at 21 days tended to be less than at 7 days (Figure 6G), perhaps related to tumor shrinkage after longer treatment. However, the amount of type IV collagen was about the same as at 7 days, indicating persistence of vascular basement membrane for at least 21 days of treatment. Treatment of RIP-Tag2 tumors with VEGF-Trap for 1, 2, or 7 days resulted in qualitatively similar but quantitatively somewhat smaller reductions in type IV collagen and CD31 immunoreactivities (Figure 6H). Treatment of LLC tumors with AG013736 for 7 days reduced CD31 staining by 52% but decreased type IV collagen staining by only 3% (Figure 6I).
Treatment-Induced Decrease in Tumor Size
Treatment of RIP-Tag2 mice with AG013736 for 21 days resulted in a significant decrease in mean tumor size. In the AG013736-treated group, tumor sectional areas were reduced 54% (0.72 ± 0.12 versus 1.55 ± 0.29 mm2) and corresponding calculated volumes were reduced 70% (0.65 ± 0.15 versus 2.20 ± 0.57 mm3) in comparison to vehicle-treated mice.
Discussion
The goal of this study was to identify cellular changes in tumor vessels that reflect the action of VEGF/VEGFR inhibitors and show which vessels depend on VEGF signaling for survival. We used two inhibitors, AG013736 and VEGF-Trap, that block VEGF signaling by different mechanisms. The approach was to characterize changes in endothelial cells, pericytes, and basement membrane of blood vessels in mouse models of spontaneous and implanted tumors, with a focus on the first week of treatment. Among the most conspicuous changes in tumor vessels were early loss of patency and blood flow, almost total disappearance of endothelial fenestrations and sprouts, eventual loss of endothelial cells, and appearance of empty sleeves of blood vessel-derived basement membrane. In RIP-Tag2 tumors, the ∼20 to 30% of vessels that survived treatment for 7 days were distinctly different from those present at the outset. Surviving vessels had a superficially normal appearance and were accompanied by pericytes with a normalized phenotype, but the vessels lacked endothelial fenestrations typical of normal islet vessels and had reduced expression of VEGFR-2 and VEGFR-3. These features not only give insight into the process of blood vessel regression but also reflect the potent anti-vascular activity of VEGF/VEGFR inhibitors.
Loss of Vessel Patency and Blood Flow
Cessation of blood flow in selected blood vessels was an early sign of drug action in RIP-Tag2 tumors. The affected vessels were not stained by lectin in the bloodstream but did have CD31 immunoreactivity. Such vessels were rare before treatment, were abundant at 1 day, peaked at 2 days, and largely disappeared by 7 days. At the final time point, there was minimal mismatch between lectin staining and CD31 immunoreactivity despite the marked reduction in overall number of vessels. These results suggest that vessel closure precedes endothelial cell regression. Although the sequence of events during the first 24 hours still need to be determined, the results thus far are consistent with findings made in the developmentally transient capillary network in the papillary membrane of the eye, which regresses by synchronous apoptosis of endothelial cells after blood flow ceases.45 The wave of apoptosis after cessation of flow may be mediated by VEGF deprivation.46 VEGF/VEGFR inhibition can reduce tumor vessel diameter to the point where blood flow stops.28 Cessation of flow would eliminate the shear stress that plays a role in endothelial cell survival.47 Methods that detect changes in blood flow may prove useful in assessing early evidence of VEGF/VEGFR inhibition, but the effects would be restricted to responsive vessels, and overall tumor blood flow may not reflect the magnitude of changes in individual vessels.32
Loss of Endothelial Cell Fenestrations
Both VEGF-Trap and AG013736 sharply reduced endothelial fenestrations in blood vessels of RIP-Tag2 tumors within 24 hours and nearly eliminated them by 7 days. Considering that VEGF-Trap has selectivity for VEGF,21 the similarity of the effect of the two agents suggests that the loss of fenestrations results from inhibition of a key action of VEGF on endothelial cells. Although AG013736 acts on multiple receptors, it is more potent for VEGFR than for PDGFR-β,35 and the dose used in these studies was chosen to produce plasma concentrations in mice that favor greater inhibition of VEGFR signaling than PDGFR-β signaling (unpublished observations). These findings emphasize that endothelial fenestrations in tumor vessels are not only VEGF-dependent but also dynamic in nature. Topical application of VEGF can induce endothelial fenestrations within 10 minutes in normally nonfenestrated blood vessels of muscle.40 The dynamic nature of endothelial fenestrations in tumor vessels makes these structures potentially useful as a biomarker for assessing the activity of VEGF/VEGFR inhibitors.
The magnitude of the reduction in fenestrations was consistent with extent of vessel loss, both in RIP-Tag2 tumors and normal thyroid. It is unclear whether differences in the magnitude of the effects of the two inhibitors on thyroid vessels reflect differences in local concentration, target specificity, or pharmacokinetics. As VEGF is a known survival factor for some blood vessels18 and an inducer of fenestrations,40 abundant endothelial fenestrations in tumor vessels may be a sign of exceptional susceptibility to VEGF/VEGFR inhibition. A link between endothelial fenestrations and sensitivity to VEGF/VEGFR inhibitors could be useful in identifying tumors that are likely to respond favorably to these agents. Yet presence of endothelial fenestrations is not the only criterion governing susceptibility because more than half of the nonfenestrated vessels in LLC tumors were destroyed by AG013736.
Reduced VEGFR-2 and VEGFR-3 Immunofluorescence
Treatment with AG013736 for 7 days reduced by half the intensity of VEGFR-2 immunofluorescence in blood vessels of RIP-Tag2 tumors but caused a smaller reduction in LLC tumors. VEGF-Trap had a similar but smaller effect on RIP-Tag2 tumors. These changes were superimposed on the large reduction in vessel number. Our experiments addressed the question of whether the intensity of immunofluorescence provides a meaningful index of expression of VEGFR-2, VEGFR-3, and other endothelial cell proteins. We reasoned that changes in receptor immunoreactivity might reflect treatment-induced changes in expression. Our results are consistent with evidence from in situ hybridization and immunofluorescence histochemistry showing that inhibition of VEGF signaling down-regulates VEGFR-2 expression in tumor vessels and certain normal capillaries.31,37,42 Therefore, it may be feasible to monitor the action of VEGF/VEGFR inhibitors by following changes in receptor expression. These findings warrant further study of how angiogenesis inhibitors affect expression of their molecular targets.
Change in Pericyte Phenotype
Pericytes are present on >97% of blood vessels in the tumor models we studied, but they have multiple abnormalities including a loose association with endothelial cells.11 Defective recruitment of pericytes to tumor vessels may result from disturbances in endothelial cell signaling. Blood vessels in mice deficient in PDGF-B or PDGFR-β do not recruit pericytes and have abnormalities reminiscent of those of tumor vessels.10,48 Treatment with AG013736 or VEGF-Trap normalized the phenotype of some pericytes, as manifested by a tighter association with endothelial cells of surviving vessels. This change is consistent with previous reports of the normalization of tumor vessels by VEGF/VEGFR inhibition.32,33 Because this change in pericyte phenotype was found not only with AG013736 but also with ligand-specific VEGF-Trap, it is likely to result from inhibition of VEGF signaling. Receptor tyrosine kinase inhibitors that target PDGFRs reportedly produce pericyte loosening33 or detachment36,49 from endothelial cells of tumor vessels, arguing against a direct role of PDGFR inhibition in the changes we observed.
Blood vessels in some tumors are reportedly susceptible to VEGF deprivation because they are immature as indicated by lack of pericytes.50 This implies that VEGF/VEGFR inhibitors should eliminate pericyte-free vessels and leave ones with pericytes. However, this did not occur. Treatment with AG013736 or VEGF-Trap eliminated more than half of the vessels in RIP-Tag2 and LLC tumors despite the presence of pericytes.11 SU5416 has a similar effect in RIP-Tag2 tumors.36,51 VEGF-Trap eliminates most vessels, including those with pericytes, in intrarenal Wilms’ tumors in mice.31 These observations indicate that disruption of endothelial cell function by antagonism of VEGF signaling leads to altered pericyte function, consistent with the interdependency of endothelial cells and pericytes in angiogenesis.52 They also suggest that functional abnormalities rather than absence of pericytes may contribute to susceptibility of tumor vessels to VEGF/VEGFR inhibition.
Blood vessels still present in RIP-Tag2 tumors after a 7-day treatment differed from those in untreated tumors and from those in normal pancreatic islets. Unlike their untreated counterparts, tumor vessels surviving treatment had almost no endothelial fenestrations and reduced expression of VEGFR-2 and VEGFR-3. Therefore, any normalization induced by treatment did not include reversion of vessels to their original phenotype. We cannot exclude that some of the remaining vessels always had these features and were revealed by pruning away the susceptible ones. But the new phenotype was more likely created by transformation of vessels during treatment, as evidenced by the disappearance of endothelial fenestrations within 24 hours, well before most of the responsive vessels degenerated, and reduction of VEGFR-2 expression to levels not normally found. In any case, the treatment created tumor vessels with a distinct phenotype. The appearance of such vessels in tumors is an indicator of response to VEGF/VEGFR inhibition and evidence of a type of vessel that may be resistant to this treatment.
After treatment with AG013736 or VEGF-Trap, fewer pericytes regressed than endothelial cells. Many surviving α-SMA-positive cells were not associated with tumor vessels, presumably because their endothelial cells had degenerated, but curiously, most were still enveloped by basement membrane. This feature favors the identification of these cells as pericytes instead of myofibroblasts. The similarly small reductions in pericytes and basement membrane raise the possibility that surviving pericytes contribute to the persistence of the vascular basement membrane after endothelial cells degenerate. This possibility suggests that targeting pericytes and/or vascular basement membrane concurrently with endothelial cells may augment the destruction of tumor vessels. The complementary effects of inhibiting VEGFR and PDGFR signaling are consistent with this idea.33,36
Vessel-Like Remnants of Basement Membrane
Our experiments showed that treatment with AG013736 or VEGF-Trap destroyed many blood vessels in RIP-Tag2 and LLC tumors, but the vascular basement membrane, reflected by type IV collagen immunoreactivity, persisted after endothelial cells regressed. These findings are consistent with observations in several other experimental models of vascular regression. The tunica vasculosa lentis, which is part of the hyaloid vasculature of the developing eye, regresses after birth. Regression of the cellular components of the blood vessels leaves sleeves of basement membrane designated intercapillarystrands,44 which appear identical to those we observed in tumors. Similarly, models of injury have shown that the basement membrane of capillaries as well as that of peripheral nerves, skeletal muscle fibers, and renal tubules can persist after the parenchymal elements disappear.43 Blood vessels in tumors and in patients with diabetes mellitus have multiple layers of basement membrane, suggesting that there are recurrent cycles of vascular degeneration and regeneration in chronic conditions.12,43 In the systems we examined, the vascular basement membrane persisted for at least 10 days in the tracheal model and 21 days in RIP-Tag2 tumors. Together, these results suggest that basement membrane ghosts are a historical record of previously existing blood vessels. Thus, unlike loss of endothelial fenestrations that may be VEGF-dependent, the appearance of basement membrane sleeves may be a manifestation of blood vessel regression regardless of the mechanism.
The persistence of the vascular basement membrane after tumor vessels degenerate warrants careful consideration from a cancer therapeutic perspective because empty sleeves of basement membrane may provide a scaffold for vascular regrowth in tumors after cessation of treatment. Injury to a skeletal muscle can create capillaries with two layers of basement membrane, an outer layer formed before the injury and an inner layer formed after the injury,43 suggesting that regenerating endothelial cells use the original outer basement membrane as a scaffold for angiogenesis and form a new inner basement membrane during regrowth. Vessels in RIP-Tag2 tumors appear to regrow along basement membrane strands after treatment is stopped (unpublished observations). The process resembles axonal regeneration in peripheral nerves. Moreover, because the longer isoforms of VEGF have a heparin-binding domain,53 heparan sulfate proteoglycans in the vascular basement membrane may serve as a reservoir of VEGF. For these reasons it may be necessary to consider strategies for targeting basement membrane strands after endothelial cells have been destroyed by VEGF/VEGFR inhibitors.
Functional Significance of VEGF Dependence of Blood Vessels
A key question is whether the observed effects of AG013736 and VEGF-Trap on tumor vessels translate into therapeutic benefit. Both agents were selected because they are potent inhibitors of VEGF signaling and have documented efficacy in preclinical tumor models.21,27,31,35 Although the main focus of the present studies was to analyze direct effects on tumor vessels during the first week, prolongation of treatment to 3 weeks was indeed accompanied by significant reduction in tumor mass. Further studies are needed to evaluate in depth the relationship between vascular changes and anti-tumor efficacy. Additional studies are also necessary to determine whether changes in tumor vessels similar to those we observed are produced by agents that inhibit angiogenesis through mechanisms unrelated to VEGF signaling.
Another important question raised by these studies is the functional significance of VEGF dependence of some normal capillaries in adult animals. A more complete understanding will require further studies, but previous experiments have shown that ∼20% of normal capillaries of the mouse trachea regress after VEGF/VEGFR inhibition and thus appear to depend on VEGF for survival.37 This dependence is age-related but still present in 16-week-old mice.37 The present studies revealed VEGF dependence of an even larger proportion (∼50%) of normal thyroid capillaries in adult mice. In both the trachea and thyroid, all of the susceptible vessels were capillaries. In neither case was the loss of capillaries accompanied by weight loss or other evidence of impaired health,37 but further investigations are warranted, including studies of the dose-response characteristics of normal vessels and tumor vessels to inhibitors of VEGF signaling.
Finally, it will be important to determine whether the presence of endothelial fenestrations in capillaries is a clue to unusual sensitivity to VEGF/VEGFR inhibition. If so, tumors with fenestrated blood vessels, such as those arising in endocrine glands or the gastrointestinal tract, may be particularly sensitive to VEGF/VEGFR inhibition. Novel surrogate markers of vessels with endothelial fenestrations may help to rationalize therapy by predicting which tumors are likely to respond to inhibitors of VEGF signaling.
Conclusions
These studies of cellular changes produced in blood vessels of two mouse tumor models by AG013736 and VEGF-Trap show that these inhibitors of VEGF signaling do much more than block growth of new tumor vessels. The agents had multiple effects that may prove useful in understanding the dependence of tumor vessels on VEGF for survival, the process of blood vessel regression, and the action of angiogenesis inhibitors. Within the first 24 hours, patency was lost and flow ceased in some tumor vessels. Most endothelial fenestrations and sprouts disappeared. Responsive vessels, representing as much as 80% of the tumor vasculature, were eliminated throughout the first week. Surviving vessels had a different phenotype, with no fenestrations and reduced VEGFR-2 and VEGFR-3 expression. Tumor vessels lacking fenestrations were less responsive to the inhibitors. Additional studies will be needed to determine whether all tumor vessels with endothelial fenestrations are dependent on VEGF for survival, but our findings suggest that this phenotype of tumor vessel is exceptionally responsive to VEGF/VEGFR inhibition. Endothelial cell regression occurred despite the presence of pericytes and was not accompanied by simultaneous loss of vascular basement membrane. Sleeves of type IV collagen remained as telltale markers of destroyed vessels. Because basement membrane remnants may serve as a scaffold for tumor revascularization, they provide another potential therapeutic target. These results will hopefully lead to a better understanding of the action of VEGF/VEGFR inhibitors on tumor vessels and stimulate the development of innovative ways to assess their in vivo action and predict which tumors will be most responsive.
Acknowledgments
We thank Barbara Sennino, Michael Rochon-Duck, and Tom Le (University of California, San Francisco) for help with immunohistochemical staining; Douglas Hanahan (University of California, San Francisco) for supplying breeding pairs for our colony of RIP-Tag2 mice; Gyulnar Baimukanova for genotyping the mice; Rolf Brekken and Philip Thorpe at University of Texas Southwestern Medical Center for the VEGFR-2 antibody; Akiyoshi Uemura of Kyoto University, Japan, for the PDGFR-β antibody; and Professor Tatsuo Ushiki of Niigata University, Japan, for generously providing the facilities for scanning electron microscopy.
Footnotes
Address reprint requests to Donald M. McDonald, Department of Anatomy, S1363, University of California, 513 Parnassus Ave., San Francisco, CA 94143-0452. E-mail: dmcd@itsa.ucsf.edu.
Supported in part by the National Institutes of Health (grants HL-24136 and HL-59157 from the National, Heart, Lung, and Blood Institute and P50-CA90270 from the National Cancer Institute), Pfizer La Jolla Laboratories, and AngelWorks Foundation and the Vascular Mapping Project (to D.M.M.).
Competing interests statement: D.D.H. and D.R.S. are employed by Pfizer Global Research and Development and are holders of Pfizer company stock and stock options. G.T. and G.Y. are employed by Regeneron Pharmaceuticals and are holders of Regeneron stock and stock options.
Present address of T.I.: Department of Developmental Molecular Anatomy, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-Ku, Fukuoka 812-8582, Japan.
References
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Mullani NA, Davis DW, Hess KR, McConkey DJ, Charnsangavej C, O’Reilly MS, Kim HW, Baker C, Roach J, Ellis LM, Rashid A, Pluda J, Bucana C, Madden TL, Tran HT, Abbruzzese JL. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol. 2002;20:3804–3814. doi: 10.1200/JCO.2002.05.102. [DOI] [PubMed] [Google Scholar]
- Ellis LM. Antiangiogenic therapy: more promise and, yet again, more questions. J Clin Oncol. 2003;21:3897–3899. doi: 10.1200/JCO.2003.07.977. [DOI] [PubMed] [Google Scholar]
- McCarthy M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet. 2003;361:1959. doi: 10.1016/S0140-6736(03)13603-3. [DOI] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Foss AJ. Endothelial cells of tumor vessels: abnormal but not absent. Cancer Metastasis Rev. 2000;19:109–120. doi: 10.1023/a:1026529222845. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Bohlen P. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]
- Im SA, Gomez-Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Lee HY, Steck PA, Kyritsis AP, Yung WK. Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59:895–900. [PubMed] [Google Scholar]
- Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman CK, Kendall RL, Cabrera G, Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ, Huckle WR, Thomas KA, Curiel DT. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc Natl Acad Sci USA. 1998;95:8795–8800. doi: 10.1073/pnas.95.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Leary MA, Ng CY, Vanin EF. Gene therapy-mediated expression by tumor cells of the angiogenesis inhibitor flk-1 results in inhibition of neuroblastoma growth in vivo. J Pediatr Surg. 2001;36:30–36. doi: 10.1053/jpsu.2001.19998. [DOI] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857–865. doi: 10.1067/msy.2002.127680. [DOI] [PubMed] [Google Scholar]
- Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, Fong TA, Strawn LM, Sun L, Tang C, Hawtin R, Tang F, Shenoy N, Hirth KP, McMahon G, Cherrington JM. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- Drevs J, Muller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, Unger C, Marme D. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- Hu-Lowe D, Hallin M, Feeley R, Zou H, Rewolinski D, Wickman G, Chen E, Kim Y, Riney S, Reed J, Heller D, Simmons B, Kania R, McTigue M, Niesman M, Gregory S, Shalinsky D, Bender S. Characterization of potency and activity of the VEGF/PDGF receptor tyrosine kinase inhibitor AG013736. Proc Am Assoc Cancer Res. 2002;43:A5357. [Google Scholar]
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen RM, Davis DW, Liu W, Zebrowski BK, Wilson MR, Bucana CD, McConkey DJ, McMahon G, Ellis LM. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 1999;59:5412–5416. [PubMed] [Google Scholar]
- Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, Bucana CD, Hicklin DJ, Ellis LM. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–499. [PubMed] [Google Scholar]
- Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, New T, O’Toole K, Zabski S, Rudge JS, Holash J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci USA. 2003;100:7785–7790. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. EMBO J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Wickman G, Hallin M, Dillon R, Amundson K, Acena A, Grazzini M, Herrmann M, Vekich S, McTigue M, Kania R, Bender S, Shalinsky DR, Hu-Lowe DD. Further characterization of the potent VEGF/PDGF receptor tyrosine kinase inhibitor, AG013736, in preclinical tumor models for its antiangiogenesis and antitumor activity. Proc Am Assoc Cancer Res. 2003;44:A3780. [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in VEGF-dependency and angiopoietin-1 induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Ushiki T. Three-dimensional cytoarchitecture of angiogenic blood vessels in a gelatin sheet implanted in the rat skeletal muscular layers. Arch Histol Cytol. 2002;65:347–357. doi: 10.1679/aohc.65.347. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002;5:35–44. doi: 10.1023/a:1021540120521. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold—anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77:314–346. [PMC free article] [PubMed] [Google Scholar]
- Latker CH, Kuwabara T. Regression of the tunica vasculosa lentis in the postnatal rat. Invest Ophthalmol Vis Sci. 1981;21:689–699. [PubMed] [Google Scholar]
- Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- Meeson AP, Argilla M, Ko K, Witte L, Lang RA. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development. 1999;126:1407–1415. doi: 10.1242/dev.126.7.1407. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE, Bucana CD, McMahon G, Ellis LM. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–1468. [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure. 1998;6:637–648. doi: 10.1016/s0969-2126(98)00065-3. [DOI] [PubMed] [Google Scholar]