Abstract
Amyloid β (Aβ) protein immunotherapy lowers cerebral Aβ and improves cognition in mouse models of Alzheimer’s disease (AD). Here we show that Caribbean vervet monkeys (Chlorocebus aethiops, SK) develop cerebral Aβ plaques with aging and that these deposits are associated with gliosis and neuritic dystrophy. Five aged vervets were immunized with Aβ peptide over 10 months. Plasma and cerebral spinal fluid (CSF) samples were collected periodically from the immunized vervets and five aged controls; one monkey per group expired during the study. By Day 42, immunized animals generated plasma Aβ antibodies that labeled Aβ plaques in human, AD transgenic mouse and vervet brains; bound Aβ1–7; and recognized monomeric and oligomeric Aβ but not full-length amyloid precursor protein nor its C-terminal fragments. Low anti-Aβ titers were detected in CSF. Aβx-40 levels were elevated ∼2- to 5-fold in plasma and decreased up to 64% in CSF in immunized vervets. Insoluble Aβx-42 was decreased by 66% in brain homogenates of the four immunized animals compared to archival tissues from 13 age-matched control vervets. Aβ42-immunoreactive plaques were detected in frontal cortex in 11 of the 13 control animals, but not in six brain regions examined in each of the four immunized vervets. No T cell response or inflammation was observed. Our study is the first to demonstrate age-related Aβ deposition in the vervet monkey as well as the lowering of cerebral Aβ by Aβ vaccination in a non-human primate. The findings further support Aβ immunotherapy as a potential prevention and treatment of AD.
Alzheimer’s disease (AD) is the most common form of dementia and afflicts more than 20 million people worldwide. Currently, there is no effective way to prevent or cure this devastating disease. Deposition of extracellular amyloid β protein (Aβ) plaques within the limbic and association cortices and the presence of neurofibrillary tangles (NFT) containing paired helical filaments (PHF) composed of hyperphosphorylated tau are the two major pathological hallmarks of AD.1 Aβ is formed when the β-amyloid precursor protein (APP) is proteolytically cleaved by β- and γ-secretases principally generating 40- and 42-amino acid products.2 In humans, deposition of Aβ42-immunoreactive (IR) diffuse (non-fibrillar) plaques precedes deposition of Aβ40 into more compacted plaques; vascular deposits are more often Aβ40-IR.3,4 Neuritic plaques contain extracellular Aβ surrounded by dystrophic neurites that are often immunopositive for APP and/or phosphorylated tau proteins. Reactive astrocytes frequently surround the perimeter of the amyloid plaque, and activated microglial cells are often detected within and surrounding the core.
Aβ has become a therapeutic target for the prevention and treatment of AD because of its presence in neuritic plaques, its neurotoxicity in vitro and in vivo, and its increased levels in humans with familial AD mutations in APP or the presenilin (PS1, PS2) genes.2 Therapeutic strategies seek to inhibit the Aβ generating proteases (β- and γ-secretases), prevent Aβ aggregation, increase Aβ clearance, prevent Aβ deposition into cerebral plaques, and inhibit the inflammatory response to Aβ deposition. One such strategy involves using Aβ immunotherapy, either by direct Aβ vaccination or passive transfer of Aβ-specific antibodies, to modulate Aβ levels in the central nervous system (CNS).
Aβ vaccination by active immunization with synthetic Aβ peptide or passive transfer with Aβ antibodies has been shown to significantly reduce cerebral Aβ levels5–7 and in some studies, improve cognitive deficits in APP and APPxPS1 transgenic (tg) mice.8–11 In a seminal report, Schenk and colleagues5 demonstrated that intraperitoneal injections of Aβ1–42 peptide with complete or incomplete Freund’s adjuvant almost completely prevented plaque deposition when given before initiation of plaque formation and significantly lowered cerebral levels if given after the initiation of plaque deposition in PDAPP tg mice. Chronic passive transfer of selected Aβ antibodies achieved similar effects.7 Other formulations of Aβ vaccination have been reported and include: intranasal Aβ immunization,6,12 genetically engineered filamentous phages displaying Aβ3–6 (EFRH),13 a soluble non-amyloidogenic, non-toxic homolog of Aβ,14 microencapsylated Aβ,15 and a recombinant adeno-associated virus Aβ vaccine expressing a fusion protein containing Aβ1–42 and cholera toxin B subunit.16 Overwhelming evidence has demonstrated that the antibodies generated by active immunization with Aβ peptide recognize an epitope within the amino-terminus of Aβ protein.6,17–19 However, passive transfer with a monoclonal antibody directed at the mid-region of Aβ (Mab 266, recognizing Aβ13–28) has also been shown to lower cerebral Aβ levels while increasing Aβ levels in the blood.20 Active Aβ immunization was shown to be less effective in reducing cerebral Aβ levels in very old APP tg mice with abundant cerebral Aβ plaques.21 Passive transfer with a single dose of Aβ Mab 266 also failed to reduce Aβ levels in brain but nonetheless improved cognitive deficits in aged APP tg mice.11
A Phase IIa clinical trial in mild-to-moderate AD patients was initiated after a Phase I single and multi-dose study showed that active immunization with Aβ1–42 peptide (AN1792) and an adjuvant, QS-21, was safe and well-tolerated. Within several months, dosing in the Phase II trial was halted due to the development of CNS symptoms in a small number of patients. Follow-up studies have reported that 18 of 298 patients (∼ 6%) who received the vaccine developed meningoencephalitis as compared to 0 of 74 placebo controls; these adverse events did not correlate with the generation of anti-Aβ42 antibody titers.22 Generation of anti-Aβ42 antibodies was detected in serum of the great majority Aβ-immunized AD patients in the Zurich cohort of this trial by enzyme-linked immunoabsorbant sandwich assay (ELISA) and immunohistochemistry on APPxPS1 tg mouse brain.23 In addition, a dose-response relationship between serum antibodies and clinical efficacy was observed in those patients with Aβ antibody titers: the higher the titers, the slower the cognitive decline as measured by three tests over a 1-year period.24 Some evidence of cerebral Aβ clearance was reported for a patient from the Phase I multi-dose trial who became ill after the formulation of AN1792 was changed to include polysorbate-80 and died a year after her last injection of AN1792.25 T lymphocytes in brain parenchyma and leptomeninges and macrophages in white matter indicated that the patient had developed meningoencephalitis. The possible recognition of full-length Aβ peptide as an autoantigen has been suggested as a cause of the deleterious T cell response. Such T cell reactions have not been demonstrated to date in APP tg mice, except if followed by dosing with pertussis toxin.26
Here, we report the first evidence of Aβ clearance from the brain in a non-human primate, the Caribbean vervet (Chlorocebus aethiops), by active Aβ immunization. Unlike the situation in mice, the APP amino acid sequence is homologous between humans and non-human primates,27 and many non-human primates naturally develop extracellular Aβ plaques in brain with aging.28
Caribbean vervets (C. aethiops) are African Green monkeys imported to St. Kitts in the Eastern Caribbean from West Africa between about 1630 and 1700. Approximately 30,000 vervets currently inhabit St. Kitts. These animals are non-endangered, omnivorous, and are considered to be a major agricultural threat.29 The Behavioral Science Foundation (BSF) in St. Kitts was established by Professor Frank Ervin, M.D. (McGill University School of Medicine, Montreal, Canada) in 1968 to study the sociology, behavior, and genetics of the vervet monkeys. Adult males average 5 to 7 kilograms and live 15 to 20 years in the wild and 20 to 30 years in captivity. Caribbean vervets have a genetically “clean” background due to their lack of any of the known African pathogenic viruses.30
Materials and Methods
Animals
The vervets studied in this report were housed and immunized in the Caribbean Primate Laboratories at the Behavioral Science Foundation (BSF) in St. Kitts, Eastern Caribbean. The BSF is a fully accredited biomedical research facility with approvals from the Canadian Council for Animal Care (CCAC) and the USPHS. The ages of the monkeys in this study were estimated based on physical appearance and sexual maturity at the time of acquisition as juveniles; minimal ages are reported and are estimated to be within a 2-year range of accuracy. Initially, archival formalin-fixed, paraffin-embedded brain tissues from three aged vervets (15, 22, and 30 years) were examined for neuropathological changes, including the deposition of Aβ protein. For the Aβ immunization study, 10 animals were selected; 5 vervets (16, 16, 18, 22, 25 years; mean 19.4 years ± 1.8 SEM; 2 females, 3 males) for immunization and 5 vervets (19, 21, 22, 23, 30; mean 23 years ± 1.9 SEM; all females) as untreated controls; no significant difference in age between groups). Between Days 42 and 100, one animal from each group died of pneumonia (immunized male, 25 years; control female, 22 years). Thus, from Day 100 forward, each group consisted of four animals instead of five (immunized group, mean age 18 ± 1.4 versus control group, mean age 23.2 ± 2.4; age difference not significant). Archival unfixed, frozen brain tissue from each of 10 age-matched vervets (range, 15 to 24 years, mean 18.8 years ± 0.9 SEM; 7 females, 3 males) was obtained (courtesy of Dr. Roberta Palmour, McGill University, School of Medicine, Montreal, Canada) for comparison of Aβ levels in brain. For immunohistochemical studies, pieces of frozen cortex were thawed slowly in ice cold 10% neutral-buffered formalin, fixed for 2 hours at room temperature (RT), processed and embedded in paraffin.
Aβ Immunization and Sample Collection
A cocktail of human Aβ peptides (three parts Aβ1–40, one part Aβ1–42, Biopolymer Laboratory, Center for Neurological Diseases, Boston, MA) previously shown by us to be an effective immunogen in wild-type and APP transgenic mice,17,18,31 was diluted at 4 mg/ml in distilled water and incubated overnight at 37°C. Congo red staining revealed minimal presence of Aβ fibrils. Aliquots were frozen at −80°C and defrosted just before use. Five vervets were each given a subcutaneous (s.c.) injection containing 1 mg Aβ (750 μg Aβ1–40, 250 μg Aβ1–42) and 100 μg Complete Freund adjuvant (CFA) on Day 1. Each animal was given 7 s.c. boost injections containing 1 mg Aβ and 100 μg Incomplete Freund adjuvant (IFA) at Days 14, 30, 60, 90, 167, 192, and 265. One day before the initial Aβ immunization, 5 ml of whole blood was drawn into a tube containing 0.5 mol/L EDTA and 1 ml of cerebral spinal fluid (CSF) was collected from the cis-terna magna under anesthesia from each of the immunized and control animals; samples were collected again on Days 42, 100, 251, and 301. The blood was spun at 3000 RPM for 5 minutes, and plasma was collected. Both plasma and CSF samples were stored at −80°C.
Upon euthanasia by anesthetic overdose, the four remaining immunized monkeys and two of the four remaining age-matched control vervets (one from each group had died naturally during the course of the trial) were perfused transcardially with 1 L lactated Ringer’s saline. The brain was removed from the skull and divided in half sagittally. One hemi-brain was frozen at −80°C for biochemical studies. From the remaining hemi-brain, small blocks of tissue were dissected from six equi-distant regions (rostral to caudal), fixed in 10% neutral-buffered formalin for 2 hours at RT, and processed for paraffin sectioning. Adjacent blocks from each brain region were frozen in OCT for cryosectioning.
Characterization of Anti-Aβ Antibodies: IHC, ELISA, and Epitope-Mapping
Monkey plasma and CSF samples were initially tested for the generation of anti-Aβ antibodies using routine immunohistochemistry (IHC) on formic-acid-pretreated paraffin brain sections of human AD patients, J20 APP transgenic mice,32 and aged vervets as previously described,33 except that goat anti-monkey secondary antibody conjugated with horseradish peroxidase (HRP) (Serotec, Raleigh, NC) was used and reacted with 3,3′diaminobenzidine (DAB; Sigma Chemical, St. Louis, MO). Plasma was serially diluted from 1:100 to 1:10,000, and CSF was diluted 1:5.
To measure anti-Aβ antibody levels by ELISA, 96-well plates were coated with 2 μg/ml synthetic Aβ1–40 peptide in 50 mmol/L carbonate buffer (pH 9.6) and incubated overnight (O.N.) at 4°C. Normal monkey IgG (Sigma Chemical) was used as the standard curve for determining anti-Aβ antibody levels in monkey plasma and was incubated in additional wells O.N. at 4°C. Plates were washed twice in PBS-Tween (10 mmol/L phosphate buffer, 2.7 mmol/L KCL, 137 mmol/L NaCl, 0.05% Tween-20) and blocked in 5% goat serum, 1% bovine serum albumin (BSA) (Sigma Chemical), and 0.05% Tween-20 (Sigma Chemical) for 2 hours at room temperature (RT) followed by three washes in PBS-Tween. Monkey plasma samples were diluted in TBS and incubated for 2 hours at RT. The plates were washed three times with PBS-Tween and then incubated for 1 hour with 1 μg/ml goat anti-monkey HRP at RT. After three washes with PBS-Tween, the plates were developed with TMB peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 5 minutes and stopped with the addition of TBS stop reagent. TMB peroxidase reaction results were read at 450 nm on a Benchmark Microplate Reader and recorded in μg/ml.
To map anti-Aβ antibody epitopes by IHC, monkey plasma samples were diluted 1:100 and incubated overnight at 4°C with overlapping Aβ peptide fragments (15 μg/μl plasma) spanning the length of Aβ1–42 (Aβ1–15, Aβ1–5, Aβ1–7, Aβ3–9, Aβ3–13, Aβ6–20, Aβ7–12, Aβ11–25, Aβ16–30, Aβ21–35, Aβ26–42) and Aβ1–40. Peptide-absorbed plasma samples were applied as primary antibody on formic-acid-pretreated paraffin human AD brain sections as previously described.4 Goat anti-monkey conjugated to HRP (Serotec) was used as a secondary antibody, and brain tissue was developed using DAB. A reduction or absence of staining indicates binding of Aβ antibodies to a particular peptide. Anti-Aβ antibodies were also epitope-mapped by ELISA as previously described by Cribbs et al34 Plasma samples were incubated overnight with short overlapping peptides spanning the amino terminus of Aβ (Aβ1–15, Aβ1–7, Aβ3–9, Aβ3–13, Aβ7–12, Aβ11–25) and Aβ1–40. ELISA plates were coated with Aβ1–40 and the procedure was performed as described above. Results were calculated as a percent reduction in optical density (OD) of plasma after absorption with various peptides. A large reduction indicates binding of the anti-Aβ antibodies to the short peptides.
SDS-PAGE and Western Blot Analysis Using Vervet Anti-Aβ Antibodies
Plasma Aβ antibodies were tested for Aβ specificity and cross-reactivity to full-length APP and APP cleavage products by Western blot analysis. APP and C-terminal fragments (APP-CTFs) were extracted from 1B1 (human APP695 transfected) Chinese hamster ovary (CHO) cells. Full-length APP was also extracted from 7PA2 (human APP717 transfected) CHO cells. Both cell lines were obtained from the Selkoe lab (CND). 1B1 and 7PA2 cells were homogenized in lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl, 2 mmol/L EDTA, 1% NP-40) and spun at 14K RPM. Synthetic Aβ1–42, Aβ1–40, 1B1 cell lysate, and 7PA2 cell lysate supernatants were run under denaturing conditions on 16% Tris-glycine gels (Invitrogen, Carlsbad, CA). Proteins were transferred to 0.2-μm nitrocellulose membrane (BioRad, Hercules, CA) and blocked with 5% nonfat milk. Membranes were incubated overnight with C8 [anti-APP polyclonal antibody (Pab)], 6E10 [anti-Aβ monoclonal antibody (Mab), Signet, Dedham, MA], or plasma from control or immunized monkeys. Immunoblots were then incubated with HRP-conjugated secondary antibodies, anti-rabbit for C-8, anti-mouse (Kirkegaard and Perry Laboratories) for 6E10, or anti-monkey (Serotec) for monkey plasma, and developed with SuperSignal Chemiluminescence (Pierce, Rockford, IL).
Aβ Protein ELISA
Snap-frozen monkey brains were homogenized in four volumes of phosphate-buffered saline (PBS) containing protease inhibitor cocktail (Roche, Indianapolis, IN). Homogenates were spun at 100 × g for 30 minutes at 4°C. Supernatants were analyzed for soluble Aβ levels. The PBS pellet was resuspended in 10 volumes of guanidine buffer (5 mol/L guanidine HCL, 50 mmol/L Tris, pH 8.0). Samples were mixed for 4 hours at room temperature. Guanidine extracts were then diluted 1:10 in casein buffer (0.25% casein, 5 mmol/L EDTA, protease inhibitor cocktail in PBS), mixed, and spun at 16,000 × g for 20 minutes. Further dilutions were made in 0.5 mol/L guanidine buffer with 0.1% BSA.
Brain homogenates, plasma and CSF were serially diluted and run on an Aβ protein ELISA as previously described.35 Aβx-40 was detected using Mab 2G3 (Aβ40) as a capture antibody and Mab 266-biotin (Aβ13–28) as a reporter antibody. Aβx-42 was detected using Mab 21F12 (Aβ42) as the capture antibody and Mab 266 as the reporter antibody. Aβ1-total was detected using Mab 266 as the capture antibody and Mab 3D6-biotin (Aβ1–5) as the reporter antibody. ELISA antibodies were kindly provided by ELAN Pharmaceuticals, South San Francisco, CA. Aβ levels in plasma and CSF were normalized to control groups due to variability of the ELISA at the different sample collection time points. The average value of the control group was set at 100%. Immunized samples were reported as a percentage of the controls at each time point.
IHC and Histological Staining of Vervet Brain Sections
Each vervet brain was examined pathologically by immunohistochemical and histological methods. Paraffin sections were immunolabeled as previously described for human AD and Down syndrome brain sections4 using the following antibodies: general Aβ (Pab R1282, 1:1000, Selkoe Lab; Mab 6E10 1:1000, Signet), Aβ42 (Mab 21F12, 1:1000, ELAN Pharm.; Pab Aβ42 1:250, BioSource, Camarillo, CA), Aβ40 (Mab 2G3, 1:1000, ELAN Pharm.; Pab Aβ40 1:250, BioSource), GFAP (astrocyte Pab, 1:1000, DAKO Corp., Carpenteria, CA), HLA-DR (microglial Mab, 1:100, Neomarkers Corp., Fremont, CA), 8E5 (APP Mab, 1:10,000, ELAN Pharm.), and AT8 (phosphorylated tau Mab, 1:100, Innogenetics, Belgium). IHC using Aβ antibodies required formic acid pretreatment while HLA-DR, 8E5, and AT8 antibodies required microwave pretreatment with a citrate buffer (Biogenex, San Ramon, CA). Immunoreactivity was visualized using the Vector Elite ABC kit (Vector Laboratories, Burlingame, CA) and DAB. Sections were counter-stained with hematoxylin, differentiated in acid alcohol, cleared in Histoclear (National Diagnostics, Atlanta, GA), and cover-slipped with Permount (Fisher Scientific, Pittsburgh, PA). Paraffin sections from each animal were also stained using thioflavin S (Sigma Chemicals), a dye that binds fibrillar amyloid, and hemosiderin (2% potassium ferrocyanide, 2% hydrochloric acid) to identify vascular hemorrhage.
For T and B cell immunohistochemistry, 6-μm thick cryosections of OCT-embedded fresh frozen brain were placed onto glass slides and stored at −80°C. For staining, sections were fixed in 4% paraformaldehyde for 5 minutes and endogenous peroxidases quenched with 0.03% H2O2 and 0.1% sodium azide. Next, sections were blocked in 10% normal horse serum in TBS for 30 minutes at RT. The following antibodies were used to detect T cells CD5 1:200 (clone CD5/54/F6) and B cells CD20 (clone L26) both from DakoCytomation (Carpinteria, CA). After incubation overnight at 4°C, a biotinylated horse anti-mouse antibody 1:200 (Vector Laboratories) was added for 30 minutes. This was followed by incubation with avidin peroxidase ELITE kit (Vector Laboratories) and visualized with DAB. Positive (spleen section) and negative (normal mouse IgG) controls were included. To search for vervet immunoglobulins in brain tissue, acetone-fixed cryosections of frontal cortex were blocked in 10% goat serum in TBS O.N. at 4°C and then labeled with goat anti-monkey IgG conjugated with HRP (Serotec) O.N. at 4°C. After washing in 50 mmol/L Tris, the sections were reacted with activated DAB. Sections were counter-stained and cover-slipped as described above.
Aβ Quantification by Visual Inspection and Computer-Assisted Image Analysis
Aβ deposition into cerebral plaques was quantified by visually counting the number of Aβ42 (Mab 21F12-immunoreactive) plaques occupying four 4× fields (∼2.4 × 3 mm) in frontal cortex from each of the four immunized and 13 control vervets. In addition, for each monkey, the maximum percent area of Aβ42 immunoreactivity in the region of highest plaque burden covering a 1.2 × 1.5 mm region was quantified by computer-assisted image analysis using an IP Lab Spectrum 3.1 Image Analyzer (Fairfax, VA). The threshold of detection of immunoreactivity was established and held constant throughout the image analysis.
Peripheral Blood Mononuclear Cell Cultures and T Cell Proliferation Assay
Ten days after the Day 265 Aβ injection, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Ficoll-PlaquePlus (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Whole blood was diluted 1:3 ratio by volume in RPMI 1640 medium (Gibco-Invitrogen Corporation, Grand Island, NY) and layered over Ficoll in a 2.5:1 ratio by volume. Tubes were then centrifuged at 1700 rpm for 30 minutes. PBMCs were then harvested from the Ficoll/plasma interface, resuspended in 10 ml of RPMI 1640 medium and washed twice. PBMCs were resuspended in 5 ml of X-vivo media [RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco-Invitrogen Corporation), 12.5 mmol/L HEPES, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 1% nonessential amino acids, 100 U penicillin and 100 μg/ml streptomycin (BioWhittaker, Walkersville, MD)]. Cells were cultured at 2 × 103 cells per well in a total volume of 200 μl X-vivo media in 30 wells of a 96-well round-bottom plate (Corning Incorporate, Corning, NY) with 5 μg/ml Aβ1–42. The remaining PBMCs were frozen in 10% DMSO (Sigma Chemical)/FBS at −80°C and were used as autologous antigen-presenting cells (APC). On Day 5, 100-μl media was removed and replaced with 100-μl media containing 10 U/ml recombinant human IL-2. On Day 8, 100 μl media was replaced with IL-2-free media. On Day 10, half the cells from each well were re-stimulated with irradiated autologous PBMCs in the presence or absence of 5 μg/ml Aβ1–42 for 48 hours and then pulsed with 1 μCi [3H]thymidine per well for 18 hours. Cells were harvested and radioactivity measured (counts per minute, cpm) in a β scintillation counter. Antigen-reactive T cells were defined by a reading of >500 cpm or a stimulation index [cpm (stimulation)-cpm(background)/cpm(background)] of greater than 2.
Statistical Analyses
Statistically significant differences in Aβ levels between the Aβ immunized and control vervets were assessed using the conservative, non-parametric Mann-Whitney U and Alternate Welch’s t-tests.
Results
Neuropathology of the Aged Vervet Brain
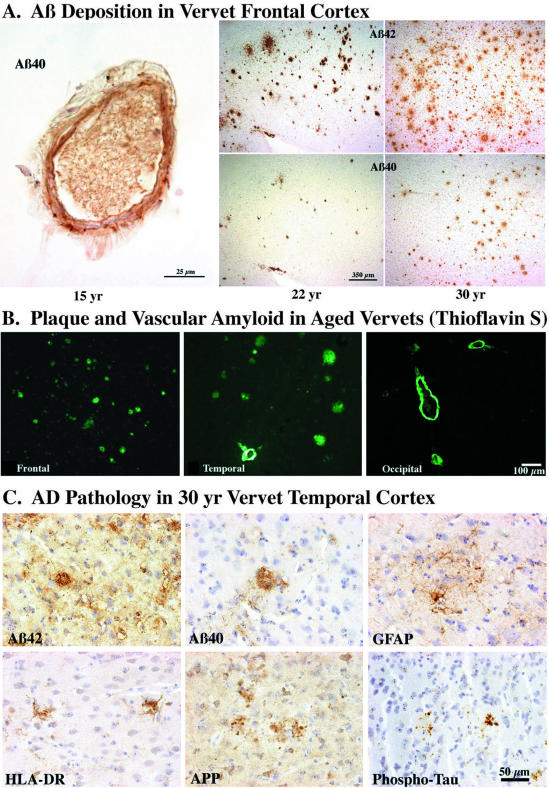
Before the initiation of an Aβ immunization trial, archival brain samples of frontal, temporal and occipital cortices from three aged vervets (15, 22, and 30 years) were examined immunohistochemically and histologically for the presence of Aβ deposition and associated neuropathologic changes. Figure 1 depicts the progression of Aβ deposition in vervet brain with age. At 15 years, Aβ immunoreactivity (IR) was observed in leptomeningeal and cortical blood vessels but not in plaques; vascular amyloid was immunoreactive predominantly for Aβ40 (Figure 1A) but Aβ42 immunoreactivity was also observed in blood vessels. Plaques were detected in all three cortices in 22- and 30-year-old vervets. As in humans, Aβ42 was much more abundant than Aβ40 in plaques in the vervet brain (Figure 1A). Fibrillar amyloid was detected by Thioflavin S in blood vessels at 15, 22, and 30 years of age, and in a subset of plaques at 22 and 30 years (Figure 1B); vascular Aβ labeling was strongest in occipital cortex. The oldest monkey (30 years) showed a full range of plaque-associated pathology, including Aβ42 and Aβ40 in plaques, reactive astrocytes (GFAP-IR) surrounding plaques, activated microglia (HLA-DR-IR), and plaque-associated dystrophic neurites (APP-IR, phospho-τ-IR) (Figure 1C). Numerous neuritic plaques, similar to the quantities seen in human AD cortex, were immunolabeled by an APP antibody in the 30-year-old vervet (data not shown). Only a subset of these neuritic plaques had phospho-tau immunoreactivity. The youngest animal (15 years) lacked glial and neuritic changes while the 22-year-old vervet showed modest plaque-associated changes.
Figure 1.
Neuropathology of the aging vervet brain: a comparison at 15, 22, and 30 years. A: Vascular but not plaque Aβ immunoreactivity (IR) was observed in the frontal cortex of a 15-year-old vervet (left). At 22 years of age, moderate numbers of Aβ42-IR and a few Aβ40-IR plaques were observed in frontal cortex (middle). At 30 years of age, Aβ42-IR plaques were abundant while Aβ40-IR plaques were found in moderate numbers (right). B: Thioflavin S histological staining revealed abundant fibrillar plaques in frontal and temporal cortices in the 30-year-old vervet. Vascular amyloid was prominent in temporal and occipital cortices. C: A full-range of plaque-associated changes was observed in the 30 year vervet including Aβ42- and Aβ40-IR plaques, GFAP-IR reactive astrocytes surrounding plaques, HLA-DR-IR activated microglia within or around plaques, and APP-IR and phospho-τ-IR dystrophic neurites within plaques. Serial sections were used to demonstrate the extent of pathology detected within and around a single plaque in temporal cortex. Bar: A: left, 25 μm, middle and right, 350 μm; B: 100 μm; C: 50 μm.
Generation and Characterization of Vervet Anti-Aβ Antibodies
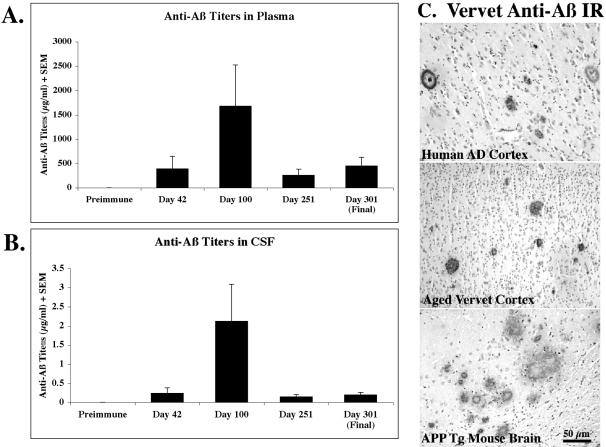
Five vervets (ages 16 to 25 years) were immunized by s.c. injection with 1 mg Aβ1–40/42 plus CFA (Day 1) or IFA (Days 14, 30, 60, 90, 167, 192, and 265). By Day 42 all of the immunized vervets had begun to generate anti-Aβ antibodies (means: 391 μg/ml in plasma, 0.2 μg/ml in CSF). Between Days 42 and 100, one animal from each group (immunized and control) expired due to natural causes associated with aging (ie, pneumonia). Thus, all anti-Aβ antibody and Aβ protein levels reported after Day 42 represent the average of four vervets per group. As shown in Figure 2, the anti-Aβ antibody levels peaked at Day 100 (1677 μg/ml in plasma, 2.1 μg/ml in CSF) in the immunized animals and dropped to relatively steady levels by Day 301 (452 μg/ml in plasma, 0.2 μg/ml in CSF). Aβ antibody levels were consistently far higher in plasma than CSF for all animals (Figure 2, A and B). The anti-Aβ antibodies in vervet plasma were able to detect immunohistochemically plaques in human AD, aged vervet, and APP transgenic mouse brain (Figure 2C). A goat anti-monkey secondary antibody conjugated to HRP was required to visualize the antibody binding to plaques; anti-human secondary antibodies did not allow detection of plaques. Robust plaque and vascular Aβ IR was observed at plasma dilutions of 1:1000 to 1:5000. Weak Aβ immunoreactivity was detected in terminal (Day 301) plasma (diluted to 1:10,000) in plaques in two of four immunized animals and in blood vessel walls in all four immunized animals. Aβ antibodies in immunized vervet CSF were also able to detect human AD plaques and vascular amyloid but required far lower dilutions (1:1 to 1:5) (data not shown).
Figure 2.
Generation of anti-Aβ antibodies in aged vervets. Data reported represent five vervets per group for pre-immune and Day 42; thereafter, data represent four animals per group (see Materials and Methods for ages). Aβ antibodies were not detected in control vervet plasma or CSF at any time point. A: Aβ antibodies were present in the plasma of all immunized monkeys by Day 42. Aβ antibody titers peaked at Day 100 and then dropped and remained relatively constant at Days 251 and 301. B: Anti-Aβ antibody levels in CSF were an order of magnitude lower than plasma but showed the same trend of peaking at Day 100, dropping and leveling off at Days 251 and 301. C: Day 301 plasma samples were diluted 1:100 and used as primary antibody on frontal cortex sections from a 76-year-old female human AD patient, a 30-year-old vervet, and an 18-month-old J20 APP tg mouse.
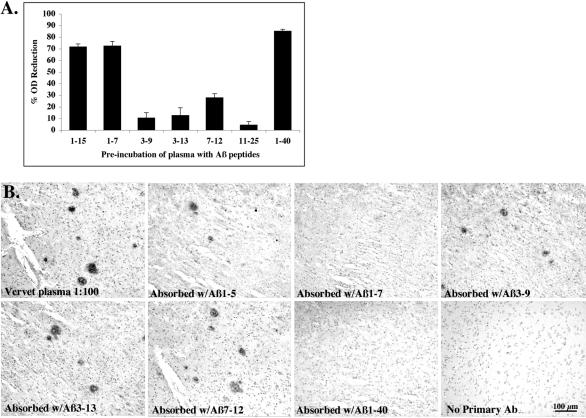
To determine the binding site for the anti-Aβ antibodies on Aβ protein, epitope-mapping was performed by pre-incubating plasma from immunized vervets with individual short Aβ fragment peptides then using the pre-incubated plasma for IHC on human AD brain or for ELISA on Aβ1–40 coated plates. A reduction in binding of the antibodies to Aβ in plaques or on the ELISA plate indicates the binding of the antibodies to the short Aβ peptides. As shown in Figure 3, the anti-Aβ antibodies consistently recognized a B cell epitope within the amino-terminus of Aβ. Pre-incubation of immunized vervet plasma with Aβ1–15, Aβ1–7, and Aβ1–40 reduced the binding of the antibodies to plate-bound Aβ1–40 by 70% or more (Figure 3A). Pre-incubation with Aβ3–9, Aβ3–13, Aβ7–12, and Aβ11–25 resulted in very little reduction in OD, indicating that the antibodies did not bind many of these short peptides and thus were available to bind the Aβ1–40 on the ELISA plate. Vervet plasma, pre-incubated with Aβ1–15, 1–5, 3–9, 3–13, 6–20, 7–12, 11–25, 26–42, or 1–40 peptides, was used as primary antibody to immunolabel plaques in human AD brain. Only pre-incubation with Aβ1–7, Aβ1–15 and full-length Aβ1–40 bound plasma Aβ antibodies and abolished plaque IR (Figure 3B). Interestingly, pre-incubation with Aβ1–5 did not abolish plaque labeling, suggesting that it did not include enough of the Aβ residues to be recognized by the antibodies. We were unable to determine the Ig isotypes of the anti-Aβ antibodies due to a lack of monkey-specific Ig isotype secondary antibodies.
Figure 3.
Epitope-mapping of vervet anti-Aβ antibodies. A: A competitive ELISA was performed by pre-incubating immunized vervet plasma (16-year-old male at Day 301) individually with short Aβ peptide fragments. Bars represent the percent reduction in OD. Pre-incubation of plasma with Aβ peptides 1–15, 1–7, and 1–40 resulted in a 70% or greater reduction in OD, indicating that these peptides bound most of the Aβ antibodies in plasma and thus were not available to bind the plate-bound Aβ1–40. B: Sections from the frontal cortex of a 76-year-old female AD patient were immunostained with the same vervet plasma after pre-incubation with each of ten different Aβ peptides (Aβ1–5, 1–7, 1–15, 3–9, 3–13, 6–20, 7–12, 11–25, 26–42, and 1–40). Figure 3B shows representative examples of the results. Plaque staining was abolished by pre-incubation of vervet plasma with Aβ1–7, 1–15, or 1–40 peptides, indicating that the plasma antibodies bound the peptides during pre-incubation and were unavailable for binding to plaque Aβ protein. Plaque staining was observed with plasma diluted at 1:100 or absorbed with Aβ1–5, 3–9, 3–13, 6–20, 7–12, 11–25, 26–42. Plaque labeling was absent when primary antibody was omitted.
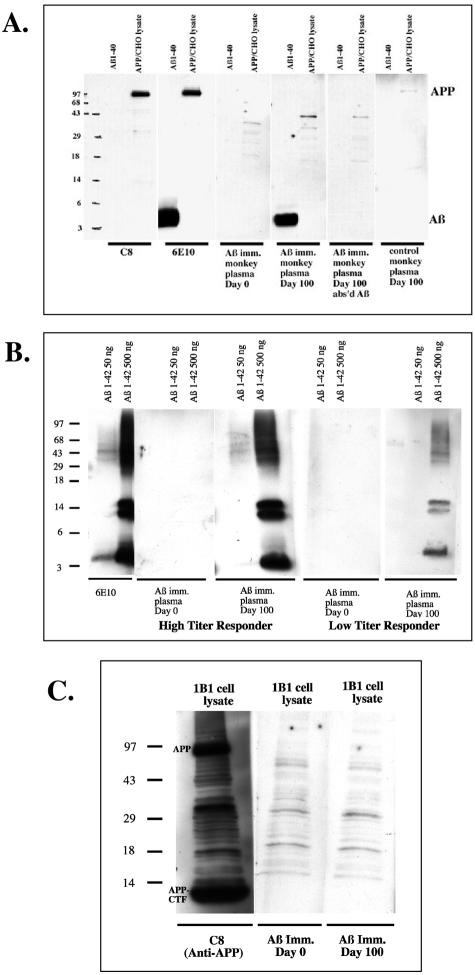
To confirm specificity of the vervet plasma antibodies for Aβ, Aβ1–40 peptide and lysates from APP transfected CHO cells were run on a gel and immunoblotted with vervet plasma (Figure 4). Pre-immune plasma did not recognize Aβ1–40 peptide or full-length APP from APP-transfected CHO lysates. However, plasma from the same animal 100 days into the Aβ immunization trial strongly labeled a 4-kd band a lane loaded with Aβ1–40 peptide (Figure 4A). The same sample did not recognize full-length APP in the APP-transfected CHO lysates. Pre-incubating the Day 100 plasma with Aβ1–40 abolished the labeling of the Aβ band. Control vervet plasma did not detect Aβ or APP (Figure 4A, far right lane) at any of the sample collection time points. In addition to recognition of monomeric Aβ by the vervet plasma antibodies, oligomeric Aβ species were also detected as illustrated in Figure 4B. Two concentrations of Aβ1–42 (50 versus 500 ng) were loaded onto a gel and then immunoblotted with pre-immune or Day 100 plasma from two immunized vervets, one low titer responder and one high titer responder. Pre-immune plasma from both monkeys failed to label Aβ1–42 monomers or oligomers. Plasma from the high titer responder labeled a 4-kd Aβ monomer as well as dimers, trimers, and higher molecular weight oligomers in the lane loaded with 500 ng Aβ1–42 but very little in the 50 ng Aβ1–42 lane. Plasma from the low titer responder showed a similar labeling pattern but the bands were much weaker in the 500 ng Aβ lane and absent from the 50 ng lane. Lastly, none of the vervet plasma samples recognized full-length APP or APP C-terminal fragments (Figure 4C).
Figure 4.
Vervet anti-Aβ specificity. A: Synthetic Aβ1–40 peptide and lysates from APP-transfected CHO lysates (7PA2 cells) were loaded onto gels and blotted with an APP C-terminal Pab, C8, an Aβ Mab, 6E10, or vervet plasma diluted 1:100. APP Pab C8 detected an APP at ∼100 kd in the CHO lysates. 6E10 detected both an Aβ band at 4kD and an APP band. Pre-immune vervet plasma did not label Aβ nor APP however, plasma from the same animal 100 days into Aβ immunization strongly labeled an Aβ band but did not label APP. The Aβ band was abolished with pre-incubation of the plasma with Aβ1–40 peptide before blotting. Control monkey plasma did not recognize Aβ nor APP. B: Two concentrations of Aβ1–42 were loaded on a gel and blotted with 6E10 or vervet plasma. 6E10 detected Aβ monomers (4 kd), dimers (8 kd), trimers (12 kd), and higher molecular weight Aβ oligomers; labeling was much stronger in the lane loaded with 500 ng Aβ peptide compared to that loaded with 50 ng. Plasma from two vervets, one with high Aβ titers and one with low Aβ titers, were used to blot the gel. Pre-immune (Day 0) plasma from both animals failed to detect Aβ. Plasma from Day 100 of the vervet with high titers strongly labeled Aβ monomers, dimers, trimers, and higher molecular weight Aβ oligomers in the lane loaded with 500 ng Aβ1–42. Very weak labeling was observed in the lane loaded with 50 ng Aβ. Plasma from Day 100 of the vervet with low titers moderately labeled Aβ monomers and oligomers in the 500 ng Aβ lane while nothing was detected in the 50 ng Aβ-loaded lane. C: APP-transfected IBI CHO cell lysates were loaded on a gel and blotted with APP Pab, C8, or vervet plasma. C8 labeled full-length APP and APP C-terminal fragments (APP-CTF). Vervet pre-immune plasma recognized neither APP nor APP-CTF. After 100 days of Aβ immunization, plasma from the same animal again failed to recognize APP or APP-CTF, whereas it did recognize Aβ1–40 peptide (A, middle).
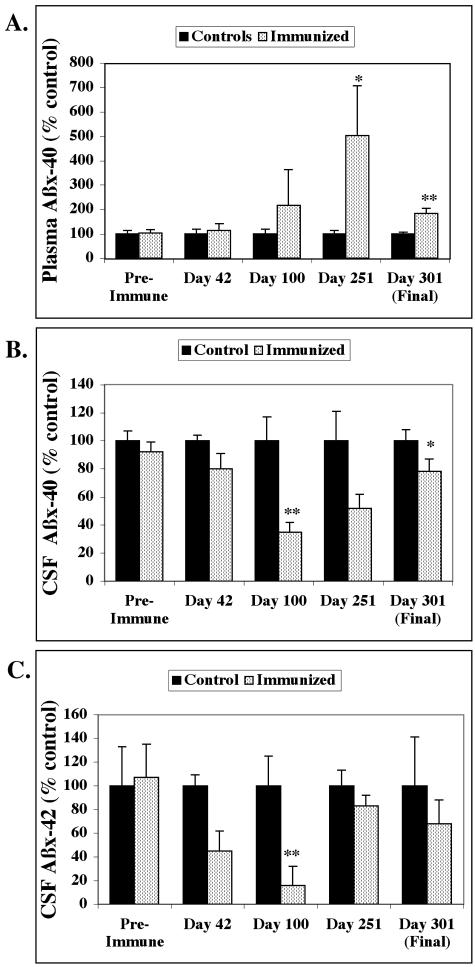
Aβ Protein Levels in Plasma and CSF
Immediately before the first Aβ injection and periodically throughout the immunization period (Days 42, 100, 251, and 301), plasma and CSF samples were collected from each of the immunized and control vervets. Aβ ELISAs were carried out on the samples and the levels normalized to the average of the controls at each time point due to variability in the absolute levels of Aβ on different days (Figure 5). Pre-immune plasma Aβx-40 levels were closely similar between immunized (366 ± 45 pg/ml) and control (354 ± 51 pg/ml) vervets. Plasma Aβx-40 levels began to rise in the immunized animals starting at Day 100 and were elevated 502% at Day 251; low animal numbers and high variability resulted in a trend (P < 0.057) toward significance using the Mann Whitney U (MWU) non-parametric test (Figure 5A). At Day 301, plasma Aβx-40 in the immunized vervets was 184% increased compared to controls (P < 0.029, MWU test). Plasma Aβx-42 levels were too low and inconsistent to obtain meaningful data.
Figure 5.
Aβ levels in plasma and CSF normalized to the average of the controls. Data for pre-immune and Day 42 samples was collected from five vervets per group; data for Days 100, 251, and 301 was collected from four vervets per group. Data are reported as a percentage of the control values. A: Plasma Aβx-40 levels were similar between the immunized and control animals before immunization but increased in the immunized group after anti-Aβ antibodies were generated. Aβ antibody titers peaked at Day 100, however, plasma Aβx-40 levels peaked at Day 251 with an increase of 502% [trend toward significance, P < 0.057 Mann Whitney U (MWU) test] compared to controls. By Day 301, plasma Aβx-40 was significantly increased 184% (P < 0.029, MWU test) in the immunized vervets compared to controls. Aβx-42 levels in plasma were too low and inconsistent to measure in plasma. B: Aβx-40 levels in CSF were similar between immunized and control vervets before immunization but were reduced in the immunized animals by 64% at Day 100 (P < 0.029 MWU test) and 22% at Day 301 (trend toward significance, P < 0.057 MWU test) compared to the controls. C: Aβx-42 levels in CSF were lower than Aβx-42 but were similar between immunized and control vervets before immunization. CSF Aβx-42 levels were reduced after the generation of anti-Aβ antibodies in the immunized monkeys at Days 42 and beyond; however, at Day 100, there was an 84% decrease which due to variability and limited sample size, only showed a trend toward significance (P < 0.057 MWU test). The decrease in CSF Aβx-42 was less dramatic at Days 251 and 301.
As with plasma, Aβ levels in CSF were the same between immunized and control vervets before immunization with Aβ peptide. Pre-immune CSF Aβx-40 was 10 ± 0.8 ng/ml for the immunized group and 10.9 ± 0.8 ng/ml for the controls. By Day 100, CSF Aβx-40 in the immunized vervets dropped a significant 64% (P < 0.029, MWU test) compared to controls (Figure 5B). Aβx-40 levels in CSF remained lower in the immunized group relative to the controls but the 22% reduction observed at Day 301 just missed significance (P < 0.057, MWU). Pre-immune CSF Aβx-42 levels were lower than Aβx-40 but were similar between the immunized (1.4 ± 0.4 ng/ml) and control (1.3 ± 0.4 ng/ml) groups. Aβx-42 in CSF was reduced in the immunized animals and reached an 84% drop at Day 100 (trend toward significance, P < 0.057 MWU test; Figure 5C).
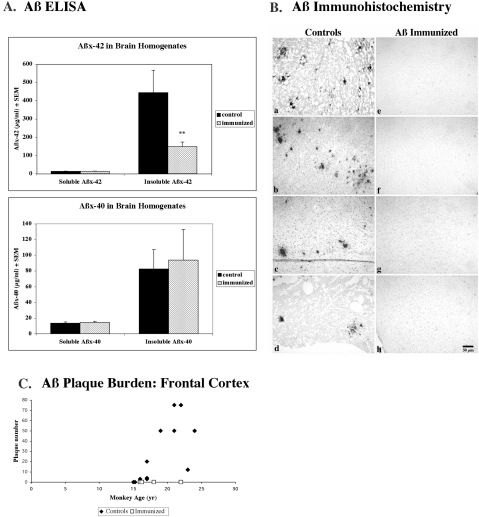
Aβ Protein Levels in Brain
At Day 301, four immunized and two control vervets were euthanized and tissues harvested. Archival, frozen brain tissue from an additional 11 aged vervets, including the control animal that expired during the study, were provided and increased the number of non-immunized age-matched animals to thirteen. The mean ages of the two groups were 18 ± 1.4 years for the immunized animals (n = 4) and 18.8 ± 0.86 years for the combined control animals (n = 13) (Table 1). Soluble and insoluble Aβ levels in brain homogenate were measured using a sensitive Aβ ELISA. No differences in the soluble levels of Aβx-42, Aβx-40, or Aβ1-total were observed between the immunized and control groups (Table 1, Figure 6A). However, insoluble Aβx-42 in brain homogenates was significantly decreased by 66% in the immunized animals compared to controls (P < 0.035 Alternate Welch’s t-test; Table 1, Figure 6A). Insoluble Aβ1-total was reduced 84% in the 4 immunized vervets (55.8 ± 34 μg/ml) compared to the 13 control vervets (346.1 ± 144) but did not quite reach significance due to inter-animal variability and the small number of animals in each group (P < 0.07, two-tailed Alternate Welch’s t-test). Insoluble Aβx-40 levels were essentially the same in the immunized vervets compared to controls.
Table 1.
Aβ Burden in Brain
| [Mean (± SEM)] | Immunized (n = 4) | Control (n = 13) | P value |
|---|---|---|---|
| Age (yr) | 18.0 (±1.4) | 18.8 (±0.86) | |
| No. plaques in frontal cortex | 0 | 26.5 (±8) | |
| Max % area of Aβ42 IR in frontal cortex by IHC | 0.004 (±0.003) | 1.53 (±1.4) | p = 0.0008* |
| Max % area of Aβ42 IR in parietal cortex by IHC | 0.0007 (±0.001) | 0.78 (±1.07) | p = 0.003* |
| Aβx-42 in brain homogenates (μg/ml): | |||
| Soluble | 14.0 (±1.3) | 13.5 (±1.6) | |
| Insoluble | 149.7 (±23.9) | 444.1 (±121.3) | p = 0.035** |
| Aβx-40 in brain homogenates (μg/ml): | |||
| Soluble | 14.4 (±1.5) | 13.6 (±1.4) | |
| Insoluble | 93.8 (±39.2) | 82.5 (±24.7) |
, Mann Whitney U test;
, Alternate Welch’s T test.
Figure 6.
Cerebral Aβ levels. A: Aβ ELISA was used to detect differences in soluble and insoluble Aβ levels in brain homogenates. No differences were observed between immunized (dotted) and control (solid) vervet soluble Aβx-42 or Aβx-40 cerebral levels. However, insoluble Aβx-42 was reduced 66% (P < 0.035, two-tailed Alternate Welch’s t-test) in the four immunized vervets compared to 13 aged age-matched controls. Insoluble Aβx-40 was much less abundant and was not significantly different between the two treatment groups. B: Aβ42 immunohistochemistry using Mab 21F12 on paraffin frontal sections revealed plaque labeling in 11 of 13 age-matched control vervets (a, 22 years; b, 21 years; c, 23 years; d, 17 years). Aβ IR plaques were not detected in frontal cortex of any of the four immunized vervets (e, 22 years; f, 18 years; g, 16 years; h, 16 years); five additional cortical regions per immunized vervet were also devoid of plaque labeling. Bar, 50 μm. C: Aβ deposition into cerebral plaques was quantified by visually counting the number of Aβ42 (Mab 21F12-immunoreactive) plaques occupying four 4× fields (∼2.4 × 3 mm) in frontal cortex from each of the four immunized (open squares) and 13 control (solid diamonds) vervets. Although the two youngest controls (15 years each) did not show any Aβ plaque labeling, all of the older animals (ages 16 to 24 years) showed some plaque labeling.
Aβ immunohistochemistry was performed on frontal and parietal cortical paraffin sections from each of the 13 control monkeys and on six brain regions (including frontal and parietal cortices) from each of the four immunized vervets (Table 1, Figure 6B). Aβ42 immunoreactivity (IR) using Mab 21F12 was observed in plaques and in some cases, blood vessels, in 11 of 13 control vervets (ages 15 to 24 years) (Figure 6B, a to d). Only the two youngest controls, both aged 15 years, were devoid of plaque labeling, but of those, one showed a modest amount of vascular Aβ positive for both Aβ40 and Aβ42. Aβ42 IR was not observed in plaques in any of the six brain regions in any of the four immunized vervets (ages 16 to 22 years) (Figure 6B, e to h). Brain sections of the immunized vervets were also devoid of Aβ plaques using antisera against Aβ40 (Mab 2G3, Pab Aβ40), generic Aβ (Pab R1282), and another Aβ42-specific antibody (Pab Aβ42) for IHC, and Thioflavin S (data not shown). Modest levels of vascular Aβ40- and Aβ42-IR as well as Thioflavin S staining were observed in one or more brain regions in two of the four immunized vervets (ages 16 and 22 years; data not shown). Aβ plaque burdens were quantified by visual inspection (Figure 6C) and computerized image analysis (Table 1). In control vervet frontal cortex, an average of 26.5 (± 8) plaques in an area ∼2.4 × 3 mm was observed by visual inspection through a microscope; plaques were absent from frontal cortex in the four immunized vervets. Computer-assisted image analysis of Aβ42 IR in frontal cortex revealed a significant difference in the maximum plaque density: 1.53% in control animals versus 0.004% (background) in immunized vervets (P < 0.0008, MWU test; Table 1). The maximum plaque density was also significantly different in parietal cortex: 0.78% in controls and 0.0007% in immunized animals (P < 0.003, MWU test; Table 1).
Neuropathological and Cellular Immune Responses to Aβ Immunization
In addition to Aβ immunolabeling, vervet brain sections were examined for the presence of gliosis, neuritic dystrophy, microvessel hemorrhage, IgG infiltration, and T and B cells (data not shown). While the overall number of plaques in the control vervet brain tissue was low-to-moderate, GFAP-IR reactive astrocytes were found associated with many of the plaques in control brain, in addition to the normal, non-pathological pattern of GFAP labeling in blood vessels and white matter. GFAP-labeled astrocytes in the immunized vervet brains were mostly associated with blood vessel walls and white matter. HLA-DR immunoreactive activated microglia were associated with only a small subset of plaques in the control brains and were not present in the brains of immunized vervets. Neuritic dystrophy was minimal in the control animals, with only a few plaques containing APP IR neurites. No τ-IR dystrophic neurites were observed in either group of animals. T cells and B cells were not detected by IHC in any of the vervet brains. Microvascular hemorrhage was not observed by hemosiderin staining in brain tissue of any of the vervets, regardless of treatment group. Lastly, there was no discernible monkey IgG immunolabeling in immunized versus control vervet brain.
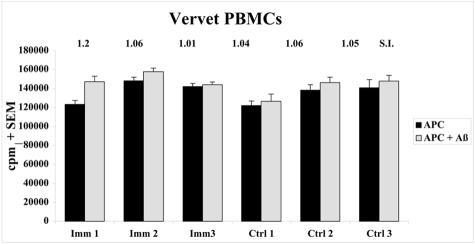
To determine whether Aβ immunization led to a systemic T cell response to Aβ, peripheral blood mononuclear cells (PBMCs) were isolated and cultured from vervet blood that was collected 10 days after the last s.c. Aβ injection. Cultured PBMCs were restimulated with irradiated autologous PBMCs in the presence or absence of 5 μg/ml Aβ1–42 and then pulsed with [3H]thymidine. As shown in Figure 7, no significant differences were observed in the rate of PBMC proliferation in response to Aβ re-stimulation or between three immunized and three control animals.
Figure 7.
Cellular immune response to Aβ peptide. Peripheral blood mononuclear cell (PBMC) cultures were isolated from whole blood collected 10 days after the last Aβ injection on Day 265. After 10 days in culture, cells were re-stimulated with irradiated autologous PBMCs (antigen presenting cells, APC) in the presence or absence of 5 μg/ml Aβ1–42 peptide for 48 hours and pulsed with [3H]thymidine for 18 hours. Cells were harvested and radioactivity measured (counts per minute, cpm). Proliferative T cells were defined by a reading of >500 cpm or a stimulation index (SI) [cpm (stimulation)-cpm(background)/cpm(background)] of greater than 2. No differences in proliferation were observed in antigen-reactive T cells on re-stimulation of PBMCs with Aβ peptide in the PBMCs of three immunized and three control vervets.
Discussion
The two major novel findings of this study are: the first demonstration of Aβ deposition in plaques and blood vessels, along with plaque-associated gliosis and neuritic dystrophy, in the Caribbean vervet monkey, and the first demonstration of the lowering of CNS Aβ by Aβ immunotherapy in a non-human primate. Cerebral plaques have been demonstrated previously in rhesus macaques, lion-tailed macaques, cynomolgus monkeys, orangutans, chimpanzees, squirrel monkeys, marmosets, and lemurs.28,36–48 To our knowledge, Aβ IR plaques have not been reported in vervet brain. As in humans,4,49 Aβ42 was detected earlier than Aβ40 in the plaques of vervet brain, with the initial plaque deposition beginning at approximately 15 to 16 years of age. Aβ40 IR was detected in a subset of plaques in older animals, typically those that were very compacted. Our finding of a predominance of Aβ42 IR plaques is similar to those reported for aged cynomolgus monkeys42 and aged marmosets;48 however, a higher number of Aβ40 IR plaques were observed in aged macaques,50 aged rhesus and chimpanzees,44 and aged orangutans.45 Genetic differences in the various primate species, differences in tissue preparation, staining technique, and in particular, the specificity of carboxyl-terminal end-specific antibodies used for immunostaining, may explain the differences observed in Aβ40 versus Aβ42 plaque detection among studies. As in humans, vascular Aβ reacted primarily for Aβ40, but some vervets also had Aβ42 IR in vessels, as reported previously for cynomolgus monkeys.42 In this cohort of vervets, vascular Aβ deposition preceded plaque deposition, as several of the younger animals (both immunized and controls) showed Aβ IR in blood vessels but not in plaques. Reactive astrocytes were found associated with many Aβ IR plaques, even in the younger animals. However, activated microglia were only observed in a subset of plaques, often compacted, in older animals (> 20 years).
NFTs have not been observed in most non-human primates, but degenerating neurites stained by silver or immunoreactive with antibodies to APP or phosphorylated neurofilament proteins have been associated with a subset of cerebral Aβ plaques in non-human primates.28,36,37,40,41,48 In our initial studies to characterize AD-like pathology in vervets, we detected many neuritic plaques containing dystrophic neurites immunopositive for APP and fewer with phospho-tau immunoreactivity in the oldest vervets (eg, 25 and 30 years), but no NFTs. In the vaccine study, phosphorylated tau IR neurites were not detected by IHC in any of the immunized or age-matched control vervet brain tissues; however, APP IR was detected in a small number of neuritic plaques in the brains of control vervets over 20 years of age, indicating that neuritic dystrophy temporally follows plaque deposition in vervets. Because aged vervet monkeys acquire many of the pathological features of human AD, they can serve as a non-transgenic non-human primate model for cerebral Aβ deposition followed by gliosis and neuritic dystrophy. Cognitive testing of aged vervets has not been performed but is currently underway.
Active Aβ immunization in vervets for 301 days led to the generation of anti-Aβ antibodies, the lowering of Aβ levels in brain and CSF, and an increase in Aβ levels in plasma. Aβ antibodies were detected in plasma, and in much lower quantities in CSF, within 42 days of the initial Aβ peptide injection. As seen with long-term Aβ immunization in APP tg mice (ETS and CAL, unpublished data), Aβ antibody titers peaked at Day 100, dropped and maintained plateau levels throughout the remainder of the study. After the initiation of Aβ antibody production, Aβ levels in CSF decreased while Aβ levels in plasma increased over time, although the limited number of vervets in the study and inter-animal variability diminished the level of significance of these results. Increased Aβ levels in blood following passive transfer of anti-Aβ antibodies and active Aβ immunization in APP transgenic mice have been previously reported11,20,31 and are confirmed by our results in vervets. As has been shown in APP tg mice, a dominant B cell epitope was observed in the amino-terminus of Aβ, specifically within Aβ1–7, for the vervet anti-Aβ antibodies. Interestingly, pre-incubation of immunized vervet plasma with Aβ1–5 peptide did not abolish detection of human AD plaques by IHC or diminish binding of the Aβ antibodies to Aβ1–40 on the ELISA plate, indicating that Aβ residues 6 and 7 were necessary for antibody recognition.
Plasma Aβ antibodies from all four immunized vervets recognized Aβ plaques and some Aβ-containing blood vessels in brain sections of human AD patients, APP tg mice, and vervet monkeys, whereas control vervet plasma did not label Aβ deposits in the adjacent sections. Importantly, anti-human secondary antibodies were unable to reveal plaque staining with the vervet plasma (data not shown); instead, an HRP-conjugated goat anti-monkey secondary antibody was needed to visualize plaques. Hock et al24 reported that tissue amyloid plaque immunoreactivity using human plasma correlated better with a slowing in cognitive decline in 24 AD patients who participated in a Phase IIa clinical trial of active Aβ1–42 vaccination than did Aβ antibody titers determined by ELISA. We found that in our vervet study, Aβ antibody titers by ELISA matched the ability of vervet plasma Aβ antibodies to label AD plaques; higher Aβ titers by ELISA corresponded to plaque detection at higher dilutions of vervet plasma by IHC. Like the anti-Aβ antibodies generated in humans in the Zurich cohort in the Phase IIa trial,23 the vervet anti-Aβ antibodies did not recognize full-length APP nor its C-terminal derivatives. It is unclear why the vervet Aβ antibodies recognize Aβ but not its precursor, however conformational differences between APP and Aβ, or the recognition of the free N-terminus of Aβ by the antibody may provide possible explanations.
Aβ levels in CNS were reduced by Aβ immunization. Following the generation of Aβ antibodies, CSF levels of Aβx-40, the dominant species of Aβ in CSF, and Aβx-42 were reduced in the immunized vervets compared to controls. Due to a high degree of inter-animal variability in Aβx-42 CSF levels, only the reduction in Aβx-40 reached significance. Very low anti-Aβ titers were observed in CSF and may have bound some of the Aβ in CSF. Alternatively, as suggested by the “peripheral sink hypothesis”,20,51 the presence of anti-Aβ antibodies in plasma and CSF may have shifted the equilibrium of Aβ efflux from the CNS to the periphery, resulting in a reduction in Aβ40 in CSF and an increase in Aβ40 in plasma. At sacrifice, insoluble Aβx-42 levels in brain homogenates of the four immunized vervets were a striking 66% lower than those in archival brain tissue from 13 age-matched control animals, confirming our earlier observations of the effects of Aβ immunization on cerebral Aβ levels in APP and PS1xAPP transgenic (tg) mice.6,31 Several possibilities may exist to explain why there was a selective reduction in insoluble Aβx-42, but not insoluble Aβx-40, compared to control levels. First, the pool of insoluble Aβ42 is much larger than insoluble Aβ40 in the aged vervet (as in aged APP tg mice) providing more molecules for binding anti-Aβ antibodies and clearing Aβ. Second, because our Aβ ELISAs measure Aβx-40 and Aβx-42, and not Aβ1–40 or 1–42, it is likely that Aβ species with heterogeneous truncated N-termini were detected. The vervet Aβ antibodies recognize an epitope within Aβ1–7 and therefore may not bind Aβ species with heterogeneous truncated N-termini as well as those beginning at Asp 1. If Aβ ending at residue 40 is more heterogeneous at the amino-terminus than Aβ ending at residue 42, one might expect less clearing of Aβ40. The same vervet brain homogenate samples, when run on an ELISA that detects Aβ beginning at Asp 1 but ending at heterogeneous Aβ C-termini (Aβ1-total), showed an even larger 84% reduction [a non-significant trend (P < 0.07) due to variability between animals in each group], confirming that the Aβ antibodies were most effective at binding and clearing Aβ starting at Asp 1. Third, several reports [including our own work in PSAPP tg mice (unpublished observation) and vervets, and that of Nicholl et al in a human case study25] have shown that Aβ immunization is not as effective at clearing vascular amyloid compared to plaque Aβ in brain. It is possible that the remaining insoluble Aβ40 and Aβ42 levels were not cleared because they existed primarily in blood vessel walls.
In addition, Aβ plaque burden was absent from six cortical regions in each of the four immunized vervets, while plaques were observed in low-to-moderate numbers in frontal and parietal cortices in 11 of 13 age-matched controls. The two youngest control vervets (15 years each) were devoid of any Aβ plaque IR, but leptomeningeal and cortical vessels had Aβ40 and Aβ42 IR in one of these two animals. The absence of plaques in the immunized vervets is likely due to the effects of Aβ immunization, but it is possible that in the two youngest immunized animals (16 years each), the absence of plaques was age-related. However, this is unlikely as one 16-year-old, four 17-year-olds, and one 19-year-old control vervets had Aβ42 plaque deposition in frontal, and in some cases, parietal cortex. This finding, combined with the reduction in Aβ in CSF on generation of Aβ antibodies in the immunized vervets compared to controls throughout the Aβ immunization period, strongly suggests a correlation between the presence of anti-Aβ antibodies and the lowering of Aβ protein in the CNS.
Several mechanisms have been proposed to explain the clearance of cerebral Aβ and improvement in cognitive deficits in APP tg mice following Aβ immunization. Aβ antibodies have been shown to cause disaggregation of amyloid fibrils or prevention of the aggregation of soluble Aβ into fibrils.52,53 It is possible that the Aβ antibodies in the vervets bound and dissolved Aβ aggregates before or shortly after plaque deposition. Aβ antibodies have been shown to bind to Fc receptors on microglial cells in the brain, inducing phagocytosis of Aβ.7 Although we did not detect activated microglia in the brains of the immunized vervets at the time of euthanization (Day 301), it is possible that the animals may have undergone transient microglial activation at the height of antibody generation (Day 100), as has been demonstrated in with both active and passive Aβ immunization in PSAPP tg mice.54,55 If so, the activated microglia may have phagocytosed the early mostly-diffuse Aβ cerebral deposits expected to exist in the immunized vervets based on observations of the age-matched control animals. In addition, Aβ antibodies in the periphery have been demonstrated to act as a “sink” by enhancing clearance of Aβ from the brain to the blood.20,31 In our study, the largest pools of Aβ (insoluble Aβx-42 in brain, and soluble Aβx-40 in CSF and plasma) were effected most strongly by the presence of the Aβ antibodies: Aβ in the CNS was reduced while Aβ was increased in the periphery (ie, plasma). The proposed mechanisms may work together or independently. Our findings of reduced Aβ levels in CNS and increased Aβ in the periphery may be supported in part by all of the aforementioned mechanisms, although our data supports the peripheral sink hypothesis most strongly. We did not observe monkey IgG labeling of plaques in the immunized vervet brain, consistent with our findings in PS1xAPP tg mice31 and those of others in APP tg mice.20 However, plaques were not detected in brain tissue of any of the immunized vervets; thus it is possible that the antibodies may have bound plaques at one point in time but were gradually cleared.
Immunizing vervets with a self-antigen, human synthetic Aβ40/42 peptide, did not elicit any detectable adverse effects. We detected no difference in proliferation of PBMCs of immunized and control vervets on restimulation with synthetic Aβ1–42 peptide. However, our methods may not be sensitive enough to detect small, Aβ-reactive T cell populations. Monsonego et al reported that multiple re-stimulation of PBMCs from blood of aged humans allowed enrichment and identification of a subset of Aβ-reactive T cells.56 Thus, it is possible that we may have missed small subsets of Aβ-reactive PMBCs in the immunized vervets. B and T cells, microvessel hemorrhage, gliosis, and neuritic dystrophy were absent from brain sections of immunized vervets. T cell lymphocyte infiltrates were reported in the brain of one human AD patient following Aβ vaccination25 but were not detected in the four immunized vervets in our study. Previously, microvessel hemorrhage was reported in an APP tg mouse model with abundant deposition of Aβ in blood vessels.57 Although vascular Aβ deposition was observed in both immunized and control vervets, no signs of microvessel hemorrhage were detected by hemosiderin staining. Overall, the immunized vervets maintained good health throughout the study and showed no overt signs of inflammation.
In conclusion, we have shown that aged Caribbean vervets may serve as a compelling model of the Aβ deposition, gliosis, and neuritic dystrophy observed in human AD brain. Cognitive assessment will be necessary to determine whether such pathological changes have an impact of the behavior of these animals. This is the first study to show modulation of Aβ levels in CNS and the periphery by active Aβ immunization in a non-human primate. Future studies in aged vervets using novel vaccine formulations and incorporating cognitive testing before and after Aβ immunization should yield important information toward the development of safe and effective vaccines for the prevention and possible treatment of AD in humans.
Acknowledgments
We thank Drs. Alon Monsonego and Victor Zota (CND, Boston) and Dr. Keith Mansfield (New England Regional Primate Research Center, NERPRC, Boston) for their advice concerning culturing PBMCs, conducting T cell proliferation studies, and tissue preparation. We also thank Drs. David Holtzman and Ronald DeMattos (Wash. U. School of Medicine, St. Louis and Eli Lily, Indianapolis, respectively) for confirming the detection of Aβ in vervet CSF and plasma before Aβ immunization. Dr. Marcel Maier (CND, Boston) is thanked for providing comments on the manuscript.
Footnotes
Address reprint requests to Cynthia A. Lemere, Ph.D., Center for Neurologic Diseases, HIM 622, 77 Avenue Louis Pasteur, Boston, MA 02115. E-mail: clemere@rics.bwh.harvard.edu.
Supported by the Foundation for Neurologic Diseases (Boston, MA) and the Behavioral Science Foundation (St. Kitts, Eastern Caribbean).
References
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Mann DM, Odaka A, Suzuki N, Ihara Y. Amyloid β protein (Aβ) deposition: aβ42(43) precedes Aβ40 in Down syndrome. Ann Neurol. 1995;37:294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Blustzjan JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid β-peptides and Apo E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Disease. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vendevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A β peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A β peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a transgenic model of Alzheimer’s disease. J Neuroscience. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart J-C, Bales K, Gannon K, Greene S, DeMattos R, Mathis C, DeLong C, Wu S, Wu X, Holtzman D, Paul S. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nature Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal Aβ treatment induces anti-Aβ antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann NY Acad Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Katz O, Solomon B. Immunization against Alzheimer’s β-amyloid plaques via EFRH phage administration. Proc Natl Acad Sci USA. 2000;97:11455–11459. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. Am J Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden D, Templeton L, McClean S, Barbour R, Huang J, Nguyen M, Ahern D, Motter R, Johnson-Wood K, Vasquez N, Schenk D, Seubert P. Encapsulation in biodegradable microparticles enhances serum antibody response to parenterally delivered β-amyloid in mice. Vaccine. 2001;19:4185–4193. doi: 10.1016/s0264-410x(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W. A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and β-amyloid plaques in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2003;14:365–379. doi: 10.1016/j.nbd.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Spooner E, Desai R, Mori C, Leverone J, Lemere C. The generation and characterization of potentially therapeutic Aβ antibodies in mice: differences according to strain and immunization protocol. Vaccine. 2002;21:290–297. doi: 10.1016/s0264-410x(02)00464-4. [DOI] [PubMed] [Google Scholar]
- Lemere C, Spooner E, Leverone J, Mori C, Clements J. Intranasal immunotherapy for the treatment of Alzheimer’s disease: Escherichia coli LT and LT(R192G) as mucosal adjuvants. Neurobiol Aging. 2002;23:991–1000. doi: 10.1016/s0197-4580(02)00127-6. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead M, Tian X, Phinney A, Manea M, French J, Lambermon M, Darabie A, Brown M, Janus C, Chishti M, Horne P, Westaway D, Fraser P, Mount H, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- DeMattos R, Bales K, Cummins D, Dodart J-C, Paul S, Holtzman D. Peripheral anti-Aβ antibody alters CNS and plasma clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Murphy M, Younkin L, Younkin S, Golde T. Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Orgogozo J-M, Gilman S, Dartigues J-F, Laurent B, Puel M, Kirby L, Jouanny P, Dubois B, Eisner L, Flitman S, Michel B, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies for β-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, deq Uervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Nicoll J, Wilkinson D, Holmes C, Steart P, Markham H, Weller R. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with amyloid-β peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003;126:285–291. doi: 10.1093/brain/awg031. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Tolan D, Selkoe DJ. Homology of the amyloid β-protein precursor in monkey and human supports a primate model for β-amyloidosis in Alzheimer’s disease. Am J Pathol. 1991;138:1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Bell D, Podlisny MB, Cork LC, Price DL. Conservation of brain amyloid proteins in aged mammals and in humans with Alzheimer’s disease. Science. 1987;235:873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Palmour R, Mulligan J, Howbert J, Ervin F. Insights from model systems. Of monkeys and men: vervets and the genetics of human-like behaviors. Am J Hum Genet. 1997;61:481–488. doi: 10.1086/515526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MT. The St. Kitts vervet (Cercopithecus aethiops). J Med Primatol. 1974;3:285–297. doi: 10.1159/000460030. [DOI] [PubMed] [Google Scholar]
- Lemere C, Spooner E, LaFrancois J, Malester B, Mori C, Leverone J, Matsuoka Y, DeMattos R, Holtzman D, Clements J, Selkoe D, Duff K. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Khan K, Saido TC, Haass C, Masliah E, Selkoe DJ, Games D. Temporal sequence of deposition of Aβ peptides bearing heterogeneous N-termini and APO J in PD-APP transgenic mice. Soc Neurosci. 1997;23:534. [209.535] [Google Scholar]
- Cribbs D, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman C, Agadjanyan M. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A β42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973;32:566–584. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- Struble RG, Price DL, Jr, Cork LC, Price DL. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985;361:267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Schwam E, Buckward F, Garcia F, Sepinwall J, Price DL. Senile plaques in aged squirrel monkeys. Neurobiol Aging. 1987;8:291–296. doi: 10.1016/0197-4580(87)90067-4. [DOI] [PubMed] [Google Scholar]
- Cork L, Masters C, Beyreuther K, Price D. Development of senile plaques: relationships of neuronal abnormalities and amyloid deposits. Am J Pathol. 1990;137:1383–1392. [PMC free article] [PubMed] [Google Scholar]
- Martin L, Sisodia S, Koo E, Cork L, Dellovade T, Weidemann A, Beyreuther K, Masters C, Price D. Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci USA. 1991;88 doi: 10.1073/pnas.88.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N, Mestre N, Petter A. Senile plaques and neurofibrillary changes in the brain of an aged lemurian primate, Microcebus murinus. Neurobiol Aging. 1991;13:99–105. doi: 10.1016/0197-4580(92)90016-q. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tamaoka A, Sawamura N, Shoji S, Nakayama H, Ono F, Sakakibara I, Yoshikawa Y, Mori H, Goto N, Doi K. Carboxyl end-specific monoclonal antibodies to amyloid β protein (Aβ) subtypes (Aβ40 and Aβ42(43)) differentiate Aβ in senile plaques and amyloid angiopathy in brains of cynomolgus monkeys. Neuroscience Letters. 1995;201:151–154. doi: 10.1016/0304-3940(95)12160-9. [DOI] [PubMed] [Google Scholar]
- Uno H, Alsum P, Dong S, Richardson R, Zimbric M, Thieme C, Houser W. Cerebral amyloid angiopathy and plaques, and visceral amyloidosis in aged macaques. Neurobiol of Aging. 1996;17:275–281. doi: 10.1016/0197-4580(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra S. Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol of Aging. 1996;17:903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra S. β-amyloid (Aβ) Deposition in the brains of aged orangutans. Neurobiol of Aging. 1997;18:139–146. doi: 10.1016/s0197-4580(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Nakayama H, Goto N, Ono F, Sakakibara I, Yoshikawa Y. Histopathological studies of senile plaques and cerebral amyloidosis in cynomolgus monkeys. J Med Primatol. 1998;27:244–252. doi: 10.1111/j.1600-0684.1998.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Maclean C, Baker H, RIdley R, Mori H. Naturally occurring and experimentally induced β-amyloid deposits in the brains of marmosets (Callithrix jacchus). J Neural Transmission. 2000;107:799–814. doi: 10.1007/s007020070060. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, W C-K. Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol. 2002;103:48–58. doi: 10.1007/s004010100429. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina H, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific A β monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, Iwatsubo T, Ihara Y. Comparable amyloid-β (Aβ) 42(43) and Aβ40 deposition in the aged monkey brain. Neuroscience Letters. 1996;214:196–198. doi: 10.1016/0304-3940(96)12893-7. [DOI] [PubMed] [Google Scholar]
- DeMattos R, Bales K, Cummins D, Paul S, Holtzman D. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc Natl Acad Sci USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frenkel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Gordon MN, Ugen KE, Gottschall PE, DiCarlo G, Dickey C, Boyett KW, Jantzen PT, Connor KE, Melachrino J, Hardy J, Morgan D. Number of Aβ inoculations in APP+PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA and Cell Biol. 2001;20:731–737. doi: 10.1089/10445490152717596. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Aβ antibodies reduce β-amyloid deposition by mechanisms both independent and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer’s disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews P, Jucker M. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]