Abstract
Diabetes increases susceptibility to chronic skin ulceration. The etiology of chronic wound formation in diabetic individuals is multifactoral but may be accelerated by changes in the structure and function of the skin secondary to impaired fibroblast proliferation, decreased collagen synthesis, and increased matrix metalloproteinase (MMP) expression. This study explored the effects of all-trans-retinoic acid (RA) on cellular and biochemical features of diabetic human skin in organ culture. Two-mm skin biopsies from hip or ankle were obtained from diabetic subjects and incubated for 9 days in the absence or presence of 2 μmol/L RA. Hip skin from non-diabetic individuals served as control. Following organ culture incubation, untreated and RA-treated tissue was examined histologically after staining with hematoxylin and eosin. In parallel, organ culture-conditioned medium collected on days 5 and 7 was assayed for levels of active and total MMP-1 (interstitial collagenase) and MMP-9 (gelatinase B). The same organ culture fluids were assayed for the presence of soluble collagen. In comparison with skin from non-diabetic individuals, diabetic skin demonstrated no major differences in overall epidermal thickness or collagen production (both were increased in RA-treated tissue as compared to non-RA-treated tissue). In contrast, levels of MMP-9 (active forms) were elevated in organ culture fluid from diabetic skin as compared to non-diabetic control skin. In the presence of RA, active forms of both MMP-1 and MMP-9 were reduced. Together, these data suggest that RA has the capacity to improve structure and function of diabetic skin, and that a major effect is on reduction of collagen-degrading MMPs.
Lower limb ulceration in diabetic patients is a common and disabling complication that results in significant morbidity and frequently leads to amputation of the leg. More than 16 million people in the United States have diabetes and 15% of those can be expected to develop one or more lower leg or foot ulcers during their lifetimes.1–3 Ulcer formation in the lower limbs is thought to be a consequence of impaired wound healing. The mechanisms responsible for impaired wound healing in diabetic patients are only incompletely understood. Loss of the peripheral microvasculature appears to be an underlying cause in many cases, leading to atrophic changes in the skin as a consequence.4–13 In diabetic skin, there is impaired growth capacity of dermal fibroblasts.14–16 Decreased fibroblast proliferation is associated with reduced collagen synthesis and increased matrix metalloproteinase (MMP) production.17–22 In this regard, the skin of individuals with diabetes has features in common with chronologically aged skin23 and with severely photodamaged skin.24,25
Treatment of photodamaged skin with topical retinoids is known to improve the clinical appearance of the skin.26,27 Improved appearance is associated with reduced levels of collagen-degrading MMPs24,25 and with increased collagen synthesis.28,29 More recently, it has been shown that retinoids function to reverse the adverse consequences of the chronological aging process in the skin.23 Specifically, retinoid treatment stimulates fibroblast proliferation and new collagen synthesis while reducing MMP production in aged skin. This is accompanied by an increase in epidermal thickness, reflecting increased keratinocyte proliferation. Whether diabetic human skin would respond to topical retinoid treatment in a similar fashion is not known. To begin addressing this question, biopsies of diabetic skin were incubated in organ culture and treated with all-trans-retinoic acid (RA). Retinoid responses in both the epidermis and dermis were assessed. Findings from this study demonstrated that diabetic skin responds to retinoid treatment with increased thickening, increased collagen production, and, most dramatically, decreased levels of collagen-degrading MMPs.
Materials and Methods
Source of Skin Biopsies and Organ Culture Procedure
Duplicate 2-mm punch biopsies were obtained from hip skin of 16 diabetic patients (10 male and 6 female) seen in the Diabetes Complications Clinic at the University of Michigan. Eleven of the patients were diagnosed with type 1 diabetes and five with type 2 diabetes. The median age was 54 years and the mean age was 53 years (range, 30–88 years). The median duration of disease was 30 years (range, 9–50 years). Complications included peripheral neuropathy (16 patients), proliferative retinopathy (2 patients), retinopathy (11 patients), nephropathy (8 patients), and a history of lower leg ulceration (2 patients). From six of the same individuals, duplicate 2-mm punch biopsies were obtained from the ankle as well. Ankle biopsies were handled exactly as biopsies from the hip (see below). Initially, the data from the ankle biopsies were kept separate. However, no statistical difference between biopsies from hip and ankle were seen and data obtained from skin of the two sites was eventually merged. Ten non-diabetic volunteers (7 males and 3 females) made up the control group. The median age for this group was 46 years and the mean age was 45 years (range, 29–64 years). There was no report of any systemic illness by individuals in the non-diabetic control group. The participation of human subjects in this study was approved by the University of Michigan Institutional Review Board and all subjects provided written informed consent before their inclusion in the study.
The punch biopsies from each individual were incubated in wells of a 24-well dish (one tissue piece per 250 μl of culture medium). Culture medium consisted of keratinocyte basal medium (KBM) (Cambrex Biologicals, Walkersville, MD) supplemented with calcium chloride to a final Ca2+ concentration of 1.4 mmol/L. One well was left as control while the other was treated with 0.75 μg/ml RA (approximately 2 μmol/L). This concentration of RA has been shown to be optimal for preservation of histological features in adult sun-protected skin.30 Fresh culture medium was provided at 2- to 3-day intervals. Organ culture-conditioned medium collected on days 5 and 7 was used for assessment of MMP levels as described below. Enzyme levels collected on the 2 days were virtually indistinguishable from each other and data from the two time-points were pooled. At the end of incubation period (day 9), tissue was fixed in 10% buffered formalin for histology. In certain experiments, tissue inhibitor of metalloproteinase-1 (TIMP-1) (1 μg/ml), soybean trypsin inhibitor (SBTI), (50 μg/ml) and catalase (10 μg/ml) were included in the culture medium throughout the incubation period. TIMP-1 was obtained from (Oncogene Research Products, San Diego, CA). Catalase and SBTI were obtained from Sigma Chemical Co. (St. Louis, MO).
Histological Evaluation
At the end of the incubation period, organ-cultured tissue was fixed in 10% buffered formalin and embedded in paraffin. Five-μm-thick sections were cut and stained with hematoxylin and eosin. Representative sections of each biopsy were selected for histological evaluation and photographed.
Substrate Embedded Enzymography
Substrate embedded enzymography (zymography)31 was used in these studies to identify and characterize MMPs. Briefly, SDS-PAGE gels were prepared for minigels from 30:1 acrylamide/bis with the incorporation of either gelatin (1 mg/ml) or β-casein (1 mg/ml) before casting. The gelatin gels were routinely 7.5% acrylamide, whereas casein gels were routinely cast at 10% acrylamide. Samples of organ culture fluid and molecular weight standards were electrophoresed at constant voltage of 150 V under non-reducing conditions. Volumes of 5 to 35 μl of undiluted specimen were normally used for these assays. After electrophoresis, gels were removed and subjected to the following wash protocol: twice for 15 minutes in 50 mmol/L Tris buffer containing 1 mmol/L Ca2+ and 0.5 mmol/L Zn2+ with 2.5% Triton X-100. The gels were then incubated overnight in Tris buffer with 1% Triton X-100 and stained with Brilliant Blue R concentrate the following day. After destaining, zones of gelatin and casein hydrolysis were detected as clear areas against a dark background. Zymographic images were digitized. Negative images were created and quantified by scanning densitometry. Using the zymographic images from gelatin and β-casein gels, respectively, values for latent and active MMP-9 and MMP-1 bands were obtained. These were used to compare culture fluids from non-retinoid-treated and retinoid-treated tissues.
Soluble Collagen Assay
The Sircol collagen Assay (Biocolor Ltd., Newton Abbey, UK) was used as a way to assess soluble collagen levels in the same organ culture fluids. This is a quantitative dye binding method designed for the analysis of acid soluble collagen released into culture medium by mammalian cells during in vitro culture. Organ culture fluid, collected at different intervals as described above, was analyzed. Briefly, a collagen standard at four concentrations (5, 10, 25 and 50 μg of the supplied reference collagen solution) and 180 μl of test samples in separate 1.5 ml conical microcentrifuge tubes were mixed with 1.0 ml of the Sircol dye reagent at room temperature for 30 minutes on a mechanical mixer. The tubes were then centrifuged for 10 minutes at 10,000 × g and the supernatants discarded. The remaining pellets were mixed with 1.0 ml of alkali reagent and resuspended using a vortex mixer. The absorbance of samples, standards and assay blanks were measured using a spectrophotometer at the wavelength of 540 nm after 10 minutes. The values obtained with the culture fluids were compared directly with the values obtained from the control wells and the standard curve.
Type I Procollagen Assay
Selected culture fluids were also assayed for type I procollagen by enzyme-linked immunosorbent assay (ELISA) (Pan Vera Corp., Madison, WI) as described previously.23
Tissue Inhibitor of Metalloproteinase-1 Assay
Culture fluids were assayed for tissue inhibitor of metalloproteinase-1 (TIMP-1) by ELISA using a commercially-available assay kit (R&D Systems, Minneapolis. MN).
Statistical Analysis
Differences between groups experiments with multiple groups were analyzed for statistical significance by analysis of variance followed by paired-group comparisons. Where there were only two groups, the Student’s t-test was used.
Results
Effects of RA on Histological Features of Non-Diabetic and Diabetic Human Skin in Organ Culture
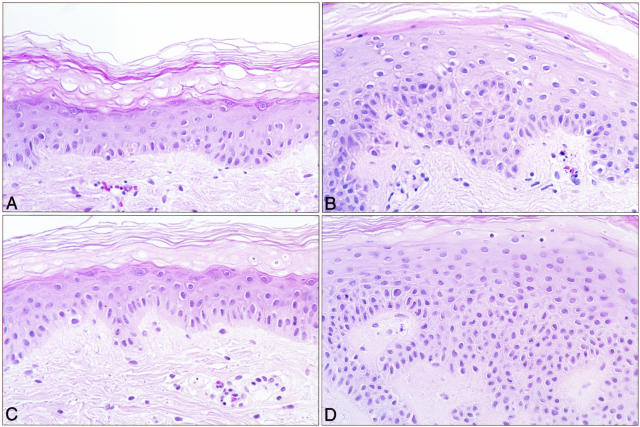
Figure 1 shows typical histological features of non-treated and RA-treated (hip) skin from non-diabetic and diabetic volunteers after 9 days in organ culture. Epidermal hyperplasia in the RA-treated skin from both individuals is evident. Although the degree of hyperplasia varied from individual to individual, a retinoid response was observed in all of the tissues treated with 0.75 μg/ml RA. The retinoid response in skin from non-diabetic volunteers was quantitatively similar to the response in diabetic skin. The retinoid response seen in either diabetic or non-diabetic skin was consistent with what has been reported previously for normal skin.30,32 Thus, it appears that skin from non-diabetic and diabetic patients demonstrates an equivalent epidermal proliferative response to treatment with RA in organ culture.
Figure 1.
Histological features of untreated and RA-treated non-diabetic and diabetic skin in organ culture. Tissue from normal and diabetic (hip) skin was maintained in organ culture for 9 days under serum-free, growth factor-free conditions in the absence or presence of 0.75 μg/ml RA. At the end of the incubation period, the tissue was fixed in 10% buffered formalin and examined at the light microscopy level after sectioning and staining with hematoxylin and eosin. A: Normal control. B: Normal RA-treated. C: Diabetic control. D: Diabetic RA-treated. Epidermal hyperplasia can be seen in the RA-treated skin from either normal or diabetic skin (×320).
Effects of RA on Elaboration of MMP-9 by Non-Diabetic and Diabetic Human Skin in Organ Culture
Organ culture fluid from each of the non-diabetic and diabetic skin samples (both RA-treated and control) was collected at days 5 and 7 and assayed for MMP-9 by gelatin zymography. The Level of total enzyme (active + latent forms) in the culture fluid from untreated diabetic skin was not significantly different from the enzyme level in culture fluid from untreated non-diabetic skin (24 ± 1 units for diabetic skin versus 19 ± 2 units for non-diabetic skin; means ± standard errors; n = 16 and 10, respectively). In comparison to untreated skin, the level of total MMP-9 in the culture fluid from RA-treated skin was also not statistically different (19 ± 2 vs. 17 ± 2 units in non-diabetic skin and 24 ± 1 vs. 20 ± 1 units in diabetic skin). It should be noted that although levels of total MMP-9 in organ culture from untreated and RA-treated skin were not significantly different, the major source of MMP-9 in organ-cultured skin is the epidermis.33 Since the number of epithelial cells is greater by 2 to 2.5-fold after RA treatment,30 (Figure 1), the reduction on a per cell basis in the presence of RA may be much greater than shown here.
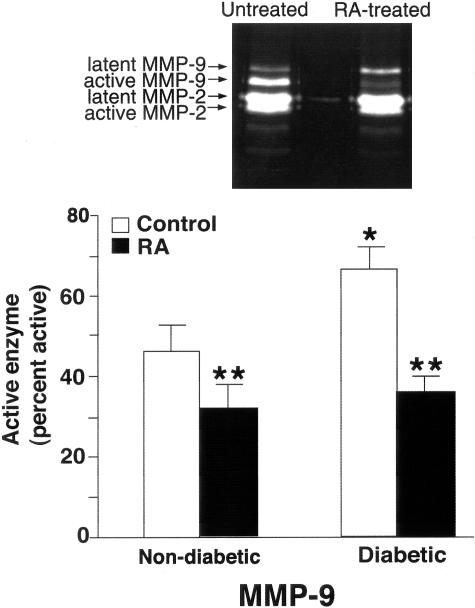
Figure 2 shows active MMP-9 levels in organ culture fluid collected from untreated non-diabetic and diabetic skin and the corresponding skin samples from RA-treated tissue. The percentage of enzyme in the active form was higher in culture fluid from diabetic skin than from non-diabetic skin. It can also be seen from Figure 2 that levels of active MMP-9 in organ culture fluid from RA-treated skin (both non-diabetic and diabetic) were reduced as compared to levels in organ culture fluid from the corresponding untreated skin.
Figure 2.
MMP-9 elaboration in untreated and RA-treated non-diabetic and diabetic skin in organ culture. Organ culture fluid was collected on days 5 and 7 and assayed for MMP-9 by gelatin zymography. Zymographic images were scanned and digitized, and negative images quantified. Upper panel: Representative gelatin zymogram demonstrating MMP-9 (and MMP-2) in organ culture fluid from untreated and RA-treated diabetic skin (day 5). A higher percentage of MMP-9 in the active form can be seen in culture fluid from untreated skin as compared to RA-treated skin. MMP-2 latent and active forms can also be seen but there is little difference between control and RA-treated samples. Lower panel: Active enzyme expressed as a percentage of total enzyme (densitometry values from active forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures from 10 normal and 16 diabetic volunteers. Statistical significance of the differences among the four groups was determined by analysis of variance followed by paired-group comparisons. *P < 0.05 relative to non-diabetic skin. **P < 0.01 relative to non-RA-treated skin of same group.
The inset for Figure 2 shows typical gelatin zymographic profiles from non-RA-treated and RA-treated (diabetic) skin. Differences in the relative amounts of latent and active MMP-9 forms are apparent. It can also be appreciated from the zymograms that there was little difference between the lanes in regard to MMP-2 levels. This was observed among the whole group of specimens examined. Specifically, total MMP-2 levels in the absence and presence of RA were 34 ± 3 and 38 ± 2 units, respectively, while the percentage of enzyme in the active form was 30 ± 3% and 34 ± 2%, respectively (means and standard errors, based on pooled data from 16 hip and 6 ankle samples). This is consistent with past studies showing that MMP-2 is not regulated in the same fashion as MMP-9.24
Effects of RA on Elaboration of MMP-1 by Non-Diabetic and Diabetic Human Skin in Organ Culture
In addition to measuring MMP-9, the same organ culture fluids from untreated and RA-treated (non-diabetic and diabetic) skin were assessed for MMP-1. Similar to what was observed with MMP-9, levels of total MMP-1 were not significantly different in diabetic skin than in non-diabetic skin (14 ± 2 units in non-diabetic skin versus 18 ± 2 units in diabetic skin; n = 10). Although the difference between the two groups was slight, fibroblast numbers in skin are known to decrease as a function of age.23 The fact that the non-diabetic population was slightly younger than the population of diabetic patients (mean age, 45 vs. 53 years) would tend, therefore, to bias the data against the increase seen in the diabetic patients.
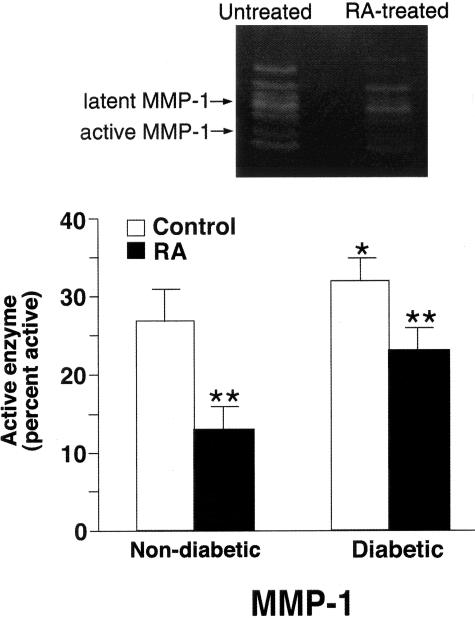
Consistent with the effects on MMP-9, RA treatment did not reduce the level of total MMP-1 in either non-diabetic or diabetic skin (12 ± 3 vs. 14 ± 2 in non-diabetic skin and 15 ± 2 vs. 18 ± 2 units in diabetic skin; not statistically different). Also consistent with the effects on MMP-9, RA treatment significantly reduced the level of active MMP-1 in both non-diabetic and diabetic skin (Figure 3).
Figure 3.
MMP-1 elaboration in untreated and RA-treated non-diabetic and diabetic skin in organ culture. Organ culture fluid was collected on days 5 and 7 and assayed for MMP-1 by β-casein zymography. Zymographic images were scanned and digitized, and negative images quantified. Upper panel: Representative β-casein zymogram demonstrating MMP-1 in organ culture fluid from untreated and RA-treated diabetic skin (day 5). A higher percentage of MMP-1 in the active form can be seen in culture fluid from untreated skin as compared to RA-treated skin. Lower panel: Active enzyme expressed as a percentage of total enzyme (densitometry values from active forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures from 10 normal and 10 diabetic volunteers. Statistical significance of the differences among the four groups was determined by analysis of variance followed by paired-group comparisons. *P < 0.05 relative to non-diabetic skin. **P < 0.01 relative to non-RA-treated skin of same group.
MMP Activation in the Presence of Oxidant, Serine Proteinase, and MMP Inhibitors
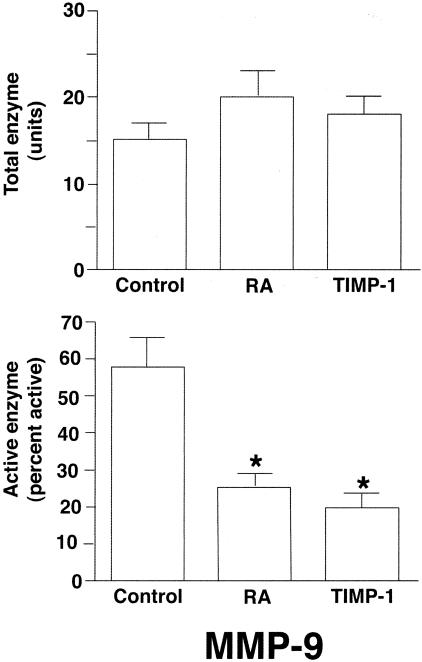
A series of experiments were conducted in which organ cultures of diabetic skin were treated with catalase (10 μg/ml) to suppress oxidant effects, SBTI (50 μg/ml) to suppress serine proteinase activity and TIMP-1 (1 μg/ml) to suppress MMP function. Culture fluids collected on days 5 and 7 were assessed for the presence of latent and active MMP-9 by gelatin zymography. None of the three inhibitors significantly affected total enzyme levels. Catalase and SBTI also had no effect on the level of active enzyme. The percentage of active MMP-9 in the presence of the two inhibitors was indistinguishable from the percentage seen in untreated culture fluid (60 ± 7% active MMP-9 in culture fluid from control organ cultures vs. 62 ± 5% and 58 ± 6% in the presence of catalase and SBTI, respectively; mean ± standard errors; n = 3). In contrast, the active enzyme form was significantly reduced in the presence of TIMP-1 (Figure 4) and the latent form was correspondingly increased. Results obtained with TIMP-1 were similar to results obtained with RA (Figure 4). Thus, treatment of organ cultures with RA produced changes in the MMP-9 expression profile that were similar to changes seen in the presence of TIMP-1.
Figure 4.
Comparison of RA and TIMP-1 for inhibition of MMP-9 activation. Organ culture fluid from control, RA-treated or TIMP-1 (1 μg/ml)-treated skin was collected on days 5 and 7 and assayed for MMP-9 by gelatin zymography. Zymographic images were scanned and digitized, and negative images were quantified. Upper panel: Total enzyme (densitometry values from combined latent and active forms). Lower panel: Active enzyme expressed as a percentage of total enzyme (values from active forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures of hip skin from four diabetic volunteers. Statistical significance of the differences among the three groups was determined by analysis of variance followed by paired-group comparisons. *P < 0.05 relative to untreated control group.
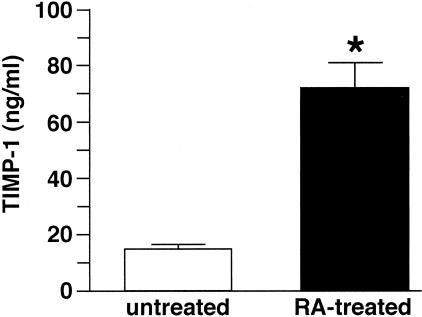
Based on these findings, two additional sets of experiments were conducted. First, day-5 culture fluids from untreated diabetic skin organ cultures (containing high levels of active MMP-9) were mixed with RA (0.75 μg/ml) for 30 minutes and then examined by gelatin zymography to determine whether the presence of RA would directly interfere with enzyme function. It did not. There was no apparent difference in the zymographic profile of the untreated and RA-“spiked” culture fluid (47 ± 5% active vs. 52 ± 6%; means and ranges based on n = 2 separate experiments). A second experiment was conducted in which organ culture fluids (days 5 and 7) from untreated and RA-treated tissue were assayed for TIMP-1 by ELISA. TIMP-1 levels were significantly elevated in the culture fluid from RA-treated skin relative to levels in untreated skin (Figure 5).
Figure 5.
TIMP-1 elaboration in organ cultures of untreated and RA-treated diabetic skin. Organ culture fluid was collected on days 5 and 7 from untreated and RA-treated diabetic skin and assayed for TIMP-1 by ELISA. Values shown are means and standard errors, based on organ cultures from four individuals. Statistical significance of the difference between untreated and RA-treated samples was determined using the Student’s t-test. *P < 0.01.
Effects of RA on Collagen Production by Non-Diabetic and Diabetic Human Skin in Organ Culture
In a final set of experiments, organ culture fluid from untreated and RA-treated diabetic skin was analyzed for soluble collagen. Soluble collagen was increased in RA-treated skin relative to control (17 ± 4 vs. 9 ± 3 μg/ml; means and standard errors; n = 7; P < 0.05). A similar trend was observed with untreated and RA-treated tissue from non-diabetic volunteers, but due to the small number of samples, the increase did not reach statistical significance (not shown). Previous studies have demonstrated that type I procollagen is increased in aged skin following retinoid treatment, and this can be seen at both the mRNA and protein levels.23 To determine whether the increase in soluble collagen noted here was a reflection of increased procollagen production, six organ culture fluid samples (three containing low levels of soluble collagen and three containing high levels) were assayed for type I procollagen. Results from the two assays were correlated. In the control samples with low soluble collagen, 24 ± 6 ng/ml of type I procollagen was present while in the retinoid treated samples with high soluble collagen, the average level of type I procollagen was 58 ± 9 ng/ml.
Discussion
Skin atrophy is a consequence of peripheral microvascular damage in diabetic skin. Atrophic changes include decreased growth capacity in the major cellular elements (keratinocytes and fibroblasts), accompanied by decreased collagen production and increased levels of collagen-degrading MMPs.14–22 Atrophy of diabetic skin is thought to underlie decreased wound-healing capacity and the concomitant increased risk of chronic ulcer formation. Extensive efforts to prevent ulcer formation in diabetic skin and to improve the healing of wounds that occur have been made. Despite these efforts, formation of non-healing ulcers occurs in approximately 15% of diabetic patients, resulting in foot amputation in many cases.2,3 Clearly, additional efforts are needed.
In the present studies, we examined the capacity of RA to reverse atrophic changes in diabetic human skin. Using 2-mm punch biopsies of diabetic skin maintained in organ culture, our results showed that epidermal thickness was increased following RA treatment. Concomitantly, there was a reduction in the levels of two tissue-destructive MMPs and increased collagen production in retinoid-treated skin. Together, these findings suggest that retinoid treatment does, in fact, have the capacity to reverse (at least some of) the cellular and biochemical events that underlie atrophy in diabetic skin. At present, we do not know if long-term treatment of “at-risk” skin will prevent or reduce the formation of non-healing ulcers in diabetic patients. Our own recent studies have shown, however, that treatment of diabetic rat skin in organ culture with RA brings about the same changes noted here with human skin34 and others have shown that retinoid treatment of rodents with diabetes increases the healing capacity of experimentally induced skin wounds.35,36
Several findings described in this report deserve additional comment. The first relates to the observed differences between non-diabetic and diabetic skin in the absence of retinoid treatment. Even though diabetic skin from the hip is not considered to be ulcer-prone, there were measurable differences between diabetic and non-diabetic hip skin in levels of active MMP-9 and MMP-1. Active forms of both enzymes were increased in diabetic skin relative to non-diabetic skin. In contrast, we did not detect significant differences in collagen synthesis between non-diabetic and diabetic skin. Thus, it appears that elevation of tissue-destructive MMPs precedes deficits in collagen production. Previous studies have demonstrated a similar relationship between elevated MMP expression and decreased collagen production in chronological aging23 and in photoaging.24,25,28,29,37 Specifically, in both chronological aging and photoaging, MMP-mediated collagen breakdown occurs over years or decades.28,29 It is only after significant damage has occurred, however, that procollagen synthesis is negatively affected. Our recent studies suggest that reduced procollagen production in chronologically aged skin and (especially) in photodamaged skin reflects fibroblast interaction with damaged matrix components and the inability of cells in such a milieu to generate sufficient mechanical tension to maintain the synthetic phenotype.37–40 The situation in skin damaged as a consequence of diabetes could be similar. Of interest in this regard, it has been demonstrated in past studies that fibroblasts isolated from extensively photodamaged skin had similar in vitro growth capacity as cells isolated from sun-protected skin of the same individuals. In vitro collagen production by cells from the two sites was also similar.37 Parallel findings have been made with skin fibroblasts from rats made diabetic with streptozotocin.34
What accounts for increased enzyme activity in diabetic skin relative to non-diabetic skin and decreased activity in RA-treated skin (either diabetic or non-diabetic) relative to untreated skin is not fully understood. On the one hand, when total enzyme (latent + active forms) was assessed, differences between diabetic and non-diabetic skin were not observed. Likewise, the effect of RA treatment on total MMP-1 and MMP-9 levels in both diabetic and non-diabetic skin were minimal. With regard to the differences between diabetic and non-diabetic skin, it should be pointed out that although we attempted to age-match the control population as much as possible to the diabetic group, the average age of the individuals in the non-diabetic group was slightly lower (45 vs. 53 years). This is of interest because, on the one hand, MMP levels increase as a function of age but, on the other, cellularity in both epidermis and dermis decrease with age.23 The loss of cellularity outweighs the increase in enzyme production per cell, such that enzyme levels tend to decrease slightly as a function of age. Based on this, it might be inferred that levels of total MMP-1 and MMP-9 in diabetic skin relative to (age-matched) non-diabetic skin would actually be somewhat higher than measured here. In the same way, cellularity increases dramatically in the skin following retinoid treatment.23,32 Thus, the decrease in enzyme levels on a per cell basis in RA-treated skin would be much greater (relative to untreated skin) than measured here.
In contrast to total enzyme levels, the levels of active MMP-1 and MMP-9 were significantly lower in RA-treated tissue than in non-treated tissue. The mechanism by which enzyme activation is blocked in the presence of RA is not known. Past studies have shown that oxidants,41 serine proteinases,42 and other MMPs43 all have the capacity to activate latent MMPs. RA could, in theory, prevent MMP activation by suppressing oxidant function or the action of one or more proteolytic enzyme. This is not likely to be the full story, however. Retinoids are weak antioxidants, and actually enhance elaboration of serine proteinases (eg, plasminogen activators).33 Furthermore, when either an antioxidant (catalase) or a serine proteinase inhibitor (SBTI) was included in organ culture fluid, it failed to inhibit MMP activation.
Another possibility is an effect on MMP inhibitor function. TIMP-1 is the major MMP inhibitor in skin.44,45 TIMP-1 functions by binding to the active site of the MMP as the enzyme undergoes the enzyme-activating conformational shift.46 As a result, one typically sees in zymography or Western blotting, a lack of activated enzyme forms and an increased amount of latent enzyme (ref. 47 and present report) in the presence of exogenous TIMP-1. Zymograms of culture fluid from RA-treated organ cultures demonstrated a similar profile. Thus, it might be that RA suppresses MMP function indirectly, through an effect on MMP inhibitor production. In support of this, TIMP-1 levels were substantially higher in organ culture fluid from RA-treated tissue than in organ culture fluid from untreated tissue. The ability of RA to stimulate TIMP-1 elaboration, it should be pointed out, has been shown with cells in monolayer culture.48,49 Ultimately, the mechanism by which RA inhibits MMP activation is still an open question. Regardless of mechanism, the reduction in active MMP levels could be expected to mitigate connective tissue damage in the RA-treated tissue.
In summary, diabetes is one of the major predisposing factors for the development of non-healing skin wounds, particularly in the lower extremities.1–3 Atrophic changes in the connective tissue of the skin are thought to play an underlying role in sensitizing the skin to chronic wound formation. In other situations where there is dermal atrophy, ie, in aged or photoaged skin50,51 or after long-term treatment with corticosteroids,52 sensitivity to wound formation also increases. To the extent that dermal atrophy predisposes to chronic wound formation, prevention of atrophic features with topical retinoid treatment should be beneficial. To date, this has not been established clinically, although studies in experimental animals are supportive.35,36 Given the data presented here, we believe that studies to determine whether topical treatment with RA will lead to reduced wound formation or improved healing of wounds in diabetes are warranted.
Footnotes
Address reprint requests to James Varani, Ph.D, Department of Pathology, The University of Michigan, 1301 Catherine Road, Box 0602, Ann Arbor, MI 48109. E-mail: varani@umich.edu.
Supported in part by grants DK59169 (to J.V.) and DK52391 (to M.J.S.) from the U.S. Public Health Service, by a Veterans Administration Career Development Award (to M.J.S.), and by a grant from the Juvenile Diabetes Research Association (to M.J.S.).
References
- Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Bethesda, MD: National Diabetes Data Group, National Institutes of Health,; Diabetes in America, (ed. 2) 1995:pp 409–428. [Google Scholar]
- Reiber GE. The epidemiology of diabetic foot problems. Diabetic Med. 1998;13:S6–S11. [PubMed] [Google Scholar]
- Margolis D, Hoffstad O. Diabetic neuropathic foot ulcers. Diabetes Care. 2002;25:10. doi: 10.2337/diacare.25.10.1835. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment: a meta-analysis. Diabetes Care. 1999;22:692–695. doi: 10.2337/diacare.22.5.692. [DOI] [PubMed] [Google Scholar]
- Eagelton WP. Influence of old age, diabetes, arterial sclerosis and gout on the healing of wounds. Am Med. 1902;4:898–904. [Google Scholar]
- Allen FM. The treatment of diabetes. Boston Med J. 1915;172:241–246. [Google Scholar]
- Greene JA, Swanson AL, Jacobs CA. Control of diabetes mellitus in relation to the healing of clean and infected wounds and the incidence of infection in clean wounds. JAMA. 1940;115:1518–1523. [Google Scholar]
- Cruse PJE, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206–210. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- Klenerman L, McCabe C, Cogley D, Crerand S, Laing P, White M. Screening for patients at risk of diabetic foot ulceration in a general diabetic outpatient clinic. Diabetic Med. 1996;13:561–563. doi: 10.1002/(SICI)1096-9136(199606)13:6<561::AID-DIA112>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Prakash A, Pundit PN, Sharma KL. Studies on wound healing in experimental diabetes. Int Surg. 1974;59:25–30. [PubMed] [Google Scholar]
- Strigini L, Ryan T. Wound healing in elderly human skin. Clin Dermatol. 1996;14:197–206. doi: 10.1016/0738-081x(95)00155-9. [DOI] [PubMed] [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(Suppl 2A):26s–38s. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- Laing P. The development and complications of diabetic foot ulcers. Am J Surg. 1998;176(Suppl 2A):11s–19s. doi: 10.1016/s0002-9610(98)00182-2. [DOI] [PubMed] [Google Scholar]
- Loots MAM, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291:93–99. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- Mendez MV, Stanley A, Phillips T, Murphy M, Menzoian JO, Park H-Y. Fibroblasts cultured from distal lower extremities in patients with venous reflux display cellular characteristics of senescence. J Vasc Surg. 1998;28:1040–1050. doi: 10.1016/s0741-5214(98)70030-8. [DOI] [PubMed] [Google Scholar]
- Hehenberger K, Hansson A, Heilborn JD, Abdel-Halim SM, Ostensson C-G, Brismar K. Impaired proliferation and increased lactate production of dermal fibroblasts in GK-rat, a spontaneous model of non-insulin dependent diabetes mellitus. Wound Repair Regen. 1999;7:65–71. doi: 10.1046/j.1524-475x.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- Bizot-Foulon V, Bouchard B, Hornebeck W, Dubertret L, Bertaux B. Uncoordinate expression of type I and III collagens, collagenase and tissue inhibitor of matrix metalloproteinase 1 along the in vitro proliferative lifespan of human skin fibroblasts: regulation by all-trans retinoic acid. Cell Biol Int Rep. 1995;19:129–135. doi: 10.1006/cbir.1995.1053. [DOI] [PubMed] [Google Scholar]
- Furth JJ. The steady-state levels of type I collagen mRNA are reduced in senescent fibroblasts. J Gerontol. 1991;46:B1224–B1225. doi: 10.1093/geronj/46.3.b122. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Page RC, Narayanan AS, Pieters HP. Effect of donor age on protein and collagen synthesis in vitro by human diploid fibroblasts. Lab Invest. 1986;55:490–496. [PubMed] [Google Scholar]
- Burke EM, Horton WE, Pearson JD, Crow TM, Martin GR. Altered transcriptional regulation of human interstitial collagenase in cultured skin fibroblasts from older donors. Exp Gerontol. 1994;29:37–53. doi: 10.1016/0531-5565(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Millis AJ, Sorttle TJ, Hoyle M, Mann DM, Diemer V. Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol. 1989;24:559–573. doi: 10.1016/0531-5565(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Maroni P, Ozer N, Zingg J-M, Azzi A. Age-dependent increase of collagenase expression can be reduced by α-tocopherol via protein kinase C inhibition. Free Rad Biol Med. 1999;27:729–737. doi: 10.1016/s0891-5849(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung J, Wang ZQ, Datta SH, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang Z-Q, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature (London) 1996;379:335–338. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Engl J Med. 1977;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15:836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Weiss JS, Ellis CN, Voorhees JJ. Topical tretinoin improves photoaged skin: a double blind, vehicle-controlled study. JAMA. 1988;259:527–532. [PubMed] [Google Scholar]
- Griffiths CEM, Russman G, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). New Engl J Med. 1993;329:530–534. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- Varani J, Fligiel SEG, Schuger L, Perone P, Inman DR, Griffiths CEM, Voorhees JJ. Effects of all-trans retinoic acid and Ca2+ on human skin in organ culture. Am J Pathol. 1993;142:189–198. [PMC free article] [PubMed] [Google Scholar]
- Gibbs DF, Apel IJ, Warner RO, Weiss SJ, Johnson KJ, Varani J. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1999;20:1136–1144. doi: 10.1165/ajrcmb.20.6.3483. [DOI] [PubMed] [Google Scholar]
- Varani J, Perone P, Griffiths C, Inman D, Fligiel S, Voorhees J. All-trans retinoic acid stimulates events in organ cultured human skin that underlie repair. J Clin Invest. 1994;94:1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Inman DR, Burmeister W, Schollenberger SB, Sitrin RJ, Johnson KJ. Human skin in organ culture: elaboration of proteolytic enzymes under growth factor-free and growth factor-containing conditions. Am J Pathol. 1995;146:210–217. [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Merfert M, Larkin D, Stevens M. All-trans retinoic acid improves structure and function of Diabetic rat skin in organ culture. Diabetes. 2002;51:3510–3516. doi: 10.2337/diabetes.51.12.3510. [DOI] [PubMed] [Google Scholar]
- Seifter E, Rettura G, Padawer J, Stratford F, Kambosos D, Levenson SM. Impaired wound healing in streptozotocin diabetes: prevention by supplemental vitamin A. Ann Surg. 1981;194:42–50. doi: 10.1097/00000658-198107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano Y, Yoshimura K, Uchida G, Sato K, Harii K. Pretreatment with topical all-trans retinoic acid is beneficial for wound healing In genetically diabetic mice. Arch Dermatol Res. 2001;293:512–521. doi: 10.1007/pl00007466. [DOI] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P, Fligiel SEG, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Fligiel SEG, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 2002;119:122–129. doi: 10.1046/j.1523-1747.2002.01810.x. [DOI] [PubMed] [Google Scholar]
- Fligiel SEG, Varani J, Datta SH, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photoaged skin in vivo and after exposure to MMP-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Schuger L, Dame MK, Leonard C, Fligiel SEG, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photo damaged skin. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinase/phosphatidylinositol 3-kinase /NF-κB pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- Davis GE, Allen KAP, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2000;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Baramova EN, Manaut C, Maquoi E, Frankenne T, Foidart JM, Noel A. Expression of membrane type I matrix metalloproteinase in A2058 melanoma cells is associated with MMP-2 activation and increased tumor growth and vascularization. Int J Cancer. 2002;98:23–28. doi: 10.1002/ijc.10134. [DOI] [PubMed] [Google Scholar]
- Chi Y, Zeigler ME, Walker J, Perone P, Varani J. Elaboration of matrix metalloproteinase inhibitors by human skin in organ culture and by human skin cells in monolayer culture: relationship to invasion. Invasion Metastasis. 1999;18:27–34. doi: 10.1159/000024496. [DOI] [PubMed] [Google Scholar]
- Varani J, Hattori Y, Schmidt T, Perone P, Zeigler ME, Fader DJ, Johnson TM. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of the skin: comparison with normal skin. Br J Cancer. 2000;82:657–665. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP): structure, function and biological functions. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Zeigler ME, Dutcheshen NT, Gibbs DF, Varani J. Growth factor-induced epidermal invasion of the dermis in human skin organ culture: expression and role of matrix metalloproteinases. Invasion Metastasis. 1996;16:11–18. [PubMed] [Google Scholar]
- Bigg HF, McLeod R, Waters JG, Cawston TE, Clark IM. Mechanisms of induction of human tissue inhibitor of metalloproteinases-1 (TIMP-1) gene expression by all-trans retinoid acid in combination with basic fibroblast growth factor. Eur J Biochem. 2000;267:4150–4156. doi: 10.1046/j.1432-1327.2000.01459.x. [DOI] [PubMed] [Google Scholar]
- Braunhut SJ, Moses MA. Retinoids modulate endothelial cell production of matrix-degrading proteases and tissue inhibitors of metalloproteinases (TIMP). J Biol Chem. 1994;269:13472–13479. [PubMed] [Google Scholar]
- Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979;73:559–566. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- Lavker RM. Cutaneous aging: chronologic versus photoaging. Gilchrest BA, editor. Cambridge, MA: Blackwell Science,; Photoaging, 1995:pp 123–135. [Google Scholar]
- McMichael AJ, Griffiths CEM, Talwar HA, Finkel LJ, Rafal ES, Hamilton TA, Voorhees JJ. Concurrent application of tretinoin (retinoic acid) partially protects against corticosteroid-induced epidermal atrophy. Br J Dermatol. 1996;135:60–64. [PubMed] [Google Scholar]