Abstract
Recently, we have revealed the existence of a “brain-hair follicle axis” in murine skin and have identified the neuropeptide substance P (SP) as a key mediator of stress-induced hair growth inhibition in vivo. Published evidence suggests that increased numbers of SP-immunoreactive sensory fibers, as seen in the dermis of stressed mice in anagen-catagen transition, are a result of transient high levels of nerve growth factor (NGF). Thus, we now aimed at dissecting the role of NGF in stress-triggered hair growth termination in our murine model. By real time PCR and immunohistochemistry, stress-exposed mice showed an up-regulation of NGF and its low-affinity receptor p75NTR; the NGF high-affinity receptor TrkA was moderately down-regulated. On neutralization of NGF, premature onset of catagen, apoptosis, and increased number/activation of perifollicular mast cells and antigen-presenting cells, which reflects the skin response to stress, was significantly abrogated. Stress or subcutaneous injection of recombinant NGF (to mimic stress) resulted in an increased percentage of SP+ neurons in dorsal root ganglia, as measured by retrograde tracing. Taken together, these data suggest that NGF is a central element in the perifollicular neurogenic inflammation that develops during the murine skin response to stress and antagonizing NGF may be a promising therapeutic approach to counter the negative effect of stress on hair growth.
Recently, we have introduced a mouse model launching experimental evidence that stress-induced hair loss is fact, and not fiction, as every so often imputed by a number of dermatologists. This mouse model provides new insights into the pathophysiology of stress-induced hair growth inhibition and permits exploration of various strategies for therapeutic intervention.1,2 In this model, exposure to sonic stress inhibits the growth of a hair shaft producing (anagen) hair follicle by premature induction of hair follicle regression (catagen) and up-regulated keratinocyte apoptosis. At the same time, it induces neurogenic inflammation characterized by perifollicular mast cell degranulation and accumulation of antigen-presenting cells, eg, activated macrophages.3 The neuropeptide substance P (SP) was identified as a key mediator of stress-induced hair growth inhibition, since increased number of SP-immunreactive nerve fibers were present in the skin of stressed mice and hair growth inhibition could be blocked by SP-receptor antagonists.1,3 This is in line with previous findings that SP is a potent hair growth modulator in mice, since significant changes in the SP skin concentration, as well as in the number of SP-positive sensory nerve fibers are associated with murine hair follicle cycling.4,5 Taken together, these findings point to the existence of a “brain-hair follicle axis” as a key element in the hair follicle’s response to stress, which constitutes a prerequisite for generalized hair loss (telogen effluvium).
Thus far, the mechanisms by which stress perception, usually elicited by unexpected and intractable environmental stimuli, affect the skin remain ill-characterized.6 Stress perception generally affects the equilibrium of nervous, endocrine, and immune systems.7–11 Besides SP and classical stress-related neurohormones like cortisol-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and prolactin (PRL),9,12 nerve growth factor (NGF) is now recognized as a decisive factor involved in stress responses.13,14 Recent findings indicate that circulating levels of NGF undergo significant variations after exposure to stressful events.15–17 In the context of the “brain-hair follicle axis”, NGF is particularly interesting since it is known to increase the number of peptidergic nerve fibers and induce the release of sensory neuropeptides such as SP.18 Therefore, it is interesting to ascertain whether the increased number of SP+ nerve fibers observed in the skin of stressed mice3 is indeed related to a stress-associated up-regulation of NGF-mediated signaling.
The biological effects of NGF, the most extensively characterized member of a family of neurotrophic factors collectively called neurotrophins (NT),19 on target cells are mediated by two different receptors: the high-affinity NGF receptor tyrosine kinase A (TrkA), which is a classical tyrosine kinase receptor activated in response to NGF binding, and the low-affinity p75NTR receptor, a member of the tumor necrosis factor (TNF) receptor-related superfamily.20 Besides its function as an important trophic factor for neuropeptidergic and sympathetic neurons in the skin, NGF is increasingly recognized as a potent immunomodulator, promoting “cross-talk” between neuronal and immune cells.20–22 Further, NGF acts as an autocrine and paracrine factor in the development and regulation of immune cells.23 NGF may be produced by immune cells, eg, mast cells, which express functional NGF receptors. Another action of NGF as immunomodulator may be the facilitation of monocyte/macrophage migration through vascular endothelium.24
The presence of NGF in murine skin has recently been described,25,26 alluding to its involvement in the control of apoptosis-driven hair follicle regression (catagen).27–29 Specifically, selected NTs accelerate catagen most likely by stimulating the p75NTR receptor, which is expressed in the regressing outer root sheath (ORS) and is known to mediate apoptosis when activated alone.27,30–32
Based on these recent insights into the role of NTs in hair growth control and the central role of NGF in stress-induced neurogenic inflammation, we hypothesized that NGF production in the skin is up-regulated following stress exposure, which in turn promotes up-regulation and release of neuropeptides, eg, SP from sensory c-fibers. Thus, NGF directly and/or via SP release triggers mast cell degranulation and induces neurogenic inflammation, thus causing premature anagen termination and catagen induction.
To probe this hypothesis, we used the C57BL/6 mouse model for hair research, since it is the best characterized mouse strain in terms of hair biology. Our experiments focused on three issues. First, we investigated changes in the hair follicle expression of NGF and its receptors p75NTR and TrkA after exposure to sonic stress. Secondly, we dissected whether inhibiting NGF activity by application of NGF- neutralizing antibody blocks stress-induced premature catagen development of C57BL/6 mouse back skin, inhibits stress-triggered premature apoptosis of hair follicle cells in situ in mice during the anagen/catagen transformation, or affects the number and activation status of perifollicular macrophages and/or mast cells in stressed mice, since these cells are recognized key elements of the neurogenic inflammatory skin response to stress.33–35 Thirdly, we examined whether stress exposure or subcutaneous injection of recombinant NGF (to mimic stress) affects the number of SP-positive sensory neurons in dorsal root ganglia innervating the skin. For this, we used retrograde tracing, a method which has long been fundamental to map connectivity in the nervous system.36,37
Materials and Methods
Animals
Six- to 8-week-old female C57BL/6 mice were purchased from Charles River (Sulzfeld, Germany) since mice at this age show the most reliable and profound stress-response1 and are in the telogen stage of the hair cycle. The animals were housed in community cages at the animal facilities of the Charité, Virchow Hospital (University Medicine Berlin, Germany) with 12-hour light periods, and were fed water and mouse chow ad libitum. Animal care and experimental procedures were followed according to institutional guidelines and conformed to the requirements of the state authority for animal research conduct (LaGetSi, Berlin, Germany).
Anagen Induction
Anagen was experimentally induced by depilation, as previously published.38,39 Briefly, mice were anesthetized with intramuscular injection of ketamine hydrochloride (Ketanest, Parke-Davis, Freiburg, Germany, 10 mg/kg body weight) and xylazine hydrochloride (Rompun, Bayer, Leverkusen, Germany, 10 mg/kg). Then, a wax/rosin mixture was applied to the dorsal skin of mice with all hair follicles in telogen, as evidenced by the pink back skin color. Peeling off the wax/rosin mixture removes all hair shafts and immediately induces a highly synchronized hair growth.38,40,41
Application of Stress
Two groups of C57BL/6 mice were exposed to sonic stress for the duration of 24 hours starting on day 14 post-depilation (p.d.), when all back skin hair follicles were in late anagen. The sound stress was emitted by a rodent repellant device (Conrad Electronics, Berlin, Germany) at a frequency of 300 Hertz in intervals of 15 seconds. The stress device was placed into the mouse cage so that the mice could not escape sound perception.1,2
Application of Anti-NGF
Intraperitoneal application of 200 μl polyclonal rabbit anti-mouse NGF antibody (Sigma-Aldrich, Munich, Germany) at a dilution of 1:1500 in PBS was performed twice in two respective groups of control or stressed mice on days 14 and 15 p.d. According to the manufacturer, a 1:4000 dilution of this antibody blocks bioactivity of 5 ng/ml NGF. The dosage of anti-NGF at 1:1500 we used was based on previously published data, where an increase of serum NGF in male mice on stress challenge (introducing a male intruder led to an increase of serum NGF initially up to 100 ng/ml, followed by 30 ng/ml on repeated exposure) has been described.42 Since we expected a less dramatic increase of serum NGF with the moderate stressor we employ and to avoid neurotoxicity on anti-NGF injection, we decided on the rather low dosage of anti-NGF at a dilution of 1:1500.
Tissue Preparation
On day 16 p.d., at the time when control mice are just about to spontaneously enter the anagen/catagen transformation of their depilation-induced hair cycle,43 all mice were sacrificed. Skin specimen from the neck region of murine back skin were harvested parallel to the vertebral line to obtain longitudinal sections through the hair follicle, which is an essential requirement for quantitative histomorphometry of the hair cycle.44 Specimens were snap-frozen in liquid nitrogen and then covered with embedding medium, as described in detail in by Müller-Röver et al.39 Cryosections were then processed for immunohistochemistry and TUNEL staining. For molecular biology studies, approximately 100 mg of skin tissue from control and stressed mice (n = 7/group) were snap-frozen in liquid nitrogen and stored at −80°C until RNA isolation.
Fluorescence Immunohistochemistry for Skin Tissue
Fluorescence immunohistochemistry (FIH) was performed for detection of NGF and NGF receptors, TrkA and p75NTR, in murine skin tissue according to published protocols.27 Briefly, the sections were washed with Tris-buffered saline solution (TBS, pH = 7.4) for 15 minutes and blocked with 2% normal goat serum (Dako) diluted in TBS for 30 minutes at room temperature (RT) to reduce non-specific binding. The cryosections were then incubated overnight at RT in a humidity chamber with the corresponding primary antibody at a dilution of 1:50 for NGF (Santra Cruz), 1:100 for TrkA (Santra Cruz) and 1:100 for p75NTR (Chemicon, Hofheim, Germany). The tissues were washed, followed by an incubation of 1 hour at 37°C with tetramethylrhodamine-isothiocyanate (rhodamine)-conjugated F(ab)2 fragments of goat anti-rabbit IgG (Jackson ImmunoResearch) at a TBS dilution of 1:200 in 2% mouse normal serum. After incubation, each section was rinsed three times in 0.1 mol/L TBS (pH 7.4) for 15 minutes. All sections were mounted and stored at −20°C until analyzed. Negative controls were performed by replacing the primary antibody with 10% of normal goat serum (Dako). Negative controls did not reveal specific immunoreactivity.
RNA Isolation, DNase Treatment, and Reverse Transcription (RT)
Pieces of skin tissue (about 100 mg/mouse), which were prepared for molecular biology studies, were treated with 1 ml Trizol (Invitrogen, Karlsruhe, Germany) and disaggregated using a mortar. The RNA was then extracted using a standard protocol, as previously described.45 After RNA preparation, the total RNA was treated with DNase I 1U/1 μg-RNA (Roche Diagnostics, Mannheim, Germany) for 15 minutes at room temperature followed by inactivation with EDTA (Sigma, Munich, Germany). After the DNase treatment, there was no detection of gDNA via PCR. First-strain cDNA synthesis was performed using Superscript II reverse transcriptase (Gibco, Karlsruhe, Germany) as previously published.45
Real Time RT-PCR for NGF
Real time PCR was used to obtain quantitative data on differences between NGF mRNA of the control group and mice exposed to stress. This assay exploits the 5′ nuclease activity of AmpliTaq Platin (Invitrogen, Karlsruhe, Germany) DNA Polymerase to cleave a fluoregenic probe designed for NGF (TipMolBiol, Berlin, Germany) and, to normalize our samples, a fluorogenic probe for the housekeeping gene hypoxanthine phosphoribosyl transferase (HPRT) was used in real time PCR. The sets of primers and probes were designed as listed in Table 1.
Table 1.
Sequence of Primers and Probes Used for Real Time PCR to Detect NGF, Normalized to HPRT
| NGF analysis | Sequence |
|---|---|
| NGF probe | 6FAM-gAT Cgg CgT ACA ggC AgA ACC gTA CAC AgA T XT A-PH |
| Reverse primer | 5′-Tag TCC AgT ggg CTT CAg ggA |
| Forward primer | 5′-TTT gCC AAg gAC gCA gCT T |
| HPRT analysis | Sequence |
|---|---|
| HPRT probe | 6FAM-TTg CAg ATT CAA CTT gCg CTC A XT CTT-PH |
| Reverse primer | 5′-CAC Agg ACT AgA ACA CCT gC |
| Forward primer | 5′-gTT ggA TAC Agg CCA gAC TTT gT |
The real time PCR reactions were developed as previously published45 except that we used a 0.2-μl probe at 20 μmol/L. Each analysis was normalized to HPRT, by calculating the difference between the CT for HPRT and the CT for NGF as Δ CT = CT HPRT − CT NGF. “Fold increase” of mRNA was calculated on the basis that Δ CT = 1 equals a 2.93-fold increase.
Quantitative Histomorphometry of Hair Follicle Cycling
Standardized techniques for the classification of hair cycle stages used published detailed guidelines, which allows accurate classification of all hair follicles with respect to their exact stage in the transition from anagen VI via catagen I-VII to telogen.39,44
Immunohistochemistry
Immunohistochemistry (IHC) was performed following published protocols.46 Briefly, anti-mouse major histocompatibility complex (MHC) class II antibody (ER-TR, BMA, Augst, Switzerland) was used in a 1:100 dilution for immunodetection of MHC-II+ cell cluster within murine skin. This antibody labels MHC class II+ antigen-presenting cells (dendritic cells, B cells, and macrophages). Cryostat sections (10 μm) were fixed in acetone (10 minutes at −20°C) and preincubated with ready-to-use blocking solution (Immunotech, Marseille, France). The rat anti-mouse MHC antibody was incubated for 45 minutes at room temperature, followed by biotinylated goat anti-rat secondary antibody (1:200, 30 minutes, Jackson ImmunoResearch), and developed using the alkaline phosphatase method.
Giemsa Staining
The presence of mast cells in murine back skin was detected using Giemsa staining (Merck, Darmstadt, Germany) In brief, Giemsa was applied at a 1:10 dilution with 2% sodium borate solution for 45 minutes at room temperature. Differentiation was achieved using 0.02% acetic acid under microscopic control, slides were dehydrated and mounted. Mast cells were classified as “degranulated” when eight or more granula could be found outside the cell membrane.
TUNEL Staining
To evaluate apoptotic cells in murine hair follicles, we used the TUNEL staining method as described before.47 Ten-μm cryostat sections of murine back skin were freshly prepared and fixed in formalin, post-fixed in ethanol/acetic acid, and incubated with digoxigenin-dUTP in the presence of terminal desoxynucleotidyl transferase (TdT), using a commercially available kit (Intergen, Oxford, UK). TUNEL-positive cells were visualized by anti-digoxigenin fluorescein isothiocynate (FITC)-conjugated F(ab)2 fragments, then counterstaining was performed using DAPI dye (1 μg/ml methanol; Roche, Mannheim, Germany) in a subsequent incubation step. Finally, sections were mounted using VectaShield (Vector Laboratories, Burlingame, CA). Negative controls for the TUNEL staining were made by omitting TdT, according to the manufacturer’s protocol.
Retrograde Tracing of Dorsal Root Ganglia (DRG), Tissue Preparation, and SP-FIH in DRG
An additional group of animals (n = 12) were again anesthetized 9 days p.d. and a fluorogold-like tracer (hydroxystilbamidine; Biotium) was applied in five subcutaneous injections with a total volume of 20 μl at a concentration of 1.25% (100 mg/800 μl distilled H2O) in the dorsal skin tissue right below the scapula, covering a total tissue area of about 1 cm2. All animals recovered undisturbed for a postoperative period of 5 days, until day 14 p.d. Then, on day 14 p.d., we followed our above protocol and exposed some of these mice to stress (n = 4). Another subgroup (n = 4) was injected subcutaneously in the dorsal skin with murine NGF (7S; Roche, Mannhein, Germany) with 10 μl/mouse at a concentration of 100 μg/ml PBS. We included this subcutaneous application of NGF to mimic stress-triggered increase of skin NGF. Lastly, the remaining subgroup of mice out of the 12 mice, in which we retrogradely traced the DRG (n = 4) and injected with PBS alone, served as a non-stressed control. On day 16 p.d., the animals were perfusion-fixed retrogradely through the left ventricle with freshly prepared Zamboni’s solution (2% paraformaldehyde, 15% picrinic acid, 0.1 mol/L PBS, pH 7.4) for 5 minutes. Dissection of the Zamboni-fixed cervical and thoracal DRG (C4 to Th10) was performed by rinses in 0.1 mol/L phosphate buffer (pH 7.4) and cryoprotection with 18% sucrose in 0.1 mol/L phosphate buffer overnight. On average, 30 DRGs were harvested per mouse and screened for traced neurons by preparing cryosections of each DRG. Traced DRG could predominantly be identified from C5 to Th3, thus, on average, 14 traced DRG/mouse were available for the preparation of serial sections, and approximately 40 consecutive slides were cut per DRG. Sections, cut at 7 μm and air dried for 30 minutes, were incubated overnight with a monoclonal rat anti-mouse SP antibody (Chemicon) at a dilution of 1:400. Several washing steps were followed with an incubation of 1 hour at 37°C with rhodamine-conjugated F(ab)2 fragments of goat anti-rabbit IgG (Jackson ImmunoResearch) at a TBS dilution of 1:200 in 2% mouse normal serum.
Quantitative Histomorphometry and Statistical Analysis
Sections to evaluate hair follicle cycling, IHC, and Giemsa staining were analyzed under a Zeiss Axioscope light microscope (Zeiss, Jena, Germany) and photo documentation was performed using KS400 camera (Zeiss, Jena, Germany). Individual hair follicles per mouse were assessed from the neck region of back skin since catagen development occurs there first. The total number of mice studied per group is listed in the figures.
A fluorescence microscope (Axioplan; Zeiss, Jena, Germany) with appropriate excitation emission filter systems for fluorescence induced by DAPI, FITC, and rhodamine was used to analyze FIH-labeled sections and TUNEL staining. Fluorogold-positive neurons were identified through a wide-band UV filter. Analysis and photo documentation was performed by a digital image analysis system (Spot advanced software, version 3.5.2; Visitron Systems; Puchheim, Germany). Differences were judged as significant if the P value was ≤0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***), as determined by the Mann-Whitney U/Wilcoxon Rank test.
Results
Stress Up-Regulates Epithelial Expression of NGF and Its Receptors p75NTR in Epidermis and Hair Follicle
The observation that stress can produce significant alterations in circulating NGF levels13–17 raised the question as to whether exposure to stress also affects the local expression of NGF and its receptors, p75NTR and TrkA, in murine hair follicles.
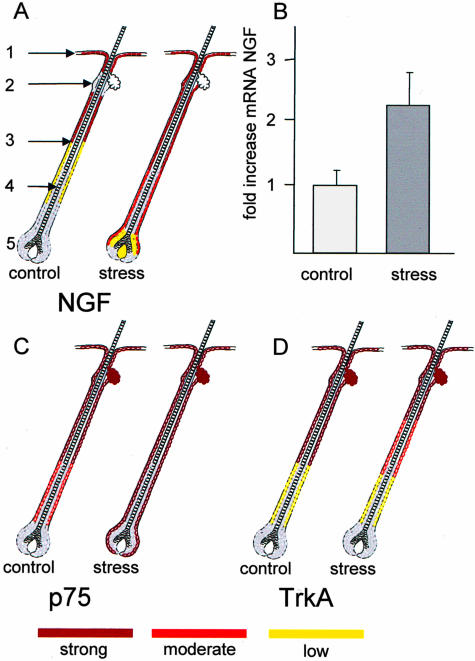
As depicted in Figure 1A, in follicles of control mice, a strong NGF signal could be detected for outer root sheath (ORS) of the upper third of the hair follicle, whereby the bulge region did not show NGF expression. Overall, NGF intensity faded along the ORS toward complete NGF negativity of the bulb. Interestingly, we observed a much stronger expression of NGF in hair follicles of stressed mice, compared to the non-stressed control, ranging from the epidermis along the bulge region/ORS and, although more moderately expressed, the suprabulb region (Figure 1A). The increase of NGF protein expression with stress, as evaluated by FIH, was supported by quantitative PCR on skin samples, where a 1.23-fold increase of NGF mRNA could be detected in stressed mice (Figure 1B). Figure 2 shows a representative area of NGF-labeling in the ORS (Figure 2A) and the bulb (Figure 2C) in control mice, and for stressed mice in Figure 2, B and D, respectively.
Figure 1.
A: Schematic drawing of NGF expression in distinct areas of the hair follicle. Areas marked in red depict strong labeling for NGF, pink show moderate expression, and orange represent low expression of NGF. Areas evaluated included the epidermis (marked as 1), bulge region (2), outer root sheath (ORS, 3), inner root sheath (IRS, 4), and bulb (5), applicable for all schematic drawing in Figure 1. Results were calculated from five mice per group with an average of 100 to 120 hair follicles evaluated per mouse. B: Results of real time PCR for NGF with mRNA (mean of delta CT) obtained from back skin tissue of mice either exposed to stress (n = 7) or non-stressed control (n = 7). Schematic drawing of NGF receptors: p75NTR expression in hair follicle areas of control and stressed mice is depicted in (C) and TrkA expression in (D), scoring of expression, number of mice used, and hair follicle is as described in (A).
Figure 2.
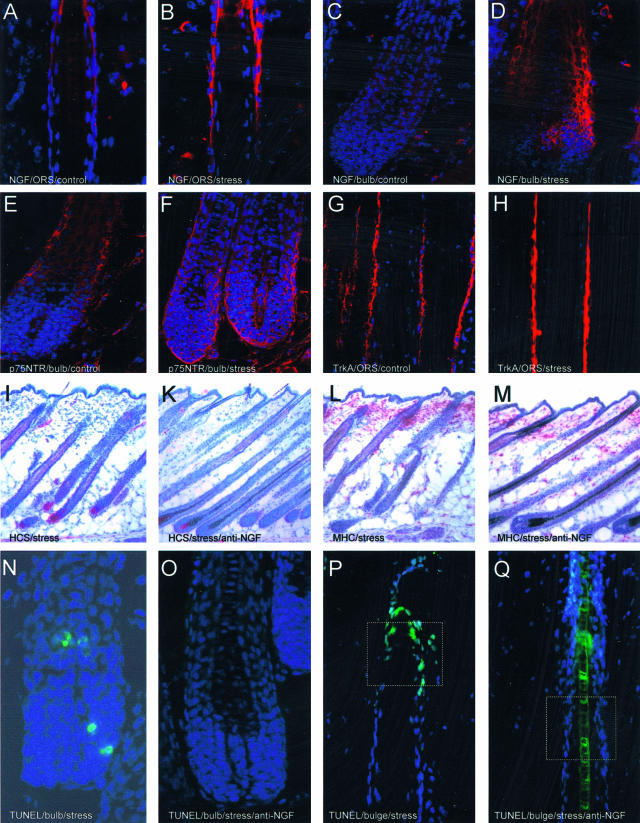
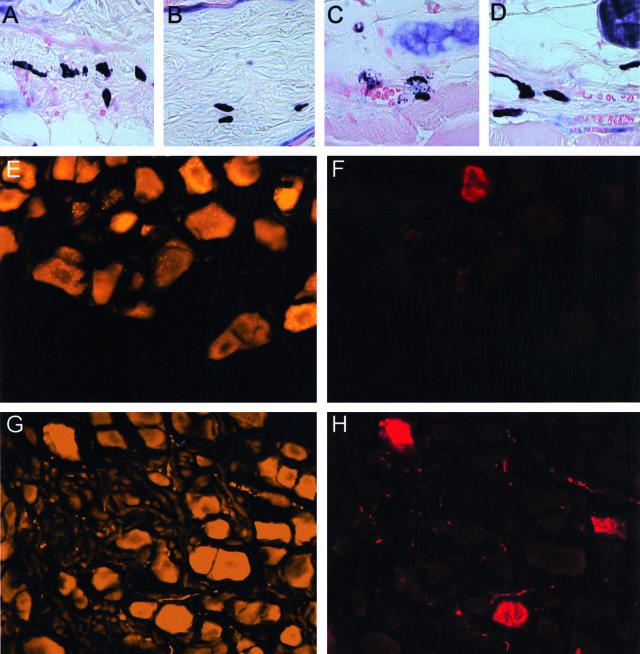
Histology of murine skin tissue used in the present study. A—D: Representative examples for NGF labeling in the ORS of control mice (A), compared to the ORS of hair follicle in stressed mice (B). Please note that NGF-labeling appears in bright red; blue was generated by DAPI-induced counterstaining to facilitate orientation. C: An example of NGF staining in the bulb of control mice, compared to a more intense labeling, which includes the dermal papilla, in stressed mice, as depicted in (D). E: Representative example for p75NTR labeling of the bulb region in hair follicle from control mice, compared to (F), which shows the NGF receptor p75NTR expression of the bulb region in stressed mice. G: Representative example for NGF-receptor TrkA labeling of the ORS of hair follicle from control mice, compared to (H), which shows the TrkA expression of the ORS in stressed mice. The effect of stress on the hair cycle stage is depicted in I and K. I: Representative area of stressed mice 16 days p.d. with the majority of hair follicle in catagen IV (CIV) or catagen V (CV). K: Representative example for hair cycle stage of stressed, anti-NGF-treated mice is depicted with hair follicle in anagen VI (AVI), compared to the non-stressed control (no picture shown). L and M: Effect of stress on MHC II+ cells in murine skin. L: Distribution of MHC-II+ cells in parafollicular dermis of stressed mice, compared to (M), where fewer cluster were detectable after stress exposure when mice were injected with anti-NGF. N: Premature onset of catagen after application of stress (I) was independently confirmed by intrafollicular apoptosis, which is a hallmark of catagen development. O: Representative histology for the bulb region of stressed mice treated with anti-NGF, where apoptosis was abrogated. P: Apoptosis of the bulge region in stressed mice. Since an important focus of research on stress-induced hair growth inhibition must be to identify vulnerable, apoptosis-prone targets of the hair follicle, we suggest that one such target might be the bulge region (white square), which is the residence of stem cells in the skin. Interestingly, as depicted in (Q), no signs of apoptosis were detectable in the bulge region (white square) of mice exposed to stress and treated with anti-NGF. Magnification, ×400 (A–D, N, O); ×50 (I–M).
Next, we analyzed the expression of the two different receptors, TrkA and p75NTR, through which the biological activity of NGF is mediated. Expression of p75NTR receptor was intensely positive in epidermis, infundibulum, and bulge region in both groups. While the bulb region of hair follicles of the control group was negative for p75NTR, we detected strong labeling of the regressing ORS and the bulb region of hair follicles from mice exposed to stress (Figure 1C). Figure 2E depicts a representative labeling for p75NTR in control mice, and Figure 2F for stressed mice. As also depicted in Figure 2F, we were able to differentiate staining of the bulb from a possible staining of the connective tissue sheath (CTS); since cell nuclei of the bulb were enclosed by the labeling, staining of the CTS would have resulted in a wider labeling. Similar to p75NTR, expression of TrkA receptor was intensely positive in epidermis, infundibulum, and bulge region in both groups. However, expression of TrkA receptor was diminished in the middle section of the proximal ORS of stressed mice compared to control mice, although differences between control and stressed mice were rather subtle (Figure 1D; Figure 2G for ORS in control and Figure 2H for stressed mice).
Neutralizing NGF Inhibits Stress-Induced Premature Catagen Development
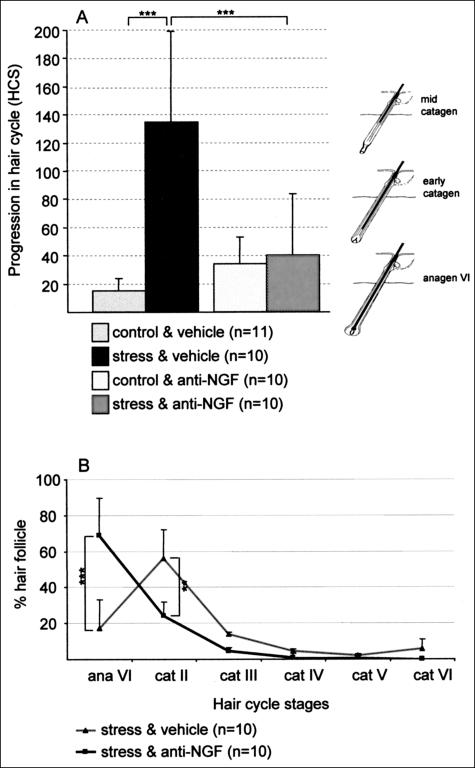
It is likely that the most severe immediate effect of stress on murine hair growth is the premature induction of catagen in depilation-induced anagen hair follicles in vivo.3 As depicted in Figure 3A, we observed a significant increase in the progression of the hair cycle from a predominance of anagen VI follicles in non-stressed control mice toward a predominance of early catagen follicles in stressed mice. Figure 2I displays such a premature hair cycle stage in stressed mice. Interestingly, when stressed mice were treated with the neutralizing NGF antibody, the stress-triggered premature onset of catagen was partially inhibited (Figure 3, A and B, which provides more details with respect to the distinct hair cycle stages for stressed mice with and without NGF neutralization; Figure 2K shows predominantly anagen VI hair follicles of stressed, anti-NGF-treated mice).
Figure 3.
A: Increased vulnerability of hair follicle toward an advanced catagen progression was detectable in stressed mice, compared to non-stressed control. This stress-induced premature onset of catagen could be abrogated by neutralizing NGF. Hair cycle score (HCS) was assessed as previously published in 39,40. Briefly, the y-axis depicts the mean ± 1 SEM of histometric score, assessed on day 16 after anagen induction. For every mouse a minimum of 100 individual hair follicles was assigned to distinct hair cycle stages. On the right of the graph, representative hair cycle stages for each HCS are depicted, ie, anagen VI is the dominant hair cycle stage with a score of 0.5. ***, P < 0.001. B: More detailed description of distinct hair cycle stages in stressed and stressed, anti-NGF-treated mice, to visualize more clearly the redistribution of the majority of hair follicle from catagen II toward anagen VI on anti-NGF treatment in stressed mice.
Neutralizing NGF Changes Stress-Triggered Pathophysiological Patterns of Apoptosis
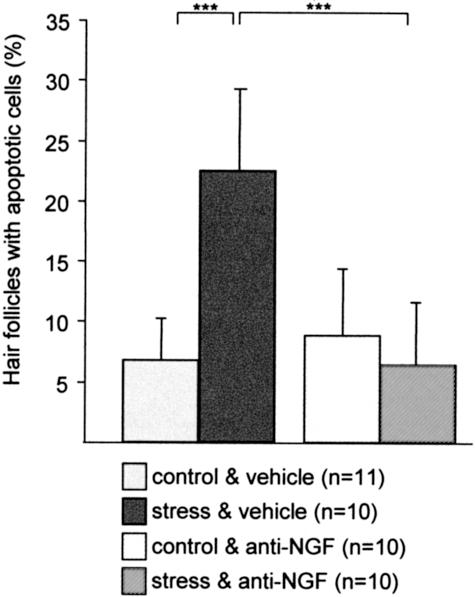
This premature onset of catagen after application of stress was independently confirmed by measuring the intrafollicular patterns of apoptosis, since the massive, yet temporospatially highly controlled occurrence of intraepithelial apoptosis in the hair follicle is a hallmark of catagen development.47 As shown in Figure 4, exposure to stress 2 days before the assessment of hair follicle on day 16 p.d. resulted in a significant increased percentage of hair follicle units with signs of apoptosis (TUNEL+ cells) in C57BL/6 mice (Figure 2N shows the representative histology for the bulb region, Figure 2P for bulge), whereby mice which received NGF-neutralizing antibody in addition to stress did not show signs of apoptosis (Figure 4, for histology see Figure 2O for bulb and Figure 2Q for bulge).
Figure 4.
The effect of stress on the percentage of hair follicle with TUNEL+ cells (mean ± SEM). A fluorescence microscope (Zeiss, Germany) with excitation emission filter systems for fluorescence induced by DAPI and FITC was used for the detection of apoptotic cells. A hair follicle was considered to be TUNEL+, if one or more apoptotic cell bodies were present. We then calculated the percentage of such TUNEL+ hair follicles to the total number of hair follicles. Mean percentages per group ± SEM are depicted in this Figure. Exposure to stress 2 days prior assessment led to a significant increase of apoptotic cells/hair follicle. The effect of stress on hair follicle apoptosis could be significantly abrogated by treatment with anti-NGF antibody. Significance of differences was determined by the Mann-Whitney U/Wilcoxon Rank test, ***, P < 0.001.
Neutralizing NGF Decreases the Number and Activation Status of Perifollicular Immune Cells in Stressed Mice
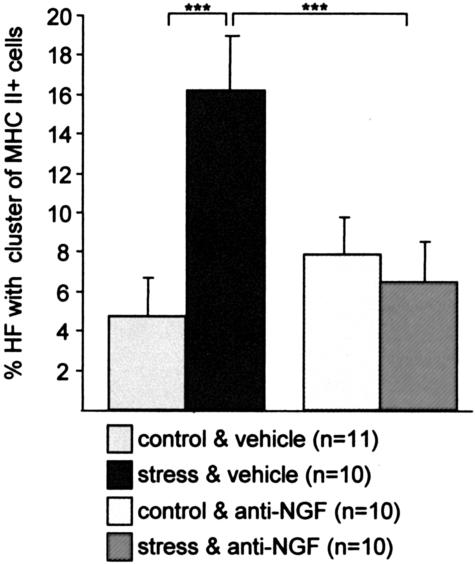
MHC class II+ cell clusters largely represent antigen-presenting cells, eg, activated macrophages, and are associated with a substantially higher risk of hair follicles to be “immunosurgically” eliminated by a process termed “programmed organ deletion”.46 The current study revealed a significant decrease of hair follicles displaying prominent perifollicular MHC class II+ cell clusters (> 5 cells) in stressed mice after treatment with NGF-neutralizing antibody (Figure 5). Figure 2L shows representative IHC of hair follicles in stressed mice and Figure 2M in anti-NGF treated, stressed mice.
Figure 5.
The effect of stress on the percentage of hair follicle with MHC-II+ perifollicular cell cluster in C57BL/6 mice (mean ± SEM). Exposure to stress 2 days prior assessment led to a significant increase of MHC-II+ cells cluster (***, P < 0.001). Injection of anti-NGF antibody abrogated the effect of stress on the percentage of hair follicle with MHC-II+ cells cluster in stressed mice, ***, P < 0.001.
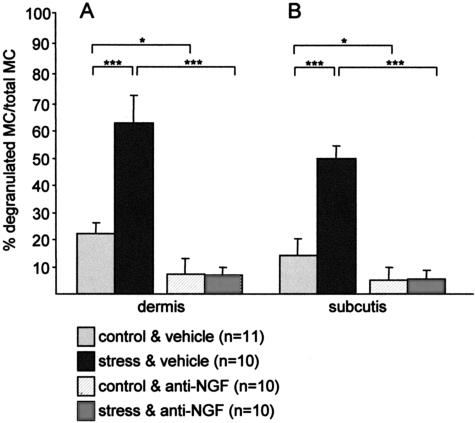
Mast cells have been shown to play a key role in the neuroimmunological linkage of stress to defined inflammatory events.33 We therefore evaluated activated (degranulated) mast cells in the current stress model. Interestingly, a significantly increased percentage of degranulated mast cells was seen in the dermis (Figure 6A, Figure 7A for histology, note the extracellular granula) and in the subcutis (Figure 6B; histology in Figure 7C) of stressed C57BL/6 mice. Strikingly, this stress-triggered activation could be abrogated by application of neutralizing NGF antibody, as evaluated in dermis (Figure 6A; Figure 7B for histology), as well as in subcutis (Figure 6B; Figure 7D for histology).
Figure 6.
The effect of stress on the percentage of degranulated (activated) mast cells, calculated from the total number of mast cells (mean ± SEM) in dermis (A) and subcutis (B) in C57/Bl6 mice. Exposure to stress 2 days prior assessment led to a significant increase of degranulated mast cells in murine dermis and subcutis, and injection of anti-NGF counteracted this activation of mast cells in stressed mice and also reduced the percentage of activated mast cells in control mice.
Figure 7.
Representative example of mice exposed to stress, which caused an increase in activated (degranulated) mast cells (dark purple staining with more than 8 extracellular granula) in dermis (A) and subcutis (C). Mostly non-activated mast cells were detectable in dermis (B) and subcutis (D) of stressed, anti-NGF-treated C57BL/6 mice, where no extracellular granula are present. E: Representative example of a fluorescent, fluorogold-traced dorsal root ganglia taken from C4 to Th10 of a control mouse. Fluorogold-induced tracing is visualized by bright yellow staining. F: The corresponding area to (E) using the filter for rhodamine, which allows to identify SP-positive neurons, as depicted in bright red color, among the total number of traced neurons. G: Spontaneous fluorescence in traced DRG of NGF-injected mice, (H) depicts the same visual field using the filter for rhodamine and is also representative for SP-positivity of neurons in DRG of stressed mice. Magnification, ×400 (A–D); ×200 (E–H).
Subcutaneous Injection of Recombinant NGF or Stress Exposure Affects Number of SP-Positive Neurons in Spinal Ganglia
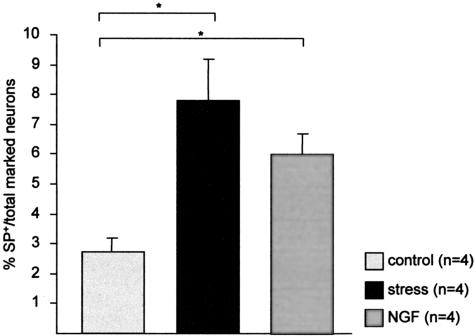
Sensory nerves are derived from the dorsal root ganglion and are present in all parts of the skin, representing the initial somatic portion of the afferent sensory pathway. The cutaneous sensory nervous system comprises a network of fine C fibers within the skin that innervate multiple target structures and play an important role in inflammation. Therefore, we used the retrograde tracing technique of skin nerve fibers to cervical and thoracal DRG. Our experiments, presented in Figure 8, clearly indicate that the percentage of SP-positive neurons among the total number of traced neurons significantly increased in mice exposed to stress. More intriguingly, subcutaneous injection of NGF to mimic stress effects, based on the findings depicted in Figure 1, A and B, also resulted in an increased percentage of SP-positive neurons in DRG. Figure 7E shows the spontaneous fluorescence on application of the fluorogold-like tracer in DRG of control mice while Figure 7F depicts the same visual field using the filter for rhodamine. Figure 7G shows the spontaneous fluorescence in traced DRG of NGF-injected mice while Figure 7H depicts the same visual field using the filter for rhodamine; this figure is also representative for stressed mice.
Figure 8.
Percentage of SP-positive neurons among the total number of marked neurons in the dorsal root ganglia taken from C4 to Th10 in non-stressed control mice, stressed mice, and non-stressed mice subcutaneously injected with NGF. On average, 14 ganglia were taken per mouse, and 40 consecutive slides were evaluated to obtain the present data, which are depicted as mean ± SEM. *, P < 0.05.
Taken together, these experiments support our working hypothesis and suggest that stress-induced neurogenic inflammation in murine skin is significantly promoted by NGF-dependent pathways which subsequently recruit SP as a key stress-associated neuropeptide, thus leading to premature hair growth termination (ie, anagen inhibition and catagen induction).
Discussion
With our present work we deliver convincing experimental evidence demonstrating that stress exposure results in NGF increase in murine skin. Increased NGF levels in turn influence hair follicle cycling, induced/accompanied by deleterious inflammatory events. This stress-triggered release of NGF chains previously published data, obtained in manifold experimental settings of stress exposure: eg, in mice and humans, exposure to acute or chronic stress leads to significant alterations of circulating and brain NGF and BDNF levels.14–16,48,49 Further, stress has been shown to influence the basal activity of nerve, immune, and endocrine cells expressing NGF receptors.21
Our recent observations now render us to the question if and how the equilibrium of the immune and nervous system in the skin is challenged by the up-regulation of NGF on stress exposure. Botchkarev et al27 and others previously reported that NGF is detectable in the skin, along with the other members of the NT family. Further, NGF can significantly enhance the percentage of hair follicles in advanced catagen stages.27,28,50 This is in line with our present in vivo observations of premature catagen development in stressed mice, where levels of NGF are up-regulated.
Interestingly, as demonstrated by Botchkarev et al,27 in the presence of p75NTR antagonist, NGF, NT-3, and BDNF failed to promote catagen development, suggesting that the catagen-promoting action of NT is significantly mediated by p75NTR and can be antagonized by NGF-neutralizing antibodies, as applied in the present study. Noteworthy, the p75NTR receptor, which is predominantly expressed in epidermis, infundibulum, ORS, and the outermost layer of bulb cells in hair follicles of stressed mice, mediates apoptosis.27,30–32 This is convincingly in accord with the increased percentage of hair follicles with signs of apoptosis we reported in stressed mice, which was predominantly located in the bulge and bulb region3 and could be abrogated by neutralizing NGF. p75NTR expression depends on the balance of bone morphogenic protein 4 (BMP4) and its antagonist noggin, both of which are closely regulated during the hair growth cycle.51 For the duration of the growth phase, BMP4 up-regulation of p75NTR is antagonized by noggin. Inflammatory processes have been linked to BMP4-up-regulation.52,53 We thus hypothesize, that stress-induced inflammation may shift the BMP4/noggin balance toward BMP4, thereby increasing the p75NTR expression. Moreover, BMP4 is able to induce SP expression in naive neurons54,55 thereby contributing to the increase of SP+ neurons observed in stressed catagen skin.3
Apart from directly inducing apoptosis of hair follicle keratinocytes, p75NTR also down-regulates growth-promoting keratinocyte growth factor (KGF) effects on keratinocyte proliferation and differentiation by down-regulation of the respective high-affinity receptor fibroblast growth factor receptor (FGFR-2) receptor.56 KGF also provides significant cytoprotection to growing hair follicles in the murine model of chemotherapy57 and may also do so under inflammatory conditions. Lack of KGF/FGFR-2 signaling thus negatively affects the growing hair follicle in two places.
Since an important focus of research on stress-induced hair growth inhibition must be to identify vulnerable, apoptosis-prone targets of the hair follicle, we suggest that one such target might be the bulge region, the residence of stem cells in the skin, where we observed a stress-triggered increase of apoptotic cells. Stem cells are vital for the homeostasis of self-renewing tissues and, in the skin, stem cells have the ability to produce a new hair.58,59 The permanent risk of permanent hair follicle loss by stress-triggered programmed organ deletion may be further promoted by the imbalance of the dimished presence of the high-affinity NGF receptor TrkA, opposed by an increased presence of the low-affinity receptor p75NTR, through which NGF then induces apoptosis.
Further, induction of apoptosis by other mediators besides NGF has been an intensive area of research for several years, and insights in the mammalian apoptotic machinery constitute a promising blueprint of the molecular network governing the fate of all living cells.60 Interestingly, on injection of recombinant TNF-α, another potent inducer of apoptosis, a significantly higher number of apoptotic cells within the murine epidermis could be observed.61 In the present study, we did not investigate levels of TNF-α, however, using the sonic stress-exposure model, which we use in various experimental settings, revealed an increase of TNF-α-positive cells on stress exposure in the uterus.62 In the context of alternate death pathways, it is also noteworthy that experiments in mice with disrupted genes for Fas or Fas ligand suggest an important pathogenetic role for apoptosis induced through the Fas/Fas ligand system in hair follicle melanocyte apoptosis in chemotherapy-induced hair loss and alopecia areata.63,64
The next question raised by our findings is how this release of NGF triggers or exacerbates deleterious inflammatory events such as mast cell activation or migration of MHC II+ antigen-presenting cells, eg, activated macrophages. Indeed, NGF has been reported to directly stimulate degranulation (activation) of mast cells, similar to the stress effects we observed in the present study.65 Mast cells express functional NGF receptors and produce NGF, which would be an additional contribution to the cutaneous neurogenic inflammation.23,66 Further, mast cells secrete proteinases, eg, tryptase and trypsin, which are not only enzymes that degrade proteins, but are also capable of mediating important inflammatory effects such as cytokine release, cell migration, recruitment of leukocytes, and endothelial cell activation.66,67 This may provide an explanation for the increased migration of MHC+ cell cluster in stressed mice, as observed in the present study.
These inflammatory effects are, at least in part, mediated by cleavage and activation of proteinase-activated receptors (PARs),68,69 indicating that, in neurogenic inflammation, proteinases may activate PARs on sensory neurons to stimulate release of SP, which, in turn, additionally enhances the inflammatory response. Moreover, the release of other neuropeptides, eg, calcitonin gene related peptide (CGRP) may also be up-regulated on stress exposure with subsequent PAR activation (Kuhlmei A,unpublished observations). Tryptase-releasing mast cells can be found in close proximity to PAR-2-expressing cells such as keratinocytes and dermal endothelial cells or C-fibers during inflammation,70 and we previously reported an increase in SP+ nerve fibers in stressed mice and observed that neurogenic inflammation may be abrogated by application of the SP high-affinity neurokinin-1 (NK1) receptor.1,3 In the present study, cervical and thoracal sensory neurons innervating the dorsal skin were identified by retrograde transport of a tracer dye, subsequently providing insights into SP expression in skin-specific sensory neurons.
NGF is also implicated in the hypothalamic-pituitary-adrenal (HPA) axis,70–73 and experimental evidence indicates that the skin possesses its own HPA axis and skin cells produce CRH and express functional CRH receptors, supporting the concept that such a distinct dermal neuroendocrine pathway may serve as a skin stress response system which translates stressful conditions into physiopathological responses.
Interestingly, when stress is induced by fear or maternal separation in distinct animal models, behavioral deficits in adult life have been described in addition to the significant alteration of brain and circulating NGF levels present postnatally, immediately upon stress exposure.74–77 Hence, it is tempting to postulate that impaired stress coping abilities of individuals may be rooted in early postnatal stress perception and result in adult pathophysiologies.
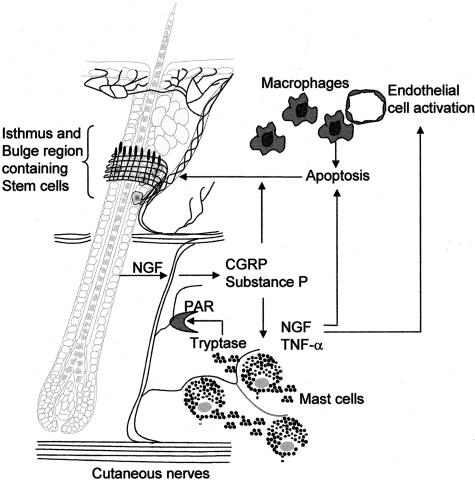
In conclusion, our data indicate that an interactive communication network between sensory nerves and immune cells in the skin is promoted by stress-triggered release of NGF and results in mast cell activation and migration of macrophages in the vicinity of the hair follicle (Figure 9). Via PARs, neuropeptide secretion, ie, SP, is enhanced, and the release of such proinflammatory neuropeptides elicits additional imbalances within the network of neurogenic inflammation. In consequence, p75NTR receptor-mediated apoptosis of hair follicles and hair follicle stem cells is increased in stressed mice. Hence, neutralization of NGF and/or antagonizing p75NTR receptor may be a clinically interesting and innovative tool to pharmacologically address stress-triggered NGF-mediated telogen effluvium.
Figure 9.
Hypothetical scenario of NGF action in stress-triggered neurogenic inflammation of the skin with consecutive hair growth inhibition: on stress exposure, NGF is increasingly present in the skin, either in the ORS and bulb region of the hair follicle, or secreted by activated mast cells. Proteases, ie, tryptase secreted by activated mast cells induce the release of inflammatory neuropeptides, eg, substance P (SP) or calcitonin gene-related peptide (CGRP) via proteinase-activated receptor (PAR)-dependent pathways, resulting in an autocrine/paracrine mechanism of mast cell activation with consecutive NGF production besides other inflammatory cytokines (ie, TNF-α), as well as macrophage activation, whereby macrophages may be recruited into the skin based on endothelial cell activation on NGF. Besides TNF-α, NGF induces apoptosis via the p75NTR receptor, which then diminishes stem cells residing in the bulge area and the cycling of hair follicle by premature onset of catagen. Thus, the skin looses the ability to produce new hair follicle due to diminished number of stem cells in addition to the increased telogen effluvium triggered by the premature catagen.
Acknowledgments
We thank R. Pliet, P. Moschansky, and P. Busse for their excellent technical assistance and Dr. Karl-Anton Kreuzer for designing the NGF probe.
Footnotes
Address reprint requests to Petra Arck, M.D., at Charité, Campus Virchow Klinikum, Medizinische Klinik/Biomedizinisches Forschungszentrum, Raum 2.0549, Augustenburger Platz 1, 13353 Berlin, Germany. E-mail: petra.arck@charite.de.
Supported by grants from the German Science Foundation (Deutsche Forschungsgemeinschaft Ar 232/14–1, Pa 345/11–1, Pe 890/1–1) and the Charité (UFF 99–648).
References
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a “brain-hair follicle axis (BHA)”: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. EMBO J. 2001;5:2536–2538. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EMJ, Hagen E, Klapp BF, Paus R. Topical minoxidil counteracts stress-induced hair growth inhibition in mice. Exp Dermatol. 2003;12:580–590. doi: 10.1034/j.1600-0625.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EMJ, Peter A, Hagen E, Fischer A, Klapp BF, Paus R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czarnetzki BM. Hair growth induction by substance P. Lab Invest. 1994;71:134–140. [PubMed] [Google Scholar]
- Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin and hair growth modulation by neuropeptides. J Invest Dermatol. 2001;116:236–245. doi: 10.1046/j.1523-1747.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- Paus R. Stress and the skin. Exp Dermatol. 2001;10:367. doi: 10.1034/j.1600-0625.2001.100507-8.x. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Arimura A, Takaki A, Komaki G. Interactions between cytokines and the hypothalamic-pituitary-adrenal axis during stress. Ann NY Acad Sci. 1994;739:270–281. doi: 10.1111/j.1749-6632.1994.tb19829.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response: The 1997 Hans Selye Memorial Lecture. Ann NY Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Ader R. On the development of psychoneuroimmunology. Eur J Pharmacol. 2000;405:167–176. doi: 10.1016/s0014-2999(00)00550-1. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Arck PC. Stress and pregnancy loss: role of immune mediators, hormones, and neurotransmitters. Am J Reprod Immunol. 2001;46:117–123. doi: 10.1111/j.8755-8920.2001.460201.x. [DOI] [PubMed] [Google Scholar]
- Aloe L, Alleva E, Böhm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci USA. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Alleva E, De Simone R. Changes of NGF level in mouse hypothalamus following inter-male aggressive behavior: biological and immunohistochemical evidence. Behav Brain Res. 1990;39:53–61. doi: 10.1016/0166-4328(90)90120-4. [DOI] [PubMed] [Google Scholar]
- Aloe L, Tirassa P, Alleva E. Cold water swimming stress alters NGF and low affinity NGF receptor distribution in developing rat brain. Brain Res Bull. 1994;33:173–178. doi: 10.1016/0361-9230(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Aloe L, Musi B, Micera A, Santucci D, Tirassa P, Alleva E. NGF antibody production as a result of repeated psychosocial stress in adult mice. Neurosci Res Commun. 1995;1:19–28. [Google Scholar]
- Lakshmanan J. Nerve growth factor levels in mouse serum: variations due to stress. Neurochem Res. 1987;12:393–397. doi: 10.1007/BF00993250. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Garrett NE, Tomlinson DR. Nerve growth factor treatment increases stimulus-evoked release of sensory neuropeptides in the rat spinal cord. Eur J Neurosci. 1997;9:1101–1104. doi: 10.1111/j.1460-9568.1997.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B. Excessive growth of the sympathetic ganglia evoked by a protein isolated from the mouse salivary gland. Proc Natl Acad Sci USA. 1960;46:373–384. doi: 10.1073/pnas.46.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52:883–894. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Aloe L, Alleva E. A role for nerve growth factor in nervous, endocrine, and immune systems. Prog Neuroendocr Immunol. 1990;3:1–10. [Google Scholar]
- Aloe L, Fiore M, Probert L, Turrini P, Kollias G, Tirassa P. Overexpression of tumour necrosis factor α in the brain of transgenic mice differentially alters nerve growth factor levels and choline acetyltransferase activity. Cytokine. 1998;11:45–54. doi: 10.1006/cyto.1998.0397. [DOI] [PubMed] [Google Scholar]
- Bonini S, Rasi G, Bracci-Laudiero ML, Procoli A, Aloe L. Nerve growth factor: neurotrophin or cytokine? Int Arch Allergy Immunol. 2003;131:80–84. doi: 10.1159/000070922. [DOI] [PubMed] [Google Scholar]
- Flügel A, Matsumuro K, Neumann H, Klinkert WE, Birnbacher R, Lassmann H, Otten U, Wekerle H. Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis: inhibition of monocyte transendothelial migration. Eur J Immunol. 2001;31:11–22. doi: 10.1002/1521-4141(200101)31:1<11::AID-IMMU11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212). J Clin Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Luftl M, Czarnetzki BM. Nerve growth factor modulates keratinocyte proliferation in murine skin organ culture. Br J Dermatol. 1994;130:174–180. doi: 10.1111/j.1365-2133.1994.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Welker P, Albers KM, Botchkareva NV, Metz M, Lewin GR, Bulfone-Paus S, Peters EM, Lindner G, Paus R. A new role for neurotrophin-3: involvement in the regulation of hair follicle regression (catagen). Am J Pathol. 1998;153:705–719. doi: 10.1016/S0002-9440(10)65621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. EMBO J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- Peters EMJ, Botchkarev VA, Müller-Röver S, Paus R, Arck PC: NGF and pro-NGF: two sides of the same sword in the control of murine hair growth? Exp Dermatol, in press [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, Gilchrest BA. Binding of β-amyloid to the p75 neurotrophin receptor induces apoptosis: a possible mechanism for Alzheimer’s disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Bienenstock J, Stead RH. Mast cells: the neuroimmune connection. Chem Immunol. 1995;61:208–235. [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Kuypers HGJM, Castsman-Berrevoets CE, Loewe H, Dann O. Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett. 1980;18:25–30. doi: 10.1016/0304-3940(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A. Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides. 2003;37:245–250. doi: 10.1016/s0143-4179(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Chase HB. Physical factors which influence the growth of hair. Montagna W, Ellis RA, editors. New York: Academic Press; The Biology of Hair Growth. 1953:pp 435–440. [Google Scholar]
- Müller-Röver S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122:777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D, Lukiewicz S. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102:862–869. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Paus R, Müller-Röver S, Botchkarev VA. Chronobiology of the hair follicle: hunting the “hair cycle clock”. J Invest Dermatol Symp Proc. 1999;4:338–345. doi: 10.1038/sj.jidsp.5640241. [DOI] [PubMed] [Google Scholar]
- Knackstedt MK, Zenclussen AC, Hertwig K, Hagen E, Dudenhausen JW, Clark DA, Arck PC. Th1 cytokines and the prothrombinase fgl2 in stress-triggered and inflammatory abortion. Am J Reprod Immunol. 2003;49:210–220. doi: 10.1034/j.1600-0897.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- Eichmüller S, van der Veen C, Moll I, Hermes B, Hofmann U, Müller-Röver S, Paus R. Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem. 1998;46:361–370. doi: 10.1177/002215549804600310. [DOI] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression. Am J Pathol. 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M, McGuire L, Duchemin AM, Laskowski B, Kiecolto-Glaser J, Glaser R. Changes in plasma nerve growth levels in older adult associated with chronic stress. J Neuroimmunol. 2001;116:102–106. doi: 10.1016/s0165-5728(01)00278-8. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Winblad B, Ebendal T, Lärkfors L. Environmental influence on behavior and nerve growth factor in the brain. Brain Res. 1990;528:62–72. doi: 10.1016/0006-8993(90)90195-h. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Welker P, Metz M, Lewin GR, Subramaniam A, Bulfone-Paus S, Hagen E, Braun A, Lommatzsch M, Renz H, Paus R. A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. EMBO J. 1999;13:395–410. doi: 10.1096/fasebj.13.2.395. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA. Noggin is required for induction of the hair follicle growth phase in postnatal skin. EMBO J. 2001;15:2205–2214. doi: 10.1096/fj.01-0207com. [DOI] [PubMed] [Google Scholar]
- Rosendahl A, Pardali E, Speletas M, Ten Dijke P, Heldin CH, Sideras P. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27:160–169. doi: 10.1165/ajrcmb.27.2.4779. [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. doi: 10.1006/mcne.1999.0798. [DOI] [PubMed] [Google Scholar]
- Hall AK, Dinsio KJ, Cappuzzello J. Skin cell induction of calcitonin gene-related peptide in embryonic sensory neurons in vitro involves activin. Dev Biol. 2001;229:263–270. doi: 10.1006/dbio.2000.9966. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Botchkarev VA, Chen LH, Lindner G, Paus R. A role for p75 neurotrophin receptor in the control of hair follicle morphogenesis. Dev Biol. 1999;216:135–153. doi: 10.1006/dbio.1999.9464. [DOI] [PubMed] [Google Scholar]
- Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, Pierce GF. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation: normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995;147:145–154. [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano CA. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- Mak TW, Yeh WC. Signaling for survival and apoptosis in the immune system. Arthritis Res. 2002;4:S243–S252. doi: 10.1186/ar569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert R, Lindner G, Bulfone-Paus S, Paus R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br J Dermatol. 2000;143:1036–1039. doi: 10.1046/j.1365-2133.2000.03784.x. [DOI] [PubMed] [Google Scholar]
- Blois SM, Joachim R, Kandil J, Margni R, Tometten M, Klapp BF, Arck PC. Depletion of CD8+ cells abolishes the pregnancy-protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172:5892–5899. doi: 10.4049/jimmunol.172.10.5893. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Botchkarev V, Kissling S, Wenzel E, Sundberg JP, Happle R, Hoffmann R. Fas-deficient C3. MRL-Tnfrsf6(lpr) mice and Fas ligand-deficient C3H/HeJ-Tnfsf6(gld) mice are relatively resistant to the induction of alopecia areata by grafting of alopecia areata-affected skin from C3H/HeJ mice. J Invest Dermatol Symp Proc. 2003;8:104–108. doi: 10.1046/j.1523-1747.2003.12182.x. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Li GZ, Palkina TN, Sharova TY, Gilchrest BA, Botchkarev VA. Fas and c-kit are involved in the control of hair follicle melanocyte apoptosis and migration in chemotherapy-induced hair loss. J Invest Dermatol. 2003;120:27–35. doi: 10.1046/j.1523-1747.2003.12022.x. [DOI] [PubMed] [Google Scholar]
- Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol. 1999;162:4271–4276. [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, D’Andrea MR, Mayer EA, Wallace JL, Hollenberg MD, Andrade-Gordon P, Bunnett NW. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, Stone SR, Howells GL. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998;94:356–362. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U, Baumann JB, Girard J. Stimulation of the pituitary-adrenocortical axis by nerve growth factor. Nature. 1979;282:413–414. doi: 10.1038/282413a0. [DOI] [PubMed] [Google Scholar]
- Snider WD, Johnson EM. Neurotrophic molecules. Ann Neurol. 1989;26:489–506. doi: 10.1002/ana.410260402. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Cigliana G, Nicolai R, Muscolo LA, Porcu A, Navarra D, Perez-Polo JR, Angelucci L. Hypothalamic involvement in the activation of the pituitary-adrenocortical axis by nerve growth factor. Neuroendocrinology. 1993;58:202–209. doi: 10.1159/000126534. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Micera A, Alleva E, Aloe L. Early maternal separation increases NGF expression in the developing rat hippocampus. Pharmacol Biochem Behav. 1998;59:853–858. doi: 10.1016/s0091-3057(97)00512-1. [DOI] [PubMed] [Google Scholar]
- Kalin NH. The neurobiology of fear. Sci Am. 1993;268:94–101. doi: 10.1038/scientificamerican0593-94. [DOI] [PubMed] [Google Scholar]
- Manni L, Micera A, Pistillo L, Aloe L. Neonatal handling in EAE-susceptible rats alters NGF levels and mast cell distribution in the brain. Int J Dev Neurosci. 1998;16:1–8. doi: 10.1016/s0736-5748(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Meaney M, Aitken D, Bhatnager S, Vanberkel C, Sapolsky R. Effects of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–769. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]