Abstract
Previously, we have shown that caspase-6 but not caspase-3 is activated by serum deprivation and induces a protracted cell death in primary cultures of human neurons (LeBlanc AC, Liu H, Goodyer C, Bergeron C, Hammond J: Caspase-6 role in apoptosis of human neurons, amyloidogenesis and Alzheimer’s disease. J Biol Chem 1999, 274:23426–23436 and Zhang Y, Goodyer C, LeBlanc A: Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci 2000, 20:8384–8389). Here, we show with neoepitope antibodies that the p20 subunit of active caspase-6 increases twofold to threefold in the affected temporal and frontal cortex but not in the unaffected cerebellum of Alzheimer’s disease brains and is present in neurofibrillary tangles, neuropil threads, and the neuritic plaques. Furthermore, a neoepitope antibody to caspase-6-cleaved Tau strongly detects intracellular tangles, extracellular tangles, pretangles, neuropil threads, and neuritic plaques. Immunoreactivity with both antibodies in pretangles indicates that the caspase-6 is active early in the pathogenesis of Alzheimer’s disease. In contrast to the nuclear and cytosolic localization of active caspase-6 in apoptotic neurons of fetal and adult ischemic brains, the active caspase-6 in Alzheimer’s disease brains is sequestered into the tangles or neurites. The localization of active caspase-6 may strongly jeopardize the structural integrity of the neuronal cytoskeletal system leading to inescapable neuronal dysfunction and eventual cell death in Alzheimer’s disease neurons. Our results suggest that active caspase-6 is strongly implicated in human neuronal degeneration and apoptosis.
Considerable debate has been raised around the type of neuronal damage or cell loss in Alzheimer’s disease (AD). We have shown that caspase (Csp)-6 but not Csp-3 is activated by serum deprivation and induces a protracted cell death in primary cultures of human neurons.1,2 Recently, the implication of caspases has been considered for AD neuronal cell death because the caspases are a group of cysteinyl proteases of which three members, Csp-3, Csp-6, and Csp-7 are the executioners of cellular apoptosis. The caspases reside in the cytosol in an inactive proenzyme form and are activated by dimerization or proteolytic cleavage.3 Once activated, these caspases proteolytically degrade a number of proteins thereby altering cellular structure and function. In vitro, caspases cleave proteins associated with AD; the amyloid precursor protein,1,4–7 tau,8–10 presenilin, and calsenilin.11–13 Caspases also indirectly induce overproduction of amyloid β peptide (Aβ).1,4,14 However, the identification of an active executor caspase in AD has remained elusive.
Studies using neoepitope antibodies to active caspases or caspase-cleaved proteins are strong indicators of the activation of caspases in tissues. Because of the central role of Csp-3 in cellular apoptosis and developmentally regulated neuronal cell death,15 most studies and tools are directed at this caspase. In well controlled experiments, the CM1 anti-active Csp-3 antibody immunoreacts with granulovacuolar degeneration (GVD) of the hippocampus but not senile plaques or neurofibrillary tangles (NFTs).16–18 However, convincing evidence for caspase activation is presented with immunodetection of caspase-cleaved actin, fodrin, amyloid precursor protein, or AMPA receptor subunits in AD synapses, NFTs, or senile plaques.4,19–21 The lack of correlation and co-localization between active Csp-3 and caspase substrates suggests that a caspase other than Csp-3 may be responsible for neuronal damage and cell death in AD brains. In apoptotic human neurons in primary cultures, Csp-6 but not Csp-3 is activated.1 Furthermore, Csp-6 but not Csp-3, -7, or -8 induces human neuronal cell death when recombinant active caspases are directly microinjected into the cytosol.2 Although the role of Csp-6 in neuronal cell death has not been extensively studied, in mice, Csp-6 has also been shown to increase in the aging brain cortex and to decrease in a learning paradigm.22,23 In the present study, we investigate the presence of active Csp-6 in AD and age-matched control short postmortem interval brains with neoepitope antibodies to the p20 subunit of human active Csp-6 (p20Csp-6Ab) and to Csp-6-cleaved tau (tauΔCsp-6Ab). Strong immunoreactivity to p20Csp-6 in the nuclei and cytosol of human fetal and adult neurons in ischemia validates the activation of Csp-6 in human neuronal apoptosis. We find a twofold to threefold increase of the p20 subunit of Csp-6 in AD frontal and temporal cortex compared to control tissues. The p20Csp-6Ab also strongly immunostains degenerating neurites and NFTs in AD brains. Furthermore, tauΔCsp-6Ab detects a similar profile of immunoreactivity and confirms the active nature of the Csp-6. Interestingly, p20Csp-6Ab and TauΔCsp-6Ab immunostain pretangle-containing neurons indicating that Csp-6 is involved in the early stage of tangle formation. We conclude from these results that Csp-6 is activated in human fetal and adult neuronal apoptosis and could contribute significantly to neuritic degeneration in AD.
Materials and Methods
Tissue Collection and Characterization
The frozen tissues of 15 AD and 8 control non-AD brains were obtained from the Canadian Brain Tissue Bank (Toronto, Ontario, Canada). The frontal, temporal, and cerebellar tissues were collected while keeping the frozen brain on dry ice. After dissections, the tissue was kept at −80°C until protein extraction. The mean postmortem interval was 8.82 ± 4.69 hours (range, 1.5 to 20 hours) for AD and 11.69 ± 6.91 hours (range, 2.5 to 20 hours) for controls, the mean age at death was 79.2 ± 6.67 years for AD (range, 63 to 90 years) and 72.75 ± 16.96 years for controls (range, 45 to 91 years), the duration of the disease was from 2 to more than 10 years when indicated in the pathology report (6 of 15 cases). Time of postmortem intervals are not significantly different between AD and non-AD brain cases (P > 0.05). Of these same cases, frontal and temporal cortices, hippocampi, and cerebellum fixed tissues were obtained from nine AD and four non-AD brains. The mean postmortem interval was 11.2 ± 3.4 hours (range, 7 to 16 hours) for AD and 9.8 ± 5.7 hours (range, 4 to 17.5 hours) for controls, the mean age at death was 78.0 ± 7.5 years for AD (range, 63 to 90 years) and 80.5 ± 5.8 years for controls (range, 72 to 85 years). All cases according to clinical and pathological assessment were considered severe AD. CERAD type semiquantitative scoring was used for the pathological assessment.
Antibodies
The C-terminal six amino acids of the p20 subunit of human Csp-6, PLDVVD, was synthesized with a N-terminal cysteine and conjugated with keyhole limpet hemocyanin. Similarly, the Csp-6-cleaved tau antibody was generated to the C-terminal peptide KSPVVSED of Csp-6-truncated tau. The peptide synthesis, conjugation, and rabbit sera production were made at ResGen (Huntville, AL) for the p20Csp-6Ab no. 1277 and Sigma Genosys (The Woodlands, TX) for anti-p20Csp-6Ab no. 10630 and for anti-TauΔCsp-6Ab no. 10635. The Bax antibody was the N-20 antiserum from Santa Cruz Biotechnology (Santa Cruz, CA), and the Csp-3 polyclonal antiserum CPP32 was a kind gift from D. Nicholson (Merck Frosst, Quebec, Canada). The PHF-1 tau antibody and the purified PHF-1 were a kind gift from Dr. Peter Davies (Albert Einstein College of Medicine, New York, NY). The Tau T5530 monoclonal antibody was bought from Sigma (St. Louis, MO). The Pharmingen (La Jolla, CA) anti-p10 antibody was used to detect full-length Csp-6. The Tau monoclonal antibody 14 (Tau mAbT14) was obtained from Zymed (San Francisco, CA).
Characterization of the Neoepitope Antibodies
The antisera generated were screened for immunoreactivity by Western blotting, immunoprecipitation, and immunocytochemistry. For Western blotting of 1277 or 10630, active Csp-6, pro-Csp-6, catalytically inactive C163A Csp-6, and Bax proteins were synthesized from a cell-free Escherichia coli lysate using the Rapid Translation System-500 (RTS-500; Roche, Indianapolis, IN) or by transforming E. coli. The pro-Csp-6 was cloned from human neuronal cDNAs and the active construct was made by inverting the p10 and p20 subunits of the Csp-6 as described.24 The cDNAs were inserted into the pIVEX vector provided with the RTS-500 system. Purified recombinant Csp-3 or Csp-6 were obtained from Pharmingen. Proteins were solubilized in sodium dodecyl sulfate loading buffer (125 mmol/L Tris, pH 6.8, 325 mmol/L glycerol, 4% sodium dodecyl sulfate, 100 mmol/L 2-mercaptoethanol, 0.05% bromophenol blue, and 0.08 g/ml dithiothreitol) and separated on a 15% sodium dodecyl sulfate polyacrylamide gel, followed by Western blotting with the antiserum. The 1277, 10630, and 10635 antisera were diluted at 1/1000, 1/5000, and 1/5000, respectively. Immunoprecipitations were performed from E. coli protein extracts containing recombinant active or inactive Csp-6. The proteins were extracted in Nonidet P-40 buffer (50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1% Nonidet P-40, 5 mmol/L ethylenediaminetetraacetic acid, pH8.0, with freshly added protease inhibitor cocktail; Roche). Antisera were used at a 1/100 dilution and the immunoprecipitate isolated with protein A-Sepharose beads as described previously.25 Controls included antibody preadsorbed with recombinant Csp-6 E. coli lysates or the peptide antigen and pre-immune serum.
Immunocytochemical Analysis
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections on the Ventana Benchmark automated immunostainer (Ventana Medical Systems, Tucson, AZ) using the company’s proprietary reagent kits. Hippocampal sections from the brain of a premature newborn who had suffered from severe perinatal asphyxia were used as a positive neuronal apoptosis control for the p20Csp-6Ab antiserum. After deparaffinization and rehydration, the sections were treated using Ventana’s Cell Conditioning Solution CC1 (catalog no 950-124) for 4 minutes at 100°C. For diagnostic purposes, we used a prediluted commercial monoclonal anti-human tau antibody (clone mAbT14, Zymed). The epitope recognized by this monoclonal antibody is located between amino acids 83 to 120 of the tau protein. The anti-mouse p20 antibody was obtained from Cell Signaling Technologies (Beverly, MA). The dilutions for 1277, 10630, and 10635 were 1:1000, 1:2000, and 16000, respectively (antibodies were diluted in Ventana’s Tris-based antibody diluent). The primary antibodies were applied for 32 minutes at 42°C. Staining was detected using Ventana’s diaminobenzidine detection kit (catalog no. 760-091) according to the manufacturer’s instructions. For double immunostaining, the first primary antibody was applied and detected with diaminobenzidine. The second primary antibody was then applied and detected with V-Red. After the immunostaining procedure, the sections were dehydrated, cleared in xylene, mounted in Mikrokitt medium (Serum International, Montreal, Canada), and coverslipped with Tissue-Tek SCA coverslipping film (Sakura, Japan). Adsorption of the 10635 antiserum was done by incubating the diluted antiserum with 20 μg of antigenic peptide/ml diluted antiserum. The immunoprecipitate was removed by centrifugation and the supernatant used as adsorbed antiserum.
Immunoprecipitation of Csp-6 p20 Fragment from AD and Non-AD Age-Matched Controls
Frozen brain pieces from 15 AD and 8 control brains were first homogenized in ice-cold Nonidet P-40 buffer (50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1% Nonidet P-40, 5 mmol/L ethylenediaminetetraacetic acid, pH 8.0, protease inhibitors cocktail freshly added; Roche) on ice, sonicated 20 seconds, then freeze-thawed once. The extracts were centrifuged at 14,000 rpm for 15 minutes and the supernatant was transferred to a new tube. Protein concentration was measured by BCA protein assay (Pierce, Rockford, IL). For immunoprecipitations, 900 μg of protein were used in a 1-ml volume. Samples were precleared with 30 μl of protein A-Sepharose beads in Nonidet P-40 buffer. Immunoprecipitations were performed with protein A-Sepharose beads and 1/100 dilution of the antiserum by rotating the mixture overnight at 4°C. The immunoprecipitate was washed three times with Nonidet P-40 buffer and resuspended in loading buffer. Immunoprecipitated proteins were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel and submitted to Western blotting with the 1277 antibody. The immunoreactivity was revealed by enhance chemiluminescence and the intensity of the p20 fragment measured by densitometer.
Statistical Evaluations
The difference between AD and non-AD samples was established statistically with analysis of variance followed by Scheffé’s test.
Results and Discussion
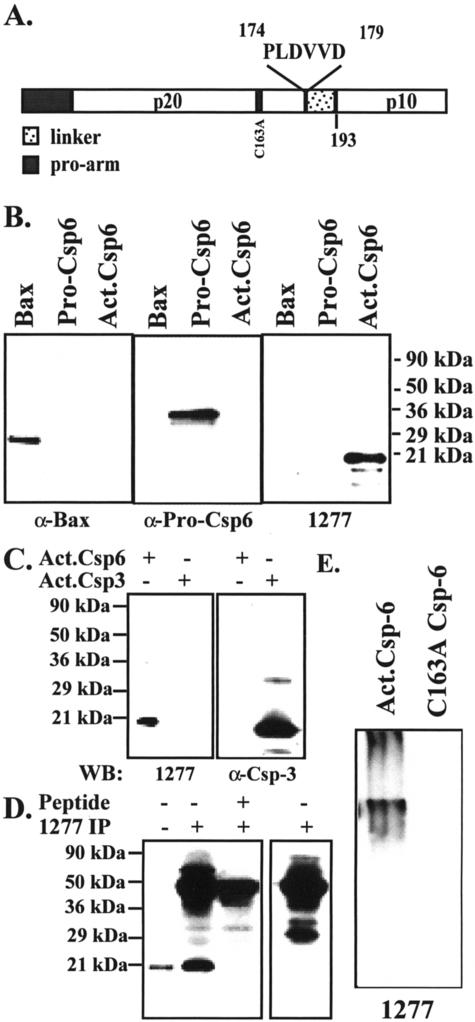
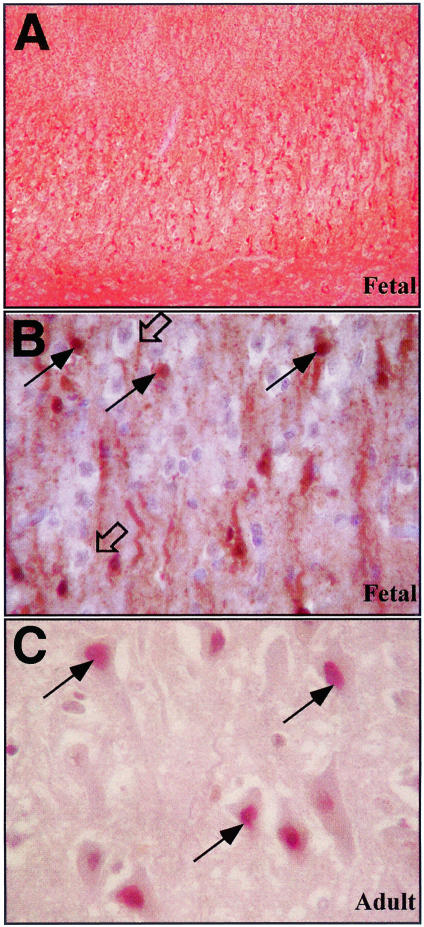
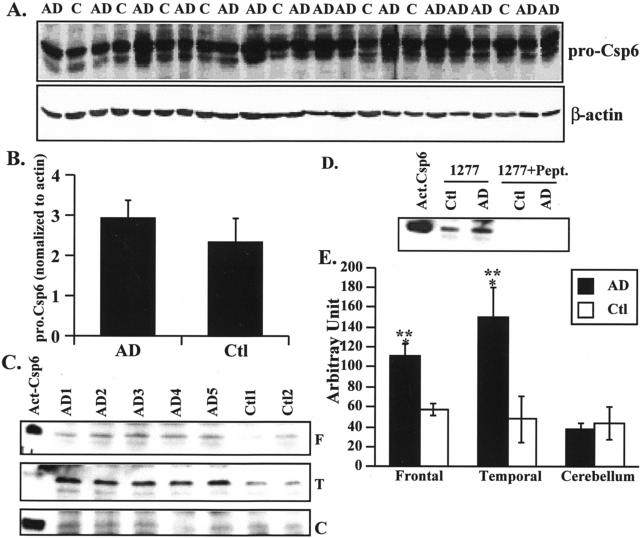
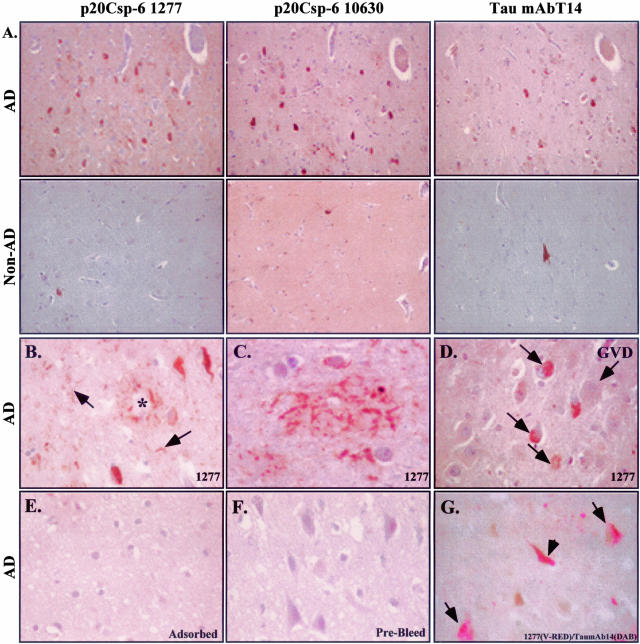
We generated a neoepitope antiserum to the p20 subunit of active Csp-6 (p20Csp-6Ab) (Figure 1A) to investigate the role of active Csp-6 in AD because Csp-6 but not Csp-3 is activated in serum deprivation and induces a protracted cell death in primary cultures of human neurons.1,2 The p20Csp-6Ab specifically recognizes active recombinant Csp-6 but not inactive Csp-6 in both native and denaturant conditions (Figure 1, B and E). The p20Csp-6Ab does not recognize active Csp-3 (Figure 1C). The p20Csp-6Ab clearly discriminates apoptotic human fetal and adult neurons from normal neurons in ischemia (Figure 2; A to C). The immunostaining in these ischemic apoptotic neurons localizes to the nucleus, cytosol, and in the neurites in fetal brain (Figure 2B) and the nucleus in adult brains (Figure 2C). By Western blot analysis, there is no difference in the level of full-length Csp-6 in AD compared to control cases (Figure 3, A and B). However, the p20Csp-6Ab specifically immunoprecipitates twofold to threefold more Csp-6 p20 subunit in the frontal and temporal cortex of 15 AD brains compared to 8 age-matched non-AD brains (Figure 3; C to E). In contrast, there is no difference in the amount of the p20 subunit of Csp-6 in the unaffected cerebellar tissue. Immunocytochemistry of the hippocampal region of nine of these AD and four of these non-AD cases to which paraffin-embedded tissues were available, shows that two independently raised p20Csp-6Abs, 1277 and 10630, stain NFTs, neuropil threads (NPTs), and degenerating neurites in senile plaques (NPs) in AD but detect only the rare tangles in non-AD controls (Figure 4; A to C). The 1277 antibody detects circumscribed globular areas of granular cytoplasmic immunostaining also positive for tau immunoreactivity that corresponds to pretangles (Figure 4, D and G). Co-localization studies often show double immunoreactivity of Tau and 1277 in tangles and pretangles. Similar results are obtained in the frontal and temporal cortex and the intensity of staining parallels the level of AD pathology. The immunoreactivity is completely adsorbed by recombinant active Csp-6 and is absent with preimmune serum (Figure 4, E and F). In contrast to observations with antibodies to the active Csp-3,16,17 there is no active Csp-6 in GVD (Figure 4D). In six of the nine AD cases, the p20Csp-6 staining intensity is less when compared to the standard tau staining. In the other three AD cases, there is significantly more p20Csp-6Ab immunoreactivity compared to anti-tau T14 (Figure 4A). The degree of staining does not correlate with the length of the illness and one of the strongly positive cases examined had manifested symptoms only 2 years before death. It is expected that neurons that have undergone apoptosis are removed efficiently and do not accumulate in the brain in chronic diseases like AD. Therefore, few apoptotic neurons would be present at any one time. Consistent with this hypothesis, our results fail to show significant amounts of p20Csp-6-positive neuronal nuclei in AD unless there is ischemic damage.
Figure 1.
The p20Csp-6 antiserum specifically recognizes the p20 subunit of active Csp-6 under denaturant and native conditions. A: Schematic diagram of pro-Csp-6 showing the sequence and location of the Csp-6 antigen PLDVVD. B: Western blot analysis of 60 μg of E. coli protein lysates from in vitro-translated recombinant Bax, pro-Csp-6, or active Csp-6 with anti-Bax, anti-pro-Csp-6, and anti-p20Csp-6Ab 1277 antisera. C: Western blot analysis of 10 μg of E. coli lysate proteins containing recombinant Csp-6 and 10 ng of purified recombinant Csp-3 with p20Csp-6Ab 1277 antiserum or anti-Csp-3 antiserum. D: Western blot analysis of nonimmunoprecipitated (lane 1) or p20Csp-6Ab 1277 immunoprecipitated p20 subunit of active Csp-6 in the absence or the presence of PLDVVD peptide (lanes 2 and 3) with p20Csp-6Ab 1277 antiserum. The right panel represents a control immunoprecipitation in the absence of E. coli lysate and recombinant Csp-6 probed with p20Csp-6Ab 1277 antiserum only to show the nonspecificity of the high-molecular weight bands. Similar results were obtained with the p20Csp-6Ab 10630 antiserum (not shown). E: Western blot analysis of active Csp-6 and catalytically inactive Csp-6 in nondenaturant conditions with p20Csp-6Ab 1277 antiserum.
Figure 2.
Immunocytochemical analysis of fetal ischemic brain with the p20Csp-6Ab 1277 antiserum. Low (A) and high (B) magnification of fetal ischemia. C: Adult ischemia. Open arrows in B identify normal nonimmunoreactive neurons and filled arrows show apoptotic immunoreactive neurons. Arrows in C identify nuclear immunoreactivity in ischemic adult neurons.
Figure 3.
The p20 subunit of Csp-6 is increased in AD frontal and temporal cortex compared to non-AD age-matched control tissues. A: Western blot analyses of proteins from AD and control (Ctl) frontal cortex with anti-full-length Csp-6 antibody (Pharmingen). B: Quantitative analysis of full-length Csp-6 shown in A. C: Western blot analysis of p20 Csp-6Ab-immunoprecipitated proteins from AD and non-AD frontal cortex (F), temporal cortex (T), and cerebellum (C) with the 1277 p20Csp-6Ab. Recombinant active Csp-6 was included as a control (Act. Csp-6). D: Western blot analysis with 1277 p20Csp-6Ab of the p20Csp-6Ab-immunoprecipitated or PLDVVD peptide-adsorbed p20Csp-6Ab-immunoprecipitated proteins from AD frontal cortex proteins. E: Quantitative analysis of the p20 Csp-6 subunit levels in the frontal, temporal, and cerebellum of 15 AD versus 8 non-AD control cases. Analysis of variance, <0.009. *, Statistically significant difference between AD and Ctl, P < 0.03. **, Statistically significant difference between cerebellum and frontal or temporal cortex, P < 0.001.
Figure 4.
The p20Csp-6 antiserum immunoreacts with NPTs, degenerating neurites in plaques, and NFTs in AD brains. A: Adjacent AD hippocampal brain section immunostained with the p20Csp-6Ab 1277 or 10630 antisera compared with monoclonal Tau T14 antibody. The second row shows the control non-AD tissue stained at the same time as the AD sections. B–D: Sections stained with 1277 p20Csp-6Ab showing immunoreactivity to plaques (*), NFT, and NPTs (arrow) in B, neuritic staining of a plaque in C and intracellular pretangles (small arrows) but not GVD in D. The p20Csp-6 immunoreactivity is adsorbed with recombinant active Csp-6 (E) and is absent in prebleed serum (F). Co-localization of active Csp-6 with Tau is shown in pretangles (arrows) and tangles (arrowhead) in G.
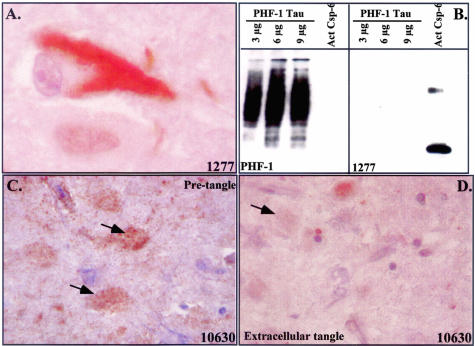
A higher magnification of the staining reveals that the immunoreactivity is sequestered in tangles and is absent from the cytosol and nucleus of the tangle-bearing neuron in contrast to the nuclear and cytosolic localization of active Csp-6 in the apoptotic ischemic neurons (Figure 5A and Figure 2). The possibility that the p20-Csp-6 antiserum cross-reacts with tangles was excluded by testing the antiserum on purified tau from paired helical filaments (Figure 5B). In other cells, the 10630 also stains noncompact granular structures reminiscent of pretangles (Figure 5C). The p20Csp-6 10630 but not the 1277 antiserum immunoreacts only very faintly with the extracellular tangles (Figure 5D).
Figure 5.
p20-Csp-6 immunoreactivity in tangles. A: High magnification showing localization of Csp-6 immunostaining in a NFT. B: Western blot analysis of purified PHF tau with PHF-1 tau antibody or p20-Csp-6 1277 antiserum. C and D: Immunoreactivity of 10630 with pretangles in C (arrows) or 10630 with extracellular tangles in D (arrow).
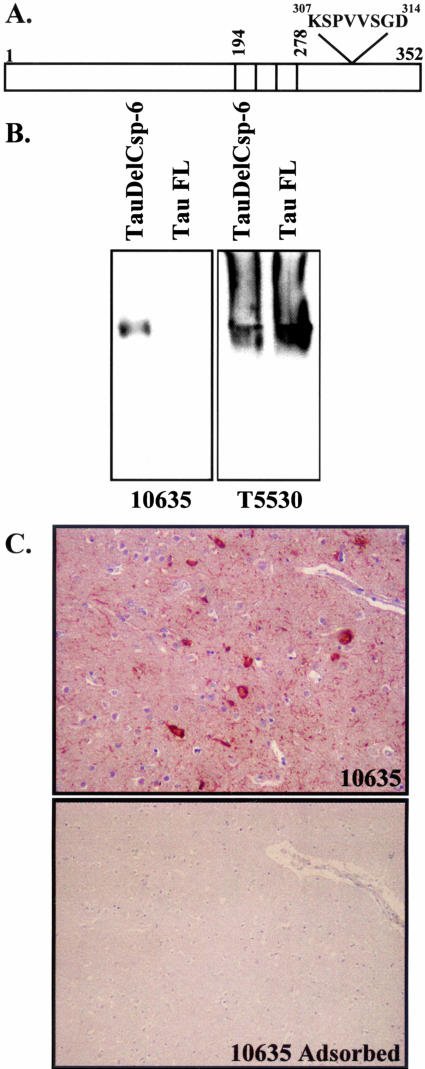
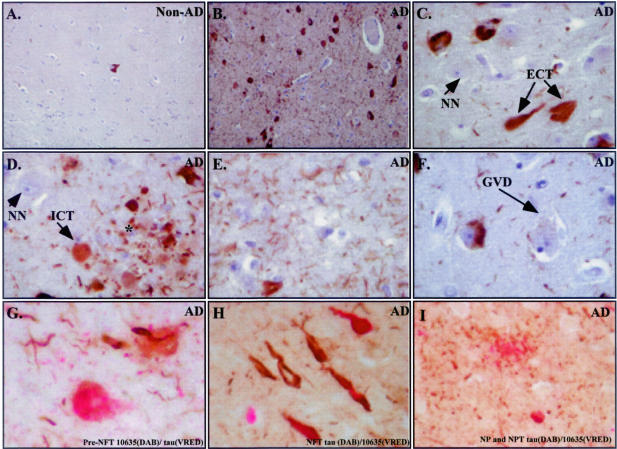
To determine whether the presence of the p20 subunit of Csp-6 represents truly active Csp-6 in vivo, we generated a second neoepitope antibody to Csp-6 cleaved tau (TauΔCsp-6Ab) (Figure 6A). TauΔCsp-6Ab antiserum specifically recognizes the Csp-6-cleaved tau (Figure 6A) under native conditions (Figure 6B). In immunocytochemistry, the 10635 TauΔCsp-6Ab strongly immunostains AD brains and the immunoreactivity is completely adsorbed with antigenic peptide (Figure 6C). TauΔCsp-6Ab does not stain the control non-AD brains except for the occasional age-dependent NFT but immunoreacts extensively in all AD cases, staining large numbers of NFTs and NPs and innumerable NPTs (Figure 7, A and B). The strongest immunoreactivity to the tauΔCsp-6Ab is seen in the same cases strongly positive for the p20Csp-6Ab. In all cases, immunoreactivity with TauΔCsp-6 is much more prominent than with the control anti-tau T14 monoclonal antibody (Figure 4A). TauΔCsp-6Ab stains extracellular tangles (Figure 7C), pretangles, and NPs (Figure 7D), and vast amounts of NPTs (Figure 7E) but not GVD (Figure 7F) or normal neurons (Figure 7, C and D). Co-immunostaining shows co-localization of the TauΔCsp-6Ab and Tau mAbT14 in pretangles (Figure 7G), tangles (Figure 7H), NPTs (Figure 7, G and I), and NPs (Figure 7I). Some tangles stain only for the T14 antibody whereas others stain only with the TauΔCsp-6Ab. (Figure 7H).
Figure 6.
Characterization of the tauΔCsp-6Ab. A: Schematic diagram showing the amino acid position of the peptide antigen used for the tauΔCsp-6Ab relative to the three microtubule binding repeats in fetal tau (352 aminoacids). B: Western blot analysis of Tau (T5530) and TauΔCsp-6 (10635) separated under nondenaturant conditions. C: Immunocytochemistry of AD brain section with 10635- and 10635-adsorbed antiserum.
Figure 7.
Csp-6-cleaved tau antibody strongly detects NPTs, neurites in senile plaques, intracellular tangles, extracellular tangles, and pretangles in AD brains. A: Immunostaining of hippocampi from non-AD (A) or AD (B) with the 10635 tauΔCsp-6Ab. Higher magnification of certain areas shows immunostaining in extracellular tangles (ECT) in C, pretangles (pre-NFT) and degenerating neurites of senile plaques (*) in D, NPTs in E, normal unstained neurons (NN) in C and D, and absence of staining in GVD in F. Co-localization of Tau and TauΔCsp-6Ab in pretangle (G), tangles (H), neuropil threads (G and I) and neuritic plaque (I).
These results suggest that active Csp-6 plays an important role in the pathophysiology of AD. First, both the p20Csp-6Ab and TauΔCsp-6Ab recognize three major pathological hallmarks of AD; NPs, NPTs, and NFTs. Second, both the p20Csp-6Ab and TauΔCsp-6Ab detect pretangles that are believed to precede the formation of tangles and thus exemplify an early event in AD pathogenesis. Third, previous results in our laboratory confirm the activation of Csp-6 in primary human apoptotic neurons.1 Fourth, the presence of active Csp-6 is sufficient to induce human neuronal cell death and this cell death is not rapid but protracted which is consistent with the slowly progressive nature of AD.2 Fifth, the active Csp-6 is not present in the nuclei of AD neurons as it is normally in apoptotic neurons of fetal and adult human ischemic brains (Figure 2) or in cell lines.26 Instead, active Csp-6 localizes to neurites and tangles where it may be inefficient in killing the neuron but still competent to cause damage by proteolytic cleavage of protein substrates. In other cell types, Csp-6 cleaves several cytoplasmic intermediate filament proteins such as cytokeratin 18,27 desmin,28 and vimentin29 in addition to the nuclear lamins.30 Therefore, disruption of the neuronal intermediate filaments could affect the structural integrity of the neuron. Furthermore, the microtubule-stabilizing protein, Tau, is cleaved by Csp-6 and Csp-6-cleaved tau may disrupt proper microtubule assembly and inhibit delivery of structural components, vesicles, and organelles to the remote regions of the neuron. As a consequence, neurons could be dysfunctional in absence of cell death.
The results also indicate that Csp-6 is a more abundant effector caspase in AD than Csp-3. The immunoreactivity to the active Csp-6 is strong in AD pathological hallmarks and absent in GVD and normal brain whereas active Csp-3 immunoreactivity is limited to a few neurons and GVD but absent in the main AD pathological lesions.16,17 The abundant amount of Csp-6-cleaved tau in AD supports the presence of active Csp-6 and the tau is probably more strongly detected than active Csp-6 because tau is both more abundant and more stable than Csp-6. The detection of Csp-3-cleaved amyloid precursor protein, fodrin, β-actin, or Tau in senile plaques, NFTs, degenerating neurites, and synapses contrast with the lack of active Csp-3 detection in AD and undeniably challenge the present hypothesis.4,20,21,31 It is possible that active Csp-3 turns over rapidly and remains undetected in AD. Alternatively, the active Csp-6 in AD could cleave at the Csp-3 substrate sites in these proteins, a situation that we observed with actin (unpublished results). Regardless, the abundant amount of the active Csp-6 in the neurites and tangles is likely to be more damaging to neuronal function than the fleeting presence of active Csp-3.
In summary, we show that the effector Csp-6 that is activated in apoptotic human neurons in primary cultures and sufficient to induce a protracted neuronal cell death is present in an active form in the degenerating neurons and neurites of AD but not in age-matched control brains. These findings strongly implicate the Csp-6 in the pathophysiology of AD and highlight the importance of understanding the mode of activation and inhibition of Csp-6 in neurons. Inhibition of Csp-6 may be an efficient treatment against the progression of AD.
Acknowledgments
We thank Dr. Hemant Paudel (McGill University, Montreal, Canada) for the recombinant Tau protein, Dr. Peter Davis (Albert Einstein College of Medicine, New York, NY) for the PHF-1 Tau and PHF-1 antibody, and Beverly Ackerman for technical assistance.
Footnotes
Address reprint requests to Andréa LeBlanc, Ph.D., The Bloomfield Center for Research in Aging, Lady Davis Institute for Medical Research, The Sir Mortimer B Davis Jewish, General Hospital, 3755 Ch. Côte Ste-Catherine, Montréal, Québec, Canada, H3T 1E2. E-mail: andrea.leblanc@mcgill.ca.
Supported by the National Institutes of Health (NS/MH40965), Canadian Institutes for Health Research, Valorisation Recherche Québec, and Fond de Recherche en Santé du Québec (to A.L.B).
H.G. and S.A. contributed equally to the work presented in this paper and should be considered as first authors.
References
- LeBlanc AC, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis and Alzheimer’s Disease. J Biol Chem. 1999;274:23426–23436. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goodyer C, LeBlanc A. Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci. 2000;20:8384–8389. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Gervais F, Xu D, Robertson G, Vaillancourt J, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman M, Clarke E, Zheng H, Van Der Ploeg L, Ruffolo S, Thornberry N, Xanthoudakis S, Zamboni R, Roy S, Nicholson D. Involvement of caspases in proteolytic cleavage of Alzheimer’s β-amyloid precursor protein and amyloidogenic β-peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, Durrwang U, Reinhard F, Zchuckert O, Evin G, Masters CL. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like enzymes. J Biol Chem. 1999;274:5823–5829. doi: 10.1074/jbc.274.9.5823. [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Passer B, Tabaton M, Ganjei K, D’Adamio L. Alternative, non-secretase processing of Alzheimer’s β-amyloid precursor protein during apoptosis by caspase-6 and -8. J Biol Chem. 1999;274:21011–21016. doi: 10.1074/jbc.274.30.21011. [DOI] [PubMed] [Google Scholar]
- Barnes N, Li L, Yoshikawa K, Schwartz L, Oppenheim R, Milligan C. Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motorneurons. J Neurosci. 1998;18:5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M, Cattaneo A. The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J Neurochem. 2000;75:624–633. doi: 10.1046/j.1471-4159.2000.0750624.x. [DOI] [PubMed] [Google Scholar]
- Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, Calissano P. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci. 1998;18:7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RW, Abraha A, Lagalwar S, LaPointe N, Gamblin TC, Cryns VL, Binder LI. Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. doi: 10.1021/bi027348m. [DOI] [PubMed] [Google Scholar]
- Kim T, Pettingell W, Jung Y, Kovacs D, Tanzi R. Alternate cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- Loetscher H, Deuschle U, Brockhaus M, Reinhardt D, Nelboeck P, Mous J, Grunberg J, Haass CHJ. Presenilins are processed by caspase-type proteases. J Biol Chem. 1997;272:20655–20659. doi: 10.1074/jbc.272.33.20655. [DOI] [PubMed] [Google Scholar]
- Choi EK, Zaidi NF, Miller JS, Crowley AC, Merriam DE, Lilliehook C, Buxbaum JD, Wasco W. Calsenilin is a substrate for caspase-3 that preferentially interacts with the familial Alzheimer’s disease-associated c-terminal fragment of presenilin 2. J Biol Chem. 2001;276:19197–19204. doi: 10.1074/jbc.M008597200. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Tanzi RE. Caspase activation increases beta-amyloid generation independently of caspase cleavage of the beta-amyloid precursor protein (APP). J Biol Chem. 2003;278:46074–46080. doi: 10.1074/jbc.M307809200. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C-Y, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Deckwerth T, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Am J Pathol. 1999;155:1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selznick L, Holtzman D, Han B, Gokden M, Srinivasan A, Johnson E, Roth K. In situ immunodetection of neuronal caspase-3 activation in Alzheimer’s disease. J Neuropathol Exp Neurol. 1999;58:1020–1026. doi: 10.1097/00005072-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Masliah E, Masliah M, Alford M. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:1041–1052. doi: 10.1097/00005072-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Chan SL, Griffin WS, Mattson MP. Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer’s disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, Cribbs DH. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol. 2001;158:189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Frautschy S, Cole G. Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and neurons and plaque microglia in Alzheimer’s disease. Am J Pathol. 1998;152:379–389. [PMC free article] [PubMed] [Google Scholar]
- Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci USA. 2001;98:1930–1934. doi: 10.1073/pnas.98.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, MacFarlane M, Luo Z, Huang Z, Fernandes-Alnemri T, Alnemri E. Generation of constitutively active recombinant caspase-3 and -6 by rearrangement of their subunits. J Biol Chem. 1998;273:10107–10111. doi: 10.1074/jbc.273.17.10107. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Papadopoulos M, Bélair C, Chu W, Crosato M, Powell J, Goodyer C. Processing of amyloid precursor protein metabolism in human primary neuron and astrocyte cultures. J Neurochem. 1997;68:1183–1190. doi: 10.1046/j.1471-4159.1997.68031183.x. [DOI] [PubMed] [Google Scholar]
- Doostzadeh-Cizeron J, Yin S, Goodrich DW. Apoptosis induced by the nuclear death domain protein p84N5 is associated with caspase-6 and NF-kappa B activation. J Biol Chem. 2000;275:25336–25341. doi: 10.1074/jbc.M000793200. [DOI] [PubMed] [Google Scholar]
- Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chang R, Trivedi M, Capetanaki Y, Cryns VL. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem. 2003;278:6848–6853. doi: 10.1074/jbc.M212021200. [DOI] [PubMed] [Google Scholar]
- Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–450. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, Earnshaw WC. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]