Abstract
In the present study, the type-1 repeats of thrombospondin-1 (TSP-1) were transfected into A431 cells. Expression of all three type-1 repeats (3TSR) and expression of just the second type-1 repeat containing the transforming growth factor (TGF)-β activating sequence KRFK (TSR2 + KRFK) significantly inhibited in vivo tumor angiogenesis and growth in nude mice. These tumors expressed increased levels of both active and total TGF-β. A431 cells expressing the second type-1 repeat without the KRFK sequence (TSR2 − KRFK) produced tumors that were slightly larger than the 3TSR and TSR2 + KRFK tumors. These tumors expressed elevated levels of active TGF-β but levels of total TGF-β were not different from control tumors. Injection of the peptide, LSKL, which blocks TSP-1 activation of TGF-β, reversed the growth inhibition observed with cells expressing TSR2 + KRFK to a level comparable to controls. Various residues in the WSHWSPW region and the VTCG sequence of both TSR2+/− KRFK were mutated. Although mutation of the VTCG sequence had no significant effect on tumor growth, mutation of the WSHWSPW sequence reduced inhibition of tumor growth. These findings suggest that the inhibition of tumor angiogenesis and growth by endogenous TSP-1 involves regulation of both active and total TGF-β and the sequences KRFK and WSHWSPW in the second type-1 repeat.
Whereas the roles of tumor oncogenes and tumor suppressor genes in tumor progression have been extensively studied, the role of the extracellular matrix has been primarily overlooked.1 Thrombospondin-1 (TSP-1) is an extracellular matrix protein that has been shown to inhibit tumorigenesis, angiogenesis, and metastasis.2–5TSP-1 is a member of a family consisting of five structurally related proteins, TSP-1 to TSP-5. TSP-1 is a 450-kd homotrimeric extracellular matrix protein expressed by both normal and tumor cells. It consists of a number of functional domains that allow it to interact with cells and other proteins. These domains include an amino terminal heparin-binding domain that interacts with proteoglycans, the integrin α3β1, and cell surface glycosaminoglycans;2–4 three type 1 repeats, which have been shown to interact with the CD36 receptor, fibronectin, and heparan sulfate proteoglycans and to activate latent transforming growth factor (TGF)-β;5–9 an RGDA sequence within the last type 3 repeat, which interacts with the integrin αvβ3; and a carboxyl terminal cell-binding domain that binds the integrin-associated protein CD47.6,10,11
TSP-1 is able to inhibit cancer cell growth and prevents metastasis in several tumor models including breast, skin, and lung carcinomas, melanoma, and malignant glioma,12–16 and its repression promotes tumor growth.17,18 Systemic injection of TSP-1 or recombinant versions of the TSRs inhibits experimental B16F10 and Lewis lung carcinoma tumor growth.19,20 The ability of TSP-1 to inhibit tumor angiogenesis contributes to its anti-tumorigenic effect.21–28 Two regions of TSP-1 have been implicated in its anti-angiogenic property: the procollagen domain and the three type 1 repeats (TSRs).21,23,24,26,29,30 Iruela-Arispe and colleagues23 have shown that the second and third TSRs are potent inhibitors of angiogenesis using the chorioallantoic membrane angiogenesis assay. Furthermore, the CD36 binding site, located in the type-1 repeats, has also been shown to confer TSP-1’s anti-tumorigenic and anti-angiogenic properties.31 Lastly, TSP-1 has also been shown to inhibit matrix metalloproteinase-2 and metalloproteinase-9 activation which may also suppress angiogenesis by blocking the release of growth factors from the extracellular matrix.32,33
The TSRs of TSP-1 are able to activate TGF-β1 bybinding to the sequence, LSKL, in the latency-associated protein and altering the conformation of TGF-β to make it accessible to its receptor.7–9,34,35 The TSR sequences, WSHWSPW and KRFK, are responsible for TSP-1’s binding to and activation of TGF-β, respectively.8,9 The WSXWSPW sequence is found in each TSR, but the KRFK sequence is only found at the start of the second TSR. Like TSP-1, TGF-β plays an important role in tumorigenesis and angiogenesis. Expression of TGF-β in cell lines in vitro and in mice decreased malignancy and inferred resistance to carcinogen-induced mammary tumor formation.36,37 Furthermore, mice with decreased or no expression of the TGF-β receptors have an increased incidence of tumor development.38–40 TGF-β also inhibits endothelial cell proliferation and migration; increases synthesis and deposition of matrix components such as fibronectin, collagen, and TSP-1; and induces apoptosis of endothelial cells.41–45
We previously demonstrated that expression of full-length TSP-1 in A431 carcinoma cells inhibited tumor growth in nude mice via an anti-angiogenic pathway.13 Here we show that A431 cells expressing either all three TSRs (3TSR) or just the second TSR with the sequence KRFK (TSR2 + KRFK) inhibited tumor angiogenesis and growth. In contrast, A431 cells expressing the second TSR without the sequence, KRFK, (TSR2 − KRFK) inhibited tumor growth to a significantly lower extent as compared to TSR2 + KRFK A431 cells. Although tumors expressing recombinant proteins displayed increased levels of active TGF-β, only the 3TSR- and TSR2 + KRFK-expressing tumors had elevated levels of total TGF-β. Furthermore, treatment with the LSKL peptide reversed the inhibition of angiogenesis and tumor growth seen with TSR2 + KRFK A431 cells whereas treatment with the SLLK control peptide had no effect. Mutations of the WSHWSPW sequence partially abrogated the inhibition of tumor growth in the TSR2 + KRFK tumors and totally abrogated the inhibition of tumor growth in the TSR2 − KRFK tumors. These findings suggest that TSP-1 TSRs inhibit growth of A431 tumors in vivo by affecting tumor angiogenesis and levels of active and total TGF-β.
Materials and Methods
Cell Culture
The human squamous cell carcinoma cell line A431 was purchased from the American Type Culture Collection (Rockville, MD). The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/L glucose (Cellgro) and supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), l-glutamine, penicillin, and streptomycin (Life Technologies, Gaithersburg, MD). Cells were harvested using Versene buffer and trypsin/ethylenediaminetetraacetic acid (EDTA) (Life Technologies).
Molecular Cloning, Cell Transfection, and Selection of Clones
Polymerase chain reaction (PCR) primers were purchased from Life Technologies. The PCR primers listed in Table 1 were used in standard PCR reactions that included human, full-length TSP-1 cDNA cloned in pBluescript, dNTPs, and TAQ polymerase (Life Technologies). A thermal cycler PCR machine (model 9600; Perkin Elmer, Emeryville, CA) was used to perform the reactions. A 519-bp product (3TSR), a 282-bp product (TSR2 + KRFK), and a 273-bp product (TSR2 − KRFK) were isolated and cloned into the pSecTag2A expression vector (Invitrogen, Carlsbad, CA) using the KpnI and AscI restriction sites built into the PCR primers. DNA sequencing was performed using the USB Sequenase Version 2.0 sequencing kit (United States Biochemical, Cleveland, OH) to confirm sequence and orientation. A431 cells were transfected with the constructs using the Superfect transfection reagent (Qiagen, Chatsworth, CA) following the manufacturer’s protocol. Clones were selected using Zeocin (Invitrogen). At least four clones were selected for each construct.
Table 1.
List of Primers Used to Generate Sequences Overexpressed in A431 Cells
| Construct | Primer 1 | Primer 2 |
|---|---|---|
| (1) Thrombospondin type-1 repeats (3TSR) | 5′ AGGCGCGCCGACTCTGCGGACGATGGC 3′ | 5′ CGGGGTACCCTAAATTGGACAGTCCTGCTT3′ |
| (2) Second type-1 repeat with KRFK sequence (TSR2+KRFK) | 5′ AGGCGCGCCAAGAGATTTAAACAGGAT 3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (3) Second type-1 repeat without KRFK sequence (TSR2-KRFK) | 5′ AGGCGCGCCAAACAGGATGGTGGCTGG 3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (4) Mutation of the tryptophan residues to threonines inTSR2-KRFK (TSR2-KRFK mut W) | 5′ AGGCGCGCCAAAGAGGATGGTGGCACGAGCCACACGTCCCCGACGTCATCT3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (5) Mutation of the tryptophan residues to threonines in TSR2+KRFK (TSR2+KRFKmut W) | 5′ AGGCGCGCCAAGAGATTTAAACAGGATGCTGGCACGAGCCACACGTCCCCGACGTCATCT 3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (6) Mutate the VTCG sequence to GSCG in TSR2-KRFK (TSR2-KRFK mut VTCG) | 5′ AGGCGCGCCAAACAGGATGGTGGCTGGAGCCACTGGTCCCCGTGGTCATCTTGTTCTGGGAGCTGTGGTGATGGT 3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (7) Mutate the VTCG sequence to GSCG in TSR2+KRFK (TSR2+KRFK mut VTCG) | 5′ AGGCGCGCCTGTGACAAGAGATTTAAACAGGATGGTGGCTGGAGCCACTGGTCCCCGTGGTCATCTTGTTCTGGGAGCTGTGGTGATGGT3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (8) Mutation of the tryptophan residues and VTCG sequence in TSR2-KRFK (TSR2-KRFK double mut) | 5′ AGGCGCGCCAAACAGGATGGTGGCACGAGCCACACGTCCCCGACGTCATCTTGTTCTGGGAGCTGGGTGATGGT 3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
| (9) Mutation of the tryptophan residues and VTCG sequence in TRS2+RFK (TSR2+KRFK double mut) | 5′ AGGCGCGCCAAGAGATTTAAACAGGATGGTGGCACGAGCCACACGTCCCCGACGTCATCTTGTTCTGGGAGCTGTGGT3′ | 5′ CGGGGTACCCTAGATGGGGCAGGCGTCTTT 3′ |
In Vitro Growth Assay
A431 cells expressing 3TSR or TSR2 with and without the KRFK and with the mutations were plated at a seeding density of 2 ×103 cells/well in 12-well tissue culture plates. At 2, 4, 6, and 8 days after plating, cells were counted using a hemacytometer. All experiments were performed in triplicates and independently at least twice.
RNA Extraction and Ribonuclease (RNase) Protection Assay
Cultured cells were harvested using trypsin/EDTA, washedin phosphate-buffered saline (PBS), and pelleted. Tumor tissues were homogenized in Qiagen lysis buffer. RNA from the cells and tissues was extracted using the Qiagen RNEasy kit (Valencia, CA). To generate the probe for RNase protection assays, the TSP-1 second type-1 repeat was cloned into pBluescript KS, the plasmid was linearized with BamHI and RNA was transcribed using T3 polymerase and including 32P-dUTP (New England Nuclear, Boston, MA) in the reaction. RNase protection was performed using the Ambion RPA II kit (Austin, TX) on 20 μg of total RNA isolated from cultured cells or tumor tissues. The 100-bp protected fragment was visualized on a Tris-boric acid-EDTA (TBE) polyacrylamide gel and exposed to film. Band intensities were measured with a densitometer and the TSP-1 TSR reading was normalized against the actin reading. β-actin (Ambion) was used as a control for RNA loading.
Growth of Tumor Xenografts in Nude Mice
Stably transfected A431 cells (3TSR, TSR2 with and without the KRFK, with the mutations, or vector alone) were trypsinized and resuspended in serum-free DMEM. Cells (2 × 106) of each type were injected intradermally into both flanks of BALB/c (nu/nu) mice (Massachusetts General Hospital, Boston, MA). Five mice were injected per group. Tumors were measured weekly using a digital caliper and tumor volumes were calculated using the following equation: volume = 4/3π × (1/2 smaller diameter)2 × (larger diameter). After 3 weeks, mice were euthanized using carbon dioxide and the tumors were removed. To inhibit TSP-1-mediated activation of TGF-β in vivo, mice received intraperitoneal injections of 500 μg of either the SLLK control peptide or the LSKL peptide (Genemed Synthesis, Inc., San Francisco, CA)9 1 day after intradermal placement of the tumor cells in the flanks and thereafter daily until day 12. All animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Except for the VTCG mutated constructs, at least two independent experiments were performed using at least two different clones for each construct.
Immunofluorescence Staining and Apoptosis
TSP-1 and CD31 immunofluorescence stainings were performed on 6-μm frozen sections of A431 tumors as described,46 using a monoclonal rat antibody against mouse CD31 (BD PharMingen, San Diego, CA) and a chicken antibody against human TSP-1 type-1 repeats (a kind gift of Dr. R.A. Swerlick, Dept. of Dermatology, Emory University School of Medicine, Atlanta, GA).
TGF-β immunofluorescence staining was performed on 6-μm formalin-fixed, paraffin-embedded or frozen tumor sections using a rabbit polyclonal antibody against active TGF-β1 (Promega, Madison, WI) following a protocol for this antibody published on Promega’s website (www.promega.com). This antibody is specific for active TGF-β in an enzyme-linked immunosorbent assay.1 All antibodies were diluted in PBS, pH 7.4, containing 0.1% bovine serum albumin, 150 mmol/L tranexamic acid, 20 μg/ml aprotinin (3 to 7 TTU/mg), 1.8 mmol/L EDTA, and 2 mmol/L iodoacetic acid. The swine anti-rabbit biotinylated F(ab)2 antibody was purchased from DAKO (Carpinteria, CA), the avidin-fluorescein isothiocyanate was purchased from Molecular Probes (Eugene, OR) and the immunofluorescence mounting medium containing propidium iodide was purchased from Vector Laboratories (Burlingame, CA). Tumor cell apoptosis was determined using the Fluorescein-FragEl DNA fragmentation kit (Oncogene, Boston, MA) according to the manufacturer’s instructions.
TGF-β Luciferase Assay Using the Mink Lung Epithelial Cells
To measure TGF-β1, -2, and -3 levels, we used mink lung epithelial cells (kindly provided by Dr. Daniel Rifkin) that were previously transfected with a construct containing part of the PAI-1 promoter that includes the TGF-β response element linked to the luciferase gene and followed a modified protocol by Abe and colleagues47 and Yang and colleagues.48 These cells were maintained in DMEM plus 10% fetal calf serum and gentamicin (200 μg/ml). On the day of the experiment, cells were placed in 24-well plates (1.5 × 105 cells/ml) in DMEM + 10% fetal calf serum and allowed to adhere for 4 hours. DMEM media containing 5 mmol/L Hepes, penicillin, streptomycin, and 0.1% bovine serum albumin (amino acid-free) replaced the previous media in the 24-well plates before adding the tumor sections. Tumors were embedded in 0.1% methyl cellulose and solidified using liquid nitrogen. Four 20-μm cryostat sections were cut and placed on 12-mm round coverslips.
To measure active TGF-β levels, coverslips were then placed tissue side down in the wells. To measure total TGF-β levels, coverslips were placed tissue side up in a 24-well plate just containing DMEM media with 5 mmol/L Hepes, penicillin, streptomycin, and 0.1% bovine serum albumin (amino acid-free) and heated at 80°C for 20 minutes. The media was allowed to cool for 20 minutes at room temperature and then was transferred onto the cells. TGF-β (37.5 to 500 pg/ml) was also placed on the cells at the same time to generate a standard curve. All samples were done in triplicate.
The next day, the cells were washed once with PBS and then lysed with 100 μl passive lysis buffer (Promega) for 20 minutes at room temperature with rocking. Ninety μl of supernatant were placed in a 96-well luciferase reader plate (Fisher, Pittsburgh, PA) and 100 μl of luciferase reagent (Promega) was added to the sample using the automated system in the luminometer. Luciferase intensity was measured in a luminometer. Relative luciferase levels were translated to pg/ml per mm3 of tumor by using the standard curve and the volume measurement of the actual tumor section (π × length × tumor width). The level of TGF-β for each tumor was normalized to control tumors by dividing it by the level of TGF-β of the control.
Computer-Assisted Morphometric Analysis of Tumor Vessels
Cryostat sections of A431 tumors were stained with the anti-mouse CD31 monoclonal antibody and representative sections obtained from at least five tumors for each cell clone were analyzed using a Nikon E-600 microscope (Nikon, Melville, NY). Images were captured using a spot digital camera (Diagnostic Instruments, Sterling Heights, IL). Morphometric analyses were performed as previously described using the IP LAB software (Scanalytics Inc., Fairfax, VA).49
Statistical Analysis
If there was a question whether groups differed statistically, a statistical analysis was performed using the computer program, InStat for Macintosh Version 2.0 (GraphPad Software). Briefly, the level of TGF-β or tumor size was entered into the program. The mean, variance, SD, and SE were calculated. Using the raw data, we performed an unpaired test and a Bonferroni multiple comparisons test. A value of P < 0.05 indicates statistical significance.
Results
Expression of TSP-1 TSRs in A431 Cells
Using PCR primers that hybridize to various regions of the TSRs of TSP-1, fragments were generated that encompassed all three TSRs (3TSR), the second TSR with the KRFK sequence at the N-terminal (TSR2 + KRFK), or the second TSR without the KRFK sequence (TSR2 − KRFK) (Figure 1A). These PCR products were cloned into the pSecTag2A expression vector and the resulting plasmids were transfected into A431 cells. At least four clones per construct were selected. To choose clones for the in vivo xenograft experiment, an in vitro growth assay was performed and cell lines with similar growth curves were selected for further studies (data not shown).
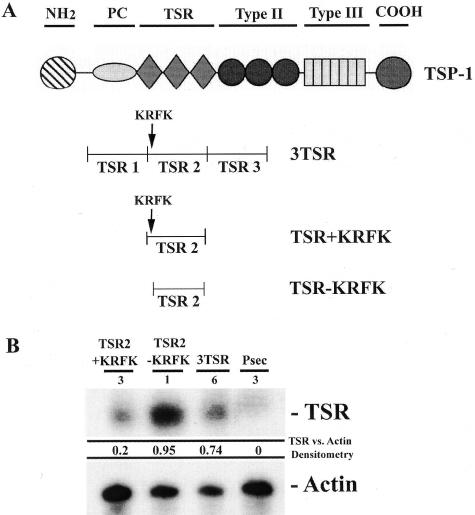
Figure 1.
A: Diagram of TSP-1 highlighting the different functional domains of the molecule. B: TSR expression level in the stably transfected A431 clones. Twenty μg of RNA isolated from one stably transfected A431 clone were probed with TSP-1 type 1 repeat and β-actin probes in a RNase protection assay.
Ribonuclease (RNase) protection assays were performed to assess the level of expression of TSRs in the cell lines using a probe corresponding to the second TSR of TSP-1. It should be noted that this probe will hybridize to endogenous TSP-1 and to RNA derived from the expression vector. A low hybridization signal was detected in pSecTag-transfected A431 control cells, corresponding to the endogenous full-length TSP-1. TSR2 + KRFK-transfected A431 cells expressed ∼20 times, TSR2 − KRFK-transfected A431 cells expressed ∼100 times, and 3TSR-transfected A431 cells expressed ∼70 times higher amounts of TSR2-related RNA as compared to pSecTag A431 control cells confirming efficient RNA production from the expression vectors (Figure 1B). Immunofluorescence using an antibody that binds to TSR2 with or without the KRFK sequence as well as endogenous TSP-1 revealed that these cells expressed higher levels of these proteins than the pSecTag control (data not shown) verifying the RNA expression data.
Expression of 3TSR or TSR2 + KRFK Inhibited Tumor Growth More Potently than TSR2 − KRFK
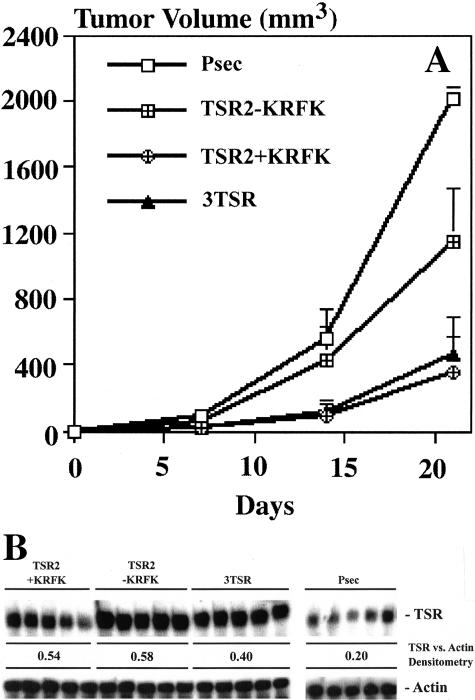
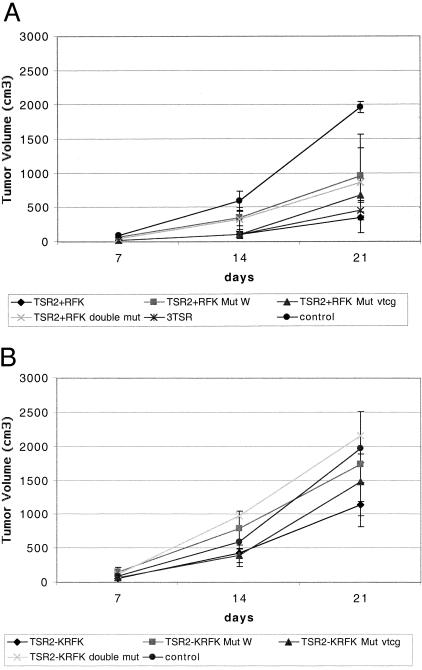
To determine how expression of the TSP-1 TSRs with and without the KRFK sequence affects tumor growth in vivo, A431 cells transfected with 3TSR, TSR2, with and without the KRFK, or pSecTag were injected intradermally into the flanks of nude mice. At day 21 after transplantation the average tumor size of the pSecTag control cells was 1968 ± 82 mm3 whereas expression of 3TSR or TSR2 + KRFK potently inhibited tumor growth (average tumor size of three clones expressing each construct was 456 ± 34 mm3 and 348 ± 22 mm3, respectively) (Figure 2A). The average size of three clones of the TSR2 − KRFK-expressing A431 tumors was between the pSecTag and KRFK-positive tumors (1142 ± 330 mm3). The level of tumor inhibition achieved with 3TSR or TSR2 + KRFK is comparable and even greater to that observed with full-length TSP-1 and TSP-2.13,50
Figure 2.
A: In vivo growth of stably transfected A431 clones in nude mice. Ten tumors from five mice were measured for each clone. Average tumor volume of two clones (pSecTag) and three clones (TSR2 with and without the KRFK sequence and 3TSR) are shown ± SD. B: TSR RNA levels in the tumors. RNA was isolated from five different tumors derived from one clone for each construct. Twenty μg of RNA isolated from tumors were probed with TSR and β-actin probes in a RNase protection assay.
Expression of Transfected TSR Constructs Is Maintained in Vivo
To confirm that the stably transfected tumor cells maintained expression of the TSRs after 21 days of in vivo growth, RNase protection assays were performed on RNA obtained from the tumors. TSR2 with and without the KRFK and 3TSR tumors expressed a significantly higher level of TSR-related mRNA than the pSecTag control tumors (Figure 2B). Using antibodies against the endothelial cell-specific marker CD31 and TSP-1 TSR, immunofluorescence confirmed increased TSR protein expression in the tumors. Higher expression of TSR protein (green) was observed in 3TSR (Figure 3D) and in TSR2 with and without the KRFK (Figure 3, C and B) tumors as compared to the pSecTag (Figure 3A) control tumor. The TSR expression was localized within the tumor tissue, indicating that TSR protein was derived from the transfected tumor cells. In contrast, immunostaining with the anti-TSR antibody only detected antigen around blood vessels (red) in the pSecTag control tumors (Figure 3A). This staining pattern probably represents endogenous TSP-1 that is secreted by stromal cells/endothelial cells because A431 cells in culture show low levels of TSP-1 production. Figure 3, E to H, depicts how expression of pSecTag, TSR2 − KRFK, TSR2 + KRFK, and 3TSR influence growth of A431 cells in mice. It is obvious that expression of either 3TSR or TSR2 + KRFK sequences inhibited tumor growth significantly. The 3TSR and TSR2 + KRFK tumors are smaller in both circumference and height compared to the TSR2 − KRFK- and pSecTag-expressing tumors.
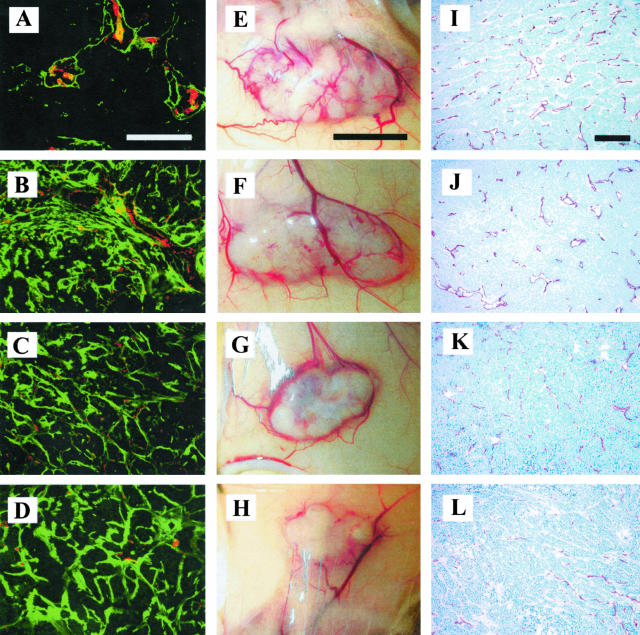
Figure 3.
A to D: Immunofluorescence double staining showing TSR (green)- and CD31 (red)-positive blood vessels in tumor sections. E to H: Macroscopic appearance of orthotopic skin tumors after 3 weeks of in vivo growth. I to L: Immunohistochemical staining of tumor blood vessels using a CD31-specific antibody. A, E, I: PSecTag; B, F, J: TSR2 − KRFK; C, G, K: TSR2 + KRFK; D, H, L: 3TSR. Scale bars: 200 μm (A); 5 mm (E); 200 μm (I).
Inhibition of Angiogenesis in 3TSR and TSR2 + KRFK Tumors
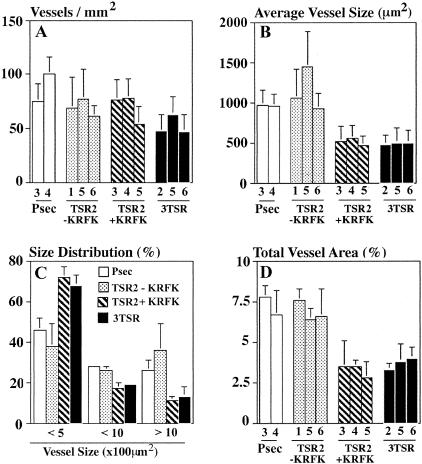
A decrease in the number and size of blood vessels was observed on the surface of 3TSR and TSR2 + KRFK tumors by visual inspection (Figure 3, H and G). CD31 immunostaining of histological sections confirmed these findings (Figure 3; I to L) and revealed that the size of tumor-associated blood vessels was reduced in the 3TSR and TSR2 + KRFK tumors (Figure 3, L and K). Morphometric analyses revealed a significant reduction in the average vessel size and the total vessel area in the 3TSR and TSR2 + KRFK tumors as compared to the TSR2 −KRFK and pSecTag control tumors (Figure 4, B and D). Analysis of the size distribution of tumor-associated blood vessels showed a reduction of large angiogenic blood vessels (size greater than 1000 μm2) and an increase in the number of smaller blood vessels (size less than 500 μm2) in 3TSR and TSR2 + KRFK tumors (Figure 4C). In contrast, vessel density remained unchanged between all of the tumors (Figure 4A).
Figure 4.
Morphometric vessel analyses. A: Vessel density; B: average vessel size; C: distribution of vessel size; D: total vessel area. CD31-stained blood vessels were counted in three different ×10 fields. For each clone, tumors from five mice were evaluated. Data are expressed as mean values ± SD.
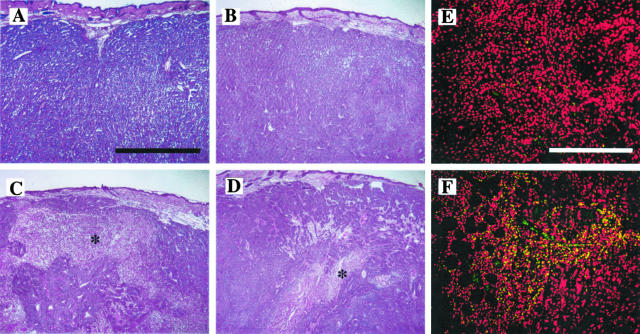
As seen in Figure 4, TSR2 − KRFK tumors did not differ significantly from control pSecTag A431 tumors in their vessel profiles suggesting that the inhibitory effect of TSR2 − KRFK on tumor growth did not change the relative ratio of vessel density to tumor cross-sectional area. Histological analysis of tumor sections showed that 3TSR- and TSR2 + KRFK-expressing tumors contained areas of central necrosis, presumably because of a lack of sufficient blood supply via decreased angiogenesis (Figure 5, C and D), whereas tumors expressing TSR2 − KRFK (Figure 5B) or pSecTag (Figure 5A) did not show signs of necrosis. We also performed terminal dUTP nick-end labeling (TUNEL) immunofluorescence staining on TSR2 + KRFK and TSR2 − KRFK tumor sections and observed an increased number of TUNEL-positive cells in TSR2 + KRFK tumor sections indicating a higher degree of apoptosis in these tumors (Figure 5, E and F).
Figure 5.
Hematoxylin stain of orthotopic tumors showing extensive areas of necrosis (asterisk) in 3TSR (C) and TSR2 + KRFK (D) tumor samples that are not present in pSecTag (A) and TSR2 − KRFK (B) tumors. TUNEL immunohistochemistry staining on TSR2 − KRFK (E) and TSR2 + KRFK (F) tumor sections. Red = propidium iodide (nuclei); green = TUNEL. Scale bars: 200 μm (A); 400 μm (E).
Blocking TSP-1 Activation of TGF-β Prevents the Inhibition of Tumor Angiogenesis and Growth by TSR2 + KRFK
To determine whether activation of TGF-β was involved in the pronounced inhibition of tumor growth observed in TSR2 + KRFK tumors, we injected either the LSKL peptide or the SLLK peptide into tumor-bearing mice. The LSKL peptide inhibits the interaction of the TSRs with latent TGF-β9 whereas the SLLK peptide serves as an inactive, scrambled control peptide.
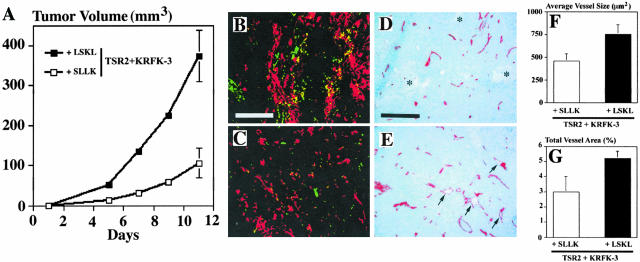
Injection of the LSKL peptide abrogated the inhibitory effect of the TSR2 + KRFK sequence on tumor growth (Figure 6A), such that the LSKL peptide-treated TSR2 + KRFK tumors were comparable in size to the pSecTag control tumors after 12 days (350 ± 58 mm3 versus 285 ± 47 mm3). There was no significant difference between the sizes of the pSecTag tumors treated with either the SLLK or the LSKL peptides (data not shown). Furthermore, the control SLLK peptide did not affect the growth of the TSR2 + KRFK tumors. The size of the tumor was similar to TSR2 + KRFK in our original experiments (107 ± 29 mm3 at day 14).
Figure 6.
A: Orthotopic tumor growth in the presence of a peptide inhibitor of TSP-1 activation of latent TGF-β, LSKL, or the control peptide, SLLK. Average tumor volume of 10 tumors from five mice ± SD. B and C: TGF-β1 immunohistochemistry staining of TSR2 + KRFK tumor treated with the SLLK peptide (B) and LSKL peptide (C). Red = propidium iodide; green = activated TGF-β1. D: CD31 staining of TSR2 + KRFK tumor treated with the SLLK peptide. Scattered areas of necrosis are marked with an asterisk. E: CD31 staining of TSR2 + KRFK tumor after LSKL peptide treatment. Large angiogenic blood vessels are marked with an arrow. Morphometric analyses on average vessel size (F) or total vessel area (G) for TSR2 + KRFK tumors treated with either LSKL or SLLK peptides. CD31-stained blood vessels were counted in three different ×10 fields. Tumors from five mice were evaluated for each clone. Data are expressed as mean values ± SD. Scale bars: 400 μm (B); 200 μm (D).
We next determined the effect of the peptide treatment on activation of TGF-β1 in these tumors. As expected, there was more active TGF-β1 present in TSR2 + KRFK tumors treated with the SLLK peptide (Figure 6B) than those treated with the LSKL peptide (Figure 6C). CD31 staining of these tumor sections showed an increased number of large, angiogenic blood vessels in the TSR2 + KRFK tumors treated with the LSKL peptide (Figure 6E) as compared with those treated with the SLLK control peptide (Figure 6D). Furthermore, the average vessel size and the total vessel area were higher in LSKL-treated TRS2 + KRFK tumors than in the SLLK-treated tumors (Figure 6, F and G). Moreover, areas of necrosis were only detected in tumors treated with the SLLK peptide, a finding similar to untreated TRS2 + KRFK tumors (Figure 5). In conclusion, treatment with the LSKL peptide results in a tumor phenotype similar to the pSecTag control tumors demonstrating that activation of TGF-β1 is an important component of the anti-tumor activity of TSP-1.
Mutations in the WSHWSPW Sequence and in the VTCG Sequence
To evaluate the role of other sequence motifs in the TSR that may play a role in TGF-β activation by TSR2 + RFK, we mutated all three of the highly conserved tryptophan residues to threonine in the WSHWSPW region of TSR2 + KRFK and TSR2 − KRFK using PCR. The WSHWSPW sequence has been implicated in TGF-β binding and inhibition of angiogenesis.23,51 We also mutated the VTCG sequence to GSCG in TSR2 + KRFK and TSR2 − KRFK to evaluate the importance of this sequence in tumor angiogenesis. The VTCG sequence has been implicated in the interaction of TSP-1 with CD36, a receptor that plays a role in TSP-1’s ability to inhibit angiogenesis.31 To determine whether other sequence motifs within the TSRs contribute to the anti-tumorigenic effect, we prepared expression vectors with both the WSHWSPW and VTCG sequences mutated.
At 21 days after injection, the tumors expressing TSR2 + KRFK with mutations in either the WSHWSPW or VTCG sequences, or in both sequences measured 959 ± 612 mm3, 682 ± 244 mm3, and 869 ± 497 mm3, respectively. Compared to the wild-type TSR2 + KRFK-expressing tumors, the WSHWSPW mutation and the double mutation resulted in an ∼2.5-fold increase in tumor size (Figure 7A). The mutated proteins still possessed some inhibitory activity in that the tumors that expressed these proteins were ∼50% smaller than control tumors. The VTCG mutation alone did not significantly abrogate tumor growth inhibition by TSR2 + KRFK. In regards to tumor angiogenesis, we did not observe any differences in vessel density or vessel size as compared to control tumors in tumor-expressing TSR2 + KRFK with mutations in the WSHWSPW, VTCG, or both sequences (data not shown).
Figure 7.
Summary of in vivo growth of stably transfected A431 clones in nude mice. Each data line represents the average tumor measurements from at least 10 tumors in five mice. Except for the VTCG mutation, experiments were performed at least twice using two different clones. A: Average tumor volume of pSecTag, 3TSR, TSR2 + KRFK, TSR2 + KRFK mutated WSXWSPW mutated, VTCG mutated, and both sequences mutated. B: Average tumor volume of pSecTag, TSR2 − KRFK, TSR2 − KRFK mutated WSXWSPW sequence, mutated VTCG sequence, and both sequences mutated.
Mutations in the WSHWSPW sequence and in both WSHWSPW and VTCG sequences in TSR2 − KRFK abrogated its ability to inhibit tumor growth because the sizes of these tumors were not significantly different from control tumors at 21 days (Figure 7B). Mutation of the VTCG sequence in TSR2 − KRFK did not significantly change TSR2 − KRFK’s ability to inhibit tumor growth (Figure 7B). Mutations in the WSHWSPW, VTCG, or both sequences of TSR2 − KRFK did not change the vessel size or density in tumors expressing these proteins (data not shown). This was not surprising because TSR2 − KRFK- and pSecTag-expressing tumors had similar vessel profiles (Figure 4).
TGF-β Expression Is Increased in 3TSR and TSR2 + KRFK Tumors
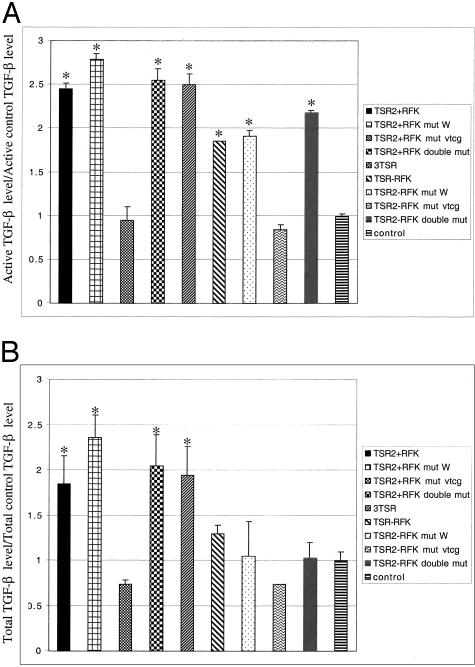
To determine whether 3TSR and TSR2 + KRFK transfection activated TGF-β in vivo, we used a luciferase assay to detect active TGF-β levels in tumor sections (Figure 8A). 3TSR and TSR2 + KRFK tumors expressed the highest levels of active TGF-β (Figure 8A). Surprisingly, expression of active TGF-β1 was moderately increased in the TSR2 − KRFK tumors as compared to the pSecTag control suggesting that alternative mechanisms for activating TGF-β were stimulated or initiated. Statistically there was a significant difference between 3TSR levels, TSR2+/ − KRFK TGF-β levels, and control active TGF-β levels as well between TSR2 + KRFK and TSR2 − KRFK. Interestingly, the total amount of TGF-β in the TSR2 + KRFK and 3TSR tumors were twofold higher than control level (Figure 8B). The TSR2 − KRFK-expressing tumors displayed a slight increase in total TGF-β as compared to the pSecTag control tumors (Figure 8B), but this difference was not statistically significant.
Figure 8.
A: Active TGF-β. B: Total TGF-β. Mink lung epithelial cells expressing a luciferase construct with a TGF-β response element were used to measure TGF-β levels in tumor cryostat sections. The experiment was done in triplicates and repeated twice using the same clone and one time using a different clone. Shown is a representational experiment with one tumor clone. Data are expressed as mean values ± SD and are normalized to pSecTag control. Asterisks indicate there is a statistical significant difference between that group and the control group (P < 0.05).
TSR2 + KRFK mut W and TSR2 + KRFK double mut had both active and total TGF-β levels equal to TSR2 + KRFK whereas TSR2 + KRFK mut VTCG had levels of TGF-β equal to control (Figure 8). Thus, the TSR2 + KRFK with the VTCG mutation conferred tumor growth inhibition without a concurrent increase in the level of TGF-β that could be detected in our assay. Bars with an asterisk above them indicate statistical significance between that group and the control group.
Mutation of the WSHWSPW sequence alone did not affect the ability of TSR2 − KRFK to increase the level of active TGF-β in tumor tissue (Figure 8A). By contrast, mutation in the VTCG sequence produced tumors in which the active TGF-β levels were equivalent to control tumors (Figure 8B). As was the case with the TSR2 + KRFK, the loss of TGF-β activity that was associated with mutation of the VTCG sequence of TSR2 − KRFK was not observed when the two mutations were combined (Figure 8A). Bars with an asterisk above them indicate statistical significance between that group and the control group.
Discussion
The data presented here demonstrate that the TSRs mediate a significant portion of the inhibition of tumor growth by TSP-1. Expression of either 3TSR or TSR2 + KRFK resulted in comparable levels of tumor growth inhibition and the degree of growth inhibition observed was similar to that seen when full-length TSP-1 was overexpressed.13 The ability of the TSRs to inhibit tumor growth depended on the KRFK and WSHWSPW sequences. Deletion of the KRFK sequence or mutation of the WSHWSPW sequence was sufficient to reduce the inhibition of tumor growth that was observed with wild-type TSR2 + KRFK. Deletion of the KRFK sequence along with mutation of the WSHWSPW sequence produced proteins that did not inhibit A431 tumor growth.
The mutations made in this study were designed to evaluate the function of sequences that have been identified as important using synthetic peptides.23,29 The loss of activity associated with each mutation indicated that these sequences were functional in the context of the intact TSR. Our structural studies indicated that neither mutation would be expected to disrupt the global folding of the TSR.52 Whereas mutation of the tryptophans would disrupt the cation-π bonds that stabilized the layered structure of the TSRs, the 11 hydrogen bonds between the a and b strands should be sufficient to stabilize the structure. Two disulfide bonds maintain the local structure of the VTCG sequence and would not be affected by mutation to GSCG. However, we do not know if the mutation would disrupt the glycosylation of the threonine residue because the serine residue located in an equivalent position is also glycosylated in some TSRs.53 Moreover, a complete consensus sequence for this glycosylation has not been determined.
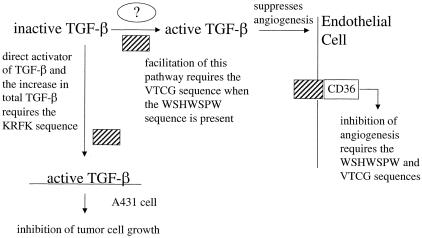
The role of the KRFK sequence in the inhibition of tumor growth was consistent with our previously published study showing that inclusion of this sequence in recombinant proteins that were delivered systemically produced higher levels of tumor growth inhibition than TSR2 − KRFK for those cell types that were responsive to TGF-β.20 Here, we have demonstrated that activation of TGF-β by the TSRs plays a crucial role in inhibiting tumor growth because treatment with the LSKL peptide, an inhibitor of TGF-β1 activation by TSP-1, resulted in a reversal of the tumor growth inhibition that was observed with the TSR2 + KRFK-transfected cells. We do not believe that LSKL peptide is promoting tumor growth by itself because the control tumors treated with the LSKL peptide were the same size as the SLLK treated. In our system, two mechanisms for activation of TGF-β by TSR2 + KRFK appeared to co-exist (Figure 9). The first mechanism probably occurred on the surface of the A431 cells and led to direct inhibition of tumor growth. The abundantly expressed TSR2 + KRFK protein activated latent TGF-β on the A431 cell surface and the relatively small amount of active TGF-β would be so rapidly internalized that changes in the level of active TGF-β would not be detected by our TGF-β luciferase assay. Therefore, we hypothesize that the bulk of the active TGF-β that is detected by our assay is generated by a second mechanism.
Figure 9.
Schematic representation of the effect of expression of the TSR-containing proteins in A431 cell tumor microenvironment. KRFK-dependent activation of TGF-β by TSR2 + KRFK (shaded box) results in suppression of tumor cell growth. TSR2 + KRFK expression also results in an increase in the level of total TGF-β that may be derived from the tumor cells or stromal cells. The increased level of active TGF-β in the TSR2 − KRFK-expressing tumors indicates that these proteins up-regulate another mechanism for activation. This activation may take place in the extracellular matrix or on the surface of stromal cells. Published data indicate that the TSR-containing proteins inhibit angiogenesis through interaction with CD36 on endothelial cells. In this study, changes in vessel morphology are only observed with 3TSR and TSR2 + KRFK indicating that active TGF-β may also contribute to the inhibition of angiogenesis.
We have not conclusively identified this second pathway, but based on our data, believe it probably occurred in the extracellular matrix or on the surface of stromal cells. It must be KRFK-independent because expression of TSR2 − KRFK led to elevated levels of active TGF-β. Furthermore, the WSHWSPW sequence does not seem to be involved because mutations in this sequence in both TSR2 + KRFK and TSR2 − KRFK did not decrease the level of active TGF-β in the tumors as measured by the luciferase assay. However, mutation in the VTCG sequence decreased levels of active TGF-β to control levels so this sequence was probably critical for this mechanism. Interestingly, the double mutant in both TSR2 + KRFK and TSR2 − KRFK had levels of active TGF-β that were equivalent to their wild-type counterparts. Thus, substituting the GSCG sequence for the VTCG sequence when the tryptophans were mutated no longer knocked out this TGF-β-activating mechanism. Even though the WSHWSPW mutant and the double mutant increased the level of active TGF-β, tumor growth was not inhibited. Perhaps the active TGF-β that was produced was either not at the tumor cell surface or insufficient to inhibit tumor cell growth. Together, the data suggest that the TSRs interact with another protein and this interaction may involve the VTCG sequence and a second sequence that is partially masked by the WSHWSPW sequence. Mutation of the WSHWSPW sequence may make this second sequence more accessible and render the VTCG sequence unnecessary. We are currently making additional mutant proteins to identify this second sequence and to test this hypothesis. The GVITRIR sequence is a candidate because it has been shown to be biologically active and to interact with the WSHWSPW sequence.52,54
In our model, we predict that these mechanisms would increase the level of active TGF-β in the TSR2 + KRFK-expressing tumor and would result in increased expression of endogenous TGF-β and TSP-1.55,56 This positive feedback loop would further increase the level of active TGF-β and result in maximal inhibition of tumor growth. In an in vitro growth assay, we found that 12.5 ng/ml of TGF-β inhibited cell proliferation throughout a 4-day time period (data not shown). TSP-1 or TSR2 + KRFK may stimulate the recruitment of other cell types that secrete TGF-β and thus increase the level of total TGF-β in the tumor microenvironment. Indeed, in tumors expressing TSR2 + KRFK, there was an increase in the level of total TGF-β as compared to controls. Interestingly, this effect was KRFK sequence-dependent because a significant increase in total TGF-β was not observed when the TSR2 − KRFK was expressed. The data presented here suggest that up-regulation of total TGF-β may be a novel mechanism for the inhibition of tumor growth by TSP-1.
TSR and TGF-β could also inhibit tumor growth by inhibiting tumor angiogenesis. A recent study reported that restoration of expression of SMAD 4, an intracellular mediator of the TGF-β superfamily, in a human pancreatic cancer cell line increased TSP-1 expression, decreased expression of vascular endothelial growth factor, and resulted in a reduced number of medium- and large-size vessels in tumors grown in nude mice.57 In our study, we observed a decrease in blood vessel size and area as well as an increase in tumor necrosis and apoptosis in the 3TSR- and TSR + KRFK-expressing A431 tumors. Interestingly, expression of full-length TSP-1 in A431 cells also affected vessel morphology in the tumors in a very similar manner.13 Furthermore, blocking TSR2 + KRFK activation of TGF-β with the LSKL peptide resulted in blood vessels with larger lumens. Because changes in vessel morphology were only observed with proteins that contained the KRFK sequence and the LSKL peptide reversed these effects, the observed changes in vessel morphology may be because of either increased levels of active TGF-β or to a synergistic effect of active TGF-β and the anti-angiogenic activity of the TSRs.
Previously published studies that used synthetic peptides have shown that the WSHWSPW sequence in the TSRs mediated the anti-angiogenic activity of TSP-1.23,29 The observation that mutation of the WSHWSPW sequence decreased the tumor growth inhibition of either TSR2 + KRFK or TSR2 − KRFK is consistent with this conclusion. The data presented here suggest that all three sequences, KRFK, WSHWSPW, and VTCG are necessary for optimal anti-angiogenic activity because no difference in blood vessel size was observed unless all three sequences were present. Whereas expression of the TSR-containing proteins in the tumor cells did not significantly affect vessel density in this study, systemic delivery of TSR recombinant proteins did specifically reduce vessel density in our previous study.20 Apparently, the local delivery of these proteins to the tumor microenvironment by the tumor cells themselves limits the ability of these proteins to inhibit angiogenesis. The fact that we did not detect any significant differences in vessel density when some of these sequences were absent or mutated does not necessarily mean that angiogenesis was not suppressed. Several other angiogenesis inhibitors were found to inhibit tumor growth without affecting vessel density in the Rip-Tag model of islet cell carcinogenesis58 and TSP-1-derived peptides administered intravenously or through subcutaneous pumps inhibited glioma tumor growth without affected vessel density.59 Apparently, both angiogenesis and tumor growth were reduced concurrently to similar degrees. We hypothesize that the inhibition of A431 tumor growth by TSR2 − KRFK is because of the inhibition of angiogenesis (Figure 9).
In conclusion, we have shown in vivo that the inhibition of tumor growth by TSP-1 involves activation of TGF-β by the TSRs. We propose that there are two mechanisms at work: TGF-β inhibitory pathway and an anti-angiogenic pathway. The combination of the two pathways during tumor progression made 3TSR and TSR2 + KRFK effective inhibitors of tumor growth in our model system. Not only does the TGF-β-activating sequence inhibit tumor growth, it also increases tumor cell apoptosis and necrosis and causes changes in vessel morphology that suggest enhanced antagonism of vascular endothelial growth factor-induced angiogenesis. Thus, in addition to the direct effects that TSP-1 has on the various cell types in the tumor microenvironment, it has indirect effects that are mediated by increases in both active and total TGF-β. Our data, in conjunction with previously published studies, indicate that expression of recombinant proteins by gene transfer into tumor cells or systematic delivery through the vasculature affect TGF-β levels in the tumor microenvironment differently.20 Identifying a TGF-β-dependent pathway by which TSP-1 inhibits tumor progression could lead to new pharmaceutical interventions in the treatment of cancer.
Acknowledgments
We thank Mark Duquette for the TSP-1 probe used in the RNase protection assay; and Hui Chen, Carole Peruzzi, and Kemin Tan for help on the project.
Footnotes
Address reprint requests to Dr. Jack Lawler, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Research North 270C, Boston MA 02215. E-mail address: jlawler@bidmc.harvard.edu.
Supported by the National Institutes of Health (grants CA86410, HL68003, CA92644, and CA69184), the American Cancer Society (research program grant 99-23901), the National Cancer Institute (National Research Service Award fellowship F32 CA84618-01 to K.O.Y.), the Aid for Cancer Research (fellowship to K.O.Y.), the Dermatology Foundation (fellowship to M.S.), and the Deutsche Forschungsgemeinschaft (to T.H.).
References
- Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159:1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clezardin P, Lawler J, Amiral J, Quentin G, Delmas P. Identification of cell adhesive active sites in the N-terminal domain of thrombospondin-1. Biochem J. 1997;321:819–827. doi: 10.1042/bj3210819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Ferro P, Duquette M. Expression and mutagenesis of thrombospondin. Biochemistry. 1992;31:1173–1180. doi: 10.1021/bi00119a029. [DOI] [PubMed] [Google Scholar]
- Merle B, Malaval L, Lawler J, Delmas P, Clezardin P. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J Cell Biochem. 1997;67:75–83. [PubMed] [Google Scholar]
- Prater CA, Plotkin J, Jaye D, Frazier WA. The properdin-like type 1 repeats of human thrombospondin contain a cell attachment site. J Cell Biol. 1991;112:1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor β. J Biol Chem. 1994;269:26783–26788. [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin-1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates αvβ3 function through integrin-associated protein. J Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuel K, Popp S, Fusenig NE, Stanbridge EJ, Boukamp P. Tumor suppression in human skin carcinoma cells by chromosome 15 transfer or thrombospondin-1 overexpression through halted tumor vascularization. Proc Natl Acad Sci USA. 1999;96:2065–2070. doi: 10.1073/pnas.96.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SW, Kyshtoobayeva AS, Kurosaki T, Jakowatz J, Fruehauf JP. Mutant p53 correlates with reduced expression of thrombospondin-1, increased angiogenesis, and metastatic progression in melanoma. Cancer Detect Prev. 1998;22:185–194. doi: 10.1046/j.1525-1500.1998.0oa18.x. [DOI] [PubMed] [Google Scholar]
- Zajchowski DA, Band V, Trask DK, Kling D, Connolly JL, Sager R. Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci USA. 1990;87:2314–2318. doi: 10.1073/pnas.87.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- Volpert OV, Pili R, Sikder H, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell. 2002;2:473–483. doi: 10.1016/s1535-6108(02)00209-x. [DOI] [PubMed] [Google Scholar]
- Watnick RS, Cheng Y-N, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increases tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W-M, Seng WL, Duquette M, Lawler P, Laus C, Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor β dependent and independent mechanisms. Cancer Res. 2001;61:7830–7839. [PubMed] [Google Scholar]
- Castle VP, Dixit VM, Polverini PJ. Thrombospondin-1 suppresses tumorigenesis and angiogenesis in serum- and anchorage-independent NIH 3T3 cells. Lab Invest. 1997;77:51–61. [PubMed] [Google Scholar]
- Iruela-Arispe L, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci USA. 1991;88:5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo B, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type-1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Vázquez F, Ortega MA. Antiangiogenic domains shared by thrombospondins and metallospondins, a new family of angiogenic inhibitors. Ann NY Acad Sci. 1999;886:58–66. doi: 10.1111/j.1749-6632.1999.tb09400.x. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Newman P, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1328–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck N. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Benelli R, Borsotti P, Rusnati M, Presta M, Giavazzi R, Rucco L, Albini A. Thrombospondin-1 inhibits Kaposi’s sarcoma (KS) cell and HIV-1 tat-induced angiogenesis and is poorly expressed in KS lesions. J Pathol. 1999;188:76–81. doi: 10.1002/(SICI)1096-9896(199905)188:1<76::AID-PATH312>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- DiPietro L, Nebgen D, Polverini PJ. Downregulation of endothelial cell thrombospondin-1 enhances in vitro angiogenesis. J Vasc Res. 1994;31:178–185. doi: 10.1159/000319585. [DOI] [PubMed] [Google Scholar]
- Guo N-H, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin-1 and type 1 repeat peptides of thrombospondin-1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bein K, Simons M. Thrombospondin-1 type 1 repeats interact with matrix metalloproteinase 2: regulation of metalloproteinase activity. J Biol Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J Biol Chem. 1994;269:26775–26782. [PubMed] [Google Scholar]
- Ribeiro SMF, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor β. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-β pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- Pierce DF, Jr, Gorska AE, Chytil A, Meise KS, Page DL, Robert J, Coffey J, Moses HL. Mammary tumor suppression by transforming growth factor β1 transgene expression. Proc Natl Acad Sci USA. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Böttinger EP, Jakowlew SB, Bagnall KM, Mariano J, Anver MR, Letterio JJ, Wakefield LM. Transforming growth factor β1 is a new form of tumor suppressor with true haploid insufficiency. Nat Med. 1998;4:802–807. doi: 10.1038/nm0798-802. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Greenhalgh DA, Bickenbach JR, Jiang A, Bundman DS, Krieg T, Derynck R, Roop DR. Expression of a dominant-negative type II transforming growth factor β (TGFβ) receptor in the epidermis of transgenic mice blocks TGFβ-mediated growth inhibition. Proc Natl Acad Sci USA. 1997;94:2386–2391. doi: 10.1073/pnas.94.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor β receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz-[a]-anthracene. Cancer Res. 1997;57:5564–5570. [PubMed] [Google Scholar]
- Fràter-Schröder M, Müller G, Birchmeier W, Böhlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986;29:295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Choi M, Ballermann B. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-β receptors. J Biol Chem. 1995;270:144–150. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Vassalli J, Orci L, Montesano R. Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res. 1993;204:1010–1016. doi: 10.1006/excr.1993.1043. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Eguchi K, Migita K, Kawabe Y, Kawakami A, Matsuoka N, Takashima H, Mizokami A, Nagataki S. Transforming growth factor β1 induces apoptotic cell death in cultured human umbilical vein endothelial cells with down-regulated expression of bcl-1. Biochem Biophys Res Commun. 1995;210:1076–1082. doi: 10.1006/bbrc.1995.1766. [DOI] [PubMed] [Google Scholar]
- Detmar M, Velasco P, Richard L, Claffey KP, Streit M, Riccardi L, Skobe M, Brown LF. Expression of vascular endothelial growth factor induces an invasive phenotype in human squamous cell carcinomas. Am J Pathol. 2000;156:159–167. doi: 10.1016/S0002-9440(10)64715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;129:443–458. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Yang L, Qiu CX, Ludlow A, Ferguson MWJ, Brunner G. Active transforming growth factor-β in wound repair. Am J Pathol. 1999;154:105–111. doi: 10.1016/s0002-9440(10)65256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Velasco P, Riccardi L, Richard L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe ML, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Tan K, Duquette M, Liu J-H, Dong Y, Zhang R, Joachimiak A, Lawler J, Wang J-H. Crystal structure of the TSp-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol. 2002;159:373–382. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem. 2001;276:6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Pearce SFA, Schneider AJ, Silverstein RL, Henkin J, Bouck N. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Ono M, Uchiumi T, Ueno H, Kohno K, Sugimachi K, Kuwano M. Up-regulation of thrombospondin-1 gene by epidermal growth factor and transforming growth factor β in human cancer cells—transcriptional activation and messenger RNA stabilization. Biochim Biophys Acta. 2002;1574:24–34. doi: 10.1016/s0167-4781(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Toriu N, Hanakawa Y, Shirakata Y, Sayama K, Takayanagi A, Ohtsubo M, Gamou S, Shimizu N, Fujii M, Miyazono K, Hashimoto K. Keratinocyte growth inhibition by high-dose epidermal growth factor is mediated by transforming growth factor β autoinduction: a negative feedback mechanism for keratinocyte growth. J Invest Dermatol. 2003;120:1030–1037. doi: 10.1046/j.1523-1747.2003.12239.x. [DOI] [PubMed] [Google Scholar]
- Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, Lüttges J, Klöppel G, Graeven Ulrich, Eilert-Micus C, Hintelmann A, Schmiegel W. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA. 2000;97:9624–9629. doi: 10.1073/pnas.97.17.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo K-M, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–811. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Bogdanov A, Marecos E, Cheng H, Chandrasekaran L, Krutzsch HC, Roberts DD, Weissleder R. Treatment of experimental brain tumors with thrombospondin-1 derived peptides: an in vivo imaging study. Neoplasia. 1999;1:438–445. doi: 10.1038/sj.neo.7900044. [DOI] [PMC free article] [PubMed] [Google Scholar]