Abstract
Glutathione transferase zeta (GSTZ1-1) is the major enzyme that catalyzes the metabolism of α-halo acids such as dichloroacetic acid, a carcinogenic contaminant of chlorinated water. GSTZ1-1 is identical with maleylacetoacetate isomerase, which catalyzes the penultimate step in the catabolic pathways for phenylalanine and tyrosine. In this study we have deleted the Gstz1 gene in BALB/c mice and characterized their phenotype. Gstz1−/− mice do not have demonstrable activity with maleylacetone and α-halo acid substrates, and other GSTs do not compensate for the loss of this enzyme. When fed a standard diet, the GSTZ1-1-deficient mice showed enlarged liver and kidneys as well as splenic atrophy. Light and electron microscopic examination revealed multifocal hepatitis and ultrastructural changes in the kidney. The addition of 3% (w/v) phenylalanine to the drinking water was lethal for young mice (<28 days old) and caused liver necrosis, macrovesicular steatosis, splenic atrophy, and a significant loss of circulating leukocytes in older surviving mice. GSTZ1-1-deficient mice showed constitutive induction of alpha, mu, and pi class GSTs as well as NAD(P)H:quinone oxidoreductase 1. The overall response is consistent with the chronic accumulation of a toxic metabolite(s). We detected the accumulation of succinylacetone in the serum of deficient mice but cannot exclude the possibility that maleylacetoacetate and maleylacetone may also accumulate.
The cytosolic glutathione transferases (GSTs) are found in almost all aerobic species and are the products of a supergene family.1 Recent studies indicate that the members of this superfamily have an extraordinary range of physiological functions. Early investigations focused on the capacity of GSTs to catalyze the conjugation of electrophilic compounds with glutathione and established their major role in the detoxication of xenobiotics.2 More recent studies have demonstrated the contribution that particular GSTs make to the metabolism of specific endobiotic compounds.1,3–6 In addition some members of the GST superfamily play a role in the modulation of cell signaling pathways and have the capacity to form and modulate ion channels.7–9
The zeta class GSTs are widely distributed in nature and are found in many species ranging from mammals to insects, plants, and fungi. GSTZ1-1 was found to be identical with maleylacetoacetate isomerase (MAAI), an enzyme that catalyzes the glutathione-dependent isomerization of maleylacetoacetate (MAA) to fumarylacetoacetate (FAA),4,10 the penultimate step in the phenylalanine and tyrosine degradation pathway (Figure 1). This catabolic pathway consists of six enzyme-catalyzed reactions that ultimately generate fumarate and acetoacetate.4 With the notable exception of GSTZ1/MAAI, deficiencies of each of these enzymes cause disease in humans. The most severe of these disorders, hereditary tyrosinemia type 1 (HT1), is caused by a deficiency of fumarylacetoacetate hydrolase (FAH), which catalyzes the last step in the phenylalanine degradation pathway.11,12 Patients with HT1 suffer from liver failure, renal tubular defects, and some also develop liver carcinoma in their teens.13,14 The deleterious symptoms that accompany FAH deficiency are thought to result from the accumulation of FAA and MAA as well as their reduced derivatives succinylacetoacetate (SAA) and succinylacetone (SA).12,15,16 FAA and MAA are electrophilic and react with intracellular nucleophiles. MAA may accumulate in GSTZ1/MAAI deficiency and may cause a tyrosinemia-like syndrome. We have previously reported polymorphic variants of GSTZ1/MAAI that show variations in their isomerase activity and in their susceptibility to inactivation by dichloroacetic acid (DCA)17–19 but a deficiency of GSTZ1/MAAI has not been clearly documented. Although a suspected case of MAAI deficiency was reported, the child died at an early age and the deficiency was never fully investigated.20 The absence of patients with MAAI deficiency may indicate that the disorder is very severe or that the lack of GSTZ1/MAAI is compensated by other enzymatic pathways. The deletion of MAAI activity in the fungus Aspergillus nidulans blocked growth on phenylalanine-supplemented media suggesting that MAA or its derivatives are toxic.4
Figure 1.
The catabolic pathway of phenylalanine and tyrosine. The enzymes referred to in the text are indicated in italics. The dotted lines indicate poorly understood steps.
In previous studies, we have shown that zeta class GSTs catalyze the biotransformation of a range of α-halo acids, including DCA, fluoroacetic acid, and 2-chloropropionate.21,22 DCA is of particular interest, because it is carcinogenic in rodents.23–25 Humans are exposed to DCA through consumption of chlorinated drinking water and as a metabolite of the solvent trichloroethylene and the sedative chloral hydrate.26,27 DCA is also used clinically for the management of congenital lactic acidosis because DCA promotes the metabolism of lactic acid by activating the pyruvate dehydrogenase complex.27 We have shown that DCA is a mechanism-based inactivator of GSTZ;19,28 consequently, it is unclear if the carcinogenicity associated with DCA in rodents is a promotional effect or is because of the accumulation of toxic metabolites after the inactivation of GSTZ1/MAAI.
To study the effects of GSTZ1/MAAI deficiency and to evaluate further the effects of DCA we have developed a BALB/c GSTZ1/MAAI-deficient mouse. During the course of the present study, Fernández-Cañón and colleagues29 reported the development of 129/Sv4 mice in which the GSTZ1/MAAI gene was inactivated. These mice remained healthy and their lack of a deleterious phenotype was attributed to a nonenzymatic glutathione-dependent bypass of the MAAI reaction. Although our results confirm the absence of a severe phenotype, as previously noted in 129/Sv4 GSTZ1/MAAI-deficient mice,29 we also observed that BALB/c GSTZ1/MAAI-deficient mice show a number of significant abnormalities including altered organ sizes, an abnormal pattern of circulating leukocytes, and the constitutive induction of hepatic alpha, mu, and pi class GSTs.
Materials and Methods
Generation of GSTZ1/MAAI-Targeted Mice
For convenience, we refer here to the mouse GSTZ1/MAAI gene as the Gstz1 gene. A mGSTZ1 cDNA clone, pmGSTZ was isolated by Dr. Angela Whittington from a λ-ZAP C57BL/6xCBA liver cDNA library (Stratagene, La Jolla, CA) with a previously described human GSTZ1 cDNA clone.10 An 885-bp fragment of the mGSTZ1 cDNA extending from −29 to 856 bp was used to screen a male mouse BALB/c liver genomic DNA library constructed in Lambda EMBL3 Sp6/T7 (no. ML1040j) (Clontech, Palo Alto, CA) Several clones were analyzed by Southern blotting with the mGSTZ1 cDNA and a 6265-bp HindIII fragment was subcloned into the phagemid vector pBluescript SK(+) and termed pZ6.5. This genomic fragment was partially sequenced and was subsequently used to generate the mGSTZ1/MAAI gene-targeting construct. Bioinformatic analysis identified a mouse BAC clone [RCPI-23-350-H8 (AQ980522)] that was obtained from BACPAC Resources, Oakland, CA, USA. Analysis of the λ phage and BAC clones allowed us to determine the organization of the mouse GSTZ1/MAAI gene (Figure 2A). Although this gene structure differs in the length of intron 1 compared with the recently published structure in 129/Sv4 mice29 we were satisfied by Southern blotting, in situ hybridization, and bioinformatic analyses (data not shown) that there is only a single GSTZ1/MAAI locus in BALB/c mice.
Figure 2.
The organization of the BALB/c mouse GSTZ1/MAAI gene and the DNA constructs made to inactivate the gene in ES cells. The position of primers listed in Table 1 are shown in D.
The steps taken to generate the targeting construct are shown schematically in Figure 2 and are described briefly below. The plasmid pZ6.5 contains exons 4 to 9 within a 6265-bp HindIII fragment and was used as the backbone of the targeting construct. Exons 6 to 8 were removed in a 1854-bp BseRI digest and replaced by a 1133-bp neomycin resistance cassette from pMC1Neo (Stratagene) and the resulting plasmid was termed pZ6.5Neo (Figure 2B). A 238-bp ClaI to Eco47III fragment was removed from pZ6.5Neo and was replaced by a 2.84-kb thymidine kinase cassette (pgkTK) to generate the final targeting cassette termed pZ6.5NeoTK (Figure 2C). A number of oligonucleotide primers were designed to identify homologous recombination and to genotype derived mice by the polymerase chain reaction. The position of these primers is shown in Figure 2D and their sequences are provided in Table 1.
Table 1.
Oligonucleotide Primers Used in Targeting mGSTZ1
| Primer name | Sequence |
|---|---|
| ZetaF2 | 5′-TGTCCCCACAGTTCACTGAGGAAT-3′ |
| ZetaF2b | 5′-AGACCCTGAACCCCATGAAGCAA-3′ |
| NeoN1 | 5′-CGATTGTCTGTTGTGCCCAGTCAT-3′ |
| NeoN2 | 5′-CGGAGAACCTGCGTGCAATCCAT-3′ |
| ZetaKOF1 | 5′-GGGAGCAGAACAGGTAAACATCTA-3′ |
| ZetaKOR1 | 5′-ACATTTGGGTACAGCAGTGTTCTT-3′ |
Refer to Figure 2D for primer binding sites.
The targeted replacement of the GSTZ1/MAAI gene was conducted by the procedure developed by Thomas and Capecchi.30 The targeting construct (pZ6.5NeoTK) was linearized by digestion with NotI and was electroporated into BALB/c embryonic stem (ES) cells, a generous gift of Dr. Birgit Ledermann (University of Zurich). Positive-negative selection was performed with G418 and gancyclovir and 43 surviving clones were screened for homologous recombination by polymerase chain reaction with ZetaF2 and NeoN1 followed by ZetaF2b and NeoN2 as nested primer pairs (Figure 2D). The Zeta F2 and Zeta F2b primers were located within the region of exon 4 removed from pZ6.5Neo and replaced with the pgkTK cassette. Proximity of the Zeta2 and NeoN primers and effective amplification of a 2101-bp fragment only occurs if homologous recombination has occurred. An ES cell colony that had undergone successful homologous recombination was selected and rechecked by Southern blotting after digestion of its genomic DNA with several restriction endonucleases (data not shown).
The targeted ES cells were injected into 3.5-day-old blastocysts from C57BL/6 mice and implanted into pseudopregnant females. Chimeric mice were bred with wild-type BALB/c females and the resulting mice genotyped to identify heterozygotes (Gstz1+/−). Heterozygotes were backcrossed to wild-type BALB/c mice for two or more generations to remove other mutations that may have arisen during the ES cell culture. Heterozygotes were intercrossed to produce isogenic Gstz1−/− mice. Mice were maintained in microisolator cages in a specific pathogen-free mouse breeding facility. All studies were undertaken with the approval of the Australian National University Animal Ethics Experimentation Committee under the guidelines established by the Australian National Health and Medical Research Committee. In accordance with those guidelines, in some experiments mice that appeared to be very distressed were sacrificed.
Genotyping GSTZ1/MAAI-Targeted Mice
Mice were genotyped by polymerase chain reaction analysis of genomic DNA obtained from tail samples. The oligonucleotide primers ZetaKOF1 and ZetaKOR1 (Table 1, Figure 2) were designed in sequences flanking the neomycin resistance gene insertion point. Gstz1+/+ mice gave rise to a single 1980-bp amplicon, Gstz1−/− mice, a single 1270-bp amplicon, and Gstz1+/− mice showed both the 1270-bp and 1980-bp amplicons after polymerase chain reaction analysis.
Northern Blotting Analysis
A mouse multiple-tissue Northern blot was obtained from Clontech and was hybridized according to the supplier’s instructions with an 885-bp cDNA fragment from pmGSTZ radiolabeled with [α-32P]dATP. The membrane was prehybridized with 5 ml of ExpressHyb solution (Clontech) at 68°C for 30 minutes. Approximately 2 to 10 ng/ml of freshly prepared 885-bp probe was denatured and hybridized to the membrane in ExpressHyb solution at 68°C for 1 hour (continuous agitation). The membrane was rinsed three times in 2× standard saline citrate and 0.05% sodium dodecyl sulfate (SDS) at room temperature followed by two washes in 0.1× standard saline citrate and 0.1% SDS at 50°C. The membrane was exposed to X-ray film at −70°C for 54 hours with an intensifying screen.
Western Blotting
Tissues from various organs (brain, heart, liver, lung, spleen, seminal glands, kidney, skeletal muscle, and testes) were rinsed in phosphate-buffered saline and homogenized with an IKA-Ultra-Turrux T25 homogenizer in homogenizing buffer (20 mmol/L potassium phosphate buffer, pH 7.4, containing 154 mmol/L KCl, 2 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L 4-(2-aminoethyl)-benzenesulfonylfluoride and 2 mmol/L dithiothreitol) at a ratio of ∼100 mg/ml. The homogenates were centrifuged for 20 minutes at 8000 × g at 4°C, and the supernatant was stored at −20°C. Proteins (100 μg) from each tissue were fractionated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) under standard reducing conditions. The fractionated proteins were electroblotted onto nitrocellulose membranes (Bio-Rad Laboratories) and additional protein-binding sites were blocked by soaking the membranes in 5% skim milk powder in 50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.5. Proteins were detected with rabbit antiserum by enhanced chemiluminescence (Amersham Biosciences). The specific antisera used in this study were previously raised in rabbits to purified recombinant human GSTs including hGSTM1-1, hGSTP1-1, hGSTA1-1, hGSTZ1-1, hGSTT2-2, and hGSTO1-1 (P. G. Board, unpublished data). Additional rabbit antiserum raised against mouse GSTA1/2, and rat NAD(P)H:quinone oxidoreductase 1 (NQO1) were generously provided by Professor John Hayes (University of Dundee).
Enzyme Activity Measurements
MAA isomerase activity in tissue extracts was determined with maleylacetone (MA) as a surrogate substrate because of the lability of MAA.31 MA was synthesized by a previously described method.32 Fumarylacetone (FA) was obtained from The Chemistry Centre (Perth, Australia). The isomerization of MA to FA was determined by a high performance liquid chromatography method modified from previously described procedures.18 The reaction mixture contained 0.01 mol/L sodium phosphate (pH 7.6), 500 μmol/L glutathione, 500 μmol/L MA, and 0.1 to 1.0 μg/ml enzyme in a final volume of 500 μl. The reaction was incubated at 25°C and was stopped after 30 seconds by addition of 100 μl of ice-cold stop solution, which contained a 1:1 mixture of 1 mol/L HCl and 5 μmol/L salicylic acid as an internal standard. The samples were chilled to 4°C and analyzed by high performance liquid chromatography to determine the quantity of FA produced. MA, FA, and salicylic acid were separated on a Waters μBondpak C18 column (3.9 × 300 mm) eluted at 1.5 ml/min. The mobile phase consisted of 40% acetonitrile, 1% triethylamine, and 59% MilliQ water; the pH of the mixture was brought to 3.1 to 3.2 with phosphoric acid, and the mixture was sparged with helium. The absorbance of the eluate was monitored at 312 nm; under these conditions, the solvent peak eluted at 1.6 minutes, MA at 2.4 minutes, FA at 3.4 minutes, and salicylic acid, the internal standard, at 4 minutes. FA formation was quantified by reference to a standard curve prepared with pure FA. The concentration of MA used in these studies was determined by conversion of a sample of MA to FA in the presence of glutathione and hGSTZ1c-1c.
The activity of GSTZ1/MAAI was also determined by measuring the formation of glyoxylate from chlorofluoroacetic acid (CFA).22 CFA (99% pure) was prepared by hydrolysis of ethyl chlorofluoroacetate (Lancaster Synthesis, Inc., Windham, NH), as previously described.21 Nonspecific GST activity was determined with 1-chloro-2,4-dinitrobenzene and glutathione as substrates as previously described.33 Succinylacetone in serum was measured indirectly by its capacity to inhibit δ-aminolevulinic acid dehydratase (δ-ALAD) activity.34
Phenylalanine Treatment
Phenylalanine was added to drinking water at a rate of 3% (w/v) and the pH was adjusted to pH 7.5 with NaOH. Rates of ingestion of phenylalanine were determined by measuring the volume consumed throughout a 3-week period. When adjusted for body weight and expressed as g phenylalanine/kg of mouse/day, the amount of phenylalanine consumed was 3.5 ± 0.09 and 4.04 ± 0.18 in male and female wild-type mice and 3.44 ± 0.16 and 3.56 ± 0.25 in male and female Gstz1−/− mice, respectively. These levels of exposure were not significantly different.
Hematological Analysis
Approximately 200 μl of blood was collected onto 10 μl of 10% (w/v) ethylenediaminetetraacetic acid (pH 8.0). The blood samples were diluted 10-fold in isotonic saline and analyzed on a Cell-Dyn 3700 blood analyzer that had been specifically calibrated for mouse blood cells. A total white blood cell count and a differential white blood cell count, as well as a range of red blood cell and platelet indices were recorded for each sample.
Light Microscopy
Samples of liver, kidney, brain, heart, lungs, pancreas, and spleen were collected for light microscopic assessment at 11 to 14 days, 28 days, 33 days, 6 weeks, and 6 months depending on the treatment group. The treatment groups included wild-type (Gstz1+/+) mice and deficient (Gstz1−/−) mice with subgroups of mice in both groups being treated with 3% phenylalanine in their drinking water. Each treatment group included six animals except for the control group treated with phenylalanine for 11 days that had four animals. In all cases the tissues were fixed immediately in 10% neutral phosphate-buffered formalin before processing and embedding into paraffin. Four-μm sections were cut from each sample and stained with hematoxylin and eosin. In addition a Perl stain (to detect iron), a Masson’s trichrome stain (to detect collagen), a periodic acid-Schiff (PAS)/diastase-PAS stains (to detect glycogen and α-1-anti-trypsin bodies) and a reticulum stain (to assess liver architecture) were performed on the liver samples.
Electron Microscopy
For electron microscopic examination, samples of kidney and liver were collected directly into 0.1 mol/L cacodylate-buffered 2% glutaraldehyde and fixed for 24 hours. Samples were then postfixed in 0.1 mol/L cacodylate-buffered 2% osmium tetroxide for 2 hours. En bloc staining preceded dehydration through a graded series of ethanol steps. Specimens were infiltrated with Spurrs resin and set overnight at 70°C.
Two levels of thin sections (100 nm) were cut from two blocks of each test mouse with two test mice examined in each group. Thin sections mounted on copper/palladium grids were stained with Reynold’s lead citrate and viewed on a Philips CM10 transmission electron microscope. Two hundred cells were examined from each level (800 cells per animal), which provided a suitable sample size for analysis.
The liver assessment included: overall preservation of tissues; the location and amount of glycogen and lipid; the morphology of the nuclei, endoplasmic reticulum, and mitochondria. The size and pleomorphism of the mitochondria were also assessed. The mitochondria were assessed as normal (1.5 ± 0.60 × 0.61 ± 0.16 μm), large (4.8 ± 0.75 × 0.52 ± 0.09 μm) or large and pleomorphic. The percentage of hepatocytes containing each type of mitochondria was recorded. The cristae of the mitochondria were also examined. In the kidney the same ultrastructural parameters were examined. In particular, the proximal and distal tubular mitochondria and the interdigitation of the proximal renal tubules were closely examined. The interdigitation was assessed as regular or irregular and the length of the mitochondrial cristae were identified as regular in length or irregular in length (50 to 300% of normal) and arrangement.
Results
Organization of the GSTZ1/MAAI Gene
A single zeta class GST gene was identified in BALB/c mice. The gene is 16.94-kb in length and is composed of nine exons (Figure 2A). The intron sizes and splice junctions are shown in Table 2. This gene has a longer intron 1 than that previously reported for the MAAI gene in 129/Sv4 mice.29 Exons 6 to 8 were replaced by a neomycin resistance gene by homologous recombination in BALB/c ES cells. The targeted ES cells (+/−) were used to ultimately derive homozygous BALB/c Gstz1−/− mice. The presence of the neomycin marker gene and homozygosity for the targeted gene (−/−) was routinely determined by polymerase chain reaction as described above.
Table 2.
Exon/Intron Sizes and Splice Donor/Acceptor Sites of mGSTZ1
| Exon | Exon (bp) | Splice donor | Intron | Intron (bp) | Splice acceptor |
|---|---|---|---|---|---|
| 1 | 70* | CCGGGAAGgtctgtgc | 1 | 10,120 | ctccacagCCTATCCT |
| 2 | 52 | TCGAATTGgtaagagc | 2 | 1135 | tctcctagCTCTGGCG |
| 3 | 68 | GGCAACAGgtaaggag | 3 | 494 | ccccacagTTCACTGA |
| 4 | 81 | TCCAGTCAgtgagtag | 4 | 333 | ctgaacagCTGGCCAT |
| 5 | 126 | CCCTTCAGgttaggca | 5 | 1301 | ctgtgcagAACCTGTC |
| 6 | 79 | CTTTAACGgtgagtca | 6 | 486 | tcccacagCTCTGGAG |
| 7 | 53 | GAGATGAGgtaagtgg | 7 | 895 | tcctttagGTATCCAT |
| 8 | 50 | GCTGAAAGgtaagaaa | 8 | 668 | tgccacagGTTCAAGG |
| 9 | 1017† |
Includes 15 bp of coding sequence plus 55 bp of 5′ noncoding sequence up to the start of translation.
Includes 127 bp of coding sequence plus 890 bp of 3′ noncoding sequence up to and including the poly(A) addition signal. Exon sequence is shown in upper case and intron sequence in lower case.
GSTZ/MAAI Was Not Expressed in Gstz1−/−-Targeted Mice
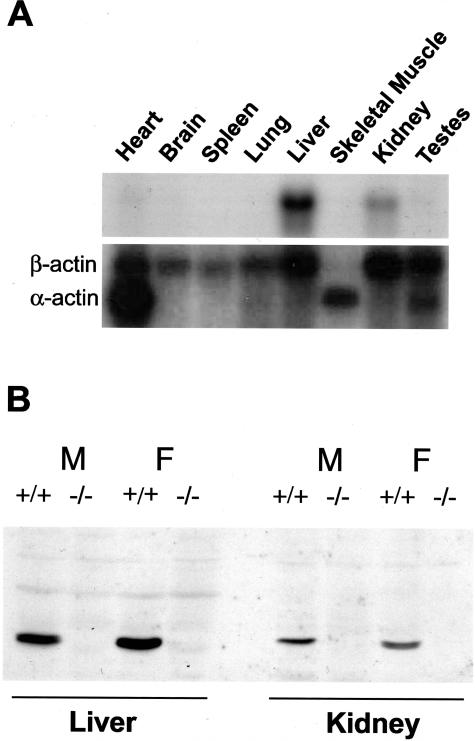
Northern blots of RNA from a range of wild-type mouse tissues demonstrated that GSTZ was expressed at a readily detectable level in liver and kidney but was relatively poorly expressed in other tissues (Figure 3A). Similar results were obtained by Western blotting with antiserum raised against human GSTZ1-1 (data not shown). Western blotting of liver and kidney extracts revealed that GSTZ1 was absent from Gstz1−/− mice but was clearly present in wild-type Gstz1+/+ mice (Figure 3B). To determine whether the targeted mutation eliminated all enzyme activity attributed to GSTZ1/MAAI, the rates of the biotransformation of CFA and the isomerization of MA were determined in liver and kidney extracts. As shown in Figure 4, the level of activity in the liver of Gstz1−/− mice was at the limit of detection and essentially zero for both reactions. There was measurable activity in the heterozygous (+/−) mice but this was significantly lower than the activity determined in the wild-type mice. It was also notable that in the liver, both wild-type and heterozygous females exhibited higher activities than male mice with both substrates. Somewhat similar results were obtained for the kidney (Figure 4) with the exception that the overall activities determined for the wild-type and the heterozygous mice were considerably lower than were measured in the liver. Although there appeared to be very low activity in the kidneys of the Gstz1−/− mice, there was variability between individuals and it was not present in all mice. Despite an attempt to concentrate and further purify the source of the residual activity this was not successful and we conclude that this low level of residual activity represents variability in the nonenzymatic blank rate at the limit of detection.
Figure 3.
The expression of GSTZ1 in different mouse tissues. A: Northern blot of mRNA hybridized with a GSTZ1 cDNA (top) and β-actin cDNA (bottom). B: Western blot of liver and kidney cytosolic proteins from male (M) and female (F) wild-type (Gstz1+/+) and deficient (Gstz1−/−) BALB/c mice probed with antiserum to recombinant human GSTZ1-1.
Figure 4.
Activity of GSTZ1/MAAI in liver and kidney cytosol. Males are shown in the filled columns and females in the open columns. The values are shown as means ± SE, n = 5 to 10 mice. *, P < 0.01 compared with the wild-type (+/+) animals. The Gstz1 genotype is given below each column. A: Activity with CFA as the substrate. B: Isomerase activity with maleylacetone as the substrate.
Detection of Succinylacetone in Serum from GSTZ1-Deficient Mice
Succinylacetone (SA) is not normally present in the urine or serum of normal humans or mice but accumulates in the serum and urine of patients with HT1 because of FAH deficiency. It is possible that SA may also be present in GSTZ1/MAAI deficiency. The concentration of SA in the serum of wild-type, heterozygous, and Gstz1−/− mice was estimated by its capacity to inhibit δ-ALAD. These experiments showed that the serum of Gstz1−/− mice contained a compound that inhibited δ-ALAD. The percent inhibition of δ-ALAD corresponded to concentration of SA in the range of 1.6 ± 0.2 μmol/L (n = 8) and 4.5 ± 0.4 μmol/L (n = 6) for Gstz1−/− mice that were given plain water or water containing 3% w/v phenylalanine for at least 35 days, respectively. There was no detectable SA in the serum of heterozygous or wild-type mice under similar conditions (Table 3).
Table 3.
Succinylacetone Concentrations in the Sera of Gstz1+/+, Gstz1+/−, and Gstz1−/− Mice (Mean ± Standard Deviation)
| Treatment | n | Days of treatment | Genotype | SA (μmol/L) |
|---|---|---|---|---|
| dH20 | 9 | — | +/+ | ND |
| dH20 | 4 | — | +/− | ND |
| dH20 | 8 | — | −/− | 1.6 ± 0.17 |
| 3% Phe | 8 | 29 to 34 | +/+ | ND |
| 3% Phe | 4 | 77 | +/− | ND |
| 3% Phe | 6 | 34 to 43 | −/− | 4.5 ± 0.44 |
ND, not detectable.
Growth and Development of GSTZ1/MAAI-Deficient Mice
The Gstz1−/− mice breed successfully and are without an immediately obvious phenotype. The total body weights of wild-type, heterozygous, and Gstz1−/− mice maintained on normal mouse chow (23% protein, 6% fat, 12 MJ/kg, 1.9% Phe + Tyr) and water ad libitum were followed for 38 weeks. Gstz1−/− male mice were consistently lighter by a small margin than the normal controls and the heterozygotes (P < 0.01) (Figure 5). This difference was not evident in females (data not shown). Throughout a 12-month period the number of deaths did not differ significantly between wild-type mice and Gstz1−/− mice.
Figure 5.
Weight gain of male BALB/c Gstz1+/+ and Gstz1−/− mice given distilled water or water containing 3% phenylalanine. The start of phenylalanine treatment at 5 weeks is indicated (↑). □, Gstz1+/+, n = 14; ▪, Gstz1+/+ mice given phenylalanine, n = 6; ○, Gstz1−/−, n = 13; •, Gstz1−/− mice given phenylalanine, n = 4.
Because deficiency of GSTZ1/MAAI is expected to disrupt tyrosine and phenylalanine metabolism, some mice were exposed to 3% phenylalanine in their drinking water. This phenylalanine load (∼3.5 × g/kg/day) was lethal in young mice (<28 days old), with most losing weight and dying within 14 days of exposure. In contrast older mice (>66 days old) appear to tolerate this treatment throughout periods of up to 1 year (Table 4). In all age groups female mice showed consistently lower rates of death than male mice (data not shown). As shown in Figure 5, the addition of phenylalanine to the drinking water of male Gstz1−/− mice caused an immediate loss of weight and effectively restricted further growth. The growth of wild-type mice fed additional phenylalanine was also slightly retarded with a reduction in weight of ∼10% after 32weeks of treatment compared to wild-type mice on water (data not shown).
Table 4.
Survival of Gstz1−/− Mice Given 3% Phenylalanine in the Drinking Water
| Age treatment began (days) | n | Sick/dead mice | Death rate (%) | Time to death (weeks) |
|---|---|---|---|---|
| 21–27 | 6 | 6 | 100 | 1–2 |
| 28–34 | 19 | 9 | 47.4 | 1–2 |
| 35–41 | 29 | 13 | 44.8 | 1–2 |
| 66–69 | 47 | 12 | 25.5 | 1–4 |
Mice that appeared to be very ill were sacrificed.
Organ Weights Are Altered by Phenylalanine Intake
Wild-type and Gstz1−/− mice were examined for changes in major organ weights as a gross indicator of possible pathologies. Mice at 9 to 10 weeks of age (at least five mice per group) were given either distilled water or 3% (w/v) phenylalanine for 28 days. As noted above, administration of phenylalanine caused a significant loss of body weight in the homozygous mutant animals. As a consequence, we expressed the organ weights as a percentage of body weight (Table 5).
Table 5.
Organ Weight as a Percentage of Body Weight in Homozygous Wild-Type (Gstz1+/+) and Gstz1−/− Mice Given Water Alone or Water Containing 3% Phenylalanine
| GSTZ1 | Phenylalanine | Body wt (g) | Liver (% body wt) | Kidney (% body wt) | Spleen (% body wt) |
|---|---|---|---|---|---|
| Males | |||||
| +/+ | — | 26.6 ± 1.0 | 5.66 ± 0.29 | 1.91 ± 0.12 | 0.33 ± 0.03 |
| +/+ | 3% | 26.5 ± 0.8 | 5.32 ± 0.30 | 1.91 ± 0.08 | 0.39 ± 0.03 |
| −/− | — | 23.1 ± 0.6 | 6.37 ± 0.23 | 2.09 ± 0.07 | 0.31 ± 0.02 |
| −/− | 3% | 14.2 ± 2.5 | 6.39 ± 0.58 | 2.37 ± 0.39 | 0.16 ± 0.07 |
| Females | |||||
| +/+ | — | 21.0 ± 0.6 | 4.93 ± 0.19 | 1.38 ± 0.08 | 0.52 ± 0.02 |
| +/+ | 3% | 19.9 ± 3.3 | 4.64 ± 0.12 | 1.42 ± 0.06 | 0.62 ± 0.11 |
| −/− | — | 20.9 ± 1.6 | 6.12 ± 0.14 | 1.59 ± 0.11 | 0.47 ± 0.03 |
| −/− | 3% | 16.8 ± 1.7 | 6.66 ± 0.54 | 1.92 ± 0.19 | 0.26 ± 0.05 |
Values are shown as means ± SD, n = 5.
When fed a standard diet the livers of Gstz1−/− mice were found to weigh significantly more than livers from wild-type mice (24.1%, P < 0.001; and 12.6%, P < 0.005 in females and males, respectively). This difference was not altered by phenylalanine treatment. In contrast, phenylalanine treatment of wild-type mice resulted in a small decrease in liver wet weight.
Both untreated male and female Gstz1−/− mice had significantly heavier kidneys than wild-type mice (9.8%, P = 0.02; and 15.3%, P = 0.01 in males and females, respectively). Treatment with phenylalanine resulted in a further increase in kidney wet weight, reaching significance in females (increase of 13.3%, P = 0.16; and 20.6%, P = 0.006 in males and females, respectively). Phenylalanine treatment had no effect on kidney weight in wild-type mice.
Untreated female Gstz1−/− mice showed a modest decrease in spleen weight of 9% (P = 0.04) with males showing a similar trend (6.7%, P = 0.2). Phenylalanine treatment of wild-type mice resulted in an increase in spleen weight in males (17.7%, P = 0.016), and showed a similar trend in females (18.6%, P = 0.1). In contrast to the increase in spleen weight of wild-type mice, treatment of Gstz1−/− mice with phenylalanine resulted in a dramatic decrease in spleen weight of 46% in females and 49.1% in males (P < 0.005 compared with untreated Gstz1−/− mice).
Pathological Changes Associated with GSTZ/MAAI Deficiency
A range of tissues was examined by light and electron microscopy to determine whether deficiency of GSTZ/MAAI generated pathological changes that were similar to those seen in FAH deficiency. Light microscopic analysis revealed that at 6 weeks of age, all six of the Gstz1−/− mice examined developed multifocal hepatitis (Figure 6B) whereas the wild-type mice did not. The multifocal hepatitis was characterized by small aggregates of neutrophils surrounding degenerating hepatocytes in hepatic lobules. At 6 months, six of seven Gstz1−/− mice examined showed focal hepatitis and three animals showed hepatocyte necrosis. The wild-type mice showed no evidence of hepatitis at any time (Figure 6A). In the eight mice that died within 7 to 21 days after the addition of 3% phenylalanine to their drinking water, five showed macrovesicular steatosis (fatty change) on light microscopy (Figure 6D) and three showed hepatocyte necrosis (Figure 6C). A mild lymphocytic infiltrate was seen in the sinusoids in some animals from all treatment groups but there was no significant difference between the groups. There was no evidence of significant anisocytosis and no hepatocyte dysplasia or hepatocellular carcinoma in any treatment group.
Figure 6.
Light microscopy showing liver and heart pathology in Gstz1−/− mice. A: Wild-type mouse given 3% phenylalanine for 6 weeks shows normal liver morphology. B: Gstz1−/− mice given water alone that show focal hepatitis (arrows and inset) at 6 weeks. C: Gstz1−/− mice given 3% phenylalanine show massive hepatic necrosis (N). D: Gstz1−/− mice given 3% phenylalanine show macrovesicular steatosis (black arrows). The red infiltrate is red blood cells. E and F: Thrombus and myocardial calcification observed in Gstz1−/− mice. E: Black arrows show calcification. F: Thrombi in the right atrium (RA) and right ventricle (RV). Original magnifications: ×200 (A, B); ×1000 (D, inset in B); ×100 (C); ×25 (E, F); ×400 (inset in E).
The spleens of all Gstz1−/− mice fed 3% phenylalanine were small with a marked reduction in both the white and red pulp. There was a marked reduction in the white-pulp mantle zone. The untreated Gstz1−/− mice and the wild-type mice showed normal spleen morphology.
Several untreated Gstz1−/− mice older than 1 year of age died or were sacrificed because of morbidity because of heart pathologies. These pathologies occurred more frequently in breeders than in nonbreeders. In a series of mice bred continuously from 8 weeks of age, 5 of 14 (36%) female and 4 of 11 (36%) male Gstz1−/− mice suffered heart lesions compared to 0 of 27 (0%) female and 1 of 10 (10%) male wild-type mice from the same experiment. Each heart lesion typically consisted of an organizing thrombus with calcification in the right atrium and/or ventricle, adhered to the wall. This was often accompanied by calcification of the myocardium, chronic inflammation, or myocyte necrosis. The average age of mice suffering these lesions was 35 weeks (range, 20 to 42 weeks) whereas the age of the healthy mice examined for these lesions was 41 weeks (range, 36 to 44 weeks) for wild-type mice and 37 weeks (range, 28 to 42 weeks) for Gstz1−/− mice. Female mice were also comparable for the number of litters produced in this time, with wild-type mice bearing 3.7 litters (range, 2 to 7 litters) and Gstz1−/− mice bearing 3.3 litters (range, 2 to 6 litters). In nonbreeding, young (3 to 12 weeks) mice, calcification of the myocardium was found in three of the eight Gstz1−/− mice fed phenylalanine that died and one of these showed an early thrombus in the right atrium associated with myocyte necrosis. Two other Gstz1−/− mice fed phenylalanine also showed myocyte necrosis. None of the nonbreeder wild-type mice or Gstz1−/− mice given water alone showed myocyte calcification or necrosis. Examples of the myocardial calcification and thrombi found in Gstz1−/− mice are shown in Figure 6, E and F. Sections of the pancreas, lungs, brain, and kidneys showed no light microscopic abnormalities in all treatment groups examined. In particular, the kidneys showed no glomerulosclerosis, significant tubular dilation, interstitial nephritis, or fibrosis.
Electron microscopic examination of hepatocytes revealed that the Gstz1−/− mice showed more large pleomorphic mitochondria than the control animals at all times examined (Figure 7). The large pleomorphic mitochondria were more abundant at 26 weeks compared with 6 weeks of age in animals given water alone (Table 6). The addition of 3% phenylalanine to the drinking water resulted in increased numbers of pleomorphic mitochondria after 3 weeks of treatment. Intramitochondrial paracrystalline inclusions were found in up to 40% of the hepatocytes of Gstz1−/− mice given phenylalanine in the drinking water (Figure 7B). Other aspects of liver ultrastructure were not altered. Examination of the liver nuclear morphology, the presence and location of lipid and glycogen, and the morphology of endoplasmic reticulum showed no changes between the wild-type and the Gstz1−/− mice at any time and in all treatment groups. There was mild dilation of the endoplasmic reticulum in 20% of the hepatocytes in response to phenylalanine in both the wild-type mice and Gstz1−/− mice.
Figure 7.

Ultrastructure of livers from two Gstz1−/− mice given 3% phenylalanine in their drinking water for 28 days. A: Large pleomorphic mitochondria. Inset: mitochondria from wild-type mouse mitochondria. B: Large pleomorphic mitochondria with a crystalline inclusion mitochondria. Original magnifications, ×11,800.
Table 6.
Differences in Ultrastructure of Mitochondria in Hepatocytes and Ultrastructural Changes in Proximal Tubular Cells in the Kidney
| Genotype | n | Age at analysis | Treatment 3% Phe | Hepatocytes
|
Kidney proximal tubule
|
||
|---|---|---|---|---|---|---|---|
| Large pleomorphic mitochondria, % | Large mitochondria, % | Irregular interdigitation of proximal tubular cells, % | Irregular mitochondrial cristae, % | ||||
| −/− | 2 | 6 weeks | — | 10 | 30 | — | — |
| +/+ | 2 | 6 weeks | — | 0 | <5 | — | — |
| −/− | 2 | 26 weeks | — | 40 | 30 | 60 | 90 |
| +/+ | 2 | 26 weeks | — | 0 | <5 | 20 | 0 |
| −/− | 2 | 12 weeks | 3 weeks | 30 | 40 | 60 | 90 |
| +/+ | 2 | 12 weeks | 3 weeks | 0 | <5 | 25 | 5 |
| −/− | 2 | 12 weeks | 8 weeks | 40 | 30 | 70 | 100 |
| +/+ | 2 | 10 weeks | 8 weeks | 0 | <5 | 20 | 5 |
The kidneys of Gstz1−/− mice showed only minor changes compared with control animals at different times and with different treatments. The interdigitation of proximal tubular epithelial cells was more irregular in the Gstz1−/− mice than in the control animals (Figure 8). Throughout both proximal and distal tubular cells, up to 70% of all mitochondria in the Gstz1−/− mice had varied cristae length, where as the control animals did not exhibit this variability (Figure 8, C and D). The cristae of the mitochondria were arranged in parallel in wild-type animals and were random in the Gstz1−/− mice. No intramitochondrial paracrystalline inclusions were found.
Figure 8.

Ultrastructure of kidneys from mice given 3% phenylalanine in their drinking water for 28 days. A: Regular interdigitation seen in wild-type animals. B: Irregular interdigitation seen in the Gstz1−/− animals. Arrows indicate some of the more irregular areas. C: Regular length and spaced parallel cristae in the mitochondria of the tubules in a wild-type animal. D: Irregular arrangement of variable length cristae of the tubules in a Gstz1−/− mouse. Original magnifications, ×8100 (A, B).
Hematological Changes Found in Gstz1−/− Mice
As a result of the dramatic change in spleen size and structure in Gstz1−/− mice after treatment with phenylalanine we investigated the hematological profile of 60- to 66-week-old mice that had been maintained on water or water containing 3% phenylalanine for a period of at least 50 weeks. There were no notable differences in the red blood cells or platelet indices of wild-type or Gstz1−/− mice given water alone or 3% phenylalanine (data not shown). In contrast, treatment of Gstz1−/− mice with phenylalanine caused a significant reduction in total white cell count compared with wild-type mice given either water alone or phenylalanine-containing water and Gstz1−/− mice given water alone (Table 7). Differential leukocyte counts indicated that all leukocyte classes were uniformly affected in homozygous deficient animals given phenylalanine.
Table 7.
Differential White Blood Cell Counts in Homozygous Wild-Type and GSTZ1/MAAI-Deficient Mice Given Water Alone or Water Containing 3% Phenylalanine
| Genotype | 3% Phe | n | White cells | Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils |
|---|---|---|---|---|---|---|---|---|
| +/+ | — | 24 | 5.6 ± 1.77 | 2.2 ± 0.67 | 2.5 ± 1.22 | 0.45 ± 0.20 | 0.21 ± 0.15 | 0.16 ± 0.22 |
| −/− | — | 20 | 6.0 ± 2.03 | 2.2 ± 0.76 | 3.0 ± 1.24 | 0.52 ± 0.23 | 0.19 ± 0.15 | 0.14 ± 0.23 |
| +/+ | +* | 14 | 5.1 ± 1.20 | 1.9 ± 0.81 | 2.3 ± 1.08 | 0.55 ± 0.70 | 0.24 ± 0.17 | 0.14 ± 0.23 |
| −/− | +* | 12 | 2.0 ± 0.60 | 0.89 ± 0.19 | 0.93 ± 0.41 | 0.13 ± 0.10 | 0.05 ± 0.07 | 0.03 ± 0.05 |
| P value* | <0.001 | <0.001 | <0.001 | 0.053 | 0.002 | 0.124 |
Data are shown as number of cells ×109/L and as means ± SD.
Induction of Other GSTs
When examining tissues for the presence of GSTZ1 protein by Western blotting (Figure 3B) duplicate samples were fractionated by SDS-PAGE and stained with Coomassie Blue to confirm equivalent protein loading in the different tracks. These gels revealed differential staining of several proteins of different sizes. Increased staining of proteins in the 25- to 30-kd range in the liver of homozygous GSTZ1-deficient mice was particularly striking (Figure 9A). The increased protein staining in this region was sufficiently distinct to permit the identification of homozygotes. Because most other soluble GSTs have subunit molecular masses that fall within this particular size range we measured the GST activity of these cytosolic extracts with 1-chloro 2,4-dinitrobenzene (CDNB) as the substrate. Female Gstz1−/− mice had CDNB activity of 8.6 ± 0.56 μmol/mg protein/minute compared with 1.8 ± 0.52 μmol/mg protein/minute in wild-type mice (mean ± SD, n = 3). This significant difference (P < 0.0001) in activity indicated that other GST(s) were induced in the liver of Gstz1−/− mice. Because CDNB is a substrate for some but not all of the GSTs that are expressed in mouse liver we used Western blotting of liver extracts with antiserum to members of the alpha, mu, omega, pi, and theta class GSTs to determine which of these isoenzymes was overexpressed. This analysis revealed that in female Gstz1−/− mice, some alpha, mu, and pi class GSTs were overexpressed relative to their levels in wild-type liver (Figure 9B). This result is consistent with the quantitative GST assay because members of the alpha, pi, and mu class GSTs accept CDNB as a substrate. Similar overexpression of GSTs was not as evident in Coomassie Blue-stained SDS-PAGE gels of cytosolic preparations from the kidney, brain, heart, lung, spleen, skeletal muscle, testes, or seminal glands, but may occur at levels that are below the level of detection of this methodology. Because some members of the alpha, mu, and pi class GSTs have anti-oxidant response elements (AREs) in their promoters and respond strongly to electrophiles, we immunoblotted NQO1, which is also strongly induced by electrophiles via an ARE. NQO1 was barely detectable in wild-type liver, however Gstz1−/− mice showed very strong expression of NQO1 (Figure 9B).
Figure 9.
Induction of other GSTs in female Gstz1−/− mice. A: SDS-PAGE of soluble proteins from the liver and kidney of wild-type (Gstz1+/+) and deficient (Gstz1−/−) mice. The arrow indicates the elevated expression of proteins in the liver of GSTZ1-deficient mice. B: Western blots of soluble liver proteins probed with antiserum to hGSTM1-1, hGSTZ1-1, hGSTP1-1, mGSTA1/2, and ratNQO1. The mouse genotype is shown below each track. Each track was loaded with the same amount of total protein.
Discussion
Characterization and Inactivation of Gstz1
Our genomic studies found evidence for a single zeta class GST gene in BALB/c mice. Comparison with a previously reported zeta class GST gene in 129/Sv4 mice29 revealed an identical organization of nine exons except for the length of intron 1, which was considerably larger in the BALB/c strain studied here. This difference may reflect a strain difference rather than the existence of multiple Gstz gene loci.
The selective removal of exons 6 to 8 from a BALB/c mouse Gstz1 gene resulted in the absence of functional GSTZ1-1 protein in homozygous (Gstz1−/−) mice. GSTZ1-1 catalyzes two distinct reactions: the isomerization of MAA to FAA and the biotransformation of α-halo acids such as CFA or DCA to glyoxylate. The absence of both activities in the liver and kidney of Gstz1−/− mice indicates that the gene was successfully inactivated and supports the contention that there is only one zeta class gene in BALB/c mice. In previous unpublished studies, we tested a range of purified recombinant human GSTs for their capacity to catalyze both the isomerase and halide-displacement reactions. None of the enzymes studied, including GSTA1-1, GSTA2-2, GSTA4–4, GSTM1-1, GSTM2-2, GSTM3-3, GSTP1-1, GSTT2-2, GSTO1-1, and GSTK, catalyzed these reactions. Thus, the absence of any isomerase or halide displacement activity in Gstz1−/− mice is consistent with the view that GSTZ1-1 is not redundant and that other GST isoenzymes cannot substitute for GSTZ1-1 activity. Neither do they seem to play a role in the glutathione-dependent metabolic bypass that has been proposed to prevent the accumulation of MAA in GSTZ1/MAAI deficiency.29
The finding that BALB/c Gstz1−/− mice survive and breed successfully confirms the observation in 129/Sv4 mice29 that deficiency of GSTZ1/MAAI is not lethal and is not associated with an overt pathology or phenotype. This presumably explains the absence of a clinical syndrome in humans. Our studies of BALB/c Gstz1−/− mice revealed several characteristics that were not observed in the 129/Sv4 mice studied by Fernández-Cañón and colleagues.29 Some of the abnormalities we observed were precipitated or exacerbated by exposure to an increased burden of dietary phenylalanine. It is probable that dietary tyrosine would cause similar effects. In our experiments only phenylalanine was used because of its solubility and ease of delivery.
Exposure to dietary phenylalanine had a range of deleterious effects on Gstz1−/− mice. Although the inclusion of 3% phenylalanine in drinking water proved lethal in 3- to 4-week-old Gstz1−/− mice, older mice were able to tolerate this challenge and survived throughout extended periods despite significant damage to several organs and cell types (see below). The tolerance that occurs with age may reflect the induction of enzymes that can further metabolize any toxic intermediates that accumulate.
Pathological Changes in Gstz1−/− Mice
The liver and kidney are important sites for the catabolism of aromatic amino acids. It is notable that Gstz1−/− mice have enlarged livers and kidneys but these organs responded differently to the addition of 3% phenylalanine to the drinking water. The differences in the response of each organ to phenylalanine presumably reflect each organ’s capacity to respond to the accumulation of a toxic metabolite(s).
The pathological changes in the liver observed by light and electron microscopy are consistent with liver toxicity because of metabolite accumulation. The presence of focal hepatitis such as that observed in the Gstz1−/− mice is seen in many metabolic disorders.35,36 The addition of phenylalanine resulted in more marked hepatocyte damage, highlighted by necrosis and macrovesicular steatosis (steatohepatitis). Macrovesicular steatosis is seen in a number of inborn errors of metabolism such as HT1 but can also be seen in drug toxicity.35,37 Necrosis, micro- and macrovesicular steatosis, and some calcification in the liver were observed on light microscopic examination of the 129/Sv4 MAAI-deficient mice.29 Electron microscopy, besides confirming the light microscopic features of steatohepatitis or acute hepatitis, demonstrated pleomorphism of the mitochondria and the presence of intramitochondrial crystalline inclusions in the liver of the Gstz1−/− mice that were exacerbated with phenylalanine treatment. These features also reflect toxic damage to the cells. Alterations in the liver mitochondria size and pleomorphism have been reported for alcoholic livers,38 adriamycin-induced stress,39 exposure to dimethylformamide,40 and nonalcoholic steatohepatitis.41 Crystalline inclusions in mitochondria have been reported in a number of tissues and conditions, including the liver of nonalcoholic steatohepatitis patients41 and one hepatitis patient,42 in atrophic deltoid muscle,42 ocular muscular dystrophy,42 onchocytic carcinoid of bronchus,43 and in the cerebral cortex.42 Crystalline inclusions with a similar ultrastructure to those observed in the liver mitochondria of phenylalanine-treated Gstz1−/− mice have also been observed in tubular epithelial giant mitochondria in a patient with nephrotic syndrome and in the liver mitochondria of a patient with hepatitis.42 Dilation of the endoplasmic reticulum in response to the known hepatotoxins paracetamol, β-galactosamine, ethanol, and the herbal protein CI-1 has been demonstrated.44 Thus, the low level of endoplasmic reticulum dilation observed in the hepatocytes of phenylalanine-treated animals may be because of hepatotoxicity. The lack of hepatocyte dysplasia in Gstz1−/− mice compared with human with HT1 may reflect differences in the type and concentration of metabolites, probably because of the presence of an alternative pathway and possibly the young age of the animals studied.
Light microscopic evidence of kidney damage was not present in Gstz1−/− mice. This probably reflects the lower metabolic flux present in the kidney relative to the liver or possibly a higher level of the proposed bypass activity.29 Electron microscopic examination, however, demonstrated some changes indicative of toxic damage of the tubule with an increase in irregularity of proximal tubule interdigitation and the length of mitochondrial cristae. Necrosis in the kidney of MAAI-deficient 129/Sv4 mice was reported29 but no further details were provided. The observed effects in both the kidney and liver may be associated with the production of toxic metabolites and may be exacerbated by exposure to phenylalanine. The dystrophic calcification seen in the myocardium is also indicative of cell damage.45–48
Unexpectedly, we noted a reduction in spleen weight in Gstz1−/− mice that was considerably greater after exposure to phenylalanine. This is consistent with systemic illness. Splenic atrophy is seen in humans in the context of some diseases.49–51 The depletion of lymphocytes in the red and white pulp is consistent with the changes noted in the hematological profile. Depletion of circulating lymphocytes and splenic atrophy have been observed in mice with experimental pancreatitis.52 The present study revealed a significant loss of circulating leukocytes in Gstz1−/− mice after exposure to phenylalanine. In contrast, the erythrocyte and platelet indices were unaffected under the same conditions. The loss of leukocytes was broad and involved cells of all lineages. This is consistent with the accumulation of a toxic metabolite in the Gstz1−/− mice. It is notable that the loss of circulating blood cells was apparently restricted to nucleated cells, suggesting the possibility of a nuclear target for the toxic metabolite. Previous studies have shown that SA has an immunosuppressive activity and strongly inhibited lymphoproliferative responses.53,54 Thus the dramatic loss of leukocytes may be a direct result of the accumulation of SA. The loss of leukocytes may increase susceptibility to infection, although increased levels of infection have not been noted in Gstz1−/− mice. However, the mice are maintained in a very clean environment and their potential exposure to pathogens and infection is limited. Further studies of these mice in a more challenging environment are warranted.
Accumulation of Succinylacetone
All of the cellular changes that have been observed in the Gstz1−/− mice were consistent with chronic, low-level toxic stress that is aggravated by the addition of phenylalanine to the diet. The source of this toxic stress is presumably metabolic intermediates such as MAA, MA, SAA, or SA that could accumulate upstream to the MAA isomerization step of the catabolic pathway (Figure 1).
It has previously been noted that SA accumulates in the serum and urine of FAH-deficient patients.15 It is thought that the SA is derived from MAA and FAA that would accumulate in the absence of FAH, but the mechanism by which this occurs is not clear. Consequently we considered the possibility that SA may also accumulate in GSTZ1/MAAI deficiency. We determined SA concentrations in serum by its capacity to inhibit δ-aminolevulinic acid dehydratase (δ-ALAD)16,34 and detected the accumulation of SA in Gstz1−/− mice fed a standard mouse diet. After exposure of Gstz1−/− mice to 3% phenylalanine there was a further increase in serum SA concentrations. Even at their highest, the levels of SA that were measured in Gstz1−/− mice were at the low end of a wide range (2 to 100 μmol/L) that has been reported in individuals with HT1 because of FAH deficiency.55 The higher concentrations of SA in FAH deficiency correlate well with the greater degree of liver and kidney pathology and the lethality that is seen in humans13,14 and in mice.56 In contrast the milder symptoms and the lack of hepatocyte dysplasia occurring in Gstz1−/− mice correlate with the relatively low serum SA concentrations. The lack of the hepatocyte dysplasia that is seen in human patients with HT1 may reflect the lower levels or different toxic metabolites, probably because of the presence of an alternative pathway and possibly the young age of the animals studied.
It is of interest to note that because SA inhibits δ-ALAD, high concentrations of SA may interfere with heme biosynthesis. Porphyria-like symptoms have been observed in HT1 patients57 and decreased erythrocyte counts and hemoglobin concentrations were observed in dogs given DCA,58 a mechanism-based inhibitor of GSTZ1-1.19 Despite the elevated concentrations of SA, heme biosynthesis does not appear to be perturbed in Gstz1−/− mice because their red cell indices, including the mean corpuscular hemoglobin concentrations, are within the normal range (data not shown).
Although the determination of SA concentrations by the inhibition of δ-ALAD is a well-established technique, it is an indirect measurement.16,34,59 Recent studies of a rat model of GSTZ1-1 deficiency show that other metabolites such as MA or FA also inhibit δ-ALAD.60 DCA is a mechanism-based inhibitor of GSTZ1-119 and treatment with DCA results in lowered GSTZ1-1 and MAAI activities in rat tissues.61,62 Specific GC-MS and LC-MS/MS assays show MA rather than SA is excreted in rats given DCA.60 It was also noted that the urine of DCA-treated rats inhibited δ-ALAD and that MA and FA are competitive inhibitors of δ-ALAD.60 Thus, it is likely that our measurements of SA concentrations in the serum of Gstz1−/− mice includes MA and possibly FA. Both MA and FA are alkylating agents that covalently modify thiol moieties and therefore have the potential to modify intracellular target molecules.63 Because increased DNA single-strand breaks and DNA hypomethylation are observed in DCA-treated rats,64,65 it will be of considerable interest to determine whether these effects are associated with the build up of MA. Because both MA and FA are cytotoxic60 and both have the potential to modify proteins and DNA, they could play a role in the pathological changes observed in the liver and kidneys and the loss of leukocytes in Gstz1−/− mice given phenylalanine. Long-term studies of these mice may reveal the consequences of long-term chronic exposure to metabolites such as MA and FA that could be elevated in individuals on a high-protein diet and exposed to DCA.
Elevated Expression of Alpha, Mu, and Pi Class GSTs
Further evidence for the accumulation of a toxic metabolite in Gstz1−/− mice was gained from the observation that the expression of alpha, mu, and pi class GSTs is significantly induced in Gstz1−/− mice. Examination of Figure 9 suggests that while the increased expression of these GSTs is a dominant feature, there appear to be other yet to be identified proteins that are also differentially expressed. The elevated expression of pi and mu class GSTs may have an impact on cell signaling pathways and thus contribute to other gene expression changes because GSTP regulates the activity of Jun N-terminal kinase,7 and apoptosis-stimulating kinase (ASK1) is regulated by a mu class GST.8 These changes in expression clearly reflect the adaptation of metabolic pathways to the altered conditions within GSTZ-deficient cells.
As there are at least four alpha class GSTs, two pi class GSTs, and possibly six mu class GSTs expressed in mice, further study will be required to identify the specific genes that are induced by GSTZ1-1 deficiency. A range of compounds modulate the expression of GSTs in rodents (for review see Hayes and Pulford66) but little is known about the mechanisms by which most compounds exert their influence. AREs and xenobiotic response elements play significant roles in the transcriptional regulation of some rodent GST genes.66,67 We noted that an alpha class GST that cross-reacts with antiserum directed against human GSTA1-1 was not induced (data not shown) whereas another mouse alpha class GST that cross-reacts with antiserum against mouse GSTA1/2 was clearly up-regulated (Figure 9B). To determine whether the selective induction of some GSTs may be mediated by the presence of AREs we examined the expression of NAD(P)H:quinone oxidoreductase 1 (NQO1) in liver from Gstz1−/− mice. NQO1 is strongly induced by electrophiles via an ARE and has significant cytoprotective functions.68 The dramatic constitutive induction of NQO1 that we observed in the liver of Gstz1−/− mice is consistent with the induction of GST genes that contain AREs. Because MAA is an electrophile it is likely that it or other related metabolites that accumulate in Gstz1−/− mice are responsible for the constitutive induction of liver GSTs. This observation has several implications that require further study. The data suggest that normal catabolites of tyrosine and phenylalanine are capable of inducing drug-metabolizing enzymes. Thus an individual’s response to therapeutic drugs could potentially be modified by their diet and constitutive induction of drug-metabolizing enzymes may be a previously unrecognized feature of patients with deficiencies of other enzymes in this catabolic pathway. It will be of interest to determine whether exposure to DCA, which inhibits GSTZ1-1 (see below), results in an induction of enzymes regulated by AREs and if DCA exposure can modulate drug metabolism.
The Gstz1−/− mice described herein provide insight into the potential clinical features of human GSTZ1/MAAI deficiency. Although it might be expected from these studies that such individuals may live a relatively normal life, they may have an abnormal capacity to metabolize some drugs and exposure to a diet high in tyrosine and phenylalanine may bring about a level of leukopenia that could increase susceptibility to infection.
Future studies of these Gstz1−/− mice will also provide information on the mechanism by which DCA and other α-halo acids exert their toxicity. DCA is a contaminant of chlorinated water supplies26 and is a metabolite of the sedative chloral hydrate and the industrial solvents such as trichloroethylene and tetrachloroethylene.27 In clinical settings DCA is used in the management of congenital lactic acidosis.27 Several studies have shown that DCA is a carcinogen in rodents.23,24,65 Although the mechanism of its carcinogenicity is not clear, it is of great interest given the human exposure that occurs. GSTZ1-1 plays a major role in the biotransformation of DCA but it is inactivated by the formation and reaction of a S-(α-chlorocarboxymethyl)glutathione adduct19,21 with a cysteine residue near the active site.19,21 Thus previous studies of the toxicity of DCA have been confounded by the variable inactivation of GSTZ1-1, the enzyme that is of prime importance in its metabolism. These Gstz1−/− mice will therefore play an important role in dissecting the mechanism of DCA-induced carcinogenesis. An understanding of this mechanism is required to properly assess the relative risk of DCA to humans and rodents.
Acknowledgments
We thank Wayne Damcevski, Helen Taylor, Mathew Newhouse, Elaine Bean, and Jean Cappello for technical assistance with various aspects of this study; Dr. Hoffman Lantum for comments on the manuscript; and Professor John Hayes for generously providing antiserum.
Footnotes
Address reprint requests to Dr. P.G. Board, John Curtin School of Medical Research, PO Box 334, Canberra ACT 2601, Australia. E-mail: philip.board@anu.edu.au.
Supported in part by grants from the National Health and Medical Research Council of Australia (grant 179818 to P.G.B. and Florey Centenary Fellowship to A.C.B.) and the National Institute of Environmental Health Sciences (grant ES03127 to M.W.A.).
References
- Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B, Danielson UH. Glutathione transferases—structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Woollatt E, Sutherland GR, Board PG. Characterization and chromosome location of the gene GSTZ1 encoding the human zeta class glutathione transferase and maleylacetoacetate isomerase. Cytogenet Cell Genet. 1998;83:109–114. doi: 10.1159/000015145. [DOI] [PubMed] [Google Scholar]
- Fernandez-Canon JM, Penalva MA. Characterization of a fungal maleylacetoacetate isomerase gene and identification of its human homologue. J Biol Chem. 1998;273:329–337. doi: 10.1074/jbc.273.1.329. [DOI] [PubMed] [Google Scholar]
- Johansson AS, Mannervik B. Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J Biol Chem. 2001;276:33061–33065. doi: 10.1074/jbc.M104539200. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Ridderstrom M, Engstrom A, Pemble SE, Mannervik B. Cloning and heterologous expression of cDNA encoding class alpha rat glutathione transferase 8-8, an enzyme with high catalytic activity towards genotoxic alpha, beta-unsaturated carbonyl compounds. Biochem J. 1992;284:313–319. doi: 10.1042/bj2840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, Shim J, Kim Y, Dong MS, Lee MJ, Kim SG, Ichijo H, Choi EJ. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- Dulhunty A, Gage P, Curtis S, Chelvanayagam G, Board P. The Glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J Biol Chem. 2001;276:3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- Board PG, Baker RT, Chelvanayagam G, Jermiin LS. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem J. 1997;328:929–935. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M. The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin Liver Dis. 2001;21:563–571. doi: 10.1055/s-2001-19035. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Lindstedt S, Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci USA. 1977;74:4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, O’Regan S. Visceral pathology of hereditary tyrosinemia type I. Am J Hum Genet. 1990;47:317–324. [PMC free article] [PubMed] [Google Scholar]
- Mitchell GA, Grompe M, Lambert M, Tanquay RM. New York: McGraw-Hill,; Hypertyrosiemia. 2001:pp 1777–1805. [Google Scholar]
- Lindblad B, Steen G. Identification of 4,6-dioxoheptanoic acid (succinylacetone), 3,5-dioxooctanedioic acid (succinylacetoacetate) and 4-oxo-6-hydroxyheptanoic acid in the urine from patients with hereditary tyrosinemia. Biomed Mass Spectrom. 1982;9:419–424. doi: 10.1002/bms.1200091003. [DOI] [PubMed] [Google Scholar]
- Sassa S, Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983;71:625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn AC, Tzeng HF, Anders MW, Board PG. Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics. 2000;10:49–57. doi: 10.1097/00008571-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Coggan M, Tzeng HF, Lantum H, Polekhina G, Parker MW, Anders MW, Board PG. GSTZ1d: a new allele of glutathione transferase zeta and maleylacetoacetate isomerase. Pharmacogenetics. 2001;11:671–678. doi: 10.1097/00008571-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphism- and species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem Res Toxicol. 2000;13:231–236. doi: 10.1021/tx990175q. [DOI] [PubMed] [Google Scholar]
- Berger R, Michals K, Galbraeth J, Matalon R. Tyrosinemia type 1b caused by maleylacetoacetate isomerase deficiency: a new enzyme defect. Pediatr Res. 1988;23:328A. [Google Scholar]
- Tong Z, Board PG, Anders MW. Glutathione transferase zeta-catalyzed biotransformation of dichloroacetic acid and other alpha-haloacids. Chem Res Toxicol. 1998;11:1332–1338. doi: 10.1021/tx980144f. [DOI] [PubMed] [Google Scholar]
- Tong Z, Board PG, Anders MW. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem J. 1998;331:371–374. doi: 10.1042/bj3310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology. 1990;63:341–359. doi: 10.1016/0300-483x(90)90195-m. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, Most BM, Olson GR. The carcinogenicity of dichloroacetic acid in the male Fischer 344 rat. Toxicology. 1996;114:207–221. doi: 10.1016/s0300-483x(96)03510-x. [DOI] [PubMed] [Google Scholar]
- Harrington-Brock K, Doerr CL, Moore MM. Mutagenicity of three disinfection by-products: di- and trichloroacetic acid and chloral hydrate in L5178Y/TK +/− (−)3.7.2C mouse lymphoma cells. Mutat Res. 1998;413:265–276. doi: 10.1016/s1383-5718(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Krasner SW, McGuire MJ, Jacangelo JG, Patania NL, Reagan KM, Aieta EM. The occurrence of disinfection by-products in U. S. drinking water. J Am Waterworks Assoc. 1989;81:41–53. [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, James MO. Clinical pharmacology and toxicology of dichloroacetate. Environ Health Perspect. 1998;106(Suppl 4):989–994. doi: 10.1289/ehp.98106s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WB, Liebler DC, Board PG, Anders MW. Mass spectral characterization of dichloroacetic acid-modified human glutathione transferase zeta. Chem Res Toxicol. 2002;15:1387–1397. doi: 10.1021/tx025553x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Canon JM, Baetscher MW, Finegold M, Burlingame T, Gibson KM, Grompe M. Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism. Mol Cell Biol. 2002;22:4943–4951. doi: 10.1128/MCB.22.13.4943-4951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Board PG, Taylor MC, Coggan M, Parker MW, Lantum HB, Anders MW. Clarification of the role of key active site residues of glutathionetransferase zeta/maleylacetoacetate isomerase by a new spectrophotometric technique. Biochem J. 2003;374:731–734. doi: 10.1042/BJ20030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J, Seltzer S. Synthesis of model compounds for maleylacetoacetic acid. J Org Chem. 1970;35:3529–3532. [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Greiner A, Lescault A. Succinylacetone. Bergmeyer HU, editor. Weinheim: Verlag Chemie,; Methods of Enzymatic Analysis. 1983:pp 79–85. [Google Scholar]
- MacSween RNM, Burt AD, Portman BC, Ishak KG, Scheuer PJ, Anthony PP. Edinburgh: Churchill Livingston,; Pathology of the Liver. 2002 [Google Scholar]
- Scheuer PJ, Lefkowitch JH. London: W. B. Saunders,; Liver Biopsy Interpretation. 2000 [Google Scholar]
- Gilbert-Barness E, Barness LA. Metabolic diseases. Natick: Eaton Publishing,; Foundations of Clinical Management, Genetics, Pathology. 2000 [Google Scholar]
- Chang ES. Ultrastructural morphogenesis of mitochondria in alcoholic liver. Acta Pathol Jpn. 1987;37:213–224. doi: 10.1111/j.1440-1827.1987.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Zeidan Q, Strauss M, Porras N, Anselmi G. Differential long-term subcellular responses in heart and liver to adriamycin stress. Exogenous L-carnitine cardiac and hepatic protection. J Submicrosc Cytol Pathol. 2002;34:315–321. [PubMed] [Google Scholar]
- Redlich CA, West AB, Fleming L, True LD, Cullen MR, Riely CA. Clinical and pathological characteristics of hepatotoxicity associated with occupational exposure to dimethylformamide. Gastroenterology. 1990;99:748–757. doi: 10.1016/0016-5085(90)90964-3. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD., Jr Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- Ghadially FN. Melbourne: Butterworth-Heinemann,; Ultrastructural pathology of the cell matrix. 1997:p 327. [Google Scholar]
- Ghadially FN, Block HJ. Oncocytic carcinoid of the lung. J Submicrosc Cytol. 1985;17:435–442. [PubMed] [Google Scholar]
- Datta S, Bhattacharyya P. Effect of a herbal protein CI-1, purified from Cajanus indicus on the ultrastructural study of hepatocytes, in models of liver failure in mice. J Ethnopharmacol. 2001;77:11–18. doi: 10.1016/s0378-8741(01)00244-6. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- Brunnert SR. Morphologic response of myocardium to freeze-thaw injury in mouse strains with dystrophic cardiac calcification. Lab Anim Sci. 1997;47:11–18. [PubMed] [Google Scholar]
- Freundlich IM, Lind TA. Calcification of the heart and great vessels. CRC Crit Rev Clin Radiol Nucl Med. 1975;6:171–216. [PubMed] [Google Scholar]
- Sood V, Chakravarti RN. Systemic stress in the production of cardiac thrombosis in hypercholesterolaemic rats. Res Exp Med (Berl) 1976;167:31–45. doi: 10.1007/BF02180286. [DOI] [PubMed] [Google Scholar]
- Dillon AM, Stein HB, English RA. Splenic atrophy in systemic lupus erythematosus. Ann Intern Med. 1982;96:40–43. doi: 10.7326/0003-4819-96-1-40. [DOI] [PubMed] [Google Scholar]
- Muller AF, Cornford E, Toghill PJ. Splenic function in inflammatory bowel disease: assessment by differential interference microscopy and splenic ultrasound. Q J Med. 1993;86:333–340. [PubMed] [Google Scholar]
- Muller AF, Toghill PJ. Splenic function in alcoholic liver disease. Gut. 1992;33:1386–1389. doi: 10.1136/gut.33.10.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Takeyama Y, Ueda T, Takase K, Nishikawa J, Kuroda Y. Splenic atrophy in experimental severe acute pancreatitis. Pancreas. 2002;24:365–372. doi: 10.1097/00006676-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Tschudy DP, Hess RA, Frykholm BC, Blaese RM. Immunosuppressive activity of succinylacetone. J Lab Clin Med. 1982;99:526–532. [PubMed] [Google Scholar]
- Winkelstein A, Hess RA, Leichtling KD, Jackson MO, Blaese RM, Weaver LD. Inhibition of human lymphoproliferative responses and altered lymphocyte membrane phenotype by succinylacetone. Immunopharmacology. 1992;24:161–170. doi: 10.1016/0162-3109(92)90072-k. [DOI] [PubMed] [Google Scholar]
- Grenier A, Lescault A, Laberge C, Gagne R, Mamer O. Detection of succinylacetone and the use of its measurement in mass screening for hereditary tyrosinemia. Clin Chim Acta. 1982;123:93–99. doi: 10.1016/0009-8981(82)90117-6. [DOI] [PubMed] [Google Scholar]
- Aponte JL, Sega GA, Hauser LJ, Dhar MS, Withrow CM, Carpenter DA, Rinchik EM, Culiat CT, Johnson DK. Point mutations in the murine fumarylacetoacetate hydrolase gene: animal models for the human genetic disorder hereditary tyrosinemia type 1. Proc Natl Acad Sci USA. 2001;98:641–645. doi: 10.1073/pnas.98.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz J, Johansson S, Lindblad B, Lindstedt S, Zetterstrom R. Exertion of delta-aminolevulinic acid in hereditary tyrosinemia. Clin Chim Acta. 1969;23:257–263. doi: 10.1016/0009-8981(69)90040-0. [DOI] [PubMed] [Google Scholar]
- Cicmanec JL, Condie LW, Olson GR, Wang SR. 90-Day toxicity study of dichloroacetate in dogs. Fundam Appl Toxicol. 1991;17:376–389. doi: 10.1016/0272-0590(91)90227-u. [DOI] [PubMed] [Google Scholar]
- Schulze A, Frommhold D, Hoffmann GF, Mayatepek E. Spectrophotometric microassay for delta-aminolevulinate dehydratase in dried-blood spots as confirmation for hereditary tyrosinemia type I. Clin Chem. 2001;47:1424–1429. [PubMed] [Google Scholar]
- Lantum HB, Cornejo J, Pierce RH, Anders MW. Perturbation of maleylacetoacetic acid metabolism in rats with dichloroacetic acid-induced glutathione transferase zeta deficiency. Toxicol Sci. 2003;74:192–202. doi: 10.1093/toxsci/kfg104. [DOI] [PubMed] [Google Scholar]
- Anderson WB, Board PG, Gargano B, Anders MW. Inactivation of glutathione transferase zeta by dichloroacetic acid and other fluorine-lacking alpha-haloalkanoic acids. Chem Res Toxicol. 1999;12:1144–1149. doi: 10.1021/tx990085l. [DOI] [PubMed] [Google Scholar]
- Lantum HB, Baggs RB, Krenitsky DM, Board PG, Anders MW. Immunohistochemical localization and activity of glutathione transferase zeta (GSTZ1–1) in rat tissues. Drug Metab Dispos. 2002;30:616–625. doi: 10.1124/dmd.30.6.616. [DOI] [PubMed] [Google Scholar]
- Lantum HB, Liebler DC, Board PG, Anders MW. Alkylation and inactivation of human glutathione transferase zeta (hGSTZ1–1) by maleylacetone and fumarylacetone. Chem Res Toxicol. 2002;15:707–716. doi: 10.1021/tx025503s. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-Jun and c-Myc protooncogenes in mouse liver: prevention by methionine. Toxicol Sci. 2000;54:399–407. doi: 10.1093/toxsci/54.2.399. [DOI] [PubMed] [Google Scholar]
- Tao L, Kramer PM, Ge R, Pereira MA. Effect of dichloroacetic acid and trichloroacetic acid on DNA methylation in liver and tumors of female B6C3F1 mice. Toxicol Sci. 1998;43:139–144. doi: 10.1006/toxs.1998.2449. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]