Abstract
There is a high prevalence of β-lactam- and macrolide-resistant Streptococcus pneumoniae in Taiwan. To understand the in vitro susceptibilities of recent isolates of S. pneumoniae to fluoroquinolones and telithromycin (which is not available in Taiwan), the MICs of 23 antimicrobial agents for 936 clinical isolates of S. pneumoniae isolated from different parts of Taiwan from 2000 to 2001 were determined by the agar dilution method. Overall, 72% of isolates were not susceptible to penicillin (with 61% being intermediate and 11% being resistant) and 92% were resistant to erythromycin. Telithromycin MICs were ≥1 μg/ml for 16% of the isolates, and for 99% of these isolates the MICs of all macrolides tested were ≥256 μg/ml; all of these isolates had the constitutive macrolide-lincosamide-streptogramin B phenotype. Eighty-eight percent of the isolates were resistant to three or more classes of drugs. The ciprofloxacin MICs were ≥4 μg/ml for six (0.6%) isolates from five patients collected in 2000 and 2001, and the levofloxacin MICs were ≥8 μg/ml for five of these isolates. Seven isolates for which ciprofloxacin MICs were ≥4 μg/ml, including one isolate recovered in 1999, belonged to three serotypes (serotype 19F, five isolates; serotype 23A, one isolate; and serotype 23B, one isolate). The isolates from the six patients for which ciprofloxacin MICs were ≥4 μg/ml had different pulsed-field gel electrophoresis profiles and random amplified polymorphic DNA patterns, indicating that no clonal dissemination occurred over this time period. Despite the increased rate of fluoroquinolone use, the proportion of pneumococcal isolates for which ciprofloxacin MICs were elevated (≥4 μg/ml) remained low. However, the occurrence of telithromycin resistance is impressive and raises concerns for the future.
For decades, the worldwide increase in the incidence of Streptococcus pneumoniae resistance to penicillin, other β-lactams, and macrolides has had a strong influence on antimicrobial therapy for pneumococcal diseases (11, 38). This problem is further complicated by the worldwide emergence of resistance to some fluoroquinolones (levofloxacin, moxifloxacin, gatifloxacin, and gemifloxacin), which have been developed in the hope of alleviating this problem (3, 7, 8, 12, 25, 32, 35, 38). The Infectious Diseases Society of America and the Centers for Disease Control and Prevention have recommended the use of some of these agents as initial treatment or the alternative treatment of choice for community-acquired pneumonia (1, 11). Although levofloxacin resistance among S. pneumoniae is rare (≤1%), a fourfold increase in the proportion of levofloxacin-resistant isolates was seen in the United States (from 0.2% in 1997 and 1998 to 0.8% in 1999) (26, 28). It should be noted, however, that these data are based on studies with two different study populations (26, 28). Moreover, a high prevalence of levofloxacin resistance (5.5%) has been reported in Hong Kong (12). The occurrence of levofloxacin-resistant S. pneumoniae strains appears to be related to selective pressure resulting from the increasing rates of fluoroquinolone (ciprofloxacin and levofloxacin) use (6, 12, 26).
In Taiwan, the rate of resistance to penicillin, other β-lactam antibiotics, trimethoprim-sulfamethoxazole, and macrolides among S. pneumoniae isolates is remarkably high (4, 10, 15-17, 30). Ciprofloxacin has been available since 1990, and levofloxacin was introduced in 2000. The latter agent has been included in the list of antibiotics in Taiwanese guidelines for the treatment of community-acquired pneumonia (23). In Taiwan, the annual cost of fluoroquinolones was $3.4 million (U.S. dollars) in 1994 (ranked fourth, by antibiotic class, after cephalosporins, penicillins, and macrolides), and this increased to $4.6 million in 1998 (ranked third, after cephalosporins and penicillins) (2). Although high incidences of ofloxacin and ciprofloxacin resistance have been observed among many common bacterial pathogens (18, 20), only one clinical isolate of S. pneumoniae has been shown to be resistant to these fluoroquinolones, and that isolate was reported in 1999 (21).
To better understand the impact of the increasing rate of fluoroquinolone use on the possible emergence of fluoroquinolone resistance and the susceptibilities of S. pneumoniae strains with preexisting high levels of β-lactam and macrolide resistance to the ketolide telithromycin, we examined the in vitro activities of newer fluoroquinolones, telithromycin, and other antibiotics against isolates recently collected from different parts of Taiwan. The serotypes and the genetic relatedness of isolates resistant to ciprofloxacin (MICs, ≥4 μg/ml) were determined.
(This study was a part of the Surveillance from Multicenter Antimicrobial Resistance in Taiwan program conducted in 2001.)
MATERIALS AND METHODS
Isolates.
From January 2000 to December 2001, a total of 936 S. pneumomiae isolates were collected for study. These isolates were recovered from various clinical specimens from patients treated at nine medical centers (each center has from 800 to 3,000 beds) located in different cities in Taiwan. These hospitals included National Taiwan University Hospital (NTUH), Taipei (478 isolates); Chang-Gung Memorial Hospital (CGMH), LinKou (94 isolates); Mackay Memorial Hospital (MMH), Taipei (80 isolates); Tri-Service General Hospital (TSGH), Taipei (25 isolates); Taichung Veterans General Hospital (VGH-Taichung), Taichung (76 isolates); China Medical College Hospital (CMCH), Taichung (36 isolates); National Cheng-Kung University Hospital (CKUH), Tainan (26 isolates); Chi-Mei Medical Center (CMMC), Tainan (36 isolates); and Kaohsiung Veterans General Hospital (VGH-Kaohsiung), Kaohsiung (83 isolates). Among these isolates, 198 (20%) were recovered from normally sterile body sites (i.e., they were invasive isolates from, e.g., blood, cerebrospinal fluid, peritoneal fluid, joint fluid, or pleural fluid), while the rest were isolated from respiratory tract secretions. Among these isolates, 206 were recovered in 2000 and 730 were recovered in 2001. All isolates were further identified by conventional methods at NTUH.
Resistance trends.
Susceptibilities to penicillin and erythromycin were determined by the routine disk diffusion method for the 1,768 isolates of S. pneumoniae recovered from patients treated at NTUH from January 1984 to December 2001. For penicillin susceptibility testing, a 10-U penicillin disk was used from 1984 to 1989 and a 1-μg oxacillin disk was used after 1990 (18). Trends for dilution susceptibility to antimicrobial agents, including fluoroquinolones, for isolates recovered from various parts of Taiwan from 1996 to 2001 were also analyzed on the basis of the results of two previous studies and this study (16, 17).
Antimicrobial susceptibility testing.
The following antimicrobial agents were provided by their manufacturers for use in this study: penicillin, erythromycin, clindamycin, tetracycline, chloramphenicol, and rifampin (Sigma Chemical Co., St. Louis, Mo.); amoxicillin-clavulanate and gemifloxacin (GlaxoSmithKline, Greenford, United Kingdom); vancomycin (Eli Lilly & Co., Indianapolis, Ind.); cefotaxime, cefpirome, teicoplanin, quinupristin-dalfopristin, and telithromycin (Aventis Pharma, Romainville, France); cefepime and gatifloxacin (Bristol-Myers Squibb, Princeton, N.J.); imipenem and ertapenem (Merck Sharp & Dohme, Rahway, N.J.); meropenem (Sumitomo Pharmaceuticals, Tokyo, Japan); azithromycin (Pfizer Inc., New York, N.Y.); tigecycline (Wyeth-Ayerst, Pearl River, N.Y.); linezolid (Pharmacia, Kalamazoo, Mich.); levofloxacin and sitafloxacin (Daiichi Pharmaceuticals, Tokyo, Japan); and ciprofloxacin and moxifloxacin (Bayer Co., Leverkusen, Germany).
The MICs of these agents for all 936 S. pneumoniae isolates were determined by the agar dilution method and were interpreted according to the guidelines established by the National Committee for Clinical Laboratory Standards (33, 34). The isolates were grown overnight on Trypticase soy agar plates supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) at 37°C in ambient air. Bacterial inocula were prepared by suspending the freshly grown bacteria in sterile normal saline and adjusting the suspension to a 0.5 McFarland standard. For susceptibility testing of these isolates, we used Mueller-Hinton agar supplemented with 5% sheep blood (BBL Microbiology Systems). By using a Steers replicator, an organism density of 104 CFU/spot was inoculated onto the appropriate plate with various concentrations of antimicrobial agents. The following organisms were included as control strains: Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and S. pneumoniae ATCC 49619.
For comparison, the susceptibilities of 60 isolates recovered from 1996 to 1999 (15 isolates per year) which had been reported previously were redetermined by agar dilution methods for comparison of the data with those for isolates collected in 2000 and 2001. Susceptibility data for isolates collected over the four time periods, 1996-1997 (16), 1998-1999 (17), and 2000 and 2001 (this study) were also compared.
Erythromycin-resistant phenotypes.
The resistance phenotypes of the erythromycin-resistant S. pneumoniae (ERSP) isolates were determined by the double-disk test with an erythromycin disk (15 μg) and a clindamycin disk (2 μg) (BBL Microbiology Systems) as described previously (22). A blunting of the clindamycin zone of inhibition proximal to the erythromycin disk for clindamycin-susceptible isolates indicated an inducible macrolide-lincosamide-streptogramin B phenotype. Resistance to both clindamycin and erythromycin without blunting of the clindamycin zone of inhibition was interpreted as the constitutive macrolide-lincosamide-streptogramin B (cMLSB) phenotype. A macrolide resistance phenotype (M phenotype) was characterized by susceptibility to clindamycin without blunting of the inhibition zone around the erythromycin disk.
Serotyping.
The serotypes of isolates resistant to ciprofloxacin were determined by the capsular swelling method (Quellung reaction) (16). All antisera were obtained from the Statens Seruminstitut (Copenhagen, Denmark).
Genotyping.
The genetic relatedness of the six isolates for which ciprofloxacin MICs were ≥4 μg/ml, the six isolates for which ciprofloxacin MICs were ≤2 μg/ml (four serotype 19F isolates and two serotype 23F isolates), and the two control strains (S. pneumoniae ATCC 49619 and the Spanish serotype 23F strain) was identified by the random amplified polymorphic DNA (RAPD) patterns generated by arbitrarily primed PCR and pulsed-field gel electrophoresis (PFGE), as described previously (19). The three random primers used in arbitrarily primed analysis were M13, ERIC1, and OPA-7 (Operon Technologies, Inc., Alameda, Calif.). The restriction enzyme used for PFGE analysis was SmaI. Interpretation of RAPD patterns and PFGE profiles (pulsotypes) was in accordance with previously described criteria (19, 37).
PCR and DNA sequencing.
The gyrA, gyrB, parA, and parC DNA fragments of the six ciprofloxacin-resistant S. pneumoniae isolates, S. pneumoniae ATCC 49619, and the Spanish serotype 23F strains were amplified and sequenced as described previously (21). These data were compared to that for a ciprofloxacin-resistant strain (strain SP39) found in 1999 (21).
RESULTS
Penicillin and erythromycin nonsusceptibility trends at a university hospital, 1984 through 2001.
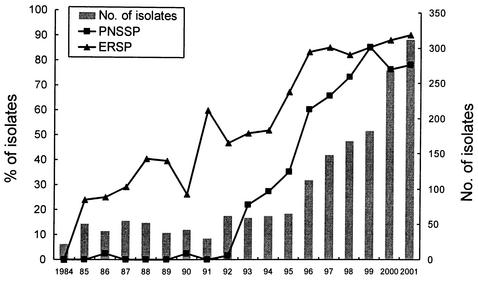
Figure 1 shows the annual incidences of penicillin-nonsusceptible S. pneumoniae (PNSSP) and ERSP isolates among the 1,768 isolates of S. pneumoniae obtained from 1984 to 2001 at NTUH. The number of organisms rose remarkably beginning in 1996, ranging from less than 70 before 1995 to more than 300 in 2001. The incidence of PNSSP rose markedly beginning in 1993 and peaked (86%) in 1999 before decreasing in 2000. A stepwise rise in the incidence of ERSP has also been seen since 1985 and reached a plateau (80 to 90%) from 1996 to 2001.
FIG. 1.
Penicillin nonsusceptibility (PNSSP) and erythromycin resistance (ERSP) trends determined by the routine disk diffusion method for 1,768 isolates of S. pneumoniae recovered from patients treated at the National Taiwan University Hospital from January 1984 to December 2001.
Dilution susceptibility data for isolates collected in 2000 and 2001.
As described in Table 1, 72% of the isolates were not susceptible to penicillin, with 61% (566 isolates) being intermediate (penicillin-intermediate S. pneumoniae [PISP]; MICs, 0.12 to 1 μg/ml) and with 11% (106 isolates) having a high level of resistance (penicillin-resistant S. pneumoniae [PRSP]; MICs, ≥2 μg/ml). Penicillin MICs were ≥4 μg/ml for 3% of the isolates. Among the invasive isolates, 68% were not susceptible to penicillin (PISP, 59%; PRSP, 9%). Seventy-three percent of the noninvasive isolates were not susceptible to penicillin (PISP, 61%; PRSP, 12%). Among the 428 isolates recovered from children (ages, ≤16 years), 88% were not susceptible to penicillin (PISP, 77%; PRSP, 11%). Sixty percent of the 508 isolates recovered from patients aged ≥17 years were not susceptible to penicillin (PISP, 47%; PRSP, 13%).
TABLE 1.
In vitro susceptibilities of 936 clinical S. pneumoniae isolated from 2000 to 2001 in Taiwan determined by the agar dilution method
| Antibiotic | MIC (μg/ml)a
|
% of isolatesb
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | I | R | |
| Penicillin | ≤0.03-4 | 0.5 | 2 | 28 | 61 | 11 |
| Amoxicillin-clavulanate | ≤0.03-4 | 0.5 | 1 | 98 | 1 | 1 |
| Cefotaximec | ≤0.03-4 | 0.5 | 1 | 68 (98) | 30 (1) | 2 (1) |
| Cefpirome | ≤0.03-2 | 0.5 | 1 | —d | — | — |
| Cefepimec | ≤0.03-8 | 1 | 1 | 51 (96) | 46 (3) | 3 (1) |
| Imipenem | ≤0.03-1 | 0.12 | 0.25 | 87 | 12 | 1 |
| Meropenem | ≤0.03-2 | 0.25 | 0.5 | 72 | 26 | 2 |
| Ertapenem | ≤0.03-4 | 0.25 | 0.5 | 98 | 1 | 1 |
| Tigecycline | ≤0.03-1 | ≤0.03 | 0.06 | — | — | — |
| Erythromycin | 0.12->128 | >128 | >128 | 6 | 2 | 92 |
| Azithromycin | 0.25->128 | >128 | >128 | 6 | 2 | 92 |
| Clindamycin | ≤0.03->128 | >128 | >128 | 38 | 1 | 61 |
| Chloramphenicol | ≤0.03-32 | 4 | 16 | 69 | 0 | 31 |
| Trimethoprim-sulfamethoxazole | 4->128 | 128 | >128 | 14 | 16 | 70 |
| Vancomycin | ≤0.03-1 | 0.25 | 0.5 | 100 | 0 | 0 |
| Teicoplanin | ≤0.03-0.25 | 0.12 | 0.12 | — | — | — |
| Quinupristin-dalfopristin | ≤0.03-8 | 1 | 2 | 71 | 23 | 6 |
| Linezolid | ≤0.03-2 | 1 | 1 | 100 | 0 | 0 |
| Ciprofloxacin | ≤0.03-≥64 | 1 | 2 | — | — | — |
| Levofloxacin | ≤0.03-32 | 1 | 2 | 99.4 | 0.1 | 0.5 |
| Moxifloxacin | ≤0.03-4 | 0.12 | 0.25 | 99.9 | 0.1 | 0.0 |
| Gatifloxacin | ≤0.03-8 | 0.25 | 0.25 | 99.6 | 0.2 | 0.2 |
| Gemifloxacin | ≤0.03-0.5 | ≤0.03 | ≤0.03 | — | — | — |
| Sitafloxacin | ≤0.03-1 | ≤0.03 | 0.06 | — | — | — |
| Telithromycin | ≤0.03-4 | 0.12 | 1 | 85e | 14 | 1 |
50% and 90%, MICs at which 50 and 90% of isolates, respectively, are inhibited.
S, susceptible; I, intermediate; R, resistant.
Susceptibility data for the treatment of meningitis (nonmeningitis) according to the interpretive criteria recommended by NCCLS (34).
—, no interpretive criteria provided by NCCLS.
Susceptibility data for telithromycin according to the European MIC breakpoints, i.e., susceptible, ≤0.5 μg/ml; intermediate, 1 to 2 μg/ml; and resistant, ≥4 μg/ml.
Erythromycin-resistant isolates (MICs, ≥1 μg/ml) and clindamycin-resistant isolates (MICs, ≥1 μg/ml) were common (92 and 61%, respectively). Thirty-three percent of the erythromycin-resistant isolates had the M phenotype. The erythromycin-resistant M phenotype was more common among PRSP isolates (46%) than among PSSP isolates (20%). Seventy-nine percent of the PRSP isolates also were not susceptible to cefotaxime.
Ciprofloxacin MICs were ≥4 μg/ml for six isolates (0.6%). Among these six isolates, all isolates (0.6%), four isolates (0.4%), and one isolate (0.1%) were also not susceptible to levofloxacin (MICs, ≥4 μg/ml), gatifloxacin (MICs, ≥4 μg/ml), and moxifloxacin (MIC, ≥4 μg/ml), respectively. All ciprofloxacin-resistant isolates had intermediate or high-level resistance to penicillin (Table 2). Levofloxacin, gatifloxacin, and moxifloxacin MICs were not ≥4 μg/ml for any of the PSSP isolates. Among the six fluoroquinolones tested, gemifloxacin and sitafloxacin were the most active, and all isolates were inhibited by 0.5 μg of gemifloxacin per ml and 1 μg of sitafloxacin per ml.
TABLE 2.
Characteristics of seven S. pneumoniae isolates for which the ciprofloxacin MIC was ≥4 μg/ml recovered from six patients in Taiwan from 1998 to 2001a
| Patient no. (location) | Age/sex | Underlying disease(s) | Clinical disease(s) | Previous exposure to quinolones | Isolate designation | Source | Date (day/mo/yr) | MIC (μg/ml)
|
Serotype/RAPD profile/PFGE profile | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | CIP | LVX | MOX | EM | TEL | |||||||||
| 1 (N) | 65/M | No | Colonization | No | A | Sputum | 19/11/1999 | 4 | 32 | 16 | 2 | >256 | 2 | 19F/a/I |
| 2 (N) | 68/F | COPD | Pneumonia | No | B | Sputum | 7/8/2000 | 1 | 16 | 16 | 4 | 32 | 0.25 | 23A/b/II |
| 3 (N) | 76/M | COPD | AECB | No | C | Sputum | 5/6/2001 | 2 | >32 | 32 | 2 | >128 | 0.03 | 23F/c/III |
| 4 (N) | 3/F | No | Bacteremia, pneumonia | No | D | Blood | 13/10/2001 | 1 | 4 | 4 | 1 | >128 | 0.5 | 19F/d/IV |
| 5 (S) | 79/M | DM, ESRD | Bacteremia, pneumonia | No | E | Blood | 3/11/2001 | 1 | 32 | 8 | 2 | 32 | 0.12 | 19F/e/V |
| 6 (C) | 72/M | DM, ESRD | Pneumonia | No | F | Sputum | 12/11/2001 | 2 | 8 | 8 | 2 | 32 | 0.12 | 19F/f/VI |
| Bacteremia | No | G | Blood | 8/12/2001 | 1 | 8 | 8 | 1 | 32 | 0.06 | 19F/f/VI | |||
Abbreviations: N, northern Taiwan; C, central Taiwan; S, southern Taiwan; F, female; M, male; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESRD, end-stage renal disease; AECB, acute exacerbation of chronic bronchitis; CIP, ciprofloxacin; EM, erythromycin; LVX, levofloxacin; MOX, moxifloxacin; PEN, penicillin; TEL, telithromycin.
Of the other agents tested, resistance to trimethoprim-sulfamethoxazole (MICs, ≥2 μg/ml for trimethoprim and 76 μg/ml for sulfamethoxazole) was the most common (76%). Some isolates (<5%) were not susceptible to extended-spectrum cephalosporins (according to the criteria for isolates from patients with meningitis or without meningitis) or carbapenems, and 8% of the isolates were resistant to quinupristin-dalfopristin. All isolates were susceptible to vancomycin (MICs, ≤1 μg/ml) and linezolid (MICs, ≤2 μg/ml).
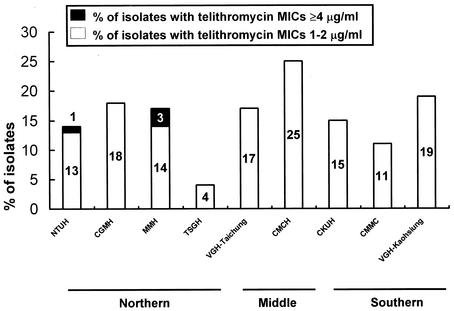
All isolates were inhibited by telithromycin at 4 μg/ml and tigecycline at 1 μg/ml. Telithromycin MICs ranged from 1 to 2 μg/ml for 15% of isolates, and telithromycin MICs were 4 μg/ml for 1% of the isolates. The MICs of all macrolides tested were ≥256 μg/ml for nearly all isolates (98.6%; 139 of 141 isolates) for which telithromycin MICs were ≥1 μg/ml; all of these isolates had the cMLSB phenotype. Isolates for which telithromycin MICs were ≥1 μg/ml were widely distributed in different parts of Taiwan, with a higher prevalence (20.5%) in the middle of Taiwan (Fig. 2). Among the 141 isolates for which telithromycin MICs were ≥1 μg/ml, 64.5% (91 isolates) were recovered in 2000 and 85.1% (120 isolates) were isolated from respiratory secretions. Telithromycin MICs were ≤0.5 μg/ml for all ciprofloxacin-resistant isolates. Eighty-eight percent of the isolates were resistant to three or more classes of drugs.
FIG. 2.
Geographic distribution of isolates for which telithromycin MICs were ≥1 μg/ml recovered from nine major hospitals located in different parts of Taiwan.
Islandwide trends in pneumococcal resistance, 1996 through 2001.
The penicillin and cefotaxime MICs for the 60 isolates evaluated in this study were identical or within ±1 dilution of the values reported from previous studies (16, 17). From 1996-1997 to 2001 (1,403 isolates), the proportion of isolates that were intermediate to penicillin increased from 28 to 60%, but the proportion of those highly resistant to penicillin decreased from 33 to 11%. The proportions of isolates intermediate (54% in 1998-1999, 39% in 2000, and 28% in 2001) or resistant (22% in 1996-1997 and 2% in both 2000 and 2001) to cefotaxime also declined.
Serotypes and genotypes of ciprofloxacin-resistant isolates.
Among the six ciprofloxacin-resistant isolates found in 2000-2001 and the one ciprofloxacin-resistant isolate found in 1999 (isolate SP39), five belonged to serotype 19F. Isolates from different patients had different RAPD patterns and PFGE profiles (Table 2). The RAPD patterns and pulsotypes of the seven ciprofloxacin-resistant isolates were different from those of the ciprofloxacin-susceptible isolates as well as that of the Spanish serotype 23F strain. The gyrA sequence data for the six isolates (isolates B to G) were identical to those for SP39, i.e., nucleotide substitutions (TCC to TTC) resulting in an amino acid change from Ser-81 to Phe in gyrA. For parC, two isolates (isolates B and E) had substitutions resulting in a change from Ser-79 to Phe, one isolate (isolate C) had substitutions resulting in both a change from Ser-79 to Phe and a change from Lys-137 to Asn, and the other three isolates (isolates D, F, and G) had no amino acid changes in parC.
Fluoroquinolone use and fluoroquinolone resistance.
The levels of consumption of the three major quinolones (norfloxacin, ofloxacin, and ciprofloxacin) increased from 1.28 tons in 1997 to 1.97 tons in 2001, and the annual level of levofloxacin consumption increased threefold from 2000 (0.33 tons) to 2001 (0.96 tons). The first isolate for which the ciprofloxacin MIC was ≥4 μg/ml (the levofloxacin MICs are unknown) was described in 1996. Despite the increased level of fluoroquinolone use, the proportions of pneumococcal isolates for which ciprofloxacin MICs were ≥4 μg/ml or for which levofloxacin MICs were ≥ 8 μg/ml remained low, ranging from 0.4 to 0.7% from 1996 to 2001 for ciprofloxacin and 0.4 to 0.6% from 1998 to 2001 for levofloxacin (Table 3).
TABLE 3.
Secular trend of ciprofloxacin and levofloxacin susceptibility among clinical S. pneumoniae isolates in Taiwan from 1996 to 2001
| Year | No. of isolates tested | No. (%) of isolates for which:
|
|
|---|---|---|---|
| Ciprofloxacin MICs were ≥4 μg/ml | Levofloxacin MICs were ≥8 μg/ml | ||
| 1996-1997 | 200 | 1 (0.5) | —a |
| 1998-1999 | 276 | 1 (0.4) | 1 (0.4) |
| 2000 | 206 | 1 (0.5) | 1 (0.5) |
| 2001 | 730 | 5 (0.7) | 4 (0.6) |
—, data not available.
Characteristics of patients harboring ciprofloxacin-resistant S. pneumoniae isolates.
Among the six patients seen in 2000-2001whose specimens were positive by culture for ciprofloxacin-resistant S. pneumoniae, five were adults and none had been exposed to any quinolones within the 2 months prior to the isolation of the resistant isolates (Table 2). One patient (patient 6) had two episodes of infections due to the same clones of ciprofloxacin-resistant S. pneumoniae. None of the five patients infected with these resistant isolates received treatment with quinolones. All the isolates were susceptible to telithromycin (MICs, ≤0.5 μg/ml).
DISCUSSION
Four aspects of this study of recent S. pneumoniae isolates from Taiwan are of particular interest. First, for a significant proportion of Taiwan isolates, particularly those with high-level resistance to macrolides (cMLSB phenotype), the telithromycin MICs were high (≥1 μg/ml). Moreover, these isolates were widely distributed throughout Taiwan. Second, despite the increasing rates of use of fluoroquinolones (particularly ciprofloxacin, ofloxacin, and levofloxacin) in clinical settings, the occurrence of ciprofloxacin and/or levofloxacin resistance among S. pneumoniae isolates did not dramatically increase. The strains most likely to be fluoroquinolone resistant were also resistant to macrolides and penicillin. Third, data from NTUH and the islandwide surveillance revealed that the proportions of both PNSSP and macrolide-resistant S. pneumoniae isolates plateaued at a high level, while the proportion of high-level penicillin-resistant isolates (penicillin MICs, ≥2 μg/ml) appeared to decline. The latter finding strongly indicates that penicillin remains the drug of choice for the treatment of infections other than meningitis due to S. pneumoniae in Taiwan (23). Finally, the high degree of polymorphism among our ciprofloxacin-resistant isolates indicates that clonal dissemination of resistant isolates does not exist.
The fact that a significant proportion of the isolates evaluated in this study were not susceptible to telithromycin is of great concern, because this agent is not yet available in Taiwan. Previous studies have shown that telithromycin is very active against macrolide-susceptible and -resistant S. pneumoniae isolates, irrespective of the macrolide resistance mechanisms, that is, whether the isolates are erm(B) positive with the MLSB phenotype or mef(A) positive with the M phenotype (5, 9, 31). The seemingly excellent activity of telithromycin makes this agent a promising alternative for the treatment of infections caused by S. pneumoniae when macrolides are indicated (9). However, several reports have demonstrated that telithromycin has decreased activities against S. pneumoniae and Streptococcus pyogenes isolates with erm(B)-mediated macrolide resistance (MICs, up to 1 and up to 64 μg/ml, respectively) (5, 9, 24, 31). The telithromycin MICs for S. pneumoniae mutants selected in vitro as a result of exposure to telithromycin were found to be high (up to 8 μg/ml) (5). Given the persistently increasing selective pressure for macrolide resistance by the use of macrolides in the community in Taiwan as well as the high proportion of macrolide-resistant isolates with the MLSB phenotype (18, 22), the need for cautious and judicious use of these agents for the treatment of pneumococcal diseases is warranted in the future. Further studies should be conducted to characterize these isolates regarding their mechanisms of resistance to telithromycin and possibly their clonalities.
The prevalence of fluoroquinolone resistance among S. pneumoniae isolates in Taiwan is relatively low compared to that among such isolates in Hong Kong and is similar to that among such isolates in the United States (3, 12-14, 27, 29, 36). In Hong Kong, a fluoroquinolone-resistant serotype 23F clone which is either identical or closely related to the Spanish 23F clone is already widespread (14). In contrast, the data presented here suggest that the ciprofloxacin-resistant isolates were genetically diverse, and clonal dissemination of these resistant isolates was not found.
In summary, Taiwan has become an epicenter for pneumococci resistant to macrolides, penicillin, and other β-lactam antibiotics. Restriction of outpatient antibiotic use through government regulations would be expected to contribute to the alleviation of the growing rates of preexisting resistance. However, some resistant domestic pneumococcal clones have further developed resistance to fluoroquinolones, and increasing numbers of isolates exhibiting high-level resistance to macrolides appear to be resistant to telithromycin, a drug that has not been used clinically in Taiwan. Thus, the implementation of appropriate measures and intervention strategies to reduce the inappropriate use of antibiotics appears to be warranted.
Acknowledgments
This work was supported in part by the National Science Council (grants NSC91-2314-B-002-171 and NSC 90-2314-B002-290) and the Center for Disease Control, Department of Health (grant DOH92-DC-115), Taiwan.
The authors are members of the SMART Study Group.
REFERENCES
- 1.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, Jr., D. M. Musher, and A. M. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, S. C., Y. C. Chen, and O. Y. P. Hu. 2001. Antibiotic use in public hospitals in Taiwan after the implementation of National Health Insurance. J. Formos. Med. Assoc. 100:151-161. [PubMed] [Google Scholar]
- 3.Chen, D. K., A. McGeer, J. C. De Azavedo, and D. E. Low for the Canadian Bacterial Surveillance Network. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Chiou, C. C. C., and M. C. McEllistrem. 2001. Novel penicillin-, cephalosporin-, and macrolide-resistant clones of Streptococcus pneumoniae serotypes 23F and 19F in Taiwan which differ from international epidemic clones. J. Clin. Microbiol. 39:1144-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, T. A., B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro development of resistance to telithromycin (HMR 3647), four macrolides, clindamycin, and pristinamycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:414-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., A. B. Brueggemann, and G. V. Doern. 2000. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg. Infect. Dis. 6:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doern, G. V., M. A. Pfaller, M. E. Erwin, A. B. Bruggemann, and R. N. Jones. 1998. The prevalence of fluoroquinolone resistance among clinically significant respiratory tract isolates of Streptococcus pneumoniae in the United States and Canada—1997 results from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 32:313-316. [DOI] [PubMed] [Google Scholar]
- 8.Empey, P. E., H. R. Jennings, A. C. Thomton, R. P. Rapp, and M. E. Evans. 2001. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann. Pharmacother. 35:687-690. [DOI] [PubMed] [Google Scholar]
- 9.Felmingham, D. 2001. Microbiological profiles of telithromycin, the first ketolide antimicrobial. Clin. Microbiol. Infect. 7(Suppl. 3):2-10. [PubMed] [Google Scholar]
- 10.Fung, C. P., B. S. Hu, S. C. Lee, P. Y. Liu, T. N. Jang, H. S. Leu, B. I. Kuo, M. Y. Yen, C. Y. Liu, Y. C. Liu, Y. J. Lau, and K. W. Yu. 2000. Antimicrobial resistance of Streptococcus pneumoniae isolated in Taiwan. An island-wide surveillance study between 1996 and 1997. J. Antimicrob. Chemother. 45:49-55. [DOI] [PubMed] [Google Scholar]
- 11.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schachat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 12.Ho, P. L., T. L. Que, D. N. C. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, P. L., W. S. Tse, K. W. Tsang, T. K. Kwok, T. K. Ng, V. C. Cheng, and R. M. Chan. 2001. Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: a case-control study. Clin. Infect. Dis. 32:701-707. [DOI] [PubMed] [Google Scholar]
- 14.Ho, P. L., W. C. Yam, T. K. Cheung, W. W. Ng, T. L. Que, D. N. Tsang, T. K. Ng, and W. H. Seto. 2001. Fluoroquinolone resistance among Streptococcus pneumoniae in Hong Kong linked to the Spanish 23F clone. Emerg. Infect. Dis. 7:906-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh, P. R., H. M. Chen, Y. C. Lu, and J. J. Wu. 1996. Antimicrobial resistance and serotype distribution of Streptococcus pneumoniae strains isolated in southern Taiwan. J. Formos. Med. Assoc. 95:29-36. [PubMed] [Google Scholar]
- 16.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, and K. T. Luh. 1999. Extremely high incidence of macrolide and trimethoprim-sulfamethoxazole resistance among clinical isolates of Streptococcus pneumoniae in Taiwan. J. Clin. Microbiol. 37:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsueh, P. R., Y. C. Liu, J. M. Shyr, T. L. Wu, J. J. Yan, J. J. Wu, H. S. Leu, Y. C, Chuang, Y. J. Lau, and K. T. Luh. 2000. Multicenter surveillance of antimicrobial resistance of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in Taiwan during the 1998-1999 respiratory season. Antimicrob. Agents Chemother. 44:1342-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsueh, P. R., C. Y. Liu, and K. T. Luh. 2000. Current status of antimicrobial resistance in Taiwan. Emerg. Infect. Dis. 8:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, and K. T. Luh. 1999. Dissemination of high-level penicillin-, extended-spectrum cephalosporin-, and erythromycin-resistant Streptococcus pneumoniae clones in Taiwan. J. Clin. Microbiol. 37:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsueh, P. R., M. L. Chen, C. C. Sun, W. H. Chen, H. J. Pan, L. S. Yang, S. C. Chang, S. W. Ho, C. Y. Lee, W. C. Hsieh, and K. T. Luh. 2002. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in Taiwan, 1981-1999. Emerg. Infect. Dis. 8:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsueh, P. R., L. J. Teng, T. L. Wu, S. W. Ho, and K. T. Luh. 2001. First clinical isolate of Streptococcus pneumoniae exhibiting high-level resistance to fluoroquinolones in Taiwan. J. Antimicrob. Chemother. 48:316-317. [DOI] [PubMed] [Google Scholar]
- 22.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, H. C. Lue, and K. T. Luh. 2002. Increased prevalence of erythromycin resistance in streptococci: substantial upsurge in erythromycin-resistant M-phenotype in Streptococcus pyogenes (1979-1998) but not in Streptococcus pneumoniae (1985-1999) in Taiwan. Microb. Drug Resist. 8:27-33. [DOI] [PubMed] [Google Scholar]
- 23.Infectious Diseases Society of the Republic of China. 1999. Guidelines for antimicrobial therapy of pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 32:292-294. [PubMed] [Google Scholar]
- 24.Jalava, J., J. Kataja, H. Seppala, and P. Huovinen. 2001. In vitro activities of the novel telithromycin (HMR 3647) against erythromycin-resistant Streptococcus species. Antimicrob. Agents Chemother. 45:789-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, R. N., and M. A. Pfaller. 2000. In vitro activity of newer fluoroquinolones for respiratory tract infections and emerging patterns of antimicrobial resistance: data from the SENTRY Antimicrobial Surveillance Program. Clin. Infect. Dis. 31(Suppl. 2):S16-S23. [DOI] [PubMed] [Google Scholar]
- 26.Jones, R. N., and M. A. Pfaller. 2000. Macrolide and fluoroquinolone (levofloxacin) resistance among Streptococcus pneumoniae strains: significant trends from the SENTRY Antimicrobial Surveillance Program (North America, 1997-1999). J. Clin. Microbiol. 38:4298-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, M. E., A. M. Staples, I. Critchley, C. Thornsberry, P. Heinze. H. D. Engler, and D. F. Sahm. 2000. Benchmarking the in vitro activities of moxifloxacin and comparator agents against recent respiratory isolates from 377 medical centers throughout the United States. Antimicrob. Agents Chemother. 44:2645-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlowsky, J. A., L. Nealy, and D. F. Sahm. 2001. Trends in ciprofloxacin nonsusceptibility and levofloxacin resistance among Streptococcus pneumoniae isolates in North America. J. Clin. Microbiol. 39:2748-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumarasinghe, G., C. Chow, and P. A. Tambyah. 2000. The emergence of resistance to levofloxacin before clinical use in a university hospital in Singapore. J. Antimicrob. Chemother. 46:862-863. [DOI] [PubMed] [Google Scholar]
- 30.Luh, K. T., P. R. Hsueh, L. J. Teng, H. J. Pan, Y. C. Chen, J. J. Lu, J. J. Wu, and S. W. Ho. 2000. Quinupristin-dalfopristin resistance among gram-positive bacteria in Taiwan. Antimicrob. Agents Chemother. 44:3374-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morosini, M. I., R. Canton, E. Loza, M. C. Negri, J. C. Galan, F. Almaraz, and F. Baquero. 2000. In vitro activity of telithromycin against Spanish Streptococcus pneumoniae isolates with characterized macrolide resistance mechanisms. Antimicrob. Agents Chemother. 45:2427-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai, K., T. A. Davies, G. A. Pankuch, B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards, 5th ed. M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 34.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing: 13th informational supplement. M100-S13 (M7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 35.Peterson, D. E., and D. F. Sahm. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 341:1546-1548. [PubMed] [Google Scholar]
- 36.Sahm, D. F., D. E. Peterson, I. A. Critchley, and C. Thornsberry. 2000. Analysis of ciprofloxacin activity against Streptococcus pneumoniae after 10 years of use in the United States. Antimicrob. Agents Chemother. 44:2521-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover, F. C., R. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]