Abstract
Intestinal antigen uptake is enhanced in inflammatory bowel disease. We analyzed transcellular transport routes of antigens in different compartments of normal enterocytes and atypical intestinal epithelial cells called “rapid antigen uptake into the cytosol enterocytes” (RACE cells). These cells constitute a recently described population of enterocyte-derived cells, which are increased in inflammatory bowel disease. Mucosa of freshly resected specimens wereincubated with the antigens ovalbumin or horseradish peroxidase. Ultrastructural labeling patterns of differentiation-dependent proteins, the brush-border enzyme sucrase-isomaltase and the cytoskeleton proteins villin and actin, were determined in enterocytes. Apoptosis was investigated biochemically and ultrastructurally by cleavage of caspase-3. Both antigens were transported to late endosomes and to trans-Golgi vesicles of enterocytes in inflammatory bowel disease and control specimens. Quantitative evaluation revealed a significantly increased transepithelial antigen transport in both compartments of RACE relative to normal enterocytes. Labeling densities for sucrase-isomaltase, villin, and actin were decreased in RACE relative to normal enterocytes. Caspase-3 was not increased in RACE cells relative to controls. RACE cells are characterized by increased antigen transport to late endosomes and the trans-Golgi network, a disassembled cytoskeleton and lower concentrations of proteins that are markers of cell differentiation.
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBD) of unknown origin. Although several studies suggest an increase of antigen transport in IBD,1 there is limited knowledge of the transcellular mechanisms of antigen transport across the epithelial barrier in CD and UC. Electron microscopic findings in IBD indicate that structural alterations of enterocytes favor the uptake of lumenal bacteria or antigens across the intestinal barrier either through the paracellular pathway via altered tight junctions or the transcellular pathway.2–4
We have recently shown that the antigens ovalbumin (OVA) and horseradish peroxidase (HRP) are taken up at the apical membrane in CD, UC, and healthy controls (HCs) and are detected in vacuoles of enterocytes and the paracellular space within minutes after administration. Both antigens were not localized within the cytosol of normal enterocytes (NE cells). In addition to ultrastructurally NEs, we demonstrated an increased number of atypical enterocytes with a decreased concentration of mitochondrial ATP synthase, shortened microvilli, an electron lucent cytosol, and high amounts of OVA or HRP in the cytosol of both CD and UC enterocytes relative to patients without inflammatory disorders as well as patients with diverticulitis, that show similar amounts of these enterocytes. These atypical enterocytes are called “rapid antigen uptake into the cytosol enterocytes” (RACE cells). Moreover the presence of RACE cells correlated with the degree of mucosal inflammation in CD and UC.5
However, the functional significance, differentiation, origin, and fate of RACE cells are still unclear. In particular, the transport pathway of antigens through endocytic cell compartments and the mechanism of the rapid cytosolic antigen uptake of RACE cells have not yet been elucidated. This study has been conducted to characterize transepithelial antigen transport within RACE cells relative to NEs using bowel specimens of patients with CD or UC. To investigate whether cytosolic arrival of antigens follows enhanced endocytosis and intracellular release of antigens into the cytosol as shown for toxins such as ricin and Shiga toxin,6 we quantified the amount of incubated OVA and HRP transported to late endosomes and vesicles of the trans-Golgi network (TGN). To investigate whether the excessive amount of antigens within the cytosol of RACE cells reflects a direct penetration of antigens throughout the surface membrane, a transport mechanism that has been described for autoantibodies,7 we examined whether the rapid translocation of OVA and HRP into the cytosol depends on the state of differentiation and maturation of enterocytes. We also analyzed the cellular distribution and expression levels of actin because actin contributes to enterocytic cell stability and mediates endocytic traffic.8
Ultrastructural features such as marginal condensation of heterochromatin within the nuclei as well as disassembly of actin filaments point to an apoptotic nature of RACE cells. This distinct recently observed enterocyte population appears to play a major role in the immunomodulatory functions and the intestinal barrier of the IBD mucosa. This is supported by observations that antigen transport into the cytosol, late endosomes, and the TGN is markedly increased and the extrusion of RACE cells into the intestinal lumen leads to enhanced permeability of the epithelial layer, ie, a direct influx of food antigens into the lamina propria.
Materials and Methods
Patients and Tissue Samples
Mucosa was obtained from macroscopically inflamed and noninflamed areas of freshly resected ileum of five patients with CD (three men, two women; mean age, 40 years; range, 25 to 61 years) and colon of five patients with UC (four men, one woman; mean age, 35 years; range, 10 to 62 years). Tissue samples with intact epithelium were exclusively taken, thus specimens with ulcerations or fissural lesions were excluded. The diagnosis of CD and UC was based on routine clinical, endoscopic, and radiological criteria and was confirmed by preoperative histological evaluation. Indications for surgery were failure of medical treatment (two CD, four UC), colorectal carcinoma (one UC), severe stenosis (one CD), and interenteric fistula (two CD). At the time of surgery three patients in the CD group and two patients in the UC group were taking corticosteroids, two patients with CD were under 5-aminosalicylic acid therapy, and three patients with UC were taking both sulfasalazine or 5-aminosalicylic acid and corticosteroids. Normal mucosa (ileum, n = 5; colon, n = 5) without inflammation from patients resected for carcinoma (>5 cm from tumor margins) served as controls. Additionally colonic tissue from three patients resected for diverticulitis (two chronic, one acute) served as an inflammatory control.
Antibodies
Primary antibodies used in this study are summarized in Table 1. Binding sites of primary antibodies were visualized at the electron microscopic level by gold-conjugated goat anti-rabbit serum (diameter 6 and 12 nm, dilution 1:50; Dianova, Hamburg, Germany), gold-conjugated goat anti-mouse serum (diameter 6 and 12 nm, dilution 1:10; Dianova) and donkey anti-goat serum (diameter 12 nm, dilution 1:10; Dianova). For immunofluorescence microscopy a fluorescein isothiocyanate-conjugated goat anti-rabbit Ig antibody (dilution 1:10; Cappel, ICN, Eschwege, Germany), and rhodamine-conjugated goat anti-mouse Ig antibody (dilution 1:10; Cappel, ICN) were used.
Table 1.
Primary Antibodies Used in This Study
| Antibody | Host | Dilution | Distributor |
|---|---|---|---|
| Polyclonal anti-OVA | Rabbit | 1:200 (IF), 1:50 (EM) | Gift from Prof. Dr. S. Strobel, London, England |
| Polyclonal anti-HRP | Rabbit | 1:100 (IF), 1:50 (EM) | DAKO, Hamburg, Germany |
| Monoclonal anti-human CD13 (aminopeptidase) | Mouse | 1:10 (IF) | Cymbus Biotechnology, Chandlers Ford-Hants, England |
| Polyclonal anti-sucrase-isomaltase | Rabbit | 1:1000 (EM) | Gift from Prof. Dr. Zweibaum, Villejuif, France |
| Monoclonal anti-actin | Mouse | 1:100 (EM) | Cedarlane, Hornby, Ontario, Canada |
| Monoclonal anti-villin | Mouse | 1:5 (EM) | Gift from Prof. Dr. Louvard, Paris, France |
| Monoclonal anti-CD 107b (LAMP-2) | Mouse | 1:30 (EM) | Pharmingen, Hamburg, Germany |
| Ulex europaeus agglutinin I | 1:50 (EM) | Vector, Burlingame, CA | |
| anti-Ulex europaeus agglutinin I | Goat | 1:150 (EM) | Vector, Burlingame, CA |
| Monoclonal anti-caspase-3 | Rabbit | 1:10 (EM) | Pharmingen, Hamburg, Germany |
| Monoclonal anti-caspase-3 | Rabbit | 1:250 (Western) | Cell Signaling Technologies, Beverly, MA |
IF, Immunofluorescence; EM, immunoelectron microscopy.
Preparation of Frozen Sections for Immunoelectron Microscopy
The method of sampling and preparation of bowel specimens has been described recently.5 Briefly, freshly resected specimens were washed out with phosphate-buffered saline (PBS). Mucosa was stripped and samples were incubated on the luminal side with the antigens OVA (molecular weight 45 kd, 10 mg/ml; Sigma, St. Louis, MO) or HRP (molecular weight 40 kd, 100 mg/ml; Sigma) in a humid chamber for different intervals (2, 5, 10, 30, or 60 minutes). Omission of OVA and HRP was used as a negative control. Samples were fixed in 5% paraformaldehyde for 60 minutes at room temperature, reduced to small pieces of 1 mm3, infiltrated with 2.1 mol/L of sucrose for 24 hours at 4°C, and frozen in liquid nitrogen at −196°C.
Sectioning and labeling of ultrathin frozen sections was performed using the technique of Tokuyasu as described in detail elsewhere.9 Thin frozen sections (50 nm) were made with an ultracryomicrotome (Leica EM FCS, Vienna, Austria) at −110°C and placed onto mesh nickel grids. At least two tissue blocks were used per patient. Frozen sections were placed on three grids per block. After quenching with 10% fetal calf serum, sections were incubated with the respective primary antibody (45 minutes), rinsed in PBS (15 minutes), incubated with the appropriate gold-conjugated secondary antibody (45 minutes), and rinsed in PBS (30 minutes, room temperature). To prevent cross-reactivity double labeling was performed using gold-conjugated antibodies from different species. Grids were contrasted and embedded in 2% methylcellulose solution (1 ml methylcellulose contained 0.1 ml 3% uranylacetate) and examined with an EM 208 S electron microscope (Philips, Kassel, Germany).
Preparation of Epon Sections for Electron Microscopy
Mucosa samples were fixed in 3% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.2, 1 hour, room temperature) and postfixed in osmium tetroxide (OsO4). Samples were dehydrated through a series of graded ethanol washes and embedded in Epon. Sections (60 to 80 nm) were cut, placed onto mesh copper grids, stained with uranyl acetate and lead salts, and examined by electron microscopy as above.
Preparation of Frozen Sections for Immunofluorescence
Semithin frozen sections (60 nm) were prepared at −110°C with an ultracryomicrotome and mounted on poly-l-lysine-coated glass coverslips. Sections were washed in PBS, blocked with fetal calf serum (dilution 1:10), and incubated with rabbit primary antibodies against OVA or HRP (Table 1) for 45 minutes and rinsed in PBS for 15 minutes. After incubation with the secondary antibody (45 minutes, room temperature), sections were washed in PBS and in H2O (15 minutes each). Sections were photographed with an Axioskop fluorescence microscope (Zeiss, Cologne, Germany).
Histological Grading of Inflammation
For routine histology samples adjacent to tissues investigated for immunoelectron, electron, and fluorescence microscopy were stained with hematoxylin and eosin. Histological grading of inflammation in CD and UC was evaluated semiquantitatively in each sample according to the score of Saverymuttu and colleagues.10
Quantitative Evaluation of Sucrase-Isomaltase, Villin, and Actin
Moderatelyinflamed mucosa (score II according to Saverymuttu and colleagues10) of patients with CD (n = 5) and UC (n = 5) was labeled with antibodies against sucrase-isomaltase and aminopeptidase, proteins of the apical brush border, as well as villin and actin, proteins of the cytoskeleton. Ten randomized electron microscopic photos of both NE and RACE cells of each patient were taken (magnification, ×16,500). Because very few RACE cells were detectable in HCs (<3%), only NE cells were examined in the control group. Labeling densities of sucrase-isomaltase and aminopeptidase on microvilli and the cytosol close to the apical membrane and the microvillous labeling density of villin were evaluated on each photo by counting gold particles per area cytosol (GP/μm2) according to Griffiths and colleagues.11 Gold particles labeling actin were counted at the terminal web and the basolateral membrane.
Quantitation of Transcellular Antigen Transport
Moderately inflamed mucosa (score II according to Saverymuttu and colleagues10) of patients with CD (n = 5), UC (n = 5), and 10 HCs (ileum, n = 5; colon, n = 5) incubated with OVA for 10 minutes was used to investigate transcellular antigen transport using immunoelectron microscopy. This time point was chosen because we have previously shown in a mouse model that OVA was directed to late endosomes and the Golgi apparatus within 10 minutes after apical administration.9 OVA was identified in late endosomes and lysosomes with antibodies against LAMP-2. The Golgi apparatus was labeled with Ulex europaeus agglutinin (UEA) and antibodies against UEA. UEA is a lectin that binds specifically proteins glycosylated with l-fucose in the TGN. Thirty electron microscopic photographs (15 of the apical cell region, 15 of the perinuclear cell region; magnification, ×16,500) were randomly taken for LAMP-2 or UEA labeling. Quantitation was performed according to Griffiths and colleagues11: the number of LAMP-2-positive vesicles and UEA-positive trans-Golgi vesicles with and without OVA loading was determined in NE cells and RACE cells per area cytosol within each photograph. Labeling density for OVA was evaluated by counting gold particles per vesicle area (GP/μm2) in OVA-loaded vesicles. To exclude that the pathway of transcellular transport was OVA-specific, control experiments were performed using the antigen HRP. In addition, tissue incubated with antigen for longer periods (30 minutes, 60 minutes) was investigated.
Caspase-3 Expression in Vitro in Apoptotic HT-29/B6 Enterocytes and in RACE Cells
In vitro experiments were performed in HT-29/B6 cells12 that grow as highly differentiated polarized epithelial monolayers. Cells were routinely cultured in 25-cm2 culture flasks in culture medium (RPMI 1640; Biochrom KG, Berlin, Germany) containing 2% stabilized l-glutamine and supplemented with 10% fetal calf serum at 37°C in an atmosphere of 95% O2 and 5% CO2. Cells were seeded on Millicell-PCF filters (effective area 0.6 cm2, PITP 01250; Millipore, Bedford, MA) with an average concentration of 7 × 105 cells/cm2.
To induce apoptosis, confluent monolayers of HT-29/B6 cells were incubated basolaterally with the topoisomerase-I inhibitor camptothecin (20 μg/ml) for 48 hours. Apoptosis was assessed morphologically (ie, nuclear and cytoplasmatic condensation, fragmentation of the nuclear chromatin, apoptotic bodies).12 Cells as well as bowel specimens were prepared for caspase-3 staining by immunoelectron microscopy as described above.
Immunoblotting for Caspase-3 in HT-29 Cells
HT-29 cells were grown to confluency as above and incubated with either OVA apically (100 mg/ml) for 2 hours or 200 U/ml of interferon-γ (kindly provided by Prof. Otto, Frauenhofer Institut IGB, Hannover, Germany) basolaterally for 24 hours. Cells were washed with PBS and lysed with extraction buffer (50 mmol/L Tris/HCl, pH 8, 0.5% Nonidet P-40. 250 mmol/L NaCl. 5 mmol/L ethylenediaminetetraacetic acid, pH 8, 50 mmol/L NaF, 0.5 mmol/L Na3VO4) containing protease inhibitors (0.5 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin). Cell lysates were centrifuged (4000 × g/15 minutes/4°C) and equivalent protein concentrations in the postnuclear lysate from control and OVA or interferon-γ-treated monolayers were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis for active caspase-3.
Statistics
Results are expressed as mean ± SEM throughout. RACE cells were compared to NE cells of CD and UC; NE cells of CD and UC were analyzed in contrast to the appropriate healthy controls (P < 0.05 considered as significant). Labeling densities were evaluated according to the Wilcoxon U-test. Chi-square test was used to determine the ratio of antigen-loaded LAMP-2 or UEA-positive vesicles.
Results
Detection of RACE Cells in Crypts and Villi of CD and UC
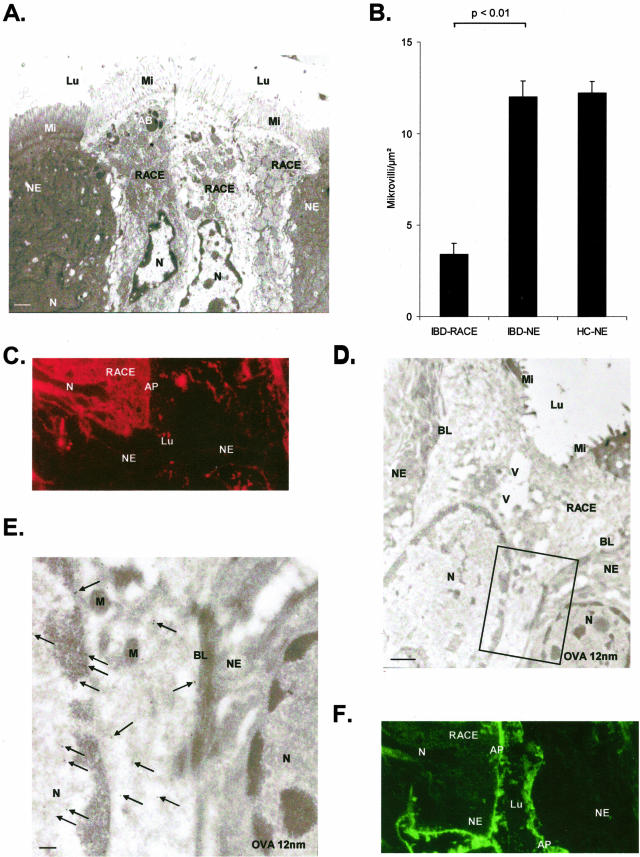
The ultrastructure of RACE cells in plastic sections was characterized by a columnar cell shape, intact tight junctions, an electron lucent cytosol with numerous vesicles, enlarged mitochondria partly with an electron lucent matrix, altered microvilli, hardly distinguishable Golgi stacks, and rough endoplasmic reticulum and nuclei with electron lucent euchromatin and marginalization of heterochromatin (Figure 1A). Number of microvilli was significantly reduced in RACE cells of IBD relative to NEs of both IBD and controls (P < 0.01, Figure 1B). By immunofluorescence OVA labeling was confined to the brush border in NE cells, but was also present in the cytosol and in nuclei of RACE cells (Figure 1; C to E). In contrast, sucrase-isomaltase was present in the cytosol of both NE and RACE cells (Figure 1F).
Figure 1.
CD (ileum OVA incubation 10 minutes). A: Electron microscopy: three RACE cells surrounded by NE cells. Epon section. B: Quantitation of microvilli in RACE and NE cells. C: Immunofluorescence: rhodamine-labeled OVA in the cytosol of RACE cells. D: Immunoelectron microscopic localization of OVA (12-nm large gold particles) within the epithelial layer. A RACE cell is situated between NE cells. E: Higher magnification of the box in D: RACE cell with moderate OVA labeling (arrows) in the cytosol and in the nucleus. NE cell without any OVA labeling in the nucleus and the cytosol. F:Fluorescein isothiocyanate-labeled SI on microvilli of NE cells and RACE cells. Labeling for SI is present in the cytosol of all RACE cells and some NE cells. AB, Apoptotic body; AP, apical membrane; Lu, lumen; Mi, microvilli; V, vesicle; BL, basolateral membrane; SI, sucrase-isomaltase; N, nucleus; M, mitochondriae. Scale bars: 1 μm (A, D); 0.1 μm (F).
Enhanced Antigen Uptake into the Cytosol of RACE Cells and into Vesicular Structures of NE Cells
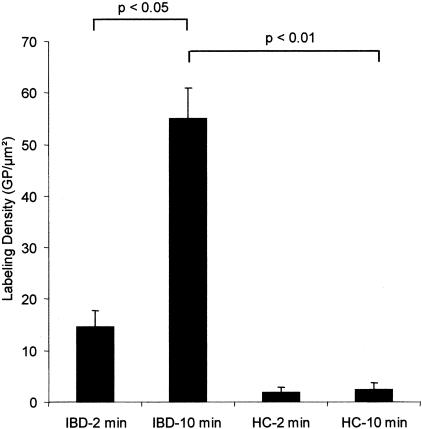
Both OVA and HRP were observed in both NE and RACE cells at the apical and basolateral membrane within a few minutes after exposure. While in NEs OVA or HRP was predominately found in the apical or in the mid/basal region after 2 or 30 minutes of antigen incubation, both antigens were distributed throughout RACE cells already after 2 minutes of incubation. Extended incubation periods showed similar findings with regard to intracellular antigen distribution in RACE cells. The amount of antigen in IBD increased with the time of antigen exposure at the paracellular space (P < 0.05) and was significantly higher in IBD relative to controls at this location site (P < 0.01, Figure 2). The junctional complexes between NE and RACE cells were preserved until RACE cells were shed into the intestinal lumen as described previously.5 After an incubation period of 10 minutes, OVA labeling density was significantly increased (P < 0.01) in nuclei of RACE cells (6.54 ± 0.76 GP/μm2) in CD and UC relative to NE cells in CD and UC (1.37 ± 0.27 GP/μm2) as well as in nuclei of HCs (0.21 ± 0.07 GP/μm2). Diverticulitis specimens revealed similar results as controls regarding the number of RACE cells and antigen distribution (data not shown). Moreover, transcellular transport of OVA and antigen-labeling density in nuclei were similar to HCs (data not shown).
Figure 2.
Quantitation of OVA at the paracellular space in IBD and controls. Graphs show mean values, error bars represent SEM.
Sucrase-Isomaltase Is Increased in the Cytosol, but Decreased at the Apical Membrane in CD and UC
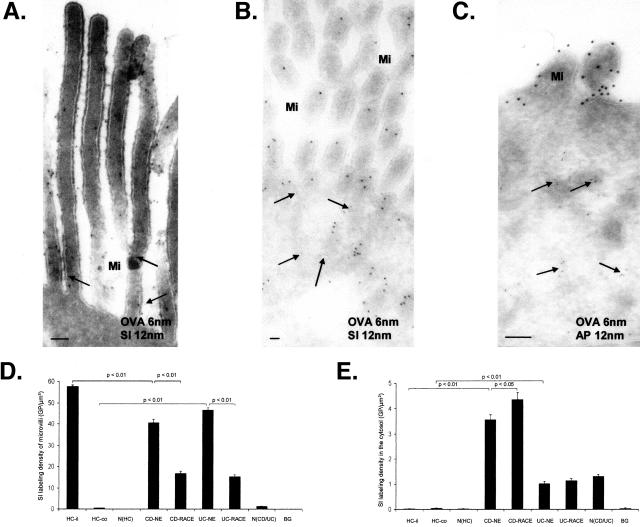
To investigate whether increased antigen uptake in RACE cells was because of immaturity, immunoelectron microscopy was used to quantify the distribution of the differentiation-dependent brush-border enzyme sucrase-isomaltase. In HCs, the majority of binding sites against sucrase-isomaltase was found at the apical membrane of microvilli. Labeling for sucrase-isomaltase was not found in the cytosol, at the basolateral membrane, or in nuclei in HCs; only a few Golgi complexes and intracellular vesicles were positive for sucrase-isomaltase in these cells (Figure 3A). However significant labeling for sucrase-isomaltase was detected in the cytosol in NE cells as well as in RACE cells of CD and UC (CD > UC) (Figure 3B). Interestingly, aminopeptidase, another brush-border enzyme, was not localized within the cytosol of NE and RACE cells (Figure 3C). Microvillous labeling density for sucrase-isomaltase was significantly higher in the ileum of HCs relative to their respective NE and RACE cells of CD and in NE cells of UC relative to their respective RACE cells. Predictably sucrase-isomaltase was not found in microvilli of colonic HCs (Figure 3D).
Figure 3.
Immunoelectron microscopic co-localization of OVA (OVA incubation 10 minutes, 6-nm gold particles) and SI or aminopeptidase (12-nm gold particles). A: Healthy control (ileum). SI and OVA (arrows) labeling on microvilli. B: CD (ileum). RACE cell with OVA labeling in the cytosol (arrows) and SI labeling on microvilli and in the cytosol. C: CD (ileum). RACE cell with OVA in the cytosol (arrows) and aminopeptidase labeling restricted to microvilli. D: Microvillous labeling density of SI. E: Cytosolic labeling densities of SI. Graphs show mean values, error bars represent SEM. N(HC), Nucleus of healthy controls; N(CD/UC), nucleus of CD and UC; HC-il, healthy ileal controls; HC-co, healthy colon controls; BG, background; Mi, microvilli; SI, sucrase-isomaltase; AP, aminopeptidase. Scale bars, 0.1 μm.
Cytosolic labeling density for sucrase-isomaltase was significantly higher in NE cells of CD relative to healthy ileum and in RACE cells of CD relative to their respective NE cells. In UC cytosolic labeling density was similar between NE and RACE cells. However labeling density was significantly increased in these cells of UC relative to healthy colon as well as in nuclei of NE and RACE cells in CD and UC relative to their respective HCs (Figure 3E).
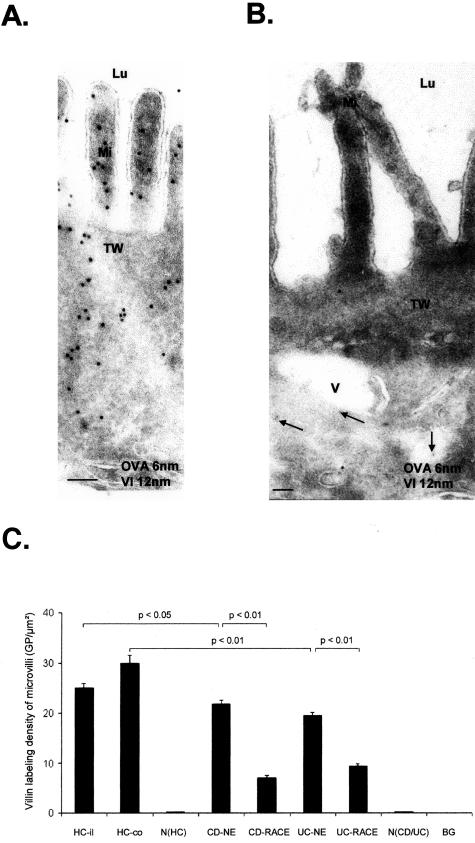
Labeling Density of Villin Is Decreased on Microvilli in CD and UC
As an additional marker for cell differentiation villin was used. A strong villin-labeling density on microvilli was observed in healthy mucosa without detection in vacuolar compartments, at the basolateral membrane, and in nuclei (Figure 4A). Villin-labeling density seemed to be reduced in RACE cells (Figure 4B). Quantitation of villin on microvilli revealed a significant reduction in NE cells of CD and UC relative to HCs and in RACE cells relative to their respective NE cells in both CD and UC. There was no villin labeling in the nucleus in CD, UC, and HCs (Figure 4C).
Figure 4.
Immunoelectron microscopic co-localization of OVA (OVA incubation 10 minutes; 6-nm gold particles) and villin (12-nm gold particles). A: Healthy control (ileum). Strong villin labeling on microvilli and in the terminal web. B: CD (ileum). RACE cell with OVA labeling (arrows) in the cytosol and weak villin labeling on microvilli and the terminal web. C: Microvillous labeling densities of villin. Graph shows mean values, error bars represent SEM. N(HC), Nucleus of healthy controls; N(CD/UC), nucleus of CD and UC; HC-il, healthy ileum controls; HC-co, healthy colon controls; BG, background; Lu, lumen; Mi, microvilli; V, vesicle; TW, terminal web. Scale bars, 0.1 μm.
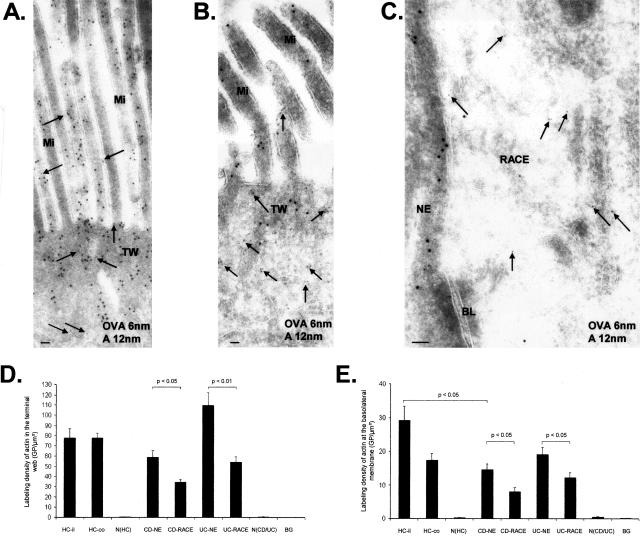
Labeling Density of Actin Is Decreased in CD and UC
Actin filaments are an important component of the epithelial cytoskeleton. HC enterocytes showed strong actin labeling within microvilli, the terminal web, and near the basolateral membrane (Figure 5A). Labeling of actin was more dense in NE cells (Figure 5B) relative to RACE cells (Figure 5C) of CD and UC in the terminal web and at the basolateral membrane. Actin labeling of NE cells in CD and UC was similar to HCs except at the basolateral membrane of CD where actin was significantly reduced relative to HCs (Figure 5, D and E).
Figure 5.
Immunoelectron microscopic co-localization of OVA (OVA incubation 10 minutes; 6-nm gold particles, arrows) and actin (12-nm gold particles). A: Healthy control (ileum). High labeling density for actin on microvilli and terminal web. B: CD (ileum). RACE cell with OVA labeling in the cytosol (arrows) and low labeling density of actin on microvilli and terminal web. C: CD (ileum). Left: NE cell with high labeling density of actin near the basolateral membrane. Right: RACE cell with OVA labeling in the cytosol (arrows) and low labeling density of actin near the basolateral membrane. D: Labeling densities of actin in the terminal web. E: Labeling densities of actin near the basolateral membrane. Graphs show mean values, error bars represent SEM. N(HC), Nucleus of healthy controls; N(CD/UC), nucleus of CD and UC; HC-il, healthy ileum controls; HC-co, healthy colon controls; BG, background; Mi, microvilli; BL, basolateral membrane; TW, terminal web. Scale bars, 0.1 μm.
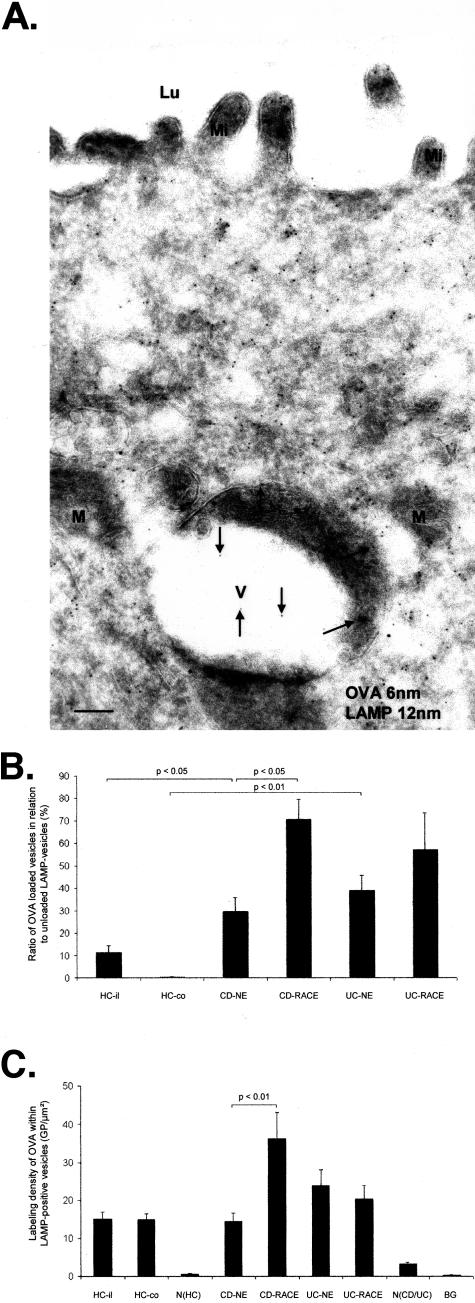
Antigen Transport to LAMP-Positive Vesicles Is Enhanced in CD and UC
To investigate whether OVA is transported to late endosomes/lysosomes, antibodies against LAMP-2 were used. The majority of LAMP-positive vesicles were observed in the apical part of enterocytes (Figure 6A). OVA-loaded LAMP-positive vesicles were increased in CD and UC relative to HCs. NE cells of CD and UC contained significantly more OVA-loaded LAMP-positive vesicles relative to HCs. The highest amount of OVA-loaded LAMP-positive vesicles was found in RACE cells in CD (Figure 6B). Labeling density for OVA within LAMP-positive vesicles did not show any significant differences in NE cells of CD and UC relative to HCs. However labeling density was significantly higher in RACE cells relative to NE cells of CD (Figure 6C). Antigen incubation for more than 10 minutes did not reveal any difference with regard to the prevalence of antigen-loaded LAMP-positive vesicles or the vesicular antigen concentration (data not shown). Control experiments with HRP-incubated specimens revealed similar results demonstrating that the transport mechanism was not OVA-specific (data not shown).
Figure 6.
Immunoelectron microscopical co-localization of OVA (OVA incubation 10 minutes; 6-nm gold particles) and LAMP (12-nm gold particles). CD (ileum). A: RACE cell with OVA-loaded (arrows) LAMP-positive vesicle. B: Antigen transport in LAMP-positive vesicles. C: Labeling densities of OVA in antigen-loaded LAMP vesicles. Graphs show mean values, error bars represent SEM. N(HC), Nucleus of healthy controls; N(CD/UC), nucleus of CD and UC; HC-il, healthy ileum controls; HC-co, healthy colon controls; BG, background; Lu, lumen; Mi, microvilli; V, vesicle; M, mitochondrium. Scale bar, 0.1 μm.
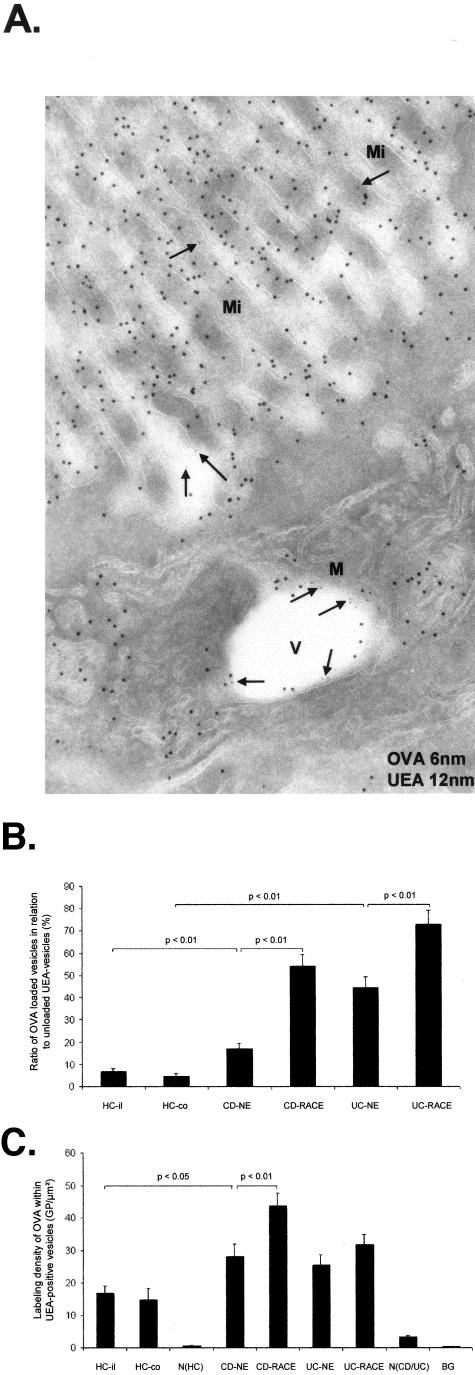
Antigen Transport to UEA-Positive Vesicles Is Increased in CD and UC
To follow antigen transport to another cell compartment, the Golgi apparatus was labeled by UEA and studied by immunoelectron microscopy. The lectin UEA, which binds to l-fucose of glycoproteins, labels the trans side of the Golgi apparatus, vesicles originating from the TGN, the apical membrane of the microvilli, and the basolateral membrane of human enterocytes. UEA-positive vesicles were localized in the apical region of enterocytes and in the basal region in the vicinity of nuclei (Figure 7A). OVA incubation induced a significant increase of OVA-loaded UEA-positive vesicles in NE cells of CD and UC relative to HCs. The highest amount of antigen-loaded UEA-positive vesicles was found in RACE cells. The difference to NE cells was significant in CD as well as in UC (Figure 7B). Moreover UEA-positive vesicles of RACE cells in CD showed a higher labeling density for OVA relative to NE cells as well as did UEA-positive vesicles of NE relative to healthy ileal controls (Figure 7C). Experiments performed with HRP-incubated mucosa showed similar results (data not shown). Incubation periods up to 1 hour showed also similar results (data not shown).
Figure 7.
Immunoelectron microscopic co-localization of OVA (OVA incubation 10 minutes; 6-nm gold particles) and UEA (12-nm gold particles). CD (ileum). A: NE cell with OVA (arrows)-loaded UEA-positive vesicle. B: Antigen transport in UEA-positive vesicles. C: Labeling densities of OVA in antigen-loaded UEA vesicles. Graphs show mean values, error bars represent SEM. N(HC), Nucleus of healthy controls; N(CD/UC), nucleus of CD and UC; HC-il, healthy ileum controls; HC-co, healthy colon controls; BG, background; Mi, microvilli; V, vesicle; M, mitochondrium. Scale bars, 0.1 μm.
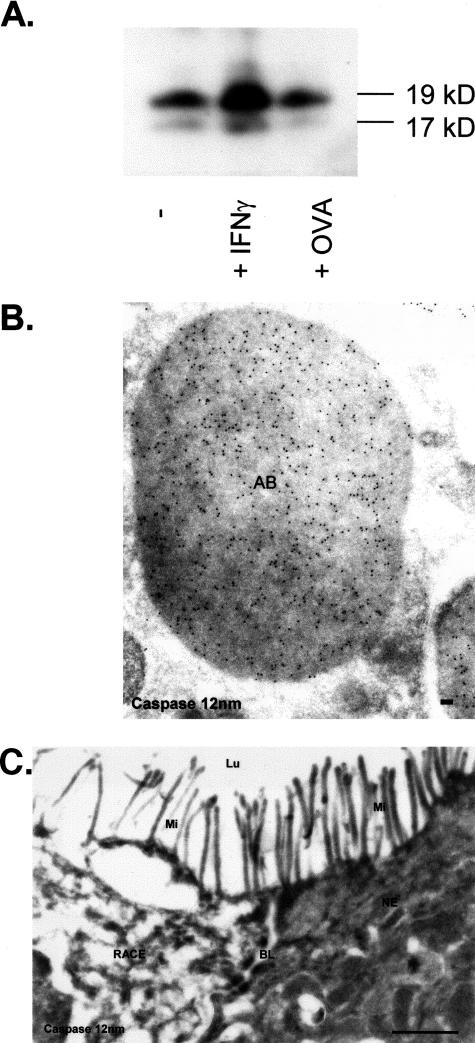
Determination of Caspase-3 in RACE Cells
To examine, whether RACE cells are cells of apoptotic origin, we determined the expression of caspase-3 in normal and RACE cells by immunoelectron microscopy. To further assess the possibility that OVA induces apoptosis, we determined caspase-3 expression in HT29 cells, that were incubated with OVA. As shown by Western blotting, caspase-3 expression in OVA-incubated monolayers was similar to controls (Figure 8A). As expected, interferon-γ-incubated monolayers showed an increased caspase-3 expression relative to controls. As shown in Figure 8B, apoptotic-induced HT29/B6 cells showed a significant labeling density in apoptotic bodies. In contrast, labeling for caspase-3 was not found in the cytosol of RACE and NE cells (Figure 8C).
Figure 8.
Detection of caspase-3 by immunoelectron microscopy and Western blotting. A: Caspase-3 expression in HT29 monolayers. B: Subcellular detection of caspase-3 (12-nm gold particles) within apoptotic HT-29/B6 enterocytes. C: Absent caspase-3 labeling in the cytosol of RACE and NE. AB, Apoptotic body; BL, basolateral membrane; Lu, lumen; Mi, microvilli. Scale bars: 0.1 μm (B); 1 μm (C).
Discussion
Under physiological conditions macromolecules such as OVA and HRP are endocytosed and transported by transcellular vesicles to the basolateral membrane.13–15 In the present study we analyzed the transcellular transport of OVA in epithelial biopsies of patients with CD, UC, and HCs to compare the transport in NE cells and RACE cells, the latter representing an enterocyte cell population described recently by our group.5 It is well established that exogenous ligands such as HRP localize under physiological conditions in early endosomes 5 minutes, in late endosomes 10 to 15 minutes, and in lysosomes 30 to 60 minutes after internalization.16,17 Although no structural continuity between endosomes and Golgi cis-ternae has been observed,18 endocytosed ligands such as the plant toxins ricin19 and Shiga-toxin B fragment20 are transported to the TGN within minutes after uptake before they are delivered to the cytosol.6 Using UEA to identify TGN vesicles and LAMP to label late endosomes and lysosomes, we could determine the amount of endocytosed OVA within these compartments of enterocytes 10 minutes after luminal loading in CD, UC, and HCs.
Quantitative evaluation resulted in three major findings: 1) OVA transport to late endosomes and TGN vesicles is increased in both NE cells and RACE cells of both CD and UC relative to healthy control cells. 2) RACE cells take up higher amounts of OVA into late endosomes than NE cells in CD but not in UC. 3) RACE cells target higher amounts of OVA to TGN vesicles than NE cells in CD as well as in UC. These results confirm our previous observations that transepithelial antigen transport is enhanced in IBD.5 The increased transport rates observed in CD relative to UC are likely because of differences in the endocytic activities of the small versus large bowel. Our data further indicate that RACE cells are more susceptible to vesicular antigen transport than NE cells. Therefore translocation of antigens into the cytosol is a process that implicates not only the apical membrane but also membranes of the endocytic pathway as well as the TGN. This is in line with the observation that several toxins such as ricin, Shiga toxin, diphtheria toxin, and Pseudomonas toxin enter the cytosol from intracellular compartments such as endosomes and TGN.6,21 Enterocytes tend to direct macromolecules into late endosomes and the TGN under certain circumstances as previously shown for toxic gliadin peptides in celiac disease22 and OVA in BALB/c mice during first antigen administration.9 Moreover, it has been shown that endocytosed OVA is transported into the cytosol by osmotic lysis of pinosomes.23
Although RACE cells internalize increased amounts of OVA and HRP into endosomes and the TGN with rapid translocation into the cytosol, it is intriguing that enterocytes in the immediate vicinity of RACE cells incorporate these antigens exclusively into endocytic compartments without further delivery into the cytosol. It is therefore important to examine structural differentiation markers in RACE versus NE cells. Sucrase-isomaltase, a differentiation-dependent integral membrane protein that is normally expressed in enterocytes along the entire length of the crypt-villus axis of the small intestine,24 was used. As expected, the highest labeling density for sucrase-isomaltase was found in healthy ileum. Significantly lower levels were observed in CD with the lowest concentrations found in RACE cells. This is in line with the finding that sucrase-isomaltase is down-regulated in human intestinal epithelial cell lines by interleukin-6,25 that has been found to be enhanced in IBD.26 We found only little staining in healthy colon controls supporting the notion that the physiological expression of sucrase-isomaltase in the large bowel occurs predominately during the fetal period. In contrast, sucrase-isomaltase was significantly found in NE and RACE cells of UC as previously observed in inflammatory, regenerative, and dysplastic mucosain UC.27The decreased apical membrane staining ofsucrase-isomaltase in RACE relative to NE cells in CD and UC suggests that RACE cells are less differentiated. Cytosolic staining of sucrase-isomaltase in IBD has been found to correlate with the presence of dysplastic changes and low level of cellular differentiation.27 This in vivo situation is comparable to an in vitro scenario when intestinal Caco-2 cells were stably transfected with cDNA encoding anti-sense villin RNA resulting in a permanent down-regulation of the endogenous villin message and dramatic effects on brush-border assembly.28 In their study, and therefore similar to RACE cells, most of sucrase-isomaltase was observed in the cytosol, whereas aminopeptidase, another integral protein, was exclusively found in vesicles and not within the cytosol, which is probably because of different trafficking mechanisms for apically sorted sucrase-isomaltase and aminopeptidase.29 In fact, sorting of sucrase-isomaltase to the apical membrane is mediated by O-glycosylation of the stalk domain and implicates its association with Triton X-100 insoluble cholesterol/sphingolipid-rich membrane microdomains or lipid rafts, whereas aminopeptidase is transported to the apical membrane by raft-independent mechanisms.29 It is interesting to note that the cytosolic concentration of sucrase-isomaltase in CD was significantly higher relative to UC with a further increase in RACE cells relative to NE cells in CD. Moreover, the proportion of sucrase-isomaltase in the cytosol versus the apically labeled protein was increased in RACE cells relative to NE cells. These findings indicate that the cytosolic translocation of sucrase-isomaltase is associated with the formation of RACE cells.
Another important protein associated with enterocytic differentiation state is villin, an actin-binding cytoskeletal protein located within the microvilli of intestinal epithelial cells.30 It is well established that the expression of villin along the crypt-villus axis increases as cells migrate from the crypt to the villus surface30,31 concomitant with an increased rate of synthesis during enterocytic differentiation and considerable posttranslational stability.32 Decreased villin levels in CD and UC relative to HCs with the lowest concentration in RACE cells our data point to a disturbance of differentiation and maturation processes in RACE cells. This could also explain why antigen uptake is increased in these cells, because immature enterocytes are known to be more susceptible to antigen uptake than fully differentiated cells.33
The most likely reason why RACE cells have not been previously described by others is because of the lack of a suitable experimental design that combines morphological and functional assessments in the same setting. Our approach using immunoelectron microscopy could unequivocally unravel the distinct ultrastructural characteristics of RACE cells and highlight an efficient uptake of luminal antigens into endocytic compartments and finally into the cytosol. The presence of villin and sucrose-isomaltose (SI) in microvilli as well as ultrastructural features such as the formation of tight junctions strongly suggest that RACE cells are of enterocytic origin. Despite few morphological similarities, such as an electron lucent cytosol and short microvilli, RACE cells differ from cup cells that are exclusively found in the small intestine and do not reveal a cytosolic antigen uptake.34 RACE cells show also some similarities to M cells such as irregular microvilli and low levels of villin and sucrase-isomaltase.35 Furthermore both cell types can present with a thin bridge of apical cytosol and enfold lymphoid cells and macrophages within invaginations of the basal membrane.36 The main difference between these two cell types lies at the functional level and is related to the uptake of luminal antigens and their transcellular transport. Although in both RACE and M cells luminal antigens such as HRP are transported in vesicles,37 cytosolic localization of transported antigens has not been demonstrated in M cells.
Our knowledge about biological, immunological, and pathophysiological functions of RACE cells is still limited. Previous results from our group using a mouse model indicate that RACE cells (called M-cell-like enterocytes) are induced after feeding with OVA independently of whether or not oral tolerance has been generated as shown in BALB/c mice, eg, in SCID mice.9 Their occurrence seems unrestricted to any defined disease entity or state because RACE cells are also occasionally found in healthy intestinal epithelium. However, RACE cells are significantly increased in severely inflamed mucosa of CD and UC.5 Because (pro)-inflammatory cytokines such as tumor necrosis factor-α and interleukin-138 have been shown to be involved in increased antigen degradation, we speculate that cytokines are also able to induce RACE cells. The transfer of exogenous antigens into the cytosol is a critical process for MHC class I presentation triggering cytotoxic immune responses, called cross presentation.23,39–41 In dendritic cells antigens gain access to the cytosolic antigen-processing machinery and to the conventional MHC class I antigen-presentation pathway by an endosome-to-cytosol transport.42,43 Therefore it would be of interest to examine whether the translocation of OVA into the cytosol induces (endogenous) antigen presentation by MHC class I proteins.
RACE cells are extruded from the epithelial layer into the intestinal lumen leaving a defect in the epithelium.5 As a gate for unrestricted antigen influx, which cannot be sealed immediately, these epithelial defects are likely to be involved in the pathogenesis of an impaired barrier function as well as enhanced paracellular flux of luminal antigens through the intestinal epithelium in CD and UC.44 Because few ultrastructural features of RACE cells such as marginal condensation of heterochromatin as well as an altered electron density of the cytosol corresponded with the morphological features of apoptotic cells, and epithelial apoptosis is enhanced in the IBD mucosa,26,45,46 we sought to determine whether RACE cells are of apoptotic origin and whether OVA induces apoptosis. Because apoptotic bodies are found only occasionally within these cells, which indicates that RACE cells may represent early stages of apoptosis, we used the apoptotic marker caspase-3 to address this question.47 However, we failed to demonstrate an increased caspase-3 labeling density in RACE cells as well as an increased caspase-3 expression in OVA-incubated HT29 cells. This suggests that OVA does not induce caspase-3-dependent apoptosis and RACE cells are not involved in caspase-dependent apoptosis. RACE cells could also be preapoptotic cells because caspases remain inactive until the death signal is received.48 Alternatively RACE cells could undergo caspase-independent apoptosis, as has been shown recently in different cancer cell lines.49 Arguments in favor of an apoptotic nature are the reduced ATP synthesis of mitochondria5,50 and the impaired structure of the cytoskeleton (loss of actin bundles)51 that we observed in RACE cells. However, because the overall cell function in RACE cells seems to be impaired, OVA could diffuse into the nucleus via nuclear pores as it has been described for this macromolecule even in healthy cells.52 We assume that (apoptotic) RACE cells contribute to increased epithelial permeability in CD and UC after extrusion into the intestinal lumen.53 RACE cells possibly enter a form of apoptosis before completing cell differentiation. To further address the apoptotic nature of RACE cells, experimental methods that could demonstrate early apoptotic processes at an ultrastructural level and simultaneously allow the cytosolic localization of antigens taken up from the intestinal lumen would be helpful.
In conclusion, antigen transport into endocytic cell compartments and finally into the cytosol is strongly enhanced in RACE cells of CD and UC. The reduced detection of villin and sucrase-isomaltase suggests that RACE cells are enterocytes that are immature and less differentiated. Further investigations of this newly described enterocyte population will promise crucial insights in the immunomodulatory functions of enterocytes within the gut-associated lymphoid tissue.
Acknowledgments
We thank Prof. Dr. S. Strobel (Institute of Child Health, London, UK) for the antibody against OVA, Prof. Dr. D. Louvard (Institut Curie, Paris, France) for the antibody against villin, Prof. Dr. A. Zweibaum (Institut National de la Sante et de la Recherche Medicale, Villejuif, France) for the antibody against sucrase-isomaltase, and Dr. Levkau (Institut fuer Arterioskleroseforschung, Muenster, Germany) for technical assistance with Western blots for caspase-3.
Footnotes
Address reprint requests to Prof. Dr. K.-P. Zimmer, Department of Pediatrics, University of Muenster, Albert-Schweitzer-Str. 33, 48149 Muenster, Germany. E-mail: zimmerp@uni-muenster.de.
Suported by grants from the University of Muenster (IMF: Sc-1-2-II/97-14 to G.S., IZKF C19, and IZKF ZPG3 to K.P.Z.) and the Deutsche Forschungsgemeinschaft (SCHU: 720/5-1 to G.S., Zi 294/6-3 to K.P.Z.).
S.K. and M.B. contributed equally to this study.
References
- Soderholm JD, Olaison G, Lindberg E, Hannestad U, Vindels A, Tysk C, Jarnerot G, Sjodahl R. Different intestinal permeability patterns in relatives and spouses of patients with Crohn’s disease: an inherited defect in mucosal defence? Gut. 1999;44:96–100. doi: 10.1136/gut.44.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, Dickersin GR. Crohn’s disease: transmission electron microscopic studies. I. Barrier function. Possible changes related to alterations of cell coat, mucous coat, epithelial cells, and Paneth cells. Hum Pathol. 1980;11:561–571. [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Peterson KH, Olaison G, Franzen LE, Westrom B, Magnusson KE, Sjodahl R. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology. 1999;117:65–72. doi: 10.1016/s0016-5085(99)70551-2. [DOI] [PubMed] [Google Scholar]
- Schurmann G, Bruwer M, Klotz A, Schmid KW, Senninger N, Zimmer KP. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis. 1999;14:41–46. doi: 10.1007/s003840050181. [DOI] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Endocytosis, intracellular transport,and cytotoxic action of Shiga toxin and ricin. Physiol Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D, Ruiz-Arguelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–164. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Zimmer KP, Buning J, Weber P, Kaiserlian D, Strobel S. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology. 2000;118:128–137. doi: 10.1016/s0016-5085(00)70421-5. [DOI] [PubMed] [Google Scholar]
- Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Simons K, Warren G, Tokuyasu KT. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl− secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol. 1991;261:C574–C582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
- Cornell R, Walker WA, Isselbacher KJ. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971;25:42–48. [PubMed] [Google Scholar]
- Berin MC, Kiliaan AJ, Yang PC, Groot JA, Taminiau JA, Perdue MH. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997;113:856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci. 1996;109:2905–2914. doi: 10.1242/jcs.109.12.2905. [DOI] [PubMed] [Google Scholar]
- Schmid S, Fuchs R, Kielian M, Helenius A, Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989;108:1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Back R, Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Griffiths G, Dean GE, Mellman I, Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci USA. 1986;83:2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Tonnessen TI, Petersen OW, Sandvig K, Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986;102:37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HR, Roberts LM, Lord JM. Toxin entry: how reversible is the secretory pathway? Trends Cell Biol. 1992;2:183–185. doi: 10.1016/0962-8924(92)90230-k. [DOI] [PubMed] [Google Scholar]
- Zimmer KP, Naim H, Weber P, Ellis HJ, Ciclitira PJ. Targeting of gliadin peptides, CD8, alpha/beta-TCR, and gamma/delta-TCR to Golgi complexes and vacuoles within celiac disease enterocytes. FASEB J. 1998;12:1349–1357. doi: 10.1096/fasebj.12.13.1349. [DOI] [PubMed] [Google Scholar]
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Poulsen MD, Noren O, Sjostrom H. Distribution of three microvillar enzymes along the small intestinal crypt-villus axis. J Submicrosc Cytol Pathol. 1994;26:453–460. [PubMed] [Google Scholar]
- Ziambaras T, Rubin DC, Perlmutter DH. Regulation of sucrase-isomaltase gene expression in human intestinal epithelial cells by inflammatory cytokines. J Biol Chem. 1996;271:1237–1242. doi: 10.1074/jbc.271.2.1237. [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Andrews CW, Jr, O’Hara CJ, Goldman H, Mercurio AM, Silverman ML, Steele GD., Jr Sucrase-isomaltase expression in chronic ulcerative colitis and dysplasia. Hum Pathol. 1992;23:774–779. doi: 10.1016/0046-8177(92)90347-6. [DOI] [PubMed] [Google Scholar]
- Costa de Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409–421. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Naim HY. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11:1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- West AB, Isaac CA, Carboni JM, Morrow JS, Mooseker MS, Barwick KW. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988;94:343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]
- Landry C, Huet C, Mangeat P, Sahuquet A, Louvard D, Crine P. Comparative analysis of neutral endopeptidase (NEP) and villin gene expression during mouse embryogenesis and enterocyte maturation. Differentiation. 1994;56:55–65. doi: 10.1046/j.1432-0436.1994.56120055.x. [DOI] [PubMed] [Google Scholar]
- Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29–18 clones. J Cell Biol. 1987;105:359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Pang KY, Walker WA. Food proteins and gut mucosal barrier. II. Differential interaction of cow’s milk proteins with the mucous coat and the surface membrane of adult and immature rat jejunum. Pediatr Res. 1984;18:1252–1257. doi: 10.1203/00006450-198412000-00005. [DOI] [PubMed] [Google Scholar]
- Madara JL. Cup cells: structure and distribution of a unique class of epithelial cells in guinea pig, rabbit, and monkey small intestine. Gastroenterology. 1982;83:981–994. [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, Colucci-Guyon E, Kraehenbuhl JP, Pringault E. Cytosolic distribution of villin in M cells from mouse Peyer’s patches correlates with the absence of a brush border. Gastroenterology. 1996;110:515–521. doi: 10.1053/gast.1996.v110.pm8566599. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- von Rosen L, Podjaski B, Bettmann I, Otto HF. Observations on the ultrastructure and function of the so-called “microfold” or “membraneous” cells (M cells) by means of peroxidase as a tracer. Virchows Arch A Pathol Anat Histol. 1981;390:289–312. doi: 10.1007/BF00496560. [DOI] [PubMed] [Google Scholar]
- Fiebiger E, Meraner P, Weber E, Fang IF, Stingl G, Ploegh H, Maurer D. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med. 2001;193:881–892. doi: 10.1084/jem.193.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141–148. doi: 10.1016/s1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Moller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–167. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- Levine AD. Apoptosis: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:191–205. doi: 10.1097/00054725-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Salvesen GS. Caspases and apoptosis. Essays Biochem. 2002;38:9–19. doi: 10.1042/bse0380009. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Caspase activation. Biochem Soc Symp. 2003;70:233–242. doi: 10.1042/bss0700233. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Kinsey GR, Bolchoz LJ, Schnellmann RG: Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther 2004, (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Comelli M, Londero D, Mavelli I. Severe energy impairment consequent to inactivation of mitochondrial ATP synthase as an early event in cell death: a mechanism for the selective sensitivity to H2O2 of differentiating erythroleukemia cells. Free Radic Biol Med. 1998;24:924–932. doi: 10.1016/s0891-5849(97)00373-0. [DOI] [PubMed] [Google Scholar]
- Bursch W, Hochegger K, Torok L, Marian B, Ellinger A, Hermann RS. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci. 2000;113:1189–1198. doi: 10.1242/jcs.113.7.1189. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Darby MK, Gerace L. A monoclonal antibody against the nuclear pore complex inhibits nucleocytoplasmic transport of protein and RNA in vivo. J Cell Biol. 1988;107:1289–1297. doi: 10.1083/jcb.107.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol. 2001;535:541–552. doi: 10.1111/j.1469-7793.2001.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]