Abstract
Support mechanisms involved in growth of androgen-independent prostate cancer are primarily unknown. Hepatocyte growth factor (HGF)/Met has been suggested to be one of them based primarily on immunohistochemical studies. We conducted a series of experiments to assess the role of the HGF/Met system in an androgen-dependent human prostate carcinoma, CWR22 and its androgen-independent derivative, CWR22R. We found that action of HGF changed from paracrine to autocrine in progression to androgen-independent state. CWR22 tumors did not express HGF but expressed Met, whereas prostate stromal cells expressed HGF at a high level. Growth of CWR22 was stimulated either by addition of HGF to the culture or by the presence of prostate stromal cells. On the other hand, CWR22R cells expressed both HGF and Met. Knockdown of Met expression by RNA interference method suppressed the growth of CWR22R cells. Our data suggest that HGF is intimately involved in growth of human prostate cancer and that progression from the androgen-dependent to the androgen-independent state is associated with an adaptive switch in support mechanism from paracrine to autocrine. Our data offer one mechanism to account for androgen-independent human cancer growth.
Prostate cancer is the most common cancer and the second most common fatal cancer in men in the United States.1 Although prostate-specific antigen (PSA)-based screening has led to the discovery of more organ-confined cancer cases than in the past, a large number of men are still discovered at either locally invasive or more advanced stage and hormonal or radiation therapy is offered as a palliative treatment. Typically, an initial response to androgen ablation therapy is observed in 70% of patients but most patients relapse within 3 years,2 and the failure of androgen ablation therapy is attributed to androgen-independent tumor growth. Mechanism(s) supporting the growth of hormone-refractory prostate cancer is under intense investigation; some of them seem to involve the androgen receptor (AR) signaling; mutated or amplified AR is activated by low doses of androgens,3–5 other steroids,6 or anti-androgens.7 Also AR activation may be regulated by other mechanisms via co-activators,8 peptide growth factors,9 cytokines,10 or aberrant methylation of AR 5′-regulatory region.11 Another proposed mechanism is a paracrine support by neuroendocrine-type carcinoma cells that are AR-negative, co-exist with conventional-type carcinoma cells, and increase in number after hormonal ablation therapy. They secrete a variety of neuropeptides that are suggested to support the growth of androgen-independent carcinoma cells by a paracrine mechanism.12,13
Hepatocyte growth factor (HGF), originally identified as a potent mitogen that stimulates the growth of hepatocytes, has multiple biological functions in different tissues.14–16 In carcinomas of various organs including the prostate,17–29 HGF has been proposed as an autocrine/paracrine factor. The HGF receptor is encoded by the Met proto-oncogene, which was initially isolated as an activated transforming gene in a human osteosarcoma cell line.30
We are interested in elucidating the role of the HGF/Met system in progression from an androgen-dependent to androgen-independent state. We have recently demonstrated that HGF produced by prostate stromal cells is a paracrine growth factor that stimulates the growth of androgen-independent human prostate cancer in vitro and in vivo.31 By immunohistochemistry, Met is expressed frequently in prostate carcinomas and its frequency increases in metastatic carcinomas.27 We and others have shown that its ligand, HGF, was expressed primarily by the prostate stromal cells but also by carcinoma cells in some cases.29,32 In that study of ours in which growth stimulatory action of HGF was demonstrated,31 the cell lines used (PC-3 and CA-7T2) expressed neither AR nor PSA. However, most of the androgen-independent human prostatic carcinomas express both AR and PSA.33 Therefore, it is appropriate to conduct studies using androgen-dependent and -independent prostate carcinomas that express both AR and PSA.
In the present study, we used human prostate cancer xenografts, CWR22 that is androgen-dependent,34,35 and CWR22R that is androgen-independent.36 Both express AR and PSA.34–36 The CWR22 xenograft was originally derived from a primary human prostate carcinoma and has been maintained in athymic nude mice by serial transplantations.34 CWR22R cells were derived from the CWR22 tumor as a regrowth after castration of tumor-bearing mice, have been adapted to grow in culture, express AR, and secrete PSA into culture medium.36 Using CWR22 and CWR22R cells, we examined the expression of Met and HGF, and tested the effect of HGF on growth of CWR22 and CWR22R cells in vitro.
Materials and Methods
Cells and Cell Culture
We used four human prostatic carcinoma cell lines CWR22R (provided by Dr. James W. Jacobberger, Case Western Reserve University, Cleveland, OH),36 LNCaP, DU145, and PC-3,37 and one xenograft CWR22 (provided by Dr. Thomas G. Pretlow, Case Western Reserve University, Cleveland, OH).34 Primary cultured CWR22 cells were established in our laboratory as follows: CWR22 tumor tissue was cut into multiple minute cubicles and dissociated at 37°C for 2 hours with 0.1% collagenase I (Worthington, Freehold, NJ). Cell suspension was filtered through a cell strainer with 70-μm nylon mesh (Becton Dickinson Biosciences, Franklin Lakes, NJ). Cells collected by centrifugation were washed three times with RPMI 1640 (Invitrogen Corp., Carlsbad, CA) fortified with 10% fetal bovine serum (FBS; Invitrogen Corp.), placed on a plastic surface, and grown in Richter’s Improved MEM (Irvine Scientific, Santa Ana, CA) containing 10 mmol/L nicotinamide (Sigma-Aldrich, St. Louis, MO), 20 ng/ml epidermal growth factor, insulin-transferrin-selenium supplement, 100 μg/ml streptomycin, 100 U/ml penicillin, 0.25 μg/ml fungizone, and 2% FBS (all from Invitrogen Corp.). Because we had confirmed earlier that primary cultured CWR22 cells expressed PSA at a high level in passages 1 to 4, cells at passages up to 2 were used in the present study.
Prostate stromal cells were derived from a cancer-free portion of a prostatectomy specimen removed for carcinoma. All of the cells except for CWR22 cells were maintained in RPMI 1640 containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone, and incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C. This is referred to as complete medium.
Isolation of Cytoplasmic RNA and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA was prepared by lysing cells in a hypotonic buffer containing Nonidet P-40 (Sigma-Aldrich), followed by removal of nuclei. Cytoplasmic RNA was reverse-transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen Corp.) at 42°C for 60 minutes with the use of random primers (5 μmol/L; Invitrogen Corp.). Subsequently, 1 μl of the products was subjected to PCR amplification. The final concentration of deoxynucleotide triphosphates (Applied Biosystems, Foster City, CA) and primers in the reaction mixture was 200 μmol/L and 1 μmol/L, respectively. TaqDNA polymerase (Applied Biosystems) was added to the mixture at a final concentration of 0.05 U/μl, and the reaction was performed in a DNA Thermal Cycler (Applied Biosystems). The primers for human Met, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and human HGF (R&D Systems, Minneapolis, MN) were purchased or synthesized as described in the previous report.31,38 PCR products were electrophoresed on 1% agarose gel and stained with ethidium bromide.
Western Blot Analysis
Cells grown in monolayers were harvested at subconfluency and lysed with CelLytic M Cell Lysis Reagent (Sigma-Aldrich) containing a protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitor cocktail I and II (Sigma-Aldrich). The samples were centrifuged at 12,000 × g for 10 minutes at 4°C and the supernatant protein samples were electrophoresed on sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with 5% nonfat dry milk (Wako, Osaka, Japan), 1× T-TBS [25 mmol/L Tris-HCl, 125 mmol/L NaCl, 0.1% Tween 20 (Sigma-Aldrich)] for 1 hour at room temperature and probed with primary antibodies overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. The immune complexes were visualized with the use of the enhanced chemiluminescence (ECL) Plus or the ECL Advance kit (Amersham Biosciences, Piscataway, NJ) according to the protocol of the manufacturer. For internal control, the blots were stripped with 62.5 mmol/L Tris-HCl (pH 6.8) buffer containing 0.7% 2-mercaptoethanol and 2% sodium dodecyl sulfate (Sigma-Aldrich) at 50°C for 30 minutes and reprobed with mouse anti-β-tubulin monoclonal antibody (Becton Dickinson Biosciences, San Jose, CA). Primary antibodies were purchased from the following commercial sources: polyclonal antibodies against phospho-Met (p-Met, Tyr1349) were from Cell Signaling Technology (Beverly, MA), polyclonal rabbit anti-human Met (C-12) antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), and secondary antibodies against rabbit or mouse IgG from Amersham Biosciences.
Three-Dimensional Collagen Gel Culture
Collagen gel was prepared as reported previously.31 In brief, 8 vol of rat tail type I collagen suspension (Becton Dickinson Biosciences) were mixed with 1 vol of 10-fold concentrated RPMI 1640 (Sigma-Aldrich) and 1 vol of reconstruction buffer (2.2 g of NaHCO3, 4.77 g of Hepes in 100 ml of 0.05 N NaOH; Sigma-Aldrich). Collagen gel with or without stromal or carcinoma cells (5 × 104 cells/well) was poured into a 24-well plate (0.5 ml/well). After incubation for 30 minutes at 37°C to permit complete gelation, a second collagen layer containing carcinoma cells (5 × 104 cells/well) was placed on top of the first layer. After gelation, RPMI 1640 or complete Richter’s Improved MEM containing 5% charcoal-stripped FBS (cFBS) was added. The medium was changed every other day. After incubation for 4, 8, or 14 days, each gel layer was mechanically removed, and cells contained in the upper layer were recovered by treatment with 0.1% collagenase I and 0.5% trypsin-5.3 mmol/L ethylenediaminetetraacetic acid (Invitrogen Corp.) and were counted with a hemocytometer. PSA protein in the culture supernatants was measured using the Tandem-E PSA immunoenzymetric assay kit (Hybritech, San Diego, CA) according to the manufacturer’s protocol. In someexperiments, mibolerone (10 nmol/L; NEN Life Science Products, Boston, MA), human recombinant HGF (20 ng/ml; R&D Systems), goat normal IgG, or goat anti-human HGF antibody (2 μg/ml; R&D Systems) was added to the above cultures.
Measurement of Secreted HGF
Cells were washed three times with Hanks’ balanced salt solution (Invitrogen Corp.) and downshifted to serum-free RPMI 1640 medium. After 48 hours, conditioned medium (CM) was collected and clarified by centrifugation. HGF protein in CM was measured by the Quantikine human HGF immunoassay kit (R&D Systems) according to the manufacturer’s protocol.
Phosphorylation of Met Protein
Cells were grown to ∼70% confluency on 100-mm dishes in complete medium. After a starving time of 48 hours in serum-free medium, CWR22R cells were incubated with goat normal IgG (2 μg/ml; R&D Systems) or goat anti-human HGF antibody (2 μg/ml) for 48 hours. PC-3 cells were stimulated with HGF (40 ng/ml) for 30 minutes in the presence or absence of goat anti-human HGF antibody (2 μg/ml). After treatment, Western analysis was performed with the use of phospho-Met antibody and then densitometric analysis was done by NIH Image 1.61.
Mutation Analysis of the Met Gene
The entire encoding sequences of the Met gene were amplified from CWR22R-derived cDNA by Platinum Pfx DNA polymerase (Invitrogen Corp.) with the use of eight oligonucleotide primer sets; 5′-CCA CTG GTT CCT GGG CAC CG-3′ and 5′-ATG AAA GGA CTT TGG CTC CC-3′, 5′-TGC TGA CAT ACA GTC GGA GG-3′ and 5′-AAG CAG TGC TCA TGA ATT GGG-3′, 5′-TCT GCC ATG TGT GTG CAT TCC CC-3′ and 5′-TCA GGC ATT CCT CCG ATC GC-3′, 5′-GCA GAC ATT TCC AGT CCT GC-3′ and 5′-GTT GAG AGG TTC TTT CCA CC-3′, 5′-ACA AGC ATC TTC AGT TAC CG-3′ and 5′-AGC GAA CTA ATT CAC TGC CC-3′, 5′-ACT TGG GTT TTT CCT GTG GC-3′ and 5′-AAT CTT TCA TGA TGA TTC CC-3′, 5′-CTT TGT TGG ACA ATG ATG GC-3′ and 5′-CCA CAC ATC TGA CTT GGT GG-3′, and 5′-CTG CCA GTG AAG TGG ATG GC-3′ and 5′-TGG CCT TTT AAA GGT CAG GC-3′. The blunt-end PCR products were directly cloned into pCR4Blunt-TOPO (Invitrogen Corp.) and then sequenced with the CEQ 2000XL (Beckman Coulter, Fullerton, CA) following the manufacturer’s protocol.
Synthetic Small Interfering RNAs (siRNAs) and Transfection
Synthetic siRNAs were purchased from Dharmacon(Lafayette, CO). The RNA duplexes were resuspended in RNase-free annealing buffer [20 mmol/L KCl, 6 mmol/L HEPES-KOH (pH 7.5), 0.2 mmol/L MgCl2] to a final concentration of 20 μmol/L. The target sequences for siRNAs used in this study were 5′-CGU ACG CGG AAU ACU UCG A-3′ for luciferase GL2, 5′-GGA CCG GUU CAU CAA CUU C-3′ for human Met, and 5′-GUA UCC UCA CGA GCA UGA C-3′ for human HGF. Luciferase GL2 siRNA labeled with Cy-3 was used for evaluating transfection efficiency and also used as a negative control for RNA interference (RNAi) study.
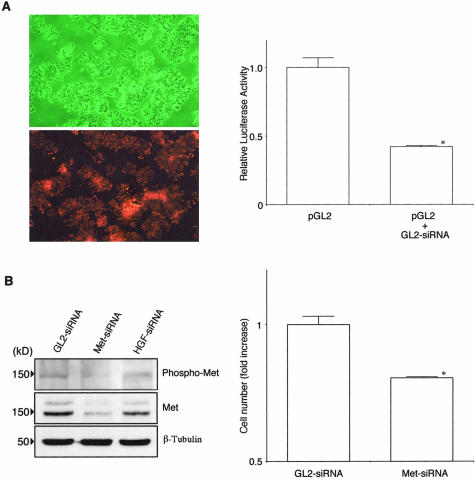
To assess transfection efficiency and function of siRNA in CWR22R cells, cells were seeded at a density of 5 × 105 per well in a 60-mm culture dish in complete medium. Transfection was performed 24 hours later with the use of Lipofectamine 2000 reagent (Invitrogen Corp.) mixed with 360 pmol of Cy-3-labeled luciferase GL2-siRNA, 3 μg of reporter plasmid pGL2 (Promega, Madison, WI), and 0.5 μg of pRL-TK Renila luciferase reporter (Promega). After 48 hours, cells were examined by a fluorescence phase contrast microscope with a Cy-3 filter (Nikon, Tokyo, Japan) and then lysed with 1× passive lysis buffer (Promega). Their luciferase activity was measured with the use of dual-luciferase reporter assay system (Promega) and a luminometer (Gene Light 55; Nition, Funabashi, Japan).
For RNAi against target genes, we transfectedCWR22R cells with synthetic siRNAs. Transfections were performed in a 100-mm culture dish at 70% confluency by using Lipofectamine 2000 reagent with 1 nmol of luciferase GL2-siRNA, Met-siRNA, or HGF-siRNA. Forty-eight hours later, cells were lysed for Western analysis or transferred to three-dimensional collagen gel cultures.
Results
Expression of Met/HGF Receptor in CWR22 and CWR22R Cells
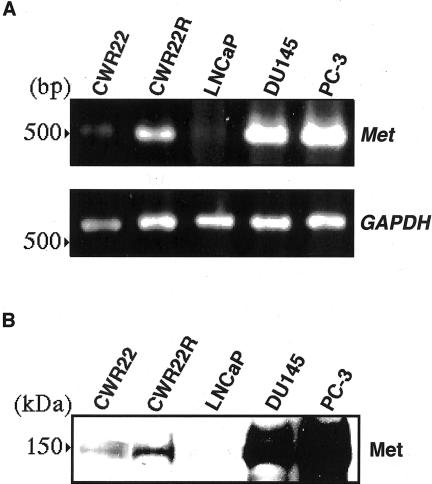
By RT-PCR and Western blotting, Met mRNA and protein were detected in both types of cells. However, CWR22R cells expressed Met mRNA and protein at a much higher level than did CWR22 cells (Figure 1, A and B). For detection of Met expression, we used DU145 and PC-3 cells as positive controls and LNCaP cells as a negative control as reported previously.28,31
Figure 1.
A: RT-PCR for the expression of Met mRNA in CWR22 and CWR22R cells. Cells used as positive controls for Met were DU145 and PC-3 cells and cells used as a negative control were LNCaP cells. Primers specific for Met and GAPDH cDNAs generated fragments of 516 bp and 782 bp in size, respectively. B: Western blot analysis for Met expression. Cell lysates were isolated and electrophoresed on 8% sodium dodecyl sulfate-polyacrylamide gel (50 μg of protein per lane). Blots were incubated with anti-Met antibodies and then were developed by the ECL kit. CWR22R cells expressed Met mRNA and protein at a much higher level than did CWR22 cells.
Effect on Growth of Exogenous HGF and Prostate Stromal Cells in Three-Dimensional Culture
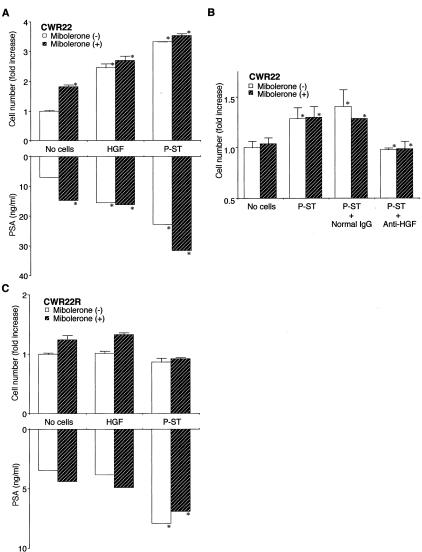
We tested the effect of exogenous HGF and prostate stromal cells on the growth of CWR22 and CWR22R cells in the presence or absence of 10 nmol/L synthetic androgen mibolerone. After a 14-day culture, exogenous recombinant human HGF (20 ng/ml) significantly stimulated the growth and PSA release of CWR22 cells by ∼2.5-fold regardless of the presence of mibolerone (P < 0.001 as compared with the control culture). Mibolerone was shown to have no additive or synergistic effects on the HGF-stimulated growth of CWR22 cells. In the 14-day culture, however, mibolerone alone enhanced the growth and PSA release of CWR22 cells. Prostate stromal cells in the collagen gel likewise enhanced the growth and PSA release of CWR22 cells by 2.8-fold (P < 0.001 over the control culture) (Figure 2A). Anti-HGF neutralizing antibody (2 μg/ml) completely abrogated the stimulatory effect of prostate stromal cells (P < 0.001) (Figure 2B). Exogenous HGF (up to 100 ng/ml) and the presence of prostate stromal cells in the collagen gel, on the other hand, had neither stimulatory nor inhibitory effect on the growth of CWR22R cells (Figure 2C). However, prostate stromal cells but not exogenous HGF induced the production of PSA in CWR22R cells (P < 0.001).
Figure 2.
Effect of exogenous HGF, prostate stromal cells (P-ST), or anti-human HGF neutralizing antibody on growth of androgen-dependent (CWR22; A and B) and -independent (CWR22R; C) human prostatic carcinoma cells in three-dimensional collagen culture. Cells were cultured in the appropriate medium containing 5% cFBS with or without 10 nmol/L of mibolerone, 20 ng/ml of human recombinant HGF, and/or 2 μg/ml of anti-human HGF neutralizing antibody. After incubation for 14 (A), 8 (B), or 4 (C) days, PSA in medium was measured, and cells were recovered and counted. Results are expressed as ratio to the control culture without mibolerone. Bars denote SD of triplicate samples. Growth of CWR22 was significantly stimulated by mibolerone, exogenous HGF, and co-cultured prostate stromal cells, and was suppressed by anti-HGF (*, P < 0.001, respective comparison), whereas growth of CWR22R cells was not. Mibolerone, however, has no additive or synergistic effects on HGF-stimulated growth of CWR22 cells.
Expression and Secretion of HGF by CWR22R Cells
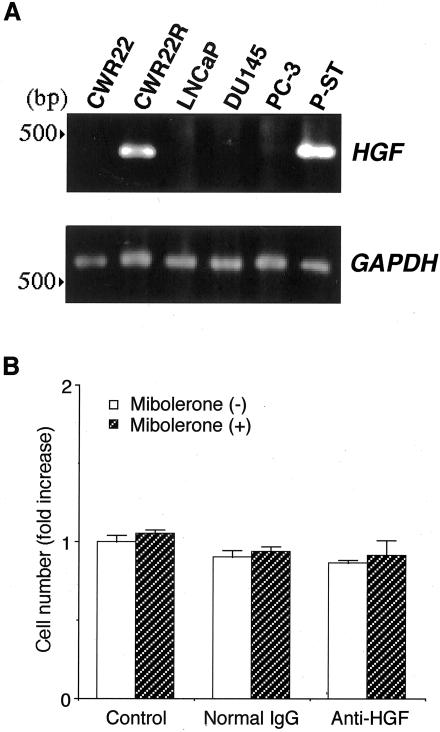
First, we examined the expression of HGF in prostatic carcinoma cells. HGF mRNA was detected only in CWR22R cells (Figure 3A). Analogous to the expression at mRNA level, only CWR22R cells released a large amount of HGF protein (Table 1). We used prostate stromal cells as a positive control for HGF expression and LNCaP, DU145, and PC-3 cells as negative controls.28,31 Next, we tested the possibility that HGF produced by CWR22R cells was an autocrine growth factor. Contrary to our expectation, anti-HGF neutralizing antibody had no effect on the growth of CWR22R cells (Figure 3B).
Figure 3.
A: RT-PCR for the expression of HGF mRNA in human prostate carcinoma cells. Cells used as a positive control for HGF were prostate stromal cells (P-ST) and cells used as negative controls were LNCaP and PC-3 cells. Primers specific for HGF and GAPDH cDNAs generated fragments of 396 bp and 782 bp in size, respectively. HGF mRNA was detected only in CWR22R cells. B: CWR22R cells were cultured in collagen gel. Anti-human HGF antibody was added into medium at a final concentration of 2 μg/ml. After incubation for 4 days, cells in the collagen layer were recovered and counted. Results are expressed as ratio to the control culture without mibolerone. Bars denote SD of triplicate samples. Anti-HGF neutralizing antibody had no effect on the growth of CWR22R cells.
Table 1.
HGF Release into CM by Human Prostate Carcinoma Cells
| Cells | HGF (pg/104 cells) |
|---|---|
| CWR22 | nm |
| CWR22R | 292 ± 2 |
| LNCaP | nm |
| DU145 | nm |
| PC-3 | nm |
| Prostate stromal cells | 677 ± 40 |
Any value under 40 pg/ml is not measurable (nm) with the assay kit used.
Biological Activity of HGF and Autocrine Activation of Met in CWR22R Cells
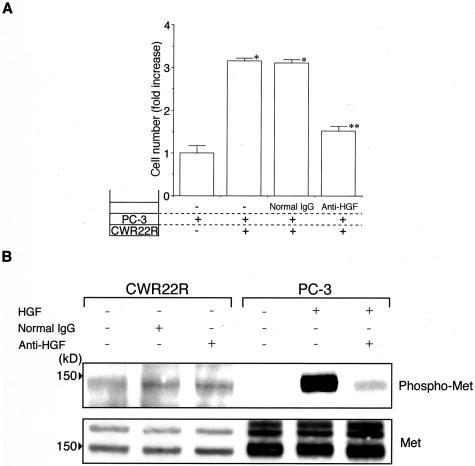
To address the question of why HGF secreted byCWR22R cells demonstrated no effect on their own growth, we examined the biological activity of CWR22R-released HGF and the phosphorylation status of Met. HGF secreted by CWR22R cells was tested for its biological activity in PC-3 cells that are known to respond to HGF.31 As compared to the control culture, the presence of CWR22R cells in the collagen gel significantly stimulated the growth of PC-3 cells, the result indicating that HGF secreted by CWR22R is physiologically active. PC-3 cells did not affect the growth of CWR22R cells at all (data not shown). Anti-HGF neutralizing antibody almost completely abrogated the stimulatory effect of CWR22R cells (P < 0.001) (Figure 4A).
Figure 4.
A: Effect of HGF secreted by CWR22R cells on growth of c-Met-expressing PC-3 cells. PC-3 (top layer) and CWR22R (bottom layer) cells were cultured in collagen gel. After incubation for 4 days, cells were counted. Anti-human HGF antibody was added to medium at a final concentration of 2 μg/ml. Results are expressed as ratio to the control culture. Bars denote SD of triplicate samples. The presence of CWR22R cells in the collagen gel significantly stimulated the growth of PC-3 cells and anti-HGF neutralizing antibody almost completely abrogated the stimulatory effect of CWR22R cells. *, P < 0.001 as compared with control culture. **, P < 0.001 as compared with positive control culture. B: Western blot analysis for phosphorylation of Met. Cell lysates were isolated and electrophoresed on 8% sodium dodecyl sulfate-polyacrylamide gel (50 μg for CWR22R and 20 μg for PC-3 of protein per lane). Blots were incubated with anti-phospho-Met (Tyr1349) antibodies and then were developed by the ECL Advance kit. Anti-HGF neutralizing antibodies drastically reduced the HGF-induced phosphorylation of Met in PC-3 cells, whereas there was no effect on the autophosphorylation of Met in CWR22R cells.
To evaluate the phosphorylation status of Met in CWR22R cells, we used the phospho-specific antibodies against Met on the tyrosine residue 1349. In Western blotting for phosphorylated Met, a specific single band was detected. In CWR22R cells, Met was constitutively phosphorylated regardless of HGF. In contrast, HGF markedly induced the phosphorylation of Met in PC-3 cells. Although addition of anti-HGF neutralizing antibodies to the medium dramatically decreased the HGF-induced phosphorylation of Met in PC-3 cells, they had no effect on the autophosphorylation of Met in CWR22R cells (Figure 4B). Met protein also was detected as two bands that indicated a precursor form (upper band) and a proteolytically processed mature form (lower band), respectively. The possibility that the enhanced tyrosine kinase activity was because of mutations39,40 was ruled out by demonstrating the normal sequence of the Met gene (data not shown). These results suggested that Met phosphorylation of CWR22R cells was regulated by an HGF autocrine loop.
Knockdown of Met Expression in CWR22R Cells by RNAi
Using the RNAi method, we examined the possibility that HGF synthesized by CWR22R cells functioned in an intracellular autocrine manner as reported in other cell systems.41,42 To evaluate transfection efficiency in CWR22R cells, we first performed co-transfection of Cy-3-labeled luciferase GL2-siRNA and the pGL2 luciferase reporter vector. As was demonstrated in Figure 5A almost all cells were transfected with the luciferase GL2-siRNA and the siRNA reduced the luciferase activity by ∼60%. Next, synthetic siRNA specific for Met or HGF was transfected into CWR22R cells. After a 48-hour culture, transfection of Met-siRNA but not HGF-siRNA reduced the phosphorylation and expression of Met protein to extremely low levels and suppressed the invasive growth by 20% (Figure 5B). HGF-siRNA reduced HGF release by 40% (Table 2). These results indicate that an HGF autocrine loop is one of androgen-independent growth support systems.
Figure 5.
A: Transfection efficiency and function of siRNAs. CWR22R cells were seeded at a density of 5 × 105 per well in a 60-mm culture dish. Twenty-four hours later, transfection was performed using lipofectamine reagent mixed with 360 pmol of Cy-3-labeled luciferase GL2-siRNA, 3 μg of the reporter plasmid pGL2, and 0.5 μg of pRL-TK Renila luciferase reporter. After 48 hours, cells were observed by a fluorescence phase contrast microscope with a Cy-3 filter and then lysed and their luciferase activity was measured. Almost all cells were transfected with the GL2-siRNA and the siRNA reduced the luciferase activity by ∼60% (P < 0.001). B: Knockdown of Met or HGF expression by RNAi. Transfections were performed in a 100-mm culture dish at 70% confluency by using lipofectamine reagent with 1 nmol of luciferase GL2-siRNA, Met-siRNA, or HGF-siRNA. Forty-eight hours later, cells were lysed for Western analysis or transferred to three-dimensional collagen gel cultures. After incubation for 4 days in the absence of androgen, cells were counted. Results are expressed as ratio to the control culture. Bars denote SD of triplicate samples. Met-siRNA but not HGF-siRNA inhibited the phosphorylation and expression of Met protein to extremely low levels and suppressed growth by 20% (P < 0.001).
Table 2.
Effect of HGF-siRNA on HGF Release by CWR22R Cells
| CWR22R cells | HGF (pg/104 cells) |
|---|---|
| GL2-siRNA | 212 ± 7 |
| Met-siRNA | 224 ± 5 |
| HGF-siRNA | 123 ± 4 |
Synthetic siRNA specific for HGF was transfected to CWR22R cells. After a 48 hour culture, HGF release into culture medium was measured. As compared to culture transfected with GL2-siRNA, HGF-siRNA reduced HGF level by 40%. Met-siRNA was also tested to demonstrate the specificity of HGF siRNA on HGF release.
Discussion
Several immunohistochemical studies including ours demonstrated that human prostate carcinoma cells but not normal secretory columnar cells of the prostatic acini express Met protein and that advanced stage prostate carcinoma cells express both Met and HGF in a high frequency.27–29,32,43 A recent study indicates that the serum HGF level in men with metastatic prostate carcinoma is significantly elevated as compared to that of men with localized carcinoma or to that of men without carcinoma.44 Although these data suggest that prostate cancer at an advanced stage is the source of HGF in circulation, the exact source or the role of HGF remains unclear. These observations, however, suggest the HGF/Met system may operate in localized (mostly androgen-dependent) as well as metastatic (androgen-dependent initially but androgen-independent after hormone therapy) prostate cancer.
To assess the role of HGF/Met in prostate cancer cells that express AR, we conducted a series of in vitro studies using androgen-dependent CWR22 and androgen-independent but still androgen-responsive CWR22R cells. The latter were derived from a CWR22 tumor as a regrowth after castration of tumor-bearing mice. Our results are summarized as follows: androgen-dependent CWR22 cells express AR and Met protein but do not produce HGF. Their growth in vitro is significantly stimulated by HGF, either exogenous or prostate stromal cell-derived. Mibolerone has no additive or synergistic effects on the HGF action. Androgen-independent CWR22R cells, on the other hand, secrete HGF in a large quantity and express Met protein at a much higher level than CWR22 cells. HGF secreted is functional, stimulating the growth of carcinoma cells that express Met. Met is constitutively phosphorylated in CWR22R cells and its knockdown by the RNAi method reduces their growth rate.
We have shown that HGF secreted by CWR22R cells is biologically active. The growth of CWR22R, however, was not inhibited by incubation with a large amount of anti-HGF antibodies. In search of literature we found a similar observation with interleukin-6 and colony-stimulating factor 1 as autocrine growth factors.41,42 In their studies, antiserum or antibodies failed to block their autocrine loop, whereas knockdown of mRNA expression by transfecting anti-sense oligonucleotide or construct inhibited hormonal actions.41,42 Our approach with RNAi technique demonstrated that knockdown of Met expression significantly suppressed cell growth. The results suggest that HGF functions via an intracellular autocrine mechanism.
The data presented in this investigation provide a strong support to the immunohistochemical observation that the HGF/Met system operates in both androgen-dependent and androgen-independent prostate cancer cells, albeit by different mechanisms, paracrine in the former and autocrine in the latter. It would be desirable to confirm our in vitro observation with additional carcinoma cell lines. To our knowledge, however, CWR22R is the only cell line to express AR, Met, and HGF. Most other prostatic carcinoma cell lines (such as PC-3 and DU145) express Met only.
Immunohistochemical data also indicate that HGF expression in advanced-stage carcinoma (73%) is not as frequent as Met protein expression (96%),32 suggesting that some carcinomas may express Met only. Therefore we should not dismiss the distinct possibility that the HGF/Met operates by a paracrine manner; the ligand, HGF, provided locally by the stromal cells at metastatic site45 or by circulation.44 We suggest that in localized cancer (mostly androgen-dependent) growth support is at least dual, namely, androgen/AR and HGF/Met but that androgen-independent cancer cells that have survived hormone therapy use the autocrine and paracrine HGF/Met loop.
Acknowledgments
We thank Dr. T.G. Pretlow for the CWR22 xenograft and Dr. J.W. Jacobberger for CWR22R cells.
Footnotes
Address reprint requests to Koh-ichi Nakashiro, Department of Oral and Maxillofacial Surgery, Ehime University School of Medicine, 454 Shitsukawa, Shigenobu-cho, Onsen-gun, Ehime 791-0295, Japan. E-mail: nakako@m.ehime-u.ac.jp.
Supported by the National Institutes of Health (grants CA 14649 and CA 33511), the Joseph L. Mayberry Sr. Research Fund, and a grant-in aid from the Ministry of Education, Science, and Culture of Japan.
References
- Parker SL, Tong T, Boden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Kozlowski JM, Ellis WJ, Grayhack JT. Andriole GL, Catalona WJ, editors. Philadelphia: WB Saunders Co.,; The Urologic Clinics of North America. 1991:pp 15–24. [Google Scholar]
- Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- Koivisto P, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest Suppl. 1996;226:S57–S63. [PubMed] [Google Scholar]
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- Nakayama T, Watanabe M, Suzuki H, Toyota M, Sekita N, Hirokawa Y, Mizokami A, Ito H, Yatani R, Shiraishi T. Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest. 2000;80:1789–1796. doi: 10.1038/labinvest.3780190. [DOI] [PubMed] [Google Scholar]
- Abrahamsson PA. Neuroendocrine differentiation in prostate carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate Suppl. 1998;8:S18–S22. [PubMed] [Google Scholar]
- Michalopoulos G, Houck KA, Dolan ML, Luetteke NC. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Russell WE, McGowan JA, Bucher NL. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984;119:183–192. doi: 10.1002/jcp.1041190207. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- Siegfried JM, Weissfeld LA, Singh-Kaw P, Weyant RJ, Testa JR, Landreneau RJ. Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res. 1997;57:433–439. [PubMed] [Google Scholar]
- Wang Y, Selden C, Morgan N, Stamp GWH, Hodgson HJF. Hepatocyte growth factor/scatter factor expression in human mammary epithelium. Am J Pathol. 1994;144:675–682. [PMC free article] [PubMed] [Google Scholar]
- Tuck AB, Park M, Sterns EE, Boag A, Elliott BE. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- Jin L, Fuchs A, Schnitt SJ, Yao Y, Joseph A, Lamszus K, Park M, Goldberg ID, Rosen EM. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer. 1997;79:749–760. doi: 10.1002/(sici)1097-0142(19970215)79:4<749::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao MS. Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol. 1995;147:799–895. [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Chung YS, Yashiro M, Nishimura S, Hasuma T, Otani S, Sowa M. Transforming growth factor-β and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res. 1997;88:152–159. doi: 10.1111/j.1349-7006.1997.tb00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Date K, Shimura H, Nakamura T. Acquisition of invasive phenotype in gallbladder cancer cells via mutual interaction of stromal fibroblasts and cancer cells as mediated by hepatocyte growth factor. Jpn J Cancer Res. 1996;87:702–710. doi: 10.1111/j.1349-7006.1996.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Weiss GH, Jin L, Fuchs A, Chowdhury S, O’Shaugnessy P, Goldberg ID, Rosen EM. Expression of scatter factor in human bladder carcinoma. J Natl Cancer Inst. 1995;87:372–377. doi: 10.1093/jnci/87.5.372. [DOI] [PubMed] [Google Scholar]
- Pisters LL, Troncoso P, Zhau HE, Li W, von Eschenbach AC, Chung LWK. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293–298. [PubMed] [Google Scholar]
- Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, Day ML. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147:386–396. [PMC free article] [PubMed] [Google Scholar]
- Kurimoto S, Moriyama N, Horie S, Sakai M, Kameyama S, Akimoto Y, Hirano H, Kawabe K. Co-expression of hepatocyte growth factor and its receptor in human prostate cancer. Histochem J. 1998;30:27–32. doi: 10.1023/a:1003262412346. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Nakashiro K, Okamoto M, Hayashi Y, Oyasu R. Hepatocyte growth factor secreted by prostate-derived stromal cells stimulates growth of androgen-independent human prostatic carcinoma cells. Am J Pathol. 2000;157:795–803. doi: 10.1016/s0002-9440(10)64593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiro K, Hayashi Y, Oyasu R. Immunohistochemical expression of hepatocyte growth factor and c-Met/HGF receptor in benign and malignant human prostate tissue. Oncol Rep. 2003;10:1149–1153. [PubMed] [Google Scholar]
- de Vere White R, Meyers F, Chi SG, Chamberlain S, Siders D, Lee F, Stewart S, Gumerlock PH. Human androgen receptor expression in prostate cancer following androgen ablation. Eur Urol. 1997;31:1–6. doi: 10.1159/000474409. [DOI] [PubMed] [Google Scholar]
- Wainstein MA, He F, Robinson D, Kung HJ, Schwartz S, Giaconia JM, Edgehouse NL, Pretlow TP, Bodner DR, Kursh ED, Resnick MI, Seftel A, Pretlow TG. CWR22: androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 1994;54:6049–6052. [PubMed] [Google Scholar]
- Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–3046. [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- Nakashiro K, Kawamata H, Hino S, Uchida D, Miwa Y, Hamano H, Omotehara F, Yoshida H, Sato M. Down-regulation of TSC-22 (transforming growth factor β-stimulated clone 22) markedly enhances the growth of a human salivary gland cancer cell line in vitro and in vivo. Cancer Res. 1998;58:549–555. [PubMed] [Google Scholar]
- Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim UR, Feltis JT, Casadevall C, Zamarron A, Bernues M, Richard S, Lips CJM, Walther MM, Tsui LC, Geil L, Orcutt ML, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson MD, Moch H, Storkel S, Lerman MI, Linehan WM, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, De Stefani A, Valente G, Giordano S, Cortesina G, Comoglio PM. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Smadja F, Stanley R, Leibovitch SA. Colony-stimulating factor 1 (CSF-1) is involved in an autocrine growth control of rat myogenic cells. Exp Cell Res. 1995;218:213–222. doi: 10.1006/excr.1995.1149. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Wolfenbarger D, DeBram-Hart M, Kanangat S, Weiss DT, Solomon A. Characterization of a novel interleukin-6 autocrine-dependent human plasma cell line. Leukemia. 1994;8:2207–2213. [PubMed] [Google Scholar]
- Knudsen BS, Gmyrek GA, Inra J, Scherr DS, Vaughan ED, Nanus DM, Kattan MW, Gerald WL, Vande Woude GF. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- Naughton M, Picus J, Zhu X, Catalona WJ, Vollmer RT, Humphrey PA. Scatter factor-hepatocyte growth factor elevation in the serum of patients with prostate cancer. J Urol. 2001;165:1325–1328. [PubMed] [Google Scholar]
- Lang SH, Clarke NW, George NJ, Testa NG. Scatter factor influences the formation of prostate epithelial cell colonies on bone marrow stroma in vitro. Clin Exp Metastasis. 1999;17:333–340. doi: 10.1023/a:1006696002497. [DOI] [PubMed] [Google Scholar]