Abstract
Development of a reliable method of isolating highly proliferative potential hepatocytes provides information crucial to progress in the field of hepatocyte transplantation. The aim of this study was to develop reliable hepatocyte transplantation using highly proliferative, eg, progenitor-like hepatocytes, based on asialoglycoprotein receptor (ASGPR) expression levels for hepatocyte transplantation. We have previously reported that mouse hepatocytes with low ASGPR expression levels have highly proliferative potential and can be used as progenitor-like hepatocytes. We therefore fractionated F344 male rat hepatocytes expressing low and high levels of ASGPR and determined the liver repopulation capacity of hepatocytes according to low and high ASGPR expression in the liver. Next, 2 × 105 cells of each type were transplanted into female liver regenerative model dipeptidyl peptidase-deficient rats, and we estimated the rate of liver repopulation by the transplanted hepatocytes in the host liver, as determined by recognition of the Sry gene on the Y-chromosome. At 60 days after hepatocyte transplantation, the transplanted hepatocytes occupied ∼76% of the total hepatocyte mass in the case of the transplantation of hepatocytes with low ASGPR expression, but accounted for ∼12% and 17% of the mass in the case of the transplantation of hepatocytes with high ASGPR expression and unfractionated hepatocytes, respectively. In conclusion, these findings suggest that hepatocytes with low ASGPR expression can result in normal liver function and a high repopulation capacity in vivo. These results provide insight into development of a strategy for effective liver repopulation using transplanted hepatocytes.
Liver transplantation is one of the most important surgical means of saving the lives of patients suffering from otherwise fatal hepatic diseases such as fulminant hepatitis and severe congenital liver failure. However, the donor shortage and the need for life-long immunosuppression, with its known side effects, are limitations of this therapy. Hepatocyte transplantation is a potential alternative to whole-organ replacement in humans. The concept behind therapeutic hepatocyte transplantation is that the reconstruction of functional parenchyma by transplanted hepatocytes supports the function of the injured liver. Several experimental studies involving transplantation of normal mature hepatocytes have achieved important therapeutic goals in a variety of metabolic liver diseases.1 However, hepatocyte transplantation has not yet been established as a reliable alternative to liver transplantation. It has been reported that the reconstruction of functional parenchyma by transplanted hepatocytes requires time, during which donor cells proliferate and then establish normal parenchymal architecture. It takes a considerable amount of time for a limited number of transplanted hepatocytes to proliferate and differentiate into fully functioning cells in vivo.2
To realize the goal of implementing hepatocyte transplantation as a reliable alternative to liver transplantation, transplanted hepatocytes must proliferate efficiently in the host liver. For the development of a successful hepatocyte transplantation therapy, it will be crucial to develop a reliable method of isolating highly proliferative potential hepatocytes. To develop a reliable strategy for hepatocyte transplantation, we considered that it would be necessary to isolate highly proliferative potential hepatocytes from the mature liver. Recently, small hepatocytes and oval cells have been proposed as important candidates for liver-specific capacity.3 It is quite difficult to isolate these minor subsets of liver cells because no novel antigenic marker has thus far been identified; the cells are identified only by their small size. It has been reported that a fraction of adult mouse hepatocytes have growth potential similar to that of hematopoietic stem cells4 and that highly proliferative hepatocytes are not necessarily small-sized cells.5 We have previously hypothesized that hepatocytes with low asialoglycoprotein receptor (ASGPR) expression levels would potentially be highly proliferative, and then reported the isolation of highly proliferative, eg, progenitor-like, hepatocytes based on ASGPR expression levels using a galactose-carrying polymer, poly[N-p-vinylbenzyl-O-β-d-galactopyranosyl-(1→4)-d-gluconamide] (PVLA) as an artificial ligand for ASGPR.6,7 ASGPR is expressed exclusively on hepatocytes. The expression of ASGPR in the liver is developmentally regulated and is reduced under physiological and pathological conditions, and ASGPR is an endocytotic and heterotrimeric receptor (hepatic lectin-1 and -2), that mediates the clearance of serum desialylated glycoproteins with terminal galactose or N-acetylgalactosamine residues. Accordingly, we investigated the reliability of hepatocyte transplantation using hepatocytes with low ASGPR expression levels.8,9
The aim of the present study was to isolate highly proliferative, eg, progenitor-like hepatocytes, based on ASGPR expression levels for hepatocyte transplantation. We performed a 90-day observation of transplanted PVLA-fractionated hepatocytes to evaluate their ability to replace hepatic mass in rats.
Materials and Methods
Cell Preparation
Rat primary hepatocytes were isolated from the livers of male DPPIV+ F344 rats (8 to 12 weeks old) (Japan SLC, Inc., Shizuoka, Japan) using the modified in situ perfusion method.7 Briefly, the dead parenchymal hepatocytes were completely removed by density gradient centrifugation using Percoll (Amersham Bioscience, Amersham, UK). The nonparenchymal cells were completely removed by centrifugation (50 × g, three times). The viable parenchymal hepatocytes were then suspended in William’s medium E (Sigma Chemical Co., St. Louis, MO) containing antibiotics (50 μg/ml penicillin, 50 μg/ml streptomycin, and 100 μg/ml neomycin). Only isolated hepatocytes with ∼99% viability were used in this study.
The Fractionation of PVLA-Bound and PVLA-Unbound Hepatocytes Using PVLA
Aliquots of 10 ng/ml PVLA (Seikagaku Corp., Tokyo, Japan) solution (25 ml) were placed in 9-cm bacteriological plastic dishes (Sterillin; Stone, Staffs, UK) for overnight incubation at 37°C, as described previously, but with slight modifications.7 After coating the dishes with PVLA, the remaining solution was decanted and the surface of the PVLA-coated dishes was blocked with Hanks’ solution containing 0.2 mg/ml bovine serum albumin (Sigma) for 2 hours at 37°C. To fractionate the hepatocytes with respectively low and high ASGPR expression levels by exploiting the interaction between the hepatocytes and the PVLA-coated dishes, we performed assays to attach the hepatocytes to the dishes. Briefly, isolated hepatocytes in William’s medium E were seeded at a density of 3.2 × 106 cells/cm2 on 10 ng/ml PVLA-coated dishes and were incubated for 60 minutes at 37°C. After incubation, hepatocytes unable to adhere to the PVLA-coated dishes, ie, the PVLA-unbound hepatocytes, were harvested from the medium. The hepatocytes able to adhere to the PVLA-coated dishes, ie, PVLA-bound hepatocytes, were detached from the PVLA-coated dishes by gentle treatment with Hanks’ solution containing 5 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (Nacalai Tesque, Inc., Kyoto, Japan) because the specific interaction between ASGPR and PVLA depended on the Ca2+ concentrations. Hepatocytes that were not separated on the PVLA-coated dishes were used as a control (unfractionated cells). After separation, the dead hepatocytes were completely removed from PVLA-fractionated hepatocytes and unfractionated hepatocytes by density gradient centrifugation using Percoll (Amersham).
Cell Staining and Flow Cytometric Analysis
After hepatocytes were separated in 10 ng/ml PVLA-coated dishes, these hepatocytes were fixed in 4% paraformaldehyde (Nacalai Tesque) for 30 minutes at 4°C. After washing three times with phosphate-buffered saline (PBS), cells were resuspended in PBS containing 0.02% bovine serum albumin, with rabbit anti-mouse ASGPR-1 antiserum and incubated for 1 hour at 4°C. It has previously been reported that this antiserum can recognize the extracellular domain of ASGPR-1.10,11 After washing, fluorescein isothiocyanate-conjugated anti-rabbit IgG (Seikagaku) was added and incubated for 30 minutes at 4°C. Finally, cells were washed and resuspended in PBS. Flow cytometric analyses were performed using FACSCalibur (Becton Dickinson Company, Ltd., Franklin Lakes, NJ).
Hepatocyte Transplantation
Recipient DPPIV− F344 rats were purchased from Charles River Japan, Inc. (Tokyo, Japan). All animals were maintained on daily cycles of alternating 12-hour light/dark with food and water available ad libitum. DPPIV− F344 rats were given two injections of retrorsine (Sigma), 30 mg/kg each, intraperitoneally, at time points that were 2 weeks apart. Four weeks after the final injection, a 70% partial hepatectomy was performed on the rats. PVLA-unbound hepatocytes, PVLA-bound hepatocytes, and unfractionated cells were transplanted into these retrorsine/partial hepatectomy rats via the portal vein. The number of transplanted cells was 2 × 105 cells in the case of all cell types, ie, PVLA-unbound hepatocytes, PVLA-bound hepatocytes, and unfractionated cells. In each experiment after transplantation, transplanted rats were sacrificed and all livers were examined to observe and analyze the transplanted hepatocytes. Each animal group received transplanted hepatocytes of each type, ie, PVLA-unbound hepatocytes, PVLA-bound hepatocytes, and unfractionated cells was established with three to five rats at each time point studied.
Detection of Transplanted Hepatocytes
The transplanted hepatocytes in the host livers were detected by DPPIV histochemical staining according to previously published techniques.3 Briefly, cryosections (10 μm thick) were prepared from the host livers and were fixed in ice-cold acetone for 5 minutes. The sections were incubated for 60 minutes in a substrate solution consisting of 0.5 mg/ml gly-pro-methoxy-β-naphthylamide (Sigma), 1 mg/ml Fast Blue BB (Sigma), 100 mmol/L phosphate-buffer (pH 6.5), and 100 mmol/L NaCl. All tissue sections were counterstained with hematoxylin.
Immunohistochemical and Histochemical Analysis
The tissue-distribution of ASGPR was detected by immunohistochemical analysis. Paraffin-embedded sections (3 μm) were prepared by standard methods. Sections were incubated overnight at 4°C with rabbit anti-mouse ASGPR-1 antiserum as the primary antibodies.10,11 After the sections were washed with PBS, they were incubated with peroxidase-conjugated anti-rabbit IgG (Zymed Laboratories, Inc., South San Francisco, CA). Peroxidase enzyme activity was detected with 3-amino-9-ethylcarbazole (Nacalai Tesque) as the substrate.
Albumin and glutamine synthetase was detected by immunohistochemical analysis. Cryosections (10 μm thick) were prepared from the host livers and were fixed in 4% paraformaldehyde for 15 minutes before being incubated overnight at 4°C with rabbit anti-rat albumin IgG (Cappel, Durham, NC) and goat anti-glutamine synthetase IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) as the primary antibodies. After the cryosections were washed with PBS, they were incubated with peroxidase-conjugated anti-rabbit IgG and peroxidase-conjugated anti-goat IgG as the secondary antibodies (Zymed). Peroxidase enzyme activity was detected with 3-amino-9-ethylcarbazole (Nacalai Tesque) and 3,3-diaminobenzidine (Vector Laboratories, Inc., Burlingame, CA) as the substrate. Glycogen was detected by periodic acid-Schiff assay. Glucose-6-phosphatase activity detected by a substrate mixture consisting of 0.025% potassium glucose-6-phosphate and 0.12% lead nitrate in 0.2 mol/L Tris-maleate buffer (pH 6.7). Cryosections (10 μm thick) were incubated with the substrate for 15 minutes, after which a sulfide solution was added. All tissue sections were counterstained with hematoxylin.
Polymerase Chain Reaction (PCR) Analysis of Transplanted Hepatocytes
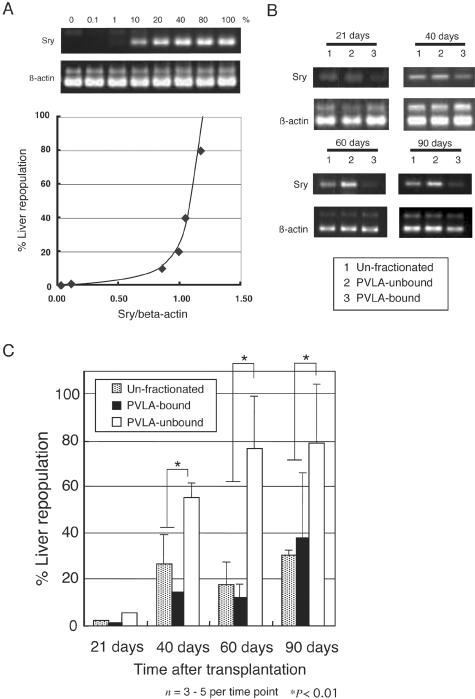
For the DNA analysis, genomic DNA from the liver was extracted by a DNeasy Tissue Kit (Qiagen, GmbH, Germany). Rat Sry gene PCR was performed using the Sry primers (sense 5′-CAGAGATCAGCAAGCATCTGG-3′; anti-sense 5′-TCTGGTTCTT-GGAGGACTGG-3′) at 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 1 minute. The rat β-actin gene was used as an internal control, with primers for β-actin (sense 5′-AGAGAAGCTGTGCTATGTTGC-3′; anti-sense 5′-GTACTCCTGCTTGCTGA-TCC-3′) being used at 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 1 minute. The repopulation percentages were calculated by dilution analyses of hepatocyte DNA. Sry DPPIV+ male rat hepatocytes were serially diluted with DPPIV− female rat hepatocytes. The PCR amplification signal was quantitated by densitometric analysis of Sry and β-actin PCR using NIH imaging. The relative values of the Sry gene in the host livers were calculated as follows: Sry/β-actin PCR amplification signal. Curve regression was obtained for serial dilutions between 0% and 100%. The percentages of the repopulation efficiency were determined using this curve regression of the slope and the relative values from the Sry gene. Data are presented as means ±SE. The unpaired Student’s t-test and Welch’s t-test were used for statistical analysis and P < 0.01 was considered significant. Each animal group transplanted hepatocytes of each type, ie, PVLA-unbound hepatocytes, PVLA-bound hepatocytes, and unfractionated cells was established with three to five rats at each time point studied.
Detection of Initial Engraftment of Transplanted Hepatocytes
The initial engraftments of 2 × 106 transplanted hepatocytes in the host livers were detected by flow cytometric analysis using anti-rat DPPIV antibody (Cosmo Bio Co., Ltd., Tokyo, Japan) at 5 days after transplantation, because the obtained engraftment was too little to analyze initial engraftment of 2 × 105 transplanted hepatocytes by PCR analysis. We transplanted 2 × 106 PVLA-fractionated and -unfractionated DPPIV+ hepatocytes into rat DPPIV− liver and at 5 days after transplantation, the hepatocytes were isolated from the host liver that had transplanted hepatocytes of each cell type. These isolated hepatocytes were fixed in 4% paraformaldehyde (Nacalai Tesque) for 30 minutes at 4°C. After washing three times with PBS, cells were resuspended in PBS containing 0.02% bovine serum albumin, with anti-rat DPPIV monoclonal antibody and incubated for 1 hour at 4°C. After washing, fluorescein isothiocyanate-conjugated anti-mouse IgG (Seikagaku) was added and incubated for 30 minutes at 4°C. Finally, cells were washed and resuspended in PBS. Flow cytometric analyses were performed using FACSCalibur (Becton Dickinson). Negative control showed the population of hepatocytes that were stained only fluorescein isothiocyanate-conjugated anti-mouse IgG as the secondary antibody.
Western Blot Analysis
Hepatocytes were lysed in 1 ml of cold lysis buffer (10 mmol/L Tris, pH 7.4,, 150 mmol/L NaCl, 1% Nonidet P-40, 1 mmol/L ethylenediaminetetraacetic acid, 20 mmol/L n-octyl-β-d-glucopyranoside, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml leupeptin, and 2 μg/ml aprotinin) (Wako Pure Chemical, Tokyo, Japan). Equal amounts of protein were mixed with 4% sodium dodecylsulfate, 1.2% β-mercaptoethanol, and 20% glycerol, and then the samples were boiled at 95°C for 10 minutes to be resolved by sodium dodecylsulfate-polyacrylamide gel electrophoresis (10%). Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA) for immunoblotting analysis with monoclonal anti-ASGPR antibody (Daiichi Kagaku Yakuhin, Tokyo, Japan), anti-β-actin antibody (Sigma), or rabbit anti-rat albumin (Cappel). The membrane was developed using the enhanced chemiluminescence method (Amersham) according to the manufacturer’s instructions. Densitometric analysis was performed using NIH imaging.
RNA Isolation and Northern Blot Analysis
Total cellular RNA was extracted with Isogen (Wako) and 10 μg RNA was loaded in each lane of a 1% agarose gel containing 18% formamide. After electrophoresis, RNA was transferred to Hybond-N membranes (Amersham) using the capillary method. After cross-linking under UV light, the membrane was hybridized with a FluoroGreen (Amersham)-labeled 0.7-kb RHL-1 (rat hepatic lectin-1; the major subunit of ASGPR) cDNA probe for 17 hours at 65°C. Hybridization and washing of the membrane were performed according to the manufacturer’s instructions. The membrane was developed using enhanced chemiluminescence methods (Amersham).
Results
Hepatocyte Isolation by PVLA
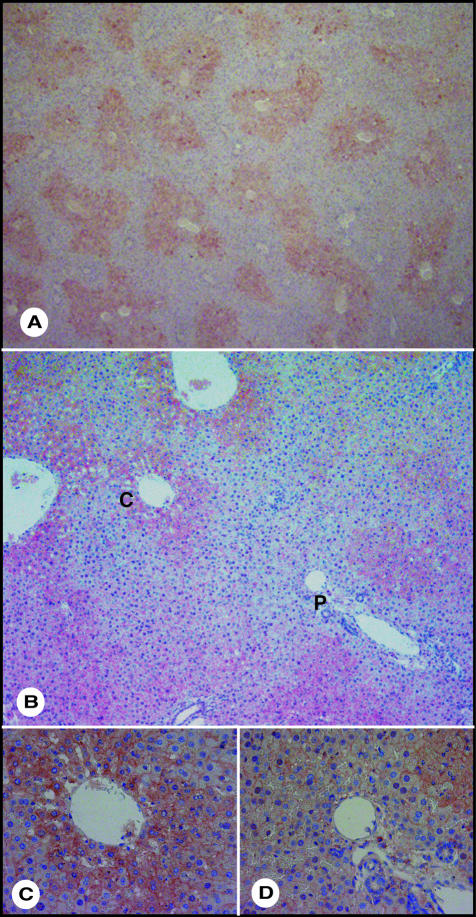
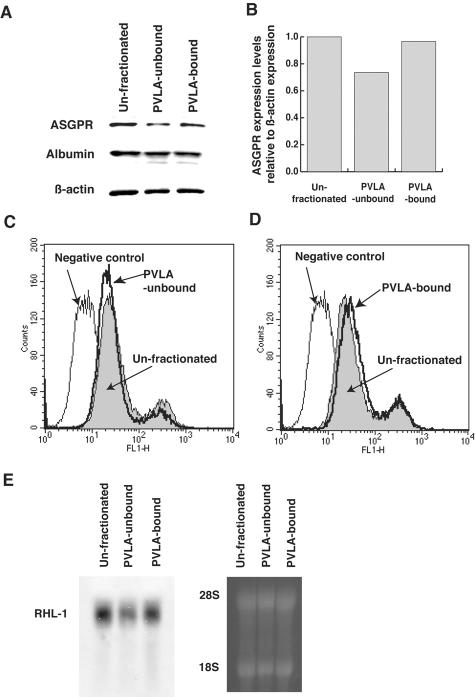
We examined the hepatic lobule distribution of ASGPR by immunohistochemical analysis. ASGPR was expressed all over the hepatic lobule, but its expression was strong in hepatocytes of the central vein area and weak in hepatocytes of portal vein area (Figure 1; A to D). The results showed that hepatocytes with low and high ASGPR expression existed in rat liver. To isolate hepatocytes with low ASGPR expression levels, we seeded rat hepatocytes on 10 ng/ml PVLA-coated dishes and incubated them for 60 minutes at 37°C. After incubation, hepatocytes that were able to adhere (PVLA-bound hepatocytes) and those unable to adhere to PVLA-coated dishes (PVLA-unbound hepatocytes) were separately harvested. The PVLA-unbound hepatocytes accounted for ∼20% of the total number of hepatocytes. We confirmed whether PVLA-bound and -unbound hepatocytes expressed high or low levels of ASGPR by Western blot analysis. The ASGPR levels were lower in the PVLA-unbound hepatocytes (lane 2) than in the PVLA-bound (lane 3) hepatocytes and were also lower than those of the controls, ie, unfractionated hepatocytes (lane 1) (Figure 2, A and B). Unfractionated, PVLA-unbound, and PVLA-bound hepatocytes expressed albumin equally; therefore, only hepatocytes, but not nonparenchymal cells, were included among the fraction of PVLA-unbound hepatocytes (Figure 2A). To confirm the difference in ASGPR expression in PVLA-separated hepatocytes, we performed flow cytometric analysis and Northern blot analysis. The expression of ASGPR was lower in the PVLA-unbound hepatocytes than in the PVLA-bound and unfractionated hepatocytes by flow cytometric analysis (Figure 2, C, and D). Total RNA was isolated from unfractionated, PVLA-unbound, and PVLA-bound hepatocytes for Northern blot analysis using an RHL-1 (rat hepatic lectin-1; the major subunit of ASGPR) cDNA probe. As shown in Figure 2E, the ASGPR message levels were considerably lower for PVLA-unbound hepatocytes (lane 2) than for unfractionated (lane 1) and PVLA-bound hepatocytes (lane 3). These results confirm that the hepatocytes that bound to the PVLA-coated dish (PVLA-bound hepatocytes) expressed high levels of ASGPR and those that did not bind (PVLA-unbound hepatocytes) expressed low levels of ASGPR.
Figure 1.
Immunohistochemical analysis for ASGPR. A: Immunological staining for ASGPR (red). B: High magnified image. p, portal area; c, central vein. C: Central vein area. D: Portal area. Original magnifications: ×40 (A); ×100 (B); ×400 (C, D).
Figure 2.
The analysis of ASGPR expression levels in control cells (unfractionated), PVLA-unbound, and PVLA-bound hepatocytes. A and B: The analysis of ASGPR expression in PVLA-fractionated hepatocytes using Western blot analysis representatively. A: ASGPR, albumin, and β-actin expression of unfractionated (lane 1), PVLA-unbound (lane 2), and PVLA-bound hepatocytes (lane 3). B: Densitometric analysis in Western blot result of ASGPR expression levels based on β-actin expression levels. C and D: The analysis of ASGPR expression in PVLA-fractionated hepatocytes using flow cytometry. C: ASGPR expression in PVLA-unbound hepatocytes (bold line). D: ASGPR expression in PVLA-bound hepatocytes (bold line). E: Northern blot analysis. RNA was collected from the unfractionated (lane 1), PVLA-unbound (lane 2), and PVLA-bound hepatocytes (lane 3) and the subsequent Northern blot analysis used the probe for RHL-1, the major subunit of ASGPR. Equivalent loading and integrity of RNA were confirmed by analyzing the bands for 28S and 18S ribosomal RNA, respectively.
Liver Repopulation by Transplantation of PVLA-Bound, -Unbound, and -Unfractionated Hepatocytes
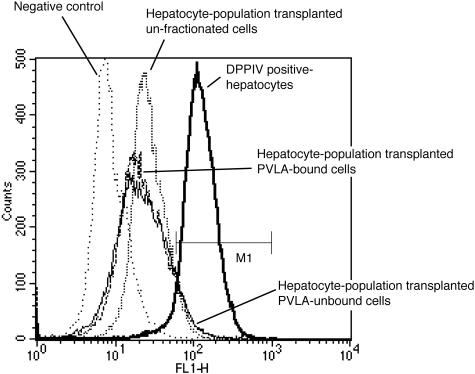
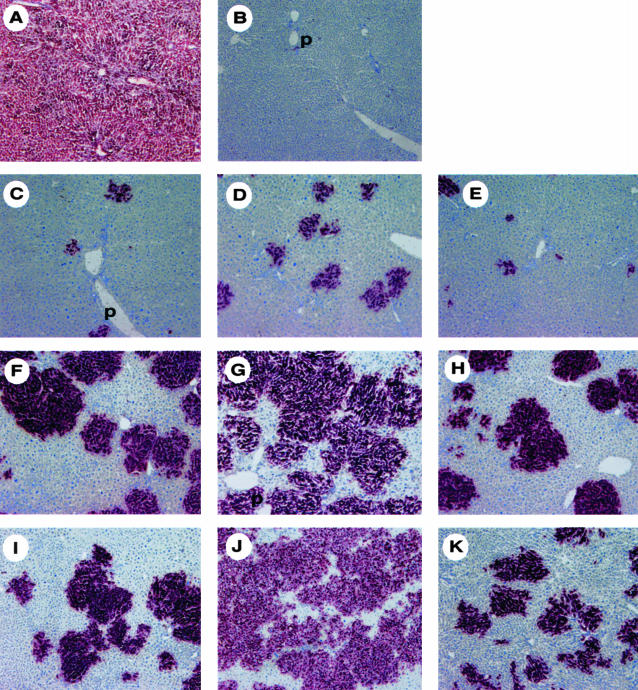
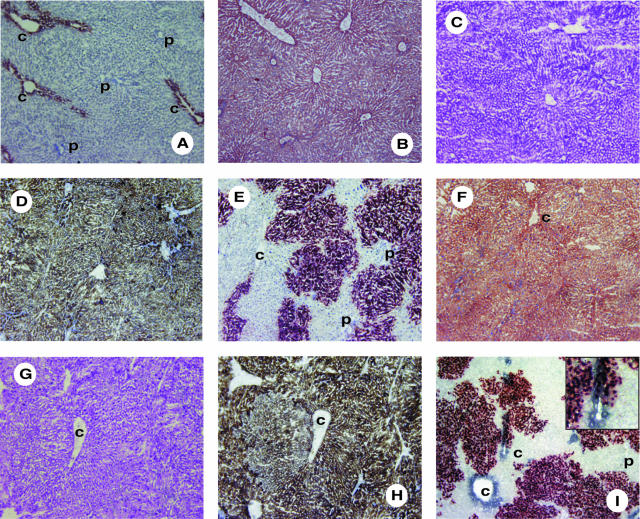
To determine whether PVLA-fractionated hepatocytes repopulated the liver in the liver regenerative model rat, we transplanted 2 × 105 PVLA-fractionated hepatocytes from male rats to the liver of retrorsine/partial hepatectomy female rats. To distinguish between donor and recipient hepatocytes, transplanted hepatocytes were isolated from DPPIV+ male F344 rats and these hepatocytes were transplanted to DPPIV− female F344 rats. To confirm whether the number of initial engrafted hepatocytes differed among unfractionated, PVLA-unbound, and PVLA-bound hepatocytes at the initial stage, we performed flow cytometric analysis for the initial engrafted DPPIV+ male hepatocytes using anti-rat DPPIV antibody at 5 days after transplantation. The percentage of engrafted hepatocytes in total hepatocytes was 4.31% for unfractionated, 6.85% for PVLA-unbound, and 6.71% for PVLA-bound hepatocytes in case of transplantation of 2 × 106 cells (Figure 3, M1), therefore we assumed that the initial engrafted hepatocytes of each type in case of 2 × 105 cells would be 0.431% for unfractionated, 0.685% for PVLA-unbound, and 0.671% for PVLA-bound hepatocytes approximately. These results showed that the number of initial engrafted DPPIV+ male hepatocytes did not differ among unfractionated, PVLA-unbound, and PVLA-bound hepatocytes. We then investigated the distribution of these transplanted hepatocytes, as demonstrated by a histochemical stain for DPPIV in the host liver. At 21, 40, and 60 days after cell transplantation, numerous large clusters of DPPIV+ donor PVLA-unbound hepatocytes were present throughout the DPPIV− recipient livers (Figure 4; D, G, and J). Moreover, a few clusters of DPPIV+ donor PVLA-bound (Figure 4; E, H, and K) and unfractionated hepatocytes (Figure 4; C, F, and I) were present. Small and large clusters were mixed in the liver sections with transplanted unfractionated hepatocytes (Figure 4; C, F, and I). Almost all of the clusters were small, but several large clusters were also observed in the liver sections with transplanted PVLA-bound hepatocytes (Figure 4; E, H, and K). Clusters of PVLA-fractionated hepatocytes were composed of approximately normal-sized hepatocytes. The location of the engrafted DPPIV+ hepatocytes did not differ among the three groups, with all being scattered in the liver lobule.
Figure 3.
Flow cytometric analysis for initial engraftments of transplanted hepatocytes of each cell type. PVLA-fractionated and unfractionated hepatocytes (2 × 106) were transplanted into rat liver and at 5 days after transplantation, the hepatocytes were isolated from the host liver that was transplanted with hepatocytes of each cell type. DPPIV-expressed hepatocytes in these host livers were analyzed by flow cytometry using anti-DPPIV antibody. Negative control showed the population of hepatocytes that were stained only with fluorescein isothiocyanate-conjugated anti-mouse IgG as the secondary antibody. M1 showed the population of DPPIV+ hepatocytes for each cell type.
Figure 4.
Histochemical stain for DPPIV in the host liver representatively after transplantation of 2 × 105 hepatocytes. The transplanted DPPIV+ hepatocytes were stained red in the host liver samples. A: Positive control of DPPIV+ male F344 rat liver. B: Negative control of DPPIV− female F344 rat liver. C to E: DPPIV stain of the host liver at 21 days after transplantation, unfractionated hepatocytes (C), PVLA-unbound hepatocytes (D), and PVLA-bound hepatocytes (E). F to H: DPPIV stain of the host liver at 40 days after transplantation, unfractionated hepatocytes (F), PVLA-unbound hepatocytes (G), and PVLA-bound hepatocytes (H). I to K: DPPIV stain of the host liver at 60 days after transplantation, unfractionated hepatocytes (I), PVLA-unbound hepatocytes (J), and PVLA-bound hepatocytes (K). p, portal area. Original magnifications, ×100.
In a parallel study, we estimated the extent of the liver repopulation by transplanted hepatocytes in the host liver by PCR quantification of the Sry gene on the Y-chromosome (Figure 5). We examined whether male DNA that was serially diluted with female hepatocytes and the relative values of Sry/β-actin PCR amplification signal were correlative or not. The results showed that there was a correlation with these relative values, and a curve regression was obtained for serial dilutions between 0% and 100% (Figure 5A). Figure 5B provides a single representative example of the Sry and β-actin PCR amplification signal for unfractionated, PVLA-unbound, and PVLA-bound hepatocytes at each time point. Figure 5C shows the percentage of repopulation efficiency for transplanted hepatocytes at each time point determined using the curve regression of the slope and the relative values from the Sry gene in host liver. At 40 days, PVLA-unbound hepatocytes occupied ∼55% of the host liver, and PVLA-bound hepatocytes and unfractionated hepatocytes occupied ∼15% and 20%, respectively (Figure 5C). At 60 days, hepatocytes with low levels of ASGPR occupied ∼76% of the host liver, and the high ASGPR hepatocytes and the unfractionated hepatocytes occupied ∼12% and 17%, respectively (Figure 5C). At 90 days, PVLA-unbound hepatocytes occupied ∼78% of the host liver, and PVLA-bound hepatocytes and unfractionated hepatocytes occupied ∼31% and 37%, respectively (Figure 5C). These results indicate that PVLA-unbound hepatocytes produced a significant liver repopulation efficiency (P < 0.01, t-test) at 40, 60, and 90 days. Each animal group transplanted hepatocytes of each type, ie, PVLA-unbound hepatocytes, PVLA-bound hepatocytes, and unfractionated cells was established with three to five rats at each time point studied.
Figure 5.
Quantitative PCR analysis of rat Sry DNA in transplanted rat livers. A: Sry PCR amplification signal and β-actin PCR amplification signal of serial dilutions from 0 to 100% of the DNA from DPPIV+ male rat with DPPIV− female rat, showing a gradual increase (top). The bottom graph is a curve regression of the genomic DNA that was serially diluted male with female hepatocytes and the relative values of Sry/β-actin PCR amplification signal (bottom). B: A single representative example of Sry and β-actin PCR amplification signal of unfractionated (lane 1), PVLA-unbound (lane 2), and PVLA-bound hepatocytes (lane 3) at 21 ∼ 90 days. C: Genomic liver PCR quantification of liver repopulation at 21 ∼ 90 days. Results obtained from the amplification of rat Sry gene relative to the amplification of rat β-actin gene based on serial dilutions from 0 ∼ 100% of Sry gene. Each column represents the group mean ± SE of three to five rats for each time point. *, P < 0.01.
Biochemical Function of Transplanted Hepatocytes
To study the ability of these transplanted hepatocytes to perform biochemical functions unique to hepatocytes, albumin synthesis, glycogen storage, and glutamine synthetase expression were examined by immunohistochemical analysis. In host liver 60 days after transplantation of 2 × 105 PVLA-unbound hepatocytes, many clusters of hepatocytes that expressed DPPIV also demonstrated a distribution of albumin, homogenous storage of glycogen, and glucose-6-phosphatase activity (Figure 6; F to H); in addition, glutamine synthetase was expressed only in hepatocytes at the periphery of the central vein in the host liver using double staining for DPPIV and glutamine synthetase (Figure 6I). In addition, DPPIV+ hepatocytes got to express glutamine synthetase at the periphery of the central vein (Figure 6I, inset). These results demonstrate that in the liver sections, 2 × 105 PVLA-unbound hepatocytes were successfully transplanted. It was also shown using normal liver sections as a positive control that similar normal liver lobule architecture and similar liver function were achieved in reconstituted liver lobule.
Figure 6.
Hepatocytic biochemical function of DPPIV+ transplanted hepatocytes in DPPIV− mutant rat liver. A to D: Normal liver sections used as a positive control, immunohistological staining for glutamine synthetase (red) (A), albumin (red) (B), glycogen (periodic acid-Schiff staining, violet) (C), and glucose-6-phosphatase activity (brown) (D). E to H: Serial sections of liver 60 days after transplantation of 2 × 105 DPPIV+ PVLA-unbound hepatocytes, showing expression of DPPIV (red) (E), albumin (red) (F), glycogen (periodic acid-Schiff staining, violet) (G), and glucose-6-phosphatase activity (brown) (H). I: Double staining for DPPIV (red) and glutamine synthetase (blue) of liver section 60 days after transplantation of 2 × 105 DPPIV+ PVLA-unbound hepatocytes. Inset in I shows a higher magnification of a field around the central vein area. p, portal area; c, central vein. Original magnifications, ×100.
Discussion
We have developed a strategy for hepatocyte transplantation using hepatocytes with low ASGPR expression levels. We consider hepatocyte transplantation using this type of hepatocytes to be an effective strategy, because hepatocytes with low ASGPR expression levels were shown to have high liver repopulation activity. We were able to obtain hepatocytes with low ASGPR expression levels as PVLA-unbound hepatocytes with the help of an asialoglycoprotein model using PVLA as an artificial ligand for ASGPR. To separate hepatocytes with low ASGPR expression, we considered that 10 ng/ml PVLA is a suitable concentration to fractionate hepatocytes with low and high ASGPR expression; therefore hepatocytes with low ASGPR expression levels cannot bind to 10-ng/ml PVLA-coated dish. We consider that PVLA-unbound hepatocytes cannot bind to the PVLA-coated dish because of the low-ASGPR expression of hepatocytes, because mRNA expression levels of ASGPR are lower for PVLA-unbound hepatocytes than for PVLA-bound and unfractionated hepatocytes. We considered that PVLA-bound, -unbound, and -unfractionated hepatocytes did not become traumatized during the separating process. There is no reason that PVLA-unbound hepatocytes cannot bind the PVLA-coated dish because of damage of cellular membrane during isolation of hepatocytes. PVLA-unbound and -bound hepatocytes accounted for ∼20%, 4 × 107 cells, and 80%, 1.6 × 107 cells of the total number of hepatocytes, ∼2 × 108 cells, respectively. We considered that hepatocytes with low ASGPR expression levels were ∼20%, hepatocytes with high ASGPR expression levels were 60%, and hepatocytes with very high ASGPR expression levels were 20% of total number of hepatocytes, according to immunohistochemical analysis using anti-ASGPR antibody. In a cytogram of forward and side scatter from flow cytometrical analysis, the size distribution was equal among unfractionated, PVLA-unbound, and PVLA-bound population (data not shown). Moreover, we were sure that the nonparenchymal cells were completely removed by density gradient centrifugation using Percoll. As far as we observed with a microscopical analysis, these PVLA-separated hepatocytes would hardly include the nonparenchymal cells and there was no reason for including nonparenchymal in PVLA-unbound population cells more than in unfractionated and PVLA-bound cells.
We have previously reported the isolation of highly proliferative PVLA-unbound hepatocytes, ie, progenitor-like hepatocytes, from the mouse liver using PVLA. We found that this type of hepatocyte expresses higher levels of epidermal growth factor receptor (EGF-R), CD29 (β1 integrin), and CD49f (α6 integrin), as well as lower levels of glutamine synthetase than do ordinary hepatocytes.7 These findings suggested that hepatocytes with a reduced ability to adhere to PVLA because of their low level of ASGPR expression could be potential candidates for progenitor-like hepatocytes because of their high proliferative capacity; as such, low levels of ASGPR expression could provide a unique marker for progenitor-like hepatocytes.7 We considered that the characteristics of rat hepatocytes with low ASGPR expression were similar to those of mouse hepatocytes with low ASGPR expression. We expected that PVLA-unbound hepatocytes would have different characteristics from PVLA-bound and unfractionated hepatocytes as a result of Northern blot, flow cytometric analysis, and immunohistochemical analysis for ASGPR. In the present study, we found that PVLA-unbound hepatocytes, ie, hepatocytes with low ASGPR expression level, are not similar to small hepatocytes and oval cells, likely because, as shown by flow cytometric analysis, the PVLA-unbound hepatocytes are normal in size. PVLA-separated populations might include some small-sized hepatocytes equally. We consider that the small-sized hepatocytes do not necessarily have highly proliferative activity, because it is reported that the small-sized hepatocytes do not have highly proliferative activity always.4
It was possible to isolate highly pure low ASGPR-expressing population of hepatocytes using high concentrated PVLA-coated dish, such as 100 ng/ml PVLA-coated dish, because the adhesion of hepatocytes to PVLA-coated dish was depended on PVLA-coating concentration. However, the number of highly pure low ASGPR-expressing hepatocytes is very few. It is necessary to obtain as many highly proliferative hepatocytes, ie, hepatocytes with low ASGPR expression levels, as possible using low concentrated PVLA-coated dishes, to develop a method of reliable hepatocyte transplantation. We therefore suggest that it would be more useful to get many highly proliferative hepatocytes, including progenitor-like cells, rather than only a few hepatic stem cells and progenitor cells, because of our ability to transplant these hepatocytes from a single donor liver to many patients.
We confirmed that the number of initial engrafted PVLA-unbound, PVLA-bound, and unfractionated hepatocytes were similar using flow cytometric analysis at 5 days after transplantation. The initial engraftment of 2 × 106 cells was 4 to 6% of the total hepatocytes by flow cytometric analysis. We considered that this amount as the initial engraftment might be rather high. The total number of hepatocytes in DPPIV− F344 female rat liver at 8 weeks old was ∼1.6 to 2 × 108 cells and the liver weight of these rats was ∼4 to 5 g. We assumed that the ∼0.5 to 1% of total hepatocytes as the initial engraftment of 2 × 106 cells was a reasonable rate. We presumed that the peak shift of DPPIV+ hepatocytes as a positive control might be a little low level in flow cytometric histogram, because DPPIV+ hepatocytes might not be stained strongly by anti-DPPIV antibody. Therefore the DPPIV+ rate (Figure 3, M1) of transplanted hepatocytes from flow cytometrical analysis might be rather high apparently. However, we considered that the initial engraftment for transplanted hepatocytes among the three groups was almost similar and there was a small possibility that PVLA-fractionated hepatocytes were likely or hard to be rejected by the host liver.
In the DPPIV histochemical analysis, the engrafted hepatocytes of the three groups were scattered all over the liver lobule. The numerous large clusters of DPPIV+ hepatocytes were present in the DPPIV− recipient liver in which PVLA-unbound hepatocytes were transplanted. Quantification of liver repopulation for transplanted hepatocytes in the host liver was performed by rat Sry gene PCR from genomic DNA in the liver. The male genomic DNA that was serially diluted with female hepatocytes and the relative values of Sry/β-actin PCR amplification signal were found to be correlative, and curve regression was obtained for serial dilutions between 0% and 100%. It therefore appeared that this PCR quantification for liver repopulation is reasonable and that PCR quantitative analysis is relative to the number of DPPIV histochemically stained hepatocytes. According to PCR quantitative analysis, at 60 days after only 2 × 105 hepatocytes transplantation, unfractionated and PVLA-bound hepatocytes occupied ∼10% each of the host liver and PVLA-unbound hepatocytes occupied ∼80% of the liver. It has previously been reported that liver repopulation achieved by transplantation of 2 × 106 hepatocytes into these liver regenerative model rats is ∼90% at 2 months, and, in the case of transplantation of 5 × 106 hepatocytes into another kind of liver regenerative model rats, liver repopulation is only 25% at 63 days.12,13 The ability to repopulate the liver with transplanted PVLA-unbound hepatocytes was approximately six times greater than that observed with either unfractionated hepatocytes or PVLA-bound hepatocytes, ie, the extent of liver repopulation achieved by transplantation of only 2 × 105 PVLA-unbound hepatocytes was ∼80% at 60 days. In the DPPIV histochemical analysis performed at 60 days after transplantation, we observed several large clusters of transplanted hepatocytes in liver sections showing transplanted PVLA-bound hepatocytes; at 90 days, the corresponding extent of liver repopulation of PVLA-bound hepatocytes was 37%. The fraction of PVLA-bound hepatocytes was thought to include hepatocytes with low ASGPR expression because the fractionation of PVLA-unbound hepatocytes, ie, hepatocytes with low ASGPR expression, might not have been complete. We therefore suggested that hepatocytes with low ASGPR expression levels have a high repopulating potential because of their similar initial engraftment for transplanted hepatocytes among the three groups.
Albumin synthesis, glycogen storage, and glucose-6-phosphatase activity were observed uniformly all over the liver lobule in the host liver. Glucose-6-phosphatase is one of the most important enzymes in intermediary carbohydrate metabolism. The expression of glutamine synthetase was found to be localized to hepatocytes, and the colonies of DPPIV+ hepatocytes expressed glutamine synthetase at the periphery of the central vein. These results demonstrated that transplanted PVLA-unbound hepatocytes perform normal biochemical functions, and that they proliferate normally in the host liver. In addition, the successfully transplanted hepatocytes formed normal liver lobule architecture; because transplanted hepatocytes got to express glutamine synthetase at the periphery of the central vein. These findings suggest that PVLA-unbound hepatocytes, ie, hepatocytes with low ASGPR expression levels, could provide high repopulation capacity in vivo. The colonies of hepatocytes with low ASGPR expression expressed ASGPR normally (data not shown). ASGPR was expressed all over the hepatic lobule homogeneously in liver sections that were transplanted with these hepatocytes. These liver sections did not have heterogeneous expression of ASGPR, such as ASGPR expression of normal liver. We considered that the formation of ASGPR zonation in these liver sections might need a long time.
According to immunohistochemical analysis for ASGPR, ASGPR was expressed all over the hepatic lobule, but the intensity of its expression is heterogeneous in hepatic lobule. The expression of ASGPR was found to be at high levels in the perivenous area and at low levels in the portal area. We have previously reported that hepatocytes with low ASGPR expression levels isolated from mouse liver expressed EGF-R highly and did not express glutamine synthetase, but hepatocytes with high ASGPR expression levels from mouse expressed EGF-R low and expressed glutamine synthetase highly. Previous observations also have revealed that ASGPR expression was decreased in proliferating hepatocytes, suggesting dependence of ASGPR expression on the state of proliferation and differentiation of the cells.11 Moreover, highly proliferative hepatocytes are reported to express low levels of glutamine synthetase, to be localized in the periportal area and to be highly sensitive to stimulation by growth factors, such as EGF and hepatocyte growth factor in addition these hepatocytes expressed high levels of EGF-R.14 Therefore, we suggested that the hepatocytes with low ASGPR expression levels, ie, PVLA-unbound hepatocytes, are localized around the portal area but not the perivenous area. Moreover the isolation of periportal cells via low ASGPR expression using PVLA would be a new method and much cleaner than the other method using double perfusion with detergents.
Currently, hepatocyte transplantation therapy would fail to cure patients with familial hypercholesterolemia or Crigler-Najjar syndrome because of the limited extent to which liver repopulation is possible.15,16 However, if transplantation therapy using highly proliferative hepatocytes such as PVLA-unbound hepatocytes, as shown here, would be indicated for these diseases, far superior results might be achieved.
In conclusion, we have demonstrated in this study that PVLA-unbound hepatocytes have a high repopulating potential in vivo; the present results suggest that hepatocyte transplantation using this type of cell would be a promising strategy for achieving effective clinical applicability.
Acknowledgments
We thank Prof. Nobuyoshi Shiojiri (Shizuoka University) for his many fruitful suggestions; Dr. Tadashi Sasagawa (Tokyo Institute of Technology) for his helpful discussions; and Ms. Kazuko Misawa and Mr. Hideki Nonaka for their excellent technical assistance.
Footnotes
Address reprint requests to Hirohiko Ise, Ph.D., Department of Organ Regeneration, Institute of Organ Transplants, Reconstructive Medicine and Tissue Engineering, Shinshu University Graduate School of Medicine., 3-1-1 Asahi, Matsumoto 390-8621, Japan. E-mail: ise@sch.md.shinshu-u.ac.jp.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- Gupta S. Hepatocyte transplantation. J Gastroenterol Hepatol. 2002;17:S287–S293. doi: 10.1046/j.1440-1746.17.s3.15.x. [DOI] [PubMed] [Google Scholar]
- Braun KM, Degen JL, Sandgren EP. Hepatocyte transplantation in a model of toxin-induced liver disease: variable therapeutic effect during replacement of damaged parenchyma by donor cells. Nat Med. 2000;6:320–326. doi: 10.1038/73179. [DOI] [PubMed] [Google Scholar]
- Katayama S, Tateno C, Asahara T, Yoshizato K. Size-dependent in vivo growth potential of adult rat hepatocytes. Am J Pathol. 2001;158:97–105. doi: 10.1016/S0002-9440(10)63948-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Finegold M, Grompe M. The repopulation potential of hepatocyte populations differing in size and prior mitotic expansion. Am J Pathol. 1999;155:2135–2143. doi: 10.1016/S0002-9440(10)65531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kobayashi A, Akaike T. Culturing hepatocytes on lactose-carrying polystyrene layer via asialoglycoprotein receptor-mediated interactions. Methods Enzymol. 1994;247:409–418. doi: 10.1016/s0076-6879(94)47032-4. [DOI] [PubMed] [Google Scholar]
- Ise H, Sugihara N, Negishi N, Nikaido T, Akaike T. Low asialoglycoprotein receptor expression as markers for highly proliferative potential hepatocytes. Biochem Biophys Res Commun. 2001;285:172–182. doi: 10.1006/bbrc.2001.5139. [DOI] [PubMed] [Google Scholar]
- Stocket RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- Dini L, Conti-Devirgiliis L, Russo-Caia S. The galactose-specific receptor system in rat liver during development. Development. 1987;100:13–22. doi: 10.1242/dev.100.1.13. [DOI] [PubMed] [Google Scholar]
- Kakegawa T, Ise H, Sugihara N, Nikaido T, Negishi N, Akaike T, Tanaka E. Soluble asialoglycoprotein receptors reflect the apoptosis of hepatocytes. Cell Transplant. 2002;11:407–415. [PubMed] [Google Scholar]
- Hirose H, Ise H, Uchiyama M, Cho CS, Akaike T. Regulation of asialoglycoprotein receptor expression in the proliferative state of hepatocytes. Biochem Biophys Res Commun. 2001;287:675–681. doi: 10.1006/bbrc.2001.5631. [DOI] [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gorla GR, Irani AN, Annamaneni P, Gupta S. Cell transplantation after oxidative hepatic preconditioning with radiation and ischemia-reperfusion leads to extensive liver repopulation. Proc Natl Acad Sci USA. 2002;99:13114–13119. doi: 10.1073/pnas.192365499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk A, Michalopoulos G, Weidner M, Gebhardt R. Different proliferative responses of periportal and pericentral rat hepatocytes to hepatocyte growth factor. Biochem Biophys Res Commun. 1995;207:578–584. doi: 10.1006/bbrc.1995.1227. [DOI] [PubMed] [Google Scholar]
- Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ, III, Stein EA, Lupien PJ, Brewer HB, Jr, Raper SE. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]