Abstract
Activating germ-line point mutations in the RET receptor are responsible for multiple endocrine neoplasia type 2-associated medullary thyroid carcinoma (MTC), whereas somatic RET rearrangements are prevalent in papillary thyroid carcinomas (PTCs). Some rare kindreds, carrying point mutations in RET, are affected by both cancer types, suggesting that, under specific circumstances, point mutations in RET can drive the generation of PTC. Here we describe a family whose siblings, affected by both PTC and MTC, carried a germ-line point mutation in the RET extracellular domain, converting cysteine 634 into serine. We tested on thyroid follicular cells the transforming activity of RET(C634S), RET(K603Q), another mutant identified in a kindred with both PTC and MTC, RET(C634R) a commonly isolated allele in MEN2A, RET(M918T) responsible for MEN2B and also identified in kindreds with both PTC and MTC, and RET/PTC1 the rearranged oncogene that characterizes bona fide PTC in patients without MTC. We show that the various RET point mutants, but not wild-type RET, scored constitutive kinase activity and exerted mitogenic effects for thyroid PC Cl 3 cells, albeit at significantly lower levels compared to RET/PTC1. The low mitogenic activity of RET point mutants paralleled their reduced kinase activity compared to RET/PTC. Furthermore, RET point mutants maintained a protein domain, the intracellular juxtamembrane domain, that exerted negative effects on the mitogenic activity. In conclusion, RET point mutants can behave as dominant oncogenes for thyroid follicular cells. Their transforming activity, however, is rather modest, providing a possible explanation for the rare association of MTC with PTC.
Medullary thyroid carcinoma (MTC) is a malignant tumor arising from neural crest-derived calcitonin-secreting C cells of the thyroid,1 whereas papillary thyroid carcinoma (PTC) derives from endoderm-derived follicular cells.2 MTC often occurs in the context of autosomal dominant multiple endocrine neoplasia type 2 syndromes (MEN2), whereas PTC is sporadic in most of the cases.3 This notwithstanding, some rare MEN2 kindreds are affected by both cancer types, the molecular mechanism for this association still remaining unclear.
Both MTC and PTC are strictly linked to activating mutations in the RET gene. RET is a transmembrane tyrosine kinase receptor for glial-derived neurotrophic factor (GDNF).4 RET rearrangements, caused by chromosomal inversions or translocations, are present in 20 to 40% of cases of PTC.5 These rearrangements result in the fusion of the RET cytoplasmic kinase domain to the 5′-ter of heterologous genes, generating the chimeric RET/PTC oncogenes. RET/PTC1 and RET/PTC3, the most prevalent versions, consist of the RET fusion to the H4 or RFG genes, respectively.5 Mice transgenic for RET/PTC oncogenes develop PTCs.6,7
Multiple endocrine neoplasia type 2 (MEN2A, MEN2B, and FMTC) syndromes predispose to MTC, pheochromocytoma, and other tumors (OMIM: no. 171400). Point mutations in RET are found in virtually all MEN2 kindreds.4 Most MEN2B patients carry the M918T substitution in the P+1 loop of the RET kinase. The majority of MEN2A and FMTC mutations affects cysteines of the extracellular domain of RET, cysteine 634 being the most frequently affected. Less frequently, FMTC is associated with changes in the N-terminal (E768D, L790F, Y791F, V804L, V804M) or C-terminal lobe (S891A) of the RET kinase. Somatic mutations of V804, M918, and E768 are found in sporadic MTC.8 Both RET/PTC and RET/MEN2 are ligand-independent constitutively active oncoproteins. In the case of RET/PTC, fusion with protein partners displaying protein-protein interaction motifs provides the RET kinase with dimerizing interfaces. This results in constitutive dimerization, autophosphorylation of the chimeric oncoproteins, and constant up-regulation of signaling. Also MEN2-associated RET point mutants are constitutively active, through different mechanisms depending on the amino acid change.9–11
Similarly to MEN2 carriers, the rare kindreds affected by both MTC and PTC carry germ-line point mutations in RET. Accordingly, K603Q,12 M918T,13,14 and V804L15 point mutations have been described in such families. Given its familial distribution, it is unlikely that PTC occurs in these families simply by chance. Furthermore, one particular transgenic mice line bearing the RET(C634R) allele under the control of the calcitonin promoter developed both MTC and PTC.16 Finally, full-length RET proteins are expressed not only in MTC17 but also at low levels in PTC, regardless of the occurrence of RET rearrangements.18,19 Despite this data, the oncogenic activity of RET point mutants for epithelial thyroid follicular cells has never been investigated.
The identification of a novel family with affected siblings showing both MTC and PTC and carrying the RET(C634S) mutation, prompted us to analyze thetransforming activity of RET(C634S), RET(K603Q), RET(C634R), and RET(M918T) in PC Cl 3 thyroid follicular cells. Here we show that the various RET point mutants induce hormone-independent proliferation of PC Cl 3 cells, although with a significantly lower efficiency compared with RET/PTC1. These findings suggest that specific point mutations in RET may play a causative role in MEN2-associated PTC cases.
Materials and Methods
Tissue Samples
Tissue samples were analyzed from all three family members (the father and two monozygotic twin daughters) of a MEN2A kindred with both MTC and PTC. Pathological examination of surgically resected material and tumor diagnosis followed established criteria.20 Serial sections of formalin-fixed, paraffin-embedded thyroid tissue from the PTCs, the MTCs, and from the nonneoplastic gland of the three patients were cut for immunohistochemistry, microdissected, and separately processed for RNA extraction to analyze RET rearrangements. Peripheral blood samples were obtained from all three patients to determine the germline RET mutational status. Processing of samples and clinical information proceeded in accordance with review board-approved protocols.
RET Mutational Analysis
DNA was extracted from white blood cells from the three patients and DNA sequencing was performed for exons 10, 11, 13, 14, 15, and 16 of RET according to standard procedures.21 One single mutation changing codon 634 from TGC to TCC (cysteine to serine) in exon 11 was found in the three patients. The mutation was confirmed with two independent polymerase chain reactions (PCRs) from two independent DNA samples in both directions.
RET Expression
Total RNA was extracted from formalin-fixed paraffin-embedded tissue with the RNeasy Kit (Qiagen, Crawley, West Sussex, UK) and subjected to on-column DNase digestion with the RNase-free DNase set (Qiagen) following the manufacturer’s instructions. The RNA quality was verified by electrophoresis through 1% agarose gel and visualization with ethidium bromide. Levels of β-actin transcripts were used as a control for equal RNA loading. To screen for RET expression, reverse transcriptase (RT)-PCR was performed using reaction conditions and primers previously described22 (Table 1). For the identification of RET/PTC1 and RET/PTC3, multiplex RT-PCR was used using a common downstream primer, TK3(−) and upstream primers H4(+) and RFG(+). Additional primers for the rare PTC2, -5, -6, -7, and -8 rearrangements were also used (Table 1). Approximately 100 ng of RNA were reverse-transcribed with the Gene Amp RNA PCR Kit (Perkin-Elmer, Corp., Wilton, CT) according to the indications of the manufacturer. PCR was then performed after the addition of upstream primers. Each 30-μl tube included a final concentration of 0.1 μmol/L for each primer, 100 μmol/L each dNTP, 0.8 U AmpliTaq polymerase (Perkin-Elmer, Corp.) in Buffer II containing 2.0 mmol/L MgCl2. After a 12-minutes hot start at 94°C, nine cycles of touchdown amplification were performed (progressively lowering the annealing temperature from 61°C to 55°C), followed by 40 cycles of amplification (94°C for 30 seconds, 55°C for 45 seconds, and 72°C for 45 seconds) with a Hybaid OmniGene thermal cycler (Sun Biosciences, Madison, CT). The amplified products were analyzed on a 3% agarose gel and hybridized with a probe covering the tyrosine-kinase domain of RET. RNA extracted from the RET/PTC1-positive TPC1 or the RET/PTC3-positive PC-PTC3 cell lines23 were used as positive controls. Amplification without previous reverse transcription, in the absence of any RNA or in the presence of normal placental RNA was used as a negative control.
Table 1.
Primers Selected for RET Analysis
| Name | Gene | Position* | Sequence | Primer size |
|---|---|---|---|---|
| TM(+) | RET | 1922–1941 | CTG TCC TCT TCT CCT TCA TC | 20 mer |
| TK1(+) | RET | 2149–2168 | TGG GAA TTC CCT CGG AAG AA | 20 mer |
| TK2(−) | RET | 2364–2383 | TGC AGG CCC CAT ACA ATT TG | 20 mer |
| TK3(−) | RET | 2185–2202 | TTC GCC TTC TCC TAG AGT | 18 mer |
| H4(+) | H4 | 335–351 | GCA AAG CCA GCG TTA CC | 17 mer |
| RFG(+) | RFG | 746–763 | CCC CAG GAC TGG CTT ATC | 18 mer |
| PTC2(+) | RIα | 602–619 | TAT CGC AGG AGA GAC TGT | 18 mer |
| PTC5(+) | Golgin | 1469–1486 | TAC TAG AAT ACT GCA ATC | 18 mer |
| PTC6(+) | HTIF1 | 1089–1106 | GCT CTA CTG CAT CAG TTA | 18 mer |
| PTC7(+) | HTIFγ | 1096–1113 | CAT TTT GCA GCT ACT CAG | 18 mer |
| PTC8(+) | KTN1 | 2760–2777 | ACA GGG AAG TGG TTA CAG | 18 mer |
The positions of TM, TK1, TK2, and TK3 are according to the standard RET sequence with the numeration beginning from the start codon of RET, according to this numeration the breakpoint in both RET/PTC1 and RET/PTC3 is at the nucleotide 2136; the positions of H4(+) (accession number M31213), RFG(+) (accession number X77548) and PTC2, 5, 6, 7, and 8 primers are according to current sequences of the NCBI database.
Antibodies and Immunohistochemistry
For immunohistochemical analysis we used anti-RET polyclonal rabbit antibodies, anti-RET(TK), directed againstthe tyrosine kinase domain of RET (amino acids 738 to 1058); their features and specificity have been previously characterized.22,24 Antibodies were affinity-purified by sequential chromatography on RET- and GST-coupled agarose columns and tested by immunoblotting of protein lysates obtained from NIH3T3 cells expressing wild-type RET or RET/PTC1. Immunohistochemistry was performed and scored similar to previous protocols24 using a 1/100 dilution. Negative controls were performed on all cases by omitting the primary antibody. Sections of MTC and of previously characterized PTCs24 were used as positive controls. Positive immunoreactivity was abolished by preadsorption with a molar excess of the RET protein. Immunohistochemistry was also performed with the antibodies anti-calcitonin (DAKO, Copenhagen, Denmark) and anti-thyroglobulin (DAKO) following standard procedures.
Cell Culture and Transfection Experiments
PC Cl 3, a differentiated thyroid epithelial cell line derived from 18-month-old Fischer rats, was cultured in Coon’s modified Ham F12 medium supplemented with 5% calf serum and a mixture of six hormones (6H): thyrotropin (10 mU/ml), hydrocortisone (10 nmol/L), insulin (10 μg/ml), apo-transferrin (5 μg/ml), somatostatin (10 ng/ml), and glycyl-histidyl-lysine (10 ng/ml) (Sigma Chemical Co., St. Louis, MO) according to Fusco and colleagues.25 Cells (5 × 105) were plated 48 hours before transfection in 60-mm tissue culture dishes. The medium was changed to Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) containing 5% calf serum and 6H. Three hours later, calcium-phosphate DNA precipitates were incubated with the cells for 1 hour. DNA precipitates were removed, and cells were washed with serum-free Dulbecco’s modified Eagle’s medium and incubated with 15% glycerol in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered saline for 2 minutes. Finally, cells were washed with Dulbecco’s modified Eagle’s medium and incubated in Coon’s modified F12 medium supplemented with 5% calf serum and the 6H. For the colony formation assay, two dishes of PC Cl 3 were transfected with each plasmid. After 2 days, G418 (neomycin) was added to one dish, while the other dish was kept in medium containing 5% calf serum in the absence of 6H. After 15 days, cells colonies were fixed in 11% glutaraldehyde in phosphate-buffered saline, rinsed in distilled water, stained with 0.1% crystal violet in 20% methanol for 15 minutes and counted. The percentage of hormone-independent colonies with respect to the total number of G418-resistant colonies was calculated as the average of three independent determinations ± SD. ARO cells26 derived from an anaplastic carcinoma negative for RET/PTC rearrangements, were cultured in RPMI supplemented with 10% fetal calf serum, 2 mmol/L l-glutamine, and 100 U/ml penicillin-streptomycin (Life Technologies, Inc.).
All of the constructs used in this study encode theshort (RET-9) RET spliced form and were cloned inpCDNA3(Myc-His) (Invitrogen, Groningen, The Netherlands). The wild-type RET, RET/PTC1, RET(M918T), and RET(C634R) constructs have been described previously.23 RET/PTC1-JM was engineered by PCR inserting the intracellular juxtamembrane domain of RET (residues 658 to 712) between H4 (residues 1 to 101) and RET kinase (residues 713 to 1072). RET(C634S) and RET(K603Q) were generated by site-directed mutagenesis using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA). All mutations were confirmed by DNA sequencing.
Protein Studies
Anti-pY1062, anti-pY1015, and anti-pY905 are affinity-purified polyclonal antibodies raised against RET peptides containing phosphorylated Y1062, Y1015, or Y905 residues.27 Anti-MAPK (no. 9101) and anti-phospho-MAPK (no. 9102) were obtained from New England Biolabs (Beverly, MA), monoclonal anti-tubulin antibodies were from Sigma Chemical Co. Secondary antibodies coupled to horseradish peroxidase were obtained from Amersham Pharmacia Biotech (Little Chalfont, UK). Immunoprecipitation and immunoblotting were performed according to standard procedures. Briefly, cells were lysed in a buffer containing 50 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; pH 7.5), 1% (v/v) Triton X-100, 50 mmol/L NaCl, 5 mmol/L EGTA, 50 mmol/L NaF, 20 mmol/L sodium pyrophosphate, 1 mmol/L sodium vanadate, 2 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin. Lysates were clarified by centrifugation at 10,000 × g for 15 minutes. Lysates containing comparable amounts of proteins, estimated by a modified Bradford assay (Bio-Rad, Munchen, Germany), were immunoprecipitated with the required antibody or subjected to direct Western blot. Immune complexes were detected with the enhanced chemiluminescencekit (Amersham Pharmacia Biotech). For the RET in vitrokinase assay, cells were solubilized in lysis buffer as described above. Then, 1 mg of proteins were immunoprecipitated with anti-RET(TK); immunocomplexes were recovered with protein A-Sepharose beads, washed five times with kinase buffer, and incubated (20 minutes at room temperature) in kinase buffer containing 200 μmol/L poly(l-glutamic acid-l-tyrosine) (poly-GT) (Sigma), 2.5 μCi [γ-32P] ATP, and unlabeled ATP to a final concentration of 20 μmol/L. Samples were spotted on Whatman 3MM paper (Springfield Mill, UK) and 32P incorporation was measured with a β-counter scintillator (Beckman Instrument, Inc, Palo Alto, CA).
Soft Agar Growth Assay
Soft agar colony assay was performed as previously reported.9 Briefly, cells (10,000 cells/dish) were seeded on 60-mm dishes in 0.3% agar in complete medium on a base layer of 0.5% agar. Complete medium was added every 3 days to the top layer. Colonies larger that 64 cells were counted 15 days later and results were expressed as efficiency ratio (number of colonies formed/number of plated cells × 100). As a positive control, ARO cells were used.
Differentiation Studies
Total RNA from the indicated cell cultures was prepared using the RNeasy kit (Qiagen) as described above. The quality of RNA from each sample was verified by electrophoresis through 1% agarose gel and visualization with ethidium bromide. Transcript levels of the indicated genes were assayed by RT-PCR. Total RNA (2.5 μg) was denatured and cDNA was synthesized using the GeneAmp RNA PCR core kit system (Applied Biosystems) following the manufacturer’s instructions. Subsequent PCR amplification was performed using 2.5 μl of the RT product in a reaction volume of 25 μl. To exclude DNA contamination, each PCR reaction was also performed without previous reverse transcription. The levels of the housekeeping β-actin transcript were used as a control for equal RNA loading. Primers were designed with the program Primer 3 (www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) and were as follows: thyroglobulin (TG) forward: 5′-GAGTGATGCTCCCAGCTTCT-3′; thyroglobulin (TG) reverse: 5′-AGTTCCTGGTGGCTGAAATG-3′; PAX-8 forward: 5′-AGCAGCAGTAGTGGTCCTCG-3′; PAX-8 reverse: 5′-CCGTCATCCAGGGTACTGTT-3′; β-actin forward: 5′-GTCAGGCAGCTCATAGCTCT-3′; β-actin reverse: 5′-TCGTGCGTGACATTAAAGAG-3′. Each RT-PCR product was loaded on 2% agarose gel, stained with ethidium bromide (0.5 μg/ml), and the corresponding image saved by the Typhoon 8600 laser-scanning system (Amersham Pharmacia Biotech).
Results
Identification of a Family, with Both MTC and PTC, Carrying a Germ-Line Point Mutation in RET
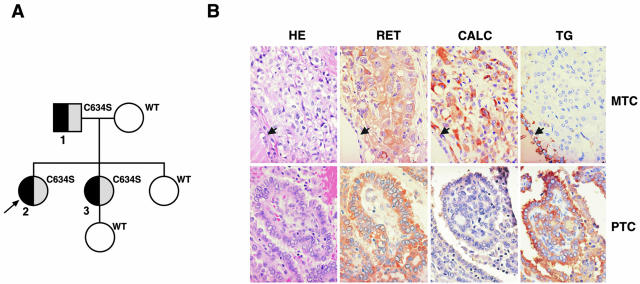
The index patient, a 32-year-old Caucasian woman (patient 2 in Figure 1A, arrow), presented with hypertension, palpitations, and pallor. Work up revealed bilateral pheochromocytomas and elevated plasma calcitonin levels with MTC. This prompted family screening for MEN2 syndrome, which was diagnosed in the patient’s monozygotic twin sister (patient 3 in Figure 1A) and in her asymptomatic father (patient 1 in Figure 1A). The three patients carried a heterozygous missense point mutation in exon 11 (TGC to TCC) converting cysteine 634 to serine. The mother and older sister of the index case, and the daughter of patient 3 were all negative for this mutation. The mother had no evidence for thyroid carcinoma.
Figure 1.
A: Pedigree of the family displaying both MTC and PTC and carrying the RET C634S mutation. Gray boxes, MTC; black boxes, PTC; arrow, index case. Patients carrying mutant or wild-type RET are indicated. B: Medullary (first line images) and papillary (second line images) thyroid carcinomas in the index patient (patient 2) stained with conventional H&E and immunostained with RET (RET), calcitonin (CALC), and thyroglobulin (TG) antibodies. The MTC in the first line images shows positive (brown) immunoreactivity for RET and calcitonin but no reactivity for thyroglobulin. The arrows point to nonneoplastic thyroid follicular epithelium that is positive for thyroglobulin but negative for RET and calcitonin, as expected. The PTC in the second line images shows positive (brown) immunoreactivity for RET and thyroglobulin but no reactivity for calcitonin. Omission of the primary antibody and competition with a molar excess of recombinant RET protein were used as negative controls (not shown).
Both twin sisters and the father underwent bilateral adrenalectomy for pheochromocytoma and total thyroidectomy. Pathological examination confirmed the clinical diagnosis of bilateral pheochromocytoma in all three patients. In addition, the father had multifocal MTC involving both thyroid lobes with no MTC lymph-node metastases (pT2, pN0, M0, stage II, AJCC 2002) and an occult PTC, follicular variant, smaller than 10 mm of diameter (microcarcinoma) with bilateral PTC lymph node metastases (pT1, pN1a, M0, stage III, AJCC 2002). The index patient had multifocal MTC (Figure 1B) involving both thyroid lobes with lymph-node metastases (pT2, pN1a, M0, stage III, AJCC 2002), multifocal PTC (Figure 1B), classical type, with lymph node metastases (pT3, pN1a, M0, stage I, AJCC 2002), and severe chronic lymphocytic thyroiditis. The index patient’s twin sister had MTC in the left thyroid lobe accompanied by metastatic MTC in three of four mediastinal lymph nodes (pT1, pN1b, M0, stage IVA, AJCC 2002), diffuse C cell hyperplasia, and PTC, follicular variant, in the right lobe (pT1, pN0, M0, stage I, AJCC 2002) with a background of widespread chronic lymphocytic thyroiditis. As of November 2003, clinical follow up has shown no evidence for recurrent papillary thyroid cancer in these three patients. I131 whole-body scanning was negative in the father in 2000, and negative in the sisters in 2001. Calcitonin levels have remained just above normal in all three patients with no clinically evident recurrent MTC. Both the sisters have been diagnosed with metastatic pheochromocytomas (to the liver in patient 2 and to the liver, skeleton, and soft tissues in patient 3). Despite this, they have had few hyperadrenergic symptoms and normal blood pressures.
Anti-thyroglobulin and anti-calcitonin immunostaining was performed on tissue sections to confirm the histological diagnosis. In all of the three patients, MTC foci stained for calcitonin whereas PTC areas were positive for thyroglobulin with no detectable overlap (Figure 1B). More importantly, both PTC and MTC areas showed strong immunostaining with anti-RET antibodies directed against the tyrosine-kinase domain (Figure 1B). Competition with a molar excess of recombinant RET protein was used as a negative control (not shown).
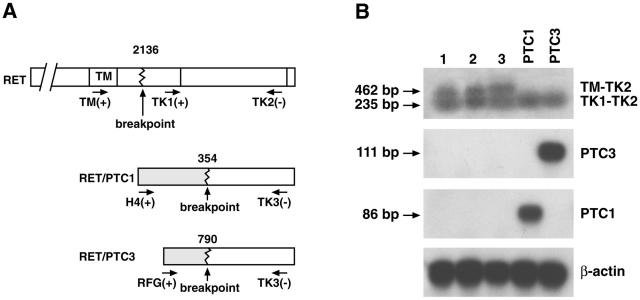
To confirm RET expression and to investigate the presence of RET/PTC rearrangements, RNA was separately extracted from MTC and PTC foci. The RET tyrosine kinase and extracellular domains were separately amplified with two specific primer pairs (TK1-TK2 and TM1-TK2, respectively). In the case of full-length RET expression, the two primer pairs give amplified products of comparable intensity, whereas in the case of rearranged RET/PTC expression the kinase domain should result in a stronger signal with respect to the extracellular domain (Figure 2A). As a control, we used RNAs extracted from cell lines expressing RET/PTC1 and 3 (Figure 2B). As in the case of MTC samples (lane 1 and data not shown), PTC tissues from the three patients expressed similar levels of both extracellular and intracellular RET domains (Figure 2B, lanes 2 to 3). Furthermore, DNA sequencing of the RT-PCR products confirmed the expression of messenger RNAs containing the C634S mutation (not shown). To further exclude the presence of RET/PTC rearrangements in the PTC tissues, tumor RNA was amplified with upstream primers specific for the most common RET/PTC rearrangements (Table 1). Neither the highly prevalent RET/PTC1 and RET/PTC3 (Figure 2B) nor the rare RET/PTC2–8 (not shown) transcripts were detected in the PTC tissues, whereas the controls scored strongly positive. All together, these findings demonstrate the expression of a RET allele carrying the C634S mutation in the PTC tissues of the patients.
Figure 2.
A: A schematic of the primers selected for the RT-PCR. B: Detection of RET, RET/PTC1, and RET/PTC3 mRNA expression by RT-PCR. Lane 1, MTC tissue of patient 2; lane 2, PTC tissue of patient 2; lane 3, PTC tissue of patient 3: both transmembrane and tyrosine kinase domains are amplifiable, indicating the presence of full-length RET mRNA. Lanes PTC1 and PTC3, positive controls represented by cell lines expressing either one of the rearranged oncogenes: only the tyrosine kinase domain is amplifiable, indicating the presence of RET rearrangements.
Biological Activity of RET Point Mutants in Thyroid Follicular Cells
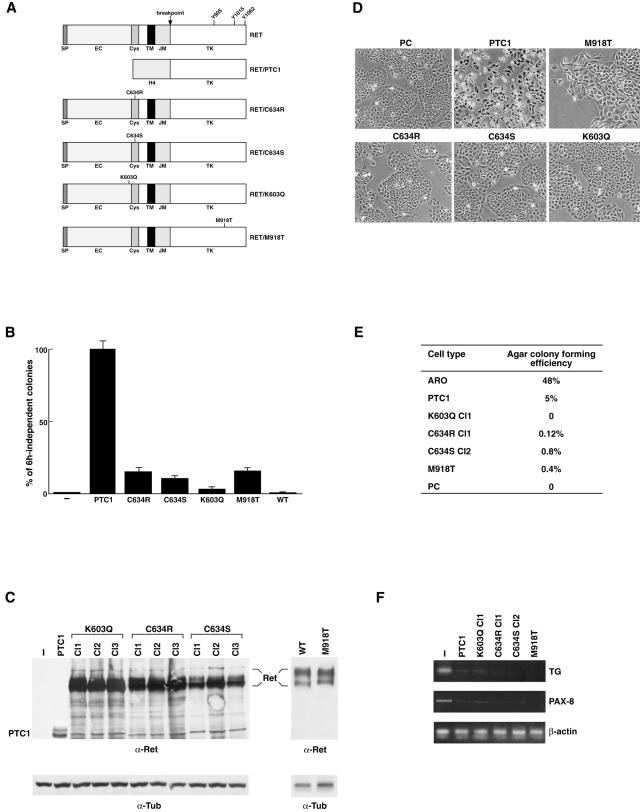
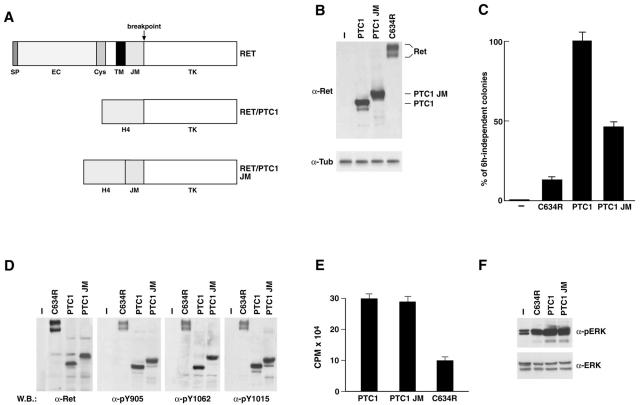
To verify whether RET point mutants exert transforming ability for thyroid follicular cells, we generated eukaryotic expression vectors for RET(C634R), RET(M918T), RET(C634S), RET(K603Q), and RET/PTC1 (Figure 3A). When adoptively expressed in thyroid follicular cells, RET/PTC oncogenes cause hormone-independent proliferation, morphological transformation, and loss of differentiation markers expression.23,28 The transforming ability of RET point mutants either in cysteine 634 or methionine 918 is well documented in fibroblasts,9–11 but never studied in thyroid follicular cells. Biological activity of the K603Q mutant has never been reported. The constructs were transfected in PC Cl 3 thyroid follicular epithelial cells that normally require a mixture of six hormones (6H), including TSH and insulin, for proliferation. The formation of colonies growing in the absence of 6H was measured in comparison to RET/PTC1 and to wild-type RET. All of the various point mutants were able to induce a certain degree of autonomous proliferation, forming 6H-independent colonies. However, such mitogenic effects were markedly reduced (more than fivefold) with respect to those mediated by RET/PTC1 (Figure 3B). No 6H-independent colonies were observed with transfection of wild-type RET. For each construct, at least three clones and one mass population were isolated by marker (G418) selection. Thus, clones with comparable expression levels (K603Q-Cl.1, C634R-Cl.1, C634S-Cl.2, and M918T) were selected (Figure 3C). Results obtained for one representative clone are shown thereafter but they were confirmed in the other cell clones as well as in the mass population. The various constructs had remarkably different effects. PC-RET/PTC1 cells showed a transformed morphology with many spindle-shaped and refractile cells that were scattered on the surface of the culture dish, whereas cells transfected with the various point mutants showed only minor changes with respect to parental cells, retaining an epithelial morphology with solid nests of polygonal and regularly shaped cells (Figure 3D). Growth curves, performed either in the presence or in the absence of 6H, confirmed these results (not shown). To confirm these findings, we tested the capacity of the various cell clones to grow in semisolid medium, a hallmark of neoplastic transformation. As a positive control, we used the highly malignant ARO cells. The average results of three independent experiments are reported in Figure 3E. PC-PTC1 cells displayed a low but detectable ability to grow in semisolid medium. Consistently with their modestly transformed phenotype, cell clones expressing the various RET point mutants exerted a strong reduction in the ability of forming colonies in soft agar. Loss of differentiation is another hallmark of neoplastic transformation. We have previously shown that the expression of RET/PTC1 abolishes differentiated gene expression in PC Cl 3 cells.28 To further evaluate the effects exerted by the various RET mutants, the expression of two representative thyroid differentiation markers, thyroglobulin (TG) and PAX8, a tissue-specific transcription factor, was studied by semiquantitative RT-PCR. As previously reported,28 PC-PTC1 cells displayed a complete loss of thyroglobulin and PAX8 expression (Figure 3F). Of note, PC Cl 3 cells expressing RET point mutants displayed a similar behavior (Figure 3F). This supports the concept that point mutations in RET can exert transforming effects in thyroid follicular cells and indicate that loss of differentiation is achieved also by RET mutants with low intrinsic kinase activity.
Figure 3.
A: Schematic representation of RET constructs used in this study. SP, signal peptide; EC, extracellular domain; CYS, cysteine-rich region; TM, transmembrane region; JM, juxtamembrane domain; TK, tyrosine-kinase domain. Autophosphorylated RET tyrosines 905, 1015, and 1062 are shown. B: The hormone-independent proliferation of the various PC Cl 3 transfectants and empty-vector transfected cells (−) is reported as percentage of G418-resistant cells forming colonies after 15 days of incubation in the absence of 6H. Average results of three independent experiments ± SD. C: Expression of the various RET proteins in transfected or empty vector-transfected (−) PC Cl 3 cell clones. Fifty μg of protein lysates were analyzed by Western blot with anti-RET(TK) antibodies. Full-length RET proteins formed a doublet of 170 kd and 150 kd of relative molecular mass. Antibodies directed against tubulin were used for normalization. D: Parental and transfected PC Cl 3 cells were photographed by using a phase-contrast light microscope. E: The anchorage-independent growth of the various PC Cl 3 transfectants and parental cells was evaluated in comparison to the ARO cell line, used as a positive control. F: The expression of thyroid differentiated genes in parental and transfected PC Cl 3 cells was evaluated by semiquantitative RT-PCR. Reverse-transcribed cDNAs were PCR amplified with primers for TG and PAX-8. β-Actin levels are shown to confirm equal cDNA content. Original magnification, ×150 (D).
Kinase Activity and Signaling Properties of RET Point Mutants
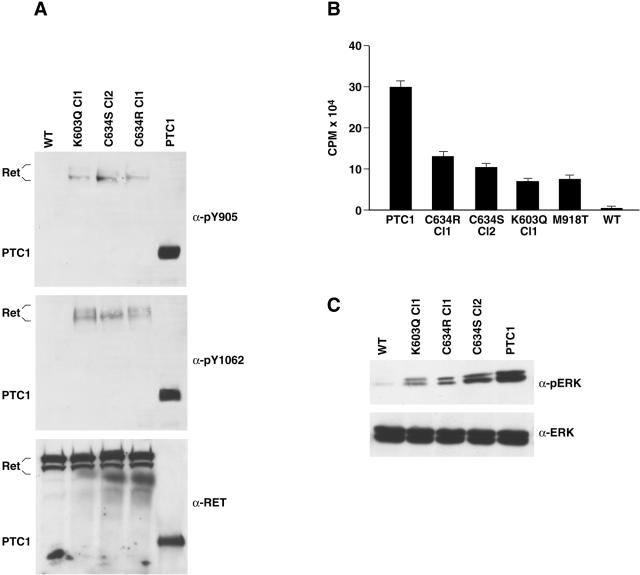
Oncogenic activation of RET results in the constitutive activation of the kinase which, in turn, causes autophosphorylation, recruitment of intracellular substrates, and activation of diverse signaling pathways.4 On phosphorylation, tyrosine 1015 behaves as a docking site for PLCγ. Tyrosine 1062 is a multiple-effector docking site, recruiting Grb2-Sos complexes leading to Ras/MAPK activation. Tyrosine 905, in the catalytic core, stabilizes the active conformation of the kinase. Initially, in vivo tyrosine phosphorylation levels of the different mutant proteins were assessed by phospho-RET antibodies specific for phosphorylated forms of Y905 and Y1062. Phosphorylation levels of the chimeric RET/PTC1 protein scored significantly (approximately threefold) higher than those of the RET point mutants in representative cell clones (Figure 4A). No detectable levels of phosphorylation were observed in wild-type RET (Figure 4A). To assess whether the different phosphorylation levels were because of variations in the intrinsic catalytic activity of the RET proteins, we performed an in vitro immunocomplex kinase assay. The four point mutants had similar activities and were more active with respect to wild-type RET. However, the level of kinase activity of the point mutants was significantly lower (>2.5-fold) than that of chimeric RET/PTC1 (Figure 4B). Stimulation of MAPK is a hallmark of RET activation.1 Thus, as a read-out of RET signaling, we measured MAPK phosphorylation levels by blotting with phospho-specific antibodies. Expression of the different RET oncogenes in PC Cl 3 cells was accompanied by constitutive phosphorylation of MAPK; however, MAPK activation levels were approximately fourfold higher in RET/PTC than in RET point mutants expressing cell clones (Figure 4C).
Figure 4.
A: Equal amounts (100 μg) of protein extracts of transfected PC Cl 3 cells were immunoblotted with phosphorylation-specific anti-RET antibodies. The blots were reprobed with anti-RET antibody. The results are representative of at least three independent assays (M918T cells are not shown). B: In vitro poly-GT phosphorylation assay: protein extracts were immunoprecipitated with anti-RET and subjected to a kinase assay with poly-GT as a synthetic substrate and [γ-32P] ATP. The phosphorylated poly-GT was spotted on 3MM Whatman paper and counted by scintillation. The results of four independent experiments were averaged and presented as poly-GT phosphorylation levels. Standard deviations are shown. C: Cell lysates (100 μg) were immunoblotted with anti-phospho-ERK (p44 and p42). Anti-ERK antibody was used for normalization. These findings are representative of at least three independent experiments (M918T cells are not shown).
Inhibitory Effects of the Juxtamembrane Domain on Mitogenic Signaling of RET Oncoproteins
To get further insights in the reduced transforming ability of RET point mutants with respect to RET/PTC, we studied the role of the juxtamembrane (JM) domain of RET. Indeed, as shown in Figure 5A, the exon encoding the RET JM domain is lost on RET/PTC rearrangements. It has been shown that the JM domain exerts negative feedback control in several tyrosine kinase receptors.29–31 We engineered a chimeric molecule (RET/PTC1-JM) by adding back the JM domain to the H4-RET chimeric oncogene (RET/PTC1) and compared its mitogenic ability to those exerted by rearranged and nonrearranged RET versions (Figure 5A). To this end, we tested hormone-independent proliferation of transfected PC Cl 3 cells. At comparable expression levels (Figure 5B), RET/PTC1-JM had levels of mitogenic activity that were intermediate between those exerted by RET/PTC1 and RET(C634R) (Figure 5C), proving that indeed JM exerts inhibitory activity. In vivo tyrosine phosphorylation levels, intrinsic kinase activity and capacity to stimulate ERK phosphorylation were evaluated. Surprisingly, despite its reduced mitogenic effects, the stoichiometry of autophosphorylation, catalytic function, and ERK activation exerted by RET/PTC-JM were indistinguishable from those of RET/PTC1 (Figure 5; D to F). Thus, although JM is inhibitory for the RET mitogenic activity, this negative effect is not mediated by down-regulation of either the RET kinase or signaling to ERKs.
Figure 5.
A: Schematic representation of RET constructs. B: Expression levels of the various RET proteins in mass populations of transfected or parental (−) PC Cl 3 cells. Fifty μg of protein lysates were analyzed by Western blot with anti-RET(TK) antibodies. Antibodies directed against tubulin were used for normalization. C: The hormone-independent proliferation of the various PC Cl 3 transfectants is reported as percentage of G418-resistant cells forming colonies after 15 days of incubation in the absence of 6H. Average results of three independent experiments ± SD. D: Equal amounts (100 μg) of protein extracts of transfected PC Cl 3 or parental (−) cells were immunoblotted with anti-RET or with phosphorylation-specific anti-RET antibodies. The results are representative of at least three independent assays. E: In vitro poly-GT phosphorylation assay. The results of three independent experiments were averaged ± SD. F: Cell lysates (100 μg) were immunoblotted with anti-phospho-ERK and anti-ERK.
Discussion
Oncogenic activation of RET in thyroid tumors can be the end-result of gene rearrangements or point mutations. Thus far, the generally accepted paradigm is that RET gene rearrangements are found in PTCs, while point mutations are specific for MTCs.4 Nonetheless, there are rare patients carrying germ-line RET point mutations of the type associated with FMTC and MEN2 syndromes with both MTC and PTC in their thyroid gland (this study).12–15 This suggests that point mutations in RET can predispose to PTC, as well. Should this be true, however, the question remains of why RET point mutations are so rarely associated with PTC.
The occurrence of PTC in patients carrying MTC and germ-line point mutations in RET may be explained by indirect (for instance, release of paracrine growth factors from transformed C-cells acting on follicular cells) or direct transforming effects of RET point mutants for follicular cells. To discriminate between the two possibilities, we made use of an in vitro model system to test biological and biochemical effects of RET point mutants on thyroid follicular cells. The various RET point mutants had constitutive activation of the kinase. Consistently, thyroid cells expressing RET point mutants displayed loss of the differentiated phenotype and hormone-independent growth in comparison to cells transfected with wild-type RET. However, mitogenic activity and levels of kinase activity were significantly lower in RET point mutants than in RET/PTC. Furthermore, RET point mutants were virtually unable to induce anchorage-independent proliferation of PC Cl 3 cells.
The reasons for such different activity are at least twofold: reduced catalytic activity and presence of the JM domain. RET point mutants, although more active than wild-type RET, had a ligand-independent catalytic activity that was significantly lower than that of RET/PTC. Several possible mechanisms can account for this difference: 1) a different efficiency of dimerization; 2) the removal of negative constraints imposed by the extracellular or transmembrane domains in RET/PTC; 3) impaired coupling of cytosolic RET/PTC with plasma-membrane-located tyrosine phosphatases, such as LAR, that have been shown to down-regulate RET kinase.32 Moreover, the presence of the JM domain negatively affected RET mitogenic activity, independently from the enzymatic activity of the receptor. It has been shown that the JM domain can mediate coupling of receptor tyrosine kinases to negative signal transducers such as RasGap in the case of EphB233 and Cbl in the case of Met.34 Accordingly, we postulate that coupling with negative intracellular signaling pathways might explain the negative effects exerted by JM on RET mitogenic ability.
Whatever the mechanism, our findings indicate that a positive selection may exist to activate RET in thyroid follicular cells by gene rearrangements rather than point mutations. Nonetheless, some specific RET point mutants are able to drive autonomous proliferation and reduced differentiation of thyroid follicular cells and, therefore, can be implicated in the occurrence of familial PTC cases. The modest activity of these RET point mutants on thyroid follicular cells may account for the rare occurrence of PTC in families carrying germ-line point mutations in RET. The biological activity of RET point mutants in thyroid follicular cells raises also the possibility that somatic point mutations in RET may be responsible for sporadic PTC as well. To our knowledge, one single case of somatic point mutation in RET associated to PTC (a mixed tumor in that case) has been reported so far.14 A detailed screening for RET point mutations in PTC samples positive for RET expression will be necessary to address this point.
In conclusion, we hypothesize that RET point mutants behave as conditional oncogenes, able to predispose to PTC only under specific circumstances such as, for instance, high level expression of the mutated allele (of note is the recent identification of two promoter polymorphisms influencing RET expression35) in follicular cells or concomitant cooperating genetic lesions. Whatever the case, these findings imply that the recently discovered small molecule kinase inhibitors of RET27,36 may exert efficacy toward MTC-associated PTC cases.
Acknowledgments
We thank Drs. Fred Gebhardt, Sandra L. Butchart, and Philip Mount of the Department of Pathology, Franklin Square Hospital Center, Baltimore, MD, for contributing the pathology material; and Dr. Mauro Papotti for helpful discussion.
Footnotes
Address reprint requests to Massimo Santoro, Dipartimento di Biologia e Patologia Cellulare e Molecolare, Facoltà di Medicina e Chirurgia, Università di Napoli “Federico II”, via S. Pansini 5, 80131 Naples, Italy. E-mail: masantor@unina.it.
Supported by the Associazione Italiana per la Ricerca sul Cancro, the Progetto Strategico Oncologia of the CNR/the Italian Ministero per l’Istruzione, Università e Ricerca Scientifica (MIUR), MIUR, the BioGeM s.c.ar.l. (Biotecnologia e Genetica Molecolare nel Mezzogiorno d’Italia), the Italian Ministero della Salute, and also in part by the Ricerca Fondamentale Orientata (ex quota 60%)-MIUR funds (to G.T.).
References
- Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114–118. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ED. Thyroid cancer: pathologic and natural history. Recent Results Cancer Res. 1980;73:47–55. doi: 10.1007/978-3-642-81325-2_5. [DOI] [PubMed] [Google Scholar]
- Eng C. Familial papillary thyroid cancer—many syndromes, too many genes? J Clin Endocrinol Metab. 2000;85:1755–1757. doi: 10.1210/jcem.85.5.6632. [DOI] [PubMed] [Google Scholar]
- Manie S, Santoro M, Fusco A, Billaud M. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 2001;17:580–589. doi: 10.1016/s0168-9525(01)02420-9. [DOI] [PubMed] [Google Scholar]
- Fagin JA. Perspective: lessons learned from molecular genetic studies of thyroid cancer—insights into pathogenesis and tumor-specific therapeutic targets. Endocrinology. 2002;143:2025–2028. doi: 10.1210/endo.143.6.8832. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Jr, Russell J, Nibu K, Li G, Rhee E, Liao M, Goldstein M, Keane WM, Santoro M, Fusco A, Rothstein JL. The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998;58:5523–5528. [PubMed] [Google Scholar]
- Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/endo.137.1.8536638. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, Di Fiore PP. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Ito S, Iwashita T, Asai N, Murakami H, Iwata Y, Sobue G, Takahashi M. Biological properties of Ret with cysteine mutations correlate with multiple endocrine neoplasia type 2A, familial medullary thyroid carcinoma, and Hirschsprung’s disease phenotype. Cancer Res. 1997;57:2870–2872. [PubMed] [Google Scholar]
- Pasini A, Geneste O, Legrand P, Schlumberger M, Rossel M, Fournier L, Rudkin BB, Schuffenecker I, Lenoir GM, Billaud M. Oncogenic activation of RET by two distinct FMTC mutations affecting the tyrosine kinase domain. Oncogene. 1997;15:393–402. doi: 10.1038/sj.onc.1201199. [DOI] [PubMed] [Google Scholar]
- Rey JM, Brouillet JP, Fonteneau-Allaire J, Boneu A, Bastie D, Maudelonde T, Pujol P. Novel germline RET mutation segregating with papillary thyroid carcinomas. Genes Chromosom Cancer. 2001;32:390–391. doi: 10.1002/gcc.1205. [DOI] [PubMed] [Google Scholar]
- McIver B, Goellner JR, Hay ID. Mixed medullary-papillary thyroid carcinoma in a patient with multiple endocrine neoplasia type 2B. (MEN-2B). Thyroid. 1996;6(Suppl 1):16. [Google Scholar]
- Orlandi F, Chiefari E, Caraci P, Mussa A, Gonzatto I, De Giuli P, Giuffrida D, Angeli A, Filetti S. RET proto-oncogene mutation in a mixed medullary-follicular thyroid carcinoma. J Endocrinol Invest. 2001;24:51–55. doi: 10.1007/BF03343809. [DOI] [PubMed] [Google Scholar]
- Papi G, Corrado S, Pomponi MG, Carapezzi C, Cesinaro A, LiVolsi VA. Concurrent lymph node metastases of medullary and papillary thyroid carcinoma in a case with RET oncogene germline mutation. Endocr Pathol. 2003;14:269–276. doi: 10.1007/s12022-003-0020-4. [DOI] [PubMed] [Google Scholar]
- Reynolds L, Jones K, Winton DJ, Cranston A, Houghton C, Howard L, Ponder BA, Smith DP. C-cell and thyroid epithelial tumours and altered follicular development in transgenic mice expressing the long isoform of MEN 2A RET. Oncogene. 2001;20:3986–3994. doi: 10.1038/sj.onc.1204434. [DOI] [PubMed] [Google Scholar]
- Santoro M, Rosati R, Grieco M, Berlingieri MT, D’Amato GL, de Franciscis V, Fusco A. The ret proto-oncogene is consistently expressed in human pheochromocytomas and thyroid medullary carcinomas. Oncogene. 1990;5:1595–1598. [PubMed] [Google Scholar]
- Bunone G, Uggeri M, Mondellini P, Pierotti MA, Bongarzone I. RET receptor expression in thyroid follicular epithelial cell-derived tumors. Cancer Res. 2000;60:2845–2849. [PubMed] [Google Scholar]
- Fluge O, Haugen DR, Akslen LA, Marstad A, Santoro M, Fusco A, Varhaug JE, Lillehaug JR. Expression and alternative splicing of c-ret RNA in papillary thyroid carcinomas. Oncogene. 2001;20:885–892. doi: 10.1038/sj.onc.1204161. [DOI] [PubMed] [Google Scholar]
- Rosai J, Carcangiu ML, DeLellis RA. Tumors of the thyroid gland. Washington: Armed Force Institute of Pathology,; Atlas of Tumor Pathology, series 3. 1992 [Google Scholar]
- Machens A, Niccoli-Sire P, Hoegel J, Frank-Raue K, van Vroonhoven TJ, Roeher HD, Wahl RA, Lamesch P, Raue F, Conte-Devolx B, Dralle H. European Multiple Endocrine Neoplasia (EUROMEN) Study Group: early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. 2003;349:1517–1525. doi: 10.1056/NEJMoa012915. [DOI] [PubMed] [Google Scholar]
- Santoro M, Papotti M, Chiappetta G, Garcia-Rostan G, Volante M, Johnson C, Camp RL, Pentimalli F, Monaco C, Herrero A, Carcangiu ML, Fusco A, Tallini G. RET activation and clinicopathologic features in poorly differentiated thyroid tumors. J Clin Endocrinol Metab. 2002;87:370–379. doi: 10.1210/jcem.87.1.8174. [DOI] [PubMed] [Google Scholar]
- Melillo RM, Santoro M, Ong SH, Billaud M, Fusco A, Hadari YR, Schlessinger J, Lax I. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol Cell Biol. 2001;21:4177–4187. doi: 10.1128/MCB.21.13.4177-4187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini G, Santoro M, Helie M, Carlomagno F, Salvatore G, Chiappetta G, Carcangiu ML, Fusco A. RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res. 1998;4:287–294. [PubMed] [Google Scholar]
- Fusco A, Berlingieri MT, Di Fiore PP, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Basolo F, Castellone MD, Melillo RM, Fusco A, Santoro M. Efficient inhibition of RET/papillary thyroid carcinoma oncogenic kinases by 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2). J Clin Endocrinol Metab. 2003;88:1897–1902. doi: 10.1210/jc.2002-021278. [DOI] [PubMed] [Google Scholar]
- Santoro M, Melillo RM, Grieco M, Berlingieri MT, Vecchio G, Fusco A. The TRK and RET tyrosine kinase oncogenes cooperate with ras in the neoplastic transformation of a rat thyroid epithelial cell line. Cell Growth Differ. 1993;4:77–84. [PubMed] [Google Scholar]
- Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106:745–757. doi: 10.1016/s0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Chan PM, Ilangumaran S, La Rose J, Chakrabartty A, Rottapel R. Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region. Mol Cell Biol. 2003;23:3067–3078. doi: 10.1128/MCB.23.9.3067-3078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna E, Gramaglia D, Longati P, Bardelli A, Comoglio PM. Loss of the exon encoding the juxtamembrane domain is essential for the oncogenic activation of TPR-MET. Oncogene. 1999;18:4275–4281. doi: 10.1038/sj.onc.1202791. [DOI] [PubMed] [Google Scholar]
- Qiao S, Iwashita T, Furukawa T, Yamamoto M, Sobue G, Takahashi M. Differential effects of leukocyte common antigen-related protein on biochemical and biological activities of RET-MEN2A and RET-MEN2B mutant proteins. J Biol Chem. 2001;276:9460–9467. doi: 10.1074/jbc.M008744200. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschard P, Fournier TM, Lamorte L, Naujokas MA, Band H, Langdon WY, Park M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell. 2001;8:995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- Fitze G, Appelt H, Konig IR, Gorgens H, Stein U, Walther W, Gossen M, Schreiber M, Ziegler A, Roesner D, Schackert HK. Functional haplotypes of the RET proto-oncogene promoter are associated with Hirschsprung disease (HSCR). Hum Mol Genet. 2003;12:3207–3214. doi: 10.1093/hmg/ddg354. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62:7284–7290. [PubMed] [Google Scholar]