Abstract
Severity of fibrosis after injury is determined by the nature of the injury and host genetic susceptibility. Metabolism of collagen, the major component of fibrotic lesions, is, in part, regulated by integrins. Using a model of glomerular injury by adriamycin, which induces reactive oxygen species (ROS) production, we demonstrated that integrin α1-null mice develop more severe glomerulosclerosis than wild-type mice. Moreover, primary α1-null mesangial cells produce more ROS both at baseline and after adriamycin treatment. Increased ROS synthesis leads to decreased cell proliferation and increased glomerular collagen IV accumulation that is reversed by antioxidants both in vivo and in vitro. Thus, we have identified integrin α1β1 as a modulator of glomerulosclerosis. In addition, we showed a novel pathway where integrin α1β1 modulates ROS production, which in turn controls collagen turnover and ultimately fibrosis. Because integrin α1β1 is expressed in many cell types this may represent a generalized mechanism of controlling matrix accumulation, which has implications for numerous diseases characterized by fibrosis.
Glomerulosclerosis, the process by which glomerular tissue is replaced by extracellular matrix (ECM), is the final common pathway for loss of functioning glomeruli. All three major cell types that constitute the glomerulus contribute to this process. Podocytes and endothelial cells are likely critical for initiation of sclerosis; however, mesangial cells are the major contributor to progression.1 The normal glomerular response to injury is rapid synthesis of ECM components (mainly collagens) that are remodeled as the injured glomerulus undergoes repair. In glomerulosclerosis, ECM accumulation, particularly collagens, is uncontrolled.
The synthesis of ECM is, in part, regulated by integrins.2 Integrin α1β1, a major collagen receptor, is expressed in all cell types in the glomerulus.3,4 This integrin has been associated with renal disease and is overexpressed in proliferating mesangium in glomerulonephritis.5,6 In addition, anti-integrin α1 antibodies reduced scarring in rat models of glomerular injury by inhibiting integrin α1β1-dependent (VLA-1) leukocyte function with consequent immune response dampening.7 Despite these results, one would expect that lack of integrin α1β1 might predispose to increased glomerulosclerosis because integrin α1β1 is critical for the support of cell proliferation and survival on collagen substrata,8 as well as for sensing extracellular collagen levels and down-regulating endogenous collagen synthesis.9 Consequently, loss of α1β1 function results in increased collagen expression9 and an inability of α1-null cells to proliferate on collagenous substrata.8
To determine the role of integrin α1β1 in mediating the host response to renal injury, we compared the response of wild-type and integrin α1-null mice to adriamycin (ADR)-induced nephropathy. This is a nonimmunologically mediated injury induced by generation of reactive oxygen species (ROS) that is characterized by mild mesangial sclerosis in BALB/c mice.10–12 We demonstrate that α1-null mice on the BALB/c background developed severe glomerular injury, characterized by mesangiolysis within 24 hours of ADR administration and consequent relentless mesangial sclerosis. Excessive ROS production was the major stimulus for the unwarranted excessive synthesis of collagen by both α1-null mice and primary mesangial cells, at steady state levels and after treatment with ADR. Excessive ROS-mediated collagen deposition could be ameliorated, both in vivo and in vitro, by treatment with antioxidants. Together these results demonstrate that integrin α1β1 is a critical modulator of glomerular injury and its functional loss leads to increased sclerosis primarily by stimulating ROS production.
Materials and Methods
Mice and Experimental Procedure
All experiments were performed according to institutional animal care guidelines. Wild-type and integrin α1-null BALB/c male mice (5 weeks old, ∼20 g body weight) received a single intravenous injection of ADR (10 mg/kg; Sigma, St. Louis, MO) as described.10 Mice were sacrificed 0, 24, and 72 hours, or 1, 2, 4, 6, and 8 weeks after ADR injection. Seven mice/genotype/treatment were used for this set of experiments. Three independent experiments were performed.
When antioxidants were used, mice were treated daily with α-tocopherol acetate (100 mg/kg daily i.p., Sigma) and ascorbic acid (10 mg/ml in drinking water daily, Sigma) beginning 7 days before ADR (ADR + AOX group) until sacrifice (1 week after ADR injection). Some mice received ADR only (ADR group) and others only the antioxidants (AOX group). One week after ADR injection was chosen as an end point because matrix deposition and hyalinosis were primarily observed in α1-null, but not wild-type mice, at this time (Figure 1). Ascorbic acid and α-tocopherol acetate were used at the indicated doses because they have been shown to ameliorate ROS-mediated injury in rodents.13,14 Four mice/genotype/treatment were used for this set of experiments. Two independent experiments were performed.
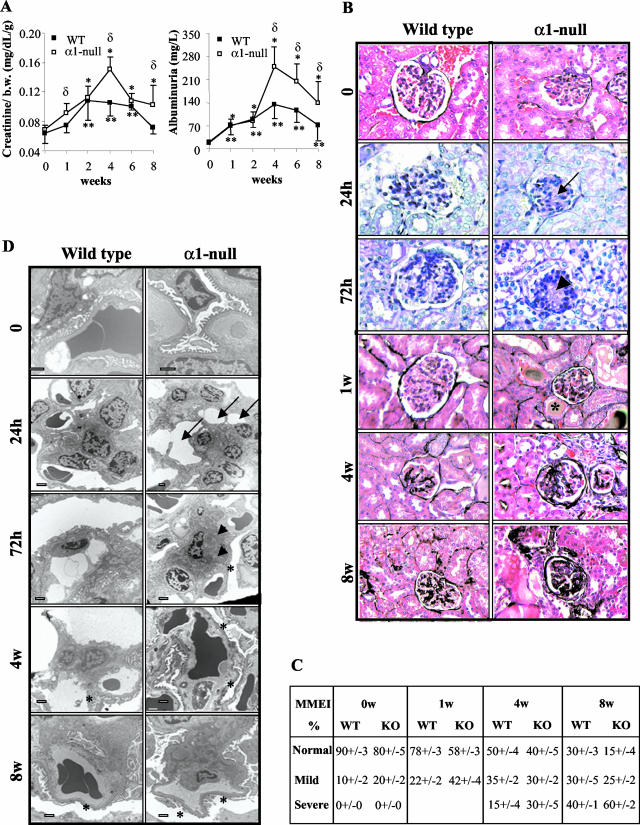
Figure 1.
Integrin α1-null mice develop severe glomerular injury after ADR injection. A: Creatinine (mg/dL/g) and albuminuria (mg/L) were measured from urine or serum obtained from untreated or ADR-treated mice immediately at sacrifice. Data are presented as the mean and SD of seven wild-type (WT) and α1-null (KO) mice. Differences between untreated and treated α1-null mice (*) or untreated and treated wild-type mice (**) or wild-type and α1-null mice within the same treatment group (δ) were significant with P < 0.05. B: Five-week-old male mice received a single intravenous injection of ADR (10 mg/kg) and kidneys were evaluated at the time points indicated. Signs of early mesangiolysis (arrow), increased matrix deposition (arrowhead), and hyalinosis (*), were evident primarily in the α1-null mice 24 hours, 72 hours, and 1 week after ADR injection (periodic acid-Schiff). At later stages, increased ECM accumulation and sclerosis were more severe in the α1-null mice compared to their treated wild-type counterparts (Jones’ staining). C: One hundred glomeruli/kidney from seven untreated and ADR-treated mice were evaluated and the degree of matrix mesangial expansion (MMEI) scored and expressed as described in Materials and Methods. D: Ultrastructural examination of kidney sections revealed early mesangiolysis (arrow), matrix accumulation (arrowhead), and FPE (*) primarily in the α1-null mice 24 hours and 72 hours after ADR injection. FPE was also visible in wild-type mice 4 weeks after ADR injection, although it was much more severe in α1-null. Scale bar, 50 μm. Original magnifications, × 400 (B).
Clinical Parameters and Morphological Analysis
Urine was collected 24 hours before sacrifice and urinary albumin was measured by the ELISA Albuwell M test kit (Exocel Inc., Philadelphia, PA) and expressed as mg/L. F2-isoprostanes were measured in urine using stable isotope dilution methods, gas chromatography negative ion chemical ionization-mass spectrometry,15 and expressed as ng/mg urine creatinine. Blood was collected immediately at sacrifice and serum creatinine was measured using a commercially available colorimetric kit (Sigma) and expressed as mg/dL/g body weight.
Kidneys, removed immediately at sacrifice, were either fixed in 4% formaldehyde and embedded in paraffin for both morphological and immunohistochemistry analysis, or depleted of medulla and frozen for mRNA analysis. Paraffin tissue sections were stained with periodic acid-Schiff or Jones’ for the evaluation of glomerular injury. Mesangial matrix expansion index (MMEI) was evaluated in 100 glomeruli/kidney by a single blinded observer (G.M.) and expressed by percentage as normal, mild, and severe (0%, 1 to 30%, and >30% of glomerular tuft with visible expanded mesangial matrix, respectively). Kidneys (n = four or seven/genotype) were evaluated and the MMEI expressed as mean ± SD for each group.
Electron Microscopy
Portions of renal cortex were fixed in 2.5% glutaraldehyde in phosphate buffer. Samples were postfixed in OsO4, dehydrated in ethanol, and embedded in resin. Thin sections were assessed for mesangiolysis, mesangial matrix deposition, and podocyte injury. Foot process effacement (FPE) was semiquantitatively assessed (percent of effacement in 10 peripheral capillary loops/glomerulus with a total of seven kidneys/genotype evaluated) and expressed as mean ± SD for each study group.
Immunohistochemistry and Immunofluorescence
Immunohistochemistry on paraffin kidneys sections was done using rabbit anti-mouse collagen IV antibodies (1:100; Biodesign, Saco, ME), or rabbit anti-mouse proliferating cell nuclear antigen (PCNA) (1:100; Santa Cruz, Santa Cruz, CA), followed by horseradish peroxidase-conjugated goat secondary antibody to rabbit IgG (1:200; Jackson Immunoresearch, West Grove, PA) and Sigma Fast DAB chromogenic tablets (Sigma). The glomerular proliferation index was expressed as (number of PCNA-positive glomerular cells/total number of glomerular cells) × 100. Glomerular apoptosis was evaluated by staining paraffin section with the Dead End colorimetric terminal dUTP nick-end labeling (TUNEL) system (Promega, Madison, WI) using diaminobenzidine as the chromogenic substrate. The glomerular apoptotic index was expressed as (number of TUNEL-positive glomerular cells/total number of glomerular cells) × 100. Ten glomeruli/kidney were evaluated with a total of seven kidneys/genotype/treatment.
To determine collagen deposition by mesangical cells, 3 × 104 cells were plated for 48 hours on uncoated four-well chamber slides in the presence of Dulbecco’s modified Eagle’s medium (DMEM) containing 2% fetal calf serum (FCS) and 0, 0.1, 0.5, and 1 μmol/L ADR in the presence of the antioxidants TEMPOL (10 μmol/L) and DPI (1 μmol/L) (both from Sigma). The doses of TEMPOL, a superoxide mimetic, and DPI, a specific NADPH oxidase inhibitor, were chosen because they represent the maximum dose able to inhibit ROS generation in wild-type and α1-null mesangial cells without cytotoxic effects (data not shown). Immunofluorescence on acetone-fixed cells was done using the rabbit anti-mouse collagen IV antibodies described above (1:100) followed by fluorescein isothiocyanate-conjugated goat secondary antibody to rabbit IgG (1:100; Jackson) and 2 μg/ml 4,6-diamidino-2-phenylindole to visualize nuclei.
Immunoblotting
To determine collagen deposition by mesangial cells, 3 × 105 cells were plated on uncoated six-well plates in the presence of DMEM containing 2% FCS and in the absence or presence of TEMPOL (10 μmol/L) and DPI (1 μmol/L). After 48 hours, cells were scraped, suspended in 50 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, and centrifuged 10 minutes at 14,000 rpm. Cell lysates were resolved by 5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Gels were either stained with Coomassie Blue to evaluate equal loading, or transferred to Immobilon-P membranes (Millipore, Billerica, MA). Membranes were incubated with a rabbit anti-collagen IV antibody (Biodesign) and immunoreactive proteins visualized using a peroxidase-conjugated goat anti-rabbit and an ECL kit (Pierce, Rockford, IL).
RNA Analysis
Total RNA from renal cortices (n = seven kidneys/genotype) was isolated using a single-step isolation kit (TRIzol; Invitrogen, Carlsbad, CA). Ten μg of total RNA was separated in formaldehyde-containing 1% agarose gels, transferred to nylon membranes (Nytran Supercharge; Schleicher & Schuell, Keene, NH), and hybridized with 32P-labeled cDNAs for mouse α1(IV) collagen. Mouse β2-tubulin was used for normalization. Collagen IV and tubulin bands were quantified by densitometry analysis using an Alpha Imager 2000 (Alpha Innotech, San Leandro, CA) and collagen IV signal expressed as collagen IV/tubulin.
Isolation of Primary Mesangial Cells and Proliferation Assays
Primary mesangial cells were isolated and characterized as previously described.16 For proliferation assays, 5 × 103 primary mesangial cells were plated in DMEM containing 2% FCS on to 96-well plates coated with 10 μg/ml fibronectin or collagen IV (both from Sigma). In some experiments, mesangial cells were cultured in DMEM containing 2% FCS in the presence of different concentrations of ADR (0, 0.1, 0.5, 1, and 5 μmol/L) alone or in combination with TEMPOL (10 μmol/L) and DPI (1 μmol/L). After 2 days the medium was replaced, the cells were then pulsed for an additional 48 hours with 3H-thymidine (1 μCi/well) and processed as described.17 Manual cell counts paralleled the results of 3H-thymidine incorporation (data not shown).
Flow Cytometry
A suspension of wild-type or α1-null mesangial cells was incubated with monoclonal antibodies of the appropriate integrin (ie, anti-α1, -α2, -α5 and -β1 integrins all from Pharmingen, San Diego, CA) (1:100 dilution), followed by incubation with the appropriate secondary antibodies (fluorescein isothiocyanate-coupled rabbit anti-rat or hamster immunoglobulin) (1:100 dilution). Flow cytometry was performed with a FACScan instrument (Becton Dickinson, Franklin Lakes, NJ). Cell suspensions incubated with secondary antibody only were used as a negative control for integrin expression. Two independent experiments were performed in duplicate.
Adhesion Assay
Microtiter plates (96-well) were coated with fibronectin or collagen IV at the indicated concentrations in phosphate-buffered saline (PBS) for 1 hour at 37°C. Plates were then washed with PBS and incubated with PBS containing 1% bovine serum albumin for 60 minutes to block nonspecific adhesion. One hundred μl of single-cell suspensions (5 × 105 mesangial cells/ml) in serum-free DMEM containing 0.1% bovine serum albumin were added in quadruplicate to 96-well plates and incubated for 60 minutes at 37°C. In some experiments, cell suspensions were preincubated with anti-integrin antibodies (anti-α1, -α2, and -β1 integrins) (10 μg/ml final concentration) on ice for 30 minutes before the assay. Nonadherent cells were removed by washing the wells with PBS. Cells were then fixed with 4% formaldehyde, stained with 1% crystal violet, solubilized in 20% acetic acid, and then read at 570 nm. Background cell adhesion to 1% bovine serum albumin-coated wells was subtracted from the values obtained on ECM proteins. Two independent experiments were performed in quadruplicate.
Detection of ROS Produced by Mesangial Cells
ROS in mesangial cells were measured using the substrate dihydro-rhodamine (Molecular Probes, Eugene, OR).18 Briefly, 15 × 104 mesangial cells were plated in six-well plates coated with 10 μg/ml of fibronectin or collagen IV in DMEM containing 10% FCS. After 24 hours, cells were incubated for a total of 6 hours with DMEM containing 2% FCS, 30 U/ml horseradish peroxidase, 2 μmol/L dihydro-rhodamine, and different concentrations of ADR (0, 0.1, 1, and 5 μmol/L) alone or together with TEMPOL (10 μmol/L) and/or DPI (1 μmol/L). Cells were then trypsinized, washed twice in PBS and the generation of fluorescent rhodamine 123 was analyzed with a FACScan (λex = 488 nm, λem = 525 nm) as described.18 Three independent experiments were performed in duplicate.
Statistical Analysis
We used the t-test for comparisons between two groups, and analysis of variance using Sigma-Stat software for statistical differences between multiple groups. P ≤ 0.05 was considered statistically significant.
Results
Integrin α1-Null Mice Develop Severe Glomerular Injury after ADR Injection
A single injection of ADR (10 mg/kg), previously shown to induce renal injury in BALB/c mice,11,12 was given to both wild-type and integrin α1-null mice on the BALB/c background. The wild-type mice developed an increase in serum creatinine 2 weeks after ADR injection (Figure 1A), which persisted until week 6 and started to recover by week 8. These mice also developed significant proteinuria that peaked 4 weeks after ADR but was still present at week 8. In the α1-null mice, the levels of serum creatinine and albuminuria were significantly worse when compared to wild-type mice at all time points (Figure 1A).
Although integrin α1-null mice develop normally and have no overt renal phenotype,19 their glomeruli are smaller with reduced glomerular tuft area and evidence of mild mesangial matrix accumulation (in 20% of glomeruli), when compared to wild-type controls (Figure 1, B and C). Within 24 hours of ADR injection, kidneys from α1-null mice, but not wild-type mice, showed evidence of mesangial expansion, focal mesangiolysis with multiple small electron-lucent mesangial lesions, and increased FPE (31 ± 6.7% in the α1-null versus 10 ± 6.5% in wild-type; P < 0.007; n = 3) (Figure 1, B and D). By 72 hours the mesangial expansion and FPE had further increased in α1-null versus wild-type mice (67 ± 12% in the α1-null versus 30 ± 10% in wild-type; P < 0.01; n = 3) (Figure 1, B and D).
At 1 week glomerular matrix deposition and hyalinosis became evident in α1-null mice, but not in their wild-type counterparts. Although wild-type kidneys showed mesangial expansion because of both mesangial cell proliferation and increased mesangial matrix deposition (measured by MMEI) by week 4 as previously reported,11 mesangial expansion was far more severe in the α1-null mice at this time (Figure 1, B and C). By week 8 glomerulosclerosis was well developed in the wild-type mice and 40% of glomeruli demonstrated severe MMEI scores (Figure 1, B and C). The pathology in the α1-null mice was significantly worse at the same time point and 60% of glomeruli showed severe MMEI scores (Figure 1, B and C). There was also worse interstitial damage of the kidneys in the α1-null mice particularly evident at 4 and 8 weeks (Figure 1B).
Ultrastructural examination of kidneys from wild-type mice showed segmental FPE 4 weeks after ADR administration, which became more widespread by week 8 (Figure 1D). In contrast, there was severe diffuse FPE involving all of the capillary loops in the α1-null mice at week 4 and by week 8, the FPE was near complete (Figure 1D). Although there was evidence of increased mesangial ECM deposition in wild-type mice at 4 weeks that progressed by week 8, the increase in ECM accumulation in α1-null mice was significantly greater at both these time points.
ADR-Treated α1-Null Mice Develop Mesangial Expansion Primarily Because of Increased Collagen IV Deposition
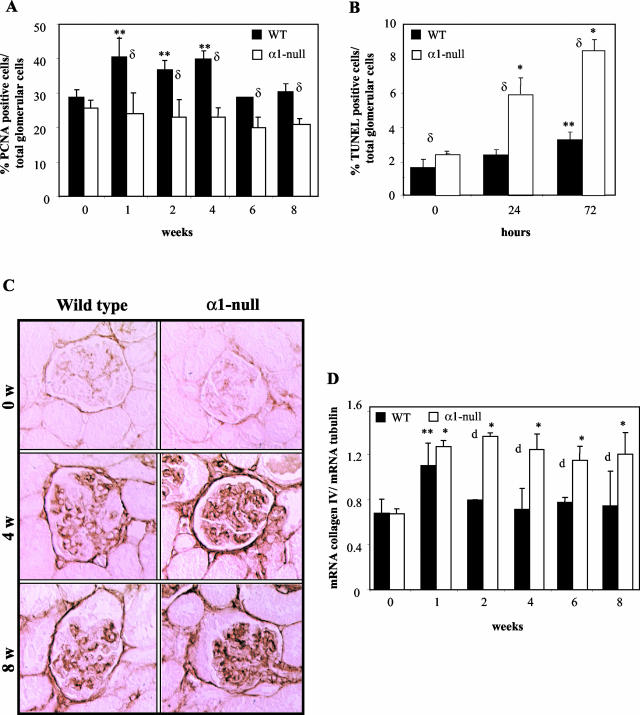
To determine the etiology of the mesangial expansion in the ADR-treated mice we initially assessed the proliferation and apoptosis index in glomeruli of untreated and ADR-treated mice. We found a significant increase in the number of PCNA-positive cells primarily in the glomeruli of wild-type mice. This increase was evident 1 week after ADR injection (40 ± 7% in wild-type versus 25 ± 8% in α1-null) and persisted throughout the duration of the study period (Figure 2A). Because leukocyte infiltration has been described in the ADR-induced injury model,11,12 and integrin α1-null mice show a defect in leukocyte infiltration to the site of injury,20,21 we assessed whether the differences in number of PCNA-positive cells could be because of lymphocyte and/or macrophage infiltration. Both macrophage and lymphocyte infiltration (assessed by staining kidney sections with anti-F4/80 and CD3 antibody, respectively) within glomeruli was minimal in both genotypes and no significant differences were observed at any time point examined (data not shown). Because early mesangiolysis was observed within 24 hours of ADR administration in the α1-null background, we assessed apoptosis at this early time point in both genotypes. As shown in Figure 2B, there was significantly more apoptosis in the α1-null mice compared to their wild-type counterparts at early time points. At later stages (weeks 1 to 8) apoptotic cells were difficult to visualize in the glomeruli of both genotypes. Taken together, these results suggest that increased glomerular proliferation of mesangial cells was not the etiology of the excessive mesangial expansion in the α1-null mice.
Figure 2.
Excessive collagen IV expression is the cause of the increased mesangial expansion in the α1-null mice after ADR treatment. A and B: Kidney sections from wild-type and integrin α1-null mice were stained with anti-PCNA antibody (A) or with the Dead End colorimetric TUNEL system (B), and the percentage of proliferating or apoptotic cells expressed as (number of PCNA-positive or apoptotic cells in the glomerulus/total number of cells in the glomerulus) × 100. Ten glomeruli/kidney were evaluated. Data are presented as mean and SD of seven kidneys/genotype. C: Collagen IV staining of kidney sections from wild-type and integrin α1-null mice after ADR treatment. Note the increased collagen deposition in the glomeruli of α1-null mice at 4 weeks after ADR treatment. Maximum difference between the two genotypes was observed at 8 weeks (anti-collagen IV). D: α1(IV) collagen mRNA levels in renal cortices of wild-type and integrin α1-null mice were normalized to the β-2 tubulin mRNA levels and expressed as mRNA collagen IV/mRNA tubulin. Data are mean and SD of seven kidneys/genotype. Increased collagen IV levels were observed in kidneys of both wild-type and α1-null mice at 1 week after ADR treatment, but persisted only in the α1-null mice throughout the study period. *, **, and δ are as in Figure 1A. Original magnifications, ×400 (C).
One of the characteristics of ADR-induced glomerular injury is increased expression and accumulation of collagen type IV in the glomerular tuft and Bowman’s capsule.10 Because integrin α1 is a primary receptor for collagen IV19 and is critical in down-regulating endogenous collagen synthesis,9 we analyzed and compared collagen IV deposition in the glomeruli of ADR-treated wild-type and α1-null mice. Collagen IV mRNA and protein accumulation in α1-null mice was increased 1 week after ADR injection and persisted throughout the study (Figure 2, C and D). Significantly less collagen IV synthesis and deposition were observed in wild-type mice at all time points examined (Figure 2, C and D). There was little or no increase in glomerular collagen I deposition in either the wild-type or α1-null mice at any of the time points (data not shown), suggesting that the primary cause of the increased mesangial expansion in the α1-null mice was because of excessive collagen IV expression.
Mesangial Cell Adhesion on Collagen IV Is Integrin α1β1-Dependent
Our in vivo observation that ADR leads to early mesangiolysis with increased apoptosis and consequent mesangial expansion and glomerulosclerosis in α1-null mice (Figure 1, B and D; Figure 2B) strongly suggested that mesangial cells are a target for ADR.18 We therefore isolated primary mesangial cells to investigate the mechanisms for the increased ADR-induced mesangial injury in α1-null mice.
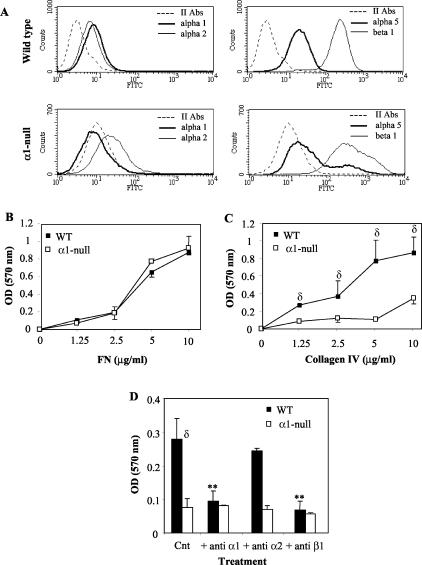
We initially determined the role of integrin α1β1 on mesangial cell adhesion to collagen IV. We established that mesangial cells express both integrins α1β1 and α2β1 and integrin α1-null cells do not compensate for lack of α1β1 by overexpressing the other major collagen receptor α2β1 (Figure 3A). Furthermore, the expression of integrin α5 (fibronectin binding receptor) and β1 subunits was comparable in both genotypes (Figure 3A). We then tested the ability of mesangial cells of both genotypes to adhere to collagenous substrata. As demonstrated in Figure 3B, no differences in adhesion were observed between wild-type and integrin α1-null cells plated on fibronectin (an integrin α5β1-dependent ligand) (Figure 3B). In contrast, despite expressing the integrin α2 subunit, α1-null mesangial cells showed significantly reduced adhesion on collagen IV compared to their wild-type counterparts (Figure 3C), suggesting that integrin α1β1 is the major collagen IV-binding receptor in the mesangium. To further verify that mesangial cell adhesion on collagen IV was mediated by integrin α1β1, mesangial cells of both genotypes were plated on collagen IV in the absence or presence of anti-α1, -α2, and -β1 integrin antibodies. As shown in Figure 3D, adhesion of wild-type mesangial cells was significantly impaired after incubation with anti-α1 or -β1 but not anti-α2 antibodies and was similar to that of α1-null mesangial cells. Taken together, these results suggest that mesangial cell adhesion to collagen IV is an integrin α1β1-dependent event.
Figure 3.
Mesangial cell adhesion on collagen IV is integrin α1β1-dependent. A: Integrin expression by wild-type and integrin α1-null mesangial cells. Cells were incubated with antibodies to α1, α2, α5, or β1 integrin subunits. Integrin expression is displayed by a shift in mean fluorescent intensity compared with no primary antibody incubation using fluorescence-activated cell sorting analysis. B and C: Primary mesangial cells (5 × 104) were plated onto 96-well plates coated with fibronectin (B) or collagen IV (C) at the concentrations indicated for 1 hour in serum-free medium. Adherent cells were then stained with crystal violet, lysed, and the OD measured. Data represent the mean and SD of quadruplicate samples of 10 pooled wild-type and integrin α1-null mice. D: Mesangial cells were plated in the presence or absence of blocking antibodies (10 μg/ml) to the integrin subunits indicated on collagen IV (5 μg/ml) for 1 hour in serum-free medium. Adhesion was then determined and expressed as described above. Note that adhesion of mesangial cells to CIV is primarily integrin α1β1-mediated. Data are expressed as in B.
ADR Reduces Mesangial Cell Proliferation that Can Be Rescued by Antioxidants
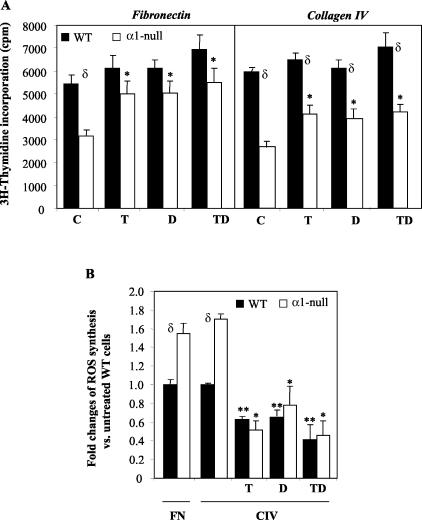
We subsequently tested the ability of mesangial cells to proliferate on both collagenous and noncollagenous substrata, by assaying 3H-thymidine incorporation.17 Wild-type mesangial cells proliferated 1.7- and 2.2-fold more than α1-null cells on fibronectin and collagen IV, respectively (Figure 4A). The results on collagen IV paralleled our previous observation that collagens promoted growth of wild-type cells, while they failed to promote that of α1-null cells.8 The differences in proliferation on fibronectin (in the absence of the natural ligand of integrin α1β1) were surprising and suggested that at the time of the assay (72 hours) either the α1-null mesangial cells secreted enough collagen IV that could act as an inhibitory ECM, or that lack of integrin α1β1 decreased cell growth by a mechanism that was autonomous of integrin α1β1-dependent cell-ECM interactions.
Figure 4.
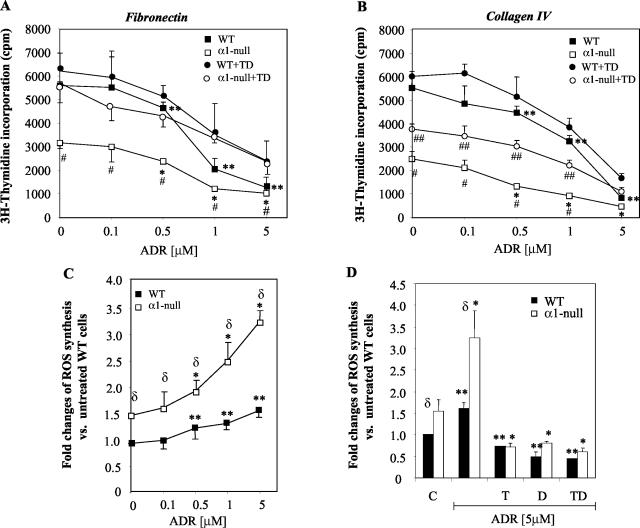
α1-Null mesangial cells show reduced proliferation on both collagenous and noncollagenous substrata. A: Primary mesangial cells (5 × 103) were plated on 10 μg/ml of fibronectin or 10 μg/ml of collagen IV in the absence or presence of 10 μmol/L TEMPOL (T), 1 μmol/L DPI (D), or a mixture of TEMPOL and DPI (TD). Incorporation of 3H-thymidine was then evaluated as described in Materials and Methods. Data represent the means and SD of quadruplicate samples derived from 10 pooled wild-type and integrin αl-null mice. B: Mesangial cells were cultured on 10 μg/ml of fibronectin (FN)- or collagen IV (CIV)-coated dishes in the absence or presence of 10 μmol/L TEMPOL (T), 1 μmol/L DPI (D), or a mixture of TEMPOL and DPI (TD) together with 2 μmol/L dihydro-rhodamine. Six hours later, ROS generation was determined by FACS as described in Materials and Methods. Data represent the mean and SD of three experiments performed in duplicates. *, **, and δ are as in Figure 1A.
Because mesangial cells produce large amounts of ROS18 and ROS can modulate cell growth,22 we determined whether alterations in ROS production might explain the differences in growth between wild-type and α1-null mesangial cells on both fibronectin and collagen IV. Using the fluorescent probe dihydro-rhodamine,18 we initially demonstrated that α1-null mesangial cells synthesize more ROS than their wild-type counterparts, which was independent of the substrate on which cells were plated (Figure 4B). To assess whether increased levels of ROS accounted for the decreased proliferation of α1-null mesangial cells, we determined the effect of the antioxidants TEMPOL (a superoxide dismutase mimic) and diphenyleneiodonium (DPI, a NADPH oxidase-specific inhibitor)23,24 on both ROS generation and mesangial cell proliferation. Incubation with TEMPOL, DPI, or a combination of the two antioxidants significantly reduced ROS generation in mesangial cells from both genotypes plated on either fibronectin (data not shown) or collagen IV (Figure 4B). The antioxidants rescued α1-null mesangial cell proliferation on fibronectin returning α1-null cell growth to wild-type levels (Figure 4A). However, the rescue of α1-null mesangial cell proliferation on collagen IV was only partial (Figure 4A). These data suggest that the mechanism for decreased cell proliferation of integrin α1-null mesangial cells on fibronectin is primarily related to increased ROS production as the proliferative signal from the ECM can be transduced by integrin α5β1. However, when α1-null mesangial cells are grown on collagen IV, decreased proliferation is related to both increased ROS production and loss of an integrin α1β1-dependent proliferative stimulus.8
To determine the effect of ADR on cell proliferation, mesangial cells were cultured on fibronectin- or collagen IV-coated dishes in the presence of increasing concentration of ADR (Figure 5, A and B). As expected there was a significant difference in proliferation between untreated wild-type and α1-null mesangial cells when plated on both fibronectin and collagen IV. Increasing concentrations of ADR reduced the percentage of cell proliferation by the same amount (slope of the curves are approximately equal) in both phenotypes in the presence of low concentrations of ADR (0.1 to 0.5 μmol/L on fibronectin and 0.1 to 1 μmol/L on collagen IV). Treatment with antioxidants rescued the proliferation of mesangial cells of both genotypes to the same degree when they were cultured on fibronectin (Figure 5A). In contrast, the rescue effects of the antioxidants on growth of the α1-null mesangial cells plated on collagen IV was only partial (Figure 5B). To obtain direct evidence that decreased proliferation in both wild-type and α1-null mesangial cells exposed to ADR was at least in part because of increased ROS production, mesangial cells grown on fibronectin (data not shown) or collagen IV (Figure 5C) were incubated with different concentrations of ADR, and ROS generation was measured. Although increased ROS production was observed in both genotypes when exposed to ADR, the α1-null cells produced significantly more ROS (Figure 5C) than their wild-type counterparts. This increase in ADR-induced ROS generation was inhibited after addition of TEMPOL and DPI either alone or in combination (Figure 5D, only 5 μmol/L shown). The rescue effect of antioxidants on proliferation was more evident when cells were treated with low (0.1 to 1 μmol/L) rather than high (5 μmol/L) concentrations of ADR (Figure 5, A and B), despite almost complete inhibition of ROS production by antioxidants in cells treated with 5 μmol/L ADR (Figure 5D). This result suggested that ADR might prevent cell proliferation in a ROS-independent mechanism when given at high concentrations for long periods of time, as previously suggested.25 Together these results show that α1-null mesangial cells produce more ROS in response to ADR compared to their wild-type counterparts and the relative increase of ROS production is proportional to the increased base line production of the α1-null mesangial cells. The direct correlation between ROS production and decreased cell proliferation supports the notion that a significant increase in ROS production by α1-null mesangial cells plays a critical contributory role in the control of mesangial cell proliferation. This mechanism is in addition to the lack of an integrin α1β1-dependent proliferative stimulus evident when α1-null mesangial cells are plated on collagen IV.8
Figure 5.
ADR reduces mesangial cell proliferation that can be rescued by antioxidants. A and B: Mesangial cells were plated on 10 μg/ml fibronectin (A)- or 10 μg/ml collagen IV (B)-coated dishes in the presence of ADR at the concentration indicated in the absence of presence of mixture of 10 μmol/L TEMPOL and 1 μmol/L DPI (TD). 3H-thymidine incorporation was then evaluated as described in Materials and Methods. Data represent means and SD of quadruplicate samples of mesangial cells derived from 10 pooled wild-type and integrin αl-null mice. C and D: Wild-type and α1-null mesangial cells were plated on 10 μg/ml collagen IV in the presence of ADR at concentrations indicated (C) or with 5 μmol/L ADR and 10 μmol/L TEMPOL (T), 1 μmol/L DPI (D), or a mixture of TEMPOL and DPI (TD) (D). ROS generation was determined after incubation with 2 μmol/L dihydro-rhodamine for 6 hours as described in Materials and Methods. Data represent the mean and SD of three experiments performed in duplicates. *, **, and δ are as in Figure 1A. Differences between ADR-treated α1-null and ADR + antioxidant-treated α1-null cells (#) or wild-type and α1-null cells within the same ADR + antioxidants group (##) were significant with P < 0.05.
ADR Induces Increased Collagen IV Synthesis and Deposition in α1-Null Mesangial Cells
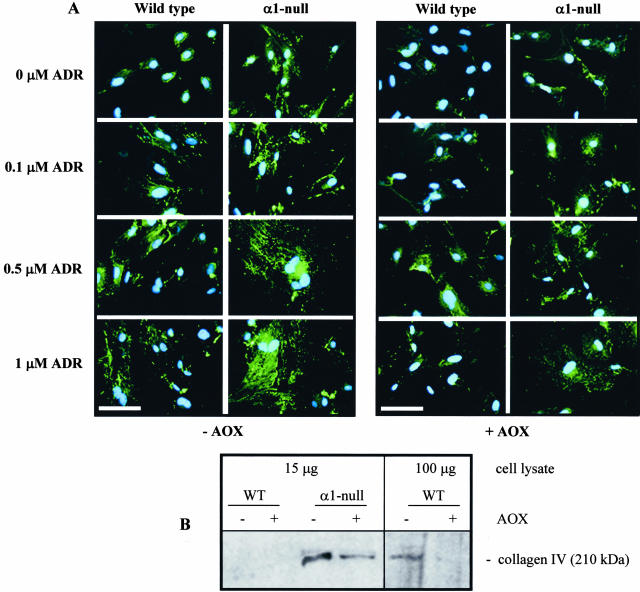
Because ADR leads to increased ECM synthesis in vivo (Figure 2, B and C),26 we used immunofluorescence and Western blot analysis to determine the effect of ADR on collagen IV deposition/accumulation in wild-type and integrin α1-null mesangial cells. At baseline α1-null mesangial cells expressed significantly more collagen IV than wild-type cells (Figure 6, A and B). Interestingly, although treatment with antioxidants completely inhibited collagen IV deposition in wild-type cells at baseline (Figure 6, A and B) only a partial reduction in collagen accumulation was observed in α1-null mesangial cells exposed to antioxidants (Figure 6, A and B). These results suggest that although increased ROS generation by α1-null mesangial cells is a major contributor to increased collagen production at baseline, there is also an integrin α1-dependent/ROS-independent mechanism that controls collagen deposition/accumulation. After ADR exposure, both genotypes showed increased accumulation of collagen IV (Figure 6A), although α1-null mesangial cells produced considerably more collagen IV than wild-type cells. Addition of antioxidants to both wild-type and α1-null mesangial cells markedly reduced collagen deposition after ADR treatment (Figure 6A) suggesting that ADR induces ECM deposition in a ROS-dependent manner.9
Figure 6.
Collagen deposition in wild-type and integrin α1-null mesangial cells. A: Mesangial cells were plated on uncoated chamber slides and treated for 48 hours with ADR at μmol/L concentrations indicated in the absence (left) or presence of 10 μmol/L TEMPOL and 1 μmol/L DPI (right). Indirect immunofluorescence was then performed to evaluate collagen IV synthesis and deposition. α1-Null mesangial cells synthesize more collagen IV than their wild-type counterparts and this synthesis is enhanced after ADR treatment. Although treatment with antioxidants (AOX) (right) reduced collagen deposition in both untreated and ADR-treated cells, α1-null mesangial cells still produced more collagen IV than their wild-type counterparts. B: Cell lysates were prepared from untreated or antioxidant-treated mesangial cells cultured as described in A and Western blot analysis was performed using anti-mouse collagen IV-specific antibodies. Note that α1-null mesangial cells produce more collagen IV than their wild-type counterparts, and treatment with antioxidants only partially rescues collagen synthesis. In contrast, antioxidant treatment completely inhibited collagen synthesis in wild-type cells, paralleling the results obtained with immunofluorescence (A). Note that collagen IV is detectable in wild-type cells only when 100 μg of total cell lysate were used for analysis. Scale bar, 20 μm (A).
In Vivo Treatment with Antioxidants Ameliorates ADR-Induced Glomerular Injury
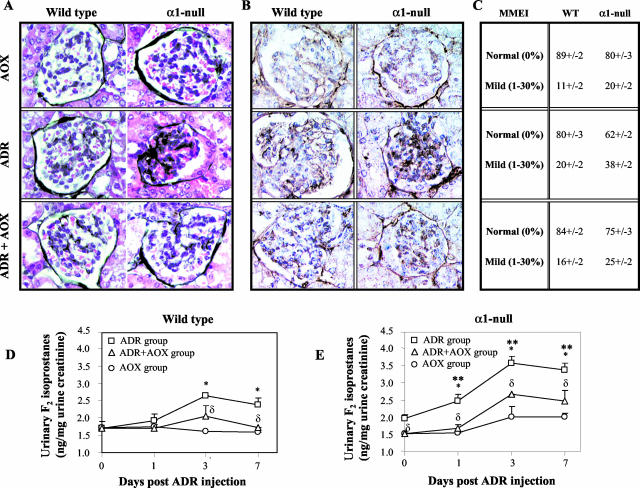
Because antioxidants rescued α1-null mesangial cell proliferation and collagen deposition in vitro (Figures 5 and 6), we investigated their effects in vivo. Pretreatment of α1-null mice with vitamins C and E significantly decreased ADR-induced matrix deposition, collagen IV accumulation, and MMEI (Figure 7; A to C). Despite this improvement, increased collagen deposition and mesangial expansion was still evident in α1-null compared to wild mice (Figure 7, A to C; ADR + AOX group), suggesting that lack of integrin α1β1 per se plays a role in the host response to injury. To demonstrate a correlation between increased ROS production and the degree of glomerular injury we measured F2-isoprostanes as a biomarker of free radical oxidative stress.27 Urinary (Figure 7, D and E) and isolated kidney (data not shown) F2-isoprostanes were significantly increased both at baseline and after ADR administration in the α1-null mice when compared to their wild-type counterparts. In vivo treatment with antioxidants (which ameliorated glomerular injury) decreased F2-isoprostanes production in both ADR-treated wild-type and α1-null mice. These results strongly suggest that, in addition to the lack of integrin α1β1 per se, increased ROS production by α1-null mice plays a significant contributory role in glomerular abnormalities at baseline, as well as in the severe acute injury and consequent fibrosis after ADR administration.
Figure 7.
In vivo treatment with antioxidants ameliorates ADR-induced renal injury. Five-week-old male mice (n = four/genotype) received ADR, antioxidants (AOXs), or a combination of ADR and AOX as described in Materials and Methods, and kidneys were analyzed 1 week after ADR injection. The increased ECM deposition, determined by both Jones’ (A) and collagen IV (B) staining, evident in ADR-treated α1-null mice, was significantly ameliorated by administration of AOXs. C: MMEI in glomeruli from ADR-, AOX-, and ADR + AOX-treated mice was evaluated as described in Materials and Methods. D and E: F2-isoprostanes were measured in the urine as described in Materials and Methods and expressed as ng/mg urine creatinine. Values represent the mean and SD calculated for four samples/treatment. F2-isoprostanes levels in the urine of untreated α1-null mice were significantly increased compared to their untreated wild-type counterparts (P < 0.05). Pretreatment with AOXs significantly reduced the excretion of this biomarker for oxidative stress in the α1-null mice (P < 0.05). ADR injection led to increased excretion of F2-isoprostanes in both wild-type and integrin α1-null mice (*, P < 0.05 relative to untreated mice), although it was significantly higher in the latter group, starting 1 day after ADR injection (**, P < 0.05 between wild-type and α1-null mice within the same group). AOX treatment in ADR-treated mice significantly reduced F2-isoprostanes excretion in both genotypes (δ, P < 0.05 between ADR-treated and ADR + AOX-treated mice). Original magnifications, ×400 (A, B).
Discussion
The role of integrin α1β1 in the host response to renal injury is unclear. Functional inhibition of integrin α1β1 (VLA1) on leukocytes decreases glomerular damage in models of crescentic glomerulonephritis7 and Alport’s syndrome.28 In contrast, integrin α1β1 is critical for down-regulating collagen synthesis,9 suggesting its functional loss could predispose the host to increased sclerosis after injury. In our study we demonstrated that injury after ADR injection was much worse in integrin α1-null mice compared to controls. This increased injury was primarily ascribed to excessive ROS production by α1-null mesangial cells both at baseline as well as in response to ADR. Treatment with antioxidants significantly decreased collagen IV synthesis in α1-null mesangial cells and ameliorated renal injury in α1-null mice in vivo. These results confirm that the major changes seen both in vivo and in vitro are dependent on increased ROS generation and are not simply mediated by impairing the ability of the host to down-regulate collagen. Thus, lack of integrin α1β1 may be a critical susceptibility determinant in glomerulosclerosis by making the glomerulus far more vulnerable to acute injury via the regulation of ROS production by mesangial cells.
Integrin α1-null mice showed a much worse response to the injury at all time points assessed and many of the α1-null mice kept longer than 8 weeks after ADR injection were severely nephrotic and died from renal failure (data not shown). The significant increase in glomerulosclerosis, decreased glomerular cell proliferation, and increased apoptosis in the α1-null mice are consistent with the findings that integrin α1β1 is a negative regulator of collagen synthesis at both transcriptional and translational levels9 and is critical to support proliferation on collagenous substrata.8 Our data are however in contrast to the observation that blocking integrin α1β1 leads to reduced glomerular scarring during crescentic glomerulonephritis.7 The crescentic glomerulonephritis model is an immunologically mediated injury induced by exposure to anti-glomerular antibodies. Integrin α1β1 is highly expressed on leukocytes, in addition to renal cells, and it is likely that anti-α1 antibodies prevent leukocyte infiltration, thus ameliorating glomerular scarring during crescentic glomerulonephritis. Consistent with this data, deletion of integrin α1 results in decreased accumulation of macrophages in the kidneys of Alport mice and delays disease progression.28,29 Decreased leukocyte infiltration, with consequent dampening of the inflammatory response and tissue damage, has been also observed in integrin α1-null mice in other model systems such as contact hypersensitivity and arthritis.21 In the ADR mouse model we did not observe any discrepancy in leukocyte infiltration between the α1-null mice and wild-type controls suggesting that the differences in response to injury was mediated by cells found within the glomerulus.
Significantly increased renal accumulation of collagen IV in the glomerulus (Figure 2C) and collagen I in the interstitium (data not shown) in integrin α1-null mice is consistent with the anatomical distribution of these ECM proteins. Integrin α1β1 negatively regulates collagen synthesis and increased collagen I accumulation is observed in skin19 as well as bone calluses of integrin α1-null mice.30 Accumulation of collagen in bones30 and kidneys (present study) of α1-null mice leads to scarring, which is unlike that observed in the skin of these animals after wounding.19 In the wounded skin, dermal collagen production is compensated for by increased synthesis of MMPs, namely MMP13,19 MMP2, and MMP9.17 In contrast, no compensatory increase in MMP expression is observed in the kidneys (data not shown) or bones.30 Thus, the severity of glomerulosclerosis observed in ADR-treated integrin α1-null mice is explained by increased collagen synthesis with no compensatory up-regulation of MMPs. These results suggest that the response to injury in integrin α1-null mice is organ- and possibly even cell type-specific.
The rapidity of the renal injury in α1-null mice, characterized by mesangiolysis within 24 hours and accumulation of mesangial ECM by 72 hours after ADR injection, was remarkable. Mesangiolysis is a sign of severe injury that is associated with loss of glomerular structure established and maintained by interactions of mesangial cells with the adjacent ECM. In α1-null mice at baseline there are probably sufficient compensatory mechanisms for the glomeruli to function normally despite impairment of α1β1 integrin-dependent interactions with surrounding ECM. However, after injury, this compensation may be insufficient to maintain tissue integrity, resulting in loss of attachment of mesangial cells to the mesangial matrix resulting in mesangiolysis and subsequent accelerated ECM deposition during the repair process. A similar, but much less marked phenotype occurs when integrin α8-null mice are exposed to mechanical stress induced by hypertension.31 Like integrin α1β1, integrin α8β1 appears to be critical for normal formation of the mesangium where it interacts with its ECM ligands.31
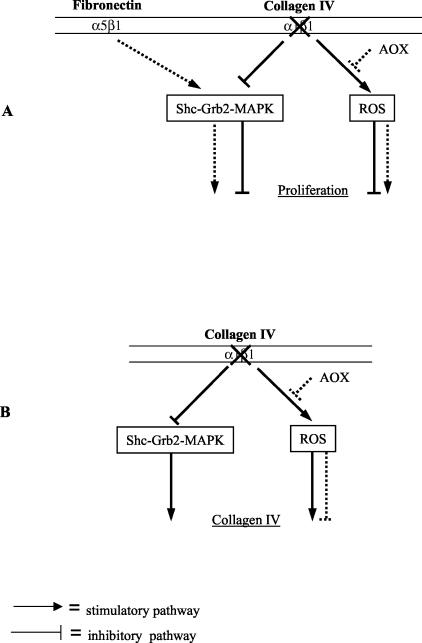
We demonstrated that decreased growth of α1-null mesangial cells on collagen IV was because of both an increase in ROS production as well as lack of integrin α1-dependent mesangial cell-ECM interactions. We previously demonstrated that integrin α1β1 is required to support cell proliferation on collagens via activation of the Shc/Grb2/MAPK pathway and failure to activate this pathway in integrin α1-null cells resulted in decreased proliferation of fibroblasts plated on collagenous substrata.8 Presumably a similar mechanism is occurring in the integrin α1-null mesangial cells grown on collagen substrata, which would explain why addition of antioxidants resulted in only a partial rescue of α1-null mesangial cell growth on collagen. In contrast antioxidants completely rescued proliferation of α1-null mesangial cells on fibronectin. In this scenario other integrins (such as α5β1) interact with ECM and probably result in activation of the Shc/GRB2/MAPK pathways.8,32 A schematic illustration of how cell growth might be regulated in α1-null mesangial cells is proposed in Figure 8A. The mechanism by which integrin α1β1 controls ROS generation is currently unknown, however, integrin α2β1, another major collagen binding receptor, is a positive modulator of NADPH-regulated ROS-dependent cellular functions. When integrins α1β1 and α2β1 are expressed on the same cell, α1β1 negatively regulates many integrin α2β1-dependent functions, such as collagen and MMP synthesis.9,33 Therefore, lack of integrin α1β1 may result in uncontrolled α2β1-mediated ROS production at baseline, which is further accentuated by exposure to ADR both in vivo and in vitro.
Figure 8.
Regulation of cell proliferation and collagen synthesis in α1-null mesangial cells. Schematic illustration of how cell proliferation (A) or collagen synthesis (B) might be regulated in α1-null mesangial cells. Integrin α1β1 is the only collagen-binding receptor able to support cell proliferation and down-regulate endogenous collagen synthesis on collagens via activation of the Shc/Grb2/MAPK pathway.8,33 In the absence of integrin α1β1, cells plated on collagen substrata develop up-regulated synthesis of ROS and decreased activation of the Shc/Grb2/MAPK pathway that could lead to reduced cell proliferation (A) and increased collagen synthesis (B). When cells are plated on collagen, addition of antioxidants (AOXs) could only partially rescue α1-null mesangial cell growth (A) or partially decrease collagen synthesis (B) because of the persistent lack of MAPK activation. In contrast, treatment with antioxidants could completely rescue proliferation of α1-null mesangial cells on fibronectin (A) as on this matrix, other integrins (ie, α5β1) could promote cell proliferation via activation of the Shc/Grb2/MAPK pathways.8,32
ROS mediate numerous physiological cellular functions at low levels but they become cytotoxic at higher concentrations. Increased ROS production by α1-null mice and mesangial cells, at baseline and in response to ADR, results not only in decreased cell growth, but also up-regulated collagen synthesis. The observation that treatment with antioxidants only partially down-regulate collagen synthesis in the α1-null cells, suggests that although increased ROS generation by α1-null mesangial cells is a major contributor to increased collagen production, there is also an integrin α1-dependent/ROS-independent mechanism that controls collagen synthesis. This would agree with our previous findings that integrin α1 modulates collagen synthesis at both transcriptional and translational levels9 (Figure 8B). ROS generation is directly correlated to many diseases characterized by fibrosis in which they induce up-regulation of profibrotic factors, such as transforming growth factor-β34 and connective tissue growth factor.35 Therefore, increased ROS production may induce synthesis of these growth factors that contribute significantly to the severe scarring phenotype seen in the α1-null mice after ADR-induced injury.
In conclusion, we have demonstrated that lack of integrin α1 predisposes mice to severe acute damage and excessive sclerosis after nonimmunologically induced renal injury. This response to the insult is mediated by both the loss of direct interactions of integrin α1β1 with its ligand collagen IV, as well as increased production of ROS. Integrin α1β1 may be regarded as a potent modifier for injury-induced glomerulosclerosis in particular, and possibly organ fibrosis in general.
Acknowledgments
We thank Ellen Donnert and Catherine Allen (Veterans Administration Flow Cytometry Unit) for excellent technical assistance and Dr. Billy Hudson for his critical review of the manuscript.
Footnotes
Address reprint requests to Ambra Pozzi, Dept. of Medicine, Div. of Nephrology and Hypertension, Vanderbilt University, Medical Center North, B3109, 1161 21st Ave., Nashville, TN 37212. E-mail: ambra.pozzi@vanderbilt.edu.
Supported by the National Institutes of Health (National Cancer Institute grant R01 CA94849–01 to A.P.; grant DK59975 to G.M.; National Institute of Diabetes and Digestive and Kidney Diseases Pediatric O’Brien Center grants P50 DK44757 and DK56942 to A.B.F.; National Institute of Diabetes and Digestive and Kidney Diseases O’Brien Center grant P50 DK39261-16 to R.Z., R.C.H., A.P., A.B.F.; grants GM15431, CA77839 and DK48831 to J.D.M.); the Department of Veterans Affairs (an advanced career development to R.Z. and a merit award to R.C.H. and R.Z.); the American Heart Association (grant-in-aid to R.Z.); and the Burroughs Wellcome Fund (clinical scientist award in translational research grant to J.D.M.).
R.Z. is a Clinician Scientist of the National Kidney Foundation of the USA.
References
- Fogo AB. Mesangial matrix modulation and glomerulosclerosis. Exp Nephrol. 1999;7:147–159. doi: 10.1159/000020595. [DOI] [PubMed] [Google Scholar]
- Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Voigt S, Gossrau R, Baum O, Loster K, Hofmann W, Reutter W. Distribution and quantification of alpha 1-integrin subunit in rat organs. Histochem J. 1995;27:123–132. doi: 10.1007/BF00243907. [DOI] [PubMed] [Google Scholar]
- Korhonen M, Ylanne J, Laitinen L, Virtanen I. Distribution of beta 1 and beta 3 integrins in human fetal and adult kidney. Lab Invest. 1990;62:616–625. [PubMed] [Google Scholar]
- Shikata K, Makino H, Morioka S, Kashitani T, Hirata K, Ota Z, Wada J, Kanwar YS. Distribution of extracellular matrix receptors in various forms of glomerulonephritis. Am J Kidney Dis. 1995;25:680–688. doi: 10.1016/0272-6386(95)90542-1. [DOI] [PubMed] [Google Scholar]
- Kuhara T, Kagami S, Kuroda Y. Expression of beta 1-integrins on activated mesangial cells in human glomerulonephritis. J Am Soc Nephrol. 1997;8:1679–1687. doi: 10.1681/ASN.V8111679. [DOI] [PubMed] [Google Scholar]
- Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol. 2002;161:1265–1272. doi: 10.1016/S0002-9440(10)64403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH. Experimental focal segmental glomerulosclerosis in mice. Nephron. 1998;78:440–452. doi: 10.1159/000044974. [DOI] [PubMed] [Google Scholar]
- Deman A, Ceyssens B, Pauwels M, Zhang J, Houte KV, Verbeelen D, Van den Branden C. Altered antioxidant defence in a mouse adriamycin model of glomerulosclerosis. Nephrol Dial Transplant. 2001;16:147–150. doi: 10.1093/ndt/16.1.147. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, Bertram JF, Ryan GB. Antioxidants protect podocyte foot processes in puromycin aminonucleoside-treated rats. J Am Soc Nephrol. 1994;4:1974–1986. doi: 10.1681/ASN.V4121974. [DOI] [PubMed] [Google Scholar]
- Naziroglu M, Cay M, Ustundag B, Aksakal M, Yekeler H. Protective effects of vitamin E on carbon tetrachloride-induced liver damage in rats. Cell Biochem Funct. 1999;17:253–259. doi: 10.1002/(SICI)1099-0844(199912)17:4<253::AID-CBF837>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates JA, Roberts LJ. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- Striker GE, Killen PD, Farin FM. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980;12:88–99. [PubMed] [Google Scholar]
- Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zent R, Ailenberg M, Waddell TK, Downey GP, Silverman M. Puromycin aminonucleoside inhibits mesangial cell-induced contraction of collagen gels by stimulating production of reactive oxygen species. Kidney Int. 1995;47:811–817. doi: 10.1038/ki.1995.123. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharra EJ, Schon M, Hassett D, Parker C, Havran W, Gardner H. Reduced gut intraepithelial lymphocytes in VLA1 null mice. Cell Immunol. 2000;201:1–5. doi: 10.1006/cimm.2000.1630. [DOI] [PubMed] [Google Scholar]
- Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131–2139. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- Yamada J, Yoshimura S, Yamakawa H, Sawada M, Nakagawa M, Hara S, Kaku Y, Iwama T, Naganawa T, Banno Y, Nakashima S, Sakai N. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res. 2003;45:1–8. doi: 10.1016/s0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AM, Lee PJ. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol. 2003;28:305–315. doi: 10.1165/rcmb.2002-0156OC. [DOI] [PubMed] [Google Scholar]
- Vichi P, Tritton TR. Adriamycin: protection from cell death by removal of extracellular drug. Cancer Res. 1992;52:4135–4138. [PubMed] [Google Scholar]
- Soose M, Wenzel S, Padur A, Oberst D, Stolte H. Fibronectin expression in human mesangial cell cultures and its alterations by adriamycin. Cell Biol Toxicol. 1995;11:51–63. doi: 10.1007/BF00769992. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson NS, Ryan ST, Enke DA, Cosgrove D, Koteliansky V, Gotwals P. Global gene expression analysis reveals a role for the alpha 1 integrin in renal pathogenesis. J Biol Chem. 2001;276:34182–34188. doi: 10.1074/jbc.M102859200. [DOI] [PubMed] [Google Scholar]
- Ekholm E, Hankenson KD, Uusitalo H, Hiltunen A, Gardner H, Heino J, Penttinen R. Diminished callus size and cartilage synthesis in alpha 1 beta 1 integrin-deficient mice during bone fracture healing. Am J Pathol. 2002;160:1779–1785. doi: 10.1016/s0002-9440(10)61124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner A, Cordasic N, Klanke B, Muller U, Sterzel RB, Hilgers KF. The alpha8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am J Pathol. 2002;160:861–867. doi: 10.1016/s0002-9440(10)64909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Heino J, Lopez-Otin C, Kahari VM. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:2446–2455. doi: 10.1074/jbc.274.4.2446. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- Park SK, Kim J, Seomun Y, Choi J, Kim DH, Han IO, Lee EH, Chung SK, Joo CK. Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem Biophys Res Commun. 2001;284:966–971. doi: 10.1006/bbrc.2001.5058. [DOI] [PubMed] [Google Scholar]