Abstract
Distinct subpopulations of fibroblasts contribute to lung fibrosis, although the mechanisms underlying fibrogenesis in these subpopulations are not clear. Differential expression of the glycophosphatidylinositol-linked protein Thy-1 affects proliferation and myofibroblast differentiation. Lung fibroblast populations selected on the basis of Thy-1 expression by cell sorting were examined for responses to fibrogenic stimuli. Thy-1 (−) and Thy-1 (+) fibroblast populations were treated with platelet-derived growth factor-BB, interleukin-1β, interleukin-4, or bleomycin and assessed for activation of transforming growth factor (TGF)-β, Smad3 phosphorylation, and α-smooth muscle actin and fibronectin expression. Thy-1 (−) fibroblasts responded to these stimuli with increased TGF-β activity, Smad3 phosphorylation, and expression of α-smooth muscle actin and fibronectin, whereas Thy-1 (+) fibroblasts resisted stimulation. The unresponsiveness of Thy-1 (+) cells is not because of defective TGF-β signaling because both subsets respond to exogenous active TGF-β. Rather, Thy-1 (−) fibroblasts activate latent TGF-β in response to fibrogenic stimuli, whereas Thy-1 (+) cells fail to do so. Defective activation is common to multiple mechanisms of TGF-β activation, including thrombospondin 1, matrix metalloproteinase, or plasmin. Thy-1 (−) lung fibroblasts transfected with Thy-1 also become resistant to fibrogenic stimulation, indicating that Thy-1 is a critical biological response modifier that protects against fibrotic progression by controlling TGF-β activation. These studies provide a molecular basis for understanding the differential roles of fibroblast subpopulations in fibrotic lung disease through control of latent TGF-β activation.
Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrotic lung disorder with a poor prognosis. It is characterized by excessive proliferation of fibroblasts and accumulation of excessive amounts of extracellular matrix proteins, with subsequent abnormal lung remodeling within fibroblastic foci.1 Although the pathogenesis of IPF remains incompletely understood, a recently developed paradigm supports a model of IPF as an abnormal wound healing response, in which excessive fibroblast migration and proliferation, progressive extracellular matrix accumulation, increased activity of fibrogenic cytokines such as transforming growth factor (TGF)-β, continuous epithelial cell death, and abnormal re-epithelialization, as well as decreased myofibroblast apoptosis are involved.2–4
The fibroblast, particularly the myofibroblast, is the primary source of extracellular matrix in the development of lung fibrosis.5–7 The myofibroblast, characterized by expression of α-smooth muscle actin (α-SMA), is a highly contractile and proliferative cell that actively produces collagen and cytokines.5,8,9 Myofibroblasts are present in the fibrotic foci of IPF and have been shown to be important for fibrosis of multiple organs including kidney and liver.10–13 In vitro, TGF-β, interleukin (IL)-1β, and IL-4 can induce the myofibroblast phenotype.14–16 Although there is evidence that there are lung fibroblast subpopulations that actively drive the fibrogenic phenotype, little is actually known about the molecular mechanisms that determine whether a fibroblast responds to injury by fibrogenesis or by normal wound healing. Several molecules, including Thy-1, COX-2, and telomerase, are differentially expressed by subsets of normal fibroblasts and are suggested to be differentially associated with the fibrogenic phenotype.17,18 However, it is not clear to what extent these molecules correlate with the myofibroblast phenotype and whether they are direct molecular modulators of this phenotype.
Thy-1 is a glycophosphatidylinositol-linked outer membrane leaflet glycoprotein expressed on subsets of neurons, lymphocytes, and fibroblasts. In vitro, lung fibroblasts that do not express Thy-1 [Thy-1 (−)] express increased levels of connective tissue growth factor, a cytokine important in fibrotic processes, as compared to fibroblasts that express Thy-1 [Thy-1 (+)].19 Significantly higher levels of platelet-derived growth factor (PDGF)-α receptor are present on Thy-1 (−) fibroblasts.20 As a result, Thy-1 (−) fibroblasts show an increased proliferative response to PDGF-AA.20 In addition, primary cultures of lung fibroblasts from fibrosis-prone rats exhibit significantly fewer Thy-1-expressing cells than those from a more resistant strain.21 In a bleomycin (BLM)-induced model of lung fibrosis, mice in which the Thy-1 gene was disrupted developed more severe lung fibrosis than wild-type animals (J.S. Hagood et al, submitted manuscript). Moreover, analysis of human lung sections reveals that fibroblasts from normal lungs are predominantly Thy-1 (+), whereas most fibroblasts in the fibroblastic foci of IPF lungs do not express Thy-1 (J.S. Hagood et al, submitted manuscript). These results suggest that the presence of Thy-1 expression on lung fibroblasts limits the development of lung fibrosis.
There is abundant evidence that TGF-β is a key mediator of lung fibrosis.22–24 TGF-β stimulates fibroblast proliferation and migration,25 induces synthesis of extracellular matrix proteins,26–28 and promotes myofibroblast differentiation.29 In addition, TGF-β protects myofibroblasts from apoptosis.30 Although TGF-β is a potent inducer of lung fibrosis, large amounts of TGF-β protein are present in the lungs of healthy adults without apparent effect. This is in part because of the fact that TGF-β is initially synthesized as an inactive latent precursor. Latent TGF-β must be activated to elicit biological effects through its widely expressed signaling receptors.31 Using an adenovirus-mediated transfection approach, Sime and colleagues22 showed that overexpression of latent form of TGF-β failed to stimulate fibrosis in rat lung, whereas overexpression of a constitutively active form of TGF-β resulted in extensive and persistent lung fibrosis. Studies in bronchoalveolar lavage fluid from human lung also revealed that alveolar macrophages from lungs with IPF secreted biologically active TGF-β, whereas alveolar macrophages from normal lungs secreted only latent TGF-β.32 These results indicate that control of latent TGF-β activation is a critical checkpoint for developing lung fibrosis. To date, at least two mechanisms have been suggested for regulation of latent TGF-β activation in fibrogenic lung injury. Alveolar macrophages activate latent TGF-β through a mechanism that involves interaction of the latent complex with thrombospondin 1 (TSP1) bound to its CD36 receptor on the macrophage and subsequent cleavage by plasmin.33 Alveolar epithelial cells activate TGF-β through interaction of the latent complex with the epithelial cell-specific αvβ6 integrin.34 Mechanism(s) regulating latent TGF-β activation by interstitial lung fibroblasts have not been addressed.
In this study, we examined whether there is a differential ability of Thy-1 (+) and (−) lung fibroblasts to activate latent TGF-β in response to fibrogenic cytokines (PDGF-BB, IL-1β, and IL-4) and the fibrogenic pulmonary toxin BLM. We showed that Thy-1 (−) lung fibroblasts, but not Thy-1 (+) lung fibroblasts, respond to profibrotic cytokines or BLM by stimulating activation of latent TGF-β. Resistance of Thy-1 (+) fibroblasts to activation was not limited to a specific TGF-β activation mechanism. Our findings suggest that the expression of Thy-1 affects fibroblast responses in the development of pulmonary fibrosis through regulating TGF-β activation and myofibroblast differentiation.
Materials and Methods
Cytokines, Growth Factors, Media, and Other Reagents
IL-4, aprotinin, and α2-anti-plasmin were purchased from Sigma (St. Louis, MO); IL-1β and recombinant human TGF-β from R&D Systems (Minneapolis, MN); PDGF-BB and neomycin sulfate (G418) from ICN Biomedicals (Aurora, OH); GM6001 from Biomol (Plymouth Meeting, PA); BLM from Calbiochem (La Jolla, CA); Zeocin from Invitrogen (Carlsbad, CA); Luciferase 1000 assay system from Promega (Madison, WI); nitrocellulose membranes from Schleicher and Schuell (Keene, NH); Western Lightning Chemiluminescence Reagent Plus from Perkin-Elmer Life Science (Boston, MA); Dulbecco’s modified Eagle’s medium (DMEM) with 25 mmol/L HEPES from Life Technologies, Inc. (Grand Island, NY); fetal bovine serum (FBS) from HyClone (Logan, UT); F12K from Cellgro (Herndon, VA); and Bio-Rad protein assay reagent from Bio-Rad Laboratories (Hercules, CA)
Proteins, Antibodies, and Peptides
Antibodies were purchased from the following vendors: anti-α-SMA antibody from Biocarta (San Diego, CA), anti-phospho-Smad2 (Ser465/467) from Cell Signaling (Beverly, MA), anti-Smad2/3 and anti-fibronectin antibody from BD Transduction Laboratories (San Diego, CA), anti-β-tubulin from Santa Cruz Biotechnology (Santa Cruz, CA), anti-TGF-β neutralizing antibody from R&D Systems. Nonimmune mouse IgG was purchased from Sigma. Mouse anti-TSP 133 antibody (Mab133) raised against TSP1 depleted of associated TGF-β was developed by our lab in a joint effort with the University of Alabama at Birmingham Hybridoma Core Facility. LSKL, SLLK, GGWSHW, and GGASHA peptides were synthesized and purified by AnaSpec, Inc. (San Jose, CA).
Primary Rat Lung Fibroblast Purification, Cell Sorting, and Transfection
Lung fibroblasts from Lewis rats were isolated and cultured as described previously.21 Single cell suspensions were labeled with fluorescein isothiocyanate-conjugated anti-CD90.1 antibody (OX-7, BD Pharmingen) and labeled cells were washed and sorted using a FACSTARPlus Cell Sorter under sterile conditions (Becton-Dickinson, Mountain View, CA). The original population of rat lung fibroblasts contained 78% Thy-1 (+) cells and 22% Thy-1 (−) cells. Fibroblasts positive and negative for Thy-1 expression were subcultured and resorted one to two times until subpopulations of > 90% purity were obtained. Sorted fibroblasts were used within four passages. Thy-1-transfected cells (RFL-6 CD90) were generated as described.35 Briefly, full-length murine Thy-1.2 cDNA was ligated into the mammalian expression vector pCDNA3.1 (Invitrogen), sequenced for verification and orientation, then transfected into RFL-6 cells using TransIT-LT1 transfection reagent (Invitrogen), and selected in 200 μg/ml Zeocin. Transfectants were selected using flow cytometry. Empty-vector-transfected RFL-6 cells (RFL-6 EV) were used as controls. All cells for experiments were demonstrated free of Mycoplasma contamination using MycoAlert mycoplasma detection kit (Cambrex Bio Science, Rockland, ME).
Cell Culture and Treatment
The sorted fibroblasts were seeded at a density of 5 × 104 cells/well in six-well plates and allowed to grow in DMEM containing 25 mmol/L HEPES and 15% FBS for 2 to 3 days until 70 to 80% confluence. Cells were then rendered quiescent in 2.5 ml of DMEM with 0.1% FBS for 48 hours and in serum-free DMEM for 2 hours. After removal of quiescent media, 1 ml of fresh serum-free media was added in the presence or absence of PDGF-BB (0 to 40 ng/ml), IL-1β (0 to 20 ng/ml), IL-4 (0 to 20 ng/ml), or BLM (0 to 3 μg/ml). Cells were treated for 24 hours. For treatment of cells with antibodies, peptides, or inhibitors, cells were seeded in 24-well plates and grown to 70 to 80% confluence. Quiescent cells were treated with 25 μg/ml of Mab133 antibody, 25 μg/ml of nonimmune mouse IgG, 1 μg/ml of anti-TGF-β antibody, 1 μmol/L LSKL peptide, 1 μmol/L SLLK peptide, 20 μmol/L GGWSHW peptide, 20 μmol/L GGASHA peptide, 200 μg/ml of aprotinin, 64 nmol/L α2-antiplasmin, or 25 μmol/L GM6001 for 24 hours. RFL-6 CD90-transfected cells and RFL-6 EV-transfected cells were seeded in six-well plates and cultured in F12K media supplemented with 10% FBS, 1% penicillin-streptomycin, and 1 μg/ml Zeocin until 70 to 80% confluent. Cells were made quiescent with media containing 0.1% FBS for 24 hours and treated with cytokines or BLM for 24 hours.
Preparation of Conditioned Media and Cell Lysates
At the end of the treatments, culture supernatants were harvested in siliconized tubes on ice, centrifuged at 300 × g for 10 minutes at 4°C to remove cell debris. Cell-free conditioned media were aliquoted and stored at −80°C until used. For collection of cell lysates, cells were washed with cold phosphate-buffered saline twice and lysed with RIPA (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid, 1% Nonidet P-40, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 0.5 μg/ml leupeptin, 0.5 μg/ml pepstatin, 100 μmol/L sodium vanadate) for 1 minute on ice. The cell lysates were harvested in siliconized tubes and sonicated for 5 seconds to shear DNA. After centrifugation at 4000 × g for 1 minute at 4°C to remove cell debris, cell lysates were aliquoted and stored at −80°C until used.
Bioassay of TGF-β Activity
Active and total TGF-β levels were quantified by the PAI-1 promoter luciferase reporter assay.36 Briefly, mink lung epithelial cells stably expressing the TGF-β response element of the plasminogen activator inhibitor-1 promoter fused to the firefly luciferase reporter gene (a generous gift from Dr. D. B. Rifkin, New York Medical Center, New York, NY) were plated in 24-well plates at a density of 2 × 105 cells/well with DMEM containing 10% FBS, 2 mmol/L l-glutamine, 1% penicillin-streptomycin, and 200 μg/ml G418. Cells were allowed to attach for 4 hours and then washed with serum-free media. For measurement of active TGF-β, 0.5 ml of conditioned media were directly added to each well. For assay of total TGF-β, 0.05 ml of conditioned media were first heat-activated for 3 minutes at 100°C, then mixed with 0.45 ml of serum-free media and a final volume of 0.5 ml of media was incubated with reporter cells for 18 hours at 37°C, mink lung epithelial cell lysates from each well were prepared using reporter lysis buffer (Promega). Luciferase activity was measured as relative light units using an Orion microplate luminometer from Berthold (Pforzheim, Germany), and converted to TGF-β activity (picomoles) using a standard curve generated by human recombinant TGF-β1. All assays were performed in triplicate.
SDS-Polyacrylamide Gel Electrophoresis (PAGE) and Immunoblotting
Protein concentration was measured using the Bio-Rad protein assay. Equal amounts of protein from conditioned media or cell lysates were loaded onto SDS-polyacrylamide gels under reducing conditions. After electrophoresis, the proteins were electrophoretically transferred from the gels to nitrocellulose at 100 V for 2 hours at 4°C. Equal loading and transfer of protein samples were verified by staining the blots with Ponceau S. Membranes were incubated with 5% bovine serum albumin in Tris-buffered saline/Tween-20 (0.1%) (TBS-T) for 1 hour at room temperature to block nonspecific protein-binding sites present in the membranes. Membranes were then incubated with primary antibodies diluted in TBS-T (anti-fibronectin antibody at 1:5000 for 18 hours at 4°C, Mab 133 at 0.05 μg/ml for 18 hours at 4°C, anti-phospho-Smad2/3 at 1:3000 for 18 hours at 4°C, anti-α-SMA antibody at 1:1000 for 1 hour at room temperature, anti-Smad2/3 at 1:2000 for 1 hour at room temperature, and anti-β-tubulin at 1:500 for 1 hour at room temperature). After extensive washing, membranes were incubated with appropriate peroxidase-conjugated secondary antibodies (0.1 μg/ml) diluted in TBS-T for 1 hour at room temperature. Immunodetection was performed by chemiluminescence.
Densitometry
Immunoblots were scanned and bands were quantified by Scanalytic’s One-Dscan version 1.31, and fold increases in fibronectin (FN) and TSP1 were normalized to total amounts of protein in conditioned media. Fold increases in Smad3 phosphorylation and α-SMA were normalized to total Smad3 and β-tubulin, respectively.
Statistical Analysis
Experiments were repeated with cells from multiple rats over different isolations. Results were expressed as means ± SD for three separate experiments. Statistical differences among treatment conditions were determined using one-way analysis of variance. The analysis was performed with SigmaStat 3.0 software (SPSS Inc., Chicago, IL). Values of P < 0.05 or P < 0.01 were considered significant.
Results
Thy-1 (+) Lung Fibroblasts Are Resistant to TGF-β Activation by Fibrogenic Stimuli
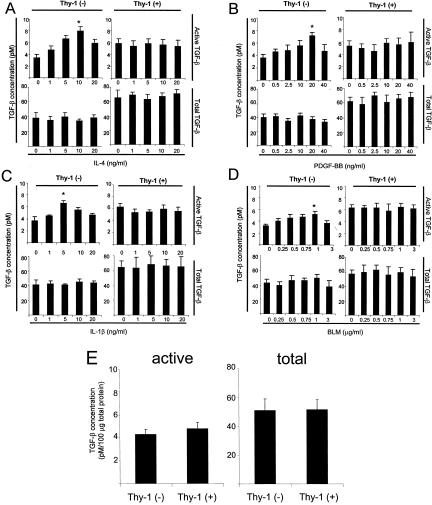
Sorted Thy-1 (+) and Thy-1 (−) rat lung fibroblasts were treated with fibrogenic cytokines (IL-4, PDGF-BB, IL-1β) or BLM. Levels of active and total TGF-β from the conditioned media were measured. Treatment of Thy-1 (−) cells with IL-4, PDGF-BB, IL-1β, or with BLM stimulated a dose-dependent increase in active TGF-β levels (Figure 1; A to D). In contrast, active TGF-β levels were not increased by these treatments in the conditioned media of Thy-1 (+) cells. Levels of total TGF-β activity were unaffected in either cell type by any of these treatments, indicating that there is no induction of latent TGF-β production. The specificity of cytokine/BLM-induced increase in TGF-β activity was demonstrated by preincubation of conditioned media from Thy-1 (−) cells with TGF-β neutralizing antibody, which led to abrogation of increases in luciferase activity (data not shown). Heat activation of latent TGF-β to measure total TGF-β protein showed that Thy-1 (+) cells have similar levels of latent TGF-β as do Thy-1 (−) cells, suggesting that resistance to TGF-β activation is not because of a defect in expression of the latent TGF-β molecule itself. When TGF-β activity was normalized by cell number, Thy-1 (+) cells showed higher levels of basal active and total TGF-β activity than Thy-1 (−) cells (Figure 1; A to D). Preincubation with TGF-β neutralizing antibody reduced luciferase activity to similar levels for both the Thy-1 (+) and Thy-1 (−) cells, ruling out that the higher basal levels of TGF-β observed in Thy-1 (+) cells result from different levels of nonspecific luciferase activity (data not shown). However, because Thy-1 (+) and (−) cells have different size and morphology, the protein per cell differs between the two cell types. When data are normalized to cell protein, levels of basal active and total TGF-β are equivalent between the two cell types (Figure 1E). Taken together, our data suggest that Thy-1 (+) and (−) lung fibroblasts differ in their ability to activate latent TGF-β in response to fibrogenic stimuli and that Thy-1 (+) cells are resistant to stimulation of TGF-β activation.
Figure 1.
Thy-1 (+) fibroblasts are resistant to additional TGF-β activation by fibrogenic stimuli. Thy-1 (+) and Thy-1 (−) rat lung fibroblasts were seeded in six-well plates and grown until 70 to 80% confluent. After quiescence, cells were cultured in fresh serum-free DMEM in the presence or absence of increasing concentrations of IL-4 (A), PDGF-BB (B), IL-1β (C), or BLM (D) for 24 hours. Conditioned media were harvested and cell number in each well was determined by cell counting. Conditioned media were assayed for active TGF-β activity (top) or heated for 3 minutes at 100°C to activate latent TGF-β for measurement of total TGF-β activity (bottom) with the PAI-1 promoter luciferase reporter assay. Values of TGF-β activity were corrected for cell number. Results are shown as mean ± SD of triplicate wells. *, P < 0.01 for basal active TGF-β activity versus 10 ng/ml IL-4-, 20 ng/ml PDGF-BB-, 5 ng/ml IL-1β-, or 1 μg/ml BLM-induced active TGF-β activity in Thy-1 (−) cells. E: Measured active and total TGF-β activity from Thy-1 (+) and (−) cells under basal conditions were corrected by total protein levels in cell lysates. Results are shown as mean ± SD of triplicate wells.
Thy-1 (−) Fibroblasts Increase Smad3 Phosphorylation in Response to Cytokines or BLM
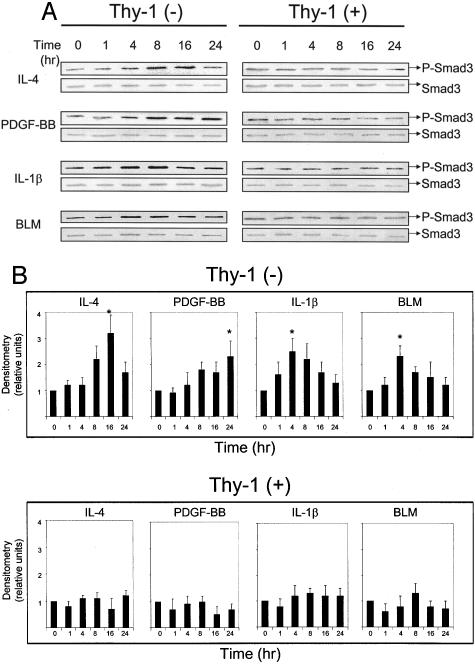
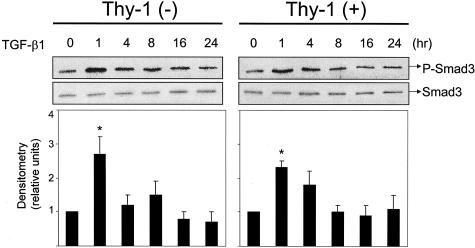
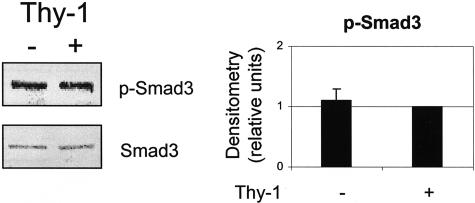
To investigate differences in autocrine TGF-β signaling, we examined whether intracellular TGF-β signaling was triggered in response to extracellular TGF-β activation. We evaluated levels of Smad2/3 phosphorylation in stimulated Thy-1 (+) and (−) fibroblasts by immunoblotting of cells lysates with a polyclonal antibody that recognizes Smad2 and Smad3 phosphorylated on ser465/467. This antibody recognizes the phosphorylated forms of both Smad2 and Smad3, which can be distinguished by their different electrophoretic mobilities. Consistent with data from the TGF-β activity assay, we observed that Thy-1 (−) cells increase Smad phosphorylation in response to cytokine or BLM treatment, whereas Thy-1 (+) cells are refractory (Figure 2, A and B). The electrophoretic mobility of the bands in these blots is consistent with Smad3 phosphorylation. Maximal levels of Smad phosphorylation were observed at 4 to 24 hours after stimulation with cytokines or BLM, in contrast to a rapid induction (1 to 4 hours) with exogenous active TGF-β1 (Figure 8). Under basal conditions, Smad3 phosphorylation levels were equivalent in both cell types (Figure 3).
Figure 2.
Thy-1 (−) fibroblasts increase Smad3 phosphorylation in response to cytokines or BLM. A: Quiescent cells were treated with 10 ng/ml of IL-4, 20 ng/ml of PDGF-BB, 5 ng/ml of IL-1β, or 1 μg/ml of BLM. Cell lysates were collected at 0, 1, 4, 8, 16, and 24 hours. Levels of phosphorylated Smad3 (p-Smad3) and total Smad3 were detected in samples (equal protein loading) by immunoblotting. B: Relative p-Smad3 levels were determined by scanning densitometry of the blots and normalized to total Smad3 levels. The level of p-Smad3 at 0 hours of each treatment was set to 1. Results are the means of three independent experiments each performed in triplicate ± SD. *, P < 0.01 for basal control versus IL-4 at 16 hours, PDGF-BB at 24 hours, IL-1β at 4 hours, or BLM at 4 hours in Thy-1 (−) cells.
Figure 8.
Smad-dependent TGF-β signaling is intact in Thy-1 (+) fibroblasts. Thy-1 (+) cells and Thy-1 (−) cells were treated with 8 pmol/L TGF-β1. Cell lysates were collected at 0, 1, 4, 8, 16, and 24 hours. Equal amounts of protein were separated on 10% SDS-PAGE. Levels of phosphorylated Smad3 and total Smad3 were detected by immunoblotting. Relative phosphorylated Smad3 levels were determined by scanning densitometry of the blots and normalized to total Smad3 levels. The level of p-Smad3 at 0 hours was set to 1. Results are the means of three independent experiments ± SD. *, P < 0.01 for basal control versus TGF-β1 at 1 hour in Thy-1 (+) or (−) cells.
Figure 3.
Basal levels of Smad3 phosphorylation are similar in Thy-1 (+) and (−) fibroblasts. Quiescent Thy-1 (+) cells and Thy-1 (−) cells were cultured in 1 ml of fresh serum-free media for 24 hours. Cell lysates were harvested and equal amounts of protein were subjected to electrophoresis on 10% SDS-PAGE under reducing conditions. Levels of p-Smad3 and total Smad3 were detected by immunoblotting and quantified by scanning densitometry setting Thy-1 (+) p-Smad3 to an arbitrary value of 1. Results are the means ± SD of three separate experiments.
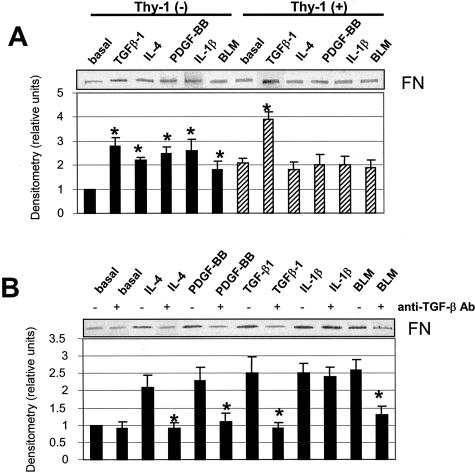
Thy-1 (−) Fibroblasts Increase TGF-β-Dependent Soluble FN Expression in Response to Cytokines or BLM, but Thy-1 (+) Fibroblasts Do Not
Increased expression of matrix proteins including FN has been identified as a hallmark of the fibrogenic activity of TGF-β.1,37 Further experiments were performed to determine whether the differential ability of these cell populations to activate latent TGF-β affects expression of FN. Treatment of Thy-1 (−) cells with cytokines or BLM increased FN in the conditioned medium. In contrast, Thy-1 (+) cells are resistant to stimulation of FN expression (Figure 4A). Stimulation of FN in Thy-1 (−) cells was because of TGF-β activity because anti-TGF-β antibody blocked up-regulation of FN by BLM, IL-4, and PDGF-BB. Basal and IL-1β-stimulated FN expression is independent of TGF-β activity (Figure 4B). Although cytokines or BLM fail to stimulate Thy-1 (+) cells to synthesize FN, addition of exogenous TGF-β to Thy-1 (+) cells induces FN expression (Figure 4A), suggesting that the inability to activate latent TGF-β correlates with the failure to up-regulate FN by Thy-1 (+) cells.
Figure 4.
Thy-1 (−) fibroblasts increase TGF-β-dependent soluble FN expression in response to cytokines or BLM, but Thy-1 (+) fibroblasts do not. A: Quiescent cells were treated with 8 pmol/L TGF-β1, 10 ng/ml IL-4, 20 ng/ml PDGF-BB, 5 ng/ml IL-1β, or 1 μg/ml BLM for 24 hours. Conditioned media were harvested and equal amounts of protein were subjected to electrophoresis on 8% SDS-PAGE under reducing conditions. Levels of FN were detected by immunoblotting. B: Thy-1 (−) cells were treated with cytokines or BLM in the absence or presence of 1 μg/ml of anti-TGF-β neutralizing antibody (anti-TGF-β) for 24 hours. Levels of FN in supernatant of cultures were detected by immunoblotting. Relative FN protein levels were determined by scanning densitometry of the blots. The basal level of FN expressed in Thy-1 (−) cells was set to 1. Results are the means of three independent experiments ± SD. *, P < 0.01 for basal control versus TGF-β1, IL-4, PDGF-BB, IL-1β, or BLM in Thy-1 (−) cells, basal control versus TGF-β1 in Thy-1 (+) cells, IL-4 versus IL-4 + anti-TGF-β, PDGF-BB versus PDGF-BB + anti-TGF-β, TGF-β1 versus TGF-β1 + anti-TGF-β, and BLM versus BLM + anti-TGF-β in Thy-1 (−) cells.
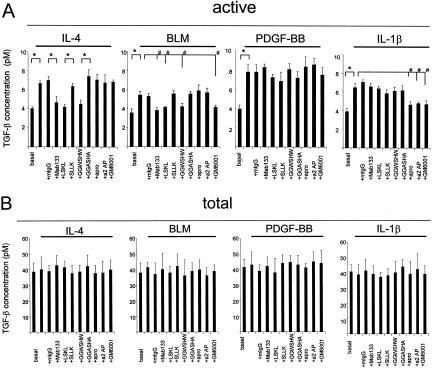
Differential Susceptibility to TGF-β Activation in Thy-1 Subpopulations Involves Multiple Activation Mechanisms
Latent TGF-β can be activated by diverse mechanisms. To determine whether Thy-1 resistance to latent TGF-β activation is associated with defects in a particular activation mechanism, we examined which mechanisms fibroblasts might use to regulate TGF-β activation. Previously, we and others33,38–42 identified the matricellular protein TSP1 as an important mediator of TGF-β activation both in vivo and in vitro. By use of peptide and antibody antagonists that block TSP1-mediated TGF-β activation, we examined whether TSP1 is involved in the cytokine- or BLM-induced TGF-β activation by Thy-1 (−) fibroblasts. In addition, we also tested the possible involvement of plasmin- or matrix metalloproteinase (MMP) in TGF-β activation using the plasmin-specific inhibitors, aprotinin and α2-anti-plasmin, or the MMP-specific inhibitor, GM6001. As shown in Figure 5A, preincubation of cells with Mab133 or either of the TSP1 antagonist peptides completely blocked TGF-β activation by either IL-4 or BLM, whereas the same concentration of nonimmune mouse IgG or control peptides (SLLK and GGASHA) had no inhibitory effect, suggesting that both IL-4 and BLM activate latent TGF-β through a TSP1-dependent mechanism. Stimulation of TGF-β activity by IL-1β was inhibited by either aprotinin or α2-anti-plasmin, suggesting that plasmin is required for IL-1β-induced activation. The MMP inhibitor GM6001 also blocked activation by both BLM and IL-1β. These data suggest that the agents use more than one mechanism to activate latent TGF-β. Interestingly, stimulation of TGF-β activity by PDGF-BB was not blocked by TSP1, plasmin, or MMP inhibitors, suggesting an alternate activation mechanism is involved. Total TGF-β levels were unaffected by any of the inhibitors (Figure 5B).
Figure 5.
Differential susceptibility to activation in Thy-1 subpopulations involves multiple activation mechanisms. Thy-1 (−) cells were cultured in 24-well plates until 70 to 80% confluent. Quiescent cells were treated with 10 ng/ml of IL-4, 20 ng/ml of PDGF-BB, 5 ng/ml of IL-1β, or 1 μg/ml of BLM in the presence or absence of 25 μg/ml of nonimmune mouse IgG (mIgG), 25 μg/ml of Mab133, 1 μmol/L LSKL peptide, 1 μmol/L SLLK control peptide, 20 μmol/L GGWSHW peptide, 20 μmol/L GGASHA control peptide, 200 μg/ml of aprotinin (apro), 64 nmol/L α2-anti-plasmin (a2AP), or 25 μmol/L GM6001 for 24 hours. Conditioned media were harvested. Cell number in each well was determined by cell counting. Conditioned media were assayed for active TGF-β activity (A) and total TGF-β activity (B). Results are the means of three separate experiments ± SD, each performed in triplicate. *, P < 0.01; and #, P < 0.05 for comparisons as indicated.
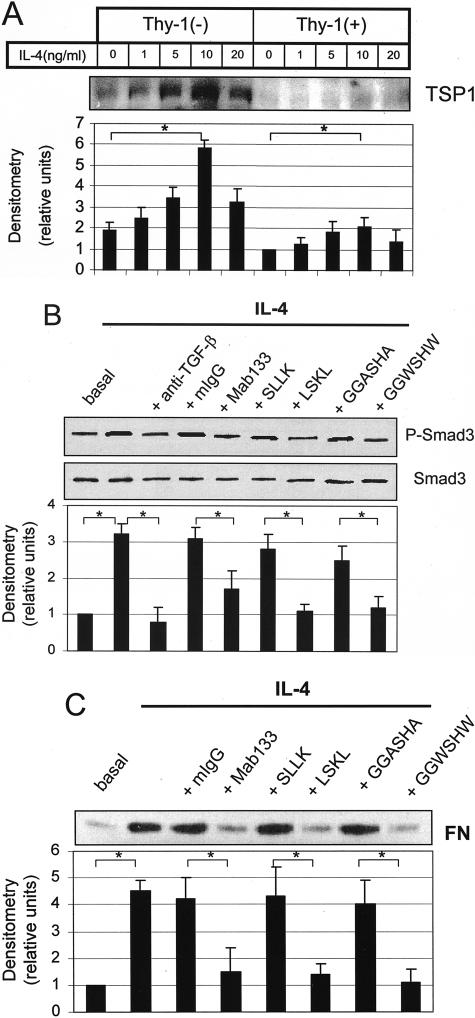
IL-4 Stimulates TSP1 Expression and TSP1 Antagonists Block IL-4 Stimulation of Smad Phosphorylation and FN Synthesis
To further evaluate the involvement of TSP1 in IL-4 stimulation of TGF-β activation, we first investigated the effect of IL-4 on TSP1 expression. As shown in Figure 6A, IL-4 treatment dramatically increases TSP1 protein expression in Thy-1 (−) cells as compared to Thy-1 (+) cells. Treatment of Thy-1 (−) cells with TSP1 antagonist peptides or antibody blocks IL-4 stimulation of Smad phosphorylation (Figure 6B) and FN synthesis (Figure 6C). These data provide evidence that TSP1 is involved in IL-4 stimulation of TGF-β activity by Thy-1 (−) fibroblasts. Incidentally, TSP1 protein is also increased by PDGF-AA, PDGF-BB, and BLM treatment of Thy-1 (−) cells (data not shown).
Figure 6.
IL-4 stimulates TSP1 expression and TSP1 antagonists block IL-4 stimulation of Smad phosphorylation and FN synthesis. A: Thy-1 (+) cells and Thy-1 (−) cells were treated with increasing concentration of IL-4. Conditioned media were harvested and equal amounts of protein were subjected to electrophoresis on 8% SDS-PAGE under reducing conditions. Protein levels of TSP1 were detected by immunoblotting with Mab133 anti-TSP1 antibody. Relative TSP1 protein levels were determined by scanning densitometry of the blots. The level of TSP-1 expressed in Thy-1 (+) cells without IL-4 treatment was set to 1. Results are the means of three independent experiments ± SD. B: Thy-1 (−) cells were treated with 10 ng/ml of IL-4 in the presence or absence of 1 μg/ml of TGF-β antibody (TGF-β Ab), 25 μg/ml of mIgG, 25 μg/ml of Mab133, 1 μmol/L LSKL, 1 μmol/L SLLK, 20 μmol/L GGWSHW, or 20 μmol/L GGASHA. Cell lysates were harvested at 16 hours and subjected to immunoblotting to detect p-Smad3 and total Smad3. Blots were stripped and reprobed for total Smad3. Levels of p-Smad3 were normalized to total Smad3. The protein level without IL-4 treatment was set to 1. C: Cells were treated as in B and conditioned media were harvested at 24 hours and subjected to immunoblotting to detect FN. Relative protein levels were determined by scanning densitometry of the blots. Results are the means of three independent experiments ± SD. *, P < 0.01 for comparisons as indicated.
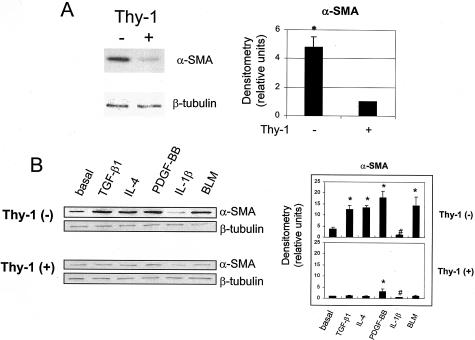
Thy-1 (−) Cells Express Increased α-Smooth Muscle Actin and Further Increase Expression in Response to Fibrogenic Stimuli
α-SMA is a marker for myofibroblast differentiation.43 To evaluate the potential ability of Thy-1 (+) and (−) lung fibroblasts to differentiate into myofibroblasts, we compared basal and stimulated expression of α-SMA in Thy-1 (+) and (−) fibroblasts. As shown in Figure 7, under basal conditions, Thy-1 (−) cells express 4.8-fold more α-SMA than do Thy-1 (−) cells (Figure 7A). When stimulated with IL-4, PDGF-BB, or BLM, Thy-1 (−) cells significantly increased α-SMA, whereas Thy-1 (+) cells were primarily refractory, except for a slight stimulation with PDGF-BB treatment. Decreased α-SMA levels were observed in both cell types after IL-1β treatment, confirming the previous finding that IL-1β dose dependently down-regulated both α-SMA protein and mRNA in lung fibroblasts.43 Interestingly, Thy-1 (+) cells did not increase α-SMA when treated with active TGF-β. Because directly adding active TGF-β to the cultures bypasses the requirement for activation, this result suggests that Thy-1 expression renders lung fibroblasts resistant to the myofibroblast phenotype. Alternately, these findings might indicate that Thy-1 (+) cells are unable to signal in response to active TGF-β because of receptor or Smad defects or the presence of inhibitors. To test this possibility, we added active TGF-β to Thy-1 (+) and (−) cultures and examined cell lysates for Smad phosphorylation (Figure 8) and conditioned media for FN synthesis (Figure 4, A and B). Thy-1 (+) cells respond to the addition of active TGF-β with increased Smad phosphorylation and increased FN expression, comparable to Thy-1 (−) cells, suggesting that Smad-dependent TGF-β signaling is intact in Thy-1 (+) fibroblasts.
Figure 7.
Thy-1 (−) cells express increased α-SMA and increase expression in response to fibrogenic stimuli. A: Thy-1 (+) and (−) fibroblasts were made quiescent in DMEM with 0.1% FBS for 48 hours and in serum-free DMEM for 2 hours. Cell lysates were collected and subjected to electrophoresis on 10% SDS-PAGE under reducing conditions. Levels of α-SMA were detected by immunoblotting. To verify equal loading, the same blot was stripped out and reprobed with anti-β-tubulin antibody. B: Quiescent cells were treated with 8 pmol/L TGF-β1, 10 ng/ml of IL-4, 20 ng/ml of PDGF-BB, 5 ng/ml of IL-1β, or 1 μg/ml of BLM for 24 hours. Cell lysates were collected and subjected to immunoblotting. Relative α-SMA protein levels were determined by scanning densitometry of the blots and were normalized to β-tubulin. The level of α-SMA expressed in Thy-1 (+) cells was set to 1. Results are the means of three independent experiments ± SD. *, P < 0.01 for basal level of α-SMA in Thy-1 (−) cells versus basal level of α-SMA in Thy-1 (+) cells, basal control versus TGF-β1, IL-4, PDGF-BB, or BLM in Thy-1 (−) cells, and basal control versus PDGF-BB in Thy-1 (+) cells. #,P < 0.05 for basal control versus IL-1β in both Thy-1 (+) cells and Thy-1 (−) cells.
Thy-1 Is Mechanistically Important for Differential Regulation of TGF-β Activation
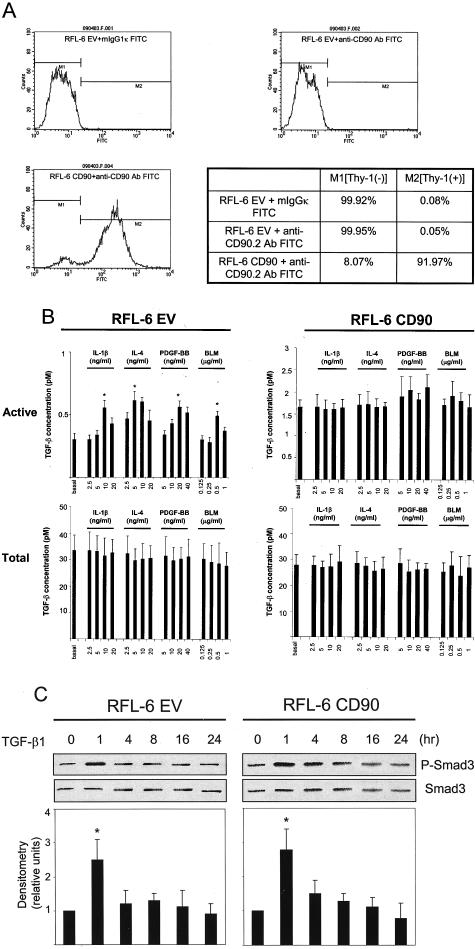
Finally, we addressed whether or not Thy-1 itself is a biological response modifier or rather a molecular marker for resistance to TGF-β activation. We generated Thy-1-expressing fibroblasts by transfecting rat lung fibroblasts (RFL-6) that lack Thy-1 expression with Thy-1.2 (RFL-CD90) (Figure 9A). RFL-6 cells transfected with empty vector (RFL-6 EV) were used as controls. The two cell populations were stimulated with cytokines or BLM and evaluated for active and total TGF-β. As for the sorted fibroblast populations, RFL-6 CD90 cells were refractory to stimulation of TGF-β activation, whereas RFL-6 EV cells responded to cytokine/BLM stimulation with increased TGF-β activation (Figure 9B). In addition, restoration of expression of Thy-1 in lung fibroblasts did not impair TGF-β signaling (Figure 9C). These data indicate that Thy-1 is a molecular biological response modifier that down-regulates activation of TGF-β in response to fibrogenic stimuli.
Figure 9.
Thy-1 is mechanistically important for differential regulation of TGF-β activation. A: Thy-1-transfected RFL-6 CD90 cells express Thy-1 on the cell surface, whereas empty vector-transfected RFL-6 EV cells do not. RFL-6 EV (top right) and RFL-6 CD90 cells (bottom left) were harvested and incubated with fluorescein isothiocyanate-conjugated anti-CD90.2 antibody. Expression of Thy-1 on the cell surface was determined by flow cytometry. RFL-6 EV cells treated with fluorescein isothiocyanate-conjugated mIgG1κ (top left) were used as a control. B: RFL-6 EV and RFL-6 CD90 cells were cultured in six-well plates until 70 to 80% confluent. Cells were made quiescent in F12K media with 0.1% FBS for 24 hours and in serum-free media for 2 hours. Cells were treated with increasing concentrations of IL-4, PDGF-BB, IL-1β, or BLM for 24 hours. Conditioned media were harvested and assayed for active TGF-β activity and total TGF-β activity. Cell number in each well was determined by cell counting. Values of TGF-β activity were normalized for cell number. Results are shown as mean ± SD of triplicate wells. *, P < 0.01 for basal active TGF-β activity versus 5 ng/ml IL-4-, 20 ng/ml PDGF-BB-, 10 ng/ml IL-1β-, or 0.5 μg/ml BLM-induced active TGF-β activity in Thy-1 (−) cells. C: Expression of Thy-1 in RFL-6 CD90 cells does not abrogate increased phosphorylation levels of Smad3 in response to exogenous TGF-β1. RFL-6 EV and RFL-6 CD90 cells were treated with 8 pmol/L TGF-β1 for 1 to 24 hours as indicated. Levels of phosphorylated Smad3 and total Smad3 were determined by immunoblot. Phosphorylated Smad3 levels were normalized to total Smad3. Results are the means of three independent experiments ± SD. *, P < 0.01 for basal control versus TGF-β1 at 1 hour in RFL-6 CD90 cells or RFL-6 EV cells.
Discussion
Because lung fibroblasts separated on the basis of Thy-1 expression have distinct morphologies, proliferative characteristics, expression of class II major histocompatibility complex (MHC) antigens, as well as production of extracellular matrix proteins,44–46 it is reasonable to speculate that these functionally distinct lung fibroblast subsets may have differing roles in the progression of lung fibrosis. In this study, we addressed whether Thy-1 expression differentially regulates TGF-β activity of lung fibroblasts in response to fibrogenic stimuli. Our results suggest that Thy-1 expression is a molecular modulator of TGF-β activation. In the absence of Thy-1 expression, lung fibroblasts respond to profibrotic stimuli by activation of autocrine latent TGF-β, leading to signaling of its fibrogenic properties and expression of α-SMA consistent with the myofibroblastic phenotype. In contrast, Thy-1 (+) expression renders cells resistant to TGF-β-dependent profibrotic sequelae.
Stimulation of Thy-1 (−) lung fibroblasts with fibrogenic cytokines or the pulmonary toxin BLM resulted in increased active TGF-β in the conditioned media without affecting total TGF-β, suggesting that the stimulated increase of TGF-β activity occurs at the level of latent TGF-β activation rather than at the level of latent TGF-β synthesis. Stimulation of TGF-β activation triggered intracellular signal transduction and induction of fibrogenic responses to TGF-β. In contrast, Thy-1 (+) fibroblasts are resistant to activation of TGF-β. It is well established that expression of TGF-β protein is in itself inadequate to stimulate fibrosis and that activation of the latent growth factor is critical for the development of fibrotic sequelae.22,47
Our studies showed that the resistance of Thy-1 (+) fibroblasts is not associated with one particular mechanism of TGF-β activation. IL-4 stimulation induced significantly greater expression of TSP1 in Thy-1 (−) lung fibroblasts than in Thy-1 (+) lung fibroblasts. This may be because of the fact that Thy-1 (−) lung fibroblasts have been shown to express higher levels of IL-4 receptor than do Thy-1 (+) cells.48 Pretreatment of Thy-1 (−) fibroblasts with antagonists of TSP1-dependent TGF-β activation abrogates IL-4 induction of TGF-β activation, signal transduction, and FN synthesis, suggesting that IL-4-induced TGF-β activation in lung fibroblasts is mediated through TSP1. TSP1 has been previously shown to participate in TGF-β activation in alveolar macrophages after lung injury.33 In this model, plasmin is required for cleavage of active TGF-β from latent TGF-β-TSP1 complex bound to the cell surface receptor CD36 on alveolar macrophages. However, preincubation of fibroblasts with plasmin inhibitors did not inhibit IL-4-induced TGF-β activation, suggesting that plasmin is not involved in the IL-4-stimulated TGF-β activation by lung fibroblasts. BLM-stimulated TGF-β activation involves both TSP1 and MMP activity. On the other hand, plasmin and MMP activity are important for IL-1β-induced TGF-β activation. Because IL-1β is able to up-regulate urokinase-type plasminogen activator (uPA) and uPA receptor (uPAR),49 and increased expression of uPA and uPAR has been found in fibroblasts in fibrotic lung tissues,14 it will be of interest to investigate whether up-regulation of uPA or uPAR by IL-1β results in activation of TGF-β in Thy-1 (−) lung fibroblasts.
A common feature of pulmonary fibrosis in humans and animal models is the appearance of myofibroblasts in the fibroblastic foci.50 Myofibroblasts are characterized by the expression of α-SMA. Consistent with the recent finding that fibroblasts/myofibroblasts in fibrotic foci of IPF are predominantly Thy-1 (−) cells (J.S. Hagood et al, submitted manuscript), we found that Thy-1 (−) lung fibroblasts constitutively express significantly more α-SMA than do Thy-1 (+) fibroblasts. Treatment of Thy-1 (−) fibroblasts with TGF-β1, IL-4, PDGF-BB, or BLM induced further increases of α-SMA expression, whereas the same treatments induce only a slight increase (PDGF-BB) or no increases (TGF-β1, IL-4, and BLM) in Thy-1 (+) cultures. These data suggest that lung fibroblasts that lack Thy-1 expression have a greater potential to differentiate into a myofibroblastic phenotype.
The role of Thy-1 expression in myofibroblastic differentiation is complex. Koumas and colleagues51 recently reported that human Thy-1 (+) myometrial and orbital fibroblasts constitutively express α-SMA, whereas Thy-1 (−) myometrial and orbital fibroblasts do not. Furthermore, the Thy-1 (+) myometrial and orbital fibroblasts express increased α-SMA when stimulated by active TGF-β. Neither latent TGF-β activation nor Smad signaling were addressed in this study. The reason for the apparent discrepancy between our findings in rat lung fibroblasts and those of Koumas and colleagues51 in human myometrial and orbital fibroblasts is not clear, but may support the concept of tissue-specific fibroblasts whose functions and characteristics vary according to their anatomical location and the cellular and extracellular matrix environment to which they are exposed. This concept is also supported by recent studies demonstrating distinct and characteristic gene expression patterns in fibroblasts isolated from different tissues.52
The mechanisms by which Thy-1 expression confers resistance to TGF-β activation and α-SMA induction in response to cytokine or BLM stimuli are currently unknown. Interestingly, treatment of Thy-1 (+) cells with exogenous active TGF-β stimulated Smad-dependent TGF-β signaling, with consequent up-regulation of FN expression. These data argue against intrinsic defects of TGF-β receptors or signaling cascades or elevated levels of secreted TGF-β inhibitors such as decorin or α2-macroglobulin in Thy-1 (+) fibroblasts, at least under basal conditions. Furthermore, the resistance of Thy-1 (+) cells to fibrogenic stimuli does not seem to be because of altered TGF-β protein synthesis or synthesis of defective latent protein that cannot be activated because levels of total protein expression between cell types did not differ significantly and latent protein was able to be activated by standard conditions (heat). It is possible that Thy-1 expression regulates expression of TGF-β modulatory proteins such as the latent TGF-β-binding protein or of soluble inhibitors under stimulated conditions. Cell-matrix interactions and cytoskeletal organization regulate cell function and Thy-1 has been shown to associate with cytoskeletal elements.53–55 Recent findings indicate that Thy-1 expression modulates cytoskeletal organization and focal adhesion formation in fibroblasts.35 Thus it is possible that cell shape and cell-matrix interactions are determinants of TGF-β activation. Such a possibility would be consistent with integrin-dependent mechanisms of TGF-β activation that require that the actin cytoskeleton be organized.34,56–58
IPF is a progressive, fatal disease for which there is no effective treatment. TGF-β activity has been shown through in vitro studies, animal models, and in clinical data to play a critical role in the lethal progression of fibrotic replacement of the alveolar wall. The abnormal myofibroblast is a key cell type involved in fibrotic remodeling of the lung interstitium. We reported novel findings identifying a differential ability between normal lung fibroblasts expressing the glycophosphatidylinositol-anchored protein, Thy-1, and the myofibroblast-like lung fibroblasts that lack Thy-1 expression to activate latent TGF-β in response to fibrogenic stimuli. These data indicate that profibrotic Thy-1 (−) cells respond to fibrogenic stimuli by activating latent TGF-β, leading to signaling of its fibrogenic properties. In contrast, Thy-1 expression confers a resistance to TGF-β activation by fibrogenic stimuli, protecting cells from TGF-β-dependent fibrotic sequelae. Although the significance of TGF-β activity for disease progression is well-established and multiple mechanisms of latent TGF-β activation in the lung have been identified, our observations are the first to correlate susceptibility or resistance to TGF-β activation with either specific fibrotic phenotypic characteristics or with specific cellular subpopulations in lung fibroblasts. Furthermore, we have identified Thy-1 as a specific cell surface molecule capable of modulating fibroblast susceptibility to TGF-β activation by multiple mechanisms. Resistance or susceptibility to TGF-β activation is potentially a significant determinant of normal resolution of wound healing or fibrotic disease progression, respectively. As such, molecules that modulate TGF-β activation represent potent therapeutic targets.
Acknowledgments
We thank Dr. Thomas H. Barker for his assistance in construction of transfected lung fibroblasts and Mark W. MacEwen for his technical assistance.
Footnotes
Address reprint requests to Joanne E. Murphy-Ullrich, Ph.D., Department of Pathology, Volker Hall 668, 1670 University Blvd., Birmingham, AL 35294-0019. E-mail: murphy@path.uab.edu.
Supported in part by the National Institutes of Health (grants DK606058 to J.E.M.-U. and HL65348 to J.S.H.) and the American Lung Association (career investigator award to J.S.H.).
References
- Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol. 2002;34:1534–1538. doi: 10.1016/s1357-2725(02)00091-2. [DOI] [PubMed] [Google Scholar]
- Lasky JA, Brody AR. Interstitial fibrosis and growth factors. Environ Health Perspect. 2000;108(Suppl 4):S751–S762. doi: 10.1289/ehp.00108s4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca C, Abraham D, Black CM. Lung fibrosis. Springer Semin Immunopathol. 1999;21:453–474. doi: 10.1007/s002810000036. [DOI] [PubMed] [Google Scholar]
- Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(Suppl 3):S87–S92. [PubMed] [Google Scholar]
- Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwartz DA, Schultz GS, Wagner CR, Musson RA. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2002;166:236–246. doi: 10.1164/rccm.2201069. [DOI] [PubMed] [Google Scholar]
- Noble PW. Idiopathic pulmonary fibrosis. New insights into classification and pathogenesis usher in a new era therapeutic approaches. Am J Respir Cell Mol Biol. 2003;29(Suppl 3):S27–S31. [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(Suppl 6):S286–S289. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Mangalwadi A, Guo B, MacEwen MW, Salazar L, Fuller GM. Concordant and discordant interleukin-1-mediated signaling in lung fibroblast Thy-1 subpopulations. Am J Respir Cell Mol Biol. 2002;26:702–708. doi: 10.1165/ajrcmb.26.6.4547. [DOI] [PubMed] [Google Scholar]
- Kmiec Z: Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 2001, 161:III-XIII, 1–151 [DOI] [PubMed] [Google Scholar]
- Zhang G, Kim H, Cai X, Lopez-Guisa JM, Alpers CE, Liu Y, Carmeliet P, Eddy AA. Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2003;14:1254–1271. doi: 10.1097/01.asn.0000064292.37793.fb. [DOI] [PubMed] [Google Scholar]
- Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int. 1996;54:S39–S45. [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast α-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1β. J Immunol. 1997;158:1392–1399. [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Nomura A, Uchida Y, Noguchi Y, Sakamoto T, Ishii Y, Goto Y, Masuyama K, Zhang MJ, Hirano K, Mochizuki M, Ohtsuka M, Sekizawa K. Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding. Am J Respir Cell Mol Biol. 2001;24:1–11. doi: 10.1165/ajrcmb.24.1.4040. [DOI] [PubMed] [Google Scholar]
- Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Lasky JA, Nesbitt JE, Segarini P. Differential expression, surface binding, and response to connective tissue growth factor in lung fibroblast subpopulations. Chest. 2001;120:S64–S66. doi: 10.1378/chest.120.1_suppl.s64. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Miller PJ, Lasky JA, Tousson A, Guo B, Fuller GM, McIntosh JC. Differential expression of platelet-derived growth factor-α receptor by Thy-1(−) and Thy-1(+) lung fibroblasts. Am J Physiol. 1999;277:L218–L224. doi: 10.1152/ajplung.1999.277.1.L218. [DOI] [PubMed] [Google Scholar]
- McIntosh JC, Hagood JS, Richardson TL, Simecka JW. Thy1 (+) and (−) lung fibrosis subpopulations in LEW and F344 rats. Eur Respir J. 1994;7:2131–2138. doi: 10.1183/09031936.94.07122131. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor β on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST, Giri SN. Reduction of bleomycin induced lung fibrosis by transforming growth factor β soluble receptor in hamsters. Thorax. 1999;54:805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier T, Degen E, Baschong W. Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res Exp Med (Berl) 1993;193:195–205. doi: 10.1007/BF02576227. [DOI] [PubMed] [Google Scholar]
- Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Transforming growth factor-β increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen RP, Kobayashi S, Bornstein P. Transforming growth factor β increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci USA. 1988;85:1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor β modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6:1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β 1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β(1). Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. EMBO J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Khalil N, Parekh TV, O’Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-β 1 by activation of latent TGF-β 1 and differential expression of TGF-β receptors (T β R-I and T β R-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor β-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGF β 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–496. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Xu YD, Hua J, Mui A, O’Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-β1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol. 2003;285:L527–L539. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J Biol Chem. 1994;269:26775–26782. [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast α-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–537. [PMC free article] [PubMed] [Google Scholar]
- Phipps RP, Penney DP, Keng P, Quill H, Paxhia A, Derdak S, Felch ME. Characterization of two major populations of lung fibroblasts: distinguishing morphology and discordant display of Thy 1 and class II MHC. Am J Respir Cell Mol Biol. 1989;1:65–74. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- Derdak S, Penney DP, Keng P, Felch ME, Brown D, Phipps RP. Differential collagen and fibronectin production by Thy 1+ and Thy 1− lung fibroblast subpopulations. Am J Physiol. 1992;263:L283–L290. doi: 10.1152/ajplung.1992.263.2.L283. [DOI] [PubMed] [Google Scholar]
- Penney DP, Keng PC, Derdak S, Phipps RP. Morphologic and functional characteristics of subpopulations of murine lung fibroblasts grown in vitro. Anat Rec. 1992;232:432–443. doi: 10.1002/ar.1092320312. [DOI] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol. 2000;23:204–212. doi: 10.1165/ajrcmb.23.2.4089. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–3614. [PubMed] [Google Scholar]
- Hasegawa T, Sorensen L, Dohi M, Rao NV, Hoidal JR, Marshall BC. Induction of urokinase-type plasminogen activator receptor by IL-1 β. Am J Respir Cell Mol Biol. 1997;16:683–692. doi: 10.1165/ajrcmb.16.6.9191470. [DOI] [PubMed] [Google Scholar]
- Kuhn C. Pathology. Phan S, Thrall R, editors. Marcel New York: Dekker,; Pulmonary Fibrosis. 1995:pp 59–83. [Google Scholar]
- Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woda BA, Woodin MB. The interaction of lymphocyte membrane proteins with the lymphocyte cytoskeletal matrix. J Immunol. 1984;133:2767–2772. [PubMed] [Google Scholar]
- Richter-Landsberg C, Greene LA, Shelanski ML. Cell surface Thy-1-cross-reactive glycoprotein in cultured PC12 cells: modulation by nerve growth factor and association with the cytoskeleton. J Neurosci. 1985;5:468–476. doi: 10.1523/JNEUROSCI.05-02-00468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Newton MR, Shotton DM. Cytoskeletal involvement in the sequential capping of rat thymocyte surface glycoproteins. J Cell Sci. 1988;89:309–319. doi: 10.1242/jcs.89.3.309. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel JG, Schultz-Cherry S, Murphy-Ullrich JE, Rifkin DB. Tamoxifen and estrogen effects on TGF-β formation: role of thrombospondin-1, αvβ3, and integrin-associated protein. Biochem Biophys Res Commun. 2001;284:11–14. doi: 10.1006/bbrc.2001.4922. [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163:533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]