Abstract
Plaques composed of amyloid β (Aβ) have been found within days following brain trauma in humans, similar to the hallmark plaque pathology of Alzheimer’s disease (AD). Here, we evaluated the potential source of this Aβ and long-term mechanisms that could lead to its production. Inertial brain injury was induced in pigs via head rotational acceleration of 110° over 20 ms in the coronal plane. Animals were euthanized at 3 hours, 3 days, 7 days, and 6 months post-injury. Immunohistochemistry and Western blot analyses of the brains were performed using antibodies specific for amyloid precursor protein (APP), Aβ peptides, β-site APP-cleaving enzyme (BACE), presenilin-1 (PS-1), caspase-3, and caspase-mediated cleavage of APP (CCA). Substantial co-accumulation for all of these factors was found in swollen axons at all time points up to 6 months following injury. Western blot analysis of injured brains confirmed a substantial increase in the protein levels of these factors, particularly in the white matter. These data suggest that impaired axonal transport due to trauma induces long-term pathological co-accumulation of APP with BACE, PS-1, and activated caspase. The abnormal concentration of these factors may lead to APP proteolysis and Aβ formation within the axonal membrane compartment.
A series of investigations suggest a link between traumatic brain injury and Alzheimer’s disease (AD). Several epidemiological studies have found that even a single incident of brain trauma is a significant risk factor for developing AD.1–6 In addition, plaques composed of amyloid β (Aβ) have been found within days following a single incident of brain trauma in humans, similar to the hallmark plaque pathology of AD.7,8 However, it has remained unknown how Aβ is so rapidly produced after brain injury or how long this process persists.
In AD, the primary mechanism for production of Aβ peptides is thought to be via transmembrane cleavage of amyloid precursor protein (APP) by β- and γ-secretases.9–12 Following brain trauma in humans and several experimental animal models, a marked accumulation of APP has been found in damaged axons, suggesting that there may be ample substrate for Aβ production. We have previously identified extensive co-accumulation of Aβ with APP in swollen axons within days following injury in a pig model of diffuse axonal injury (DAI).13 Likewise, we have recently found widespread axonal Aβ accumulations associated with Aβ plaques in brain-injured humans with DAI.14 These observations suggest that damaged axons serve as a key source of Aβ following brain trauma. Although intra-axonal proteolysis of APP to Aβ is not a typical process proposed for AD, a recent study has demonstrated Aβ production within the axon membrane compartment of peripheral nerves.15,16 This process was mediated by β-site APP-cleaving enzyme (BACE) and a catalytic component of γ-secretase, presenilin-1 (PS-1).10,17
Here, we used the pig model of DAI to explore the relationship of Aβ accumulation in damaged axons with proposed mediators of Aβ production. Due to the long-term neurodegenerative changes induced by brain trauma,18–21 we examined the accumulation of APP, Aβ, BACE, and PS-1 in the brain over 6 months following injury. In addition, we evaluated potential caspase activation and caspase-mediated proteolysis of accumulating APP.
Materials and Methods
This study was conducted in accordance with the animal welfare guidelines set forth in Guide for the Care and Use of Laboratory Animals, U.S. Department of Health and Human Services Publication Number 85–23, 1985. All animal procedures were previously approved by the University of Pennsylvania Institutional Animal Use and Care Committee.
Pre-Injury Preparation
Fifteen young adult (6 months old) miniature swine (Hanford strain, Sinclair Research Center, Inc., Columbia, MO), both male and female, 17 to 20 kg, were used for this study. The animals were fasted for 12 hours, following which anesthesia was induced with an initial injection of midazolam (400 to 600 mg/kg). Once sedated, animals received 2 to 4% isoflurane via snout mask until they reached a plane of surgical anesthesia. A venous catheter was then inserted in the ear, and the animals were endotracheally intubated and maintained on 1.5 to 2% isoflurane. Physiological monitoring and apparatus included noninvasive ECG electrode leads affixed to the chest and extremities, a pulse oximeter placed on the skin of the tail, a rectal thermometer, and sampling tubes for end tidal CO2 measurement attached to the endotracheal tube. Arterial blood gasses were also periodically evaluated pre- and post-injury. The pigs were continuously monitored and all data from physiological monitoring were collected on a computer-driven storage system. Intracranial pressure monitoring was not performed since previous studies demonstrated only small transient changes using the injury parameters applied in this study.20
Brain Injury
Brain trauma was induced via head rotational acceleration as previously described in detail.22,20 Briefly, the animals’ heads were secured to a padded snout clamp, which, in turn, is mounted to the linkage assembly of a pneumatic actuator, or HYGE device, that converts the linear motion to an angular (rotational) motion. For these experiments, the linkage was adjusted to produce a pure impulsive head rotation of 110° in the coronal plane, with the center of rotation close to the brain center of mass. Head rotational acceleration was biphasic with a predominant deceleration phase. Triggered release of pressurized nitrogen rotates the linkage assembly the full 110° in 20 msec. Following brain injury, animals’ heads were released from the clamp. Sham (control) animals received identical treatment without injury.
Tissue Preparation
For histopathological analyses, animals were sacrificed at 3 days (n = 3), 7 days (n = 3), and 6 months (n = 3) post-injury or sham treatment (n = 2 at 6 months of age, n = 1 at 11 months of age). After animals received an overdose of pentobarbital (150 mg/kg i.v), the descending aorta was clamped and the animals were transcardially perfused with 4 liters of saline followed by 10 liters of 4% paraformaldehyde. The brains were carefully removed, post-fixed in 4% paraformaldehyde solution for 2 hours, and stored in phosphate buffer solution (Millonig’s buffer). Fixed brains were subsequently blocked into 0.5-cm coronal sections for gross examination and photography. All blocks were cut into coronal slices 2- to 4-mm thick and processed for paraffin-embedding in an automated tissue processor (Shandon Hypercenter XP, Shandon Scientific Instruments, Pittsburgh, PA). Serial sections of 6 μm were cut on a Leitz rotary microtome (Leica, Malvern, PA) and mounted on poly-L-lysine-coated slides.
For protein analysis, animals were deeply anesthetized and perfused with heparinized saline at 3 hours (n = 3), 3 days (n = 3), and 7 days (n = 3) following brain trauma. Sham animals (n = 2) were perfused in the same manner. Surgical resection of 2 cm3 of brain tissue from the left frontal lobe was performed before transcardial perfusion. Gray and white matter were rapidly dissected from the tissue on crushed dry ice and stored at −80°C.
Immunohistochemistry
Immunohistochemistry (IHC) staining was performed on serial paraffin-embedded sections that were coded to hide the injury and age status of the animals to blind the analysis. We performed both single- and double-label IHC on the tissue to detect accumulations of APP, neurofilament (NF) proteins, Aβ, BACE, PS-1, caspase-3, caspase-mediated cleavage of APP (CCA), and kinesin, and to identify macrophages. We used a well-characterized panel of primary antibodies for these studies listed in Tables 1, including their specificities, dilutions, and references. One antibody, “249”, was created specifically for this study to detect CCA. This antibody was raised in a rabbit to react with the amino-terminal fragment of APP generated by caspase-3 cleavage. The peptide corresponding to the carboxy-terminus of this APP fragment, CGVVEVD, was synthesized by Research Genetics and its sequence confirmed by mass spectrometry. It was coupled covalently through its cysteine residue to maleimide-activated KLH, and the conjugate was used to immunize mice and generate the Ab249 antiserum.
Table 1.
Summary of Antibodies Used for Immunohistochemistry
| Antibody | Epitope protein/amino acids | Type | Dilution | Company | Reference |
|---|---|---|---|---|---|
| 22C11 | APP/60-100 | M | 1:40 | Chemicon | 47 |
| APP | APP/C terminal & C99 fragments | P | 1:200 | ProSci | 48, 49 |
| APP | APP/N terminal | P | 1:00 | Serotec | 50 |
| 249 | caspase cleavage of APP | P | 1:2000 | ** | 51 |
| 6E10 | Aβ/1–17 | M | 1:500 | Signet | 57 |
| 6F/3D | Aβ/8–17 | M | 1:100 | DAKO | 52, 53 |
| 10Δ5 | Aβ1–28 | M | 1:50 | * | 54–56 |
| 4G8 | Aβ17–24 | M | 1:1000 | * | 57, 58 |
| Aβ1–40 | Aβ1–40 | P | 1:100 | Chemicon | 59, 60 |
| Aβ1–40 | Aβ1–40 | P | 1:1000 | Signet | 59, 60 |
| 70 | Aβ1–40 | P | 1:1000 | ** | 61 |
| Aβ17–42 | Aβ17–42 | P | 1:100 | Chemicon | 62–64 |
| Aβ1–42 | Aβ1–42 | P | 1:1000 | Signet | 62–64 |
| 13335 | Aβ1–42 | P | 1:1000 | * | 14, 65 |
| BCO5 | Aβ1–42(43) | M | Wako Pure | 66, 67 | |
| Aβ1 | Aβ & C99 fragments | P | 1:1000 | ** | 68, 69 |
| N52 | NF-H | M | 1:400 | Sigma | 70, 71 |
| P20 | Procaspase-3 | P | 1:100 | Santa Cruz | 72, 73 |
| Large subunit | |||||
| BACE2/ | β-secretase | P | 1:500 | Alpha | 12, 74 |
| Asp1 | |||||
| PS-1 | C-terminal | P | 1:200 | Zymed | 75 |
| Presenilin | |||||
| PS-1 | N-terminal | P | 1:50 | Chemicon | 76 |
| Presenilin | |||||
| L1 | kinesin-L | M | 1:200 | Chemicon | 77, 78 |
| H1 | kinesin-H | M | 1:200 | Chemicon | 79 |
| OX42 | CD11b | M | 1:100 | Serotec | 80 |
, antibodies from Dr. V.M.-Y. Lee;
, antibodies from Dr. Robert Siman; M, monoclonal antibody; P, polyclonal antibody.
Single-Label IHC Examination
Single-label examinations were performed using the panel of primary antibodies listed in Table 1. Sections were deparaffinized and endogenous peroxidase activity was quenched with 5% H2O2 (Sigma, St. Louis, MO) in methanol (Fisher, Pittsburgh, PA) for 30 minutes. Sections were then blocked using 2% normal horse serum (NHS) (Sigma) in 0.1 mol/L Tris, pH 7.4 for 30 minutes and incubated overnight with a primary monoclonal antibody (mAb) or polyclonal antibody (pAb) at 4°C. Sections were then incubated at room temperature for 1 hour each with the appropriate biotinylated secondary antibodies and ABC kit (1:1000, Vector Laboratories, Inc., Burlingame, CA), followed by DAB peroxidase substrate kit (Vector). Antibodies were diluted in 0.1 mol/L Tris buffer with 2% NHS and 0.1% Triton X-100, and tissue sections were washed in 0.1 mol/L Tris buffer. Sections were counter-stained with hematoxylin, dehydrated, and cover-slipped. For the single-label immunostaining, omission of primary antibody or application of control serum instead of primary antibody on selected sections of pig tissue provided a negative control. Paraffin-embedded sections from confirmed human AD brain tissue served as positive control for Aβ accumulation (including Aβ accumulation in neurons and Aβ plaques). Additional pig brain sections were stained with hematoxylin and eosin (H&E) and cresyl violet for verification of and comparison with previously established of traumatic neuron loss and pyknosis.20
Double-Label IHC Examination
To examine co-accumulation of proteins in pathological profiles in the brain tissue, sections were incubated in combinations of primary antibodies listed in Table 2. Processing of each primary antibody on the sections was as described above. Incubations of fluorescent secondary antibodies were performed in a darkroom. The secondary antibodies were, respectively, Alexa-594-conjugated goat anti-rabbit IgG (1:200, Molecular Probes, Inc, Eugene, OR), fluorescein-conjugated goat anti-rabbit IgG (1:200, Molecular Probes), Texas red-conjugated horse anti-mouse IgG (1:200, Vector Laboratories), fluorescein-conjugated horse anti-mouse IgG (1:200, Vector), TRITC-conjugated rabbit anti-goat IgG (1:200, Sigma) and fluorescein-conjugated rabbit anti-goat IgG (1:200, Jackson ImmunoReseach Laboratories, Inc., West Grove, PA) (Table 2). In general, IHC staining was performed sequentially; ie, one primary antibody was applied followed by its respective secondary antibody and the procedure repeated using secondary staining sequence. As a negative control, each pair of primary antibodies was processed separately to confirm the lack of cross-reactivity between the two secondary and primary antibodies. All sections were examined with light and fluorescence microscopy using a Nikon Microphoto SA with an UFX-DX camera system (Optical Apparatus, Ardmore, PA) and appropriate filters. Some images were captured with a Nikon Eclipse E600 with spot RT digital camera (Diagnostic Instruments Inc, Sterling Heights, MI).
Table 2.
Antibodies for Immunohistochemical Double Staining
| Primary antibody | Target | Source | Type | Secondary antibody | Source | Type | Chromogen |
|---|---|---|---|---|---|---|---|
| 13335 | Aβ | Rabbit | P | Anti-rabbit IgG | Goat | P | Alexa-594 |
| 6F/3D | Aβ | Mouse | M | Anti-mouse IgG | Horse | P | Fluorescein |
| N52 | NF200 | Mouse | M | Anti-mouse IgG | Horse | P | Texas red |
| APP | APP | Rabbit | P | Anti-rabbit IgG | Goat | P | Fluorescein |
| 22C11 | APP | Mouse | M | Anti-mouse IgG or | Horse | P | Fluorescein |
| anti-mouse IgG | Horse | P | Texas red | ||||
| 249 | CCA | Rabbit | P | Anti-rabbit IgG | Goat | P | Alexa-594 |
| P20 | Caspase-3 | Goat | P | Anti-goat IgG | Rabbit | P | Tritc |
| BACE | BACE | Rabbit | P | Anti-rabbit IgG or | Goat | P | Alexa-594 |
| anti-rabbit IgG | Goat | P | Fluorescein | ||||
| PS-1 | presenilin | Rabbit | P | Anti-rabbit IgG or | Goat | P | Alexa-594 |
| anti-rabbit IgG | Goat | P | Fluorescein | ||||
| L-1 | Kinesin | Mouse | M | Anti-mouse IgG | Horse | P | Texas red |
Thioflavin S and Congo Red Stain
Brain sections were also stained with Thioflavin S (ICN Biomedicals, Inc, Aurora, OH) and Congo red (ICN Biomedicals) to detect protein aggregation. Sections were deparaffinized and hydrated through xylene, alcohol to water, fixed in 10% PBS-buffered formalin for 1 hour (for Thioflavin S) or 10 minutes (for Congo red), rinsed in PBS, and then stained with 0.0125% Thioflavin S in 40% alcohol in 60% PBS for 5 minutes or with 0.5% Congo red in 50% alcohol for10 minutes. Slides were differentiated in 50% alcohol in 50% PBS for 2 × 10 seconds (for Thioflavin S) or in 80% alcohol until pink stain (for Congo red). In addition, double-labeling of tissue sections was performed with Thioflavin S and the anti-Aβ mAb, 4G8, which was visualized with Texas red-conjugated goat anti-mouse antibody. After rinsing with PBS for 3 times × 5 minutes, sections stained for Thioflavin S alone or Thioflavin S and Aβ were cover-slipped using anti-fade medium (Southern Biotechnology Associates Inc, Birmingham, AL). The edges were sealed with clear acrylic nail polish and viewed under fluorescence microscopy. Sections stained for Congo red were dehydrated, cover-slipped with Cytoseal (Stephens Scientific Division of Comwell Corporation, Riverdale, NJ), and viewed under fluorescence microscopy using an excitation of 530 to 550 nm, followed by examination using polarized light to evaluate reduction of green birefringence.
Semi-Quantitative Analysis for Immunohistochemistry
Semi-quantitative analysis for axonal profiles was performed to determine the extent and distribution of axonal injury throughout the brain in the sections of NF, APP, and Aβ immunohistochemical staining by using an MCID Imaging Research image analysis system (Imaging Research, Inc., St. Catharines, Canada). For each stain, two sections from each block of tissue were examined and the frontal and parietal lobes and basal ganglia regions were evaluated by an observer blinded to the age and injury status of individual animals. Based on preliminary studies of brains of animals, these regions had a relatively high density of axonal injury and Aβ deposition. Axonal profiles (darkly immunoreactive varicose axonal swellings and axonal bulbs) were identified in the coronal brain section by their morphology and were counted manually using light microscopy at ×200 magnification. Anatomical regions were based on the Atlas of the Brain of Domestic Animals.23 The mean number of axonal profiles per brain region for each animal in different time point following brain trauma was calculated.
Western Blot Analysis
Brain tissue was lysed in RIPA buffer (1X PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with a cocktail of protease inhibitor (Roche Diagnostic, Indianapolis, IN) at 4°C. After homogenization on ice, tissue lysates were centrifuged at 45000 rpm for 30 minutes. Protein concentration was determined by DC protein assay (Bio-Rad Laboratories, Hercules, CA). The samples were boiled in 2X SDS sample buffer for 5 minutes and run on high performance 12% and 4 to 12% NuPAGE Bis-Tris gels and 7% NuPAGE Tris-Acetate gel (Invitrogen, Carisbad, CA). The polypeptides were electrotransferred to Immobilon-P PVDF membrane. Non-specific binding was blocked using 5% nonfat milk in PBST (9.1 mmol/L dibasic sodium phosphate, 1.7 mmol/L monobasic sodium phosphate, 150 mmol/L NaCl, and 0.1% Tween 20) for 30 minutes at room temperature. Aβ, APP (full-length APP and C-terminal fragment), BACE, PS-1, caspase-3, CCA, and kinesins were determined by primary antibodies as follows: pAb Aβ1–40 (1:500), pAb 70 (1:2000), pAb Aβ17–24 (1:500), pAb Aβ1 (1:1000), pAb APP-CT (1:500), pAb BACE2 (1:1000), pAb PS-1 (1:1000), pAb P20 (1:100), pAb 249 (1:2000), mAb L1 (1:1000), and mAb H1 (1:000) overnight at 4°C, and then by biotinylated secondary antibodies (1:500–1:1000, Vector Laboratories) as well as Vectastain ABC kit (1:1000, Vector Laboratories) for 1 hour each at room temperature. Before the products were visualized by DAB (Vector Laboratories), the membranes were washed 3 times with TTBS (100 mmol/L Tris, 0.9% NaCl, and 0.1% Tween 20) at room temperature, each time for 5 minutes.
In addition to Western blot detection of Aβ from unprocessed tissue homogenates, immunoprecipitation of Aβ from the tissue was also performed, using rabbit pAb 13335, specific for Aβ1–42. Individual samples of 750 μl (1 μg/μl) were incubated with 1 μg normal horse IgG (Sigma) and 20 μl protein A/G PLUS-Agarose (Santa Cruz Biotechnology Inc, Santa Cruz, CA) for 30 minutes at 4°C. After centrifugation (1,000 × g for 5 minutes), Aβ peptides in the supernatant were precipitated with 20 μl of pAb 13335 and 20 μl of protein A/G PLUS-Agarose overnight at 4°C. The pellets were collected by centrifugation at 1,000 × g for 5 minutes and then washed four times with RIPA buffer, each time repeating the centrifugation step. Protein transfer and visualization were as described above.
Sandwich ELISA Detection of Aβ
Tissue samples taken immediately before transcardial perfusion of animals surviving 3 hours or 7 days after injury were evaluated and compared to uninjured brains (n = 2 to 3 per group). To detect soluble and insoluble Aβ, brain tissue was homogenized with either RIPA buffer alone or RIPA buffer with 70% formic acid at a W/V ratio 1:5 and centrifuged at 45,000 rpm at 4°C for 1 hour (Beckman, Palo Alto, CA). Supernatant was subjected to sandwich ELISA. The pellet was resuspended in 70% formic acid (1:1) with sonication until clear. The formic acid samples were then neutralized by adding 1 mol/L Tris base (1:20). Samples were evaluated with sandwich enzyme linked immunosorbent assays (ELISA) for Aβ peptides. A microplate (Costar, Corning, NY) was pre-coated with anti-Aβ monoclonal antibody 6E10 that recognizes residues 1–17 of Aβ (1:500, Signet, Dedham, MA) in coating buffer pH 9.6 (0.1 mol/L NaHCO3 and 0.1 mol/L Na2CO3) for 24 hours at 4°C. After incubation, wells were probed with biotinylated rabbit polyclonal antibodies to Aβ1–42 (1:1000, Signet) for 24 hours at 4°C and with HRP-conjugated streptavidin (1:5000, Pierce, Rockford, IL) for 1 hour at room temperature. 3,5,3′,5′-tetramethylbenzidine (TMB, KPL, Gaihersburg, MD) was used as the chromogen and read at 450 nm on the EL340 Bio Kinetics microplate reader (Bio-TEK Instrument, Winooski, Vermont) using CK4 software. Synthetic Aβ1−42 (American Peptide, Vista, CA) was used as standard. The obtained value was corrected with the wet weight to each tissue sample and expressed as pmol/gram. Analysis was always performed in duplicate.
Statistical Analysis
For immunoblotting, quantification of the density in a given protein band was performed with NIH image software on pictures captured by digital camera. For quantification of protein concentration in the brain tissue, the microplate was read by the Bio Kinetics microplate reader automatically. All values are presented as means ± SE. Statistical analysis was performed using StatView (Abacus Concepts, Berkeley, CA). Differences between mean densities of protein expression and Aβ levels for each group were assessed using two-way analysis of variance (analysis of variance) followed by Fisher’s protected least significant difference (FPLSD) post-hoc two-tailed test. A P value <0.05 was considered significant.
Results
Physiology and Behavior Following Brain Trauma
Immediately following trauma, no substantial changes were noted in arterial blood gasses, pulse oximetry, or end tidal CO2 following injury. All animals began to wake within 15 minutes and were able to ambulate typically within 1 hour post-injury. The animals appeared to have slightly sluggish responses to sensory stimuli (startle reflex, tactile response) for up to 8 hours following trauma. By 24 hours post-injury, all of the animals appeared completely normal based on gross neurosensory examination (normal startle reflexes, gait, rooting behavior, eating, and drinking).
Long-Term Axonal and Neuronal Pathology
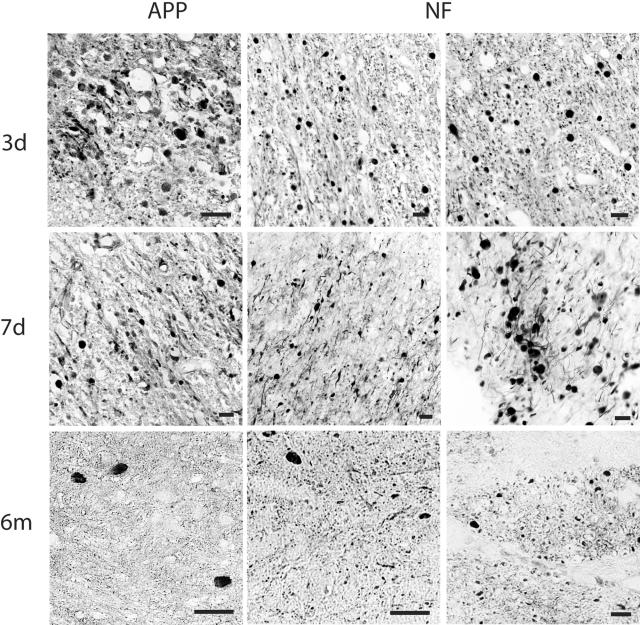
In sham animals, no axonal injury or any other overt pathological changes were found at any time point. In injured animals, as previously noted,20,24 a high density of axonal bulbs and axonal swellings were observed throughout the brain, most commonly in the roots of gyri at the interface of the gray and white matter of frontal and parietal lobes and in basal ganglia, at 3 and 7 days following trauma. Most axonal bulbs had the classic appearance of discrete spherical profiles surrounded by a halo where the bulb pulled away from the tissue. These profiles were up 40 μm in diameter, connected to an almost normal diameter proximal portion of the axon. The morphology of these profiles was distinct from elongated swellings that spanned up to several hundred μm of a single axon shaft with the appearance of varicosities. According to the averaged sector score/brain section, we found the mean number of axonal profiles in the frontal lobe to be 71 ± 7, 88 ± 6 in the parietal lobe, and 110 ± 5 in basal ganglia at 3 days post-injury. By 7 days post-injury, the mean number of axonal profiles was 53 ± 3 in the frontal lobe, 72 ± 5.7 in the parietal lobe, and 102 ± 9.4 in basal ganglia. Compared to brains at 7 days post-injury, the mean number of axonal profiles was reduced by 6 months following trauma. Nonetheless, the mean number of damaged axons remained relatively high in some brain regions despite the chronic state of the injury. Here, the mean number of axonal profiles was 17 ± 2 in the frontal lobe (77% reduction), 19 ± 4 in the parietal lobe (74% reduction), and 46 ± 2 in basal ganglia (58% reduction) (Figure 1). Swollen axon profiles were identified by antibodies targeting APP (22C11) and NF protein (N52).
Figure 1.
Representative photomicrographs demonstrating APP and NF protein accumulation in damaged axons in the subcortical white matter and basal ganglia detected by antibodies to 22C11/APP N-terminal and N52/NF200 at 3, 7 days, and 6 months following injury in the pig. Dark staining represents immunoreactivity of APP and NF proteins in swollen axons. Bar, 25 μm.
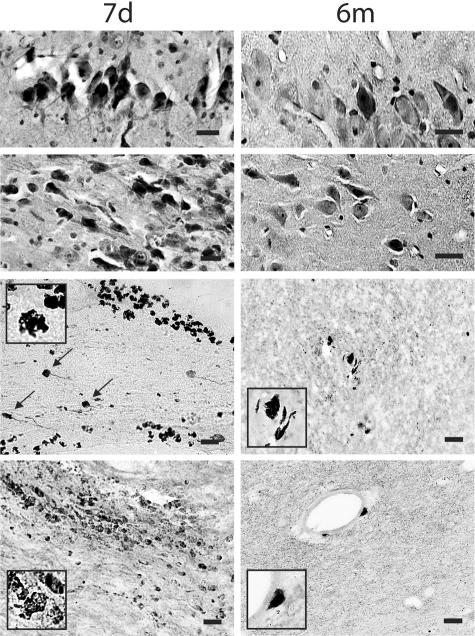
A modest number of pyknotic neurons were found in CA1 and CA3 subfields of the hippocampus and cerebellum (H&E) at 3 to 7 days and even by 6 months post-injury (Figure 2, top four panels).
Figure 2.
Representative photomicrographs revealing a modest number of pyknotic neurons in CA1 and CA3 subfields of the hippocampus (top panels) and foamy macrophage infiltrations within subcortical white matter, basal ganglia and lining blood vessels. Axonal bulbs were close to macrophage infiltrations (black arrows), detected by antibody OX42 at 7 days and 6 months post-injury (bottom panels). Bar, 25 μm.
At 3 days post-injury, foamy macrophages were abundantly present within injured brain tissue lining and in the parenchyma near damaged axons and large blood vessels, as well as in injured tissue nonadjacent to blood vessels. By 6 months post-injury, macrophages were still observed in the parenchyma and around the vessels, although in far fewer numbers than during the sub-acute time points (Figure 2, bottom four panels).
Accumulation of Aβ, BACE, PS-1, Caspase-3, CCA, and Kinesin
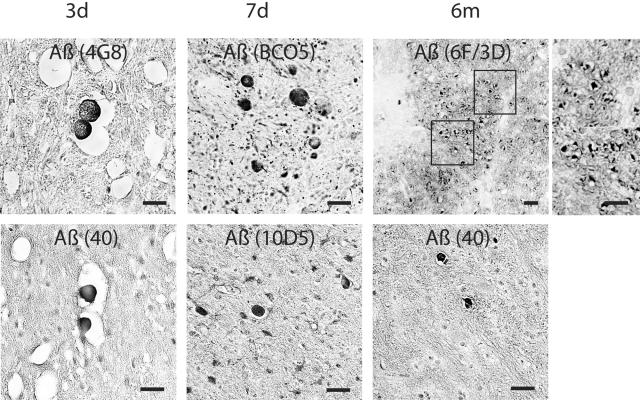
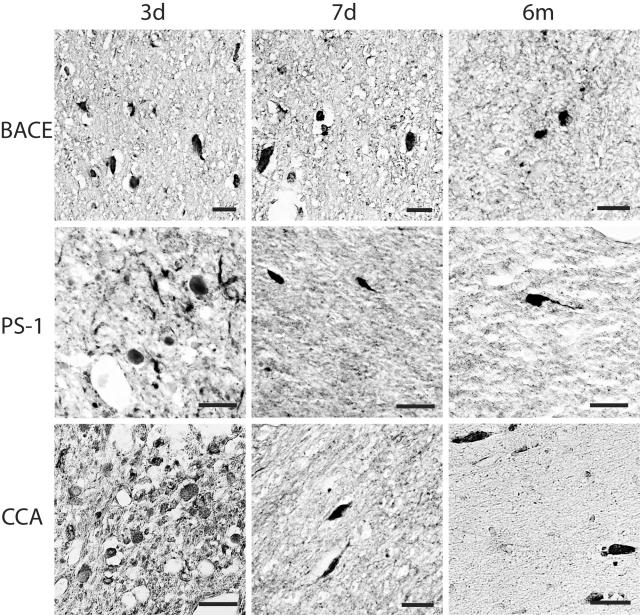
Immunoreactivity demonstrating accumulation of Aβ, mediators and markers of APP cleavage (BACE, PS-1, CCA, caspase-3) and kinesin, was found in all injured animals. Overwhelmingly this staining highlighted swollen axonal profiles with antibodies specific to Aβ (10Δ5, 4G8, Aβ1–40, 13335, Aβ17–24, BCO5 and 6F/3D), BACE (BACE2/Asp1), PS-1 (PS/NT and PS/CT), caspase-3 (P20), CCA (249), and kinesin (L1 and H1) (Figures 3 and 4). Immunoreactivity for these proteins and peptides in damaged axons persisted up to 6 months after brain trauma and followed the same general distribution as those stained with APP and NF, particularly in the subcortex and basal ganglia. Axon swellings stained with Aβ appeared to be signature profiles of axonal bulbs at the terminal ends of disconnected axons. However, elongated varicose swellings of non-disconnected axons typically did not show accumulation of Aβ despite accumulation of all other proteins examined. We also observed accumulation of Aβ (Figure 5), APP, BACE, PS-1, caspase-3, and CCA in a limited number of neurons of the cortex, hippocampus, and cerebellum and in glial cells of white matter at 3 and 7 days and 6 months after trauma.
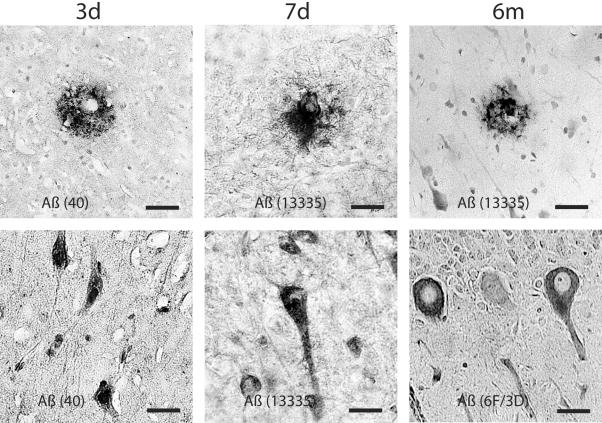
Figure 3.
Representative photomicrographs revealing Aβ accumulation in axonal bulbs in the basal ganglia at 3, 7 days, and 6 months after brain injury in the pig. Aβ was identified by antibodies to 4G8, BCO5, 6F/3D, Aβ1–40, 10Δ5. Bar, 25 μm.
Figure 4.
Representative photomicrographs demonstrating BACE, PS1 and CCA in damaged axons in the subcortical white matter and basal ganglia at 3 days to 6 months following brain injury in the pig. Accumulations in axons were detected by antibodies BACE-2, PS-1, and 249. Bar, 25 μm.
Figure 5.
Representative photomicrographs showing Aβ-containing plaque-like profiles in the gray and white matter. Occasionally, perivascualr Aβ depostis were found (top, middle). Aβ accumulation was also found in cortical and cerebellar neurons at 3 days to 6 months following brain trauma. Aβ was identified by antibodies, 6F/3D, Aβ1–40, 13335. Bar, 25 μm.
Aβ-containing plaque-like profiles were found in both gray and white matter within days and at 6 months following injury (Figure 5), similar to those previously observed within 10 days post-trauma.13 Most of the plaques found in white matter were in close proximity to axonal pathology. These plaques were not very numerous (the most plaques found in a coronal section were 10), and were found in only approximately half of all injured animals. In a few cases, perivascular Aβ deposits were found (Figure 5). Although plaque-like profiles were also detected at 6 months post-trauma, the total number did not appear to increase over time.
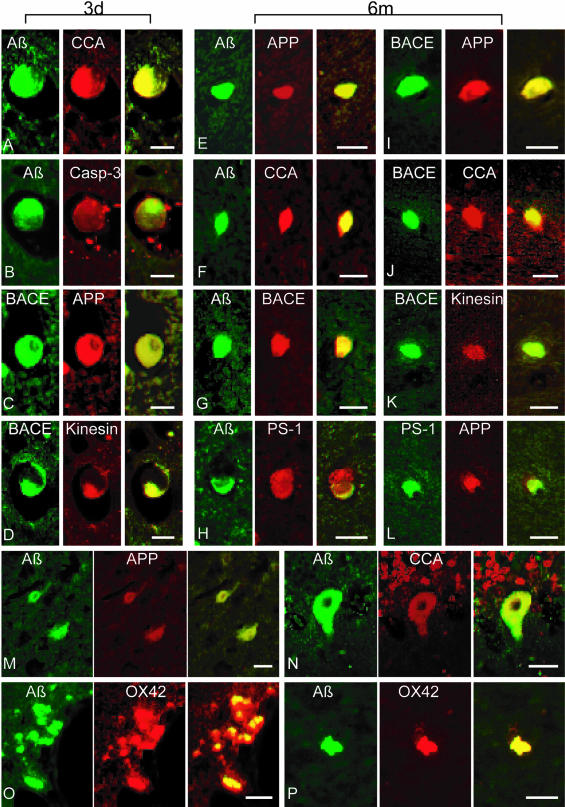
Using double-fluorescence immunohistochemistry, we consistently found co-immunoreactivity of combinations of APP, Aβ, BACE, PS-1, caspase-3, CCA and kinesin-L in axonal bulbs at 3 days and 6 months after injury (Figure 6). Double-labeling could also be found in a few neurons even by 6 months post-injury. Surprisingly, Aβ staining was found in macrophages also stained with OX42 at 3 days and 6 months post-injury (Figure 6). No co-localization of GFAP with Aβ was observed in injured or sham animals (data not shown).
Figure 6.
Representative double-immunofluorescence photomicrographs demonstrating co-accumulations of proteins in damaged axons (A–L), neurons (M–N) and macrophages (O–P) at 3 days and 6 months post-injury. Merged green and red fluorescence shown in yellow. In axon bulbs in the white matter, co-accumulation Aβ (antibodies 6F3D and 13335/Green) was found with CCA (249/Red) in (A) and (F), caspase-3 (P20/Red) in (B), BACE (BACE-2/Red) in (G), APP (22C11/Red) in (E), and PS-1 (PS-1/Red) in (H). Co-accumulation of BACE (Green) was found with APP (Red) in (C) and (I), kinesin (L1/Red) in (D) and (K), and CCA (Red) in (J). Co-accumulation of APP (Red) was found with PS-1 (Green) in (L). In neurons, Aβ (Green) co-accumulated with APP (Red) in (M) and CCA (Red) in (N). Macrophages demonstrated co-immunoreactivity of Aβ (13335/Green) with OX42 (CD11b/Red) in (O) and (P). Bar, 25 μm.
Thioflavin S and Congo Red Positive Staining
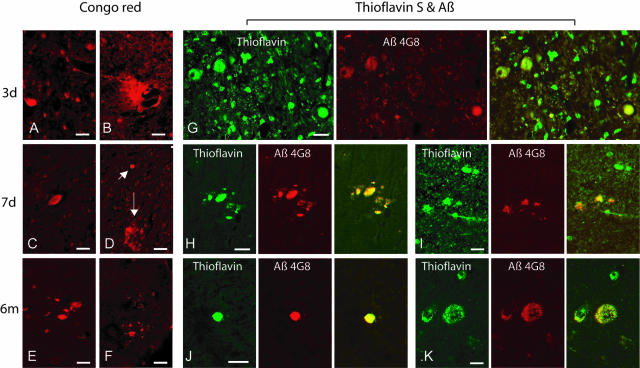
Positive staining for Thioflavin S alone and double-staining for Thioflavin S and Aβ was observed in axonal bulbs in the subcortical white matter of the frontal and parietal lobes as well as the basal ganglia at 3 days to 6 months post-injury. In addition, a few plaque-like profiles were observed in both gray and white matter of the frontal and parietal lobes at 3 days to 7 days post-injury (Figure 7).
Figure 7.
Representative photomicrographs showing Congo red staining in damaged axons in subcortical white matter at 3 days, 7 days and 6 months post-injury (A, C, D, E, and F), and in plaque-like profiles in the white matter at 3 and 7 days post-injury (B and D). Congo red stained plaque-like profiles (D, downward arrow) could be found in proximity with axonal bulbs (D, diagonal arrow). Double-staining with Thioflavin S and an anti-Aβ antibody shows co-localization in swollen axons in the subcortical white matter (G–J) at 3 days to 6 months after brain trauma, as well as in a few plaque-like profiles in the gray matter (K) at 6 months following brain injury. Bar, 25 μm.
Positive staining for Congo red was observed in axonal bulbs and neurons and a limited number of plaque-like profiles in the same areas and at the same time points detected by Thioflavin S and antibodies for Aβ peptides. Using polarized-light microscopy, limited green birefringence was observed in damaged axons and plaque-like profiles (Figure 7).
Identification of Aβ, APP, BACE, PS-1, Caspase-3, CCA, and Kinesin by Immunoblotting
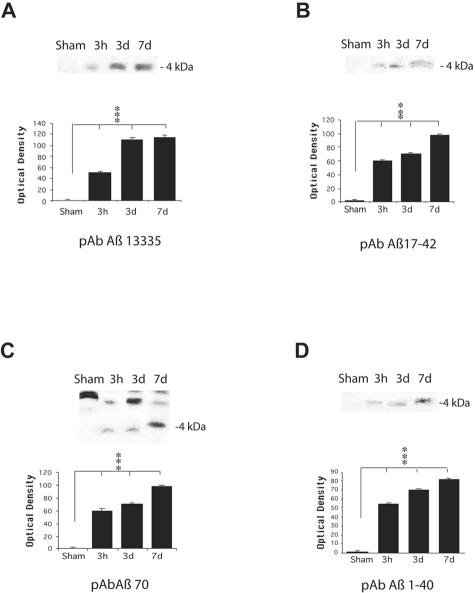
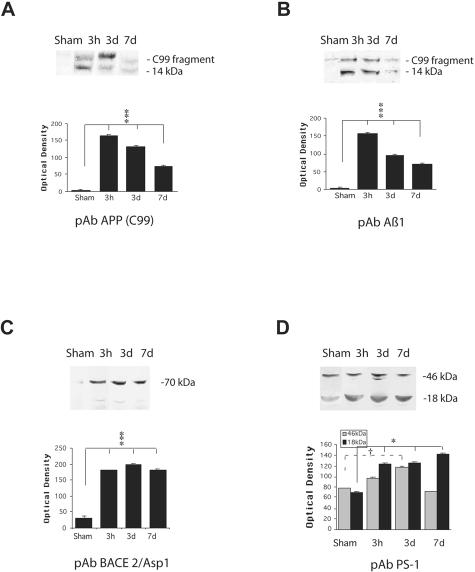
In corroboration of immunohistochemical identification of Aβ, APP, BACE, PS-1, CCA, caspase-3, and kinesin production and accumulation in injured brains, Western blot analysis identified Aβ in injured white matter, but not in sham brains. APP, BACE, PS-1, CCA, caspase-3, and kinesin were also observed in the white matter of injured brains from 3 hours to 7 days following TBI. On Western blots, anti-Aβ antibodies (Aβ1–40, Aβ 70, specific for Aβ1–40 and Aβ17–42) revealed a band of protein with a molecular weight of 4 kd, consistent with Aβ (Figure 8, B–D). We found that the expression of Aβ in the brain tissue samples appeared to follow the same temporal pattern as was found with immunohistochemical analysis. The staining for Aβ on the blots was relatively light at 3 hours following injury and distinct staining was found by 1 week following injury compared to virtually no expression of Aβ in sham tissue (P < 0.001). Examination of immunoprecipitated Aβ with anti-Aβ antibody 13335, specific for Aβ1–42, also revealed a slightly stained band at 4 kd at 3 hours post-injury and a strongly stained band at 7 days post-injury. No similar band was observed in sham tissue (Figure 8A, P < 0.001).
Figure 8.
Western blot analysis of Aβ in injured and sham brain white matter. Immunoreactive bands at the molecular weight of Aβ peptides (approximately 4 kd) were found at all post-injury time points by all anti-Aβ antibodies used (primary antibodies listed below graphs). Graphic representation of quantitative analysis of the optical density of the bands used mean values and SE from two separate experiments. P values refer to comparison between sham and groups of injured animals at different time points. ***, P < 0.001.
Antibodies specific for APP (APPCT and Aβ1) showed cleavage of APP into a 14-kd C99 fragment (Figure 9, A and B). The strongest staining on the blots was 3 hours after injury, compared to no expression of C99 fragment in the tissue of sham (P < 0.001).
Figure 9.
Western blot analysis of APP C99 fragment (14 kd), BACE, and PS-1 in injured and sham brain white matter. Primary antibodies used are listed below graphs. Quantitative analysis of the protein expression is shown graphically, with mean values and SE of optical density of immunostaining from two separate experiments. P values refer to comparison between groups of injured animals and sham. *, P < 0.05; ***, P < 0.001; †, P < 0.05.
Staining with BACE antibody revealed bands in the range of 70 kd. The level of BACE was relatively low in sham brain, compared to expression of BACE protein in the injured tissues (P < 0.001, Figure 9C). PS-1 antibody, reactive with the C-terminal region of presenilin-1, labeled the 46 kd PS-1 protein and also the 18 kd C-terminal fragment (CTF). Levels of the CTF increased following injury compared to sham (P < 0.05, Figure 9D).
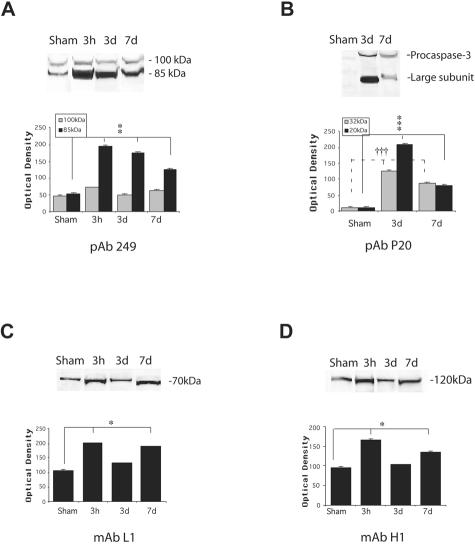
Anti-caspase-3 antibody reacted with the 32 kd procaspase-3 polypeptide, and also the 17 to 20 kd active large subunit. The active caspase-3 level was high at 3 days and relatively low by 7 days following injury (P < 0.001, Figure 10B). In the injured brain, an antibody to CCA identified APP cleavage to an 85 kd fragment (Figure 10A). The relatively strong staining on the blots was found in injured tissues compared to slight staining of 85 kd fragment in sham tissue (P < 0.01). Antibodies to kinesins (L1, specific for kinesin-L and H1, specific for kinesin-H) labeled polypeptides of 70 kd and 120 kd, respectively, consistent with kinesin-L and kinesin-H proteins (Figure 10, C and D; P < 0.05).
Figure 10.
Western blot analysis of caspase-mediated APP proteolysis, caspase-3, and kinesins in injured and sham brain white matter. (A) Banding representing CCA, (B) the 20 kd large subunit of caspase-3 and procapase, (C) Kinesin-L, and (D) Kinesin-H. Primary antibodies listed below graphs. Quantitative analysis of the protein expression of CCA, caspase-3, and kinesins is shown graphically with mean values and SE of optical density of immunostaining from two separate experiments. P values refer to comparison between groups of injured animals at different time points and sham. *, P < 0.05; **, P < 0.01; ***, P < 0.001; †††, P < 0.001.
ELISA
The levels of soluble and insoluble Aβ1–42 in white matter of frontal lobe from sham animals was found to be relatively low (97.39 and 143.65 pmol/g, respectively). Compared to this baseline level, at 3 hours post-injury, a 2.4-fold increase in the concentration of soluble Aβ1–42 and 2.6-fold increase in the concentration of insoluble Aβ1–42 (232.67 and 369.65 pmol/g respectively) were found in frontal lobe white matter (P < 0.01). By 7 days post-injury, a 7.6-fold increase in the concentration of soluble Aβ1–42 and 7.9-fold increase in the concentration of insoluble Aβ1–42 (744.43 and 1128.99 pmol/g, respectively) were observed (P < 0.01) from tissue taken from the same brain region.
Discussion
In contrast to previously held doctrines of axonal degeneration following DAI, we found that axons continue to swell and degenerate for at least 6 months following inertial brain trauma in the pig. Immunohistochemical analysis revealed that this long-term process resulted in the accumulation of APP and Aβ primarily in the swollen ends of disconnected axons. In addition, BACE, PS-1, activated caspase-3, CCA, and kinesin were all found co-accumulating with APP and Aβ in damaged axons. Western blot analysis of white matter confirmed a total tissue increase of all of these factors following brain trauma. These data suggest that damaged axons serve as a long-term source of Aβ following brain trauma. Furthermore, extensive co-accumulation of BACE, PS-1, activated caspase-3, and CCA with APP in damaged axons suggests a unique mechanism of Aβ production within the axonal membrane compartment following trauma due to impaired transport and pathological accumulation.
It is well recognized that axonal injury is one of the most common and important pathological features of brain trauma.24–28 However, based on seminal characterizations of DAI in humans, it is generally believed that axonal swelling and disconnection is a limited event following traumatic brain injury, persisting for no more than a few months.26 Here, we found that axons continue to swell and disconnect for at least 6 months following trauma. It remains unclear why axons fail after such a protracted time following brain trauma. Possible mechanisms include delayed neuron death with deafferentation. However, we only found limited evidence of extensive neuron death following brain trauma in the pig. Alternatively, axons damaged at the time of brain trauma may continue to function relatively normally until they are exposed to an additional stress, such as persistent post-traumatic inflammatory changes that have been previously noted in a rodent model of brain trauma.29 Regardless of the mechanisms of rapid and long-term axonal damage, impaired axonal transport may represent a watershed event leading to the production and/or accumulation of toxic proteins, peptides, and their aggregates following trauma.
Failed axonal transport is increasingly being implicated in neurodegenerative disease mechanisms from AD to tauopathies and beyond.30,31 Likewise, our data suggests that impaired axonal transport due to trauma can induce both rapid and long-term accumulations of several key axonal proteins that normally do not encounter each other in such high concentrations. Although these proteins are usually transported in distinct cargoes, their pathological concentration in DAI may lead to abnormal proteolysis and aggregation.
With lysis or leakage of the swollen axons, protein accumulations would be released into the surrounding tissue and cerebrospinal fluid, potentially initiating further pathology. This process may partially account for recent reports describing large increases in Aβ peptides in the CSF of brain-injured patients.32,33 Conceivably, released axonal Aβ could aggregate in the tissue parenchyma, resulting in the formation of Aβ plaques, which have been found in large numbers within days following brain trauma in humans.7,8 Although a similar process may occur over years in spontaneous neurodegenerative diseases in humans, it is accelerated as a consequence of DAI, potentially due to the massive number of axons that are acutely injured.
In the present study, at 6 months following injury in the pig, we also found Aβ plaques based on both immunohistochemical evidence and staining for Congo red and Thioflavin S. However, the total number of plaques remained relatively low, similar to that found at early post-injury time points.13 Thus, despite a continuing source of Aβ from damaged axons, a progressive increase in plaques was not detected. This may reflect a persistent mechanism for the removal of Aβ from the tissue following brain trauma in pigs. There may be either turnover of Aβ plaques over time and/or elimination of Aβ from swollen axons before plaque formation, which would limit the number of plaques found at any post-injury time point.
Although we did not find any direct evidence of Aβ plaque turnover, we did observe a potential mechanism of Aβ removal from the tissue. We found that brain trauma induced long-term microgliosis and that the microglia were consistently found in close proximity with axonal pathology. Moreover, the microglia were immunoreactive for Aβ even by 6 months following brain trauma. While this observation may represent microglial phagocytosis of Aβ from axons or plaques, this process remains to be determined. It has long been suspected that accumulated APP in damaged axons could provide ample substrate for Aβ production.18,34,35 Indeed, immunohistochemical detection of APP accumulation in axonsthroughout the white matter has become a standard method to identify DAI in human brains.36–40 However, axonal Aβ has only recently been identified in DAI in humans and pigs, as well as in rat models of head impact.13,14,21,41
Here, we found that Aβ accumulation in swollen axons persists even six months following injury throughout the subcortical white matter and basal ganglia. At all time points examined, Aβ staining was typically identified in the discrete swellings at the terminal ends of disconnected axons, but not in elongated varicose axonal swellings that were otherwise visualized with APP staining and other transported proteins. Accordingly, impaired axonal transport alone does not appear to account for the accumulation of Aβ, since Aβ was not identified in all axons where other proteins are found accumulating. Rather, axon disconnection may favor processes that lead to the production of Aβ within the axonal membrane compartment, such as enhanced proteolysis of APP.
Intra-axonal production of Aβ following trauma is suggested here by co-accumulation of Aβ in damaged axons with key modulators of Aβ formation in AD, BACE, and PS-1. Additionally, we identified corresponding increases of BACE and PS-1 in white matter homogenates at all time points up to 6 months following brain trauma in the pig. Transmembrane BACE cleaves APP to produce a 99-residue C-terminus fragment (C99).11,17 In turn, PS-1 is a catalytic component of γ-secretase, which proteolyzes the transmembrane domain of C99 to produce 4 kd Aβ peptides.17 Although the axonal membrane compartment is not a typical location proposed for Aβ formation in AD, a recent study identified intra-axonal proteolytic processing of APP to Aβ mediated by BACE and PS-1 in peripheral nerves.15 This study further demonstrated that APP functions as a kinesin-I membrane receptor, remarkably mediating fast axonal transport of BACE and PS-1. Here, we also found extensive co-accumulation of kinesin with APP, BACE, and PS-1 in axonal bulbs. Thus, transport interruption in damaged axons can lead to pathological accumulation of kinesin, APP and the secretases known to proteolyze APP to Aβ. We have yet to find direct evidence of the role of accumulating BACE and PS-1 in damaged axons. However, our observation that axons are a primary location of Aβ accumulation suggests localized processing of APP within the axonal membrane compartment. If proven, this would represent a fundamentally novel process of traumatic brain injury.
Another potential means of APP proteolysis following injury is caspase activation. Caspase-3 is the predominant caspase involved in APP cleavage,42–44 consistent with its marked elevation in dying neurons of AD brains and co-localization of its APP cleavage products with Aβ in senile plaques. Stone and colleagues41 have recently observed caspase-mediated APP cleavage products co-accumulating with Aβ in damaged axons in a rat model of brain impact. In the present study, we found long-term activated caspase-3 and accumulation of caspase-mediated APP cleavage products co-localizing with Aβ in damaged axons up to at least 6 months following injury. Likewise, large increases in the levels of activated caspase-3 and caspase-mediated APP cleavage products were found in the white matter following injury. Caspases have been suggested to contribute to the complex proteolytic processing of APP to Aβ peptides by cleaving APP at predominantly (P4) VEVD 720 (P1)/A, which may corrupt the normal intracellular processing of APP.43 This process may provide one mechanism whereby intra-axonal APP could be processed to form Aβ following injury. However, cleavage at this site does not directly produce amyloidigenic Aβ peptides, Aβ1–40 and Aβ1–42, and there is current debate whether further intracellular processing of caspase-mediated APP cleavage products ultimately results in amyloidigenic Aβ production.45,46
In all, persistent accumulation of Aβ in axons due to impaired transport following traumatic brain injury suggests a long-lasting pathological process (Figure 11). Although the primary axonal location of Aβ formation and accelerated rate of production may be unique to brain trauma, our present data suggests that the processing of APP shares similarities with Aβ formation in AD. Ongoing release of this large pool of axonal Aβ following injury may contribute to long-term Aβ plaque formation and toxicity. Ultimately, this process could play a role in the observed link between a history of brain trauma and an increased risk of developing AD.
Figure 11.
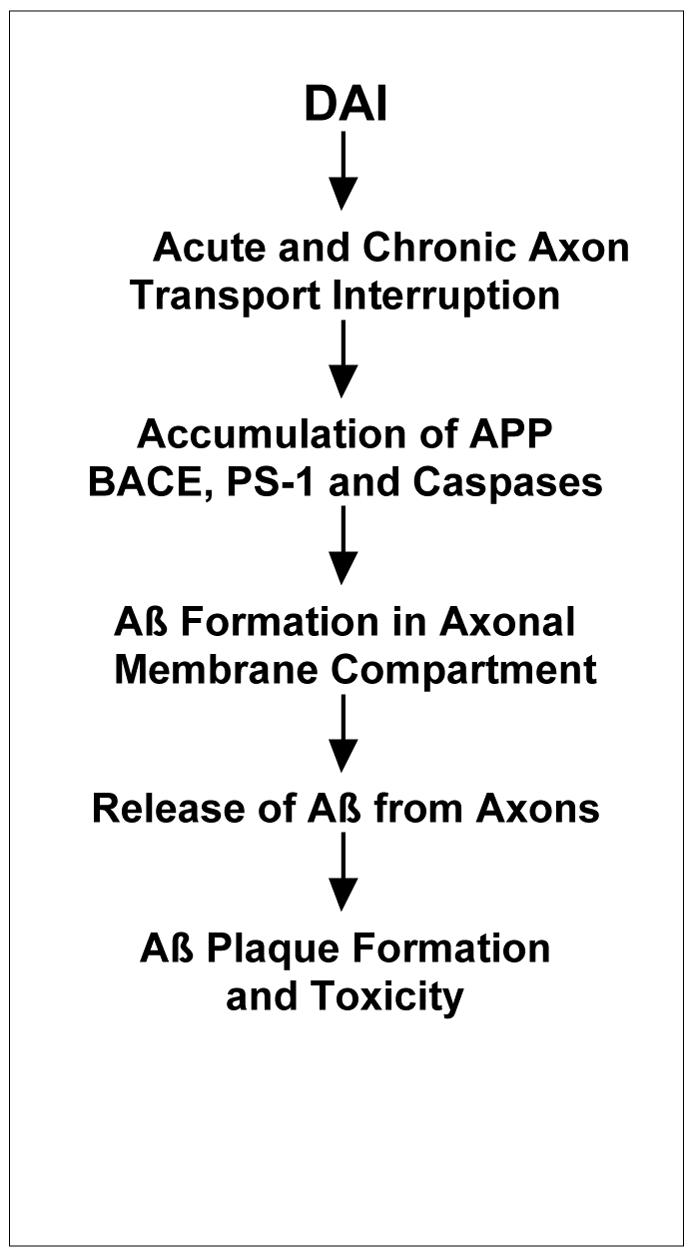
Proposed pathway of Aβ production and dispersal in diffuse axonal injury.
Acknowledgments
We thank Dr. V.M.-Y Lee for supplying antibodies.
Footnotes
Address reprint requests to Douglas H. Smith, M.D., Department of Neurosurgery, University of Pennsylvania, 105c Hayden Hall, 3320 SmithWalk, Philadelphia, PA 19104-6316. E-mail:smithdou@mail.med.upenn.edu.
Supported by National Institutes of Health (NIH) grants NS38104 and NS08803 (to D.H.S.) and AG11542 (to J.Q.T.).
References
- Rasmusson DX, Brandt J, Martin DB. Head injury as a risk factor in Alzheimer’s disease. Brain Inj. 1995;9:213–219. doi: 10.3109/02699059509008194. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Tang M, Marder K. Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemetz PN, Leibso C, Naessens JM. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips BA, Gau BA, Welsh-Bohmer KA, Burke JR, Guralink JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease dementias. Neurology. 2000;8:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, Litvan I. Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer’s disease. Eur J Neurol. 2001;8:707–710. doi: 10.1046/j.1468-1331.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI. β A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- Graham DI, Gentleman SM, Lynch A, Roberts GW. Distribution of β-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol. 1995;21:27–34. doi: 10.1111/j.1365-2990.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. In search of γ-secretase: presenilin at the cutting edge. Proc Natl Acad Sci USA. 2000;97:5690–5692. doi: 10.1073/pnas.97.11.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;22:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Nunan J, Small DH. Regulation of APP cleavage by α-, β-, and γ-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, Leoni MJ, Xu BN, Wolf JA, Meaney DF. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:982–992. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Iwata A, Graham DI. Amyloid-β accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases: new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Pierce JE, Trojanowski JQ, Graham DI, Smith DH, McIntosh TK. Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and β-amyloid peptide after experimental brain injury in the rat. J Neurosci. 1996;16:1083–1090. doi: 10.1523/JNEUROSCI.16-03-01083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Pierce JE, Wolf JA, Trojanowski JQ, Graham DI, McIntosh TK. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997A;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Xu BN, McIntosh TK, Gennarelli TA, Meaney DF. Characterization of diffuse axonal pathology and selective hippocampal damage following inertial brain trauma in the pig. J Neuropathol Exp Neurol. 1997B;56:822–834. [PubMed] [Google Scholar]
- Iwata A, Chen XH, McIntosh TK, Browne KD, Smith DH. Long-term accumulation of amyloid-β in axons following brain trauma without persistent up-regulation of amyloid precursor protein genes. J Neuropathol Exp Neurol. 2002;61:1056–1068. doi: 10.1093/jnen/61.12.1056. [DOI] [PubMed] [Google Scholar]
- Meaney DF, Bean N, Shreiber D, Miller R, Smith DH, Ross DT, Gennarelli TA. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. 1995;4:689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- Felix B, Léger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP, Duclos A, Fort F, Gougis S, Costa M, Duclos N. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–138. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Chen XH, Meaney DF, Xu BN, Nonaka M, McIntosh TK, Wolf JA, Saatman KE, Smith DH. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:588–596. doi: 10.1097/00005072-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP. Fate of reactive axonal swellings induced by head injury. Lab Invest. 1985;52:540–552. [PubMed] [Google Scholar]
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Adams JH, Graham DI, Gennarelli TA, Maxwell WL. Diffuse axonal injury in non-missile head injury. J Neurol Neurosurg Psychiatry. 1991;54:481–483. doi: 10.1136/jnnp.54.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Meaney DF. Axonal damage in traumatic brain injury. Neuroscientist. 2000;6:483–495. [Google Scholar]
- Nonaka M, Chen XH, Pierce JE, Leoni MJ, McIntosh TK, Wolf JA, Smith DH. Prolonged activation of NF-κB following traumatic brain injury in rats. J Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Lee VM, Trojanowski JQ. Tau and axonopathy in neurodegenerative disorders. Neuromolecular Med. 2002;2:131–150. doi: 10.1385/NMM:2:2:131. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer’s disease. NeuroMolecular Med. 2002;2:89–100. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- Raby CA, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, Mehta PD, Spiegel K, Kuo YM, Roher AE, Emmerling MR. Traumatic brain injury increases β-amyloid peptide 1–42 in cerebrospinal fluid. J Neurochem. 1998;7:2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- Emmerling MR, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, Mehta PD, Spiegel K, Kuo YM, Roher AE, Raby CA. Traumatic brain injury elevates the Alzheimer’s amyloid peptide A β 42 in human CSF: a possible role for nerve cell injury. Ann NY Acad Sci. 2000;903:118–122. doi: 10.1111/j.1749-6632.2000.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Kraydieh S, Green EJ, Dietrich WD. Temporal and regional patterns of axonal damage following traumatic brain injury: a β-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol. 1997;56:1132–1141. doi: 10.1097/00005072-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Lewen A, Li GL, Nilsson P, Olsson Y, Hillered L. Traumatic brain injury in rat produces changes of β-amyloid precursor protein immunoreactivity. Neuroreport. 1995;26:357–360. doi: 10.1097/00001756-199501000-00032. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. β-amyloid precursor protein (β APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- McKenzie KJ, McLellan DR, Gentleman SM, Maxwell WL, Gennarelli TA, Graham DI. Is β-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol. 1996;92:608–613. doi: 10.1007/s004010050568. [DOI] [PubMed] [Google Scholar]
- Lambri M, Djurovic V, Kibble M, Cairns N, Al-Sarraj S. Specificity and sensitivity of βAPP in head injury. Clin Neuropathol. 2001;20:263–271. [PubMed] [Google Scholar]
- Gorrie C, Oakes S, Duflou J, Blumbergs P, Waite PM. Axonal injury in children after motor vehicle crashes: extent, distribution, and size of axonal swellings using β-APP immunohistochemistry. J Neurotrauma. 2002;19:1171–1182. doi: 10.1089/08977150260337976. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for β-amyloid precursor protein. Acta Neuropathol (Berl) 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid β peptide in traumatic axonal injury. J Neurotrauma. 2002;19:601–614. doi: 10.1089/089771502753754073. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, Durrwang U, Reinhard FB, Schuckert O, Evin G, Masters CL. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J Biol Chem. 1999;26:5823–5829. doi: 10.1074/jbc.274.9.5823. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-β precursor protein and amyloidogenic A β peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Uetsuki T, Kuwako K, Hara T, Kawakami T, Aimoto S, Yoshikawa K. Cell death induced by a caspase-cleaved transmembrane fragment of the Alzheimer amyloid precursor protein. Cell Death Differ. 2002;9:199–208. doi: 10.1038/sj.cdd.4400931. [DOI] [PubMed] [Google Scholar]
- Soriano S, Lu DC, Chandra S, Pietrzik CU, Koo EH. The amyloidogenic pathway of amyloid precursor protein (APP) is independent of its cleavage by caspases. J Biol Chem. 2001;276:29045–29050. doi: 10.1074/jbc.M102456200. [DOI] [PubMed] [Google Scholar]
- Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J Neurochem. 2002;82:283–294. doi: 10.1046/j.1471-4159.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg B, Davis K, Wallace W, Lieberburg I, Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988;11:525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Hilbich C, Monning U, Grund C, Masters CL, Beyreuther K. Amyloid-like properties of peptides flanking the epitope of amyloid precursor protein-specific monoclonal antibody 22C11. J Biol Chem. 1993;15:26571–26577. [PubMed] [Google Scholar]
- Barnes NY, Li L, Yoshikawa K, Schwartz LM, Oppenheim RW, Milligan CE. Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motoneurons. J Neurosci. 1998;1:5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc Natl Acad Sci USA. 1996;9:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y, Saito N, Fujii A, Yokotani J, Takakura T, Nishimura T, Esaki H, Yamada T. A pH-dependent conformational transition of Aβ peptide and physicochemical properties of the conformers in the glial cell. Biochem J. 2002;1:547–556. doi: 10.1042/0264-6021:3610547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Tanzi RE, Marzloff K, Barbour R, Schenk D. Kunitz protease inhibitor-containing amyloid β protein precursor immunoreactivity in Alzheimer’s disease. J Neuropathol Exp Neurol. 1992;51:76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Balass M, Solomon B. N-terminal EFRH sequence of Alzheimer’s β-amyloid peptide represents the epitope of its anti-aggregating antibodies. J Neuroimmunol. 1998;1:85–90. doi: 10.1016/s0165-5728(98)00098-8. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Cacabelos R. β-amyloid (1–40)-induced neurodegeneration in the rat hippocampal neurons of the CA1 subfield. Acta Neuropathol (Berl) 1998;95:455–465. doi: 10.1007/s004010050825. [DOI] [PubMed] [Google Scholar]
- Jung SS, Gauthier S, Cashman NR. β-amyloid precursor protein is detectable on monocytes and is increased in Alzheimer’s disease. Neurobiol Aging. 1999;20:249–257. doi: 10.1016/s0197-4580(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Arai T, Akiyama H, Ikeda K, Kondo H, Mori H. Immunohistochemical localization of amyloid β-protein with amino-terminal aspartate in the cerebral cortex of patients with Alzheimer’s disease. Brain Res. 1999;27:202–206. doi: 10.1016/s0006-8993(98)01366-3. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;17:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh K, Cole GM, Yang F, Savage MJ, Greenberg BD, Gentleman SM, Graham DI, Nicoll JA. β-amyloid (Aβ) 42(43), aβ42, aβ40, and apoE immunostaining of plaques in fatal head injury. Neuropathol Appl Neurobiol. 2000;26:124–132. doi: 10.1046/j.1365-2990.2000.026002124.x. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Kawooya JK, Pinsker LP, Emmons T, Mistretta S, Siman R, Greenberg BD. elevated Aβ levels in Alzheimer’s disease brain is associated with selective accumulation of Aβ42 in parenchymal amyloid plaques and both Aβ40 and Aβ42 in cerebrovascular deposits. Amyloid: Int J Exp Clin Invest. 1995;2:234–240. [Google Scholar]
- Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid β-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer’s disease. EMBO J. 2002;16:805–813. doi: 10.1096/fj.01-0732com. [DOI] [PubMed] [Google Scholar]
- Yang F, Mak K, Vinters HV, Frautschy SA, Cole GM. Monoclonal antibody to the C-terminus of β-amyloid. Neuroreport. 1994;27:2117–2120. doi: 10.1097/00001756-199410270-00032. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Forno LS, Higgins L, Scardina JM, Eng LF, Cordell B. Development of a monoclonal antibody specific for the COOH-terminal of β-amyloid 1–42 and its immunohistochemical reactivity in Alzheimer’s disease and related disorders. Am J Pathol. 1994;144:1082–1088. [PMC free article] [PubMed] [Google Scholar]
- Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral, and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A β 42(43) and A β 40 in senile plaques with end-specific A β monoclonals: evidence that an initially deposited species is A β 42(43). Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, Sawamura N, Odaka A, Suzuki N, Mizusawa H, Shoji S, Mori H. Amyloid β protein 1–42/43 (A β 1–42/43) in cerebellar diffuse plaques: enzyme-linked immunosorbent assay and immunocytochemical study. Brain Res. 1995;8:151–156. doi: 10.1016/0006-8993(95)00162-j. [DOI] [PubMed] [Google Scholar]
- Howland DS, Savage MJ, Huntress FA, Wallace RE, Schwartz DA, Loh T, Melloni RH, Jr, DeGennaro LJ, Greenberg BD, Siman R. Mutant and native human β-amyloid precursor proteins in transgenic mouse brain. Neurobiol Aging. 1995;16:685–699. doi: 10.1016/0197-4580(95)00078-s. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Howland DS, Trusko SP, Savage MJ, Lang DM, Greenberg BD, Siman R, Scott RW. Enhanced amyloidogenic processing of the β-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer’s disease mutations and a “humanized” Aβ sequence. J Biol Chem. 1996;20:23380–23388. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- Shaw G, Osborn M, Weber K. Reactivity of a panel of neurofilament antibodies on phosphorylated and dephosphorylated neurofilaments. Eur J Cell Biol. 1986;42:1–9. [PubMed] [Google Scholar]
- Harris J, Ayyub C, Shaw G. A molecular dissection of the carboxyterminal tails of the major neurofilament subunits NF-M and NF-H. J Neurosci Res. 1991;30:47–62. doi: 10.1002/jnr.490300107. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;23:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;1:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Li J, Xu M, Zhou H, Ma J, Potter H. Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell. 1997;5:917–927. doi: 10.1016/s0092-8674(00)80356-6. [DOI] [PubMed] [Google Scholar]
- Wen PH, Friedrich VL, Jr, Shioi J, Robakis NK, Elder GA. Presenilin-1 is expressed in neural progenitor cells in the hippocampus of adult mice. Neurosci Lett. 2002;25:53–56. doi: 10.1016/s0304-3940(01)02485-5. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Richards B, Brady ST. Regulation of kinesin: implications for neuronal development. Dev Neurosci. 2001;23:364–376. doi: 10.1159/000048720. [DOI] [PubMed] [Google Scholar]
- Vancoillie G, Lambert J, Mulder A, Koerten HK, Mommaas AM, Van Oostveldt P, Naeyaert JM. Kinesin and kinectin can associate with the melanosomal surface and form a link with microtubules in normal human melanocytes. J Invest Dermatol. 2000;114:421–429. doi: 10.1046/j.1523-1747.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Schnapp B, Inouye H, Neve RL. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990;25:3278–3283. [PubMed] [Google Scholar]
- Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]