Abstract
Drug tolerability affects compliance. We evaluated the tolerability levels of azithromycin (750-mg loading dose plus 250 mg/day; n = 148 subjects), doxycycline (100 mg/day; n = 75), and placebo (n = 77) as prophylaxis against malaria in Indonesian adults over 20 weeks. Self-reported and elicited symptoms, health perception, hearing, hematology, and biochemistry were assessed. The loading dose was well tolerated. The frequencies (number per person-years [p-yr]) of all daily reported symptoms were similar in the three arms of the study: 40.2/p-yr for azithromycin, 39.7/p-yr for doxycycline, and 38.2/p-yr for placebo. Relative to those who received placebo, azithromycin recipients complained more often of heartburn (rate ratio = 10.5 [95% confidence interval, 2.8 to 88.1]), paresthesia (2.03 [1.08 to 4.24]), and mild (1.55 [1.01 to 2.48]) and severe (11.2 [1.34 to ∞]) itching but less often of fever (0.21 [0.09 to 0.49]) and tinnitus (0.09 [0.04 to 0.21]). Azithromycin recipients showed no evidence of clinical hearing loss or hematologic, hepatic, or renal toxicity. One azithromycin recipient developed an erythematous rash. Daily azithromycin was well tolerated by these Indonesian adults during 20 weeks of treatment.
Prolonged drug use for disease prophylaxis, treatment, or suppression should be well tolerated. This is especially true for healthy recipients of prophylaxis, such as travelers and pregnant women, for whom the risk-to-benefit ratio requires both safe and easily tolerable regimens.
Azithromycin has several clinical indications that include the treatment and prophylaxis of Mycobacterium avium-M. intracellulare complex (MAC) infection and the treatment of community-acquired pneumonia and genital or ocular Chlamydia trachomatis infection (25). The standard doses of azithromycin (30 mg/kg of body weight for children and 1.5 g total dose for adults) are well tolerated. Up to 12% of patients report any symptom, <10% report gastrointestinal symptoms, and 0.7 to 1.3% discontinue therapy. Such tolerability compares favorably to that of other antibiotics (7, 11, 12, 22). High doses of azithromycin, 1 g/kg for adults and 20 mg/kg for children, are also well tolerated (2, 16). However, doses used for MAC infections (1.2 g/week or 300 to 600 mg/day) are less well tolerated (3, 9, 10, 17). Gastrointestinal symptoms are reported by a high proportion of patients (e.g., by 71 [78.9%] of 85 recipients of weekly azithromycin, resulting in six [7%] withdrawals [17]). Hearing loss in the speech frequency range has also been reported in 13 to 17% of patients with MAC infections. It is dose dependent and recovers within 2 to 11 weeks after discontinuation of the drug (3, 9, 23, 24). Irreversible high-frequency-hearing loss has also been reported following treatment with 750 mg of oral azithromycin in a 37-year-old woman (19). Biochemical and hematological assessments in large clinical series have been unremarkable. Mild elevations of liver enzymes (0.3 to 1.7%) and transient neutropenia (1.5%) or neutrophilia (1.5%) have been documented (11). Significant hepatotoxicity (e.g., hypersensitivity hepatitis, cholestasis) is rare (4, 15), as are anaphylaxis, pseudomembranous colitis, erythema multiforme, and Churg-Strauss syndrome (12, 13, 22).
One trial has previously assessed azithromycin as prophylaxis against malaria. Azithromycin was administered daily (250 mg) or weekly (1 g) for 13 weeks to Kenyan adults. The reported symptoms were similar to those of placebo recipients, and there was no evidence of toxicity on routine hematological or biochemical testing (1).
We report the tolerability of daily-administered azithromycin as prophylaxis against malaria in Indonesian adults.
MATERIALS AND METHODS
Subjects and study conduct.
This double-blind, placebo-controlled trial assessed the prophylactic efficacy and tolerability of azithromycin in men and women who had been radically cured (with concurrent quinine doxycycline and primaquine) of any preexisting malaria infection. Details of the study have been reported previously (21). Three hundred Indonesian adults (225 soldiers and 75 villagers) received either (i) azithromycin (750-mg loading dose followed by 250 mg/day) plus a doxycycline placebo (n = 148) (arm A), (ii) doxycycline (100 mg/day) plus an azithromycin placebo (n = 75) (arm D), or (iii) a double placebo (n = 77) (arm P). At the time of witnessed drug consumption, drinking water was provided and sweet biscuits were offered.
This study was conducted in accordance with the Indonesian Ministry of Health, the Indonesian Army, the U.S. Navy, the U.S. Army, and the U.S. Food and Drug Administration regulations governing the protection of human subjects in medical research. All subjects in this study gave informed consent.
Assessment and analysis of AEs.
An adverse event (AE) was defined as a new symptom, physical sign, or illness that developed during the study; AEs were classified as mild (no interference in activities of daily living), moderate (affecting activities of daily living; treatment required), or severe (bed rest or hospital admission required). Twenty-five common symptoms and less common, “other,” symptoms were recorded from two sources: (i) questionnaires administered on days 0 and 1 (loading dose tolerance) and monthly thereafter and (ii) volunteered responses from the subjects to the question asked daily, “Any symptoms?” A health questionnaire was completed at the end of the study. Hearing was assessed (Rinné and Weber tests) at enrollment and at the end of the study. Routine hematology and biochemistry were evaluated at enrollment, at week 4, and at the end of the study.
The sample size was based on the objective of estimating the prophylactic efficacy of azithromycin to rule out a prophylactic efficacy of <70% (80% power, 5% type 1 error, 1 sided) (6). Chi-square analysis and Koopman's method were used to assess differences of independent proportions (14). Incidence rates of daily reported symptoms were compared by using the exact conditional test (5). Days were subtracted if subjects were absent from questioning and/or if they developed malaria (i.e., the 7 days before the day of the positive slide result were subtracted). Laboratory data were analyzed by using analysis of variance or the t test (unpaired or paired), as appropriate. Many comparisons were performed; therefore, in Table 1, we decided to show only the results with P values of ≤0.05 and 95% confidence intervals for or rate ratio [RR] measures that exclude 1.
TABLE 1.
Incidence rates per person-year by drug arm (azithromycin, doxycycline, and placebo) for all symptoms voluntarily reported daily over the 20 weeks of the trial and significant RR comparisonsa
| Symptom | Azithromycin symptoms (rate)b | A vs P RR (95% CIc) | Placebo symptoms (rate)c | D vs P RR (95% CI) | Doxycycline symptoms (rate)b |
|---|---|---|---|---|---|
| All | 1,525 (40.22) | 416 (38.22) | 852 (39.75) | ||
| Gastrointestinal | 323 (8.51) | 104 (9.55) | 161 (7.51) | ||
| Anorexia | 104 (2.74) | 39 (3.58) | 0.56 (0.35-0.89) | 43 (2.0) | |
| Nausea | 53 (1.39) | 19 (1.74) | 35 (1.63) | ||
| Vomiting | 11 (0.29) | 6 (0.55) | 5 (0.23) | ||
| Heartburnd | 73 (1.92) | 10.48 (2.79-88.14) | 2 (0.18) | 6.35 (1.58-55.3) | 25 (1.17) |
| Mild abdominal pain | 43 (1.13) | 18 (1.65) | 25 (1.17) | ||
| Severe abdominal pain | 2 (0.05) | 0 (0) | 10.7 (1.14-∞)e | 10 (0.47)f | |
| Diarrhea | 34 (0.89) | 18 (1.65) | 18 (0.84) | ||
| Diarrhea, >5/day | 3 (0.08) | 2 (0.18) | 0 (0) | ||
| Central nervous system | 492 (12.98) | 151 (13.88) | 278 (12.97) | ||
| Mild headache | 227 (5.98) | 64 (5.88) | 119 (5.55) | ||
| Severe headache | 14 (0.37) | 8 (0.73) | 7 (0.33) | ||
| Dizzinessg | 100 (2.63) | 19 (1.75) | 2.06 (1.23-3.6) | 77 (3.59) | |
| Tinnitus | 9 (0.24) | 0.10 (0.04-0.21) | 27 (2.48) | 0.13 (0.05-0.31) | 7 (0.33) |
| Hearing loss | 9 (0.24) | 5 (0.46) | 6 (0.28) | ||
| Blurred vision | 6 (0.16) | 4 (0.37) | 7 (0.33) | ||
| Paresthesiad | 78 (2.06) | 2.04 (1.08-4.24) | 11 (1.01) | 0.23 (0.06-0.72) | 5 (0.23) |
| Difficulty sleeping | 49 (1.3) | 13 (1.2) | 1.91 (1.02-3.84) | 49 (2.29)f | |
| Hallucinations | 0 (0) | 0 (0) | 1 (0.05) | ||
| Dermatological | 160 (4.22) | 1.84 (1.20-2.92) | 25 (2.3) | 1.65 (1.04-2.69) | 81 (3.78) |
| Mild itching | 135 (3.56) | 1.55 (1.01-2.48) | 25 (2.3) | 75 (3.49) | |
| Severe itchingd | 19 (0.5) | 11.2 (1.34-∞)e | 0 (0) | 2 (0.09) | |
| Rash | 6 (0.16) | 0 (0) | 4 (0.19) | ||
| Miscellaneous | 207 (5.46) | 71 (6.52) | 144 (6.72) | ||
| Cough | 143 (3.77) | 43 (3.95) | 94 (4.39) | ||
| Fever | 11 (0.29) | 0.21 (0.09-0.49) | 15 (1.38) | 0.37 (0.15-0.87) | 11 (0.51) |
| Chills | 10 (0.26) | 2 (0.18) | 5 (0.23) | ||
| Sweats | 10 (0.26) | 4 (0.37) | 13 (0.60) | ||
| Myalgia | 33 (0.87) | 7 (0.64) | 21 (0.98) | ||
| Others | 343 (9.05) | 1.51 (1.16-2.01) | 65 (5.97) | 1.47 (1.1-1.98) | 188 (8.77) |
Person-years of follow-up were 37.91 (arm A), 10.88 (arm P), and 21.43 (arm D).
Values are total numbers of symptoms reported, with incidence rates per person-year given in parentheses.
95% CI, 95% confidence interval.
A versus D: heartburn RR = 1.6 (95% CI, 1.04-2.7), paresthesia RR = 8.8 (3.6-27.9), and itching (severe) RR = 5.3 (1.3-47.5).
A value of 0.5 was added to the numerator and denominator symptom counts to estimate the RR if the denominator count was 0; 95% confidence intervals were calculated by using the observed data (no adjustment was made to the numerator or denominator).
D versus A: abdominal pain (severe) RR = 8.85 (95% CI, 1.89-83.03), and difficulty sleeping RR = 1.77 (1.17-2.68).
Dizziness and/or muzzy head.
RESULTS
The majority of the subjects (286 of 300 [95.3%]) were young males (mean age, 27 years). Enrollment characteristics were similar in the three arms (21). All subjects had normal Rinné tests, but one (in arm A) had Weber lateralization. The median (range) follow-up times in weeks for the different arms were as follows: (i) azithromycin, 14.6 (1.3 to 20.3); (ii) doxycycline, 15.7 (5.1 to 20.3); and (iii) placebo, 7 (0.7 to 18.9). Approximately 97% of all drug doses ingested were witnessed (26,041 of 26,857).
There were eight AEs that necessitated study withdrawal. After 4 weeks of prophylaxis, a 26-year-old soldier on azithromycin developed a widespread, pruritic, erythematous, maculopapular rash, giving a risk of 0.67% (95% confidence interval, 0.034 to 3.29). This moderate AE was considered azithromycin induced. The other seven AEs were all unrelated to the study drugs: ureteric colic, dengue fever, and a motorcycle accident (in arm A); acute bronchitis with hyperventilation and subarachnoid hemorrhage (in arm D); and headache with photophobia and severe malaria (in arm P).
Loading-dose tolerance and monthly reported symptoms.
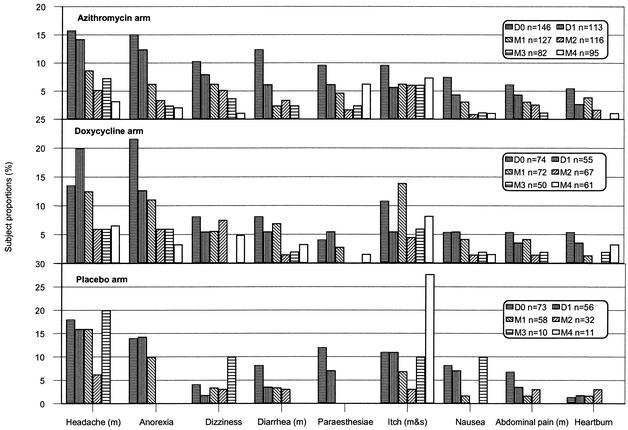
The proportions of subjects who, in response to the symptom questionnaire, reported either a new symptom (i.e., a symptom not present on day 0) or any symptom on day 1 were similar among all three arms (details not shown). Thereafter, several symptoms appeared to show a downward trend in the azithromycin and doxycycline arms, whereas itching appeared to show an upward trend in all three arms (Fig. 1). Discerning trends in the placebo arm is problematic because of the high rate of withdrawal from the study.
FIG. 1.
Trends in selected symptoms as reported by azithromycin, doxycycline, and placebo recipients on a symptom questionnaire on days 0 and 1 (D0 and D1) and at monthly intervals (M1 through M4) during a malaria prophylaxis study in Papua. These elicited symptoms represent an alternative data set to that of the daily volunteered symptoms presented in Table 1. m, moderate; m&s, mild and severe.
Self-reported daily symptoms over 20 weeks: rate comparisons
The incidence rates of any self-reported symptoms ranged from 0 to 9.05 symptoms per person-year (p-yr) (a maximum of 9 days over 1 year) (Table 1). Azithromycin recipients reported more heartburn, paresthesia, and severe itching, and doxycycline recipients reported more severe abdominal pain and difficulty sleeping. Subjective hearing loss in participants in any arm was reported with a frequency of <0.5/p-yr.
Self-reported daily symptoms within the first 4 weeks. (i) Proportional comparisons.
The percentages of subjects reporting any symptom at least once were similar in the three arms: 58.8% (arm A), 66.2% (arm P), and 61.3% (arm D). Among all the comparisons, only heartburn was an important symptom: (i) arm A versus arm P, 14 of 148 (9.5%) versus 0 of 77 (0%) (P = 0.005), and (ii) arm D versus arm P, 7 of 75 (9.3%) versus 0 of 77 (0%) (P = 0.006). Subjective hearing loss was reported by nine (3%) participants equally distributed across the three arms (χ2 [2 df] = 1.1; P = 0.57).
(ii) Rate comparisons.
The incidence rates of any self-reported symptom ranged from 0 to 7.8/p-yr. The incidences of symptoms per person-year for each arm were 35.8/p-yr (arm A), 37.5/p-yr (arm P), and 38.8/p-yr (arm D). The important symptoms in the azithromycin recipients, versus those in the placebo recipients, were heartburn (2.41 versus 0/p-yr; RR = 25.7 [95% confidence interval, 3.19 to ∞]) and myalgia (1.70 versus 0.19/p-yr; RR = 8.88 [1.41 to 368.9]). For doxycyline recipients, the important symptoms were heartburn (2.26 versus 0/p-yr, RR = 24.6 [2.77 to ∞]), myalgia (1.56 versus 0.19/p-yr, RR = 8.19 [1.14 to 358.8]), and difficulty sleeping (6.26 versus 1.91/p-yr, RR = 3.28 [1.59 to 7.40]). Subjective hearing loss in participants in any arm was reported with a frequency of <0.53/p-yr.
Health questionnaire and hearing at study end.
The health questionnaire was answered by 257 of 300 (85.6%) subjects. The majority of subjects (94.9% [244 of 257]) reported feeling healthier than they had felt at the start of the study. The proportions of subjects who recollected experiencing nausea and/or vomiting, diarrhea, or hearing impairment at any time during the study were 25.3% (65 of 257), 23.3% (60 of 257), and 5.1% (13 of 257), respectively; all proportions were not significantly different between the three arms (details not shown). Weber lateralization was detected in 7 (2.7%) of 262 subjects: 3 of 132 (2.3%) in arm A, 2 of 64 (3.1%) in arm D, and 2 of 70 (2.8%) in arm P (χ2 [2 df] = 0.14; P = 0.57). All had normal Weber tests at enrollment.
Laboratory evaluations.
Mean biochemical and hematological values at day 0, week 4, and the end of the study and their mean changes (values at study end minus values at day 0) were not significantly different between each drug arm and placebo (details not shown). Proportions of subjects with elevated aspartate transaminase (AST) levels (>40 IU/liter) at enrollment (17 of 300 [5.6%]), 1 month later (23 of 265 [8.6%]), and at the end of the study (14 of 154 [9.1%]) were not significantly different from each other (P = 0.28). Nine (6.2%) of 145 subjects with normal day 0 AST levels had mildly elevated values at the end of the study: (i) arm A, 41 to 50 IU/liter (n = 6), and (ii) arm D, 41 to 63 IU/liter (n = 3). Total white cell counts were unremarkable during follow-up: for azithromycin recipients, ≥4,500/μl, and for doxycycline recipients, ≥4,400/μl.
DISCUSSION
Azithromycin administered daily for up to 20 weeks was well tolerated by the Indonesian adult study participants and did not appreciably affect their routine hematological or biochemical measurements. Doxycycline was also well tolerated.
Clinicians assess drug toxicity by evaluating symptoms in the context of the patient's past medical history, drug history, the local disease epidemiology, physical signs, and pertinent investigations. By contrast, clinical trials focus primarily on broad assessments involving group comparisons, and these trials benefit from blinding. We have analyzed a large amount of data over 20 weeks and considered some of the issues inherent in such an analysis. We have reported symptom data as proportions and rates. Proportions are an indication of how many subjects report symptoms within a defined period of equal risk. Rates are an indication of how often these symptoms are reported. The differential attrition in the placebo arm precluded comparisons of risk, relative risk, and symptom trends over 20 weeks; therefore, risk analysis was confined to the first 4 weeks of follow-up. Analysis by incidence rates takes into account the unequal follow-up times but cannot determine whether many symptoms are reported by few subjects or vice versa (8). The second issue involves the interpretation of results obtained from multiple statistical comparisons. Determining which symptoms are important or whether differences exist based on simple hypothesis testing is challenging because of the possibility of overreporting (type I error) or failing to detect (type II error due to adjusting P values) significant symptoms (18). Cognizant of these issues, and with the view that analysis of complex symptom data are largely exploratory, we flagged only those comparisons where the P value was ≤0.05.
The most important AE was an azithromycin-induced maculopapular rash in one subject that was classified as moderate in severity. The reported risk of a rash with azithromycin from large clinical series is low at ≤1.1% (11, 12, 22). Several other symptoms were noteworthy. Heartburn, a well-recognized effect of doxycycline (20), was also reported but at a lower frequency than in the azithromycin group. In azithromycin reports, heartburn is often not reported separately from abdominal pain, making it difficult to put our heartburn data into context. At 4 weeks, itching, paresthesia, and subjectively severe abdominal pain were unremarkable. However, over 20 weeks, itching and paresthesia were reported more frequently by subjects in the azithromycin arm (versus placebo) and severe abdominal pain was reported more frequently by subjects in the doxycycline arm (versus placebo), suggesting that these symptoms emerged gradually. At 4 weeks, myalgia was reported more frequently by subjects in both drug arms but not over 20 weeks, suggesting that it developed acutely but was tolerated over time. Hearing loss was reported infrequently. At the end of the study, signs consistent with a sensineural hearing loss were detected in a small number (2.6%) of subjects from all three arms. Despite the limitations of tuning fork hearing assessment relative to audiometry, these signs are unlikely to represent appreciable auditory nerve pathology. Azithromycin-induced hearing loss generally improves with drug discontinuation or dose reduction, but clinicians should be aware that irreversible hearing loss has been reported (19). No azithromycin recipient developed leukopenia as has been reported in other studies (4, 16). A small proportion of azithromycin recipients developed mild elevations of AST by the end of the study; the peak value was 50 IU/liter. This modest increase is of doubtful clinical significance, but we cannot exclude the possibility of a mild, drug-induced hepatitis.
Our trial evaluated azithromycin in predominantly healthy and fit young soldiers under field conditions in a tropical environment. Our findings cannot be extended with confidence to children, women, pregnant women, and patients with AIDS. The small sample size precluded the detection of rare and possibly serious side effects.
The prophylactic efficacy of azithromycin against falciparum malaria was considered too low, ∼72%, to recommend it as a first-line prophylactic agent (21). However, azithromycin's antimalarial properties could be useful in drug combinations. Tolerability data from this study support further studies with azithromycin.
Acknowledgments
We thank the following for their contribution to the study: (i) the soldiers and commanders of battalion 141 and the villagers for their participation in the study; (ii) battalion commander Januar Syamsudin for his general support; (iii) L. Hadiarso (Indonesian Army) for reviewing the protocol and for facilitating the study execution; (iv) Kunto (Indonesian Army, Papua) and Kristanto (Indonesian Navy, Papua) for facilitating the study execution; (v) M. Dunne (Pfizer) for providing study drugs; (vi) J. K. Baird, E. Gomez, I. Sumawinata, Taufik, Khotib, Khairul, Krisin, and Hartono for data collection and conduct of the clinical trial; (vii) B. Subianto (Provincial Health Office) for facilitating the study execution; and (viii) S. Hoffman (U.S. Navy) and A. Zumla (London University) for critical review of the manuscript. Pfizer Central Research (Groton, Conn.) supplied all study drugs and placebos free of charge.
This study was funded by the U.S. Army Medical Materiel Development Activity and the U.S. Naval Medical Research and Development Command (Department of Defense funding reference 65807/849/QG).
The views expressed in this paper are those of the authors and do not in any way represent those of the Indonesian Army, the Indonesian Ministry of Health, the U.S. Army, or the U.S. Navy.
REFERENCES
- 1.Andersen, S. L., A. J. Oloo, D. M. Gordon, O. B. Ragama, G. M. Aleman, J. D. Berman, D. B. Tang, M. W. Dunne, and G. D. Shanks. 1998. Successful double-blinded, randomized, placebo-controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in western Kenya. Clin. Infect. Dis. 26:146-150. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, R. L., P. Arullendran, H. C. Whittle, and D. C. W. Mabey. 1993. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet 342:453-456. [DOI] [PubMed] [Google Scholar]
- 3.Brown, B. A., D. E. Griffith, W. Girard, J. Levin, and R. J. Wallace. 1997. Relationship of adverse events to serum drug levels in patients receiving high-dose azithromycin for mycobacterial lung disease. Clin. Infect. Dis. 24:958-964. [DOI] [PubMed] [Google Scholar]
- 4.Cascaval, R. I., and D. J. Lancaster. 2001. Hypersensitivity syndrome associated with azithromycin. Am. J. Med. 25:483-484. [DOI] [PubMed] [Google Scholar]
- 5.Ederer, F., and N. Mantel. 1974. Confidence limits on the ratio of two Poisson variables. Am. J. Epidemiol. 100:165-167. [DOI] [PubMed] [Google Scholar]
- 6.Farrington, C. P., and G. Manning. 1990. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat. Med. 9:1447-1454. [DOI] [PubMed] [Google Scholar]
- 7.Ferwerda, A., H. A. Moll, W. C. Hop, J. M. Kouwenberg, C. V. Tjon Pian Gi, S. G. Robben, and R. de Groot 2001. Efficacy, safety and tolerability of 3 day azithromycin versus 10 day co-amoxiclav in the treatment of children with acute lower respiratory tract infections. J. Antimicrob. Chemother. 47:441-446. [DOI] [PubMed] [Google Scholar]
- 8.Glynn, R. J., and J. E. Buring. 1996. Ways of measuring rates of recurrent events. BMJ 312:364-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith, D. E., B. A. Brown, W. M. Girard, D. T. Murphy, and R. J. Wallace. 1996. Azithromycin activity against Mycobacterium avium complex lung disease in patients who are not infected with human immunodeficiency virus. Clin. Infect. Dis. 23:983-989. [DOI] [PubMed] [Google Scholar]
- 10.Havlir, D. V., M. P. Dube, F. R. Sattler, D. N. Forthal, C. A. Kemper, M. W. Dunne, D. M. Parenti, J. P. Lavelle, A. C. White, Jr., M. D. Witt, S. A. Bozzette, and J. A. McCutchan. 1996. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N. Engl. J. Med. 335:392-398. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins, S. 1996. Clinical toleration and safety of azithromycin. Am. J. Med. 91(Suppl. 3A):40S-45S. [DOI] [PubMed]
- 12.Hopkins, S. J., and D. Williams. 1995. Clinical tolerability and safety of azithromycin in children. Pediatr. Infect. Dis. J. 14:S67-S71. [Google Scholar]
- 13.Hubner, C., A. Dietz, W. Stremmel, A. Stiehl, and K. Andrassy. 1997. Macrolide-induced Churg-Strauss syndrome in a patient with atopy. Lancet 350:563.. [DOI] [PubMed] [Google Scholar]
- 14.Koopman, P. A. 1984. Confidence limits on the ratio of two proportions. Biometrics 40:513-517. [Google Scholar]
- 15.Longo, G., C. Valenti, G. Gandini, L. Ferrara, M. Bertesi, and G. Emilia. 1997. Azithromycin-induced intrahepatic cholestasis. Am. J. Med. 102:217-218. [PubMed] [Google Scholar]
- 16.Martin, D., T. F. Mroczkowski, Z. A. Dalu, J. McCarthy, R. B. Jones, S. J. Hopkins, and R. B. Jonson. 1992. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. N. Engl. J. Med. 327:921-925. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield, E. C., J. Fessel, M. Dunne, G. Dickinson, M. R. Wallace, W. Byrne, R. Chung, K. F. Wagner, S. F. Paparello, D. B. Craig, G. Melcher, M. Zajdowicz, R. F. Williams, J. W. Kelly, M. Zelasky, L. B. Heifets, and J. D. Berman. 1998. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double-blind, placebo-controlled multicenter trial. Clin. Infect. Dis. 26:611-619. [DOI] [PubMed] [Google Scholar]
- 18.Perneger, T. V. 1998. What's wrong with Bonferroni adjustments. BMJ 316:1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees, B. D., and E. M. Gross. 2000. Irreversible sensineural hearing loss as a result of azithromycin ototoxicity. Ann. Otol. Rhinol. Laryngol. 109:435-437. [DOI] [PubMed] [Google Scholar]
- 20.Schneider, R. 1977. Doxycycline esophageal ulcers. Am. J. Dig. Dis. 22:805-807. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, W. R. J., T. L. Richie, D. J. Fryauff, H. Picarima, C. Ohrt, D. Tang, D. Braitman, G. S. Murphy, H. Widjaja, E. Tjitra, A. Ganjar, T. R. Jones, H. Basri, and J. Berman. 1999. Malaria prophylaxis using azithromycin: a double-blind placebo-controlled trial in Irian Jaya, Indonesia. Clin. Infect. Dis 28:74-81. [DOI] [PubMed] [Google Scholar]
- 22.Treadway, G., and D. Pontani. 1996. Paediatric safety of azithromycin: worldwide experience. J. Antimicrob. Chemother. 37(Suppl. C):143-149. [DOI] [PubMed] [Google Scholar]
- 23.Tseng, A. L., L. Dolovich, and I. E. Salit. 1997. Azithromycin-related ototoxicity in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 24:76-77. [DOI] [PubMed] [Google Scholar]
- 24.Wallace, M. R., L. K. Miller, M. T. Nguyen, and A. R. Shields. 1994. Ototoxicity with azithromycin. Lancet 343:241.. [DOI] [PubMed] [Google Scholar]
- 25.Zuckerman, J. M., and K. M. Kaye. 1995. The newer macrolides. Infect. Dis. Clin. N. Am. 9:731-745. [PubMed] [Google Scholar]