Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by immune complex-mediated tissue injury. Many different adhesion molecules are thought to participate in the development of SLE; however, few studies have directly examined the contributions of these proteins. Here we demonstrate that LFA-1 plays an essential role in the development of lupus in MRL/MpJ-Faslpr mice. Mice deficient in LFA-1, but not Mac-1, showed significantly increased survival, decreased anti-DNA autoantibody formation, and reduced glomerulonephritis. The phenotype of the LFA-1-deficient mice was similar to that observed in β2 integrin-deficient (CD18-null) MRL/MpJ-Faslpr mice, suggesting a lack of redundancy among the β2 integrin family members and other adhesion molecules. These studies identify LFA-1 as a key contributor in the pathogenesis of autoimmune disease in this model, and further suggest that therapeutic strategies targeting this adhesion molecule may be beneficial for the treatment of SLE.
The primary events believed to lead to the development of vascular injury and tissue damage in systemic lupus erythematosus (SLE) patients include loss of immunological tolerance, generation of autoantibodies, immune complex formation and deposition, endothelial cell and complement activation, and leukocyte emigration and activation.1,2 A complex interplay among cytokines, chemokines, adhesion molecules, and other inflammatory mediators is thought to be necessary for disease pathogenesis.3–7 However, despite numerous investigations, few of the key molecules that mediate organ inflammation in SLE have been identified. Discovery of such molecules is essential to develop specifically targeted therapies having improved efficacy in SLE or reduced side effects.
Leukocyte and endothelial cell adhesion molecules, such as the selectins, integrins, and members of the immunoglobulin family of adhesion receptors mediate many different immune and inflammatory responses and have been implicated in the development of SLE and other inflammatory diseases.7–9 Only a small number of studies, though, have addressed the contributions of these molecules and their ligand interactions in SLE. MRL/MpJ-Faslpr (Tnfrsf6lpr) mice develop a systemic autoimmune disease with similarities to SLE.10 Our previous analyses, as well as other studies of MRL/MpJ-Faslpr mice, suggest that ICAM-1 plays important roles in the pathogenesis of inflammatory disease in this model.11–14 ICAM-1-deficient MRL/MpJ-Faslpr mice showed increased survival, as well as significant inhibition of glomerulonephritis and vasculitis compared to control MRL/MpJ-Faslpr mice.11,12 However, loss of ICAM-1 did not block the production of autoantibodies or reduce immune complex deposition in the kidneys.11,12
ICAM-1 interacts with several different ligands on leukocytes, including the β2 integrins LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18), and p150/95 (CD11c/CD18).15,16 At this time, it is not clear whether the phenotype observed in ICAM-1-deficient MRL/MpJ-Faslpr mice was because of loss of ICAM-1-dependent interactions with one or more of these receptors, and whether the β2 integrins serve additional, ICAM-1-independent roles in SLE. To address the specific functions of the β2 integrins, we generated MRL/MpJ-FasLpr mice having null mutations in CD11a, CD11b, or CD18. We found that loss of LFA-1, but not Mac-1, significantly protected mice from the development of murine lupus. LFA-1-deficient mice showed increased survival, attenuated autoantibody formation, and inhibited development of glomerulonephritis compared to Mac-1 mutants and control MRL/MpJ-FasLpr mice. CD18-deficient MRL/MpJ-FasLpr mice, which do not express any of the β2 integrins, showed a phenotype similar to that of CD11a mutants. These results strongly indicate that LFA-1 plays a dominant role in the initiation and progression of disease, and that other adhesion molecules are not able to compensate for the loss of this adhesion molecule. Furthermore, they show that LFA-1 interactions with ICAM-1, as well as other ligands, are necessary for the development of autoimmunity in this model. Finally, these findings suggest that therapies targeting LFA-1 may be beneficial for the treatment of SLE.
Materials and Methods
Generation of CD18, CD11a, and CD11b Mutant MRL-MpJ-Faslpr Mice
MRL/MpJ-Faslpr (Tnfrsf6lpr) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The CD18-, CD11a-, and CD11b-null mutations from a mixed 129/Sv × C57BL/6 strain background were sequentially backcrossed 7 to 10 generations with MRL/MpJ-Faslpr mice.17–19 Mice were genotyped by Southern blot analysis or the polymerase chain reaction to identify double-mutant mice and verify the Faslpr mutation. MRL/MpJ-Faslpr inbred mice were used as controls, and approximately equal numbers of males and females were used for all studies. Animal housing, care, and all experimental manipulations were conducted according to the Guide for the Care and Use of Laboratory Animals and with approval of the Institutional Animal Care and Use Committee.
Survival Analysis and Kidney Function
Survival analysis and kidney function tests were performed as previously described.11 Survival data were determined using Kaplan-Meier curves and analyzed by the generalized Wilcoxon test. Serum blood urea nitrogen (BUN) concentrations were determined using an automated clinical chemistry analyzer by Analytics, Inc. (Gaithersburg, MD).
Flow Cytometry
T-cell populations in lymph nodes of 12-week-old CD11b−/− and control MRL/MpJ-Faslpr mice were analyzed by flow cytometry. Cells were first stained with anti-CD4-FITC (L3T4), anti-CD8-PE (53-6.7), anti-CD3-PECy5 (145-2C11), and anti-B220-biotin (RA3-6B2), all from eBiosciences (San Diego, CA). Avidin-APC (Pharmingen, La Jolla, CA) was used to detect biotinylated anti-B220 and cells were then analyzed using a FACSCalibur (Becton Dickinson, Mountain View, CA) flow cytometer. The frequency of CD4−CD8− double-negative T cells that co-express B220 was determined in CD3-gated cells.
Histopathology and Electron Microscopy
Sex- and age-matched mutant and control mice were examined at 20 weeks of age or at death. At death specimens were taken from mice observed to be overtly ill or that died spontaneously without premonitory signs. The ages of these mice approximated the median survival age for each group. Tissue was obtained from all major organs, fixed in 70% ethanol:10% saturated formalin:20% deionized water for 24 hours, and then transferred to buffered 10% formalin. Trimmed tissue samples were processed and embedded in paraffin according to standard methods, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Duplicate 3-μm sections of kidneys were stained with periodic acid-Schiff and hematoxylin. Additional kidney tissue was fixed in glutaraldehyde, embedded in plastic, and examined ultrastructurally by transmission electron microscopy.
Renal lesions were evaluated by subjective scoring and morphometry. Scoring was done according to a modification of the method of Austin and colleagues20 by an observer blinded to the genotypes of the mice. Specific changes evaluated were glomerular cellularity (numbers of mesangial, epithelial, and capillary endothelial cells of the glomerular tuft), necrosis (nuclear fragmentation and fibrin deposition), crescent formation (including synechiae), neutrophil accumulation, capillary basement membrane changes (thickening, reduplication), sclerosis (mesangial thickening), capsular fibrosis (fibrous crescent formation, capsular sclerosis, periglomerular fibrosis), interstitial mononuclear inflammatory cell accumulation, tubular changes (atrophy, dilation), and interstitial fibrosis. Each was scored 0, 1, 2, or 3 for normal, mild, moderate, or severe, respectively. Digital images of at least 6, and up to 15, glomeruli and adjacent tubules and interstitium were evaluated from both H&E- and periodic acid-Schiff and hematoxylin-stained sections from each mouse, and equal numbers of glomeruli from superficial, mid, and deep cortex were examined. Lesion scores for each mouse were calculated as the mean of summed individual scores for each image, with scores for necrosis and crescent formation weighted by factors of 2.
Morphometry of glomeruli was done with a histomorphometry system consisting of a Leica DMR research microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany), SPOT RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI), and Image Pro Plus v4 image analysis software (Media Cybernetics, Silver Spring, MD). The area of each glomerulus, including crescents and capsular abnormalities, if present, were determined, and the color segmentation feature of the software was used to determine the area of eosinophilic or periodic acid-Schiff staining noncellular components (fibrin, basement membrane, mesangial matrix) and the area and number of basophilic objects (nuclei and nuclear fragments). Lesion scores and morphometry results were analyzed by analysis of variance for main effects of genotype and age, and Bonferroni’s test for supplemental mean comparisons. Probabilities of 0.05 or less were considered statistically significant.
Immunohistochemistry
Kidneys were snap-frozen in liquid nitrogen and OCT compound, and 5-μm cryostat sections were prepared and fixed in acetone. Sections were then treated with 3% H2O2, blocked with 10% rabbit serum, and incubated with rat anti-mouse CD3 monoclonal antibody (17AZ) (BD PharMingen). The sections were then incubated with a biotin-conjugated anti-rat IgG (Vector Laboratories, Burlingame, CA) and processed with a streptavidin-biotin immunoperoxidase kit (Vector Laboratories). 3,3′-Diaminobenzidine tetrahydrochloride was used as the peroxidase substrate and hematoxylin as the nuclear counterstain.
For comparisons of CD3+ T-cell infiltration, digital images of at least six glomeruli from stained frozen sections from each mouse (n = 5 for CD11a−/− and n = 8 for control MRL/MpJ-Faslpr mice) were captured using a Diagnostic Instruments SPOT Insight camera and a Nikon no. 600 microscope using the ×40 objective (Nikon, Melville, NY). T cells in each glomerulus were counted according to the criteria that the cells were located within the tissue of the glomerular tuft and were not within the lumina of glomerular capillaries; the cells clearly had the morphology of lymphocytes, with round, centrally located nuclei and narrow rims of basophilic cytoplasm; and immunostaining was most intense peripherally, as would be expected for a cell surface marker. Average numbers of T cells per glomerulus were analyzed statistically by one-way analysis of variance with supplemental mean comparisons by Tukey’s test.
Autoantibody Titers and Serum Immunoglobulin Measurements
IgG anti-DNA antibody titers, and total serum IgG and IgM were determined as previously reported.21 Briefly, enzyme-linked immunosorbent assay plates were coated with 100 μg/ml of either single- or double-strand calf thymus DNA (Sigma-Aldrich, St. Louis, MO). After blocking with 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS) overnight at 4°C, serial dilutions of sera were added to the wells in duplicate and incubated at 37°C for 1 hour. Plates were washed and a secondary goat anti-mouse IgG alkaline phosphatase-labeled antibody (Southern Biotechnology Associates, Birmingham, AL) was added at a 1:2000 dilution. Plates were incubated for 1 hour at 37°C and washed. The plates were developed by a 15-minute incubation of para-nitrophenyl phosphate (pNpp). Anti-DNA antibody titers were determined by identifying the highest dilution that was twice the amount of background absorbance. For the analysis of total serum immunoglobulins, IgG- and IgM enzyme-linked immunosorbent assay plates were prepared by precoating 2.5 μg/ml of goat anti-mouse Ig (Southern Biotechnology Associates) overnight at 4°C. Wells were blocked with 1% BSA/PBS for 1 hour at 37°C. Known Ig standards ranging from 50 ng/ml to 0.781 ng/ml and individual samples were added in duplicate and incubated at 37°C for 2 hours. Wells were washed three times with 0.5% BSA/PBS solution and the secondary goat anti-mouse IgG or IgM horseradish peroxidase antibody (Southern Biotechnology Associates) added and incubated for 1 hour at 37°C. Wells were washed three times and developed for 15 minutes using ABTS substrate (Sigma-Aldrich). Absorbencies for all measurements were done using a Tecan Spectrafluor Plus plate reader (Tecan, US Research, Triangle Park, NC). All anti-DNA antibody measurements were performed with n = 25, and IgG and IgM measurements performed with n = 20. Data between different genotypes were analyzed using a nonpaired Student’s t-test at time intervals of 12, 16, 20, or 24 weeks for anti-DNA antibody analyses, or 12, 20, and 28 weeks for total serum Ig and IgM measurements.
Results
Loss of LFA-1 Reduces Lymphadenopathy and Prolongs Survival
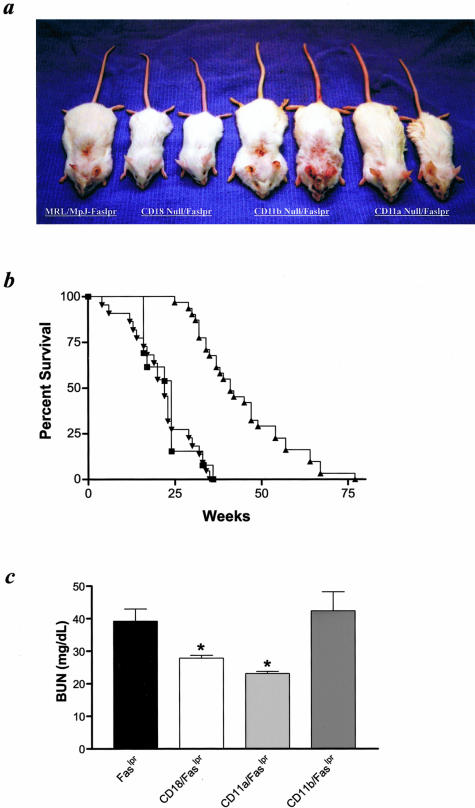
MRL/MpJ-Faslpr mice are a well-described model of SLE and are characterized by autoantibody formation, lymphadenopathy, immune complex deposition, vasculitis, and glomerulonephritis.10 These mice also show accelerated lethality and generally die between 6 to 10 months of age because of renal failure and complications from vasculitis. To assess the role of the β2 integrins in the development of autoimmune disease in this model, we backcrossed the CD11a-, CD11b-, and CD18-null mutations seven or more generations onto the MRL/MpJ-Faslpr background.17–19 CD11a−/− MRL/MpJ-Faslpr and CD18−/− MRL/MpJ-Faslpr mice failed to develop significant lymphadenopathy, consistent with previous findings in mice that showed that loss or inhibition of these adhesion molecules inhibited lymphocyte homing and recirculation.19 Surprisingly, CD11b−/− MRL/MpJ-Faslpr mice frequently showed exaggerated lymphadenopathy (Figure 1a). The histological pattern of these extremely enlarged nodes, however, did not differ from that of other mutants or of control MRL/MpJ-Faslpr mice, and was characterized by lack of differentiation of cortex and medulla and presence of sheets of small to medium lymphocytes interspersed with focal collections of plasma cells, among which were varying numbers of Mott cells (data not shown). However, CD11b−/− MRL/MpJ-Faslpr mice did contain a significantly higher frequency of CD4− CD8−, B220+ (double-negative) T cells in lymph nodes compared to control mice (74.2 ± 7.2% versus 40.8 ± 0.2%, respectively; P < 0.05). At this time, the mechanism by which loss of Mac-1 leads to this increased accumulation of double-negative cells in this model is not known, but may involve a previously undefined role for this adhesion molecule in regulating lymphocyte trafficking or possibly cell survival.
Figure 1.
Phenotypic characteristics of mutant MRL/MpJ-Faslpr mice. a: Twenty-week-old control MRL/MpJ-Faslpr (male), CD18−/− (male and female), CD11b−/− (male and female), or CD11a−/− (male and female) are shown from left to right. b: Kaplan-Meier survival analysis between control MRL/MpJ-Faslpr (▪, n = 25), CD11a−/− (▴, n = 30), and CD11b−/− (▾, n = 24) mice. c: BUN measurements from five male and five female, 20-week-old mice. *, P < 0.05.
CD11a−/− and CD18−/− MRL/MpJ-Faslpr mice, unlike CD11b homozygotes, also showed reduced signs of cutaneous vasculitis throughout their lifetime compared to control MRL/MpJ-Faslpr mice (Figure 1a). These findings are similar to our previous observations in ICAM-1-deficient MRL/MpJ-Faslpr mice, and suggest that loss of ICAM-1/LFA-1 interactions protects against the development of vasculitis in this model.11 CD11a deficiency also resulted in a significant increase in survival, in contrast to both CD11b−/− and control MRL/MpJ-Faslpr mice (median ± SEM survival of CD11a−/− MRL/MpJ-Faslpr mice = 41 ± 3 weeks versus CD11b−/− MRL/MpJ-Faslpr mice = 22 ± 2 weeks and control MRL/MpJ-Faslpr mice = 24 ± 2 weeks, P < 0.001; Figure 1b). Survival studies of CD18−/− MRL/MpJ-Faslpr mice were not performed, because several mice generated died before 18 weeks of age. Although a cause of death was not determined for all CD18-deficient mice, common necropsy findings included multifocal necrotizing and suppurative inflammation with intralesional bacteria in lymph nodes, spleen, and other organs, indicating disseminated bacterial disease. The submandibular lymph nodes frequently were affected, suggesting that the infections were of periodontal or other oral origin. These findings are consistent with previous observations of the CD18-null mutation on other strain backgrounds.19 However, a number of CD18−/− mice survived longer than 20 weeks of age, including some that lived to more than 40 weeks of age.
CD11a−/− MRL/MpJ-Faslpr Mutant Mice Show Significant Inhibition of Glomerulonephritis
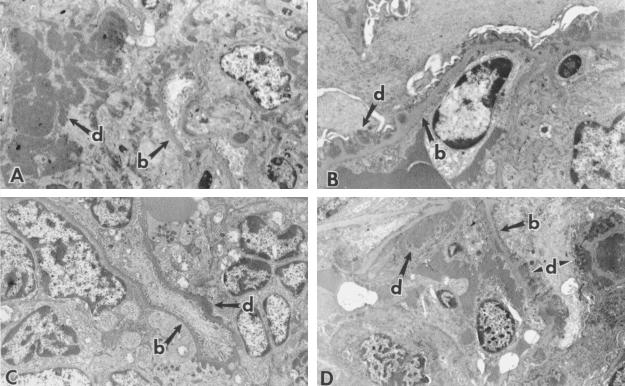
The increased survival observed in CD11a−/− mice, but not CD11b−/− MRL/MpJ-Faslpr mutant mice was associated with improved renal function as assessed by BUN concentrations in serum collected from 20-week-old mice (Figure 1c). Both CD11a−/− and CD18−/− MRL/MpJ-Faslpr mice showed a significantly lower serum BUN compared to control and CD11b−/− mice. These findings suggest that complete deficiency of all β2 integrins or LFA-1 may inhibit the initiation or progression of glomerulonephritis. Light microscopic examination of kidney sections from 20-week-old mice, though, showed similar glomerular changes in all groups (data not shown). Glomerular lesions at this time point were characterized by mild to moderate hypercellularity, neutrophil accumulation, and mesangial thickening, with little or no necrosis, fibrin deposition, or capsular changes, and mean lesion scores for CD11a−/−, CD11b−/−, and CD18−/− mice were not significantly different from the mean score for MRL/MpJ-Faslpr control mice (data not shown). Ultrastructural differences were evident, however. Glomeruli from control MRL/MpJ-Faslpr mice had abundant electron-dense deposits (Figure 2A). These deposits were identified predominantly within the mesangial regions; however, both subendothelial and intramembranous deposits were also noted. The deposits were large, confluent, and occupied extensive areas within the mesangium. The mesangial matrix and cellularity were both increased moderately. The foot processes from the visceral epithelial cells were effaced. Vascular and tubular electron-dense deposits were not identified.
Figure 2.
Glomerular ultrastructure. A: Control MRL/MpJ-Faslpr representative glomerulus from a 20-week-old mouse. Extensive electron-dense deposits (d) within the markedly expanded mesangium of the glomerulus were observed (b = basement membrane). B: CD18−/−, 20-week-old mouse. Marked reduction in glomerular deposits with distinct, nonconfluent, small electron-dense deposits within the basement membrane. C: CD11a−/−, 20-week-old mouse. Occasional linear electron-dense deposits were noted in the paramesangial areas abutting the basement membranes and within the glomerular basement membranes. D: CD11b−/−, 20-week-old mouse. Abundant confluent electron-dense deposits were readily seen within the mesangial regions (arrows). Epimembranous and membranous deposits (arrowheads) were also present within glomerular basement membranes. Original magnifications, ×5000.
In marked contrast, CD18−/− mice possessed infrequent electron-dense deposits (Figure 2B). Dense deposits were present within the basement membranes of the glomerular capillaries, while less frequent mesangial deposits were also seen. The deposits with the CD18−/− mice were also relatively small, nonconfluent, and distinct from adjacent normal basement membrane and mesangial matrix. The foot processes still tended to be attenuated and flattened. Electron-dense deposits were not seen with the vessels or tubules. Similar findings were noted with CD11a−/− mice (Figure 2C). CD11b−/− mice, unlike CD18−/− and CD11a−/− mice, showed massive mesangial electron-dense deposits and were indistinguishable from MRL/MpJ-Faslpr controls (Figure 2D). To further characterize the decreased electron-dense deposits in CD11a−/− and CD18−/− mice, sections from selected mice were assessed by immunofluorescent staining using anti-IgG and anti-C3 antibodies and compared to controls. Results indicated deposition of both IgG and C3 in irregular multifocal patterns consistent with the distribution of electron-dense deposits, and with considerably reduced C3 staining in the LFA-1−/− and CD18−/− mice (data not shown).
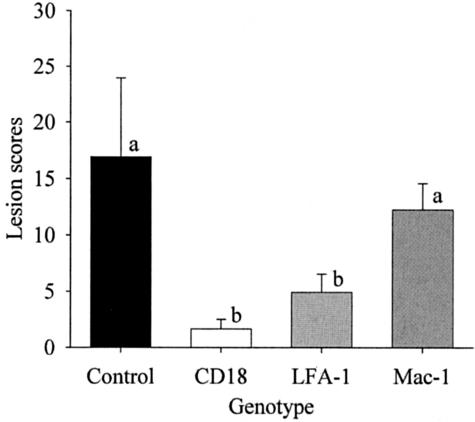
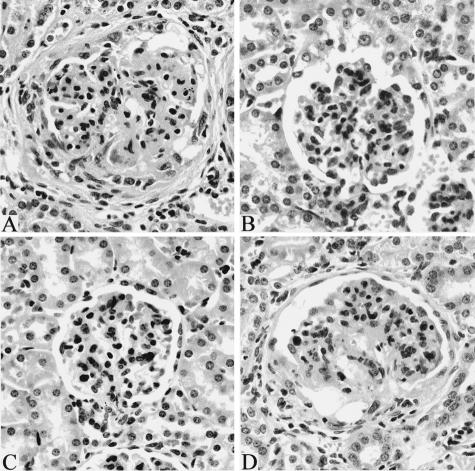
Among older (at death) groups of mice, mean lesion scores for CD11a−/− and CD18−/− mice were significantly lower than those for control MRL/MpJ-Faslpr mice and for CD11b−/− mice (Figure 3). Lesions in control MRL/MpJ-Faslpr mice were severe, and included glomerular enlargement, hypercellularity, mesangial thickening, basement membrane reduplication, accumulation of neutrophils, necrosis, and fibrin deposition, accompanied by capsular proliferation and fibrosis (Figure 4A). In contrast, lesions in CD18−/− and CD11a−/− mice were characterized primarily by mild to moderate hypercellularity, neutrophil accumulation, and mesangial thickening (Figure 4, B and C). Lesions in older CD11b−/− mice were similar to those in control MRL/MpJ-Faslpr mice (Figure 4D). In addition, mean lesion scores for these groups were not significantly different. Thus, deficiency of either CD11a−/− or CD18−/−, but not CD11b−/−, prevented progression of glomerulonephritis from the mild to moderate form evident in 20-week-old mice to the severe, destructive disease typical of older MRL/MpJ-Faslpr mice.
Figure 3.
Renal lesion scores for control MRL/MpJ-Faslpr mice (n = 6), CD18−/− mice (n = 3), CD11a−/− mice (n = 6), and CD11b−/− mice (n = 4). Means ± SD. Bars designated with different letter codes are significantly different (P < 0.05).
Figure 4.
Glomerular histopathology. A: Control MRL/MpJ-Faslpr mouse at death (23 weeks old). Severe glomerulitis with neutrophil accumulation, necrosis, sclerosis, crescent formation with synechia, and capsular fibrosis. B: CD18−/− mouse at death (34 weeks old). Mild neutrophil accumulation. C: CD11a−/− mouse at death (43 weeks old). Mild neutrophil accumulation. D: CD11b−/− mouse at death (24 weeks old). Severe glomerulitis similar to that in A. H&E. Original magnifications, ×20.
Results of morphometric analyses were similar to those of lesion scores. Total glomerular area and glomerular noncellular area correlated most highly with lesion scores (r = 0.997 and r = 0.940, respectively), and statistical analysis of these data gave results identical to those of analysis of lesion scores (data not shown). We also analyzed scores for individual component lesions. Glomerular neutrophil score means for all (both 20 week and older) CD11b−/− mice (1.88) and all control MRL/MpJ-Faslpr mice (1.72) were not significantly different (P > 0.05). Mean neutrophil scores for all CD11a−/− mice (1.02) and all CD18−/− mice (0.30) were significantly different (P < 0.05) both from those for CD11b−/− mice and control MRL/MpJ-Faslpr mice and from each other, the latter suggesting that loss of CD11a alone is less inhibitory to neutrophil emigration into the glomerulus than loss of all β2 integrin expression. Finally, we investigated whether loss of LFA-1 also led to a reduction in T-lymphocyte infiltration into the kidney during the development of glomerulonephritis. Immunohistochemical analysis revealed a significant reduction in the mean number of glomerular CD3+ T cells in CD11a−/− mice compared to controls at 20 weeks of age (0.37 ± 0.11 versus 1.3 ± 0.21 CD3+ T cells, respectively; P < 0.009).
LFA-1 Attenuates Autoantibody Formation
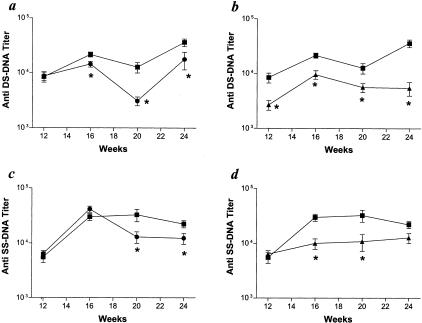
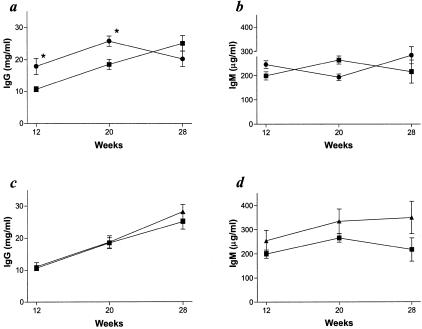
Decreased neutrophil emigration is a major mechanism by which LFA-1 and CD18 deficiencies would be expected to inhibit the development of glomerulonephritis, and our histopathological and ultrastructural findings are consistent with this possibility.18,19 However, the reduction in the severity of glomerulonephritis may also be influenced by other factors, including decreased autoantibody production. MRL/MpJ-Faslpr mice develop prominent hypergammaglobulinemia with increased serum IgG and IgM, and autoantibody formation against a wide variety of antigens including double- and single-stranded DNA.10 LFA-1 has been reported to be an important co-stimulatory molecule for immune cell activation through its participation in immune synapse formation and its ability to influence Th1 versus Th2 cytokine responses.22–26 To assess whether loss of LFA-1 or all four β2 integrins inhibited antibody production, we compared anti-double-stranded and anti-single-stranded DNA antibodies, and total serum IgG and IgM in CD11a−/−, CD18−/−, and control MRL/MpJ-Faslpr mutant mice. In general, the titers of both IgG anti-double-stranded and anti-single-stranded DNA antibodies were lower in both CD18−/− and CD11a−/− mice than in control MRL/MpJ-Faslpr mice (Figure 5). The reduction in autoantibody production was not because of decreased immunoglobulin production overall. Total serum levels of IgG in CD18−/− mutant mice were actually increased compared to control MRL/MpJ-Faslpr mice; however, total serum IgM levels were not significantly different (Figure 6, a and b). This is consistent with the previous characterizations of the CD18-null mutation on other strain backgrounds, and may be because of a compensatory response related to the increased susceptibility to infection associated with CD18 deficiency.19 CD11a−/− mice did not demonstrate any significant difference in total serum IgG or IgM compared to control mice (Figure 6, c and d). These data directly demonstrate that LFA-1 plays an important role in facilitating the development of autoimmunity in this model.
Figure 5.
Serum IgG anti-double-stranded and single-stranded DNA antibodies. a: IgG anti-double-stranded DNA antibody titers between control MRL/MpJ-Faslpr (▪, n = 25) and CD18−/− (•, n = 25) mice. b: IgG anti-double-stranded DNA antibody titers between control MRL/MpJ-Faslpr (▪, n = 25) and CD11a−/− (▴, n = 25) mice. c: IgG anti-single-stranded DNA antibody titers between control MRL/MpJ-Faslpr (▪, n = 25) and CD18−/− (•, n = 25) mice. d: IgG anti-single-stranded DNA antibody titers between control MRL/MpJ-Faslpr (▪, n = 25) and CD11a−/− (▴, n = 25) mice. *, P < 0.01.
Figure 6.
Total serum immunoglobulin levels. a: Total serum IgG concentrations between control MRL/MpJ-Faslpr (▪, n = 25) and CD18−/− (•, n = 25) mice. b: Total serum IgM concentrations between control MRL/MpJ-Faslpr (▪, n = 25) and CD18−/− (•, n = 25) mice. c: Total serum IgG concentrations between control MRL/MpJ-Faslpr (▪, n = 25) and CD11a−/− (▴, n = 25) mice. d: Total serum IgM concentrations between control MRL/MpJ-Faslpr (▪, n = 25) and CD11a−/− (▴, n = 25) mice. *, P < 0.01.
Discussion
SLE is a polygenic autoimmune disease that can affect many different organ systems.1,27 Despite intensive studies, the pathogenetic pathways that lead to the initiation and progression of tissue inflammation in SLE remain primarily undefined. Our findings directly demonstrate that LFA-1 plays a central role in the development of the SLE-like phenotype in MRL/MpJ-FasLpr mice. We observed that loss of LFA-1, but not Mac-1, significantly inhibited the development of inflammatory disease and increased survival in this model. Previous investigations in humans with SLE and in animal models have implicated LFA-1 in various stages of disease pathogenesis.28–31 For example, LFA-1 antibody treatment of NZB/NZW F-1 mice was previously shown to inhibit autoantibody production in this lupus model, although the effects of this treatment on the development of glomerulonephritis were not evaluated.31 In addition, Yung and colleagues32 found that transfer of a T-cell line overexpressing LFA-1 into normal mice led to the development of a lupus-like condition characterized by anti-DNA autoantibodies and immune complex-mediated glomerulonephritis. Interestingly, T cells from SLE patients have been shown to overexpress LFA-1, which may augment autoantibody production, as well as promote T-cell recruitment to inflammatory sites.33 This increased expression of LFA-1 seems to be the direct consequence of DNA hypomethylation in the LFA-1 promoter.34,35
LFA-1- and CD18-deficient mice, unlike Mac-1 mutants, had decreased severity of glomerular disease, according to each of several criteria, including functional (BUN values), immune complex deposition (electron-dense deposits), complement fixation (C3 immunofluorescence), neutrophil accumulation, T-cell infiltration, and glomerular injury (light microscopic and ultrastructural pathology). We regard this as one of the most striking findings from this study, for two reasons. First, LFA-1 and CD18 deficiencies ameliorated disease to a similar degree, indicating that LFA-1 is the dominant β2 integrin in this model. This was somewhat unexpected, because Mac-1 was previously shown to participate in leukocyte activation, recruitment, and the development of immune complex glomerulonephritis in several models.36,37 Second, loss of LFA-1 did not completely prevent glomerulonephritis, but did prevent progression to severe disease with extensive glomerular destruction, which is characteristic of MRL/MpJ-FasLpr mice. We previously examined the role of β2 integrin ligand, ICAM-1 in this model.11 ICAM-1 mutant mice, like LFA-1 mutants, showed increased survival and significant attenuation of glomerulonephritis. Together, these findings suggest that LFA-1 interactions with ICAM-1 on vascular endothelium are essential for the recruitment of leukocytes during the development of renal disease. Furthermore, these data indicate that in the absence of LFA-1, other adhesion pathways, such as VLA-4/VCAM-1, cannot fully compensate for the lack of this adhesion molecule in facilitating glomerulonephritis.13
Our findings support the hypothesis that LFA-1 also plays an important role in autoantibody production in this model.31 Both the CD18 and CD11a mutations resulted in a significant decrease in serum titers of both anti-double- and single strand DNA autoantibodies. This may lead to reduced immune complex deposition and C3 fixation in the kidney, as was observed in both CD18- and LFA-1-deficient mice, and contribute to the overall reduction in the severity of glomerulonephritis. However, these data also show that loss of LFA-1 cannot completely inhibit autoantibody production and the development of autoimmunity in this lupus model. Decreased production of autoantibodies has been reported in MRL/MpJ-Faslpr mice having mutations in the CD40, B7.1, or B7.2 genes, suggesting that these molecules, as well as others, may partially compensate for the loss of LFA-1.38–41 In our previous study of ICAM-1-deficient MRL/MpJ-Faslpr mice, we did not observe a significant reduction in autoantibody formation.11 This suggests that engagement of LFA-1 with other ligands such as ICAM-2 may provide a sufficient accessory signal for T and B cells in the absence of ICAM-1.23,42,43
There has been a longstanding interest in using anti-adhesion molecule therapies, including β2 integrin antagonists, for the treatment of patients with chronic inflammatory diseases.44 In the majority of studies, little benefit to patients was reported, most likely because of redundancies among different adhesion molecules, use of murine origin monoclonal antibodies, or high rates of clearance of inhibitors from the circulation. However, a humanized monoclonal antibody directed against LFA-1 has now been tested in several clinical trials involving psoriasis patients, with a significant number of patients showing reduced disease severity.45–47 Our findings also implicate LFA-1 as a potential therapeutic target for SLE. Blocking LFA-1 interactions with its ICAM ligands can potentially target several pathways implicated in the development of tissue inflammation and damage in SLE patients, including autoantibody production and leukocyte emigration. Other nonantibody inhibitors, including lovastatin, have recently been characterized and selectively inhibit the activity of LFA-1.48–50 Thus, these inhibitors, as well as anti-LFA-1 antibodies, may be useful for the treatment of SLE patients.
Acknowledgments
We thank Alex Szalai for technical expertise and critical reading of this manuscript.
Footnotes
Address reprint requests to Daniel C. Bullard, Ph.D., Department of Genetics, Hugh Kaul Human Genetics Building (KHGB) 640A, University of Alabama at Birmingham, 720 South 20th St., Birmingham, AL 35294. E-mail: pike@uab.edu.
Supported by the National Institutes of Health (grant RR-017009 to D.C.B.) and the Arthritis Foundation (to D.C.B.).
References
- Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mageed RA, Prud’homme GJ. Immunopathology and the gene therapy of lupus. Gene Ther. 2003;10:861–874. doi: 10.1038/sj.gt.3302016. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Hultman P, Kono DH. Using single-gene deletions to identify checkpoints in the progression of systemic autoimmunity. Ann NY Acad Sci. 2003;987:236–239. doi: 10.1111/j.1749-6632.2003.tb06053.x. [DOI] [PubMed] [Google Scholar]
- Cravens PD, Lipsky PE. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immunol Cell Biol. 2002;80:497–505. doi: 10.1046/j.1440-1711.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- Sturfelt G. The complement system in systemic lupus erythematosus. Scand J Rheumatol. 2002;31:129–132. [PubMed] [Google Scholar]
- Kelley VR, Wuthrich RP. Cytokines in the pathogenesis of systemic lupus erythematosus. Semin Nephrol. 1999;19:57–66. [PubMed] [Google Scholar]
- McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Bullard DC. Roles of leukocyte/endothelial cell adhesion molecules in the pathogenesis of vasculitis. Am J Med. 1999;106:677–687. doi: 10.1016/s0002-9343(99)00132-1. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Bullard DC. Philadelphia: Lippincott Williams and Wilkins,; Cell Adhesion Molecules in the Rheumatic Diseases. 2001:pp 478–489. [Google Scholar]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–391. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Bullard DC, King PD, Hicks MJ, Dupont B, Beaudet AL, Elkon KB. Intercellular adhesion molecule-1 deficiency protects MRL/MpJ-Faslpr mice from early lethality. J Immunol. 1997;159:2058–2067. [PubMed] [Google Scholar]
- Lloyd CM, Gonzalo JA, Salant DJ, Just J, Gutierrez-Ramos JC. Intercellular adhesion molecule-1 deficiency prolongs survival and protects against the development of pulmonary inflammation during murine lupus. J Clin Invest. 1997;100:963–971. doi: 10.1172/JCI119647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale JF, Harari OA, Marshall D, Haskard DO. TNF-a and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. J Immunol. 1999;163:3993–4000. [PubMed] [Google Scholar]
- Marshall D, Dangerfield JP, Bhatia VK, Larbi KY, Nourshargh S, Haskard DO. MRL/lpr lupus-prone mice show exaggerated ICAM-1-dependent leucocyte adhesion and transendothelial migration in response to TNF-alpha. Rheumatology (Oxford) 2003;42:929–934. doi: 10.1093/rheumatology/keg251. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- Lu H, Smith CW, Perrard J, Bullard DC, Tang L, Beaudet AL, Entman ML, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil transmigration in Mac-1 deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin HA, III, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE. Delayed lupus onset in (NZB x NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- Dustin ML. The immunological synapse. Arthritis Res. 2002;3(Suppl 4):S119–S125. doi: 10.1186/ar559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]
- Ni HT, Deeths MJ, Li W, Mueller DL, Mescher MF. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–5189. [PubMed] [Google Scholar]
- Moy VT, Brian AA. Signaling by lymphocyte function-associated antigen 1 (LFA-1) in B cells: enhanced antigen presentation after stimulation through LFA-1. J Exp Med. 1992;175:1–7. doi: 10.1084/jem.175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff M, Koch A, Rollinghoff M. Two signals are involved in polyclonal B cell stimulation by T helper type 2 cells: a role for LFA-1 molecules and interleukin 4. Eur J Immunol. 1992;22:599–602. doi: 10.1002/eji.1830220246. [DOI] [PubMed] [Google Scholar]
- Kelly JA, Moser KL, Harley JB. The genetics of systemic lupus erythematosus: putting the pieces together. Genes Immun. 2002;1(Suppl 3):S71–S85. doi: 10.1038/sj.gene.6363885. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Amano K, Sekine H, Koide J, Abe T. Upregulated expression and function of integrin adhesive receptors in systemic lupus erythematosus patients with vasculitis. J Clin Invest. 1993;92:3008–3016. doi: 10.1172/JCI116924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y, Abe K, Tokano Y, Hashimoto H. The expression of LFA-1, ICAM-1, CD80 and CD86 molecules in lupus patients: implication for immunotherapy. Intern Med. 1999;38:175–177. doi: 10.2169/internalmedicine.38.175. [DOI] [PubMed] [Google Scholar]
- Kootstra CJ, Van Der Giezen DM, Van Krieken JH, De Heer E, Bruijn JA. Effective treatment of experimental lupus nephritis by combined administration of anti-CD11a and anti-CD54 antibodies. Clin Exp Immunol. 1997;108:324–332. doi: 10.1046/j.1365-2249.1997.3641266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly MK, Kitchens EA, Chan B, Jardieu P, Wofsy D. Treatment of murine lupus with monoclonal antibodies to lymphocyte function-associated antigen-1: dose-dependent inhibition of autoantibody production and blockade of the immune response to therapy. Clin Immunol Immunopathol. 1994;72:198–203. doi: 10.1006/clin.1994.1130. [DOI] [PubMed] [Google Scholar]
- Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, Yang J, Chang S, Hemati N, Richardson B. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866–2871. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BC. Role of DNA methylation in the regulation of cell function: autoimmunity, aging and cancer. J Nutr. 2002;132:2401S–2405S. doi: 10.1093/jn/132.8.2401S. [DOI] [PubMed] [Google Scholar]
- Lu Q, Kaplan M, Ray D, Zacharek S, Gutsch D, Richardson B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1282–1291. doi: 10.1002/art.10234. [DOI] [PubMed] [Google Scholar]
- Lu Q, Ray D, Gutsch D, Richardson B. Effect of DNA methylation and chromatin structure on ITGAL expression. Blood. 2002;99:4503–4508. doi: 10.1182/blood.v99.12.4503. [DOI] [PubMed] [Google Scholar]
- Tang T, Rosenkranz A, Assmann KJM, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma J, Saito T, Ootaka T, Sato H, Abe K. Intercellular adhesion molecule-1, intercellular adhesion molecule-3, and leukocyte integrins in leukocyte accumulation in membranoproliferative glomerulonephritis type I. Am J Kidney Dis. 1996;28:685–694. doi: 10.1016/s0272-6386(96)90249-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Tesch G, Schwarting A, Maron R, Sharpe AH, Kelley VR. Costimulation by B7-1 and B7-2 is required for autoimmune disease in MRL-Faslpr mice. J Immunol. 2000;164:6046–6056. doi: 10.4049/jimmunol.164.11.6046. [DOI] [PubMed] [Google Scholar]
- Liang B, Gee RJ, Kashgarian MJ, Sharpe AH, Mamula MJ. B7 costimulation in the development of lupus: autoimmunity arises either in the absence of B7.1/B7.2 or in the presence of anti-b7.1/B7.2 blocking antibodies. J Immunol. 1999;163:2322–2329. [PubMed] [Google Scholar]
- Liang B, Kashgarian MJ, Sharpe AH, Mamula MJ. Autoantibody responses and pathology regulated by B7-1 and B7-2 costimulation in MRL/lpr lupus. J Immunol. 2000;165:3436–3443. doi: 10.4049/jimmunol.165.6.3436. [DOI] [PubMed] [Google Scholar]
- Ma J, Xu J, Madaio MP, Peng Q, Zhang J, Grewal IS, Flavell RA, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- Damle NK, Klussman K, Aruffo A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J Immunol. 1992;148:665–671. [PubMed] [Google Scholar]
- Carpenito C, Pyszniak AM, Takei F. ICAM-2 provides a costimulatory signal for T cell stimulation by allogeneic class II MHC. Scand J Immunol. 1997;45:248–254. doi: 10.1046/j.1365-3083.1997.d01-391.x. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Gottlieb A, Krueger JG, Bright R, Ling M, Lebwohl M, Kang S, Feldman S, Spellman M, Wittkowski K, Ochs HD, Jardieu P, Bauer R, White M, Dedrick R, Garovoy M. Effects of administration of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasis. J Am Acad Dermatol. 2000;42:428–435. doi: 10.1016/s0190-9622(00)90214-7. [DOI] [PubMed] [Google Scholar]
- Papp K, Bissonnette R, Krueger JG, Carey W, Gratton D, Gulliver WP, Lui H, Lynde CW, Magee A, Minier D, Ouellet JP, Patel P, Shapiro J, Shear NH, Kramer S, Walicke P, Bauer R, Dedrick RL, Kim SS, White M, Garovoy MR. The treatment of moderate to severe psoriasis with a new anti-CD11a monoclonal antibody. J Am Acad Dermatol. 2001;45:665–674. doi: 10.1067/mjd.2001.117850. [DOI] [PubMed] [Google Scholar]
- Dedrick RL, Walicke P, Garovoy M. Anti-adhesion antibodies efalizumab, a humanized anti-CD11a monoclonal antibody. Transpl Immunol. 2002;9:181–186. doi: 10.1016/s0966-3274(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Gadek TR, Burdick DJ, McDowell RS, Stanley MS, Marsters JC, Jr, Paris KJ, Oare DA, Reynolds ME, Ladner C, Zioncheck KA, Lee WP, Gribling P, Dennis MS, Skelton NJ, Tumas DB, Clark KR, Keating SM, Beresini MH, Tilley JW, Presta LG, Bodary SC. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science. 2002;295:1086–1089. doi: 10.1126/science.295.5557.1086. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G. Lymphocyte function-associated antigen-1 blockade by statins: molecular basis and biological relevance. Endothelium. 2003;10:43–47. doi: 10.1080/10623320303360. [DOI] [PubMed] [Google Scholar]