Abstract
Cyclooxygenase-2 (COX-2) and the prostaglandin products generated as a result of COX-2 activity mediate a variety of biological and pathological processes. Scarless healing occurs in fetal skin in the first and second trimesters of development. This scarless healing process is known to proceed without a significant inflammatory response, which appears to be important for the lack of scarring. Because the COX-2 pathway is an integral component of inflammation, we investigated its role in the fetal repair process using a mouse model of scarless fetal wound healing. COX-2 expression in scarless and fibrotic fetal wounds was examined. In addition, the ability of exogenous prostaglandin E2 to alter scarless fetal healing was evaluated. The results suggest that the COX-2 pathway is involved in scar production in fetal skin and that targeting COX-2 may be useful for limiting scar formation in adult skin.
The cutaneous wound healing process is known to differ between fetal and adult skin. Wound repair in adult skin begins with an acute inflammatory phase and ends with the formation of a permanent scar. In contrast, early gestation fetal wounds (first and second trimester) heal in a near perfect fashion, rapidly and without the production of a scar.1–4 There has been much interest in characterizing the key factors responsible for the switch from scarless healing to an adult-like, scar-producing phenotype typical of skin past the second trimester of gestation. Identification of differences in the two types of healing could identify factors that promote scar tissue generation. This correlation between factors identified as reduced in scarless healing and the inhibition of those factors in adult wounds to reduce scarring has been especially true for transforming growth factor-β (TGF-β). This cytokine was one of the first mediators found to be differentially regulated in scarless healing and was shown to promote scar tissue deposition when introduced into scarless wounds.5–12 As a result of these findings and others implicating TGF-β in fibrosis,13 the effect of down-regulating this molecule was tested in adult skin and found to reduce scar formation.14–16
A key feature of scarless fetal healing appears to be a lack of inflammation in response to the wounding event.6–8,17–25 In contrast, the early phases of wound healing in late fetal and adult skin are characterized by a robust inflammatory response, and eventually a permanent scar in the wound area.26,27 While the interleukins IL-6, IL-8, and IL-10 have been studied in fetal wound repair,23–25 the role of other classic inflammatory mediators in scarless healing is not known.
Metabolites and enzymes of the arachidonic acid cascade, including the cyclooxygenase-2 (COX-2) enzyme and its enzymatic product prostaglandin E2 (PGE2), are known to be critical mediators of the inflammatory response. COX-2 has received much attention recently as it is involved in diseases associated with dysregulated inflammatory conditions, such as rheumatoid and osteoarthritis, cardiovascular disease, and the carcinogenesis process.28–32 COX-2 undergoes immediate-early up-regulation in response to an inflammatory stimulus,33 such as a wound. It functions by producing prostaglandins that control many aspects of the resulting inflammation, including the induction of vascular permeability and the infiltration and activation of inflammatory cells.34 Interest in the role of the COX-2 pathway and other aspects of inflammation in the adult wound repair process is increasing35 as these early events have been shown to regulate the outcome of repair.36–38
Based on the involvement of COX-2 in inflammation and the recent demonstration that it contributes to several aspects of adult wound repair,36,39,40 we examined the role of COX-2 in the fetal wound healing process. These studies demonstrate differential expression of the COX-2 enzyme in early and late gestation fetal wounds. Furthermore, PGE2, a COX-2 product shown to mediate many processes in the skin, caused a delay in healing and the production of a scar when introduced into early fetal wounds. These data further our understanding about the fundamental differences between scarless healing and normal repair, and suggest the involvement of COX-2 in the production of scar tissue.
Materials and Methods
Fetal Surgeries
A murine model of fetal wound healing was used to examine the COX-2 pathway in scarless and fibrotic repair. All work was approved by the Ohio State University animal use committee. Female and male mice were mated, and surgery was performed on the pregnant female 15 or 18 days after the detection of a vaginal plug, designated day 0. These time points represent ages at which scarless (embryonic day 15, E15) or fibrotic (embryonic day 18, E18) healing take place (see Figure 1). After preparation of the abdomen for aseptic surgery, a midline laparotomy was performed under isoflurane anesthesia. Incisions were made in the uterine wall and amniotic sac overlying each fetus, and a full-thickness incisional wound, approximately 2 mm in length, was made on the dorsum of the fetus using microsurgical scissors. One μl of India ink (Fisher Scientific, Pittsburgh, PA) diluted to 10% in sterile phosphate-buffered saline (PBS) (Invitrogen Corporation, Carlsbad, CA) was introduced subcutaneously into the wound site to allow visualization of the area after healing had occurred.10 The uterine incision was closed using 6−0 nylon suture. After approximately four fetuses were wounded in this manner, the muscle and skin layers of the pregnant mouse were sutured closed. Xylazine (3 mg/kg, Phoenix Scientific, St. Joseph, MO) was given subcutaneously for sedation and analgesia and to preclude cannibalism of the pups by the dam. Fetal skin was harvested at 6 hours, 24 hours, or 7 days post-wounding to examine inflammation and scar tissue formation. The skin was either fixed in formalin for subsequent histological analysis or snap-frozen for protein or RNA isolation. Due to the small size of the wounds, several wounds had to be pooled for each protein and RNA sample to obtain a sufficient yield for analysis. Normal E15 and E18 skin were harvested as controls. A minimum of 10 wounds for each experimental group at each time point were analyzed for histology and two pooled samples per group were used for protein and RNA analysis.
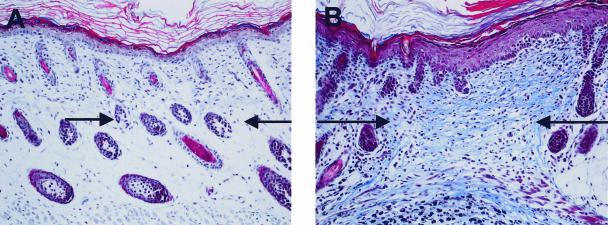
Figure 1.
Differential scar formation in fetal skin. Masson’s trichrome stains were performed on 7 day skin wounds that had been generated at either 15 (A, E15) or 18 (B, E18) days of gestation. The wound/scar margins are marked with arrows (magnification, ×20).
Using the same surgical techniques, E15 fetuses were wounded and injected with 1 μl of the ink solution (PBS) or the ink solution containing 2 μg PGE2 (Sigma, St. Louis, MO). Wounds were harvested at 24 hours, 3 days, or 7 days post-wounding. The skin was either fixed in formalin for subsequent histological analysis or snap-frozen for protein isolation. Digital photographs using a Nikon Coolpix 2100 camera (Nikon Corp., Japan) were taken before sacrifice to document scarring. Due to the small size of the wounds, several wounds were pooled for each protein sample. At least 10 wounds for each experimental group at each time point were analyzed for histology and two pooled samples per treatment group were used for enzyme immunoassays.
Protein Analysis and Enzyme Immunoassays
For Western blot analysis, tissue samples were ground in liquid nitrogen using a mortar and pestle, and sonicated in Laemmli buffer (BioRad, Hercules, CA). For cultured fibroblasts, cell pellets were dispersed in Laemmli buffer and sonicated. After centrifugation, the total protein concentration of each sample was determined using the BCA (bicinchoninic acid) protein assay kit (Pierce Biotechnology, Rockford, IL). Twenty-five μg of sample or 0.5 μg of COX-2 protein standard (Cayman Chemical Company, Ann Arbor, MI) were separated on 10% SDS-polyacrylamide gels in the presence of 0.1% SDS. Proteins were then electroblotted onto Immobilon-P membranes (Millipore, Bellerica, MA). The membranes were blocked with 10% nonfat dry milk in TBST (Tris-buffered saline containing 0.1% Tween 20). The membranes were incubated with affinity-purified COX-2 rabbit polyclonal antibody (1:500 dilution, Cayman Chemical) for 1 hour at room temperature. Bound antibody was then probed with a horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) for 1 hour at room temperature. After extensive washes with TBST, proteins were visualized using an ECL chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ). The developed films were digitized and the bands were quantitated using Kodak 1D image analysis software (Eastman Kodak, Rochester, NY).
For enzyme immunoassays, tissue samples were ground in liquid nitrogen. Protein was isolated in buffer containing protease inhibitors and total protein concentrations were determined as previously described.36 PGE2 (Amersham-Pharmacia, Piscataway, NJ) and TGF-β 1 (R & D Systems, Minneapolis, MN) levels were assessed using commercial kits as specified by the manufacturer. Ten μg (PGE2) or 40 μg (TGF-β1) of total protein was used for the assays.
RNA Isolation and Semi-Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Gene expression levels of COX-2 were determined using RT-PCR as previously described.34 After separation of the PCR products in agarose gels and visualization with ethidium bromide, images were captured using a Kodak DC290 camera and bands were quantitated using Kodak 1D image analysis software (Eastman Kodak). Levels of COX-2 were normalized to the housekeeping gene HPRT, and the data are expressed as a percentage of the values in age-matched unwounded skin.
Histology and Immunostaining
Tissues were processed and subjected to Masson’s trichrome staining to detect collagenous scar tissue as previously described.36 Stained sections were photographed and assessed for the presence of scar tissue. Additionally, immunostaining for COX-2 was performed. After deparaffinization and rehydration, samples were washed in 1X Automation Buffer (Biomeda Corp., Foster City, CA). Sections were steamed in pre-warmed antigen unmasking fluid (Vector Laboratories, Burlingame, CA) for 3 minutes, washed with automation buffer, then blocked with casein solution (Vector Laboratories) for 30 minutes. Sections were then incubated overnight at 4°C with an antibody specific for COX-2 (1:200 dilution in casein, Cayman Chemical Co). After washing with automation buffer, sections were incubated with rabbit link and rabbit label solutions (BioGenex, San Ramon, CA) for 30 minutes each, then chromogenic detection was performed using DAB (Vector Laboratories). Sections were counterstained with hematoxylin, dehydrated, and mounted.
Fetal Fibroblast Culture and Proliferation Assessment
Normal embryonic day 15 and 18 skin were harvested for fibroblast cultures as outlined by Scheid et al.41 Briefly, excised fetal skin was placed into Dulbecco’s modified Eagle’s medium (high glucose, Invitrogen/Life Technologies, Carlsbad, CA) supplemented with 20% heat-inactivated fetal calf serum (Gibco, Invitrogen Corp.), 100 U/ml penicillin, 100 μg/ml streptomycin, non-essential amino acids, 2 mmol/L L-glutamine, and 1 mmol/L sodium pyruvate (Life Technologies) at 37°C and 5% CO2. Following 8 to 14 days of cultivation under daily renewal of media, sufficient fibroblasts had migrated out of the skin specimens to allow first passaging with trypsin-EDTA. Tissue culture experiments were performed within the third to eighth passage. Cells were seeded in 96-well culture plates at a density of 5 × 102 cells per well. Twenty-four hours later, cells were treated with media containing increasing doses of PGE2 (Sigma), including 0 nmol/L (untreated), 1 nmol/L, 10 nmol/L or 100 nmol/L in the presence of a 10% total volume of alamar blue (Biosource International, Camarillo, CA) to determine proliferation rates. Plates were read spectrophotometrically at 570 and 600 nm at 8, 24, 48, and 72 hours after treatment. These values were used to calculate the percent reduction of the alamar blue dye, which corresponds to proliferation, as specified by the manufacturer.
Statistical Analysis
Statistical differences between the treatments were determined using a Student’s t-test generated using StatView software (Abacus Concepts, Berkeley, CA).
Results
Comparison of Scar Tissue Production in Fetal Wounds During Development
Masson’s trichrome staining, used to stain collagenous scar tissue blue, illustrates the healing differences between E15 and E18 skin wounds at 7 days post-wounding (Figure 1). Restoration of the normal tissue architecture can be seen in wounds generated at E15 (Figure 1A) compared to evident scars identified by the loss of hair follicles and dense collagen in wounds generated at E18 (Figure 1B). These results recapitulate what has been found previously in fetal wound healing studies and validate the murine model used in the present studies. This disparity in scar tissue production seen in late versus early gestation fetal wounds has been shown to correlate with differences in the levels of inflammation in these wounds.
Differential COX-2 Expression in Embryonic Wounds
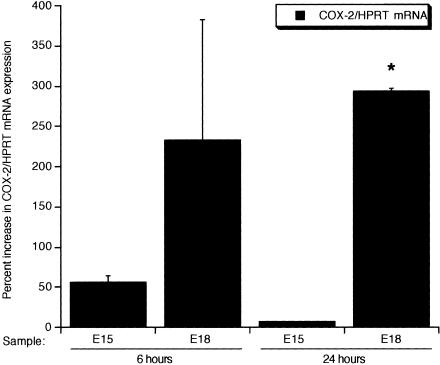
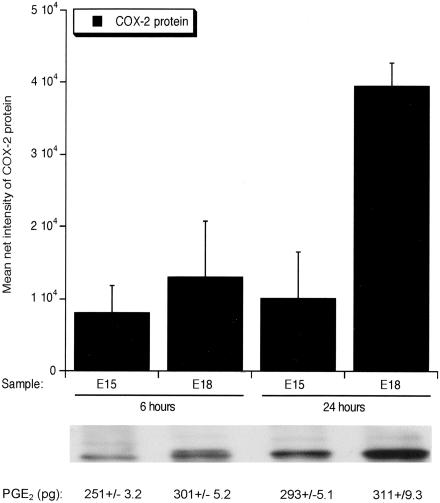
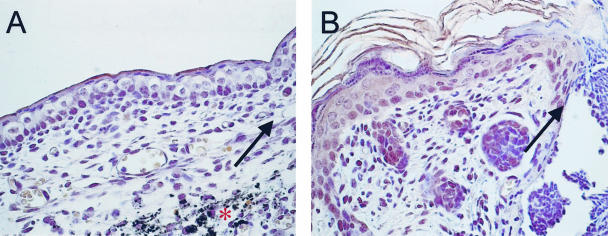
Because COX-2 and its prostaglandin products, particularly PGE2, are known to be involved in cutaneous inflammation and inflammation is thought to contribute to the switch from scarless to fibrotic healing in fetal skin, we examined the expression of COX-2 and the production of PGE2 in early and late gestation fetal wounds. COX-2 mRNA (Figure 2) and protein (Figures 3 and 4) levels as well as PGE2 levels were higher in E18 wound tissue compared to wounds introduced at E15, with 24 hours being the peak of expression (Figures 2 to 4). Twenty-four hours post-wounding, immunohistochemical staining demonstrated COX-2 protein expression in basal keratinocytes, inflammatory cells, and stromal cells in wounds made in E18 skin (Figure 4B) but not in wounds made at E15 (Figure 4A). By 7 days, COX-2 protein levels in wound tissue were undetectable, as seen in normal unwounded skin (data not shown). The differential expression of COX-2 in E15 scarless and E18 fibrotic wounds suggest that COX-2 may be involved in the regulation of scar tissue production.
Figure 2.
COX-2 mRNA expression during fetal wound healing. Semi-quantitative RT-PCR analysis was used to examine COX-2 mRNA levels in E15 and E18 wounds. The net intensity values for COX-2 bands were normalized to the housekeeping gene HPRT, and the data are presented as the percent difference in COX-2/HPRT gene expression from the age-matched unwounded values (mean ± SEM, *, P < 0.05).
Figure 3.
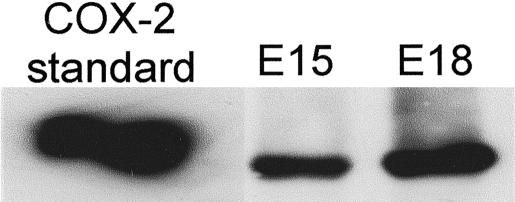
COX-2 protein and PGE2 levels in fetal wounds. COX-2 protein was detected by Western blot and levels were analyzed using image analysis software. The mean net intensities of the bands ± SEM were plotted and are shown along with representative images of the protein bands. Corresponding PGE2 levels (pg) ± SEM are also shown.
Figure 4.
Immunohistochemical localization of COX-2. Localization of COX-2 protein expression in E15 (A) and E18 (B) wounds was assessed by immunohistochemistry. The wound margins are marked with arrows and the * denotes India ink used to mark the wound site, which can be seen in A (magnification, ×60).
Enhanced COX-2 Expression in Late Fetal Fibroblasts
In addition to wound tissue, the levels of COX-2 protein in cultured fetal fibroblasts, the cell type responsible for collagen deposition and scar tissue production, were also examined (Figure 5). Fibroblasts were isolated from E15 or E18 skin. Net intensity values generated from Western blot analysis revealed 33% higher levels of COX-2 protein detected in fibroblasts isolated from E18 skin, which heals with a scar, compared to those from E15 skin, which heals without a scar. Taken together with the data in Figures 2 to 4, these results suggest that a baseline increase in COX-2 expression in E18 wound tissue and fibroblasts may be critical to the presence of inflammation and subsequent scar formation characteristic of late gestation fetal repair.
Figure 5.
COX-2 protein levels in cultured fetal fibroblasts. Western blot analysis of COX-2 protein was performed on fibroblasts cultured from E15 or E18 skin. COX-2 protein is shown as a positive control.
Effect of Exogenous PGE2 on Scarless Healing
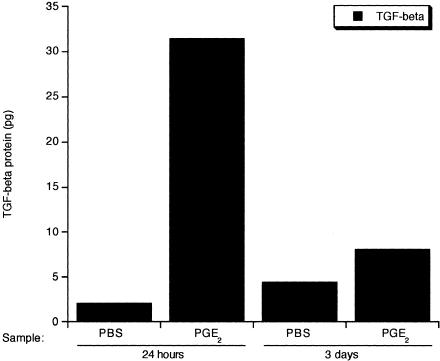
To further investigate the function of the COX-2 pathway in fetal wound repair, we characterized the early fetal healing response in the presence of the COX-2 enzymatic product and inflammatory modulator PGE2. E15 wounds, which represent scarless healing, were injected with either the India ink solution alone (PBS) as a control or the India ink solution containing 2 μg of PGE2. The ability of PGE2 to alter the repair process was evaluated. An early increase in TGF-β 1 protein levels were detected in PGE2-treated wounds 24 hours after wounding compared to PBS control wounds (Figure 6). While the levels of TGF-β 1 in PGE2-treated wounds dropped by day 3 post-wounding, the levels were still twice those of control wounds. TGF-β 1 levels returned to control levels by 7 days after wounding (data not shown).
Figure 6.
TGF-β 1 protein levels in E15 fetal wounds. The total protein levels of the pro-fibrotic factor TGF-β 1 in fetal wound tissues were assessed by ELISA. The mean levels of TGF-β 1 were determined in control wounds (PBS) and PGE2-treated wounds at 24 hours and 3 days post-wounding.
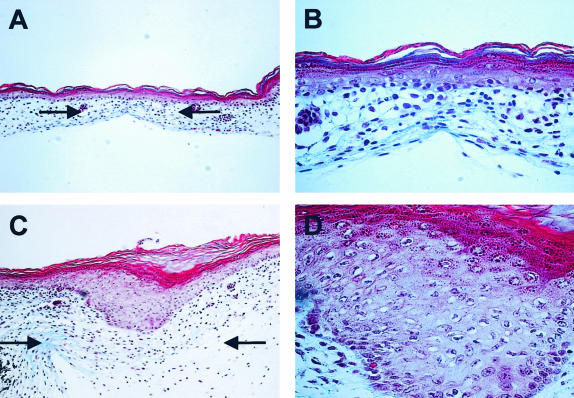
Three days after the wounds were created, a delay in healing was evident in wounds injected with PGE2. At this point, approximately half of the PGE2 injected wounds were not closed, compared to control wounds which were completely healed at this stage. Histological examination of the closed wounds demonstrated that those exposed to PGE2 displayed a hyperproliferative epithelium (Figure 7, C and D), suggesting a delay in the re-epithelialization process at this stage. In contrast, the control wounds contained an intact epithelial layer similar to age-matched unwounded skin (Figure 7, A and B).
Figure 7.
The effect of PGE2 on the healing of E15 skin. Representative Masson’s trichrome stained tissue sections from control wounds [magnification, ×20 (A); magnification, ×60 (B)] and PGE2-treated wounds [magnification, ×20 (C); and magnification, ×60 (D)] 3 days after wounding demonstrates delayed healing in wounds treated with PGE2. Arrows are used to indicate the wound site in A and C.
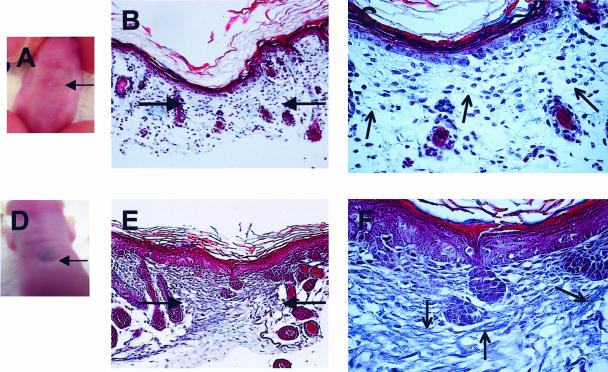
In addition to decreasing healing rates, PGE2 treatment of E15 wounds also resulted in the production of scar tissue at day 7 (Figure 8, D to F) compared to control E15 wounds (Figure 8, A to C) which lacked scar formation. This was evident both macroscopically, with a scar that could be seen by eye (Figure 8D), and also microscopically, where the presence of scar tissue and dense collagen fibers can be seen in trichrome-stained tissue sections (Figure 8, E and F). These results further support the idea that the COX-2 pathway can alter the outcome of wound repair.
Figure 8.
Gross and histological appearance of scar tissue deposition. At day 7 post-wounding, digital pictures were taken of fetuses that had either been injected with ink alone (A, PBS) or with 2 μg PGE2 at age E15 (D). Representative Masson’s trichrome-stained tissue sections also illustrate the lack of scar tissue production in control wounds [magnification, ×20 (B); magnification, ×60 (C)] and the presence of a scar in wounds injected with PGE2 (magnification, × 20 (E); magnification, ×60 (F)). Scars/wound sites are marked with filled arrows (B and E). Open arrows highlight collagen within the wound (C and F).
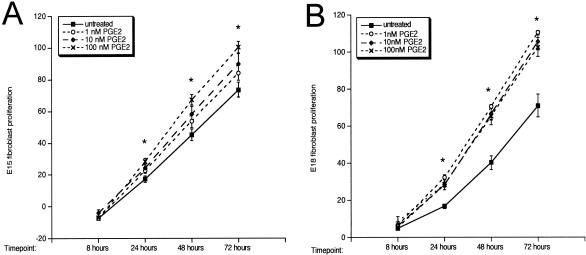
PGE2 Promotes Fetal Fibroblast Proliferation
One component of healing thought to regulate scar tissue deposition is the proliferative rate of dermal fibroblasts, the cell type responsible for the deposition and remodeling of scar tissue. A mechanism by which PGE2 could be promoting scar tissue deposition in E15 scarless fetal wounds could be by increasing the rate of fibroblast proliferation. Therefore, we examined the effects of the addition of exogenous PGE2 on cultured fetal fibroblast proliferation (Figure 9). The addition of PGE2 resulted in a dose-dependent increase in the proliferation of fibroblasts cultured from E15 skin (Figure 9A, *, P < 0.05 compared to untreated cells). A similar increase in fibroblast proliferation in response to PGE2 treatments was detected in fibroblasts cultured from E18 (Figure 9B) and adult skin (data not shown). These data implicate an increase in fibroblast proliferation as a possible mechanism for the induction of scar tissue production in fetal wounds as a consequence of PGE2 exposure (Figure 8).
Figure 9.
Fetal fibroblast proliferation. The proliferation rates of cultured E15 (A) and E18 (B) fetal fibroblasts in response to PGE2 treatment were determined using alamar blue. The fibroblasts responded to PGE2 in a dose-dependent manner. Treatment with 100 nmol/L PGE2 resulted in a significant increase in proliferation compared to untreated cells (*, P < 0.05).
Discussion
The COX-2 enzyme and its product PGE2 are known to modulate inflammation in the skin in response to stimuli such as ultraviolet light exposure34,42 and topical administration of the tumor promoter TPA (12-O-tetradecanoylphorbol-13-acetate).43,44 While there have been reports that COX-2 inhibition can be detrimental to gastric healing45,46 and bone repair,47 this does not appear to be true for healing in the skin. Recent studies have shown that reducing PGE2 with the use of inhibitors specific for the COX-2 enzyme does not have an effect on wound re-epithelialization or tensile strength,36,48–50 although it has been suggested that reducing PGE2 by inhibiting COX-1, the “constitutive” homologue of COX-2, may have an effect.48 While it seems that COX-2 inhibition does not deter the proper re-epithelialization of wounds of the skin, several studies now implicate the COX-2 pathway in the regulation of the inflammatory phase of cutaneous wound repair.36,51 Inhibition of this inflammatory pathway has also been suggested to reduce scar formation.36 This observation fits with fetal wound healing studies demonstrating a lack of inflammation in wounds that heal without a scar.
While it has been known for some time that early fetal wounds do not exhibit acute inflammation as a reaction to a cutaneous wound, most of the studies to date have focused on differences in the fetal environment, the extracellular matrix of fetal wounds, or the well-studied TGF-β, rather than differences involved in the suppression of inflammation in these wounds. Classic inflammatory mediators that have been examined in fetal wound repair include, IL-6, IL-8, and IL-10,23–25 as well as other substances that can induce inflammation in fetal skin.6–8,20–22 As an absence of inflammation seems to be particularly important for scarless healing to take place, a better understanding of inflammatory regulation in fetal wounds is warranted. The studies described here demonstrate differential expression of COX-2 in scarless versus scar-forming fetal skin wounds. The involvement of the COX-2 pathway in scar formation is further highlighted by the fact that increasing PGE2 levels in scarless wounds results in the conversion of a scarless healing process into one of repair with the generation of a scar.
Besides the wounds themselves, fetal fibroblasts, thought to be an important regulator of scarless healing,52 were also assessed for COX-2 content. Similarly to what was found in wound tissue, higher COX-2 levels were also detected in cultured fetal fibroblasts isolated from E18 skin compared to fibroblasts isolated from E15 skin. High COX-2 expression and PGE2 production are believed to contribute to the uncontrolled proliferation of tumor cells, as well as to mobilization and invasion, so it is conceivable that higher COX-2 expression in fibroblasts could stimulate their own activation, migration, and/or proliferation, augmenting scar tissue production. A case for COX-2 and its product, PGE2, being important for scarring has also been made in adult wound healing studies36,53 and in reports indicating an increase in collagen deposition and proliferation by fibroblasts in response to PGE2.54,55 It has been suggested that the mitogenic effects of PGE2 on fibroblasts is mediated by signaling through the EP1 receptor.56 While PGE2 induced the proliferation of fibroblasts in our studies and previous studies have shown that PGE2 can augment proliferation and collagen deposition of fibroblasts,54,55 it appears that fibroblasts from other organs do not respond in the same manner. For example, PGE2 is known to reduce proliferation and collagen deposition by lung fibroblasts,57–59 suggesting that PGE2 can have variedeffects on cells depending on the type and origin ofthe cell.
While the present studies are the first to report on relative COX-2 levels and the effects of PGE2 on scarring in the murine fetus, the ability of PGE2 to induce acute inflammation in fetal rabbit wounds has been previously documented. In these studies, the introduction of PGE2 and another prostaglandin, PGF2α, induced inflammation in fetal wounds,60 although their effect on collagen deposition or fibrosis was not examined. Whether PGE2 displays immunosuppressive or anti-inflammatory properties or instead acts as a pro-inflammatory molecule most likely results from differences in the expression or activity of the receptors for PGE2. These receptors, EP1 to EP4, display varied properties after binding PGE261. Interestingly, one of the PGE2 receptors, EP4, was found to be differentially expressed in scarless wounds.62 Studies on the regulation and function of these receptors in the skin are just beginning, and differences in EP receptor expression and activity may very well influence not only fetal wound healing, but adult wound repair as well. Future studies will be needed to fully characterize the role of each of these receptors in the cutaneous repair process.
There are several plausible mechanisms by which PGE2 could be inducing scar formation in fetal wounds. PGE2 could be enhancing acute inflammation, already known to interfere with scarless healing, thereby indirectly promoting scar formation through the recruitment and activation of inflammatory cells. PGE2 treatment could be both delaying healing and promoting scar tissue deposition through increases in the pro-fibrotic TGF-β.13,63 Disruption of the TGF-β signaling pathway in smad3-deficient mice has been shown to speed the rate of healing,62 and extensive data demonstrates restricted TGF-β levels are crucial to scarless healing.5–12 Lastly, our data demonstrating increased fibroblast proliferation in response to PGE2 suggests that PGE2 could be directly stimulating fibroblasts to proliferate, amplifying collagen production and scarring. This idea is also supported by previous studies demonstrating an increase in collagen deposition and proliferation by fibroblasts following exposure to PGE2.54,55
The data presented here further advance our understanding about the conditions necessary for scarless healing by suggesting that low levels of COX-2 expression and PGE2 may be necessary for the scarless repair of fetal skin. We have previously demonstrated a link between decreased COX-2 activity and reduced scarring in adult murine skin.36 The current study provides evidence that PGE2 induces scar formation in fetal skin, further supporting a role for the COX-2 pathway in scar formation. With the demonstration that COX-2 and its products enhance scarring, the availability of COX-2 inhibitors offers a potential way to mediate scar tissue production in adult skin. The use of COX-2 inhibitors to reduce scar tissue production has implications for alleviating both the cosmetic and the functional problems associated with excessive scarring.
Acknowledgments
We thank Jeanne Green as well as the rest of the staff in the Wiseman Hall Vivarium Surgery and Mary Ross for their help in carrying out the described experiments.
Footnotes
Address reprint requests to Tatiana M. Oberyszyn, Ph.D., The Ohio State University, Department of Pathology, 1645 Neil Avenue, 129 Hamilton Hall, Columbus, OH 43210. E-mail: oberyszyn.1@osu.edu.
References
- Chin GS, Stelnicki EJ, Gittes GK, Longaker MT. Characteristics of fetal wound repair. Garg HG, Longaker MT, editors. New York: Marcel Dekker,; Scarless wound healing. 2000:pp 239–257. [Google Scholar]
- Ehrlich HP. Collagen considerations in scarring and regenerative repair. Garg HG, Longaker MT, editors. New York: Marcel Dekker,; Scarless wound healing. 2000:pp 99–114. [Google Scholar]
- Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MW, Harrison MR. Studies in fetal wound healing: VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. 68–69. [DOI] [PubMed] [Google Scholar]
- Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11:424–431. [PubMed] [Google Scholar]
- Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, Cohen IK, Diegelmann RF. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Lanning DA, Nwomeh BC, Montante SJ, Yager DR, Diegelmann RF, Haynes JH. TGF-beta1 alters the healing of cutaneous fetal excisional wounds. J Pediatr Surg. 1999;34:695–700. doi: 10.1016/s0022-3468(99)90358-5. [DOI] [PubMed] [Google Scholar]
- Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg. 1995;222:146–154. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo C, Beanes SR, Hu FY, Zhang X, Dang C, Chang G, Wang Y, Nishimura I, Freymiller E, Longaker MT, Lorenz HP, Ting K. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am J Pathol. 2003;163:2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelnicki EJ, Bullard KM, Harrison MR, Cass DL, Adzick NS. A new in vivo model for the study of fetal wound healing. Ann Plast Surg. 1997;39:374–380. doi: 10.1097/00000637-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Sullivan KM, Lorenz HP, Meuli M, Lin RY, Adzick NS. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg. 1995;30:198–202. doi: 10.1016/0022-3468(95)90560-x. 202–193. [DOI] [PubMed] [Google Scholar]
- Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107:1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Choi BM, Kwak HJ, Jun CD, Park SD, Kim KY, Kim HR, Chung HT. Control of scarring in adult wounds using antisense transforming growth factor-beta 1 oligodeoxynucleotides. Immunol Cell Biol. 1996;74:144–150. doi: 10.1038/icb.1996.19. [DOI] [PubMed] [Google Scholar]
- Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, Cohen IK. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Morykwas MJ, Ditesheim JA, Ledbetter MS, Crook E, White WL, Jennings DA, Argenta LC. Monodelphis domesticus: a model for early developmental wound healing. Ann Plast Surg. 1991;27:327–331. doi: 10.1097/00000637-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Morykwas MJ, Ledbetter MS, Ditesheim JA, White WL, Vander Ark AD, Argenta LC. Cellular inflammation of fetal excisional wounds: effects of amniotic fluid exclusion. Inflammation. 1991;15:173–180. doi: 10.1007/BF00918644. [DOI] [PubMed] [Google Scholar]
- Frantz FW, Bettinger DA, Haynes JH, Johnson DE, Harvey KM, Dalton HP, Yager DR, Diegelmann RF, Cohen IK. Biology of fetal repair: the presence of bacteria in fetal wounds induces an adult-like healing response. J Pediatr Surg. 1993;28:428–433. doi: 10.1016/0022-3468(93)90243-e. 433–424. [DOI] [PubMed] [Google Scholar]
- Haynes JH, Johnson DE, Mast BA, Diegelmann RF, Salzberg DA, Cohen IK, Krummel TM. Platelet-derived growth factor induces fetal wound fibrosis. J Pediatr Surg. 1994;29:1405–1408. doi: 10.1016/0022-3468(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Kumta S, Ritz M, Hurley JV, Crowe D, Romeo R, O’Brien BM. Acute inflammation in foetal and adult sheep: the response to subcutaneous injection of turpentine and carrageenan. Br J Plast Surg. 1994;47:360–368. doi: 10.1016/0007-1226(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. 872–863. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–676. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Treatment of rheumatoid arthritis and osteoarthritis with COX-2-selective inhibitors: a managed care perspective. Am J Manag Care. 2002;8:S502–S517. [PubMed] [Google Scholar]
- Altman R. Acute coronary disease athero-inflammation: therapeutic approach. Thromb J. 2003;1:2. doi: 10.1186/1477-9560-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Rodger IW, Shennib H, Hu F, Tayara L, Giaid A. Cyclooxygenase-2 (COX-2) in acute myocardial infarction: cellular expression and use of selective COX-2 inhibitor. Can J Physiol Pharmacol. 2003;81:114–119. doi: 10.1139/y03-023. [DOI] [PubMed] [Google Scholar]
- Koki A, Khan NK, Woerner BM, Dannenberg AJ, Olson L, Seibert K, Edwards D, Hardy M, Isakson P, Masferrer JL. Cyclooxygenase-2 in human pathological disease. Adv Exp Med Biol. 2002;507:177–184. doi: 10.1007/978-1-4615-0193-0_28. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- Wu KK. Cyclooxygenase 2 induction: molecular mechanism and pathophysiologic roles. J Lab Clin Med. 1996;128:242–245. doi: 10.1016/s0022-2143(96)90023-2. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Ross MS, Parrett ML, Oberyszyn TM. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat. 2000;62:367–384. doi: 10.1016/s0090-6980(00)00089-7. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Breyer MD. Inflammatory modulation and wound repair. J Invest Dermatol. 2003;120:xi–xii. doi: 10.1046/j.1523-1747.2003.12154.x. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11:25–34. doi: 10.1046/j.1524-475x.2003.11106.x. [DOI] [PubMed] [Google Scholar]
- Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU. 1 null mouse: tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Futagami A, Ishizaki M, Fukuda Y, Kawana S, Yamanaka N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab Invest. 2002;82:1503–1513. doi: 10.1097/01.lab.0000035024.75914.39. [DOI] [PubMed] [Google Scholar]
- Laulederkind SJ, Thompson-Jaeger S, Goorha S, Chen Q, Fu A, Rho JY, Ballou LR, Raghow R. Both constitutive and inducible prostaglandin H synthase affect dermal wound healing in mice. Lab Invest. 2002;82:919–927. doi: 10.1097/01.lab.0000020407.98665.98. [DOI] [PubMed] [Google Scholar]
- Scheid A, Wenger RH, Christina H, Camenisch I, Ferenc A, Stauffer UG, Gassman M, Meuli M. Hypoxia-regulated gene expression in fetal wound regeneration and adult wound repair. Pediatr Surg Int. 2000;16:232–236. doi: 10.1007/s003830050735. [DOI] [PubMed] [Google Scholar]
- Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- Griffiths RJ, Wood BE, Li S, Blackham A. Pharmacological modification of 12–0-tetradecanoylphorbol-13-acetate induced inflammation and epidermal cell proliferation in mouse skin. Agents Actions. 1988;25:344–351. doi: 10.1007/BF01965041. [DOI] [PubMed] [Google Scholar]
- Kwak WJ, Han CK, Son KH, Chang HW, Kang SS, Park BK, Kim HP. Effects of ginkgetin from ginkgo biloba leaves on cyclooxygenases and in vivo skin inflammation. Planta Med. 2002;68:316–321. doi: 10.1055/s-2002-26742. [DOI] [PubMed] [Google Scholar]
- Sun WH, Tsuji S, Tsujii M, Gunawan ES, Sawaoka H, Kawai N, Iijima H, Kimura A, Kakiuchi Y, Yasumaru M, Sasaki Y, Kawano S, Hori M. Cyclo-oxygenase-2 inhibitors suppress epithelial cell kinetics and delay gastric wound healing in rats. J Gastroenterol Hepatol. 2000;15:752–761. doi: 10.1046/j.1440-1746.2000.02242.x. [DOI] [PubMed] [Google Scholar]
- Guo JS, Cho CH, Lam Liu ES, Choy HT, Wang JY, Leung Koo MW. Antiangiogenic effect of a highly selective cyclooxygenase-2 inhibitor on gastric ulcer healing in rats. Toxicol Appl Pharmacol. 2002;183:41–45. doi: 10.1006/taap.2002.9457. [DOI] [PubMed] [Google Scholar]
- Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- Kampfer H, Brautigam L, Geisslinger G, Pfeilschifter J, Frank S. Cyclooxygenase-1-coupled prostaglandin biosynthesis constitutes an essential prerequisite for skin repair. J Invest Dermatol. 2003;120:880–890. doi: 10.1046/j.1523-1747.2003.12140.x. [DOI] [PubMed] [Google Scholar]
- Blomme EA, Chinn KS, Hardy MM, Casler JJ, Kim SH, Opsahl AC, Hall WA, Trajkovic D, Khan KN, Tripp CS. Selective cyclooxygenase-2 inhibition does not affect the healing of cutaneous full-thickness incisional wounds in SKH-1 mice. Br J Dermatol. 2003;148:211–223. doi: 10.1046/j.1365-2133.2003.05065.x. [DOI] [PubMed] [Google Scholar]
- Muller-Decker K, Hirschner W, Marks F, Furstenberger G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J Invest Dermatol. 2002;119:1189–1195. doi: 10.1046/j.1523-1747.2002.19501.x. [DOI] [PubMed] [Google Scholar]
- Muscara MN, McKnight W, Asfaha S, Wallace JL. Wound collagen deposition in rats: effects of an NO-NSAID and a selective COX-2 inhibitor. Br J Pharmacol. 2000;129:681–686. doi: 10.1038/sj.bjp.0703112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96:1251–1259. doi: 10.1097/00006534-199511000-00002. discussion 1260–1251. [DOI] [PubMed] [Google Scholar]
- Talwar M, Moyana TN, Bharadwaj B, Tan LK. The effect of a synthetic analogue of prostaglandin E2 on wound healing in rats. Ann Clin Lab Sci. 1996;26:451–457. [PubMed] [Google Scholar]
- Lupulescu A. Effect of prostaglandins on protein RNA, DNA and collagen synthesis in experimental wounds. Prostaglandins. 1975;10:573–579. doi: 10.1016/s0090-6980(75)80003-7. [DOI] [PubMed] [Google Scholar]
- Durant S, Duval D, Homo-Delarche F. Effect of exogenous prostaglandins and nonsteroidal anti-inflammatory agents on prostaglandin secretion and proliferation of mouse embryo fibroblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 1989;38:1–8. doi: 10.1016/0952-3278(89)90140-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Satoh H, Togoh M, Taniguchi S, Hashimoto Y, Kurokawa K. Positive and negative regulation of cell proliferation through prostaglandin receptors in NIH-3T3 cells. J Cell Physiol. 1996;169:401–409. doi: 10.1002/(SICI)1097-4652(199611)169:2<401::AID-JCP20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem J. 1997;321:639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn JH, Halushka PV, LeRoy EC. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980;65:543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem. 1982;257:8630–8633. [PubMed] [Google Scholar]
- Morykwas MJ, Perry SL, Argenta LC. Effects of prostaglandins and indomethacin on the cellular inflammatory response following surgical trauma in fetal rabbits. Int J Tissue React. 1993;15:151–156. [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Li HS, Hebda PA, Kelly LA, Ehrlich GD, Whitcomb DC, Dohar JE. Up-regulation of prostaglandin EP4 receptor messenger RNA in fetal rabbit skin wound. Arch Otolaryngol Head Neck Surg. 2000;126:1337–1343. doi: 10.1001/archotol.126.11.1337. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]