Abstract
Aberrant phosphorylation of the neuronal cytoskeleton is an early pathological event in Alzheimer’s disease (AD), but the underlying mechanisms are unclear. Here, we demonstrate in the brains of AD patients that neurofilament hyperphosphorylation in neocortical pyramidal neurons is accompanied by activation of both Erk1,2 and calpain. Using immunochemistry, Western blot analysis, and kinase activity measurements, we show in primary hippocampal and cerebellar granule (CG) neurons that calcium influx activates calpain and Erk1,2 and increases neurofilament phosphorylation on carboxy terminal polypeptide sites known to be modulated by Erk1,2 and to be altered in AD. Blocking Erk1,2 activity either with antisense oligonucleotides to Erk1,2 mRNA sequences or by specifically inhibiting its upstream activating kinase MEK1,2 markedly reduced neurofilament phosphorylation. Calpeptin, a cell-permeable calpain inhibitor, blocked both Erk1,2 activation and neurofilament hyperphosphorylation at concentrations that inhibit calpain-mediated cleavage of brain spectrin. By contrast, inhibiting Erk1,2 with U-0126, a specific inhibitor of Mek1,2, had no appreciable effect on ionomycin-induced calpain activation. These findings demonstrate that, under conditions of calcium injury in neurons, calpains are upstream activators of Erk1,2 signaling and are likely to mediate in part the hyperphosphorylation of neurofilaments and tau seen at early stages of AD as well as the neuron survival-related functions of the MAP kinase pathway.
Neurofilaments accumulate abnormally in several major neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, Lewy body disease, and amyotrophic lateral sclerosis.1,2 The recent observation that mutations of the low molecular weight neurofilament subunit (NF-L) are a cause of Charcot-Marie-Tooth disease type 2E, an axonopathy affecting the central and peripheral nervous systems, supports growing experimental evidence that primary alterations of the neurofilament cytoskeleton can promote neurodegeneration in various pathological settings.3–5 Neurofilaments (NF) are composed of three subunits, designated high (NF-H), middle (NF-M), and low (NF-L) molecular weight, reflecting their relative molecular mass on gels of 200 kd, 140 kd, and 70 kd, respectively. These subunits have a relatively conserved central core region involved in filament assembly and variable amino terminal and carboxy terminal domains, which may be phosphorylated by at least six different protein kinases.6–8 Human NF-M and NF-H have long carboxy terminal domains of 49 kd and 61 kd that contain 13 (NF-M) and 43 or 44 (NF-H) lysine-serine-proline (KSP) repeats in their polypeptide sequence, accounting for much of the phosphate on these proteins.9–12 In Alzheimer’s disease, the state of NF-H and NF-M phosphorylation markedly increases13 beginning at preclinical stages of AD14 and this hyperphosphorylation may be the first evidence of an altered cytoskeleton in dystrophic neurites.15,16 Moreover, the microtubule-associated protein tau, which binds to the core subunit of neurofilaments, is regulated by many of the protein kinases that phosphorylate neurofilament proteins, and also shares some phosphoepitopes in common,17–19 suggesting that some of the mechanisms that underlie tau and neurofilament pathology might overlap.
Among the neurofilament protein kinases implicated in AD pathogenesis are mitogen-activated protein kinases (MAPKs), a family of intracellular protein kinases involved in signal transduction and the regulation of cell proliferation, differentiation, and survival.20 The MAP kinases, ERK1 and 2, are activated in AD brain21 and may contribute to the hyperphosphorylation of both tau22 and neurofilament proteins1,13 by phosphorylating KSP motifs in these proteins.23 Despite evidence for Mek1,2 and Erk1,2 activation in AD brain,24,25 little is known about the events leading to activation. In this study, we establish that calpains, a family of calcium-activated neutral cysteine proteases, are activated in the neuronal populations that exhibit ERK1,2 activation, neurofilament hyperphosphorylation, and prominent neurofibrillary tangle formation in AD. We further show in primary neurons exposed to calcium injury that calpains mediate both the activation of Erk1,2 and the Erk1,2-dependent phosphorylation of neurofilament protein sites known to be altered in AD. These findings have additional implications for the AD-related dysfunction of tau, which is also regulated by Erk.
Materials and Methods
Fixed and frozen human brain tissue was obtained from the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA) and the Alzheimer’s Disease Clinical Core at New York University School of Medicine (New York, NY). Formalin-fixed prefrontal cortices from 10 moderate to severe AD cases aged 61 to 87 years with a postmortem interval (PMI) of 11.9 ± 1.97 hours and 10 non-demented cases aged 42 to 90 years with a PMI of 15.1 ± 2.47 hours were used for immunohistochemical studies. Frozen prefrontal cortices from 10 moderate to severe AD cases aged 58 to 89 years with a PMI of 8.78 ± 2.6 hours and 10 non-demented cases aged 61 to 94 years with a PMI of 9.7 ± 2.68 hours were used for Western blot analysis. The diagnosis of AD was established by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) and criteria proposed by Mirra et al26 and Braak and Braak.27 The following antibodies and chemicals were obtained as indicated: polyclonal antibodies to Erk1,2 and protein A plus G agarose beads (Santa Cruz Biotech, Inc., Santa Cruz, CA), monoclonal antibody against the phosphorylated NF-M (Zymed, San Francisco, CA), monoclonal antibody to β-tubulin (Amersham Life Sciences, Chicago, IL), monoclonal antibody to fodrin (Chemicon, Temecula, CA), monoclonal antibodies SMI-31 and SMI-32 against phospho- and dephospho-epitopes, respectively, on the NF-H and NF-M tail domains (Sternberger Monoclonal Inc., Lutherville, MD), polyclonal antibodies against phospho-Erk1,2 and phospho-independent Erk1,2 (Cell Signaling, Boston, MA), secondary antibodies conjugated to Alexa 488 and Alexa 568 (Molecular Probes, Eugene, OR), alkaline phosphatase conjugated anti-mouse and anti-rabbit secondary antibodies (Promega, Madison, WI), monoclonal antibody against μ-calpain was a kind gift from J. Elce (Queens University, Kingston, ON), rabbit polyclonal antibodies against NF-L and m-calpain were made in this laboratory, the RT-97 monoclonal antibody clone was a kind gift from Brian Anderton (Institute of Psychiatry, London, UK), recombinant Erk2 and MEK1 proteins were kind gifts from Dr. N.G. Ahn (University of Colorado, Boulder, CO), microcystin LR (Calbiochem, San Diego, CA), (γ-32P) ATP (New England Nuclear, Boston, MA), protease inhibitor cocktail (Boehringer Mannheim GmbH, Germany), ECL kit (Amersham Biotech, Chicago, IL), P81 phosphocellulose paper (Whatman, Maidstone, UK), immobilon membranes (Millipore, Bedford, MA), dialysis tubing (Spectrum, Los Angeles, CA), myelin basic protein (Sigma Chemical Company, St. Louis, MO), antisense oligonucleotides against Erk1,2, U-0126, ionomycin, and calpeptin (Biomol, Plymouth, PA). The KSPXK synthetic 14 mer peptide derived from neurofilament sequence was custom synthesized at QCB in Boston, and all cell culture reagents were purchased from Life Technologies, Inc. (Gaithersburg, MD).
Tissue Extraction
An equal quantity of brain tissue by weight from prefrontal cortex of each human brain sample was homogenized in a tissue extraction buffer (Tris-HCl pH 6.8 containing 1 mmol/L EGTA, EDTA, and 1% Triton X-100), 20 mmol/L β-glycerophosphate, 20 mmol/L sodium fluoride, 1 mmol/L sodium vanadate, and protease inhibitor cocktail in the ratio of 1:10 (W/V) using a hand-held tissue tearer at setting 5 for 30 seconds, 3 cycles. The samples were centrifuged at 15,000 rpm (30,000 × g) for 30 minutes. The tissue homogenates and supernatants were used for Western blot analysis of Erk1,2 and calpain, respectively.
Neurofilament Triplet Preparation and Purification of NF-L
The neurofilament triplets were prepared from mouse spinal cords as described earlier,23,28 and NF-L protein was purified on a DE-52 column using an FPLC system. NF-L was further purified by SDS-PAGE using a 7% 20 × 20 cm preparative gel, followed by zinc staining29 and electroelution, dialysed against 0.1 mol/L ammonium bicarbonate, and used as an antigen to generate the polyclonal antibody in rabbits.
Expression and Purification of KSPXXXK Fusion Protein
A rat NF-H tail fragment containing 156 amino acids with 24 KSPXXXK repeats, tagged to GST fusion protein was expressed and purified as described previously.23
Primary Neuronal Cultures, Treatment with Drugs and Extraction
Rat hippocampal and cerebellar granule (CG) neurons were cultured by conventional methods as described.30,31 CG neuronal cultures [7 days in vitro (DIV)] and hippocampal neurons (8 or 14 DIV) deprived of serum for 12 hours were treated with vehicle (0.1% ethanol), or ionomycin (0.5 μmol/L in ethanol), or ionomycin together with calpeptin (20 μmol/L in ethanol), or U-0126 (10 μmol/L in ethanol). Calpeptin and U-0126 were added to cultures 2 hours before the addition of ionomycin. After 24 hours of drug treatment, cells were washed twice in phosphate buffered saline (PBS; 10 mmol/L phosphate, 137 mmol/L sodium chloride, 27 mmol/L potassium chloride, pH 7.4); lysed in lysis buffer (M-Per, Pierce, Rockford, IL) containing 10 mmol/L of β-glycerophosphate, 10 mmol/L sodium fluoride, 1 mmol/L vanadate, 5 μmol/L microcystin LR, and protease inhibitor cocktail (Roche Chemicals, Indianapolis, IN); and incubated on ice for 15 minutes with frequent mixing on a vortex. The cell lysate was centrifuged at 15,000 rpm in a refrigerated microcentrifuge for 30 minutes, and the supernatant was used for the analysis of fodrin cleavage. As described,23 the Erk1,2 activity assays, Western blot analysis of Erk1,2 and neurofilament proteins were performed using cell lysates following protein determination using BCA method (Pierce) as described by the manufacturer.
Immunohistochemistry
Immunoreactivity was demonstrated on 30- to 40-μm thick vibratome sections as previously described.32 Negative controls were tissue sections incubated with only secondary antibody. Monoclonal antibodies that recognize phospho-NF-H (SMI-31) and dephospho-NF-H (SMI-32), were diluted 1:250. The anti-calpain antibody C-18, which recognizes active m-calpain, was diluted 1:500. Rabbit polyclonal antibodies recognizing Erk1,2 (Upstate, Charlottesville, VA), phospho-Erk1,2, and total m-calpains were diluted 1:100. All antibody dilutions used were made in dilution buffer (Tris-buffered saline pH 7.4 with 1% normal goat serum, 0.9% NaCl, 2% bovine serum albumin (BSA), and 0.4% Triton X-100) and signal detected using peroxidase and diaminobenzidine. Photomicrographs were obtained using a Zeiss Axioskop I Microscope.
Double-immunofluorescent labeling of brain sections was demonstrated on 6-μm paraffin-embedded sections. Slides were treated in 0.3% H2O2/methanol, rinsed in TBS and microwaved for 3 minutes in 0.01 mol/L citrate buffer, pH 6.0. Non-specific staining was blocked using 20% normal goat serum diluted in TBS. Slides were incubated overnight at room temperature with a phospho-Erk monoclonal antibody (1:200) and C-18 polyclonal antibody (1:500) in diluting buffer. Slides were incubated in biotinylated anti-mouse (1:250) for 30 minutes, rinsed, then incubated for 2 hours with streptavidin-Alexa 488 and goat-anti-mouse-Alexa 568 (Molecular Probes), each diluted 1:300. Images were captured using a Leica TCS-NT confocal microscope.
Immunocytochemistry of Neurons
Neurons fixed in 4% paraformaldehyde were permeabilized for 20 minutes in 0.2% Triton-X-100, blocked in 10% horse serum in PBS for one hour, and incubated with primary antibodies (diluted in 4% horse serum/PBS 0.05% Tween-20) against NF-L (1:250; polyclonal antisera generated in our laboratory), phospho-Erk1,2 polyclonal antibody, phosphorylated NF-M monoclonal antibody, or β-tubulin (diluted as suggested by the supplier) for 1 hour at room temperature (RT). After three washes in blocking solution, the neurons were incubated with anti-rabbit Alexa 488, Alexa 568, and streptavidin Alexa 568 (all from Molecular Probes). Secondary antibodies were diluted in the same buffer as the primary antibodies and incubated for 1 hour at RT. Cells were washed and mounted on the coverslips and analyzed by laser confocal microscopy (Leica, Wetzlar, Germany) using a TCS software program.
Immunoprecipitation of Mek1,2 and Erk1,2 Kinases
Immunoprecipitation of Mek1,2 and Erk1,2 was performed essentially as described earlier.23,33 In brief, protein A plus G agarose beads (60 μl packed volume) were washed in 1X PBS three times, suspended in 500 μl of 1X PBS, and incubated at RT for 30 minutes with 60 μl of primary antibodies to Mek1,2 and Erk1,2 (Santa Cruz). Washing was repeated as above, and cell lysates (equivalent to 250 μg of protein) were incubated with Protein A plus G agarose beads coupled to Mek1,2 or Erk1,2 polyclonal antibodies for 2 hours at 4°C. The beads were washed in buffer (50 mmol/L Tris/HCl (pH 7.4) containing 5 mmol/L EDTA, 10 mmol/L NaF, 10 mmol/L β-glycerophosphate, and 0.01% Triton X-100) and suspended in 100 μl of 1X kinase buffer.
Kinase Assay
Kinase assays were performed in duplicates for each sample, as previously described.23 Briefly, 10 μl of the immunoprecipitates of Erk1,2 were mixed in a kinase reaction mixture containing 50 mmol/L Tris/HCl, pH 7.5, with 1 mmol/L EDTA, EGTA, DTT 1 mmol/L orthovanadate, 1 μmol/L microcystin LR, myelin basic protein (0.4 mg/ml), and gamma (γ-32P) ATP (50 μmol/L). The reaction was initiated by the addition of (γ-32P) ATP, incubated at 30°C for 1 hour, and terminated by transferring an aliquot of the kinase reaction mixture to a phosphocellulose pad. The phosphocellulose pads were dried and washed in 75 mmol/L phosphoric acid five times for 15 minutes each with a final wash in 95% ethanol for 5 minutes. The pads were dried and counted using a Packard liquid scintillation counter and liquid scintillant. Each sample included four reaction sets, two for the control sample without the substrate and two for the experimental sample with the substrate. Control counts were deducted from the experimental value for the final cpm reading. The data are presented as % control versus the treated samples. For the time course of Erk2 kinase activity in the presence and absence of 20 μmol/L calpeptin, a mixture of recombinant Erk2 (0.25 μg) and Mek1 (0.1 μg) were used as the enzyme source, and a synthetic peptide derived from mouse NF-H sequence containing two KSP repeats was used as substrate.
Inhibition of Both Erk2 Activation and Phosphorylation of KSPXXXK Fusion Protein in Vitro by U-0126
Recombinant Erk2 (1 μg) and KSPXXXK fusion protein (2 μg) derived from the sequence of rat NF-H were incubated in a kinase reaction mixture described above, using 10 μl of immunoprecipitated Mek1,2 from lysates of ionomycin-treated hippocampal neurons as enzyme source. Incubations were carried out in the presence and absence of U-0126 (10 μmol/L) or calpeptin (20 μmol/L). Reactions were started by the addition of γ-32P ATP (50 μmol/L) and stopped by the addition of Laemmli’s buffer and analyzed by SDS-PAGE and autoradiography. A similar set of reactions were run in the presence of 50 μmol/L ATP and analyzed by Western blot analysis using RT-97, a monoclonal antibody that specifically recognizes the phosphorylated forms of NF-H and NF-M.
Antisense Oligonucleotide Reduction of Erk1,2 Protein Levels in Neurons
Hippocampal primary cultures obtained from day 1 postnatal rat pups were grown on laminin-coated glass coverslips for 14 days in neurobasal media with B27 supplement and with 10% fetal calf serum. Cells were starved in neurobasal media with B27 supplement and without serum for 24 hours and subsequently treated with 5 μmol/L phosphorothioated Erk1,2 antisense oligonucleotides directed against the initiation codon and the following 14 bases of the mouse P42 MAP kinase (Erk2) mRNA (5′-GCCGCCGCCGCCGCCAT-3′). This sequence is identical in rat and human P42 and P44 MAPKs. To ensure higher levels of transfection, oligofectamine reagent was used as described in the protocol provided by the manufacturer. After 48 hours, the same concentration of oligonucleotides was added and incubated for an additional 24 hours. Cells were fixed with 4% formaldehyde/PBS, permeabilized with 0.2% Triton X-100, and stained with anti-Erk1,2 goat polyclonal antibody (1:200), and subsequently with biotinylated anti-goat antibodies (1:200) and streptavidin-Alexa 568 (1:200). Anti-phospho-NF-M monoclonal antibody was followed by secondary Alexa 488 antibody and analyzed using confocal imaging.
SDS-PAGE and Western Blot Analysis
Protein concentration in each fraction was assayed by the BCA method (Pierce), and protein aliquots (10 to 20 μg) were loaded for each lane on a 7% minigel for electrophoresis unless otherwise mentioned. Proteins were electrotransferred from gels to PVDF or nitrocellulose membranes using a Genei blotter (Idea Scientific, Minneapolis, MN) and blocked using 3% nonfat dry milk (Biorad) in Tris-buffered saline (20 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 0.2% Tween 20; TBS-T) for at least 2 hours. The membranes were incubated overnight at 4°C with primary antibodies at appropriate dilutions. Blots were developed with alkaline phosphatase-conjugated secondary antibodies using the chromogenic substrate BCIP/NBT (Promega) or with chemiluminescence-based CDP star (Tropix, Bedford, MA) phosphatase substrate or peroxidase-conjugated secondary antibodies using the ECL kit (Amersham Biotech).
Results
Neurofilament Hyperphosphorylation in AD Brain Is Associated with Activation of Erk1,2 and Calpains
To understand the impact of disturbed calcium homeostasis in AD, we examined the status of the calcium-dependent neutral protease, calpain, Erk1,2 kinase, and neurofilament phosphorylation in the prefrontal cortex of 10 sets of AD and control brains.
Calpains
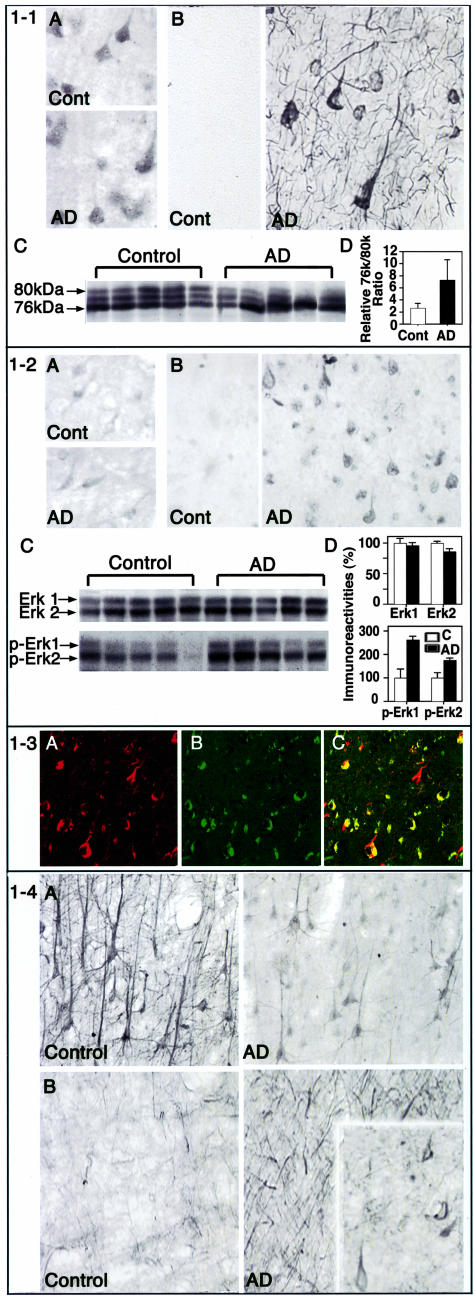
Pyramidal neurons in lamina III of AD brains were strongly immunolabeled with an antibody recognizing the active site of m-calpain (C-18), which becomes exposed when the protease is activated34 (Figure 1–1B). We previously showed, and confirm here, that C-18 does not label m-calpain in normal brain where m-calpain is predominantly in an inactive state, but it strongly immunolabels NFT and neuropil threads in AD brain (Figure 1–1B). In this study, the immunolabeling of lamina III pyramids and abnormal neurites was consistent with localization of activated calpain to these neurofibrillary lesions. In contrast to activated m-calpain, total m-calpain immunoreactivity was comparable in neurons of AD and control brains (Figure 1–1A). Although activated m-calpain cannot be detected by Western blot analysis with C-18,34 we could measure the state of activation of μ-calpain, the form of calpain that is activated at micromolar calcium levels, by using a monoclonal antibody that detects latent (80 kd) and activated (76 kd) forms of this protease.35 Activated (76 kd) μ-calpain, expressed either as absolute levels (not shown) or as a ratio of the latent 80-kd form of μ-calpain (Figure 1–1, C and D), was two- to threefold higher (P < 0.03) in AD neocortex than in control cases, consistent with our immunocytochemical evidence of m-calpain activation in affected neocortical neurons and with earlier observations34,35 indicating that both μ- and m-calpains are activated in AD brains.
Figure 1.
1-1: Calpains are activated in AD brains. Vibratome sections (30 μm) of control and AD prefrontal neocortices (Lamina III) were immunohistochemically stained as described in Materials and Methods using a polyclonal antibody that recognizes total m-calpain (A), and a polyclonal antibody (C-18) that recognizes the active form of m-calpain (B) 34. Western blot analysis was carried out on cytosolic fractions of AD and control prefrontal neocortices using a monoclonal antibody to μ-calpain. The blots were developed by alkaline phosphatase-based chemiluminescence (C). The relative densities of the bands were measured by scanning the films using O-foto software followed by quantitation using NIH image and presented in bar graph. (D; n = 10; P < 0.05. The L/H ratio reflects the relative degree of activation of calpain in AD and control brain samples35 and represents the ratio between the active calpain (low molecular weight band at 76 kd = L) and the inactive precursor at high molecular mass (high molecular weight band at 80 kd = H). 1-2: Erk1,2 Map kinases are activated in AD brains. Vibratome sections (30 μm) of control and AD prefrontal neocortices (Lamina III) were immunohistochemically stained as described in Materials and Methods using (A) a monoclonal antibody that recognizes total Erk1,2 and (B) a polyclonal antibody that recognizes the phosphorylated forms of Erk1,2. Western blot analysis was carried out on whole homogenates of AD and control prefrontal neocortices using polyclonal Erk1,2, and phospho-Erk1,2 antibodies (C). The blots were developed by alkaline phosphatase-based chemiluminescence. The relative densities of the bands were measured by scanning the films using O-foto software followed by quantitation using Scan Analysis program and shown in the bar graph (D; n = 5). The measurement of P44 Erk1 and P42 Erk2 bands was done separately using the same total area for different samples. 1-3: Active calpain and active Erk1,2 are co-localized in AD brain. Paraffin sections (6 μm) from prefrontal cortex were double-immunofluorescence-labeled as described in Materials and Methods. Using a polyclonal antibody (C-18) that recognizes the active form of m-calpain and a monoclonal antibody that recognizes total Erk1as primary antibodies. Alexa 564 (red) and Alexa 468 (green) conjugated secondary antibodies were used to probe active calpain (A; red) and active Erk1,2 (B; green). The sections were imaged using a confocal microscope. The overlay displaying yellow (C) illustrates co-localization of active Erk1,2 and active calpain. 1−4. Neurofilament phosphorylation is enhanced in neocortical pyramidal neurons in AD brain. Vibratome sections (30 μm) of control and AD prefrontal neocortex (Lamina III) were immunohistochemically stained as described in Materials and Methods using (A) SMI 32 monoclonal antibody that recognizes dephospho-epitopes on the neurofilament-H and NF-M tail domains; (B) SMI-31 monoclonal antibody that specifically recognizes the phosphorylated tail domains of these neurofilament subunits and strongly decorates neurites and some perikarya (inset) in AD brains.
Map Kinases Erk1,2
Similarly, lamina III pyramidal neurons in normal and AD cases contained comparable levels of Erk1,2 immunoreactivity (Figure 1–2A); however, Erk1,2 in these neurons and their processes in AD neocortex was considerably more activated than normal as revealed by an antibody against phosphorylated Erk1,2, the activated form of this kinase (Figure 1–2B). Phospho-Erk1,2 was also often associated with fibrous structures that were thioflavin S-positive and assumed the flame-shaped appearance of neurofibrillary tangles (Figure 1–2B). Neurons in control brains were very weakly labeled, if at all (Figure 1–2B). Confirming the immunocytochemical evidence, Western blot analyses of AD neocortex showed that levels of activated forms of Erk1,2 (pErk) were elevated 1.6-fold and 0.77-fold, respectively (P < 0.01), compared to those in age-matched control cases (Figure 1–2, C and D). In addition to this highly vulnerable neuronal population in AD brain, other susceptible pyramidal cell populations in neocortex and hippocampus displayed similar immunohistochemical patterns of activated Erk1,2 and m-calpain (data not shown). To examine whether both active Erk and active calpains occur in the same neurons, we performed double immunofluorescence labeling followed by confocal imaging, which establishes the co-localization of both active Erk1,2 and active calpain in the same neurons (Figure 1–3, A to C). In the foregoing immunocytochemical and immunoblot analyses, none of these parameters studied correlated with age, PMI, or gender in these AD and control brains.
Neurofilaments
Human NF-H and NF-M contain 43 or 44 and 13 KSP motifs, respectively, on their carboxy terminal tail domains.9,11,12,36,37 Antibodies that specifically recognize the state of phosphorylation of KSP motifs on these neurofilament proteins1 revealed markedly increased phosphorylation of neurofilaments in pyramidal neurons of lamina III in the prefrontal cortex, which are among the most vulnerable to degeneration in AD (Figure 1–4). SMI 32, which recognizes the dephosphorylated state of KSP motifs, strongly decorated neuronal perikarya and their processes in 10 control brains but only weakly immunostained pyramidal neurons and their neuritic extensions in the corresponding region of 10 AD brains (Figure 1–4A). Conversely, SMI 31, which recognizes neurofilaments when they are extensively phosphorylated at multiple KSP motifs, labeled neuropil fibers more strongly in all AD prefrontal cortex samples than in the age-matched, non-demented cases (Figure 1–4B) and decorated perikarya in a subset of these neurons (Figure 1–4B, inset). These findings confirm, in our tissue samples, previous observations by Morrison and colleagues.16,38,39
Calpains Increase Erk1,2-Mediated NF Phosphorylation in Hippocampal Neurons and CG Neurons by Activating Erk1,2
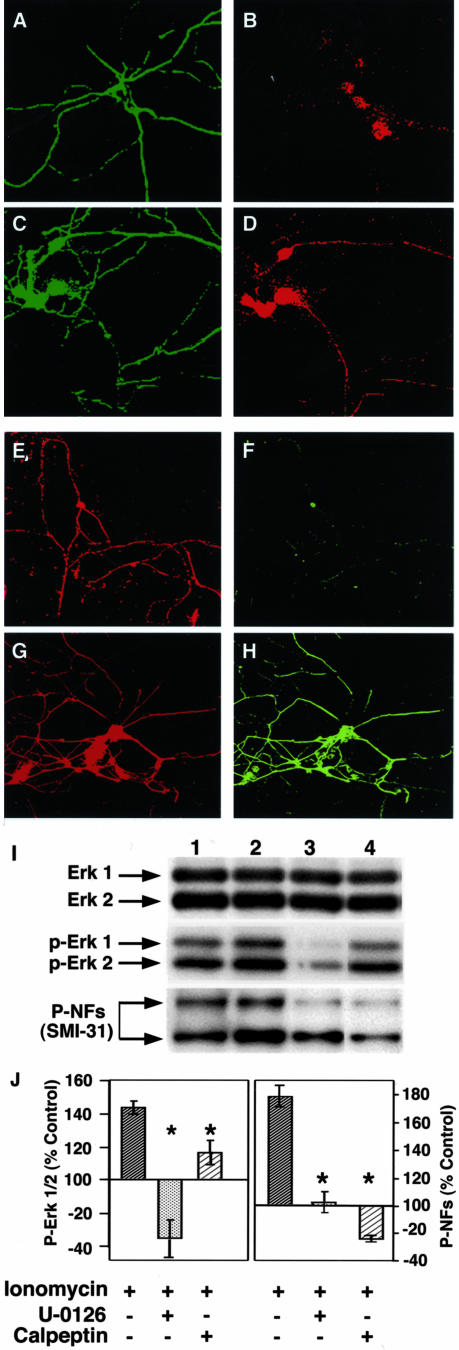
To mimic the development of neurofilament hyperphosphorylation seen in AD brain, we treated hippocampal neurons and CG neurons with the calcium ionophore ionomycin, which promotes cellular influx of calcium.40 After 24 hours of treatment with 0.5 μmol/L ionomycin, hippocampal neurons exhibited elevated levels of calpain activity as evidenced by reduced levels of uncleaved fodrin, a known calpain substrate, and by marked accumulation of a 150-kd cleavage product of brain spectrin (fodrin) known to be specifically generated by calpains (Figure 2–1, A and C). Another minor band at 120 kd specifically generated by caspase-3 cleavage of fodrin, was seen at equal immunostaining intensities in control, ionomycin- and calpeptin-treated neurons ruling out activation of caspase-3 in our experimental conditions (Figure 2–1, A and C). Calpeptin, a relatively selective and membrane-permeable inhibitor of both μ- and m-calpain, completely inhibited the ionomycin-induced proteolysis of fodrin (Figure 2–1, A and C). Ionomycin treatment of hippocampal neurons for 24 hours substantially enhanced neurofilament phosphorylation throughout the neuron and most notably in the growing neurites, which normally show relatively low levels of NF phosphorylation. For these immunocytochemical studies, we used the antibody RMO 281 (Zymed, San Francisco, CA), which recognizes the multiphosphorylation repeats on the NF-M carboxy terminal tail domain selectively, rather than using SMI 31, which cross reacts with nuclear lamins and can obscure perikaryal neurofilament labeling patterns in cultured neurons. The effects of ionomycin were exerted at the level of protein phosphorylation rather than neurofilament gene expression because the levels of immunoreactive neurofilament protein were unaltered by ionomycin treatment as judged by the unchanged NF-L levels under these treatment conditions (Figure 2–1B). We implicated calpain activation as being essential to this response by showing that calpeptin (20 μΜ), added to the cultures at a concentration that blocked calpain activity, completely inhibited ionomycin-induced hyperphosphorylation of the NF-M carboxy terminal domain (Figure 2–1, D to I). Further, to confirm that calpain activation is upstream of Erk activation in hippocampal neurons under conditions of abnormal calcium influx, we blocked Erk1,2 activation with U-0126 (a specific inhibitor of Mek1,2 which are the upstream kinases that activate Erk1,2), in neurons treated with 0.5 μmol/L of ionomycin. Although Erk1,2 activity were markedly inhibited by U0126, ionomycin-induced calpain activation, reflected by calpain-specific fodrin cleavage, was unaltered (Figure 2–2, A and B), indicating that calpain activation under these conditions of calcium injury is not downstream of Erk activation.
Figure 2.
2-1: Calpeptin inhibits ionomycin-induced calpain activation and NF-M phosphorylation in primary hippocampal neurons in culture. A: Cytosol of hippocampal neurons, untreated (lane 1) or treated with ionomycin (0.5 μmol/L; lane 2) or ionomycin with calpeptin (20 μmol/L; lane 3), were subjected to 7% SDS-PAGE followed by Western blot analysis. A α-spectrin monoclonal antibody that specifically reacts with brain spectrin (fodrin) was used at 1:1000 dilution. This antibody also reacts with a 150-kd calpain-specific cleavage product of fodrin and a caspase-3-specific cleavage product of fodrin migrating at 120 kd; fodrin breakdown products (FBP 150 and FBP 120) both indicated by arrows. B: Western blot analysis, showing unaltered NF-L in hippocampal neurons under the three experimental conditions shown in Figure 2–1A. C: The bar graph presents densitometric analyses of fodrin and the 150-kd fodrin cleavage product from multiple immunoblot analyses. The values represent the mean ± SEM from three separate experiments.*, P < 0.05. D–I: Hippocampal neurons (8 DIV), untreated (D and E) or treated with 0.5 μmol/L ionomycin (F and G) or ionomycin with calpeptin (20 μmol/L; H and I), were then immunostained with a polyclonal antibody to NF-L (D, F, H) or a monoclonal antibody to phospho-NF-M (E, G, I). The arrows in F and G point to the processes that show hyperphosphorylated NF-M. 2-2: Inhibition of Erk1,2 has no effect on ionomycin-induced calpain activation in primary hippocampal neurons in culture. A: Cytosol from hippocampal neurons, untreated (lane 1) or treated with ionomycin (0.5 μmol/L; lane 2) or ionomycin with Mek1,2 inhibitor, U-0126 (10 μmol/L; lane 3), were subjected to 7% SDS-PAGE followed by Western blot analysis, using an α-spectrin monoclonal antibody that specifically reacts with brain spectrin (fodrin) and a calpain-specific cleavage product of fodrin migrating at 150 kd. B: Densitometric analysis of the 150-kd calpain cleavage product of fodrin from immunoblot analyses as represented in A. The level of the 150-kd product is expressed as a percentage of total fodrin-related immunoreactivity to correct for unavoidable minor differences in total protein loading from different hippocampal cultures. The values represent the mean ± SEM from three separate experiments.*, P < 0.05.
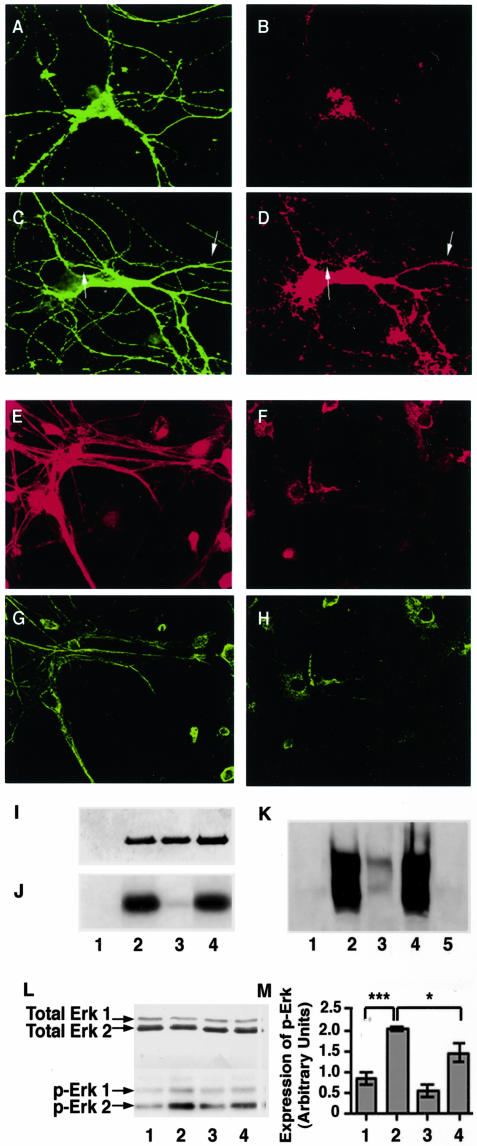
Because Erk1,2 is known to phosphorylate KSP motifs on the carboxy tail domains of NF subunits23 we investigated whether or not this protein kinase mediates ionomycin-induced NF hyperphosphorylation. Double-label confocal analyses of ionomycin-treated hippocampal neurons (Figure 3, C and D) revealed a significant increase in phospho-Erk1,2 immunoreactivity compared to that in untreated neurons (Figure 3, A and B). Similar to the phospho-NF-M immunolabeling pattern, activated Erk1,2 immunoreactivity was stronger not only in perikarya but also in neuronal processes, which normally contain low levels of phospho-Erk1,2. Treatment with U-0126, or calpeptin (which inhibits both μ-and m-calpains), significantly reduced the levels of active Erk1,2 in hippocampal neurons treated with ionomycin (Figure 3, L and M). As expected, neurons treated with U-0126 alone showed a marginal reduction in basal activation of Erk1,2 (data not shown). Addition of U-0126 to an in vitro kinase reaction mixture containing Mek1,2, Erk2, and a KSPXXXK fusion protein inhibited both Erk2 activation and KSPXXXK fusion protein phosphorylation. (Figure 3, I to K). Similarly, addition of an oligonucleotide encoding an antisense sequence around the initiation codon of the Erk2 gene to the medium of these neuronal cultures significantly diminished Erk1,2 immunoreactivity in hippocampal neurons (Figure 3, E and F) and lowered levels of phosphorylated NF-M commensurately (Figure 3, G and H).
Figure 3.
The Mek1,2 inhibitor, U-0126, calpeptin, and Erk1,2 antisense oligonucleotides attenuate Erk1,2 activation and NF-phosphorylation in hippocampal neurons. A–H: Hippocampal neurons (14 DIV) were either untreated (A and B) or treated with 0.5 μmol/L ionomycin (C and D) were immunostained with a monoclonal antibody to β tubulin (A and C) and a polyclonal antibody to phospho-Erk 1,2 (B and D). These neurons were also treated with sense (E and G) and antisense (F and H) oligonucleotides against Erk1,2 mRNA as described in Materials and Methods and were immunostained with a goat polyclonal antibody (Santa Cruz) against Erk1,2 (E and F) and with a monoclonal antibody to phospho-NF-M (G and H). I: Recombinant Erk2 (1 μg) was incubated with the immunprecipitate of Mek1,2, from lysates of ionomycin-treated hippocampal neurons, in the absence (lane 2) and presence (lane 3) of U-0126 (10 μmol/L) or calpeptin (lane 4; 20 μΜ) in a kinase reaction mixture detailed in the text, followed by SDS-PAGE and silver stain. Lane 1 represents the immunoprecipitate of Mek1,2. J: Autoradiogram of Erk2 shown in I. K: Representive image taken of a Western blot for a KSPXXXK fusion protein using the monoclonal antibody RT-97, specific for phosphorylated NF-H and NF-M. The KSPXXXK fusion protein was incubated in a kinase reaction mixture containing the immunoprecipitate of Mek1,2 and recombinant Erk2, in the absence (lane 2) and presence (lane 3) of U-0126 (10 μmol/L) or calpeptin (lane 4; 20 μΜ). Lane 1 represents the mixture of immunoprecipitate of Mek1,2 and Erk2, and lane 5 represents KSPXXXK fusion protein alone. L: Representive image taken of a Western blot for hippocampal neuron cell lysates (15 μg) in vehicle-treated (lane 1), ionomycin (0.5 μmol/L; lane 2), ionomycin and U-0126- (0.5 μmol/L and 10 μmol/L; lane 3) and calpeptin-treated (20 μmol/L; lane 4) neurons for 24 hours, that were probed for Erk1,2 and phospho-Erk1,2 expression. M: Bar graph shows the relative band intensities of p-Erk1,2. Values are expressed as mean ± SEM for three separate experiments. The ionomycin-treated neurons (lane 2) showed a significantly higher activation compared to vehicle-treated controls (lane 1; P < 0.001, n = 3) and the neurons treated with ionomycin together with calpeptin (lane 4; P < 0.05, n = 3).
To confirm these findings and to examine the interrelationship between calpains, Erk and NF phosphorylation further, we also conducted experiments in CG neurons, which yield more abundant protein for Western blot analyses than the hippocampal cultures. By immunocytochemistry, ionomycin- (0.5 μmol/L) treated CG neurons showed a stimulatory effect on neurofilament carboxy-terminal tail phosphorylation (Figure 4 E to H) and Erk1,2 activation (Figure 4, A to D) similar to that in hippocampal neurons (Figure 3, A to D, L, and M). Western blot analysis of CG neurons in cultures confirmed that ionomycin treatment for 24 hours significantly elevated the levels of activated Erk1,2 (phospho-ERK) more than 40% (P < 0.05) compared to untreated cultures, without affecting total Erk1,2 levels (Figure 4, I and J). Erk1,2 activation was associated with elevated levels of extensively phosphorylated NF-M and NF-H, detected using SMI 31. On the other hand, co-treatment of ionomycin-treated neurons with U-0126, inhibited the activation of Erk1,2 kinases and commensurately decreased the phosphorylation state of NF-H and NF-M subunits (Figure 4, I and J). Treatment of CG neurons with calpeptin also substantially reduced the ionomycin-induced activation of Erk1,2 and completely blocked the ionomycin-induced hyperphosphorylation of NF subunits (Figure 4, I and J).
Figure 4.
The Mek1,2 inhibitor, U-0126, and calpeptin attenuate ionomycin-induced Erk1,2 activation and NF-phosphorylation in cerebellar granule neurons. A–D: CG neurons (7 DIV) either vehicle-treated (A, B, E, F) or treated with 0.5 μmol/L ionomycin (C, D, G, H), were immunostained with antibodies against β-tubulin (A and C), p-Erk1,2 (B and D), NF-L (E and G), and phospho-NF-M (F and H). E: The CG neurons (7 DIV), either vehicle-treated (lane 1) or treated with ionomycin (0.5 μmol/L; lane 2) or ionomycin together with either U-0126 (10 μmol/L; lane 3) or calpeptin (20 μmol/L; lane 4) for 24 hours, were subjected to SDS-PAGE (equal protein loading, 15 μg) and Western blot analysis with either anti-Erk1,2, anti p-Erk1,2, or SMI 31 against phospho-NF-H and neurofilament M. F: Bar graph shows the relative band intensities of p-Erk1,2 (left) and phospho-NF-M (right). The values are the mean ± SEM of three to five separate experiments. *, P < 0.05.
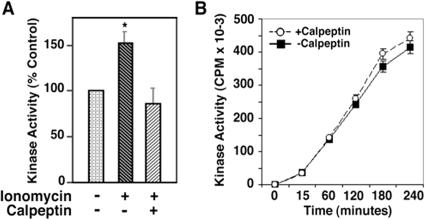
To confirm the effect of calpeptin on Erk1,2 activation, we measured Erk1,2 activity after immunoprecipitating the kinase from neuronal cultures treated with either ionomycin, calpeptin, or both. Calpeptin completely inhibited the ionomycin-induced stimulation of Erk1,2 activity (Figure 5A) while only having a modest effect on baseline activity (data not shown). Calpeptin had no direct effect on Erk2 activity when added to the in vitro kinase assay using recombinant Erk2 as enzyme (Figure 5B), indicating that its inhibitory effect on Erk1,2 activity in intact cells is through calpain inhibition rather than direct inhibition of Erk1,2.
Figure 5.
Calpeptin reverses ionomycin-induced Erk1,2 activation in CG neurons but has no direct effect on Erk1,2 activity in vitro. A: CG neurons (7 DIV), were either vehicle-treated (bar 1), or treated with ionomycin (0.5 μmol/L; bar 2), or ionomycin together with calpeptin (20 μmol/L; bar 3) for 24 hours were then lysed. Erk1,2 were immuno-precipitated using anti-Erk1,2 polyclonal antibody, and kinase activity was measured using myelin basic protein as a substrate. The relative kinase activity is expressed as a percentage of the vehicle-treated control, represented as 100%. Values are means ± SEM for five separate experiments, P < 0.05. B: A time course of Erk2 kinase activity in the presence and absence of calpeptin (20 μmol/L). In vitro kinase activity was monitored using a synthetic peptide as described in Materials and Methods. Values are means ± SEM for five determinations at each time point.
Discussion
Our findings implicate calpains as activators of the MAPK pathway leading directly to modification of the cytoskeleton where calcium influx into neurons is increased. Our results also provide evidence that neurofilament hyperphosphorylation in vulnerable neuronal populations in Alzheimer’s disease may involve a calpain-mediated rise in Erk1,2 activity. Calpains are known to regulate the activities of various enzymes, including several protein kinases and phosphatases that modify the cytoskeleton, in addition to cleaving cytoskeletal proteins directly.41–43 Based on these actions, calpain overactivation in AD35,44 has been proposed to play an important role in the development of cytoskeletal pathology and neurodegeneration, and is consistent with considerable evidence for alterations of calcium homeostasis in AD pathogenesis.44–47 Our data in human brain confirms earlier observations that activated forms of calpain are abundantly associated with neurofibrillary tangles and neuropil threads in Alzheimer’s disease and in a range of tauopathies48,49 and that the association is relatively selective for these inclusions.49
The role of calpain in the activation of Erk1,2 links together disparate recent observations on calcium signaling through the MAPK pathway and suggests that calpain-dependent Erk1,2 activation is part of a general mechanism for calcium signaling under various conditions. Our data indicate that calpain and Erk1,2 are most likely events within a single pathway. The placement of calpains upstream of Erk activation in neurons exposed to ionomycin is supported by both the effectiveness of calpeptin in inhibiting Erk activation and the lack of efficacy of MEK inhibition in blocking calpain activation. Both of these studies were substantiated by direct assessment of the calpain-specific cleavage of fodrin. These results differ from those in fibroblasts under conditions of Erk overexpression where calpain activation was dependent on Erk activity;50 however, these observations and ours are not incompatible. Calpain activity is regulated by several mechanisms, including direct activation by calcium, modulation of calpain sensitivity to a given calcium concentration, or possibly interaction with regulatory cofactors or inhibitors.51 In fibroblasts, EGF receptor signaling selectively activated m-calpain, and not μ-calpain, by a mechanism that has not been shown to require calcium fluxes.50 In our studies, the direct activation of both μ-and m-calpains by ionomycin-induced elevation of calcium is expected based on considerable evidence for the calcium-dependent conversion of latent calpain to its active form.52,53 We have also established here that calpain does not directly activate Erk1,2. While further studies are required to identify the additional intermediate steps in the mechanism of Erk1,2 activation by calpain, our results establish a pathway from calcium injury to neurofilament phosphorylation that involves calpain-dependent Erk activation. The ability of transfected μ-calpain in 293 cells to stimulate spectrin breakdown and Erk1,2 activation and the inhibition of these processes by overexpressing calpastatin, the endogenous inhibitor of calpain in 293 cells (unpublished observations) is additional strong support for the hypothesis that calpains are upstream of Erk1,2.
Our observation that the phosphorylation of neurofilament carboxy tail domains in response to increased intracellular calcium is largely mediated by Erk1,2, supports evidence that KSP motifs on neurofilament subunits are substrates for Erk1,2.8,23 The residual phosphorylation of neurofilaments observed in our studies, despite inhibition of Erks, reflects contributions from other neurofilament kinases like JNKs and cdk5. In this regard, SAPKs, members of the MAP kinase family involved in cell stress responses and Cdk5,8,54,55 are also known to phosphorylate the multiphosphorylation repeat domain of neurofilament proteins and are up-regulated in AD brain. Although it is possible that both of these protein kinases are activated after ionomycin treatment, neither appears to play a major role in calcium-induced hyperphosphorylation of neurofilament proteins, which was completely blocked by inhibiting MAP kinase activity.
Calpains are activated in various states of calcium injury, and their proteolytic actions are, in many cases, believed to be critical to neurodegeneration and neuronal cell death in these states. Activated calpain is clearly lethal to neurons or other cells when the level of activation is high.51,56,57 In this regard, Erk1,2 kinases have also been shown to be involved in neuronal death caused by several neurotoxins and experimental treatments in vitro.58–63 In each of these cases, MEK1,2 inhibition blocked cell death, suggesting that the MAPK pathway was involved in the cell death process.
Erk1,2 activation is not always essential to the cell death process after calpain activation.64–66 Low levels of calpain and Erk activation, leading to increased neurofilament phosphorylation could even be neuroprotective in some circumstances. Phosphorylation of neurofilament carboxy tail domains increases the resistance of neurofilaments to proteolysis67 and increases transcription of the low molecular weight neurofilament subunit.68 These responses, which can also be elicited by neurotrophic factors,69 are known to promote the outgrowth and stabilization of neurites.70,71 The effectiveness of the MAPK pathway in promoting either survival or death may depend on the cellular context in which it is activated, which may include the relative activities of other kinase pathways.72 With respect to AD, calpains and Erk1,2 are both activated, and neurofilament and tau hyperphosphorylation develops at early stages of AD well before neurons degenerate,24,34,35,38,39,73 suggesting that this early response might be neuroprotective in AD. Neurofilament hyperphosphorylation, however, is part of a general pathological response in AD tangle formation that includes alterations of tau phophorylation, which is also regulated by Erks.24,25,74 Ultimately, these responses may contribute to the development of tau pathology and neurofibrillary tangle formation.
Acknowledgments
We thank Hayley McAuliff, Janet Rosdil, and Gina Lardi for help with manuscript preparation, Drs. Phil Grant and ShriHari Kadkol for critical reading of the manuscript, and Joel P. Jacob for his help in hippocampal neuronal cultures.
Footnotes
Address reprint requests to Ralph A. Nixon, M.D., Ph.D., Nathan Kline Institute, New York University School of Medicine, 140 Old Orangeburg Rd., Orangeburg, NY 10962. E-mail: Nixon@nki.rfmh.org.
Supported by National Institute on Aging grants AG05604 and P01A02219.
References
- Sternberger NH, Sternberger LA, Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Walkenstein N, Lee VM. Expression of neurofilament subunits in neurons of the central and peripheral nervous system: an immunohistochemical study with monoclonal antibodies. J Neurosci. 1986;6:650–660. doi: 10.1523/JNEUROSCI.06-03-00650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J. Neurofilament functions in health and disease. Curr Opin Neurobiol. 1999;9:554–560. doi: 10.1016/S0959-4388(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Lavedan C, Buchholtz S, Nussbaum R, Albin R, Polymeropoulos M. A mutation in the human neurofilament M gene in Parkinson’s disease that suggests a role for the cytoskeleton in neuronal degeneration. Neurosci Lett. 2002;322:57–61. doi: 10.1016/s0304-3940(01)02513-7. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A, Miller C. Neurofilaments and neurological disease. Bioessays. 2003;25:346–355. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Sihag RK. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 1991;14:501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathobiology. Brain Pathol. 1993;3:29–38. doi: 10.1111/j.1750-3639.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Pant HC, Veeranna, Grant P. Regulation of axonal neurofilament phosphorylation. Curr Top Cell Regul. 2000;36:133–150. doi: 10.1016/s0070-2137(01)80006-6. [DOI] [PubMed] [Google Scholar]
- Lee V, Otvos L, Jr, Carde M, Hollosi M, Dietzschold B, Lazzarini R. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci USA. 1988;85:1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos LJ, Hollosi M, Perczel A, Dietzschold B, Fasman GD. Phosphorylation loops in synthetic peptides of the human neurofilament protein middle-sized subunit. J Protein Chem. 1988;7:365–376. doi: 10.1007/BF01024886. [DOI] [PubMed] [Google Scholar]
- Vechio J, Bruijn L, Xu Z, Brown RJ, Cleveland D. Sequence variants in human neurofilament proteins: absence of linkage to familial amyotrophic lateral sclerosis. Ann Neurol. 1996;40:603–610. doi: 10.1002/ana.410400410. [DOI] [PubMed] [Google Scholar]
- Lacoste-Royal G, Mathieu M, Gauvreau D. HincII RFLP in the human gene for the heavy neurofilament subunit (NF-H). Nucleic Acids Res. 1989;17:6434. doi: 10.1093/nar/17.15.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I. Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett. 2001;507:81–87. doi: 10.1016/s0014-5793(01)02944-1. [DOI] [PubMed] [Google Scholar]
- Dixon M, Bub D, Chertkow H, Arguin M. Object identification deficits in dementia of the Alzheimer type: combined effects of semantic and visual proximity. J Int Neuropsychol Soc. 1999;5:330–345. doi: 10.1017/s1355617799544044. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Morrison JH. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol. 1989;282:191–205. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Lewis DA, Campbell MJ, Huntley GW, Benson DL, Bouras C. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer’s disease. Brain Res. 1987;416:331–336. doi: 10.1016/0006-8993(87)90914-0. [DOI] [PubMed] [Google Scholar]
- Brion J, Smith C, Couck A, Gallo J, Anderton B. Developmental changes in tau phosphorylation: fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer’s disease. J Neurochem. 1993;61:2071–2080. doi: 10.1111/j.1471-4159.1993.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Lovestone S, Reynolds C. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- Lovestone S. Early diagnosis and the clinical genetics of Alzheimer’s disease. J Neurol Sci. 1999;246:69–72. doi: 10.1007/s004150050310. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Masliah E, Jin L, Cole G, Wieloch T, Shapiro I. Protein kinases and phosphorylation in neurologic disorders and cell death. Lab Invest. 1991;64:596–616. [PubMed] [Google Scholar]
- Rapoport M, Ferreira A. PD98059 prevents neurite degeneration induced by fibrillar β-amyloid in mature hippocampal neurons. J Neurochem. 2000;74:125–133. doi: 10.1046/j.1471-4159.2000.0740125.x. [DOI] [PubMed] [Google Scholar]
- Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Braak H, An WL, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I. Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Brain Res Mol Brain Res. 2002;109:45–55. doi: 10.1016/s0169-328x(02)00488-6. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Vinals F, Ribalta T. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration. Brain Pathol. 2001;11:144–158. doi: 10.1111/j.1750-3639.2001.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel D, Sumi S, Crain B, Brownlee L, Vogel F, Hughes J, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Veeranna, Shetty KT, Link WT, Jaffe H, Wang J, Pant HC. Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J Neurochem. 1995;64:2681–2690. doi: 10.1046/j.1471-4159.1995.64062681.x. [DOI] [PubMed] [Google Scholar]
- Dzandu J, Johnson J, Wise G. Sodium dodecyl sulfate-gel electrophoresis: staining of polypeptides using heavy metal salts. Anal Biochem. 1988;174:157–167. doi: 10.1016/0003-2697(88)90531-3. [DOI] [PubMed] [Google Scholar]
- Arancio O, Lev-Ram V, Tsien R, Kandel E, Hawkins R. Nitric oxide acts as a retrograde messenger during long-term potentiation in cultured hippocampal neurons. J Physiol (Paris) 1996;90:321–322. doi: 10.1016/s0928-4257(97)87907-7. [DOI] [PubMed] [Google Scholar]
- Oberto A, Marks N, Evans H, Guidotti A. Lead (Pb+2) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J Pharmacol Exp Ther. 1996;279:435–442. doi: 10.1163/2211730x96x00234. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Thayer CY, Bird ED, Wheelock TR, Nixon RA. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer’s disease: evidence for a neuronal origin. Brain Res. 1990;513:181–192. doi: 10.1016/0006-8993(90)90456-l. [DOI] [PubMed] [Google Scholar]
- Veeranna, Shetty KT, Amin N, Grant P, Albers RW, Pant HC. Inhibition of neuronal cyclin-dependent kinase-5 by staurosporine and purine analogs is independent of activation by Munc-18. Neurochem Res. 1996;21:629–636. doi: 10.1007/BF02527763. [DOI] [PubMed] [Google Scholar]
- Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain Res. 1997;763:145–158. doi: 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MW, Lazzarini RA, Lee VM, Schlaepfer WW, Nelson DL. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987;6:1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Collatz M, Rudel R, Brinkmeier H. Intracellular calcium chelator BAPTA protects cells against toxic calcium overload but also alters physiological calcium responses. Cell Calcium. 1997:453–459. doi: 10.1016/s0143-4160(97)90056-7. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Guttmann RP. Calpains: intact and active? Bioessays. 1997;19:1011–1018. doi: 10.1002/bies.950191111. [DOI] [PubMed] [Google Scholar]
- Ekinci FJ, Shea TB. Free PKC catalytic subunits (PKM) phosphorylate tau via a pathway distinct from that utilized by intact PKC. Brain Res. 1999;850:207–216. doi: 10.1016/s0006-8993(99)02146-0. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Banno Y, Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y, Sakai N. Crucial role of calpain in hypoxic PC12 cell death: calpain, but not caspases, mediates degradation of cytoskeletal proteins and protein kinase C-α and -δ. Neurol Res. 2001;23:522–530. doi: 10.1179/016164101101198776. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, Mohan PS, Shea TB, Beermann M. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann NY Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- O’Neill C, Cowburn R, Bonkale W, Ohm T, Fastbom J, Carmody M, Kelliher M. Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer’s disease. Biochem Soc Symp. 2001;67:177–194. doi: 10.1042/bss0670177. [DOI] [PubMed] [Google Scholar]
- Mattson M, Chan S. Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium. 2003;34:385–397. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Adamec E, Murrell J, Takao M, Hobbs W, Nixon R, Ghetti B, Vonsattel JP. P301L tauopathy: confocal immunofluorescence study of perinuclear aggregation of the mutated protein. J Neurol Sci. 2002;1–2:85–93. doi: 10.1016/s0022-510x(02)00150-8. [DOI] [PubMed] [Google Scholar]
- Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol (Berl) 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Nixon R. The calpains in aging and aging-related diseases. Aging Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Yuan Y, Jackson SP. Calpain regulation of integrin α IIb β 3 signaling in human platelets. Platelets. 2000;11:189–198. doi: 10.1080/09537100050057620. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Hosfield CM, Lim D, Elce JS, Jia Z, Davies PL. A Ca(2+) switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Li BS, Pant HC. Cyclin-dependent kinase 5 in neurofilament function and regulation. Neurosignals. 2003;12:252–264. doi: 10.1159/000074627. [DOI] [PubMed] [Google Scholar]
- Lau LF, Ahlijanian MK. Role of cdk5 in the pathogenesis of Alzheimer’s disease. Neurosignals. 2003;12:209–214. doi: 10.1159/000074622. [DOI] [PubMed] [Google Scholar]
- Lankiewicz S, Marc Luetjens C, Truc Bui N, Krohn A, Poppe M, Cole G, Saido T, Prehn J. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J Biol Chem. 2000;275:17064–17071. doi: 10.1074/jbc.275.22.17064. [DOI] [PubMed] [Google Scholar]
- Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch T, Auerswald EA, Welsh K, Reed J, Fritz H, Fuentes-Prior P, Spiess E, Salvesen G, Machleidt W. Ionomycin-activated calpain triggers apoptosis: a probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–27226. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- Park J, Koh J. Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. J Neurochem. 1999;73:450–456. doi: 10.1046/j.1471-4159.1999.0730450.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gu Z, Zhang G, Jing G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res. 2000;857:71–77. doi: 10.1016/s0006-8993(99)02364-1. [DOI] [PubMed] [Google Scholar]
- Guise S, Braguer D, Carles G, Delacourte A, Briand C. Hyperphosphorylation of tau is mediated by ERK activation during anticancer drug-induced apoptosis in neuroblastoma cells. J Neurosci Res. 2001;63:257–267. doi: 10.1002/1097-4547(20010201)63:3<257::AID-JNR1019>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Murray B, Alessandrini A, Cole A, Yee A, Furshpan E. Inhibition of the p44/42 MAP kinase pathway protects hippocampal neurons in a cell-culture model of seizure activity. Proc Natl Acad Sci USA. 1998;95:11975–11980. doi: 10.1073/pnas.95.20.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runden E, Seglen P, Haug F, Ottersen O, Wieloch T, Shamloo M, Laake J. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism. J Neurosci. 1998;18:7296–7305. doi: 10.1523/JNEUROSCI.18-18-07296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan P. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–7930. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Holtzman D. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu C, Qiu L, Hagberg H, Sandberg M, Blomgren K. Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischaemia. J Neurochem. 2003;86:351–362. doi: 10.1046/j.1471-4159.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- Abe K, Saito H. Amyloid beta neurotoxicity not mediated by the mitogen-activated protein kinase cascade in cultured rat hippocampal and cortical neurons. Neurosci Lett. 2000;292:1–4. doi: 10.1016/s0304-3940(00)01415-4. [DOI] [PubMed] [Google Scholar]
- Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–658. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentrich E, Han S, Pessoa-Brandao L, Butterfield L, Heasley L. Collaboration of JNKs and ERKs in nerve growth factor regulation of the neurofilament light chain promoter in PC12 cells. J Biol Chem. 2002;277:4110–4118. doi: 10.1074/jbc.M107824200. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Wrathall JR. Neurotrophic factors in central nervous system trauma. J Neurotrauma. 1995;12:853–870. [Google Scholar]
- Black M, Aletta J, Greene L. Regulation of microtubule composition and stability during nerve growth factor-promoted neurite outgrowth. J Cell Biol. 1986;103:545–557. doi: 10.1083/jcb.103.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, DiTella M, Feiguin F, Carri N, Caceres A. Neurotrophin-3 enhances neurite outgrowth in cultured hippocampal pyramidal neurons. J Neurosci Res. 1994;39:219–232. doi: 10.1002/jnr.490390212. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis R, Greenberg M. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Vickers J, Riederer B, Marugg R, Buee-Scherrer V, Buee L, Delacourte A, Morrison J. Alterations in neurofilament protein immunoreactivity in human hippocampal neurons related to normal aging and Alzheimer’s disease. Neuroscience. 1994;62:1–13. doi: 10.1016/0306-4522(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Lu Q, Soria J, Wood J. p44mpk MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J Neurosci Res. 1993;35:439–444. doi: 10.1002/jnr.490350411. [DOI] [PubMed] [Google Scholar]