Abstract
Human hepatocytes were transplanted into urokinase-type plasminogen activator-transgenic SCID mice (uPA/SCID mice), which are immunodeficient and undergo liver failure. The transplanted cells were characterized in terms of their in vivo growth potential and functions. The human hepatocytes progressively repopulated the murine host liver. However, the recipients died when the replacement index (RI) of the human hepatocytes exceeded 50%. The hosts (chimeric mice) survived at RI >50% when treated with a drug that has anti-human complement factor activity, and these mice developed livers with RI values as high as 96%. In total, 36 chimeric mice were generated, and the rate of successful engraftment was as high as 92%. The yield of chimeric mice with RI >70% was 32%. The human hepatocytes in the murine host liver expressed mRNAs for a variety of human cytochrome P450 (hCYP) subtypes, in a manner that was similar to the donor liver. The mRNAs for hCYP3A4 and hCYP1A1/2 were induced in the liver in a CYP type-specific manner when the mice were treated with rifampicin and 3-methylcholanthrene, respectively. These results indicate that human hepatocytes that propagate in mice retain their normal pharmacological responses. We conclude that the chimeric mouse developed in the present study is a useful model for assessing the functions and pharmacological responses of human hepatocytes.
The human liver is an important organ for pharmacological studies aimed at developing new human medicines. The availability of normal human hepatocytes would facilitate the treatment of liver failure patients with human hepatocyte-incorporated artificial liver devices or transplantation therapy involving human hepatocytes. Recently, researchers have developed humanized (chimeric) mice by partially repopulating the mouse liver with human hepatocytes. Dandri and colleagues1 transplanted fresh human hepatocytes into mice that were generated by crossing mice transgenic for urokinase-type plasminogen activator (uPA) with mice that carried a deletion of the recombinant activation gene-2 (RAG-2). The replacement index (RI) of the resulting chimeric liver was 15% at most. Human hepatitis B viruses were able to infect and replicate in the humanized uPA/RAG-2 mice.1 Humanized mice were also produced by transplanting frozen and thawed (F-T) human hepatocytes into uPA/SCID mice, and these mice have been used for infection studies with human hepatitis C viruses.2
Immunodeficient mice that undergo liver failure are useful hosts for the propagation of human hepatocytes, and humanized mice thus obtained are useful for human hepatocyte-related medical and clinical studies. However, the following issues remain unresolved: whether a mouse can be generated whose liver is replaced completely with human hepatocytes; and whether human hepatocytes that are propagated in mice metabolize chemicals and drugs in a manner that is identical to that of the liver of the human body. The resolution of these issues is crucial to facilitating the application of humanized mice in various areas of medical science, such as pathology, physiology, and pharmacology. Assuming that human hepatocytes can be propagated efficiently in a mouse so that they retain their normal spectrum of differentiation phenotypes, these cells can be used in place of human liver for studying the biological characteristics of human hepatocytes and for testing the metabolism and safety of medicines. Furthermore, the ability to propagate large numbers of human hepatocytes as cell sources for extracorporeal artificial liver devices and transplantation therapy is clinically relevant.
In the present study, we initially attempted to generate uPA/SCID mice with livers that were almost completely repopulated with human hepatocytes. Subsequently, the livers of the chimeric mice were tested for normal expression of the various forms of human cytochrome P450 (hCYP), which play central roles in metabolizing medicines in the human liver. In this context, we also examined whether the chimeric liver showed induction of the appropriate hCYP subtype when the mouse was administered a drug with known specificity for a certain CYP subtype in the human liver.
Materials and Methods
Generation of uPA/SCID Mice
The uPA/SCID mice were generated by crossing uPA mice [B6SJL-TgN(Alb1Plau)144Bri; The Jackson Laboratory, Bar Harbor, ME] with SCID mice (Fox Chase SCID C.B-17/Icr-scid Jcl; Clea Japan Inc., Tokyo, Japan). Genomic DNA was isolated from the tail tissues of mice 8 to 10 days after birth using the DNeasy tissue kit (Qiagen, Tokyo, Japan). The presence of the uPA transgene in the genomic DNA was assessed by polymerase chain reaction (PCR) amplification of the human growth hormone (hGH) sequence, which was introduced in the transgene, using the primers (hGH1) listed in Table 1. The genotypes of the SCID mice were distinguished by PCR-restriction fragment length polymorphism for the presence or absence of the point mutation in the gene for the DNA-dependent protein kinase3 using primers (SCID) listed in Table 1.
Table 1.
Oligonucleotide Primers and TaqMan Probes Used in PCR Amplifications
| Gene | Forward primers (5′-3′) [TaqMan probes (5′-3′)] | Reverse primers (5′-3′) |
|---|---|---|
| hGH1 | CGCCCAACTCTGAGTGGG | CGCCCAACTCTGAGTGGG |
| SCID | GTGTCTGACTAGAAAGCTAGAGAGA | CACAGTGAAGTGCCATACTGCTCA |
| hGH2 | GTCTTGGCTCGCTGCAATC | CGGGAGACTGAGGCAGGAG |
| [CCGCCTCCTGGGTTCAAGCGA] | ||
| mGAPDH | GGATGCAGGGATGATGTTC | TGCACCACCAACTGCTTAG |
| [CAGAAGACTGTGGATGGCCCTC] | ||
| hAlu | GGCGCGGTGGCTCACG | TTTTTTGAGACGGAGTCTCGCTC |
| mc-mos | GAATTCAGATTTGTGCATACACAGTGACT | AACATTTTTCGGGAATAAAAGTTGAGT |
| hCYP1A1 | TGATTGGGCACATGCTGAC | GCTGGCTCATCCTTGACAGT |
| hCYP1A2 | AGACTGCCTCCTCCGGG | CAGGGTTAGGCAGGTAGCG |
| hCYP2C9 | GGAGATCCGGCGTTTCTC | CGGTCCTCAATGCTCCTCTT |
| hCYP2C19 | GAAGGAGATCCGGCGTTTC | TCAATGCTCCTCTTCCCCAT |
| hCYP2D6 | GTCCAACAGGAGATCGACGA | GGCATGTGAGCCTGGTCA |
| hCYP3A4 | TGTGAGGAGGTAGATTTGGCTC | TCAGGAGGAGTTAATGGTGCTAA |
| hGAPDH | CCACCTTTGACGCTGGG | CATACCAGGAAATGAGCTTGACA |
Determination of the Zygosity of the uPA Transgene
The zygosity of the uPA+/− and uPA+/+ mice was determined by quantifying the hGH sequence, which was inserted in the uPA transgene. The level of uPA transgene in the genomic DNA was measured by real-time quantitative genomic PCR4 using genomic DNA as the template, together with the Universal PCR master mix (Applied Biosystems, Tokyo, Japan), forward and reverse primers, and TaqMan probes for hGH2 (Table 1). PCR was performed using the PRISM 7700 sequence detector (Applied Biosystems). The genes for hGH and murine glyceraldehyde 3-phosphate dehydrogenase (mGAPDH) were quantified, and the ratio of hGH2 to mGAPDH was calculated. The tested mice were divided into two groups with higher and lower hGH2:mGAPDH ratios, whereby the ratio for the higher group was approximately twofold higher than that for the lower group. We defined the mice in the higher ratio group and lower ratio group as uPA+/+ and uPA+/− mice, respectively.
Isolation of Human Hepatocytes
The present study was performed with the approval of the ethics board of the Hiroshima Prefectural Institute of Industrial Science and Technology. Liver tissues were isolated from Japanese donors who gave their consent before the operation, in accordance with the 1975 Helsinki Declaration. These tissues were used to isolate hepatocytes using the method reported previously,5 except that the disaggregated liver cells were centrifuged at 50 × g for 2 minutes instead of 1 minute, to yield parenchymal hepatocytes (PHs). Two types of hepatocytes, PHs and small hepatocytes (SHs),5–8 were prepared and used in the present study. The PHs were isolated from nine donors with ages of 3, 12, 16, 37, 47, 51, 53, 58, and 61 years, with viability of 94%, 92%, 95%, 62%, 84%, 88%, 92%, 94%, and 79%, respectively. The supernatants obtained from four of these nine donors were centrifuged at 150 × g for 5 minutes, to obtain the nonparenchymal cell (NPC) fraction. The NPC fractions isolated from the 12-, 16-, 53-, and 58-year-old donors contained SHs that could be identified by phase-contrast microscopy at rates of 93%, 95%, 36%, and 87%, respectively. The viability rates of the SHs from the 12-, 16-, 53-, and 58-year-old donors were 82%, 85%, 76%, and 87%, respectively. The NPC fraction was used as a source of SHs and was transplanted in uPA+/+/SCID+/+ mice. There were no significant differences between the RI values of the PHs and SHs at 52 to 105 days after transplantation. Thus, in the present study, we used both PHs and SHs without discrimination.
Cryopreservation and Thawing of Human Hepatocytes
Isolated PHs and NPCs were suspended at 107 hepatocytes/ml/vial in cryopreservation solution (Kurabo Industries Ltd., Osaka, Japan), and cryopreserved using the programmable freezer KRYO-10 Series III (Planner Products Ltd., Middlesex, UK), followed by storage in liquid nitrogen. The cells were thawed by immersing the vials in a 37°C water bath, followed by rapid dilution of the cells with culture medium at 4°C. The cells were washed twice to remove the cryopreservation solution. The trypan blue exclusion test was performed on the thawed cells. The viability rates of the F-T PHs from donors of age 12, 16, 53, and 58 years were 90% (mean, n = 2), 64%, 86%, and 54%, respectively. The viability rates of the F-T SHs from the 12-, 47-, 53-, and 61-year-old donors were 79 ± 3% (mean ± SD, n = 3), 39%, 60%, and 32%, respectively. We also used commercially available frozen human hepatocytes, which represent hepatocytes from a 9-month-old-Caucasian boy (IVT079; In Vitro Technologies Inc., Baltimore, MD). These hepatocytes were called 9MM in the present study. The 9MM hepatocytes were thawed as described above, and transplanted into the uPA+/+/SCID+/+ or uPA+/−/SCID+/+ mice, as detailed below; cell viability at the time of transplantation was 81 ± 6% (n = 4).
Transplantation of Human Hepatocytes
The uPA+/+/SCID+/+ mice and uPA+/−/SCID+/+ mice at 20 to 30 days after birth were injected intraperitoneally with 100 μl of 1 mg/ml anti-asialo GM1 rabbit antiserum (Wako Pure Chemical Industries Ltd., Osaka, Japan), 1 day before transplantation. Fresh or F-T hepatocytes were used as the donor cells. These mice were anesthetized with ether and injected with 5.0 to 7.5 × 105 viable hepatocytes through a small left-flank incision into the inferior splenic pole. The lower reaches of the injection site were tied with string for hemostasis. In certain instances, the host animals were injected intraperitoneally with 200 μl of 1.5 mg/ml Futhan (nafamostat mesilate, 6-amidino-2-naphthyl p-guanidinobenzoate dimethanesulfonate; gifted by Torii Pharmaceutical Co., Ltd., Tokyo, Japan).
Measurement of Human Albumin (hAb) and hC3a in Mouse Blood, and Clinical Chemistry of Mouse Serum
Blood samples (5 μl) were collected periodically from the tail vein, and the levels of hAb and human C3a (hC3a)-desArg were determined with the Human Albumin ELISA Quantitation kit (Bethyl Laboratories Inc., Montgomery, TX) and ORTEIA hC3a ELISA kit (Pharmingen, San Diego, CA), respectively. Sera were collected at sacrifice and analyzed for GPT and total albumin (tAb) using the FDC 3500 photometer (FUJIFILM Co., Ltd., Tokyo, Japan).
Futhan Administration to Human Serum-Injected uPA−/−/SCID+/+ Mice
The uPA−/−/SCID+/+ mice were injected intraperitoneally with human serum at 30 ml/kg body weight, and sacrificed at 3, 6, 9, or 12 hours after the serum injection. Blood samples were collected for determinations of hAb, hC3a, and hemolysis. The sera were separated from the blood and used for the determination of GPT. Hemolysis was assayed in blood that was diluted 10-fold with EDTA-GBV [isotonic barbital-buffered saline (pH 7.5)], which inhibits the activities of complement factors (CFs).9 Plasma, which was obtained by centrifuging the diluted blood at 4°C, was used to measure the free hemoglobin concentrations at 414 nm.9 The mice were administered with 200 μl of 1.5 mg/ml Futhan 2 hours after the serum injection, and they were sacrificed 9 hours after the injection, to allow collection of blood samples for the assays described above.
In Situ Hybridization and Immunohistochemistry
Cryosections with a thickness of 7 μm were treated with 4% paraformaldehyde, incubated with 100 ng/ml of proteinase K for 10 minutes at 37°C, and treated with the Avidin Biotin blocking kit (Vector Laboratories, Burlingame, CA). The DNA in the sections was denatured for 6 minutes at 90°C, and the sections were hybridized for 2 hours at 37°C with biotinylated human DNA probes (Dako Cytomation, Kyoto, Japan). The sections were washed with a stringent wash solution, incubated with biotin-tyramide (GenPoint System; Dako Cytomation) for 15 minutes, and then treated with peroxidase-streptavidin for 15 minutes. The hybridized DNA was visualized by reacting with diaminobenzidine for 1 to 5 minutes, and the sections were stained with hematoxylin and eosin (H&E). Cryosections (5 μm thick) were also incubated with antibodies against human-specific cytokeratins 8 and 18 (hCK8/18) (ICN Pharmaceuticals, Inc., Aurora, OH), fluorescein-labeled goat anti-human C3 (hC3) antibodies (Antibodies Inc., Davis, CA), or anti-human C5b-9 (hMAC, human membrane attack complex) antibodies (DAKO Cytomation). Immunoreactivities for hCK8/18 and hMAC were visualized with peroxidase- and dextran-conjugated anti-mouse immunoglobulins (Dako Envision+; DAKO Cytomation) using diaminobenzidine as the substrate and goat anti-mouse IgG (H+L) Alexa 488 (Molecular Probes, Eugene, OR), respectively.
Detection of Human DNA within Transplanted Mouse Livers
Human genomic DNA in the chimeric liver was detected by amplifying the primate-specific Alu gene.10 Genomic DNA samples were isolated from the liver using the DNeasy tissue kit, and used as templates (10 ng) to amplify the Alu repeats by 20 cycles of PCR (30 seconds at 95°C and 1 minute at 63°C) with Alu Sx-specific primers (hAlu; Table 1) and the Advantage 2 PCR kit (Clontech Inc., Palo Alto, CA). The liver tissues of humans and those of control (nontransplanted) uPA−/−/SCID+/+ and uPA+/+/SCID+/+ mice served as positive and negative controls, respectively. Murine genomic DNA in the host liver was detected by amplifying the mouse-specific sequence c-mos (mc-mos) by 30 PCR cycles of 30 seconds at 95°C, and 1 minute at 60°C, using 10 ng of genomic DNA and the primers designed by Mercer and colleagues2 (Table 1). The liver tissues of humans and of control uPA−/−/SCID+/+ and uPA+/+/SCID+/+ mice served as the negative and positive controls, respectively.
Western Blotting and Determination of hCYP2C9 Enzymatic Activity
The microsomal fractions were isolated from chimeric livers,11 aliquots of which (25 μg of protein) were loaded on 10% sodium dodecyl sulfate-polyacrylamide gels, electrophoresed, and transferred to nitrocellulose membranes. The membranes were incubated with antibodies against hCYP2C9 (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan) and visualized with the ECL Western blotting detection system (Amersham Biosciences Corp., Piscataway, NJ). Diclofenac 4′-hydroxylase activity was determined as reported previously,12 with slight modifications. Diclofenac (30 μmol/L) (Sigma Chemical Co., St. Louis, MO) was incubated with 0.2 mg/ml of microsomal protein for 30 minutes. Supernatants of the reaction mixtures were used for determining 4′-hydroxy diclofenac by HPLC. HPLC was performed at a flow rate of 1.0 ml/minute using the Mightysil RP-18 column (Kanto Chemical, Tokyo, Japan), 35°C-column oven (CTO 6A, Shimadzu, Kyoto, Japan), L-7100 pump, L-7200 autosampler, and D-2000 integrator (Hitachi, Tokyo, Japan). Eluates were monitored at 278 nm in the L-7405 UV detector (Hitachi). The mobile phase was 25% acetonitrile in 50 mmol/L phosphate buffer (pH 7.4). The experiments were performed in duplicate, and the results are expressed as the average value.
Quantification of hCYP mRNAs in the Livers of Chimeric Mice
The mRNAs of hCYP1A1, 1A2, 2C9, 2C19, 2D6, and 3A4, and that of hGAPDH were quantified in the liver tissues of the donor, chimeric mice, and uPA−/−/SCID +/+ control mice by real-time quantitative reverse transcriptase-PCR. The cDNAs were synthesized using 1 μg of total RNA isolated from the livers, as reported previously.13 Three control uPA−/−/SCID+/+ or chimeric mice were injected intraperitoneally with rifampicin (50 mg/kg body weight/day) or 3-methylcholanthrene (3-MC; 20 mg/kg body weight/day) for 4 days, and sacrificed 24 hours after the last injection. The hCYP genes were amplified in the PRISM 7700 sequence detector (Applied Biosystems) using the respective cDNAs as the templates, a set of human gene-specific primers (Table 1), and the SYBR Green PCR master mix (Applied Biosystems). We confirmed that these primers were capable of amplifying the hCYP genes but not the mouse CYP genes. The levels of PCR products were monitored continuously during amplification by measuring the increases in intensity of SYBR Green I that bound to the double-stranded DNA. A series of dilutions of plasmid cDNAs for each gene were used to generate the standard amplification curves. The mRNA copy number in each cDNA sample was calculated using the standard amplification curves.13 Statistical differences were evaluated by the Student’s t-test.
Results
Repopulation of Mouse Livers with Human Hepatocytes in the Absence of Complement Inhibitor
Hepatocytes were isolated from patients aged 3, 12, 51, 53, and 58 years, and were transplanted immediately after isolation. In other experiments, hepatocytes that were prepared from patients aged 12, 16, and 58 years were frozen, thawed, and transplanted as F-T hepatocytes. The hepatocytes in eight transplantation experiments engrafted the host liver and yielded multiple colonies that were positive by in situ hybridization for biotinylated human DNA probes and for immunostaining with the anti-hCK8/18 antibodies 21 days after transplantation. There were no differences in colony-forming ability between fresh and F-T donor hepatocytes. The number and size of the colonies varied among the preparations.
Seven preparations of human hepatocytes were available for transplantation experiments: fresh hepatocytes from donors at 37 and 53 years of age; F-T hepatocytes from donors at 12, 47, 53, and 61 years of age; and F-T 9MM hepatocytes. To select a high RI-yielding donor from these preparations, each preparation was transplanted into uPA+/+/SCID+/+ or uPA+/−/SCID+/+ mice. The hAb level in the blood of each host was measured periodically to estimate the RI. Because the hepatocytes from the 12-year-old boy (12YM) and from 9MM were found to promote higher hAb levels (>2 mg/ml) than the other preparations, they were used in all of the following transplantation experiments. We transplanted 5 × 105 F-T 12YM hepatocytes into the livers of four uPA+/+/SCID+/+ mice, and monitored their growth by determining the concentration of hAb in the host blood. Two of these mice did not show increases in hAb beyond 3 mg/ml, and they survived for up to 70 days after transplantation. The hAb level of the third mouse reached 3 mg/ml 34 days after transplantation, at which time point the mouse began to lose weight, and eventually died. The hAb level of the fourth mouse reached 3 mg/ml 42 days after transplantation, and increased thereafter to >3 mg/ml. This mouse survived for a further 20 days, although it continuously lost weight. The hAb level of this mouse began to decrease 60 days after transplantation, and it was about to die at 63 days after transplantation. These results suggest that chimeric mice have a high risk of mortality when the level of hAb exceeds 3 mg/ml.
Mice with >3 mg/ml hAb in the blood showed necrosis and atrophy of the kidney (Figure 1, A and B) and pancreas (data not shown), and severe hemorrhage in the lung (data not shown), although the regions of their livers that contained human hepatocytes did not display any necrosis or atrophy. Thus, we conclude that the observed cell death in the transplanted livers was not caused by human hepatocyte-induced liver failure, but was probably because of the severe insult to host tissues induced by human hepatocyte-secreted hCFs. Indeed, immunohistochemical analysis and enzyme-linked immunosorbent assay showed the presence of hC3 in the chimeric liver (data not shown) and hC3a in the blood, respectively, as detailed below. Depositions of hC3 (Figure 1C) and hMAC (Figure 1D) were also observed in the diseased kidneys. These results indicate that chimeric mice can survive in situations in which the hAb levels exceed 3 mg/ml, but only if the activities of the hCFs in the chimeric mice are inhibited effectively.
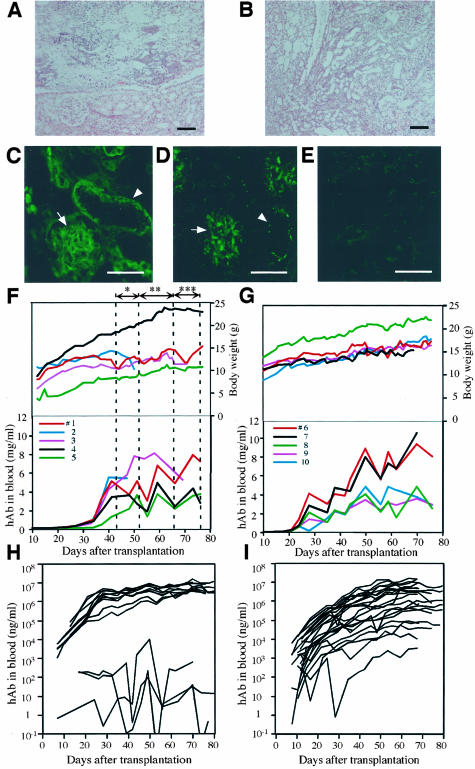
Figure 1.
Generation of humanized mice by treatment with or without a complement inhibitor. Histopathology (H&E staining) of the right kidney (A) and left kidney (B), and immunohistochemistry for hC3 (C) and hMAC (D), which was performed on the right kidney of a dying chimeric mouse that did not receive the complement inhibitor. The mouse that had a blood level of <3 mg/ml hAb and showed reduced body weight, was about to die 63 days after transplantation. Paraffin sections of the kidney were stained with H&E. A: Necrosis in renal papilla is evident in the kidney. B: Chronic pyelonephritis is observed throughout the kidney. hC3 (C) and hMAC (D) are deposited in the kidney. Photograph E represents the negative control stain for D. We confirmed that the antibodies against hC3 and hMAC did not cross-react with complement-induced necrotic tissues in the mouse ischemia/reperfusion kidney model.28 F: Relationships between body weights, hAb levels, and Futhan treatment. F-T hepatocytes of 12YM were transplanted into five mice (animals 1 to 5). The Futhan treatment schedule, which was determined by observing the changes in body weight of animal 1, was as follows: once every 2 days (*), once per day (**), and twice per day (***), as indicated by the double-arrowhead lines. The remaining four chimeric mice followed this regimen. Each line represents a chimeric mouse with the indicated animal number (#). G: Relationships between body weights, hAb levels, and Futhan regimen. The six mice in experiment 1 (Table 2) were transplanted with 12YM hepatocytes. Five of these mice were initially given Futhan on days when the hAb level was >2 mg/ml, and were administered thereafter with Futhan in the once every 2 days manner until the hAb level reached 4 mg/ml. Subsequently, Futhan was administered once per day or twice per day as long as the hAb concentration was between 4 and 6 mg/ml or >6 mg/ml, respectively. H: Humanized mice with 12 YM hepatocytes. The results obtained in experiments 1 and 2 are summarized in the graph. I: Humanized mice with 9MM hepatocytes. The uPA+/+/SCID+/+ mice were transplanted with 9MM hepatocytes (experiments 3 to 6 in Table 2). The mice were treated with Futhan according to the regimen described in G. Scale bars: 100 μm (A, B); 50 μm (C–E).
Repopulation of Mouse Livers with Human Hepatocytes in the Presence of an Anti-Complement Drug
We tested the above hypothesis by treating chimeric mice with Futhan, which is an anti-complement drug that inhibits the C5/C3 convertase.14 We performed an experiment to determine the appropriate regimen of Futhan treatment for 13 mice that were transplanted with hepatocytes from the 12YM donor. There were no consistent or significant rises in the hAb levels of 3 of 13 of these mice, although their body weights increased slowly, but steadily. Another four mice showed steadily increasing levels of hAb, although these levels remained <2 mg/ml. These mice survived and their body weights increased steadily throughout the experimental period. One animal died 52 days after transplantation; the cause of death was unclear. The remaining five mice (animals 1 to 5 in Figure 1F) showed hAb levels of >∼2 mg/ml 43 days after transplantation. Therefore, these latter five mice were chosen for analysis, and changes in their hAb levels and body weights were monitored for up to 77 days (Figure 1F). Animal 1 was used to establish the Futhan treatment regimen, such that the rate of decline in body weight of this mouse was used as an indicator of the extent of hCF assault on its host tissues. Futhan was administered to the animals once every 2 days, once per day, and twice per day, between days 43 and 51, days 52 and 65, and days 66 and 76, respectively (Figure 1F). This regimen was effective in maintaining the body weight of animal 1.
Mouse 4 and mouse 5 showed lower hAb levels than the other mice at 43 days after transplantation, and they showed steady increases in body weight (Figure 1F). The hAb levels of mouse 2 and mouse 3 were >4 mg/ml between days 43 and 52, during which period Futhan was given in the once every 2 days manner. Mouse 2 continued to lose body weight, and died on day 50. The hAb level of mouse 3 was >6 mg/ml between days 52 and 70, when the drug was given in the once per day manner. The body weight of mouse 3 started to decrease at 64 days, and continued to decrease until sacrifice. Although the fortification of Futhan treatment seemed to be not sufficient in mouse 2 and mouse 3, the results of these experiments indicate that Futhan is effective in steadily increasing the body weights of the hosts, thereby permitting them to survive under conditions of high hAb, ie, >3 mg/ml. These results suggest that an appropriate regimen of Futhan treatment is: once every 2 days, once per day, and twice per day, when the hAb concentrations are between 2 and 4 mg/ml, 4 and 6 mg/ml, and >6 mg/ml, respectively.
To test whether this Futhan regimen was generally applicable to yield chimeric mice with high RI values, six additional experiments (experiments 1 to 6 in Table 2) were performed with hepatocytes from 12YM (experiments 1 and 2 in Table 2) and 9MM (experiments 3 to 6), Each of the experiments was done with five to seven host animals (Table 2). Thus, transplantation was performed on 36 uPA+/+/SCID+/+ mice in total. Experiments 1 and 2 (Table 2) involved the transplantation of six and seven mice, respectively. One mouse in experiment 1 showed a hAb level of <2 mg/ml throughout the experimental period. The remaining five mice (animals 6 to 10) were first given Futhan on days when their hAb levels exceeded 2 mg/ml. hAb levels and body weights of these five mice increased progressively during the experimental period (Figure 1G). The hepatocytes used in experiment 2 were successfully engrafted in five mice that subsequently showed hAb levels >3 mg/ml. These animals showed progressive increases in hAb levels and body weight, as seen in experiment 1. Figure 1H summarizes the changes in the host blood levels of hAb of those 13 mice in experiments 1 and 2. When the human hepatocytes were engrafted successfully, the host animals sustained high levels of hAb, ranging from 3 to 13 mg/ml.
Table 2.
Repopulation of Human Hepatocytes in Futhan-Treated Mice
| Donor | Experiment no. | No. of animals | Cell fraction (no. of transplanted cells) | No. of animals engrafted | No. of animals alive for >69 days (no. of animals whose RI was examined) | No. of animals with hAb concentration ranged below, hAb concentration ∼70 days, mg/ml* (RI at sacrifice, %)†
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (0) | 0–1 (0–20) | 1–2 (20–35) | 2–3 (35–50) | 3–5 (50–70) | >5 (>70) | ||||||
| 12YM | 1 | 6 | SHs (5 × 105) | 5 | 6 (2) | 1 (1) | 0 (0) | 0 (0) | 3 (0) | 0 (0) | 2 (1) |
| 2 | 7 | SHs (5 × 105) | 5 | 7 (4) | 2 (0) | 0 (0) | 0 (0) | 0 (2) | 1 (1) | 4 (1) | |
| Total | 13 | 10 | 13 (6) | 3 (1) | 0 (0) | 0 (0) | 3 (2) | 1 (1) | 6 (2) | ||
| ratio (%) | 77‡ | 23§ (17) | 0 (0) | 0 (0) | 23 (33) | 8 (17) | 46 (33) | ||||
| 9MM | 3 | 7 | PHs (5 × 105) | 7 | 7 (7) | 0 (0) | 3 (3) | 0 (0) | 2 (1) | 2 (3) | 0 (0) |
| 4 | 5 | PHs (5 × 105) | 5 | 4 (2) | 0 (0) | 2 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (0) | |
| 5 | 6 | PHs (7.2 × 105) | 6 | 6 (6) | 0 (1) | 3 (1) | 0 (1) | 0 (0) | 0 (1) | 3 (2) | |
| 6 | 5 | PHs (7.5 × 105) | 5 | 4 (4) | 0 (0) | 2 (1) | 0 (1) | 0 (0) | 1 (1) | 1 (1) | |
| Total | 23 | 23 | 21 (19) | 0 (1) | 10 (5) | 0 (2) | 3 (3) | 3 (5) | 5 (3) | ||
| ratio (%) | 100 | 0 (5) | 48 (26) | 0 (11) | 14 (16) | 14 (26) | 24 (16) | ||||
| Total | 36 | 33 | 34 (25) | 3 (2) | 10 (5) | 0 (2) | 6 (5) | 4 (6) | 11 (5) | ||
| ratio (%) | 92 | 9 (8) | 29 (20) | 0 (8) | 18 (20) | 12 (24) | 32 (20) | ||||
hAb concentration was determined by ELISA at 68 to 73 days.
RI was determined by in situ hybridization with human DNA probes at sacrifice.
Obtained as 10 of 13.
Obtained as 3 of 13.
We also examined whether this Futhan regimen was applicable to the generation of chimeric mice with high RI values, using the hepatocytes of other donors. The 9MM hepatocytes were transplanted into 23 mice (experiments 3 to 6 in Table 2). The changes in the host blood levels of hAb are shown in Figure 1I. The rate of engraftment was high (100%) in this experiment, whereas the corresponding rate for 12YM hepatocytes was 77% (Table 2). Two of the chimeric mice died, one at 38 days and the other at 62 days after transplantation; the cause of death was unclear. Eleven mice were given Futhan when their hAb levels reached 2 mg/ml. Their hAb levels increased steadily throughout the experimental period, up to ∼80 days after transplantation. In the present study, we performed a total of 36 transplantation experiments with hepatocytes from two different donors. The overall rate of successful engraftment was 92% (Table 2).
Gross Appearance and Histological Examination of Chimeric Livers
The chimeric livers were characterized morphologically and histologically with respect to the extent of chimerism, as estimated by the concentrations of hAb in the host blood. The red nodules that were distributed sporadically in the livers of mice whose hAb levels were below the level of detection may represent colonies of transgene-deleted host hepatocytes, as reported previously.15 The livers of mice that had 1 to 5 mg/ml hAb in the blood were chimeric, in that they contained both white and red areas. The livers of mice with hAb blood levels of >5 mg/ml were all red-colored.
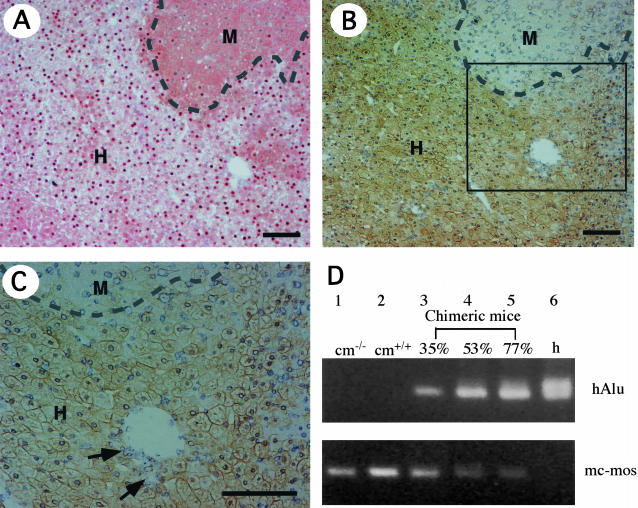
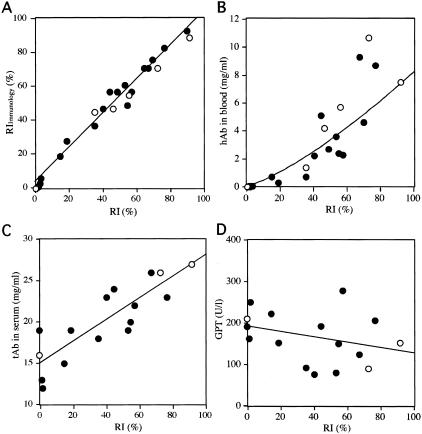
To identify human hepatocytes in the chimeric livers, serial liver sections of 25 chimeric mice were subjected to both hDNA in situ hybridization (Table 2 and Figure 2A) and hCK8/18 immunostaining (Figure 2, B and C). The regions that were positive for in situ hybridization coincided almost exactly with those that showed positive immunostaining in the corresponding serial section (Figure 2, A and B). The anti-hCK8/18 antibodies reacted specifically with hepatocytes and bile duct cells in the human livers, but did not react with the livers of the uPA+/+/SCID+/+, uPA+/−/SCID+/+, and uPA−/−/SCID+/+ mice (data not shown). The RI of the mouse liver that received the human hepatocytes was determined as the ratio of the area occupied by hDNA-positive hepatocytes to the entire area examined in the in situ hybridization sections of six of seven lobes. We also calculated the RIImmunology as the ratio of area occupied by hCK8/18-positive hepatocytes to the entire area examined in immunohistochemical sections of seven lobes. The values for RI and RIImmunology were highly coincident with each other (r2 = 0.98; Figure 3A). Both in situ hybridization and immunohistochemistry demonstrated that the liver was composed of human and mouse hepatocytes, as well as mouse bile duct cells (Figure 2C). Of the 25 mice examined for RI, 5 mice had RI > 70% (Table 2). The RI values correlated well with the hAb concentrations in the blood of chimeric mice, as shown below (r2 = 0.92; Figure 3B). The correlation graph suggests that mice with >5 mg/ml hAb in the blood should have RI > 70%. Thus, 11 of 34 mice (32%) were considered to have RI > 70% (Table 2).
Figure 2.
Demonstration of mouse liver chimerism. Histological serial sections were prepared from six liver lobes of each of 6 chimeric mice (donor, 12YM) and 19 chimeric mice (donor, 9MM), and stained with human genomic probes and anti-hCK8/18 antibodies. A: In situ hybridization of chimeric liver sections with human genomic DNA probes. Regions that contain hepatocytes with positive and negative nuclei are defined as human (H) and mouse (M) areas, respectively, the boundary being indicated by a dashed line. The RI of this chimeric mouse liver, which is calculated as the frequency of positive regions relative to that of the entire examined area in the sections, is 75%. B: CK8/18 immunostaining of a serial section of A. H and M indicate the regions that contain hepatocytes that are immunologically positive and negative, respectively, the boundary being indicated by a dashed line. The RI of this chimeric mouse liver, which is calculated as the frequency of immunopositive regions relative to that of the entire examined area in the sections, is given as RIImmunology = 77%, which is almost identical to the RI value calculated in A. C: High magnification of the region enclosed by the square in B. Bile duct cells, which are indicated with arrows, were negative for immunoreactivity. D: Chimerism determined by PCR. The hAlu sequence was amplified successfully from the liver genomic DNAs of human individuals (h, lane 6), and of chimeric mice with RI = 35% (lane 3), RI = 53% (lane 4), and RI = 77% (lane 5), but not from the control uPA−/−/SCID+/+ mouse (cm−/−; lane 1) or the control uPA+/+/SCID+/+ mouse (cm+/+; lane 2). PCR products for mc-mos were amplified from the mouse livers (lanes 1 and 2), but not from the human liver (lane 6). Scale bars, 100 μm.
Figure 3.
Improvements in host liver function after repopulation with human hepatocytes. A: Correlation between the RIs, which were calculated using in situ hybridization and the RIImmunology values obtained by immunostaining with anti-CK8/18, for 6 animals (donor, 12YM; open circles) and 19 animals (donor, 9MM; filled circles). These animals are listed in Table 2. The two RI values are almost identical. The relationship between RIImmunology (y) and RI (x) fits the formula: y = 1.0 x + 3.2, with r2 = 0.98. B: The hAb concentration is plotted against the corresponding RI for the same animals as in A. C: The serum tAb concentrations are plotted against the corresponding RI values for three animals (12YM, open circles) and 13 animals (9MM, filled circles). D: The serum GPT levels are plotted against the corresponding RI values for the same animals as in C. The solid lines represent correlative curves.
Chimerism was demonstrated by semiquantitative genomic PCR of the hAlu and mc-mos genes in the livers of control and chimeric mice, and of humans (Figure 2D). The PCR products for hAlu and mc-mos were not detected in the genomes of normal mice and human livers, respectively. The levels of hAlu and mc-mos in the chimeric mice increased and decreased, respectively, as the RI values increased.
Improvement of Host Liver Function in Chimeric Mice Containing Human Hepatocytes
We examined the effects of chimerism on the function of the host liver by measuring several different parameters. The levels of tAb (sum of the levels of human and mouse Ab, hAb + mAb) and GPT in the sera were measured as parameters of the degrees of liver function and damage, respectively. The uPA+/+/SCID+/+ mice with RI = 0% had 16 and 19 mg/ml tAb (Figure 3C), and 195 and 212 U/L GPT (Figure 3D). When the livers of these animals were humanized, the tAb level increased (r2 = 0.74; Figure 3C) and the GPT level showed a tendency to decrease as the hAb concentration increased (Figure 3D).
Histopathology of the Chimeric Mouse Liver
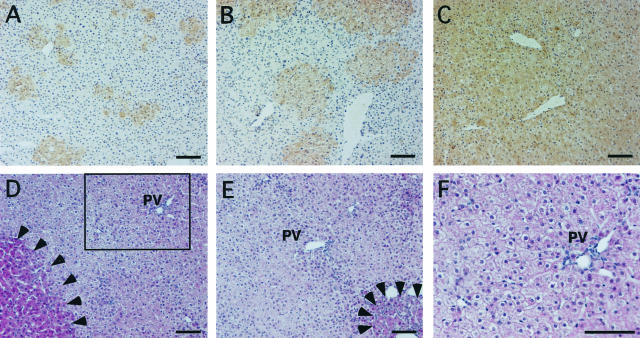
To examine the time course of replacement of the mouse liver with human hepatocytes, mice were sacrificed on days 7, 14, 20, 35, 64, and 81 after transplantation, and their liver sections were stained with the anti-CK8/18 antibodies (Figure 4; A to C). Small clusters that contained 5 to 10 hepatocytes were observed at 7 days after transplantation. The human hepatocyte colonies gradually became larger. Different chimeric mice showed almost confluent human hepatocytes at 64 days and 81 days after transplantation.
Figure 4.
Time course of repopulation of human hepatocytes in the host liver, and histopathology of the chimeric liver. Liver sections from mice that were transplanted with 9MM hepatocytes were stained with the anti-CK8/18 antibodies on days 14 (A), 35 (B), and 81 (C) after transplantation, at which time points the RIImmunology values were 10, 33, and 92%, respectively. The colonies that were derived from the human hepatocytes increased gradually in size, and were almost confluent by day 81 after transplantation. D to F: Histopathology of chimeric livers. H&E sections were prepared from the liver of a chimeric mouse with RI = 90% at 68 days after transplantation (donor 9MM). Most of the regions are populated with few eosinophilic human hepatocytes. There are a few nodules of transgene-deleted mouse hepatocytes, as indicated by the arrowheads in D. Some of the mouse hepatocytes in the degenerative regions that were basophilic originally have now become eosinophilic, as indicated by the arrowheads in E. The region enclosed by the square in D is magnified and shown in F. PV, portal vein. Scale bars, 100 μm.
Histopathology was performed on H&E liver sections of the mice that were transplanted with human hepatocytes. Eosinophilic nodules were observed occasionally in the less eosinophilic human regions (Figure 4D, enclosed by arrowhead). These nodules were probably composed of transgene-deleted mouse hepatocytes.15 Generally, the human hepatocytes were not associated with severe inflammation or necrosis in any of the examined hosts (Figure 4; D to F). The mouse hepatocytes outside the nodules were originally basophilic, degenerative, and showed single-cell necrosis, as reported previously,15 but they became more eosinophilic with time after transplantation (Figure 4E, enclosed by arrowhead).
Effect of Futhan on the Levels of Hemolysis, CFs, and GPT in Human Serum-Injected Control Mice and on the Levels of CFs in Chimeric Mice
We investigated whether Futhan, by virtue of its activity as an inhibitor of human complement, was effective in yielding chimeric mice with high RI values. It was reported previously that mice showed hemolysis when they were injected intravascularly with human sera, whereas heat treatment of the sera abolished this effect.9 In addition, the administration of Futhan inhibited the serum-induced hemolysis.9 Thus, CFs in human serum appear to be responsible for the hemolysis. We investigated whether the levels of hCFs, hemolysis, and GTP increased when mice were treated with human sera, and whether the increases in the levels of these parameters were suppressed when the mice were co-administered with Futhan. Human sera were injected intraperitoneally into uPA−/−/SCID+/+ mice at a dose of 30 ml/kg body weight. The concentrations of hC3a and hAb in the blood peaked at ∼145 ng/ml and 5 mg/ml, 3 and 9 hours after injection, respectively (Table 3). Hemolysis reached a plateau 3 hours after injection, the level at that stage being ∼3.4-fold higher than the control value. GPT leakage increased ∼17-fold, 9 hours after injection. When Futhan was given 2 hours after the serum injection, hemolysis was reduced significantly, concomitant with statistically significant (Student’s t-test) decreases in the levels of hC3a and GPT (Table 3).
Table 3.
Effects of Futhan Treatment on Cell Injury of Mice Injected with Human Serum
| No. of test animals | hAb mg/ml | hC3a ng/ml | Hemolysis OD (414 nm) | GPT U/l | |
|---|---|---|---|---|---|
| Control | 4 | 0 | 0 | 0.113 ± 0.017 | 8.8 ± 4.6 |
| Three hours after injection of human serum | |||||
| 4 | 3.71 ± 1.03 | 145 ± 33 | 0.384 ± 0.163 | 36.8 ± 5.3 | |
| Nine hours after injection of human serum | |||||
| Without Futhan | 8 | 5.22 ± 1.14 | 80 ± 33 | 0.373 ± 0.083 | 154.5 ± 106.2 |
| With Futhan | 8 | 4.58 ± 1.05 | 29 ± 22† | 0.202 ± 0.111† | 58.3 ± 48.1* |
P < 0.05.
P < 0.01 compared with “without Futhan.”
The uPA−/−/SCID+/+ mice were intraperitoneally injected with human sera, and sacrificed at 3, 6, 9, and 12 hours after the serum injection to collect blood for the determination of hAb, hC3a, and hemolysis. Sera were separated from blood and used for the determination of GPT. Hemolysis was assayed in the 10-fold diluted blood. Plasma was obtained by centrifuging the diluted blood and used to measure free hemoglobin concentrations. Mice were administered with Futhan at 2 hours after the serum injection and sacrificed at 9 hours after the injection to collect blood for measuring the above parameters.
The concentrations of hC3a in the blood of chimeric mice with hAb >3 mg/ml were high enough (60 to 200 ng/ml) to induce damages to tissues, as judged by the levels of hemolysis and the GPT in the nonchimeric control mice that were injected with human sera. We tested whether Futhan decreased the hC3a levels in chimeric mice with hAb blood levels of >2 mg/ml. Futhan was administered intraperitoneally to the chimeric mice, and hC3a was determined 1 hour later. Futhan decreased the hC3a level to 75.9 ± 15.2% (n = 7, mean ± SD; Wilcoxon signed-ranks test, P < 0.05) of the levels measured before the injection of Futhan.
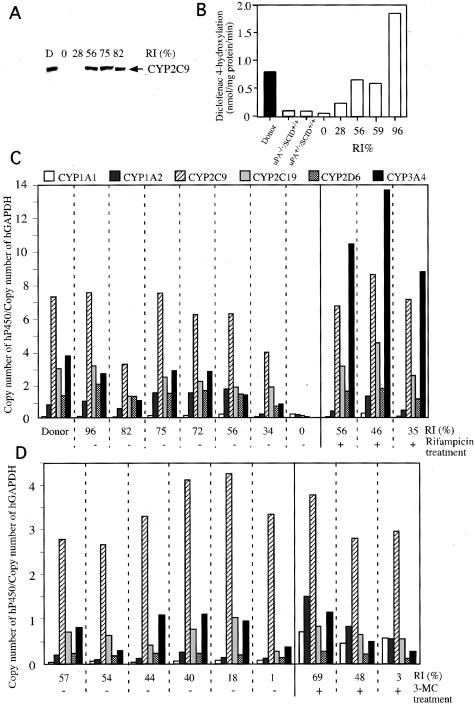
Expression of hCYP Proteins and mRNAs in the Livers of Chimeric Mice
The microsomal fractions were prepared from the liver tissues of 12YM and uPA−/−/SCID+/+ mice, as well as 10 chimeric mice that carried hepatocytes from 12YM. The extracted proteins were subjected to Western blotting for hCYP2C9. Although the hCYP2C9 protein was not detected in the microsomal fractions of the control uPA−/−/SCID+/+ mice (data not shown) or of chimeric mice with RI < 28% (Figure 5A), it was detected in six animals with RI > 34% (Figure 5A). The human liver contains four CYP isoforms, ie, CYP2C8, CYP2C9, CYP2C18, and CYP2C19, all of which are absent in mice and rats.16 CYP2C9 preferentially catalyzes diclofenac via its diclofenac 4′-hydroxylation activity.17 The microsome fractions of the control uPA−/−/SCID+/+ and uPA+/−/SCID+/+ mice showed very low levels of activity for this enzyme, as compared to those of the donor liver (Figure 5B). Treatment of mice with Futhan did not affect the level of this enzyme (data not shown). Thus, we conclude that diclofenac is an appropriate substrate for measuring the activity of human-specific CYP in chimeric mouse livers. Liver microsomes were prepared from five chimeric mice and treated with diclofenac, to test their ability to metabolize the drug. The level of diclofenac 4′-hydroxylase of the mouse with RI = 0% was similar to those of the control uPA−/−/SCID+/+ and uPA+/−/SCID+/+ mice (Figure 5B), whereas the levels of this enzyme in the remaining chimeric mice increased with increasing RI values. Thus, the mouse with RI = 96% showed higher enzyme activity than the donor. From this, we conclude that human hepatocytes in chimeric livers retain pharmacological activity against diclofenac via the production of hCYP2C9.
Figure 5.
Expression of hCYPs. A: Western blots for the detection of hCYP2C9. Western blotting was performed on the microsomes of the donor and 10 chimeric mice (donor, 12YM) using antibodies against hCYP2C9. The results are shown for the donor (D) and five chimeric mice with different RIs as examples. B: Measurements of liver diclofenac 4′-hydroxylation activities. Microsomes were isolated from the donor liver (filled bar), uPA−/−/SCID+/+, and uPA+/−/SCID+/+ control mouse livers (open bar), and five chimeric mouse livers (open bars with RI values), and were treated with diclofenac to measure their diclofenac 4′-hydroxylation activities. C and D: Profiles of the relative mRNA expression levels of six isoforms of hCYP. The ratios were obtained by dividing the copy number of each isoform mRNA in the donor and chimeric livers by that of hGAPDH mRNA in the donor and chimeric livers, respectively. The RI of each animal is indicated. C: Ten mice were transplanted with human hepatocytes (donor 12YM). Three mice (RI values of 56%, 46%, and 35%) were injected daily for 4 days with rifampicin, as indicated by +. The − designation represents mice that did not receive rifampicin treatment. D: Nine mice were transplanted with 9MM hepatocytes, three (RI values of 69%, 48%, and 3%) of which were injected daily for 4 days with 3-MC (indicated by +) before sacrifice for the measurement of CYP-specific mRNAs, whereas the other animals were not treated with 3-MC (indicated by −). The RI values indicated in A through D were calculated based on the percentage occupancy of in situ hybridization-positive regions in the histological sections of chimeric liver tissues.
Total RNA samples were isolated from the liver tissues of 12YM, seven mice that were humanized with 12YM hepatocytes (Figure 5C), and six mice that were humanized with 9MM hepatocytes (Figure 5D). The mRNAs for six hCYPs, hCYP1A1, 1A2, 2C9, 2C19, 2D6, and 3A4, were quantified by quantitative reverse transcriptase-PCR using the total RNA samples. The hCYP sequences were not amplified in the control uPA−/−/SCID+/+ mouse livers, which indicates that the primers used are specific for human genes (Table 4). The mRNA levels varied among the hCYPs, representatives of which are shown in Table 4. The mRNA level for each hCYP in each individual chimeric mouse correlated well with the RI value for that mouse. A mouse with a higher RI value generally showed much higher hCYP expression levels than a mouse with a lower RI value (Table 4). The relative copy numbers of the hCYPs were obtained by normalizing the observed copy numbers against the copy number of hGAPDH. The hCYP expression profile of each chimeric mouse was similar to that of the donor, 12YM (Figure 5C). The six hCYP genes of the donor liver were divided into three groups based on expression intensity: 1) the low-expression group, which comprised hCYP1A1; 2) the medium-expression group, which included hCYP1A2, 2C19, 2D6, and 3A4; and 3) the high-expression group, which comprised hCYP2C9. This grouping was applicable to all of the chimeric mice tested irrespective of the differences in RI values, with the exception of the mice that had RI = 0%. This suggests that the human hepatocytes in the chimeric mice express the six hCYP genes in a seminormal manner, as is the case in the human body. The above-mentioned grouping was generally applicable to the CYP expression patterns of the 9MM-chimeric liver (Figure 5D).
Table 4.
Copy Number of hCYPs in 1 ng Total RNAs of Livers of the Donor, Chimeric, and Control Mice
| hCYPs
|
hGAPDH | ||||||
|---|---|---|---|---|---|---|---|
| 1A1 | 1A2 | 2C9 | 2C19 | 2D6 | 3A4 | ||
| Donor 12YM | 4,661 | 53,038 | 502,722 | 203,573 | 92,214 | 258,081 | 68,930 |
| Chimeric mouse (RI = 82%) | 5,436 | 80,493 | 607,754 | 252,823 | 161,770 | 216,504 | 80,651 |
| Chimeric mouse (RI = 34%) | 2,519 | 13,231 | 234,166 | 110,422 | 41,284 | 48,901 | 59,610 |
| Donor 9MM | — | — | — | — | — | — | — |
| Chimeric mouse (RI = 54%) | 5,899 | 10,193 | 319,111 | 76,680 | 20,593 | 34,328 | 119,927 |
| Chimeric mouse (RI = 1%) | 371 | 584 | 16,153 | 1,314 | 668 | 1,810 | 4,841 |
| uPA−/−/SCID+/+ mice (n = 3) | 18 ± 7 | 95 ± 41 | 219 ± 45 | 18 ± 5 | 8 ± 2 | 2 ± 2 | 575 ± 104 |
| Rifampicin-treated uPA−/−/SCID+/+ mice (n = 3) | 23 ± 9 | 89 ± 67 | 155 ± 20 | 52 ± 38 | 11 ± 6 | 2 ± 2 | 525 ± 74 |
| 3-MC-treated uPA−/−/SCID+/+ mice (n = 3) | 20 ± 3 | 97 ± 25 | 333 ± 30 | 26 ± 4 | 8 ± 1 | 4 ± 1 | 577 ± 94 |
—, No data. mRNAs of 6 hCYPs, hCYP1A1, 1A2, 2C9, 2C19, 2D6, and 3A4, and hGAPDH were quantified for liver tissues of the donor 12YM, chimeric mice, and uPA−/−/SCID+/+ control mice by real-time quantitative RT-PCR. Liver tissues of the donor 9MM were not available. Three control uPA−/−/SCID+/+ mice were intraperitoneally treated with rifampicin or 3-MC for 4 days, and sacrificed at 24 hours after the last injection.
We also examined whether the chimeric liver was capable of responding to CYP-inducing chemicals. Rifampicin and 3-MC were used as specific inducers of CYP3A, and CYP1A1 and 1A2, respectively.18 Chimeric mice with hepatocytes from 12YM and 9MM were injected intraperitoneally with rifampicin and 3-MC, respectively. The mRNA levels of six hCYPs in the liver tissues were measured 24 hours later. The mean relative copy number of hCYP3A4 was 1.9 ± 1.3 (n = 6, Figure 5C) in the 12YM-transplanted chimeric livers. Rifampicin treatment enhanced the expression 5.8-fold (11.0 ± 1.3, n = 3, P < 0.001, Student’s t-test; Figure 5C). This level of induction was specific for hCYP3A4 among the six hCYPs tested. The mean relative copy numbers of hCYP1A1 and CYP1A2 in the 9MM-transplanted chimeric livers were 0.057 ± 0.018 and 0.15 ± 0.07 (n = 6, Figure 5D), respectively. The administration of 3-MC enhanced the expression levels of the CYP1A1 and CYP1A2mRNAs to 0.57 ± 0.02 (10.0-fold increase, P < 0.001, n = 3; Figure 5D) and 0.96 ± 0.49 (6.4-fold increase, P < 0.05, n = 3; Figure 5D), respectively. Neither rifampicin nor 3-MC induced the expression of any of the six hCYPs in uPA−/−/SCID+/+ mice (Table 4), which indicates that the primers used are specific for human sequences.
Discussion
Hepatocytes play key roles in drug metabolism and are an important component of research studies into new medicines. However, social and ethical considerations limit the availability of sufficient quantities of fresh human hepatocytes for research purposes, which has led researchers to use preparations of rodent hepatocytes. However, there are significant differences in terms of metabolic activities between rodent and human hepatocytes.19–21 Human hepatocytes are also required for the study of the infectious mechanisms of human hepatitis viruses, for which purpose rodent hepatocytes are not useful, because of the species specificity of the viruses.
Dandri and co-workers1 produced uPA/RAG-2 mice that carried humanized liver cells with a maximal RI value of 15%, and they used these mice in HBV infection studies. Mercer and colleagues2 generated uPA/SCID mice with humanized livers, the sera of which contained maximally 2 mg/ml hAb, which corresponds to ∼1 mg/ml hAb in the blood. The data in Figure 3B suggest that an RI value of 20% corresponds to 1 mg/ml of hAb in the blood. This level of replacement can be achieved without using any drug with anti-complement activity. The above-cited authors also determined RI by immunohistochemistry, and obtained a value of ∼50%. The data in Figure 3B in the present study suggest that a hAb level of <3 mg/ml corresponds to RI = 50%. This concentration of hAb might allow chimeric mice to survive for long periods in the absence of any inhibitor of hCFs.
In the present study, we used liver-defective, immunodeficient mice to yield chimeric mice. In this instance, the chimeric mice could not survive when the level of hAb in the host blood exceeded 3 mg/ml, which corresponded to RI > 50%. Atrophy and necrosis of the kidney and pancreas were observed in these mice. However, there was neither severe necrosis nor inflammation in the human hepatocyte-repopulated livers. Depositions of hC3 and hMAC were observed in the kidneys. The currently available data strongly suggest that chimeric mice die because of human complement-induced disorders in organs other than the liver, particularly the kidneys. The administration of Futhan, which is an inhibitor of CFs, had a remarkable effect on the chimeric mice, in that these mice survived for long periods of time (>6 months) and showed steady gains in body weight within the 2 months after transplantation. The animals appeared normal and showed high RI values (∼96% maximally). Futhan treatment also decreased the concentration of hC3a in the blood of control mice that were injected with human sera. Thus, it seems likely that Futhan functions as an anti-CF agent, as one would expect. We believe that activated hCFs produced by propagated human hepatocytes attack the cells of the host mouse. Futhan has been shown to be effective in the therapy of acute pancreatitis22 and pulmonary vascular injury,23 and in the prolongation of xenograft survival.24 Thus, it appears that Futhan not only suppresses CFs, but also functions as a serine protease inhibitor to prevent secondary injuries caused by proteases that leak from injured tissues.
We calculated the ratio of hAb to tAb (mAb + hAb), and showed that the rate of replacement of human hepatocytes did not necessarily match the rate of replacement of hAb, with the former value being generally higher that the latter. This finding suggests that repopulated human hepatocytes are less active in secreting hAb in the chimeric liver, and that murine hepatocytes that have deleted the transgene have higher levels of mAb secretion.
CYP isoforms play central roles in the metabolism of chemicals and medicines. One of the major aims of the present study was to determine whether human hepatocytes established in the murine liver would show the same spectrum of expression of CYP genes as in the human body. We developed a method to efficiently propagate human hepatocytes in mice, and we found that these propagated human hepatocytes had hCYP expression spectra that were similar in the mouse body and donor liver. The relative copy numbers of the hCYPs in the chimeric livers were similar to those in the donor liver. The copy number of each hCYP in each chimeric mouse correlated well with the RI of the individual mice. The microsomal fraction of the chimeric liver was shown to contain hCYP2C9 protein and to retain its metabolic activity. The chimeric liver was also shown to induce specific subsets of hCYPs in response to specific inducers.
Recently, researchers have produced transgenic mice that carry one of the hCYP isotypes of CYP 2E1, 4B1, and 1B1, and the functions of each of these enzymes in vivo have been investigated.25–27 However, this type of transgenic approach has limitations as an in vivo model, because it is practically impossible to introduce all of the hCYP genes that may participate in the metabolism of a specific drug. In addition, host CYPs that might affect the metabolism of a given drug cannot be deleted. On theother hand, the chimeric mice that were generated in the present study were repopulated by human hepatocytes, which could induce a set of major CYPs that were essential for a given drug. Thus, we conclude that these propagated human hepatocytes can be used in in vitro tests for drug safety and stability, as cells that can be incorporated into extracorporeal artificial liver devices, and as transplantable cells for regenerative therapy of patients with liver failure. In addition, the chimeric mice have value as humanized model animals for drug metabolism studies.
Acknowledgments
We thank Ms. K. Furumoto and Ms. S. Hamamura for breeding the uPA/SCID mice; Ms. C. Ohnishi, Ms. H. Kohno, Ms. Y. Matsumoto, Ms. S. Nagai, and Mr. Y. Kageyama for technical assistance; and Drs. S. Ohta, K. Sugihara, H. Ohdan, and K. Arihiro for valuable advice.
Footnotes
Address reprint requests to Katsutoshi Yoshizato, Ph.D., Developmental Biology Laboratory, Department of Biological Science, Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526, Japan. E-mail: kyoshiz@hiroshima-u.ac.jp.
Supported by the Hiroshima Tissue Regeneration Project, the Yoshizato Project, and Cooperative Link of Unique Science and Technology for Economy Revitalization (CLUSTER), Japan.
References
- Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JRT, Tyrrell DLJ, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Hamatani K, Matsuda Y, Araki R, Itoh M, Abe M. Cloning and chromosomal mapping of the mouse DNA-dependent protein kinase gene. Immunogenetics. 1996;45:1–5. doi: 10.1007/s002510050159. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hino H, Tateno C, Sato H, Yamasaki C, Katayama S, Kohashi T, Aratani A, Asahara T, Dohi K, Yoshizato K. Long-term culture of human hepatocytes which show a high growth potential and express their differentiated phenotypes. Biochem Biophys Res Commun. 1999;256:184–191. doi: 10.1006/bbrc.1999.0288. [DOI] [PubMed] [Google Scholar]
- Tateno C, Yoshizato K. Growth and differentiation in culture of clonogenic hepatocytes that express both phenotypes of hepatocytes and biliary epithelial cells. Am J Pathol. 1996;149:1593–1605. [PMC free article] [PubMed] [Google Scholar]
- Tateno C, Takai-Kajihara K, Yamasaki C, Sato H, Yoshizato K. Heterogeneity of growth potential of adult rat hepatocytes in vitro. Hepatology. 2000;31:65–74. doi: 10.1002/hep.510310113. [DOI] [PubMed] [Google Scholar]
- Katayama S, Tateno C, Asahara T, Yoshizato K. Size-dependent in vivo growth potential of adult rat hepatocytes. Am J Pathol. 2001;158:97–105. doi: 10.1016/S0002-9440(10)63948-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Sato T, Suzuki S, Iwaki M, Yoshikawa T. Inhibitory effects of FUT-175, a new synthetic protease inhibitor, on intravascular hemolysis by human serum in mice. Int J Immunopharmacol. 1987;9:533–537. doi: 10.1016/0192-0561(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz E, Labuda M, Sinnett D, Glorieux FH, Labuda D. Linkage mapping by simultaneous screening of multiple polymorphic loci using Alu oligonucleotide-directed PCR. Proc Natl Acad Sci USA. 1992;89:8448–8451. doi: 10.1073/pnas.89.18.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Kitamura S, Yamada T, Ohta S, Yamashita K, Yasuda M, Fujii-Kuriyama Y. Aryl hydrocarbon receptor (AhR)-mediated induction of xanthine oxidase/xanthine dehydrogenase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Biophys Res Commun. 2001;281:1093–1099. doi: 10.1006/bbrc.2001.4464. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Mimura N, Fujii H, Minami H, Sasaki Y, Shimada N, Chiba K. Role of human cytochrome P450 3A4 in metabolism of medroxyprogesterone acetate. Clin Cancer Res. 2000;6:3297–3303. [PubMed] [Google Scholar]
- Asahina K, Sato H, Yamasaki C, Kataoka M, Shiokawa M, Katayama S, Tateno C, Yoshizato K. Pleiotrophin/heparin-binding growth associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am J Pathol. 2002;160:2191–2205. doi: 10.1016/S0002-9440(10)61167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagi R, Miyata T, Maeda K, Sugiyama S, Miyama A, Nakashima I. FUT-175 as a potent inhibitor of C5/C3 convertase activity for production of C5a and C3a. Immunol Lett. 1991;27:49–52. doi: 10.1016/0165-2478(91)90243-4. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Comparisons of catalytic selectivity of cytochrome P450 subfamily enzymes from different species. Chem Biol Interact. 1997;106:161–182. doi: 10.1016/s0009-2797(97)00068-9. [DOI] [PubMed] [Google Scholar]
- Leemann T, Transon C, Dayer P. Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′-hydroxylation in human liver. Life Sci. 1993;52:29–34. doi: 10.1016/0024-3205(93)90285-b. [DOI] [PubMed] [Google Scholar]
- Bowen WP, Carey JE, Miah A, McMurray HF, Munday PW, James RS, Coleman RA, Brown AM. Measurement of cytochrome P450 gene induction in human hepatocytes using quantitative real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2000;28:781–788. [PubMed] [Google Scholar]
- Kato R. Characteristics and differences in the hepatic mixed function oxidases of different species. Pharmacol Ther. 1979;6:41–98. doi: 10.1016/0163-7258(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Green CE, LeValley SE, Tyson CA. Comparison of amphetamine metabolism using isolated hepatocytes from five species including human. J Pharmacol Exp Ther. 1986;237:931–936. [PubMed] [Google Scholar]
- Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos. 2001;29:1316–1324. [PubMed] [Google Scholar]
- Iwaki M, Ino Y, Motoyoshi A, Ozeki M, Sato T, Kurumi M, Aoyama T. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J Pharmacol. 1986;41:155–162. doi: 10.1254/jjp.41.155. [DOI] [PubMed] [Google Scholar]
- Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K. Effect of nafamostat mesilate on pulmonary vascular injury induced by lipopolysaccharide in rats. Am J Respir Crit Care Med. 1997;155:711–718. doi: 10.1164/ajrccm.155.2.9032217. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Shirakura R, Matsumiya G, Fukushima N, Nakata S, Matsuda H, Matsumoto M, Kitamura H, Seya T. Prolonging discordant xenograft survival with anticomplement reagents K76COOH and FUT175. Transplantation. 1993;55:709–713. doi: 10.1097/00007890-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- Imaoka S, Hayashi K, Hiroi T, Yabusaki Y, Kamataki T, Funae Y. A transgenic mouse expressing human CYP4B1 in the liver. Biochem Biophys Res Commun. 2001;284:757–762. doi: 10.1006/bbrc.2001.5055. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Chae KR, Shin DH, Hwang JH, Lim CH, Kim YJ, Kim BJ, Goo JS, Shin YY, Jang IS, Cho JS, Kim YK. Xenobiotic response in humanized double transgenic mice expressing tetracycline-controlled transactivator and human CYP1B1. Arch Biochem Biophys. 2001;395:32–40. doi: 10.1006/abbi.2001.2542. [DOI] [PubMed] [Google Scholar]
- De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]