Abstract
Skeletal muscle differentation is a complex process regulated at multiple levels. This study addressed the effect of glutathione (GSH) depletion on the transition of murine skeletal muscle C2C12 myoblasts into myocytes induced by growth factor inactivation. Cellular GSH levels increased within 24 hours on myogenic stimulation of myoblasts due to enhanced GSH synthetic rate accounted for by stimulated glutamate-L-cysteine ligase (also known as γ-glutamylcysteine synthetase) activity. In contrast, the synthesis rate of GSH using γ-glutamylcysteine and glutamate as precursors, which reflects the activity of the GSH synthetase, did not change during differentiation. The stimulation of GSH stores preceded the myogenic differentiation of C2C12 myoblasts monitored by expression of muscle-specific genes, creatine kinase (CK), myosin heavy chain (MyHC), and MyoD. The pattern of DNA binding activity of NF-κB and AP-1 in differentiating cells was similar both displaying an activation peak at 24 hours after myogenic stimulation. Depletion of cellular GSH levels 24 hours after stimulation of differentiation abrogated myogenesis as reflected by lower CK activity, MyHC levels, MyoD expression, and myotubes formation, effects that were reversible on GSH replenishment by GSH ethyl ester (GHSEE). Moreover, GSH depletion led to sustained activation of NF-κB, while GSHEE prevented it. Furthermore, inhibition of NF-κB activation restored myogenesis despite GSH depletion. Thus, GSH contributes to the formation of myotubes from satellite myoblasts by ensuring inactivation of NF-κB, and hence maintaining optimal GSH levels may be beneficial in restoring muscle mass in chronic inflammatory disorders.
Compromised skeletal muscle performance and muscle wasting are frequent features of patients afflicted with chronic disorders. Signs of muscle atrophy or cachexia are often seen in cancer, AIDS, and inflammatory disorders such as arthritis and chronic obstructive pulmonary disease.1–3 In many of these conditions, muscle wasting is associated with chronic elevations in circulating inflammatory cytokines, in particular tumor necrosis factor-α (TNF).4,5 Indeed, chronic increases in serum TNF can induce muscle wasting which can be ameliorated with antibodies against TNF.6,7 One of the mechanisms whereby TNF exerts its deleterious effects is through oxidative stress, and thus GSH, the most important and versatile antioxidant in cells, is known to modulate the damaging effects of TNF.8,9 Indeed, the levels of GSH are depleted in skeletal muscle of critically ill patients.10,11 Recently, we have reported the inability of muscle from patients with chronic obstructive pulmonary disease to maintain optimal GSH levels that along with enhanced TNF plasma levels may account for the reduced adaption to endurance training.12,13
The investigation of factors that modulate myogenesis may be of significance in designing therapies aimed to ensure skeletal muscle differentiation in chronic disorders. Many studies have contributed to the identification of functional players that modulate myogenesis including oxidative stress, NF-κB activation, and protein kinases. For instance, in vitro studies demonstrated that oxidative stress-induced muscle wasting can be prevented by antioxidants and reactive oxygen species (ROS) scavengers.14 ROS contribute to fatigue and decreased force generation through modification of sarcoplasmic reticulum function, alterations in mitochondrial respiration, and even direct oxidation of critical sulfhydryl groups on contractile proteins which may contribute to enhanced degradation of myofibrillar proteins.15 Moreover, a recent study reported that N-acetylcysteine, a GSH precursor, restored the formation of multinucleated myotubes from C2C12 myoblasts despite treatment with TNF.16 In addition to the regulation of myogenesis by TNF, redox-sensitive transcription factors such as NF-κB or AP-1 have been also shown to modulate myogenic differentiation.17–21 For instance, transfection of C2C12 cells with IκBα-SR, a non-degradable mutant of IκBα that prevents NF-κB activation by cytokines, enhanced myogenic expression despite exposure to TNF.17 Thus, besides its role as a master regulator of the inflammatory response, NF-κB activation regulates myogenesis by controlling the cell cycle machinery. In this regard, it has been demonstrated that cyclin D1 is transcriptionally regulated by NF-κB18 and that initiation of myogenic differentiation is preceded by exit from the cell cycle, an event that requires down-regulation of cyclin D1. Alternatively, the activity of the muscle-specific basic-helix-loop-helix transcription factors MyoD and myogenin can be repressed as a direct consequence of NF-κB activation.19 Furthermore, it has been shown that c-Jun, a component of AP-1, repressed myogenesis due to a direct physical antagonism of the activities of MyoD and myogenin.20,21
The regulation of GSH during myogenesis has been recently observed in C2C12 cells. Langen et al16 recently observed a transient increase in the levels of GSH during myogenesis, although the mechanism underlying this finding was not further examined. However, to the best of our knowledge, the regulation of myogenesis by GSH has not been examined previously. Thus, in the present study we have used the murine skeletal muscle cell line C2C12 to investigate the regulation of glutamate-L-cysteine ligase (also known as γ-glutamylcysteine synthetase, γ-GCS), the rate-limiting enzyme in GSH synthesis, and the impact of GSH depletion on both the activation of NF-κB and AP-1 and the myogenesis of C2C12 cells.
Materials and Methods
Materials
Diethyl maleate (DEM), glutahione ethyl ester (GSHEE), buthionine-sulfoximine (BSO), glycine, glutamate, cysteine, γ-glutamylcysteine and hydrogen peroxide were purchased from Sigma (Madrid, Spain). Monochlorobimane and chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate were obtained from Molecular Probes (Eugene, OR). Antibodies anti-Myo D and α-tubulin were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA) and the FITC-conjugated goat anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories, Inc, (West Grove, PA). The anti-myosin heavy chain (MyHC) antibody (MF-20) was from Developmental Studies Hybridoma Bank (University of Iowa, IA).
Cell Culture
C2C12 cells can differentiate into spontaneously contracting myotubes on exposure to growth factor inactivation or deprivation.22 C2C12 myoblasts obtained from American Type Culture Collection (ATCC, Rockville, MD) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% newborn calf serum and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) referred to as growth medium. Myoblasts were seeded at a concentration of 0.35 × 106 cells per well in 6-well plates and cultured for 24 hours to reach 100% confluence (day 0). To induce myogenic differentiation, cells were washed in PBS and cultured in low glucose DMEM supplemented with antibiotics and 10% heat-inactivated horse serum, referred to as differentiation medium. All of the experiments were carried out from day 0 to day 7 after the induction of differentiation and cells were examined daily for biochemical and morphological signs of myogenesis.
Assessment of Myogenic Differentiation
Myogenic differentiation was estimated biochemically by the determination of muscle creatine kinase (CK) activity in cellular lysates obtained at different times (day 0 to day 7) after the replacement of growth medium by differentiation medium. Cells were washed twice in cold PBS and lysed in 0.2 ml of 50 mmol/L Tris-MES (pH 7.8) and 1% Triton X-100. Lysates were centrifuged and the supernatants stored at −80°C for CK determination using a commercially available kit (Sigma CK-10, Sigma) according to the manufacturer’s instructions. Specific CK activity was calculated after correction for protein content. Alternatively, myogenesis was also evaluated by determination of MyHC and MyoD levels. For this purpose, cells were washed in PBS, and whole-cell lysates were prepared by lysis buffer (40 mmol/L Tris, 300 mmol/L NaCl, 2% (v/v) Nonidet P-40, 1 mmol/L dithiothreitol, 1 mmol/L phenylmetylsulfonyl fluoride, 10 μg/ml leupeptin, and aprotinin). Lysates were incubated on ice for 10 minutes, followed by a 30-minute centrifugation at 16,000 × g. Equal amounts of protein (20 μg) were subjected to SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. Membranes were incubated overnight with 1% goat serum/Tris-buffered saline (TBS) at 4°C to reduce nonspecific binding followed by washing in TBS-Tween20 (TBS/T, 0.05%, v/v) and incubation with antibody specific for MyHC, MyoD, or α-tubulin. The blots were incubated with a peroxidase-conjugated secondary antibody and visualized by chemiluminescence (Amersham, Madrid, Spain). Bands detected on the x-ray films were quantified using commercial software.
Confocal Microscopy
To confirm the morphological appearance of myotubes we examined the expression of MyHC relative to the number of nuclei at different times of differentiation. Cells were plated on glass coverslips and fixed with 70% ethanol:formaline:glacial acetic acid (20:2:1, v/v/v) for 1 minute at room temperature. After blocking nonspecific binding sites with 1% goat serum/TBS for 24 hours at 4°C, cells were incubated with anti-MyHC antibody (1:5 dilution) in 1% goat serum/TBS for 2 hours at room temperature. Afterward, cells were incubated with FITC-conjugated goat anti-mouse IgG (1:200 dilution) for 1 hour at room temperature. Subsequently, nuclei were labeled with ethidium bromide (40 μg/ml) for 20 minutes at room temperature. Cells were then extensively washed with TBS/T and mounted onto glass slides. The specimens were examined in a Leica TCS-NT laser scanning confocal microscope equipped with an argon-krypton laser and a ×63 Leitz Plan-Apo objective (NA 1.4). The quantitation of myotube formation was expressed as percentage nuclei in MyHC-positive cells compared to total number of nuclei in the field. More than 10 fields per condition and time point were examined on myogenic induction.
Determination of GSH and γ-GCS Activity
Cells were washed twice with PBS, scraped off culture dishes, and collected in PBS containing 5% trichloroacetic acid. The mixture was centrifugated to discard precipitated protein, and the content of GSH and GSSG was analyzed by HPLC.23 The GSH synthetic capacity was determined using GSH precursors glutamate, glycine, cysteine and cofactors, ATP and Mg2+ in the presence of monochlorobimane, as described previously in detail.24 The synthesis of GSH under these conditions reflects the contribution of γ-GCS and GSH synthetase activities. GSH synthetase activity was assayed using glycine, glutamate, and γ-glutamilcysteine instead of cysteine as precursors. The rate of GSH formation was monitored as the net rate of fluorescence increase of GSH-monochlorobimane adduct catalyzed by GSH S-transferase over time after subtracting the BSO-inhibitable fluorescence signal as characterized.24 BSO, an amino acid sulfoximine, is a potent and specific inhibitor of γ-GCS acting as an analogue of the transition-state formed in the reaction catalyzed by γ-GCS.25
Reactive Oxygen Species Determination
Hydrogen and other organic peroxides were monitored spectrofluorimetrically using chloromethyl-2′-7′-dichlorodihydrofluorescein diacetate (2μmol/L) determining the fluorescence of 2′-7′-dichlorofluorescin (DCF) as described in detail previously.26 Relative fluorescence units were normalized per mg cellular protein.
Determination of Cell Death
Cell death was determined by measuring the leakage of lactate dehydrogenase (LDH) into the medium as percentage of total cellular LDH. Cells were lysed in TBS plus 5% Triton-X100. The mixture was spun at 13,000 × g for 1 minute at 4°C and the resultant supernatant was used for LDH activity determination by measuring the rate of oxidation of NADH at 340 nm (25°C) by a standard spectrophotometric method and normalized by the protein content. To estimate the population of apoptotic cells, we monitored the exposure of phosphatidylserine in the outer leaflet of the plasma membrane using the Annexin V-FITC Apoptosis Detection Kit (BioVision). Briefly, the medium was removed and cells were washed twice with PBS and incubated with binding buffer, anexin V-FITC, and propidium iodide for 15 minutes at room temperature in the dark. The mixture was then analyzed in a FACStar flow cytometer (Becton Dickinson, San Jose, CA).
Nuclear Extract Preparation and NF-κB Activation
Nuclear extracts were prepared after lysing cells with Nonidet P-40. Samples were centrifuged, and the pellet was resuspended in ice-cold buffer (20 mmol/L HEPES, 0.4 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF, and 0.75 μg/ml each of leupeptin, antipain, and aprotinin) and then kept on ice for 15 minutes. Cells were then lysed with Nonidet P-40 (10%) and the nuclear pellet was recovered after centrifugation at 13,000 × g at 4°C for 1 minute and stored at −80°C. NF-κB DNA binding activity in nuclear extracts was assessed by electrophoretic mobility shift assays using NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′), as described previously.27 Band specificity was determined by competition experiments using a molar excess of unlabeled NF-κB to the nuclear extract before the addition of labeled probe. In some cases, supershift assays were done using anti-p65 and anti-p50 antibodies (Santa Cruz Biotechnology) before addition of the labeled probe. In some instances, differentiating cells were treated with agents that prevent NF-κB activation, such as Bay 11–7085. This agent has been shown to interfere with IκBα phosphorylation28 thus abolishing NF-κB activation.
AP-1 Activation
Activation of AP-1 was examined using a consensus oligonucleotide 5′-CGCTTGATGAGTCAGCCGGAA-3′ labeled by T4 polynucleotide kinase as described.29 Binding reactions included 10 μg of nuclear extracts in incubation buffer (10 mmol/L Tris-HCl, pH 7.5, 40 mmol/L NaCl, 1 mmol/L EDTA, and 4% glycerol), 1 μg of poly(dI-dC), and labeled oligonucleotide (30,000 cpm). The mixture was electrophoresed, and the gel was dried and autoradiographed at −70°C overnight.
Depletion of Cellular GSH
To assess the role of GSH on myogenic differentiation, cells were depleted of cellular GSH by DEM followed by BSO. Confluent C2C12 cells were washed in PBS and cultured in differentiation medium for up to 7 days. 24 hours after myogenic stimulation, cells were treated with DEM (0.8 mmol/L, 0.1 mmol/L, or 0.05 mmol/L) for 15 minutes and washed thoroughly to remove excess DEM and then incubated with BSO (1 mmol/L) for 24 hours. After this period of time, cells were washed and fresh differentiation medium added and cells cultured until day 7. In some cases, 24 hours after DEM plus BSO pretreatment, GSHEE (2 mmol/L) was added for 24 to 48 hours, removed and culture continued with fresh differentiation medium until day 7. Cells were lysed at different periods of times and assayed for CK activity, MyHC levels, MyoD expression, or NF-κB activity.
Statistics
Results are expressed as the mean ± SD (SD) of three to five independent experiments. Groups were compared among themselves using Student’s t-test for unpaired data. Differences at P < 0.05 were considered significant.
Results
Activation of γ-GCS during Myogenic Differentiation
C2C12 cells have been used as an in vitro model to understand the regulation of myogenic differentiation. This cell line can differentiate into myotubes on growth factor deprivation.22 As a validation of this well-characterized cellular system, we monitored the myogenic differentiation of C2C12 into myotubes at the biochemical and morphological levels following replacement of growth medium to differentiation medium. CK activity increased over time on stimulation of cells with differentiation medium and this outcome was mirrored by the time-dependent increase in the levels of MyHC. Moreover, the fusion of myoblasts into multinucleated myotubes was verified by confocal microscopy and video microscopy showing contraction of emerging muscle fibers 7 days after culture in differentiation medium (not shown).
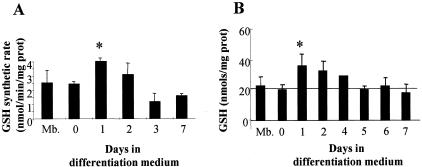
Since oxidative stress modulates myogenesis and because previous studies reported the regulation of GSH levels during myogenic differentiation of C2C12 cells,16 we analyzed the activity of γ-GCS, the rate-limiting enzyme in GSH biosynthesis,23–25,30 as this has not been previously examined. The maximal GSH synthetic rate from unlimited precursors and cofactors was examined in dialyzed cytosol prepared from cells at different times after myogenic differentiation in the presence of monochlorobimane to complex the newly synthesized GSH.23,24 As shown, there was a significant stimulation of the GSH synthetic rate in samples taken 24 hours after the induction of myogenesis declining thereafter (Figure 1A). Moreover, the GSH synthetic rate using γ-glutamylcysteine instead of cysteine and glutamate as GSH precursors did not change with respect to myoblasts at any time of differentiation (not shown). As expected and in agreement with previous observations,16 the cellular total GSH levels (GSH plus GSSG) increased with respect to basal myoblasts (day 0) within the first 24 hours after the induction of differentiation, returning to basal levels thereafter (Figure 1B). Thus, these findings show an early stimulation of the GSH synthetic capacity on myogenic differentiation of C2C12 accounted for by the activation of γ-GCS.
Figure 1.
Enhanced GSH synthetic rate during myogenesis. Myoblasts were grown to 100% confluency for 24 hours in growth medium and then the culture media switched to differentiation medium to induce differentiation. A: Cells were lysed and the cytosolic fraction was used to assess the maximal GSH synthetic rate in a cell-free system in the presence of unlimited GSH precursors (glutamate, cysteine, and glycine) and needed cofactors as described in Materials and Methods. The de novo synthesized GSH was conjugated with monochlorobimane and the fluorescence of the adduct was continuously determined over time in a fluorimeter. B: Total GSH content (GSH+GSSG) was determined in cell lysates from confluent myoblasts (Mb) and differentiating cells at different times of differentiation following culture in differentiation medium. Cell GSH content was analyzed by HPLC after derivatization with dinitrofluorobenzene as described in Materials and Methods. Data are the mean ± SD of three to six independent experiments. *, P < 0.05 versus Mb.
Role of GSH on Myogenic Differentiation
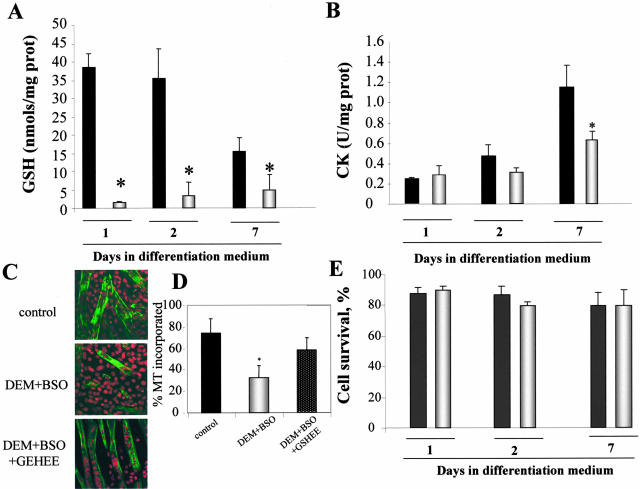
To assess the role of the stimulated GSH biosynthesis on skeletal muscle differentiation, we next evaluated the impact of GSH depletion on myogenesis. GSH levels were depleted first with DEM, a α-β unsaturated carbonyl electrophile known to be detoxified by GSH S-transferases using GSH as a cofactor. In addition, since DEM may lead to induction of γ-GCS resulting in the recovery of GSH stores,31 cells were subsequently incubated with BSO to inhibit the potential induction of γ-GCS by DEM. Incubation of cells with DEM+BSO 24 hours after switching growth medium to differentiation medium, led to depletion of GSH levels compared to differentiating cells and prevented the recovery of GSH stores at day 7 (Figure 2A). More importantly, this strategy impaired the increase in CK activity with respect to the stimulation seen in control differentiating cells (Figure 2B) as well as the formation of myotubes monitored by the expression of MyHC by confocal microscopy from 79 ± 9% to 28 ± 7% in control or DEM+BSO-treated cells, respectively (Figure 2, C and D). This effect of GSH depletion was significant at day 3 of differentiation and persisted through day 7 (not shown). To discern whether this outcome was due to a toxic effect, we monitored cell survival at different days during culture in differentiation medium. As shown, the survival of differentiating myoblasts was similar regardless of DEM+BSO pretreatment (Figure 2E). Since LDH monitors necrotic cell death, we searched for specific signs of apoptotic cell death including phosphatidylserine externalization as well as the morphological appearance of chromatin. Using an annexin V-FITC detection system and staining of cells with DNA-binding fluorochromes we did not detect signs of apoptosis despite treatment of cells with DEM+BSO (not shown).
Figure 2.
GSH depletion impairs differentiation of C2C12 cells. Myoblasts were grown in growth medium to reach confluency and 24 hours later the culture medium was switched to differentiation medium to induce myogenesis. Twenty-four hours after myogenic stimulation in differentiation medium cells were treated with DEM (0.8 mmol/L preincubation for 15 minutes) followed by BSO (1 mmol/L) for 24 hours to deplete GSH and inhibit γ-GCS activity (DEM+BSO, open bars) or not (control, closed bars), after which cells were grown in differentiation medium until day 7. A: Total GSH levels were assessed by HPLC as in Figure 1B. B: To assess the effect of DEM/BSO on differentiation and myogenesis, CK activity was determined in cell lysates at different times of culture in differentiation medium with or without DEM+BSO preincubation. C: The morphological appearance of myotubes at day 7 was examined by confocal microscopy. Nuclei were stained by propidium iodide (red) and MyHC by an anti-MyHC antibody followed by a FITC-labeled secondary antibody (green). In some cases we examined the effect of GSH replenishment by GSHEE on DEM+BSO-treated cells. D: Quantitation of myotube formation from the representative images shown in C as the percentage of nuclei in MyHC-positive cells compared to total number of nuclei in the field. E: Cell survival was determined after treatment with DEM+BSO as the percentage of LDH released into the medium and viability expressed as percentage of LDH found in cells plus medium. Data are the mean ± SD of three to five independent experiments. *, P < 0.05 versus untreated control cells cultured in DM medium.
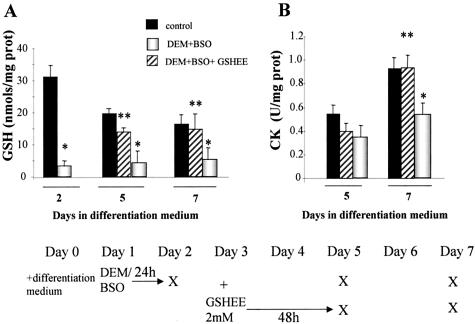
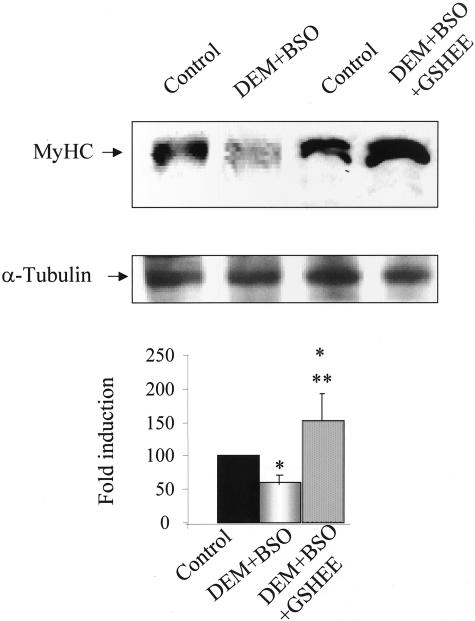
To further validate the dependence of myogenesis on appropriate GSH levels, we next assessed whether the impairment of myogenesis by GSH depletion was reversible on GSH replenishment. To this end, DEM+BSO-exposed cells were then incubated with GSHEE, a permeable form of GSH, determining GSH levels and CK activity at day 5 and 7 during culture in differentiation medium (see bottom diagram of Figure 3 for schematic experimental plan). This treatment restored GSH to levels of control differentiating cells (Figure 3A) and resumed myogenesis monitored by CK activity (Figure 3B). Furthermore, the impairment of myogenesis on GSH depletion with DEM+BSO was also translated in the lower levels of MyHC (Figure 4). Consistent with the findings on CK activity, the replenishment of GSH content of DEM+BSO-treated cells by GSHEE enhanced the expression of MyHC (Figure 4) and the extent of myotube formation from 28 ± 7% to 62 ± 8% in DEM+BSO and DEM+BSO plus GSHEE, respectively (Figure 2, C and D).
Figure 3.
Treatment with GSH ethyl ester restores differentiation of GSH-depleted C2C12 cells. Confluent myoblasts were treated with differentiation medium to induce differentiation with or without DEM+BSO treatment at day 1 for 24 hours as indicated in the bottom scheme. In some cases, DEM+BSO-pretreated cells were then exposed to GSH ethyl ester (GSHEE, 2 mmol/L) for 48 hours and cultured in fresh differentiation medium until day 7 as indicated. The X in the bottom scheme represents the time at which cells were taken for determination of total GSH levels (A) or CK activity as a marker for biochemical differentiation (B). Results are the mean ± SD of three to four independent experiments. *, P < 0.05 control; **, P < 0.04 versus DEM+BSO.
Figure 4.
Regulation of MyHC expression by GSH levels. Myoblasts were stimulated for differentiation into myotubes by switching culture medium to differentiation medium. In some cases, cells were treated with DEM+BSO with or without GSHEE (2 mmol/L) as indicated in the scheme of Figure 3. At day 7 of culture in differentiation medium, cells were lysed and levels of MyHC or α-tubulin as a loading control were determined by Western blot. The bottom panel represents the quantitive effect of DEM+BSO with or without GSHEE on MyHC levels. Results are the mean ± SD of three to four independent experiments with similar results. *, P < 0.05 versus control; **, P < 0.05 versus DEM+BSO.
GSH Depletion Impairs Myogenesis through Sustained Activation of NF-κB but not that of AP-1
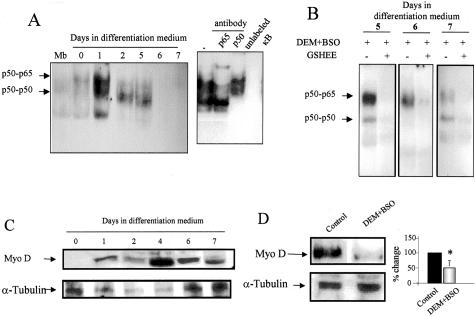
Previous findings indicated a role for NF-κB in the regulation of myogenesis.17–19 Since the activation of this transcription factor can be modulated by ROS generation at least in some cell types,32 we next examined the activation of NF-κB during myogenesis of C2C12 cells. As shown, the pattern of NF-κB activation was distinct in confluent myoblasts compared to differentiating cells with NF-κB undergoing a prominent activation during the first 24 hours of myogenic stimulation, declining thereafter (Figure 5A). To identify NF-κB homodimer and heterodimer complexes we performed supershift assays. The complexes detected at day 1 of differentiation were the p65/p50 heterodimer and the p50/p50 homodimer, with the latter being the predominant complex detected at day 2 and 5 of differentiation (Figure 5A) and this outcome was accompanied by IκB-α degradation (not shown). Consistent with the inhibitory role of DEM+BSO preincubation on skeletal muscle myogenesis, GSH depletion resulted in sustained activation of the p65/p50 heterodimer in nuclear extracts obtained from DEM+BSO pretreated cells at different times of culture in differentiation medium (Figure 5B). In addition, since NF-κB is known to regulate the activity of critical transcription factors for myogenesis,19 we examined the levels of MyoD. The expression of MyoD increased over time during differentiation (Figure 5C); however, DEM+BSO pretreatment abrogated the increase in MyoD levels observed in differentiating myoblasts (Figure 5D). Moreover, consistent with effect of GSH replenishment by GSHEE on MyoD expression (Figure 4), we tested the activation of NF-κB by GSHEE. As seen, GSHEE abolished the activation of NF-κB induced by DEM+BSO (Figure 5B).
Figure 5.
Regulation of NF-κB activation and MyoD expression during myogenesis by GSH. A: NF-κB activation was examined by electrophoretic mobility shift assay in nuclear extracts prepared from myoblasts or at different days after myogenesis induction by differentiation medium. The arrows denote the presence of the p65/p50 heterodimer and p50/p50 homodimer using anti-p65 and anti-p50 antibodies shown in the supershift assay. In addition the specificity of the complexes was ascertained by competition with a molar excess of unlabeled oligonucleotide. B: In some cases, cells were treated with DEM+BSO at day 1 of differentiation medium stimulation for 24 hours and then cultured with or without GSHEE (2 mmol/L) for 48 hours, isolating nuclear extracts from days 5 to 7 to examine NF-κB activation in nuclear extracts. C: The levels of MyoD and α-tubulin were determined in C2C12 cells at different times after culture in differentiation medium. D: Effect of DEM+BSO pretreatment at day 1 for 24 hours on the levels of MyoD and α-tubulin at day 2 of culture in differentiation medium. Data shown are representative of three independent experiments with similar results observed.
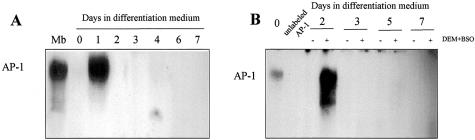
We also tested effect of GSH depletion on AP-1 activation, another redox-sensitive transcription factor32 known to regulate myogenesis20,21 whose down-regulation has been shown to precede myocyte differentiation.33 Parallel to the observations with NF-κB, AP-1 activation was seen at day 1 of differentiation and declined thereafter (Figure 6A), consistent with prior observations.33 To investigate whether the effect of GSH depletion in impairing myogenesis was related to the activation of AP-1, we examined its pattern of activation in nuclear extracts from DEM+BSO-treated cells (Figure 6B). AP-1 activation was seen at day 2 of differentiation in GSH-depleted cells which contrasts with the observations seen with NF-κB.
Figure 6.
AP-1 activation during myogenesis with or without GSH depletion. A: AP-1 activation was examined by electrophoretic mobility shift assay in nuclear extracts from C2C12 cells at different times during differentation. B: Cells were treated with DEM+BSO at day 1 of differentiation isolating nuclear extracts at different times during differentiation to examine AP-1 activation. The cellular GSH levels after DEM+BSO addition remained depleted by 88% with respect to control cells from day 1 to day 7. Data shown are representative of four independent experiments with similar results observed.
Inhibition of NF-κB Activation Resumes Differentiation Despite GSH Depletion
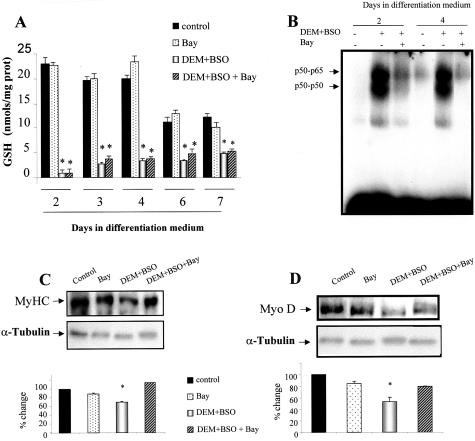
Since GSH depletion correlates with NF-κB activation, we next investigated if NF-κB inhibition, in conditions where GSH was depleted, restored myogenesis. In this setting, differentiating C2C12 cells were exposed to DEM+BSO to deplete GSH and then treated with Bay 11–7085, which prevents NF-κB activation by its ability to block IκBα phosphorylation.28 As seen in GSH-depleted cells (Figure 7A) Bay 11–7085 blocked NF-κB activation examined at day 2 and 4 (Figure 7B), resulting in stimulated differentiation monitored by MyHC (Figure 7C) and MyoD expression (Figure 7D).
Figure 7.
Inhibition of NF-κB activation restores myogenesis despite GSH depletion. Differentiating C2C12 cells were treated with DEM+BSO at day 1 with or without Bay 11–7085 (Bay) to prevent NF-κB activation. A: GSH levels at different times of differentiation were determined as indicated in Figure 1B. B: NF-κB activation was examined in nuclear extracts from cells treated with DEM+BSO with or without Bay which prevents NF-κB activation by inhibition of IκBα phosphorylation. The effect of Bay on differentiation of DEM+BSO-treated cells was examined by the expression of MyHC (C) and MyoD (D) in cell extracts taken at day 7 after culture in differentiation medium with respect to the α-tubulin levels used as loading control. The changes quantitated in the corresponding panels are the mean ± SD of three independent experiments. *, P < 0.05 versus controls.
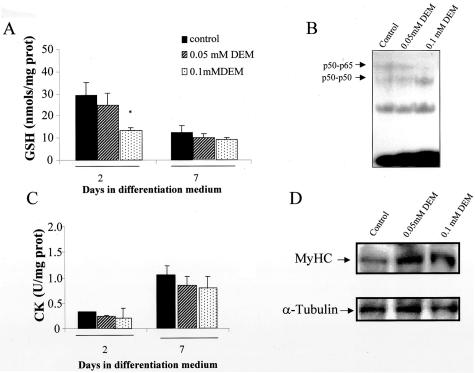
Finally, we also investigated the consequences of mild GSH depletion on myogenesis and NF-κB activation. Twenty-four hours after stimulation of differentiation, cells were treated with a milder DEM concentration so that GSH would fall to the levels seen in growing C2C12 cells. This mild depletion of GSH, however, did not impair myogenesis estimated by CK activity and MyoD levels (Figure 8). Importantly, the decrease in GSH was not accompanied by activation of NF-κB (Figure 8). Thus, these findings indicate that the differentiation of C2C12 cells correlates inversely with NF-κB activation and that the relevance of stimulated GSH homeostasis for myogenesis is to ensure inhibition of NF-κB.
Figure 8.
Mild GSH depletion does not impair myogenesis. Differentiating C2C12 cells were treated with DEM at the doses shown at day 1 for 15 minutes and then BSO was added as indicated in Figure 3. A: GSH levels were examined in differentiating cells at day 2 and 7 as indicated in Figure 1B. B: NF-κB activation was examined in nuclear extracts from cells pretreated with DEM at the doses indicated. The effect of mild GSH depletion on differentiation was examined by CK activity (C) as well as expression of MyHC with respect to α-tubulin (D).
Discussion
Cachexia or muscle wasting is a serious complication of chronic disorders and hence deciphering the responsible mechanisms may be of outmost relevance in a number of pathological conditions. Using an in vitro cellular model of skeletal muscle myogenesis, we provide compelling evidence for a critical role of GSH for the myogenic program of C2C12 cells. Although previous data indicated a transient increase in GSH levels during myogenic differentiation of C2C12 cells,16 the mechanism responsible for this observation was not further pursued. Our findings show for the first time the activation of γ-GCS early during myogenesis. Indeed, the activity of the GSH synthetase measured in cell-free extracts using γ-glutamylcysteine instead of cysteine and glutamate as precursors did not change at any time during differentiation. Consistent with the critical role of γ-GCS in the homeostasis of GSH,23,30 the maximal stimulation of both peaked at 24 hours of myogenic stimulation returning to basal values. The reason for this transient stimulation of the GSH levels and biosynthesis during myogenesis is uncertain. It may denote a different antioxidant need existing in myoblasts at different stages of differentiation as described previously,34 or it may reflect the disposal of the newly synthesized GSH stores to ensure appropriate redox environment needed for myogenesis. In regard with the former possibility, GSH is known to modulate the redox state of specific thiol residues of target proteins including protein kinases9 and reduced GSH equivalents may be used as a cofactor of GSH S-transferases to accomplish this task. For instance, extracellular signal-regulated kinase has been shown to mediate the inhibition of myogenesis by reactive species.16,35 Furthermore, the activity of Jun-D decreases on exposure of skeletal muscle to oxidative stress. Jun-D modulates the activity of the myogenin and myogenin-Jun-D complexes exhibit a high affinity toward the myosine CK enhancer.14 Moreover, a pro-oxidant environment and reduced GSH levels lead to activation of stress-activated protein kinases p38 and JNK, as shown in hepatocytes.36 A recent study reported an essential role for the p38/MAKK3 pathway in the differentiation of C2C12 cells.37 However, although we did not determine the activation of this pathway in cells following GSH depletion, its putative involvement may have not been sufficient to stimulate myogenesis in conditions of GSH depletion. On the other hand, the transient increase of GSH homeostasis seen may indicate the consumption of GSH stores to control the consequences of oxidative stress and the generation of ROS, particularly from mitochondria, during differentiation. For instance, it has been shown that the mitochondrial activity of QM7 myoblasts increases during differentiation38 and since mitochondrial metabolism generates ROS,8 it is conceivable that enhanced ROS generation may contribute in part to the lower GSH levels than would be expected from the stimulation of γ-GCS activity.
The mechanism leading to stimulation of γ-GCS and subsequent GSH homeostasis deserves further comments, in particular whether this process is specific for myogenic differentiation or a result of serum deprivation. Prior studies in various cell types (hepatocytes, lymphocytes, or fibroblasts) associate increased GSH levels, γ-GCS activity stimulation, and γ-GCS heavy chain mRNA induction with cell proliferation being the up-regulation of GSH homeostasis essential for the cell to enter the S phase.29,39 Since serum deprivation stimulates the exit of C2C12 cells from the cell cycle, a prerequisite for their differentiation, our observation of GSH homeostasis stimulation appears to be the result of the differentiation of myoblasts into myotubes, rather than to the serum deprivation itself. A final note concerns the mechanism mediating the stimulation of γ-GCS during differentiation. γ-GCS is a heterodimer made up of a catalytic (heavy) and regulatory (light) subunit of 73 kd and 30 kd, respectively. Since the activity of γ-GCS is a major determinant of the rate of GSH synthesis, most of the studies examining the stimulation of the γ-GCS activity in response to multiple stimuli ranging from oxidative stress to cytokines, or from hormones to ionizing radiation, reported the transcriptional up-regulation of the γ-GCS heavy subunit.23,29,30,31,39–42 Thus, consistent with these findings, the stimulation of γ-GCS during myogenesis of C2C12 may reflect increased levels of the catalytic subunit of γ-GCS.
ROS generation may play another role, in addition to consuming GSH equivalents. As just alluded, the catalytic activity of γ-GCS resides in its heavy subunit and it is induced by a number of stressful conditions including oxidative stress23,29,30,39 and hence an overgeneration of ROS at the time of myogenic differentiation may have contributed to the induction of γ-GCS (Figure 9). Although this remains to be confirmed, the abolishment of the stimulation of γ-GCS by BSO at the time of myogenic stimulation (day 0) results in enhanced detection of ROS, which fits with observations that addition of hydrogen peroxide (100 to 200 μmol/L) prevents differentiation of C2C12 cells16 (and our unpublished findings). Thus, along with observations in a murine model of cachexia indicating that features of muscle wasting were prevented by inhibitors of nitric oxide and non-lipophilic antioxidants,14 our findings are consistent with a role for GSH in promoting efficient differentiation program of satellite myoblasts into myotubes.
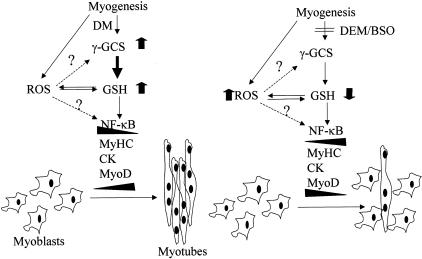
Figure 9.
γ-GCS induction during myogenesis. Schematic diagram depicting our findings. Myogenesis differentiation induces γ-GCS resulting in enhanced GSH levels. The signal and mechanism leading to induction of γ-GCS is uncertain and may include overgeneration of ROS possibly from mitochondria (see text for details). Enhanced GSH levels ensures myogenesis perhaps through down-regulation of NF-κB and subsequent induction of muscle-specific genes. On the other hand, the abolishment of stimulated GSH levels may contribute to persistent activation of NF-κB, which in turn contribute to the down-regulation of MyoD.
Our findings reveal an interesting regulation of NF-κB during myogenesis of C2C12 cells. The stimulation of myoblasts into myotubes was accompanied by a transient DNA binding activity of the p65/p50 heterodimer within the first 24 hours of differentiation (Figure 5). Our observations showing decreased NF-κB DNA binding are in agreement with observation reporting decreased NF-κB DNA binding activity that was preceded by an initial increase in the binding of the p50 homodimer.17 In another study, a transient rise in NF-κB DNA binding activity during the initial phase of myogenesis was also reported,43 although neither the transactivation nor the composition of the NF-κB complexes contributing to the increased DNA binding activity were examined. Since GSH depletion clearly abolished the expression of muscle-specific genes, we examined the regulation of NF-κB in conditions of GSH depletion. In agreement with previous findings in L6 myoblasts,44 GSH depletion by DEM+BSO enhances NF-κB activation thus contributing to the lower expression of MyoD19 and impaired myogenesis. The profile of NF-κB activation in relation with the regulation of GSH is intriguing. Enhanced DNA binding of the p65/p50 heterodimer is seen with stimulated GSH levels (day 1 of differentiation) as well as in cells with profound GSH depletion. Although the latter is consistent with the redox-dependent regulation of NF-κB, the activation of this transcription factor despite enhanced GSH levels may reflect a redox-independent event45 and may be related to the recognized role of NF-κB in the activation of survival pathways.46 The early activation of NF-κB during myogenesis may be important for the survival of committed myoblasts to differentiate, a possibility that remains to be confirmed.
Consistent with the role of AP-1 in the regulation of myogenesis, we examined the activation of AP-1 during the time course of differentiation of C2C12 and the effect of GSH depletion. Parallel to the observations with NF-κB, AP-1 activation monitored as DNA binding activity in nuclear extracts from differentiating C2C12 cells underwent a transient activation at 24 hours after myogenic stimulation that declined afterward, consistent with previous findings.33 The pattern of AP-1 activation in GSH depleting conditions was similar as in differentiating GSH-repleted cells except for the activation seen 48 hours after stimulation of differentiation. This outcome contrasts with the activation seen with NF-κB arguing against a role for AP-1 in mediating the impairment of myogenesis by GSH depletion. Interestingly, however, recent observations reported that AP-1 complexes containing Fra2, a member of the Fos family of AP-1, increased the expression of MyoD promoting myogenesis.47 Thus, although our findings establish a correlation between GSH levels and NF-κB regulation in the differentiation of myoblasts, there seems to be a threshold of GSH level below which NF-κB activation ensues, impairing myogenesis. The question then arises as to why GSH is stimulated early during differentiation if a mild depletion of GSH is permissible without leading to NF-κB activation. It is possible that in addition to regulation of this transcription factor, GSH may be used to ensure an appropriate redox environment or regulation of other factors that may contribute to myogenesis. In this regard Sir2 has been suggested to regulate C2C12 myogenesis through sensing redox state.48
In conclusion, the maintenance of adequate muscle GSH levels are of heuristic value and imply that optimal GSH content may be a key therapeutic strategy of relevance for patients afflicted from chronic disorders characterized by catabolic events and muscle wasting. The addition of GSH precursors and/or antioxidants may favor the transition of satellite myoblasts into multinucleated myotubes potentially diminishing the impact of cachexia.
Footnotes
Address reprint requests to Dr. Fernández-Checa, Liver Unit, Institut Malalties Digestives, Hospital Clinic i Provincial, C/Villarroel, 170, 08036-Barcelona, Spain. E-mail: checa229@yahoo.com.
Supported in part by grants from Fondo de Investigaciones Sanitarias, FIS 00/0281; E-Remedy (IST-2000–25146) from the European Union; Comissionat per a Universitats I Recerca de la Generalitat de Catalunya (1999 SGR 00228); Red Respira C03/11 (to J.R.), the Research Center for Liver and Pancreatic Diseases P50 AA11999, grant 1R21 AA014135–01 funded by the U.S. National Institute on Alcohol Abuse and Alcoholism, and Plan Nacional de I+D grants SAF 2003–04974 (to J.C.F-Ch.).
References
- Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223–248. doi: 10.1002/(sici)1098-1128(199905)19:3<223::aid-med3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- Zhao SP, Zeng LH. Elevated plasma levels of tumor necrosis factor in chronic heart failure with cachexia. Int J Cardiol. 1997;58:257–261. doi: 10.1016/s0167-5273(96)02873-2. [DOI] [PubMed] [Google Scholar]
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-α levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- Sherry BA, Gelin J, Fong Y, Marano M, Wei H, Cerami A, Lowry SF, Lundholm KG, Modawer LL. Anticachectin/tumor necrosis factor-α antibodies attenuate development of cachexia in tumor models. EMBO J. 1989;3:1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- Costelli P, Carbó N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argilés JM, Baccine FM. Tumor necrosis factor-α mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Checa JC, Kaplowitz N, García-Ruiz C, Colell A, Miranda M, Mari M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa JC. Redox regulation and signaling lipids in mitochondrial apoptosis. Biochem Biophys Res Commun. 2003;304:471–479. doi: 10.1016/s0006-291x(03)00619-3. [DOI] [PubMed] [Google Scholar]
- Hammarquist F, Luo J, Cotgreave IA, Andersson K, Wernerman J. Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med. 1997;25:78–84. doi: 10.1097/00003246-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Luo J, Hammarquist F, Andersson K, Wernerman J. Skeletal muscle glutathione after surgical trauma. Ann Surg. 1996;223:420–427. doi: 10.1097/00000658-199604000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, Gonzalez de Suso JM, Vilaro J, Barbera JA, Figueras M, Argiles JM, Fernández-Checa JC, Roca J. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–1118. doi: 10.1164/ajrccm.164.7.2103065. [DOI] [PubMed] [Google Scholar]
- Rabinovich RA, Figueras M, Ardite E, Carbó N, Troosters T, Filella X, Barberá JA, Fernández-Checa JC, Argilés JM, Roca J. Increased tumour necrosis factor-α plasma levels during moderate-intensity exercise in COPD patients. Eur Res J. 2003;21:789–794. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem. 1998;179:99–110. doi: 10.1023/a:1006859920875. [DOI] [PubMed] [Google Scholar]
- Langen R, Schols A, Kelders M, Van der Velden J, Wouters E, Janssen-Heininger YMW. Tumor necrosis factor-α inhibits myogenesis through redox-dependent and-independent pathways. Am J Physiol. 2002;283:C714–C721. doi: 10.1152/ajpcell.00418.2001. [DOI] [PubMed] [Google Scholar]
- Langen RCJ, Schols AMW, Kelders MCJM, Wouters EFM, Janssen-Heininger YMW. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Guttridge D, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1996;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge D, Mayo M, Madrid L, Wang C, Baldwin AS., Jr NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2365. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Li L, Chambard JC, Karin M, Olson EN. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev. 1992;6:676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM. Functional antagonism between c-Jun and Myo-D proteins: a direct physical association. Cell. 1992;68:507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature (London) 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Ardite E, Sans M, Panés J, Romero FJ, Piqué JM, Fernández-Checa JC. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000;80:735–744. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190:212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- Paris R, Morales A, Coll O, Sanchez-Reyes A, Garcia-Ruiz C, Fernández-Checa JC. Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J Biol Chem. 2002;277:49870–49876. doi: 10.1074/jbc.M208303200. [DOI] [PubMed] [Google Scholar]
- Roman J, Gimenez A, Lluis JM, Gasso M, Rubio M, Caballeria J, Pares A, Rodes J, Fernández-Checa JC. Enhanced DNA binding and activation of transcription factors NF-κ B and AP-1 by acetaldehyde in HEPG2 cells. J Biol Chem. 2000;275:14684–14690. doi: 10.1074/jbc.275.19.14684. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IκBα phosphorylation in endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Messina JP, Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;143:1974–1981. [PubMed] [Google Scholar]
- Morales A, García-Ruiz C, Miranda M, Marí M, Colell A, Ardite E, Fernández-Checa JC. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase. J Biol Chem. 1997;272:30371–30379. doi: 10.1074/jbc.272.48.30371. [DOI] [PubMed] [Google Scholar]
- Sekhar KR, Long M, Long J, Xu ZQ, Summar ML, Freeman ML. Alteration of transcriptional and post-transcriptional expression of γ-glutamylcysteine synthetase by diethyl maleate. Radiat Res. 1997;147:592–597. [PubMed] [Google Scholar]
- Sen CK, Packer L. Antioxidants and redox regulation of gene transcription. EMBO J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Lehtinen SK, Rahkila P, Helenius M, Korhonen P, Salminen A. Down regulation of transcription factors AP-1, Sp1, and NF-κB precedes myocyte differentiation. Biochem Biophys Res Commun. 1996;229:36–43. doi: 10.1006/bbrc.1996.1754. [DOI] [PubMed] [Google Scholar]
- Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Bennet AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Matsumaru K, Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- Cabane C, Englaro W, Yeow K, Ragno M, Derijard B. Regulation of C2C12 myogenic terminal differentiation by MKK3/p38α pathway. Am J Physiol. 2003;284:C659–C666. doi: 10.1152/ajpcell.00078.2002. [DOI] [PubMed] [Google Scholar]
- Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, Wrutniak C, Cabello G. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J Biol Chem. 2000;275:2733–2744. doi: 10.1074/jbc.275.4.2733. [DOI] [PubMed] [Google Scholar]
- Shaw JP, Chou I. Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J Cell Physiol. 1986;129:193–198. doi: 10.1002/jcp.1041290210. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Mari M, Morales A, Colell A, Ardite E, Fernández-Checa JC. Human placenta sphingomyelinase, an exogenous acidic pH-optimum sphingomyelinase, induces oxidative stress, glutathione depletion, and apoptosis in rat hepatocytes. Hepatology. 2000;32:56–65. doi: 10.1053/jhep.2000.8267. [DOI] [PubMed] [Google Scholar]
- Morales A, Miranda M, Sanchez-Reyes A, Colell A, Biete A, Fernández-Checa JC. Transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase by ionizing radiation. FEBS Lett. 1998;427:15–20. doi: 10.1016/s0014-5793(98)00381-0. [DOI] [PubMed] [Google Scholar]
- Rahman I, Bel A, Mulier B, Lawson MF, Harrison DJ, MacNee W, Smith CA. Transcriptional regulation of γ-glutamylcysteine synthetase-heavy subunit by oxidants in human alveolar epithelial cells. Biochem Biophys Res Commun. 1996;229:832–837. doi: 10.1006/bbrc.1996.1888. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-κB, and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Reznick AZ, Roy S, Packer L. Glutathione regulation of tumor necrosis factor-a-induced NF-κB activation in skeletal muscle-derived L6 cells. Biochem Biophys Res Commun. 1997;237:645–649. doi: 10.1006/bbrc.1997.7206. [DOI] [PubMed] [Google Scholar]
- Li N, Karin M. Is NF-κB the sensor of oxidative stress. EMBO J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Andreucci JJ, Grant D, Cox DM, Tomc LK, Prywes R, Goldhamer DJ, Rodrigues N, Bedard PA, McDermott JC. Composition and function of AP-1 transcription complexes during muscle cell differentiation. J Biol Chem. 2002;277:16426–16432. doi: 10.1074/jbc.M110891200. [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao R, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]