Abstract
Vinculin and its muscle splice variant metavinculin link focal adhesions and cell-to-cell contact sites to the actin cytoskeleton. We hypothesized that normal expression of vinculin isoforms would be essential for integrity of cardiomyocytes and preservation of normal cardiac function. We studied heterozygous vinculin knockout mice (Vin+/−) that develop and breed normally. The Vin+/− mice displayed: 1) a 58% reduction of vinculin and a 63% reduction of metavinculin protein levels versus wild-type littermates; 2) normal basal cardiac function and histology but abnormal electrocardiograms, intercalated disks, and ICD-related protein distribution; 3) increased mortality following acute hemodynamic stress imposed by transverse aortic constriction (TAC); 4) cardiac dysfunction by 6 weeks post-TAC; and 5) misalignment of α-actinin containing Z-lines and abnormal myocardial ultrastructure despite preserved cardiac function. Decreased expression of vinculin/metavinculin leads to abnormal myocyte structure without baseline physiological evidence of cardiac dysfunction. These structural changes predispose to stress-induced cardiomyopathy.
Vinculin (Vin) is a 117-kd ubiquitously expressed, membrane-associated protein that links focal adhesions (cell-substrate contacts), adherens junctions (cell-to-cell contacts), and costameres (subsarcolemmal adhesion plaques) to the actin cytoskeleton.1–3 This linkage occurs since vinculin binds to F-actin, α-actinin, α-catenin, and talin.4–6 In cardiac myocytes, vinculin is detected at the lateral sarcolemma, in transverse ribs called costameres, in Z-lines, as well as at cellular attachment sites termed intercalated disks (ICDs).7–9
Dilated cardiomyopathy has been associated with mutations in a number of structural proteins, and many such studies have shown that the link from sarcomere to cell membrane is critical for preservation of normal myocardial function and integrity.10 Relevant to the focus of the current examination is that a 124-kd splice-variant isoform of vinculin, termed metavinculin (Mvin), is expressed only in cardiac and smooth muscle.11 Alterations in metavinculin have been recently related to human cardiomyopathy.12,13
Homozygous inactivation of the murine vinculin gene caused embryonic lethality by E10.5. The embryos displayed a thin-walled myocardium and cerebral abnormalities including incomplete fusion of the neural folds.14 The heterozygous knockout mice (Vin+/−) develop and breed normally and are the focus of the current study. We hypothesized that reduced expression of vinculin/metavinculin in the intact Vin+/− mouse heart would lead to impaired cardiac function either in the basal condition or following hemodynamic stress. The strategy used to inactivate the vinculin gene in these mice leads to impaired production of vinculin as well as metavinculin.14 We found that the Vin+/− mice displayed: 1) a 58% reduction of vinculin and a 63% reduction of metavinculin protein levels versus wild-type littermates; 2) normal basal cardiac function and histology but abnormal electrocardiograms, intercalated disks, and ICD-related protein distribution; 3) increased mortality following acute hemodynamic stress imposed by transverse aortic constriction (TAC); 4) cardiac dysfunction by 6 weeks post-TAC; and 5) misalignment of α-actinin containing Z-lines and abnormal myocardial ultrastructure despite preserved cardiac function. These results indicate that reduced expression of vinculin/metavinculin in the mammalian myocardium alters myocyte structure and can predispose to stress-induced cardiac dysfunction.
Materials and Methods
Antibodies and Fluorochromes
The following antibodies were used: mouse monoclonal anti-pan-vinculin (which can detect both vinculin and metavinculin forms) (clone hVIN-1, Sigma, V9131); mouse monoclonal anti-sarcomeric-α-actinin (clone EA-53, Sigma, A7811); rabbit polyclonal anti-pan-cadherin (Sigma, C3678); rabbit polyclonal anti-connexin 43 (CX43) (Sigma, C6219), rabbit polyclonal anti-β1D integrin,15 mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Research Diagnostics Inc., TRK5G4–6C5); horseradish peroxidase (HRP) conjugated anti-mouse/anti-rabbit (Jackson Immunoresearch; 115–175-146/771–035-152), rhodamine-labeled phalloidin (Molecular Probes, R-415); Alexa Fluor 488 goat anti-mouse/anti-rabbit (Molecular Probes, A-11029/A-11034); and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma, D-9542).
Vinculin Knockout Mice
The strategy used to produce these mice was previously described.14 Briefly, exon 3 was deleted, which prevents production of both vinculin and metavinculin protein from the targeted allele. Genotypes of all mice were determined by polymerase chain reaction with primers P1, P2, P3, and P4. The sequences of these primers were: P1 (exon 3 823 forward) 5′-CCT GCG CGG GAT TAC CTC ATT GAC-3′; P2 (exon 4 1642 reverse) 5′-TGC TCA CCT GGC CCA AGA TTC TTT-3′; P3 (neomycin 479 forward) 5′-CCG GCC ACA GTC GAT GAA TC-3′; and P4 (neomycin 933 reverse) 5′-TCC GGT GCC CTG AAT GAA CT-3′. PCR with these primer sets resulted in a 474-bp neomycin product and an 843-bp exon 3/4 product from the WT allele. All mice were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care (AALAC) approved facility.
Western Blotting
Freshly isolated hearts were washed in ice-cold PBS, immediately frozen in liquid nitrogen, pulverized, homogenized in modified RIPA buffer, and analyzed via techniques as described previously.16,17 Whole heart protein lysate was resolved by SDS-PAGE and Western blots were performed to detect vinculin/metavinculin and GAPDH. Blots were incubated with primary antibodies overnight at 4°C. Dilutions were anti-vinculin (1:5000) and anti-GAPDH (1:5000- 1:30,000). Densitometric quantitation of protein bands was performed digitally with Alphaese software (ChemiImager 4400, α Innotech Corp., San Leondro, CA). All sample intensities were normalized to the GAPDH signal as a loading control.
Tissue Acquisition, Morphometry, Histology, Electron Microscopy, and Processing for Immunofluorescence Microscopy
For morphometric analyses, the animals were sacrificed at 3, 4, and 6 months of age. Postoperatively, one group of animals was sacrificed after 4 weeks and a second group after 12 weeks. At sacrifice, hearts were arrested in diastole by injection of a high potassium solution (25 mmol/L KCl and 5% dextrose in 1X PBS). Heart tissue was preserved for histology or immunohistochemistry as previously described.17 For electron microscopy (EM) studies, three samples from each genotype were analyzed. Samples were obtained from basal mice at 4 months of age. They were fixed in Karnovsky’s fixative (4% paraformaldehyde, 2.5% glutaraldehyde, 5 mmol/L CaCl2 in 0.1 mol/L Na cacodylate buffer, pH 7.4) overnight at 4°C followed by 1% OsO4 in 0.1 mol/L Na cacodylate buffer, pH 7.4, for 1 hour at room temperature. Tissue pieces were then en bloc stained with 4% uranyl acetate in 50% ethanol and subsequently dehydrated using a graded series of ethanol solutions followed by propylene oxide and infiltration with epoxy resin (Scipoxy 812, Energy Beam Sciences, Agawam, MA). After polymerization at 65°C overnight, thin sections were cut and stained with uranyl acetate (4% uranyl acetate in 50% ethanol) followed by bismuth subnitrate. Sections were examined at an accelerating voltage of 60 kV using a Zeiss EM10C electron microscope.
For immunohistochemistry, 10-μm cryosections were prepared via standard technique and stained with antibodies as outlined.16 Antibodies were diluted as follows: anti-vinculin (1:700), anti-α-actinin (1:500), anti-pan-cadherin (1:600), anti-connexin-43 (1:600), phalloidin (1:300), DAPI (1:1000) and all secondary antibodies (1:200). Control sections stained with the primary antibodies alone did not detect autofluorescence of the heart tissue and no significant background fluorescence was evident in the sections stained with secondary antibody alone. The results were visualized via standard immunofluorescent microscopy (Zeiss), confocal (Leica TSC-SP or Zeiss LSM 5 PASCAL microscopes) or deconvolution (Deltavision, Applied Precision, Inc., Seattle, WA) imaging.
Intercalated Disk Scoring
Cryosections from five wild-type and five Vin+/− hearts were analyzed. Specimens were stained with the indicated antibodies and areas with longitudinally oriented myocytes were located in each of the hearts. Five areas from each heart (25 fields per genotype) were analyzed via confocal or deconvolution microscopy (as above) in a manner similar to that described previously.18 Analysis of the digitized images was performed by two observers who were blinded to the genotype of the specimens. ICDs per field were counted and judged by their staining pattern as either normal or wide and disrupted. The percentage of normal ICDs was calculated for each genotype. The interobserver variability was not statistically significant.
Physiological, Surgical, and Echocardiographic Procedures
Wild-type and heterozygote vinculin KO mice were anesthetized with Avertin (2,2,2, tribromoethanol, 2.5% solution, 0.016 ml/g body mass, Aldrich Chemical Corp.) or isoflurane. Echocardiography (M-mode, 2-D and Doppler) was performed and then measured by investigators blinded to the genotype of the animal, as previously described.17,19
Mouse electrocardiograms (ECGs) were obtained in anesthetized animals with subcutaneous electrodes (Grass Instruments, Warwick, RI). Recording was performed with a custom-designed ECG amplifier that was optically isolated and optically coupled. Power for the amplifier was supplied by light-emitting diode photocells to reduce electronic noise. Measurements were made according to London20 as outlined in Figure 3.
Figure 3.
Electrocardiograms display widened QRS complexes in Vin+/− mice. No significant differences in heart rate or PR interval duration were seen between WT and Vin+/− mice, whereas QRS duration (measured as indicated by the arrows) was significantly prolonged in Vin+/− animals (n = 12 per group, *P < 0.007). A: Heart rate in WT and Vin+/− mice. B: PR duration (ms) in WT and Vin+/− mice. C: QRS duration (ms) in WT and Vin+/− mice. D and E: Representative ECG tracings in WT and Vin+/− mice, respectively.
Transverse aortic constriction was performed on 8-week-old male mice (28–31 g) with a 26-gauge needle to induce a fixed left ventricular pressure overload, as previously described.17,21 Sham surgery, identical except for the aortic constriction, was performed in both groups. Surgeries were performed on mice of both genotypes in a mixed/random manner by a surgeon blinded to the genotype of the animal at the time of surgery. A test group of animals underwent acute aortic constriction and the systolic pressure gradient across the surgical aortic stenosis was determined by bilateral carotid catheterization. Additional groups of animals undergoing TAC or sham operations were sacrificed at 4 or 12 weeks postoperatively. Echocardiographic evaluation was determined before and at 4, 6, 8, and 12 weeks following surgery.
Invasive hemodynamic determinations were performed at baseline and following dobutamine infusions as previously described.22 The measurements were acquired, digitized, displayed, and analyzed with HEM V3.3 software (Notocord Systems, Croissy sur Seine, France). All hemodynamic data were recorded continuously for at least of 30 minutes to ensure stable levels of pressures and heart rates.
Statistical Methods
Data were compiled and are shown as means ± standard errors of the mean (SEM). Data were evaluated using unpaired, two-tailed t-tests (95% confidence interval) or two-way repeated-measures analysis of variance with post hoc analysis using a Bonferroni test and GraphPad Prism4 software (GraphPad Inc, San Diego, CA). P < 0.05 was considered significant.
Results
Cardiac Vinculin Protein Levels Are Reduced to Less than 50% of Wild-Type Levels in Vin+/− KO Mice
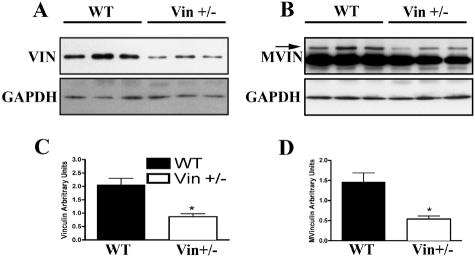
Whole heart lysates from Vin+/− (n = 6) and WT mice (n = 6) were used for Western blotting with an anti-vinculin antibody. GAPDH protein expression was used as an internal loading control. Densitometric analyses showed a 58% reduction of vinculin and a 63% reduction in metavinculin protein levels, in the Vin+/− mice, compared to wild-type mice (Figure 1).
Figure 1.
Vinculin and metavinculin protein levels are reduced in Vin+/− hearts. Whole heart lysates were analyzed by Western blotting simultaneously with antibodies to vinculin/metavinculin and GAPDH. Representative experiments are shown. A: Vin/GAPDH Western blot. B: Mvin/GAPDH Western blot. C and D: Densitometric analysis performed by normalizing vinculin or metavinculin expression to simultaneously measured GAPDH. The mean intensity per group is shown in arbitrary units. (n = 6, each group, *P < 0.003 vs. WT).
Vinculin+/− Hearts Are Histologically and Functionally Normal in the Basal State but Show Abnormal Baseline Electrocardiograms
To evaluate the consequences of reduced vinculin expression on cardiac form and function, histological and physiological assessments of the Vin+/− and control hearts were performed. Histological evaluation in the basal state showed no evidence of interstitial fibrosis, necrosis, or alterations in the myocardial architecture in mice up to 6 months of age (Figure 2, A and B). Echocardiography was performed in Vin+/− and wild-type littermate control animals up to 18 months of age to assess wall thicknesses, LV chamber dimensions and indices of cardiac function (by % fractional shortening (%FS) or velocity of circumferential fiber shortening (Vcf)). No significant differences were detected between the groups at either age. (Table 1). Invasive hemodynamics were performed at baseline and following dobutamine stimulation in 3-month-old Vin+/− and wild-type control mice via retrograde cannulation of the left ventricle using a 1.4F Millar catheter22 (Figure 2, C–F). No significant differences were detected in HR, pressures, or hemodynamic responses of the Vin+/− versus WT mice at baseline or after adrenergic stimulation. ECGs recorded in sedated animals showed no differences in heart rates or PR intervals, but widened QRS complexes were noted in Vin+/− animals (n = 12 each, P < 0.007; Figure 3).
Figure 2.
Basal cardiac function and morphology is normal in Vin+/− mice. No significant differences were detected in Vin+/− vs. WT mice when evaluated by histological analysis or closed-chest cardiac catheterization at baseline and during dobutamine infusion. (Cardiac catheterization: WT n = 4 and Vin+/− n = 5). A and B: Myocardial sections stained with hematoxylin and eosin from 3-month-old wild-type (A) and Vin+/− (B) mice revealed normal myocyte architecture and lack of fibrosis in both groups. (Magnification, ×400). C: Heart rate. D: LV systolic pressure. E: LV dP/dtmax. F: LV dP/dt min.
Table 1.
Basal Echocardiographic Findings in WT and Vin+/− Animals at 5 and 18 Months of Age
| WT (n = 12) 5 months old | WT (n = 12) 16–18 months old | Vin+/− (n = 12) 5 months old | Vin+/− (n = 16) 16–18 months old | |
|---|---|---|---|---|
| LVEDD (mm) | 4.06 ± 0.14 | 4.53 ± 0.11 | 4.00 ± 0.12 | 4.29 ± 0.1 |
| LVESD (mm) | 2.43 ± 0.13 | 2.95 ± 0.17 | 2.32 ± 0.09 | 2.60 ± 0.09 |
| IVSd (mm) | 0.66 ± 0.01 | 0.71 ± 0.01 | 0.66 ± 0.01 | 0.73 ± 0.02 |
| LVPWTd (mm) | 0.68 ± 0.01 | 0.74 ± 0.01 | 0.67 ± 0.02 | 0.77 ± 0.02 |
| % FS | 40.33 ± 1.75 | 35.36 ± 2.36 | 42.00 ± 1.57 | 39.44 ± 1.41 |
| Vcf | 7.89 ± 0.48 | 7.51 ± 0.50 | 8.64 ± 0.46 | 8.66 ± 0.34 |
| HR (bpm) | 502 ± 19 | 500 ± 19 | 525 ± 21 | 512 ± 16 |
| BW (g) | 48.42 ± 2.86 | 57.07 ± 2.77 | 44.67 ± 2.51 | 54.89 ± 3.12 |
No statistical differences were detected between WT and Vin+/− hearts. Data are displayed as means ± SEM.
LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVSd, interventricular septum in end-diastole; IVSs, interventricular septum in end-systole; LVPWTd, left ventricular posterior wall thickness in end-diastole; LVPWTs, left ventricular posterior wall thickness in end-systole; % FS, percent fractional shortening; Vcf, circumferential fiber shortening; HR, heart rate; BW, body weight.
Cytoskeletal Proteins and Intercalated Disks Are Abnormal in the Vinculin+/− Mice
Given the above findings in combination with the known cellular localization of vinculin, we evaluated the distribution of cytoskeletal/membrane-associated proteins in the myocardium of Vin+/− as compared to littermate control hearts. Vinculin itself was found to have abnormal morphology and distribution at the intercalated disks despite continued normal localization of the protein at the lateral cell membranes (Figure 4, A and B). Since vinculin is localized at cell-cell junctions and ECG abnormalities were detected in the Vin+/− mice, we next evaluated the expression pattern of intercalated disk proteins. We studied connexin 43(Cx43) expression since this is the predominant gap-junction protein in the ventricular myocardium (Figure 4, C and D).23,24 Cadherin expression was also evaluated as representative of adherens junctions (Figure 4, E and F). Using either confocal or deconvolution microscopy, localization of both Cx43 and cadherin was found to be abnormal. The expression of both of these proteins was detected more frequently in widened and disturbed ICDs in Vin+/− than WT (n = 5 mice of each genotype, P < 0.002; Figure 4G and Table 2).
Figure 4.
Altered intercalated disks are detected in Vin+/− hearts as visualized by immunomicroscopy of vinculin, connexin 43, and cadherin. Immunofluorescent staining with an anti-vinculin antibody in hearts of 4-month-old WT (A) and Vin+/− (B) mice. Vin+/− hearts showed normal costamere distribution but unorganized intercalated disk appearance via vinculin staining. Identical findings were detected with anti-Cx43 and anti-cadherin antibodies. Scoring for ICD disorganization was performed by two observers in a genotype-blinded fashion. TRITC-labeled phalloidin was used to visualize F-actin and DAPI for nuclei. A and B: Vinculin staining in WT (A) and Vin+/− (B). C and D: Cx43 (green), F-actin (red), and DAPI (blue) staining in WT (C) and Vin+/− (D). E and F: Cadherin (green) and F-actin (red) staining in WT (E) and Vin+/− (F). G: Graphical representation of ICD scoring in WT and Vin+/− hearts. ICD structure was judged by their Cx43 and cadherin staining pattern as either normal or wide. Percentage of wide ICDs is shown for each genotype (*P < 0.001 Vin+/− vs. WT, #P < 0.002 Vin+/− vs. WT).
Table 2.
Intercalated Disk Scoring in WT and Vin+/− Hearts
| Genotype (total number of ICDs) | WT Cx43 (n = 985) | Vin+/− Cx43 (n = 830) | WT pan-cadherin (n = 1070) | Vin+/− pan-cadherin (n = 899) |
|---|---|---|---|---|
| % Normal ICDs | 73.9 ± 2.2 | 39.1 ± 0.9 | 70.7 ± 1.6 | 36.7 ± 1.9 |
| (no. of normal ICDs) | (727) | (325) | (756) | (328) |
| % Wide ICDs | 26.1 ± 2.2 | 60.9 ± 0.9 | 29.3 ± 1.6 | 63.3 ± 1.9 |
| (no. of wide ICDs) | (258) | (505) | (314) | (571) |
Micrographs from five mice of each genotype were digitally acquired. The micrographs were analyzed by two observers in a genotype blinded fashion. ICDs per field were counted and ICD structure was judged by their CX43 or pan-cadherin staining pattern as either normal or wide. Percentage of normal and wide ICDs was calculated for each genotype. Results are displayed as means ± SEM.
Cardiovascular Stresses Leads to Sudden Death and Ventricular Dysfunction in the Vinculin+/− Mouse
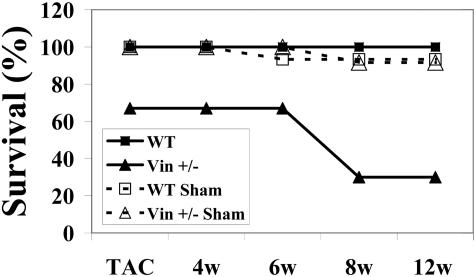
Since no functional changes were noted in the basal myocardium, we evaluated how Vin+/− mice would tolerate stressful stimuli. We investigated the effect of pressure overload using the well established model of transverse aortic constriction.17,21 First, acute aortic constriction was performed and showed no difference in pressure gradients between wild-type and Vin+/− groups (47 ± 7 mm Hg in WT vs. 49.8 ± 3.3 mm Hg in Vin+/−, n = 5 per group). Subsequently, 8-week-old male mice were used for a chronic TAC study. Initially we enrolled mice into a study where they were followed up to 12 weeks postoperatively and evaluated at distinct time points for cardiac function by echocardiography (Figure 5). At time of entry into the study, animal groups and numbers were: WT Sham (n = 7) Vin+/− Sham (n = 6), WT TAC (n = 9), Vin+/− TAC (n = 9). While all animals survived the surgery and were ambulatory, 33% of the Vin+/− TAC group died spontaneously during the first 3 to 6 hours following this initial postoperative recovery, leaving 6 in this group that were followed subsequently. (Identical perioperative mortality was seen in a second independent TAC study used for immunomicroscopic analyses; see animal numbers below.) All sham animals of both genotypes and all WT TAC mice survived through the surgery and acute postoperative period. Only 30% of Vin+/− TAC mice survived up to the full 12-week period following TAC versus 100% of the WT TAC.
Figure 5.
Vin+/− mice show increased mortality following pressure overload. Transverse aortic constriction was performed in nine Vin+/− mice and 33% (n = 3) died within 6 hours, following initial postoperative recovery. The remaining six Vin+/− mice and all other TAC and sham animals from both genotypes were then followed for up to 12 weeks after surgery. Surviving animals at the following specified time points: TAC (0w): WT Sham, (n = 7), Vin+/− Sham (n = 6), WT TAC (n = 9), Vin+/− TAC (n = 6); 4 weeks post-TAC (4w): WT Sham, (n = 7), Vin+/− Sham (n = 6), WT TAC (n = 9), Vin+/− TAC (n = 6); 6 weeks post-TAC (6w): WT Sham, (n = 6), Vin+/− Sham (n = 6), WT TAC (n = 9), Vin+/− TAC (n = 6); 8 weeks post-TAC (8w): WT Sham, (n = 6), Vin+/− Sham (n = 5), WT TAC (n = 9), Vin+/− TAC (n = 3); 12 weeks post-TAC (12w): WT Sham, (n = 6), Vin+/− Sham (n = 5), WT TAC (n = 9), Vin+/− TAC (n = 3)
Cardiac function was determined by echocardiography before surgery and at 4, 6, 8, and 12 weeks following TAC (Figure 6). At 4 weeks post-TAC, both WT mice and Vin+/− had similar increases in ventricular posterior and septal wall thicknesses (Figure 6, C and D) when compared to their sham controls. These data are consistent with an appropriate adaptive physiological response to pressure overload. At 4 weeks after surgery no detectable difference was seen between the Vin+/− TAC or WT TAC groups; however, beginning at 6 weeks, and subsequently at 8 and 12 weeks post-TAC, LV function decreased only in Vin+/− mice (Figure 6, A and B). An increased end-systolic dimension (LVESD) was detected only in Vin+/− TAC versus other groups, consistent with their contractile failure (Figure 6F).
Figure 6.
Echocardiographic analysis following chronic pressure overload showed that LV function became compromised beginning at 6 weeks post-TAC only in Vin+/− mice. Animals were evaluated by echocardiography at baseline (BL) and subsequently at 4, 6, 8, and 12 weeks post-TAC. A: Left ventricular fractional shortening (LV %FS). B: Circumferential fiber shortening (Vcf). C: Left ventricular end-diastolic posterior wall thickness (LVPWTd). D: Left ventricular end-diastolic interventricular septum thickness (IVSTd). E: Left ventricular end-diastolic diameter (LVEDD). F: Left ventricular end systolic diameter (LVESD). Baseline: WT (n = 11), Vin+/− (n = 12). 4 weeks (4w): WT-Sham (n = 5), WT-TAC (n = 6), Vin+/− Sham (n = 6), Vin+/− TAC (n = 6). 6 weeks (6w): WT-Sham (n = 5), WT-TAC (n = 6), Vin+/− Sham (n = 4), Vin+/− TAC (n = 6). 8 weeks (8w): WT-Sham (n = 5), WT-TAC (n = 7), Vin+/− Sham (n = 5), Vin+/− TAC (n = 6). 12 weeks (12w): WT-Sham (n = 4), WT-TAC (n = 7), Vin+/− Sham (n = 5), Vin+/− TAC (n = 3). P values: LV %FS: *WT TAC vs. Vin+/− TAC, P < 0.004. §Vin+/− TAC vs. Vin+/− Sham, P < 0.02. Vcf: *WT TAC vs. Vin+/− TAC, P < 0.01. §Vin+/− TAC vs. Vin+/− Sham, P < 0.03. LVPWTd: §Vin+/− TAC vs. Vin+/− Sham, P < 0.005. # WT TAC vs. WT Sham, P < 0.02. IVSd: §Vin+/− Sham vs. Vin+/− TAC, P < 0.001. #WT Sham vs. WT TAC, P < 0.003. LVESD *WT TAC vs. Vin+/− TAC, P < 0.002. §Vin+/− TAC vs. Vin+/− Sham P < 0.03. #WT TAC vs. WT Sham, P < 0.02.
α-Actinin/Z-Line Registration Is Abnormal in Vinculin+/− Mice
Since the vinculin+/− mice were relatively intolerant of TAC and vinculin is localized to costameres, Z-lines and cell-cell junctions, we evaluated the cellular structure of the myocardium in unstressed Vin+/− animals as well as in those following aortic constriction. For this study we performed (as outlined above) a second TAC study and sacrificed animals at 4 weeks postoperatively when cardiac function by echocardiography was identical in WT and Vin+/− groups. The number of animals used for this study were: WT Sham (n = 9), Vin+/− Sham (n = 6), WT TAC (n = 7), Vin+/− TAC (n = 12).
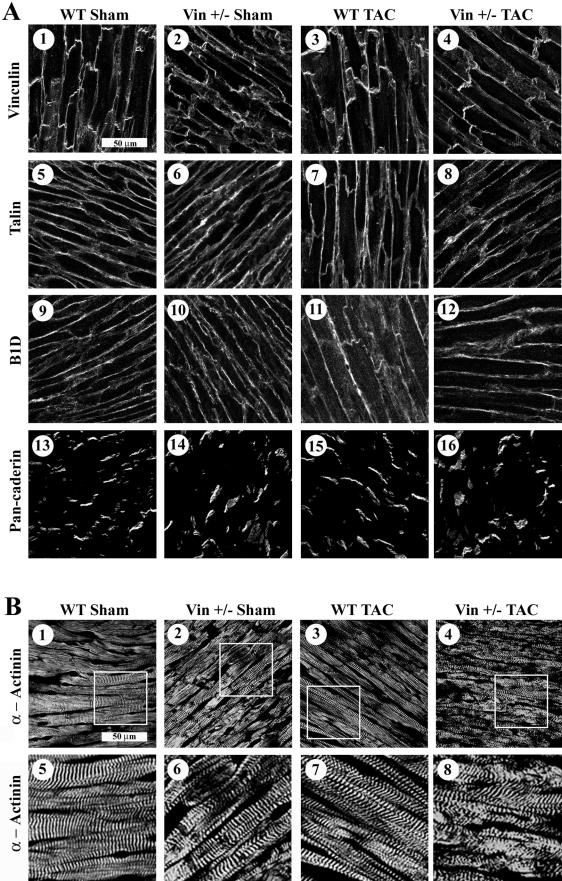
We studied a representative group of proteins (vinculin, β1D integrin, and cadherin) which could be altered within the cytoskeleton, focal adhesion or intercalated disk. We also evaluated the distribution of additional proteins which bind directly to vinculin, namely sarcomeric α-actinin and talin (Figure 7). Myocardial tissue was analyzed at 4 weeks following initiation of TAC, a time period when normal cardiac function was found in Vin+/− animals and WT controls. Random fields were evaluated from specimens that showed clearly delineated longitudinal orientation of myocytes. Confocal microscopy showed that Vin+/− hearts had abnormal vinculin and cadherin staining in widened intercalated disks both at baseline and in the post-TAC specimens (Figure 7A). Z-line registration, visualized by α-actinin staining, appeared mildly abnormal in sham-operated Vin+/− animals, as compared to sham-operated littermate WT controls. Following hemodynamic stress with TAC, misalignment of Z-lines became even more evident in the Vin+/−, while WT TAC animals continued to display normal registration (Figure 7B). These results imply that normal expression of vinculin is required for maintenance of proper architecture of the cardiac myocyte.
Figure 7.
Z-lines are disorganized in myocardial tissue from Vin+/− mice. Confocal microscopic analysis of vinculin and associated proteins was performed at 4 weeks after surgery in all groups (n = 3 per group), a time point when no physiological differences were detected in Vin+/− vs. WT animals. A: Immunostaining for vinculin (1–4) and the associated proteins, talin, (5-8), β1D-integrin (9–12), and pan-cadherin (13–16). Abnormal intercalated disk structure is evident in Vin+/− animals not WT controls as indicated by vinculin and cadherin distribution. Localization of talin and B1D were similar in all groups. B: Immunostaining for the vinculin-binding protein α-actinin (1–8). WT sham hearts (1 and 5) displayed normal Z-line structure as compared to Vin+/− sham hearts (2 and 6) which showed mild irregularities in Z-lines. Normal parallel Z-line staining continued to be seen in WT-TAC hearts (3 and 7) while the Vin+/− TAC Z-lines (4 and 8) became even more irregularly distributed. Magnified micrographs from 1 to 4 are shown below each in panels 5–8.
Electron Microscopy of Vin+/− Hearts Shows Abnormalities of Myofibril Anchorage at the Intercalated Disks and Z-Lines as well as Mitochondrial Alterations
To further investigate the abnormalities detected in the myocytes of Vin+/− mice, we performed electron microscopy on ventricular specimens from three Vin+/− hearts and three WT hearts in their basal state. Samples were obtained from 4-month-old mice so that the WT and Vin+/− animals would both have normal cardiac function. We specifically evaluated tissue which revealed longitudinally oriented myocytes. In each of the Vin+/− samples as compared to WT sections, increased separation of myofibrils was noted as clearing adjacent to intercalated disks as well as Z-lines. In addition, mitochondria in the Vin+/− mice appeared less densely packed and with swollen cristae. Examples of representative micrographs obtained from mice of each genotype are shown in Figure 8.
Figure 8.
Transmission electron microscopy of left ventricular mouse myocardium. Cardiac muscle from wild-type mice (A) shows well aligned arrays and insertions of myofibrils at Z-lines and intercalated disks. Mitochondria are visible in packed strands and are of normal size. Myofibrils of Vin+/− myocardial tissue (B) appear separated from the ICDs (arrows) as well as from Z-lines (arrowheads). Myofibrils in Vin+/− appear less densely packed than in WT muscle and were interrupted occasionally by swollen and disorganized mitochondria. C and D: Higher magnification views of A and B. Panels are representative of micrographs obtained from three animals of each genotype.
Discussion
We used heterozygous vinculin knockout mice to explore the role of this protein in the myocardium. Vin+/− mice showed reduced expression of vinculin and its muscle-specific isoform metavinculin to less than 50% of wild-type levels. This resulted in several important findings. The Vin+/− mice appeared normal at baseline and were able to breed appropriately. In the basal Vin+/− mice, we found: 1) no significant changes in ventricular function as determined by invasive catheterization or echocardiography up to 18 months of age; 2) normal cardiac morphology, morphometry, and light microscopic histology; 3) that the ECG QRS duration was greater than in WT mice; 4) immunomicroscopic analyses of vinculin itself, connexin-43, and cadherin were abnormal in the intercalated disk; and 5) electron microscopy showed disturbances of the myofibril attachment at intercalated disks and Z-lines. Ultrastructural examination of mitochondria was also abnormal in the Vin+/− mice compared to WT. With acute transverse aortic constriction, substantial mortality was noted in the Vin+/− animals but not in wild-type littermate controls. With TAC, only 67% of the Vin+/− animals survived past the perioperative period. These surviving mice initially developed identical changes in left ventricular wall thicknesses and mass as compared to the wild-type controls. By 6 weeks post-TAC, the Vin+/− group showed a decline in LV function and substantial mortality resulted only in this group. Heart specimens were analyzed from animals at 4 weeks after sham surgery or TAC when normal cardiac function remained. Z-line registration was mildly abnormal only in the Vin+/− sham group but not the WT sham, as shown by immunostaining of vinculin’s direct binding partner, α-actinin. The immunomicroscopic changes became more profound in the Vin+/− mice subjected to stress (TAC), whereas no alterations occurred in the stressed WT mice. Ultrastructural analysis of Vin+/− basal, unstressed myocardium, displayed abnormal insertion of myofibrils at Z-lines and intercalated disks, as well as disturbances of mitochondria. These results suggest that normal amounts of cardiac vinculin expression are required for myofibril anchorage into the Z-line and intercalated disk as well as preservation of normal mitochondrial architecture.
As a response to mechanical stress, cardiac myocytes remodel their anchorages from the extracellular matrix through the cytoskeleton to the sarcomere. In basal, non-stressed hearts, we detected ultrastructural abnormalities in the Vin+/− hearts that were physiologically normal by several methods. Prior work using immuno-electron microscopy had established that vinculin was located immediately adjacent to the fascia adherens region of the ICD membrane.25 The changes in myofibrillar attachment to Z-lines and intercalated disks in the Vin+/− mice could clearly predispose them to develop cardiac dysfunction when provoked by pathological conditions such as aortic constriction. It is also interesting to note that mitochondrial abnormalities were detected in the Vin+/− specimens. Mitochondrial disturbances like these were similarly detected in mice null for the intermediate filament desmin.26 It is possible that this mitochondrial problem could also have contributed to the cardiac dysfunction of the Vin+/− hearts subjected to hemodynamic stress.
In TAC-stressed Vin+/− hearts that were still physiologically normal, we also detected abnormalities in Z-line structure as indicated by α-actinin immunostaining. No similar findings were seen in identically stressed WT hearts. While we cannot exclude that this Z-line abnormality is indicative of early heart dysfunction, vinculin’s direct link to α-actinin suggests that its loss from this critical mechanosensitive linkage could be a primary initiator of the myocardial decompensation cascade.27 These immunomicroscopic findings are in agreement with the EM ones discussed above. In this location, vinculin could be crucial for maintaining normal sarcomere stability by cross-linking α-actinin to actin. Abnormalities in several Z-line proteins including desmin, actin, and α-actinin-associated LIM protein (ALP) have been previously associated with cardiomyopathies.28–31 Not only may disturbance of the Z-line lead to structural abnormalities in the cardiac myocyte, but this region may function as a critical signaling nidus for molecules such as protein kinase N (PKN) and G protein-coupled receptor kinases (GRKs).32,33
Of particular relevance for our study is that vinculin has a splice variant isoform termed metavinculin34 which is expressed exclusively in cardiac and smooth muscle, and perhaps skeletal muscle. In heart, the dominant isoform is still the ubiquitously expressed vinculin. The heterozygous knockout mice studied here have reduction in both vinculin and metavinculin. Based on the current work, it is unclear if reduction in vinculin or metavinculin is predominantly responsible for the observed phenotype. Two recent studies support an important role for metavinculin in cardiac function. Tissue sampled from a patient previously diagnosed with “idiopathic” cardiomyopathy displayed disorganized intercalated disk structures and was found to have a complete absence of metavinculin expression.12 More recently, three heterozygous metavinculin mutations (two point mutations and one deletion) were found in patients with “idiopathic” dilated cardiomyopathy.13 While in vitro analysis of these mutants showed that they still bound and cross-linked F-actin like the wild-type metavinculin, F-actin filament organization was disturbed by these mutants. Like the metavinculin deficient patient, histological analysis of a cardiac biopsy from the patient with the metavinculin Arg975Trp mutation displayed abnormal intercalated disks similar to our mice. It must be appreciated though that the analysis of the human sample was performed in a failing heart, while our analyses detailed hearts that had normal physiological function.
Vinculin is present in both cell-to-cell as well as in cell-to-matrix sites.35 Our results suggest that vinculin is critical component of the cardiac myocyte intercalated disk. Intercalated disks are specialized junctions at the ends of cardiac muscle cells that bond cells to each other, transmit forces of contraction, and provide areas of low electrical resistance for the rapid spread of excitation.36,37 Growing evidence has accumulated that ICD alterations are present in cardiac pathologies such as the transition of pressure overload hypertrophy to heart failure or in frankly cardiomyopathic hearts.38 Only in recent years has direct evidence linked such abnormalities or deficiencies in ICD proteins to cardiomyopathies, suggesting that changes in the ICD may be a primary cause of cardiac pathologies.31,39,40 This premise is illustrated by several mouse models with altered stoichiometry within the ICD, including muscle LIM protein (MLP) knockout mice40,41 as well as tropomodulin and cadherin overexpressing mice.39,42 Our results also support this concept and indicate for the first time that reduced vinculin expression can predispose the myocardium to progress from compensated hypertrophy to heart failure.
Vinculin may in part stabilize the cardiac myocyte through its indirect linkage to transmembrane integrins. In migratory cells, the recruitment of vinculin to focal adhesions is an early event in strengthening the linkage of ECM to the cytoskeleton.43,44 Whether this finding in non-cardiac cells is applicable to myocytes is not fully understood, but it is suggested by our work. In further support of this hypothesis, recent work from our laboratory has shown that a reduction of β1 integrin expression specifically in the cardiac myocyte resulted in abnormal cardiac function.17 In addition, cardiac myocyte specific dominant-negative disruption of vasodilator-stimulated phosphoprotein (VASP) and mammalian enabled (Mena) from the murine ICD also lead to dilated cardiomyopathy.45 This is of particular importance, realizing that VASP directly binds to vinculin.46
The total ablation of murine vinculin expression results in embryonic lethality with multiple defects including a thin-walled myocardium. Whether this cardiac phenotype in the null mice resulted from a primary or secondary phenomenon is not known. In contrast, the Vin+/− mice we studied had normal development and cardiac function unless stressed. Data from other species and in vitro work both contrast with and support the findings in mice. First, a homozygous vinculin knockout in Drosophila melanogaster leads to viable and fertile flies, even though no stress was placed on the flies.47 Second, F9 mouse embryonic stem cells, devoid of vinculin, mechanotransduced some biochemical signals, eg, mechanical activation of focal adhesion kinase.48 Yet, Caenorhabditis elegans vinculin mutants had disorganized muscle,49 while vinculin was still required for normal cell spreading, motility, adhesion, and morphology.50–53 Most importantly, vinculin is necessary for stabilization of focal adhesions and transfer of mechanical forces from the cell membrane to the cytoskeleton through integrins.54 Furthermore, in vitro antisense suppression of vinculin expression (to approximately 45 ± 20% of wild-type levels) caused disruption of myofibril assembly and alignment in fetal cardiac myocytes.55 These cell culture results differ from our own and highlight the importance of studies in the intact myocardium.
In summary, we have identified a mouse model with reduced expression of vinculin and metavinculin, which will allow us to gain further insight into the molecular mechanisms which predispose the myocardium to transition from compensated hypertrophy to heart failure. Further work is necessary and ongoing to determine the precise cellular and molecular mechanisms which lead to the phenotypic and physiological results in these heterozygous knockout mice.
Acknowledgments
We thank Drs. Matthew Schibler and Chris Babbit, as well as Michael Huang, for helpful advice and expertise. John Parker (UCLA Cardiovascular Research Laboratories) designed and constructed the optically isolated ECG amplifier. Patricia Reid was invaluable for EM assistance, and Dr. Michael C. Fishbein provided expertise in evaluation of the EM micrographs. The Sharon Tamor Hansen Imaging and Physiology Lab at UCSD was used for deconvolution microscopy with the excellent assistance of Dr. Antine E. Stenbit.
Footnotes
Address reprint requests to Robert S. Ross, VA San Diego Healthcare System, Cardiology Section, 111A, 3350 La Jolla Village Drive, San Diego, CA 92161, E-mail: rross@ucsd.edu.
Supported by grants from the National Institutes of Health (HL57872 and HL73393 to R.S.R.), the American Heart Association (R.S.R.) and the UCLA Laubisch Endowment Fund (K.P.R. and R.S.R.).
References
- Rudiger M, Korneeva N, Schwienbacher C, Weiss EE, Jockusch BM. Differential actin organization by vinculin isoforms: implications for cell type-specific microfilament anchorage. FEBS Lett. 1998;431:49–54. doi: 10.1016/s0014-5793(98)00723-6. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Signal transduction from the extracellular matrix—a role for the focal adhesion protein-tyrosine kinase FAK. Cell Struct Funct. 1996;21:445–450. doi: 10.1247/csf.21.445. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MH, DiLullo C, Schultheiss T, Holtzer S, Murray JM, Choi J, Fischman DA, Holtzer H. The vinculin/sarcomeric-α-actinin/α-actin nexus in cultured cardiac myocytes. J Cell Biol. 1992;117:1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Isenberg G. Vinculin and α-actinin: interaction with actin and effect on microfilament network formation. Cold Spring Harbor Symp Quant Biol. 1982;46 Pt 2:613–623. doi: 10.1101/sqb.1982.046.01.057. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Isenberg G. Interaction of α-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci USA. 1981;78:3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983;97:1081–1088. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracio L, Simpson DG, Hilenski L, Carver W, Decker RS, Vinson N, Borg TK. Distribution of vinculin in the Z-disk of striated muscle: analysis by laser scanning confocal microscopy. J Cell Physiol. 1990;145:78–87. doi: 10.1002/jcp.1041450112. [DOI] [PubMed] [Google Scholar]
- Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin JA, Bowles NE. Genetic abnormalities responsible for dilated cardiomyopathy. Curr Cardiol Rep. 2000;2:475–480. doi: 10.1007/s11886-000-0063-9. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Ornatsky OI, Kabakov AE, Glukhova MA, Koteliansky VE. Diversity of vinculin/meta-vinculin in human tissues and cultivated cells: expression of muscle specific variants of vinculin in human aorta smooth muscle cells. J Biol Chem. 1988;263:6631–6635. [PubMed] [Google Scholar]
- Maeda M, Holder E, Lowes B, Valent S, Bies RD. Dilated cardiomyopathy associated with deficiency of the cytoskeletal protein metavinculin. Circulation. 1997;95:17–20. doi: 10.1161/01.cir.95.1.17. [DOI] [PubMed] [Google Scholar]
- Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT, Jockusch BM. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105:431–437. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Pham CG, Harpf AE, Keller RS, Vu HT, Shai SY, Loftus JC, Ross RS. Striated muscle-specific β(1D)-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway. Am J Physiol Heart Circ Physiol. 2000;279:H2916–H2926. doi: 10.1152/ajpheart.2000.279.6.H2916. [DOI] [PubMed] [Google Scholar]
- Babbitt CJ, Shai SY, Harpf AE, Pham CG, Ross RS. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol. 2002;118:431–439. doi: 10.1007/s00418-002-0476-1. [DOI] [PubMed] [Google Scholar]
- Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the β1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada KA. Effects of diminished expression of connexin43 on gap junction number and size in ventricular myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H1662–H1670. doi: 10.1152/ajpheart.2000.278.5.H1662. [DOI] [PubMed] [Google Scholar]
- Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–H2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- London B. Cardiac arrhythmias: from (transgenic) mice to men. J Cardiovasc Electrophysiol. 2001;12:1089–1091. doi: 10.1046/j.1540-8167.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RS, Shai SY, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol. 2001;158:1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- Zhang JQ, Elzey B, Williams G, Lu S, Law DJ, Horowits R. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- Wang X, Osinska H, Dorn GW, Nieman M, Lorenz JN, Gerdes AM, Witt S, Kimball T, Gulick J, Robbins J. Mouse model of desmin-related cardiomyopathy. Circulation. 2001;103:2402–2407. doi: 10.1161/01.cir.103.19.2402. [DOI] [PubMed] [Google Scholar]
- Li D, Tapscoft T, Gonzalez O, Burch PE, Quinones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–464. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- Pashmforoush M, Pomies P, Peterson KL, Kubalak S, Ross J, Jr, Hefti A, Aebi U, Beckerle MC, Chien KR. Adult mice deficient in actinin-associated LIM-domain protein reveal a developmental pathway for right ventricular cardiomyopathy. Nat Med. 2001;7:591–597. doi: 10.1038/87920. [DOI] [PubMed] [Google Scholar]
- Mukai H, Toshimori M, Shibata H, Takanaga H, Kitagawa M, Miyahara M, Shimakawa M, Ono Y. Interaction of PKN with α-actinin. J Biol Chem. 1997;272:4740–4746. doi: 10.1074/jbc.272.8.4740. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Pitcher JA, Li X, Bennett V, Lefkowitz RJ. α-Actinin is a potent regulator of G protein-coupled receptor kinase activity and substrate specificity in vitro. FEBS Lett. 2000;473:280–284. doi: 10.1016/s0014-5793(00)01543-x. [DOI] [PubMed] [Google Scholar]
- Moiseyeva EP, Weller PA, Zhidkova NI, Corben EB, Patel B, Jasinska I, Koteliansky VE, Critchley DR. Organization of the human gene encoding the cytoskeletal protein vinculin and the sequence of the vinculin promoter. J Biol Chem. 1993;268:4318–4325. [PubMed] [Google Scholar]
- Critchley DR. Focal adhesions—the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disk? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs. III. Intercalated disk remodeling. J Mol Cell Cardiol. 1999;31:333–343. doi: 10.1006/jmcc.1998.0886. [DOI] [PubMed] [Google Scholar]
- Ferreira-Cornwell MC, Luo Y, Narula N, Lenox JM, Lieberman M, Radice GL. Remodeling the intercalated disk leads to cardiomyopathy in mice misexpressing cadherins in the heart. J Cell Sci. 2002;115:1623–1634. doi: 10.1242/jcs.115.8.1623. [DOI] [PubMed] [Google Scholar]
- Ehler E, Horowits R, Zuppinger C, Price RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM, Perriard JC. Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol. 2001;153:763–772. doi: 10.1083/jcb.153.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Hunter JJ, Ross JJ, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Welch S, Gude N, Khoury PR, Daniels SR, Kirkpatrick D, Walsh RA, Price RL, Lim HW, Molkentin JD. Pathogenesis of dilated cardiomyopathy: molecular, structural, and population analyses in tropomodulin-overexpressing transgenic mice. Am J Pathol. 1999;155:2101–2113. doi: 10.1016/S0002-9440(10)65528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Ginsberg MH. Talin forges the links between integrins and actin. Nat Cell Biol. 2003;5:694–697. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenthaler M, Engelhardt S, Schinke B, Kobsar A, Schmitteckert E, Gambaryan S, Engelhardt CM, Krenn V, Eliava M, Jarchau T, Lohse MJ, Walter U, Hein L. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003;285:H2471–H2481. doi: 10.1152/ajpheart.00362.2003. [DOI] [PubMed] [Google Scholar]
- Huttelmaier S, Mayboroda O, Harbeck B, Jarchau T, Jockusch BM, Rudiger M. The interaction of the cell-contact proteins VASP and vinculin is regulated by phosphatidylinositol-4,5-bisphosphate. Curr Biol. 1998;8:479–488. doi: 10.1016/s0960-9822(98)70199-x. [DOI] [PubMed] [Google Scholar]
- Alatortsev VE, Kramerova IA, Frolov MV, Lavrov SA, Westphal ED. Vinculin gene is non-essential in Drosophila melanogaster. FEBS Lett. 1997;413:197–201. doi: 10.1016/s0014-5793(97)00901-0. [DOI] [PubMed] [Google Scholar]
- Goldmann WH. The coupling of vinculin to the cytoskeleton is not essential for mechano-chemical signaling in F9 cells. Cell Biol Int. 2002;26:279–286. doi: 10.1006/cbir.2001.0854. [DOI] [PubMed] [Google Scholar]
- Barstead RJ, Waterston RH. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991;114:715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann WH, Galneder R, Ludwig M, Xu W, Adamson ED, Wang N, Ezzell RM. Differences in elasticity of vinculin-deficient F9 cells measured by magnetometry and atomic force microscopy. Exp Cell Res. 1998;239:235–242. doi: 10.1006/excr.1997.3915. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Fabry B, Tsai KY, Goldmann WH, Ingber DE. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem Biophys Res Commun. 2000;277:93–99. doi: 10.1006/bbrc.2000.3636. [DOI] [PubMed] [Google Scholar]
- Rodriguez Fernandez JL, Geiger B, Salomon D, Ben Ze’ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993;122:1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JL, Ben Ze’ev A, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG, Adamson ED. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci USA. 1995;92:9161–9165. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parasharama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- Shiraishi I, Simpson DG, Carver W, Price R, Hirozane T, Terracio L, Borg TK. Vinculin is an essential component for normal myofibrillar arrangement in fetal mouse cardiac myocytes. J Mol Cell Cardiol. 1997;29:2041–2052. doi: 10.1006/jmcc.1997.0438. [DOI] [PubMed] [Google Scholar]