Abstract
Matrix metalloproteinases (MMPs) are known to play a role in cell growth, invasion, angiogenesis, metastasis, and bone degradation, all important events in the pathogenesis of cancer. Multiple myeloma is a B-cell cancer characterized by the proliferation of malignant plasma cells in the bone marrow, increased angiogenesis, and the development of osteolytic bone disease. The role of MMPs in the development of multiple myeloma is poorly understood. Using SC-964, a potent inhibitor of several MMPs (MMP-2, -3, -8, -9, and -13), we investigated the role of MMPs in the 5T2MM murine model. Reverse transcriptase-polymerase chain reaction demonstrated the presence of mRNA for MMP-2, -8, -9, and -13 in 5T2MM-diseased bone marrow. Mice bearing 5T2MM cells were given access to food containing SC-964. The concentration of SC-964 measured in the plasma of mice after 11 days of treatment was able to inhibit MMP-9 activity in gelatin zymography. Treatment of 5T2MM-bearing mice resulted in a significant reduction in tumor burden, a significant decrease in angiogenesis, and partially protective effect against the development of osteolytic bone disease. The direct role of MMPs in these different processes was confirmed by in vitro experiments. All these results support the multifunctional role of MMPs in the development of multiple myeloma.
Multiple myeloma (MM) is a B-cell cancer characterized by the proliferation of malignant plasma cells in the bone marrow (BM) and the presence of a monoclonal immunoglobulin in the serum (paraprotein). MM cells produce osteoclast-activating factors and angiogenic factors, which result in the development of osteolytic lesions and the formation of new blood vessels (angiogenesis). The factors regulating these events are only now becoming clear but include cytokines, growth factors, adhesion molecules, and proteinases. Matrix metalloproteinases (MMPs) are a family of enzymes with ∼24 members. Members of the MMP family have been implicated in the progression of a number of malignancies.1–3 The proteinases are able to degrade many components of the extracellular matrix leading to invasion, metastasis, and angiogenesis.4–6 MMPs are also known to release matrix-bound growth factors resulting in tumor growth.7,8 In myeloma, it has been shown that tumor cells from patients with MM and from 5T33MM mice, bearing murine myeloma cells, secrete MMP-9 and that this secretion is induced by the interaction of MM cells with the BM microenvironment.9–11 BM stromal cells from patients with MM are also an important source of MMPs, mainly MMP-2.12 The demonstration that myeloma cells express MMPs raises the possibility that they play a role in certain processes in MM; homing, tumor growth, invasion, osteolytic bone disease, and angiogenesis.13 MMP inhibitors provide valuable tools to investigate the role of MMPs in these processes and might also be useful as anti-cancer agents. Endogenous tissue inhibitors of MMPs (TIMPs) tightly control MMP activity. The balance between MMPs and TIMPs regulates the net proteolytic activity. This balance is disturbed in pathological circumstances such as cancer. TIMPs are able to inhibit invasion, metastasis, tumor growth, and angiogenesis,14–16 but they have also been shown to promote tumor progression.17 Considerable effort has been put into the development of synthetic MMP inhibitors. SC-964 is a potent inhibitor of several MMPs with IC50s of 0.2 nmol/L, 56.5 nmol/L, 1.0 nmol/L, <0.1 nmol/L, and 0.2 nmol/L against MMP-2, -3, -8, -9, and -13, respectively, but was designed to be highly sparing of MMP-1 (IC50 of 3883 nmol/L) to avoid induction of the musculoskeletal syndrome.18 Recent results demonstrated that SC-964 is a potent inhibitor of angiogenesis in the mouse basic fibroblast growth factor-induced corneal micropocket model. SC-964 has been tested in several models of human cancer. Growth of the human prostate (PC3) tumor and human breast carcinoma (MDA-MB-435) was significantly inhibited by treatment of tumor-bearing mice with SC-964. SC-964 is able to decrease B16 melanoma lung metastasis. No data are available in models of hematological malignancies. The compound has not been tested in clinical trials. Because MM remains incurable, new approaches for therapy are necessary. To study the pathobiology of MM, we use the murine 5T2MM model.19–21 In the present study, we investigated the role of MMPs in the pathogenesis of MM by treating mice, bearing 5T2MM cells, with the broad-spectrum MMP inhibitor SC-964.
Materials and Methods
Mice
C57BL/KaLwRij mice were purchased from Harlan CPB (Horst, The Netherlands) and housed under conventional conditions. They were treated according to the conditions approved by the Ethical Committee for Animal Experiments, Vrije Universiteit Brussel (license no. LA1230281).
5T2MM Model
5TMM cells originated spontaneous in elderly C57Bl/KaLwRij mice.22 The disease can be transplanted by isolation of the BM of the diseased mice followed by intravenous transfer into young syngeneic mice. Using this method, several 5TMM models have been developed with similar characteristics that were found in humans. For example, the tumor cells are localized in the BM and mice have a serum paraprotein that reflects tumor load. Angiogenesis is increased and mice develop osteolytic lesions.19–21,23,24Two 5TMM lines, 5T2MM and 5T33MM have been extensively characterized by our group25 of which the 5T2MM model most closely resembles the most common form of human MM.
RNA Isolation and cDNA Synthesis
The BM of 5T2MM-diseased mice was isolated and 5T2MM cells were purified by density gradient centrifugation as previously described.26 B-end3 is an endothelioma cell line kindly provided by Dr. Y. St.-Pierre (INRS, University of Quebec, Laval, Canada). Total RNA from 5T2MM and b-end3 cells was isolated using the SV total RNA isolation system (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Heart and uterus were isolated from a C57Bl/KaLwRij mouse and total RNA was extracted using TRIzol reagent (Gibco, Life Technologies, Gent, Belgium). The concentration and purity of RNA was determined by spectrophotometric measurement (Gene Quant II; Pharmacia Biotech, Cambridge, UK). Total RNA (1 to 5 μg) was converted into cDNA using the superscript first-strand synthesis system (Gibco, Life Technologies) with random hexamers as primers.
Polymerase chain reaction (PCR) for MMP-2, -3, -8, -9, and -13 was performed with specific primers (Table 1) (kindly provided by Dr. Y. St. Pierre) in a 25-μl reaction mix containing 2.5 μl of 10× NH4 reaction buffer (Bioline, London, UK); 2 mmol/L MgCl2 (Bioline) for MMP-2, -8, -9, and -13; 1 mmol/L MgCl2 for MMP-3; 200 μmol/L dNTPs (Bioline); 300 nmol/L of primers; 0.5 U Red Taq polymerase (Bioline); and 1 μl cDNA. In the reaction mix for the MMP-3 PCR 5 μl of 5× high-specific additive (Bioline) were added. Cycle parameters for MMP-2 and -9 PCR were 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute (40 cycles); for MMP-3 PCR 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 2 minutes (40 cycles); for MMP-8 and -13 PCR 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute (35 cycles). Amplicon length for MMP-2, -3, -8, -9, and -13 was, respectively, 705, 673, 420, 380, and 559 bp.
Table 1.
Sequences of the Primers Used in PCR for MMP-2, -3, -8, -9, and -13
| Sense sequence | Anti-sense sequence | |
|---|---|---|
| MMP-2 | CTTTGCAGGAGACAAGTTCTGG | TTAAGGTGGTGCAGGTATCTGG |
| MMP-3 | ATTGCATGACAGTGCAAGGG | TGGAGGACTTGTAGACTGGG |
| MMP-8 | ACACTAACCTGACCTACTGG | GGTAGTGAATAGGTGCTGGG |
| MMP-9 | CCATGAGTCCCTGGCAG | AGTATGGATGTTATGATG |
| MMP-13 | CCTCCACAGTTGACAGGCTC | GCCAGTGTAGGTATAGATGG |
Treatment of 5T2MM-Bearing Mice with SC-964
SC-964 was prepared as an admixture in mouse chow by the Pharmacia Formulation Group. It was tested for stability in chow and shown to virtually 100% recoverable as parent after 30 days storage. Mice were given free access to food (n = 17) or food containing 1600 ppm SC-964 (Pfizer, St. Louis, MO) (n = 18) 1 day before injection of 5T2MM cells. Age- and sex-matched mice (naïve) were included as controls (n = 18). 5T2MM cells (1.5 × 106) were injected intravenously into each mouse. After 11 days of treatment, the concentration of SC-964 was measured in the plasma of the animals by mass spectrometry. Standards were prepared in mouse plasma. Plasma from mice was combined with acetonitrile containing an internal standard, mixed, centrifuged, and the supernatant was applied to a Metachem/Ansys-Metasil AQ 5μ C18 120A 050 × 020 column equilibrated with 0.1% formic acid in water and eluted with a gradient of acetonitrile in water. Eluate from the chromatography was analyzed using an Applied Biosystems Sciex 2000 triple quadrupole Mass Spectrometer (M+H = 501.1) and the data analyzed with the Sciex 2000 Analyst. On development of paralysis (11 weeks after injection of tumor cells), all mice were sacrificed. Hind and fore legs were dissected and used for determination of tumor load, microvessel density (MVD), and bone disease. For in vitro experiments, SC-964 was solubilized in dimethyl sulfoxide (DMSO) at 10 mmol/L and stored at −20°C.
Assessment of Tumor Load
The BM was flushed from a hind leg and treated with NH4Cl to lyse red blood cells. The percentage of 5T2MM cells in this cell suspension was determined by membranic flow cytometry staining with anti-idiotype monoclonal antibodies as previously described.25 Isotype-matched antibodies were used as controls.
Assessment of Angiogenesis by MVD
MVD was determined as previously described.21 Briefly, fore legs were fixed in zinc fixative for 48 hours and decalcified for 4 days. The material was embedded in paraffin and sections were cut. Slides were immunostained for CD31 to visualize the blood vessels and sinusoids in the BM. The endogenous peroxidase was quenched and trypsinization was used for antigen retrieval. The slides were preincubated with normal goat serum followed by incubation with primary antibody (PECAM-1; Becton Dickinson Pharmingen, LA) and secondary antibody (biotin-conjugated goat anti-rat-specific polyclonal immunoglobulin, 554014; Becton Dickinson Pharmingen). Tyramide signal amplification (TSA; NEN Life Science Products, Boston, MA) was used to enhance the signal intensity. Chromogenic visualization was accomplished through the use of a streptavidin-horseradish peroxidase conjugate followed by diaminobenzidine. Hot spots were selected on CD31-immunostained sections and the number of blood vessels was counted using a microscope eyepiece graticule (area = 0.20 μm2).
Assessment of Bone Disease
Bone disease was determined as previously described.27 The femur and tibia were fixed in 4% formalin and radiographed using a Faxitron X-ray system (Hewlett Packard, McMinnville, OR). X-rays were scanned using an UMAX PowerLook 1100 Scanner (Umax Systems, Willich, Germany) and images were enlarged. The number of osteolytic lesions was counted manually.
For histological and histochemical analysis, the femur and tibia were decalcified in ethylenediaminetetraacetic acid and embedded in paraffin. Sections (3 μm) were cut and stained with hematoxylin and eosin. Cancellous bone area was determined as a proportion of the total area in the distal femoral metaphysis and proximal tibial metaphysis using a Leica QWin image analysis system (Leica Microscope Systems, Milton Keynes, UK). To identify osteoclasts, sections of tibia and femur were also stained for the presence of tartrate-resistant acid phosphatase (TRAP) and counterstained with Gill hematoxylin. Because cancellous bone is almost completely removed in 5T2MM-diseased mice, only osteoclasts lining corticoendosteal bone surfaces were assessed. The number of osteoclasts present on 3 mm of each corticoendosteal surface, beginning 0.5 mm from the growth plate, was counted.
Gelatin Zymography
Serum-free RPMI 1640 media (BioWhittaker, Verviers, Belgium) conditioned for 24 hours by 5T2MM cells (1 × 106 cells/ml) were electrophoresed under nonreducing conditions using 10% sodium dodecyl sulfate-polyacrylamide gels containing 1 mg/ml of gelatin (Merck, Darmstadt, Germany). After electrophoresis, the gel was washed twice in 2% Triton X-100 for 30 minutes to remove sodium dodecyl sulfate. After overnight incubation in 0.05 mol/L of Tris buffer (pH 7.6) containing 10 mmol/L CaCl2 and 3 mmol/L NaN3, gels were stained with Coomassie brilliant blue and destained in 40% methanol/10% acetic acid. Gelatinolytic activity was identified as a clear band on a blue background. Supernatant from human fibrosarcoma HT1080 cells was used as positive control for the detection of MMP-2 and MMP-9. The 72-kd band corresponds to MMP-2, the 92-kd band to MMP-9. Mouse MMP-9 has a molecular mass of 110 kd, hence it is located higher on the gel than human MMP-9 (92 kd).
Thymidine Incorporation Assay
5T2MM cells (4 × 104 cells/well) were plated in a 96-well plate in co-culture with BM fibroblasts irradiated with 1500 rad in the presence of 0.6% DMSO (vehicle) or 6 μmol/L SC-964. Cells were pulsed with 1 μCi of (methyl-3H) thymidine (Amersham, Buckinghamshire, UK) and 16 hours later harvested with a cell harvester (Inotech, Wohlen, Switzerland) on filters (FiltermatA; Wallac, Turku, Finland). Filters were dried for 1 hour at 60°C and sealed in sample bags (Wallac) containing 4 ml of Optiscint scintillation liquid (Wallac). Radioactivity was measured using a 1450 Microbeta liquid scintillation counter (Wallac). Results are expressed as the total number of counts and corrected for the counts generated by BM fibroblasts alone.
Annexin V/Propidium Iodide Staining
5T2MM cells (106 cells/ml) were incubated with 6 μmol/L SC-964 or 0.6% DMSO for 6 and 20 hours. The percentage of apoptotic cells was determined by staining with annexin V-FITC (5 μl/105 cells; BD Pharmingen, Erembodegem, Belgium) and propidium iodide (100 ng/ml/105 cells) followed by flow cytometry.
In Vivo BM Homing
5T2MM cells were labeled with chromium-51 as described previously.28 Cells (0.5 × 105) were injected intravenously into mice pretreated for 4 days with vehicle or 1600 ppm SC-964. After 18 hours, the mice were sacrificed and kidneys, spleen, liver, lungs, ribs, vertebrae, and legs were collected. The radioactivity of each organ was measured in a gamma counter (MR480C/TP ITM; Van Hopplynus, Brussels, Belgium).
Assessment of Angiogenesis by Rat Aortic Ring Assay
The rat aortic ring assay was performed as previously described.21,29 Briefly, thoracic aortas were removed from rats and sectioned into aortic rings of 1-mm long. The ring-shaped explants were then embedded in a rat tail interstitial collagen (type 1) gel (Collagen R; Serva, Heidelberg, Germany) and allowed to polymerize in cylindrical agarose wells. Aortic rings (triplicates) were kept in culture at 37°C in 6 ml of medium conditioned for 48 hours by 5T2MM-diseased BM in the presence of 0.6% DMSO (vehicle) or 6 μmol/L SC-964. After 1 week images were taken from the triplicates under the microscope. Image analysis was performed on a Workstation Sun SPARC30, using the software Visilog5.0 from Noesis according to Blacher and colleagues.29 After generation of a binary image, the number of microvessels (Nv), the maximal microvessel length (Lmax), and the total number of branchings (Nb) were determined automatically.
Osteoclast Resorption Assay
Peripheral blood of female MF1 mice was used as a source of peripheral blood mononuclear cells. Circulating mononucleated cells were isolated by Ficoll-Histopaque (Sigma, Poole, UK) gradient centrifugation (838 × g, 20 minutes, 4°C). The mononuclear cells were plated at 1 to 2 × 105 cells on glass coverslips or dentine slices in a 96-well plate in 100 μl of α-MEM (Invitrogen, Paisley, UK) containing 10% fetal calf serum (Sigma), 1% penicillin (Invitrogen), 1% streptomycin (Invitrogen), and 1% amphotericin (Invitrogen). After 2 hours the nonadherent cells were washed away and the coverslips and dentine slices were transferred into a 24-well plate containing 1 ml of α-MEM, 10% fetal calf serum, 100 μg/ml dexamethasone (Sigma), 25 ng/ml M-CSF (R&D, Abingdon, UK), and 30 ng/ml RANKL (Peprotech, London, UK). 0.6% DMSO (vehicle) or 6 μmol/L SC-964 was added after 24 hours of incubation. The cultures were fed on days 4 and 7. The coverslips were harvested on day 7 and the cells were stained for the TRAP (Sigma). The dentine slices were incubated for 10 days, treated with trypsin for 10 minutes at 37°C, and left in NH4OH for at least 4 hours. The dentine slices were then sonicated and pits produced by resorbing osteoclasts were stained with toluidine blue. Each condition contained four dentine slices. Pits were counted in five areas of each dentine slice.
Statistical Analysis
For statistical analysis, the Mann-Whitney test was used. P < 0.05 was considered significant.
Results
Expression of MMP mRNA in 5T2MM-Bearing Mice
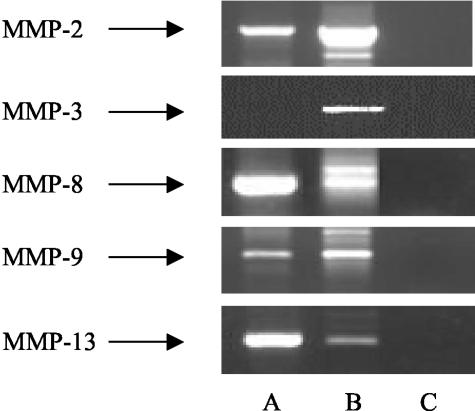
SC-964 is a potent inhibitor of MMP-2, -3, -8, -9, and -13. Therefore, we investigated whether these MMPs were expressed in the BM of 5T2MM-bearing mice. The mean purity of 5T2MM cells in the BM samples was 90 ± 3.6%. Reverse transcriptase (RT)-PCR demonstrated expression of mRNA for MMP-2, -8, -9, and -13, but not of MMP-3 (Figure 1) in BM cells isolated from 5T2MM mice.
Figure 1.
RT-PCR of MMP-2, -3, -8, -9, and -13. A: BM cells of 5T2MM-diseased mice. B: Positive control (b-end3 cells, heart, uterus, b-end3 cells, and uterus for, respectively, MMP-2, -3, -8, -9, and -13). C: Negative control (no reverse transcriptase during cDNA synthesis).
Treatment of 5T2MM-Bearing Mice with SC-964
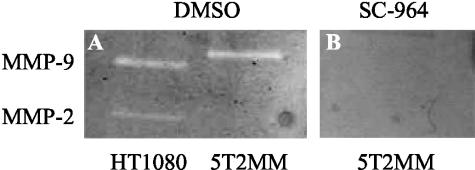
MMPs were shown to be expressed in the BM of mice bearing MM. By inhibiting MMPs it is possible to investigate their role in the development of MM. Mice injected with 5T2MM cells were given access to food or food containing 1600 ppm SC-964. After 11 days of treatment, SC-964 was detected in the plasma at a mean concentration of 6 ± 1.5 μmol/L (n = 8). To establish whether this concentration of SC-964 was able to inhibit MMP-9, gelatin zymography was performed. Overnight incubation of the gel with 0.6% DMSO (control) demonstrated MMP-9 secretion by 5T2MM cells and HT1080 cells, the positive control (Figure 2A). However, overnight incubation of the gel with 6 μmol/L SC-964 inhibited MMP-9 activity (Figure 2B). Thus, 6 μmol/L SC-964 was used for subsequent in vitro experiments.
Figure 2.
Gelatin zymography of media conditioned by 5T2MM cells. The gel was incubated overnight with 0.6% DMSO (A) or 6 μmol/L SC-964 (B). Conditioned medium of HT1080 cells was used as positive control.
MMPs Play a Role in MM Growth
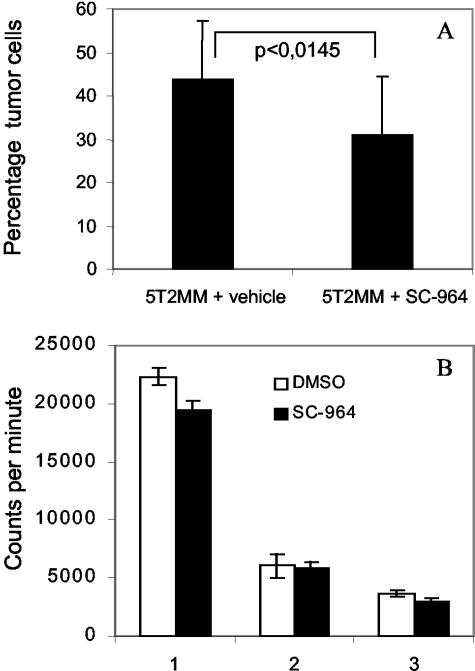
Tumor burden in the BM of mice treated with vehicle or SC-964 was determined by measuring the percentage of 5T2MM cells by flow cytometry. Flow cytometric analysis of 5T2MM idiotype-positive cells demonstrated a relative decrease of tumor load by 29% in 5T2MM-bearing mice treated with SC-964 compared to vehicle (Figure 3A). Since the treatment of mice started 1 day before injection of 5T2MM cells, the decrease in tumor burden could be because of an inhibition of homing of the 5T2MM cells to the BM. In vivo homing experiments, performed as described in Material and Methods, demonstrated no difference in homing in mice treated with vehicle (8.81 ± 3.96%, n = 2) and SC-964 (10.07 ± 3.78%). Subsequently, the influence of MMP inhibition on the proliferation of tumor cells was measured by 3H-thymidine incorporation. 5T2MM cells are dependent of the stroma and tumor cells co-cultured with BM fibroblasts are able to divide and grow.26 5T2MM cells were co-cultured with irradiated BM fibroblasts in the presence of vehicle (DMSO) or SC-964. Incubation with SC-964 for 16 hours resulted in a minor decrease in 3H-thymidine incorporation compared to incubation with vehicle (Figure 3B). Two of three experiments are statistically significant. Annexin V/propidium iodide staining followed by flow cytometry did not reveal any effect of SC-964 on the apoptosis of 5T2MM cells after 6 and 20 hours (data not shown), indicating that the decreased values of 3H-thymidine incorporation were because of decreased proliferation and not decreased survival.
Figure 3.
A: The effect of MMP inhibition by SC-964 on tumor burden in 5T2MM-bearing mice. The percentage of 5T2MM cells in the BM was measured by flow cytometry with anti-5T2MM idiotype antibodies. Each group contained 17 animals. Error bars represent SD. B: The proliferation of 5T2MM cells as measured by 3H-thymidine incorporation. 5T2MM cells were incubated with irradiated BM fibroblasts in the presence of vehicle (0.6% DMSO) or 6 μmol/L SC-964. Results represent the mean ± SD of quintuplicates. Results from three independent experiments are shown. P values of 0.009, 0.754, and 0.0209 were calculated for, respectively, experiments 1, 2, and 3.
MMPs Promote Angiogenesis
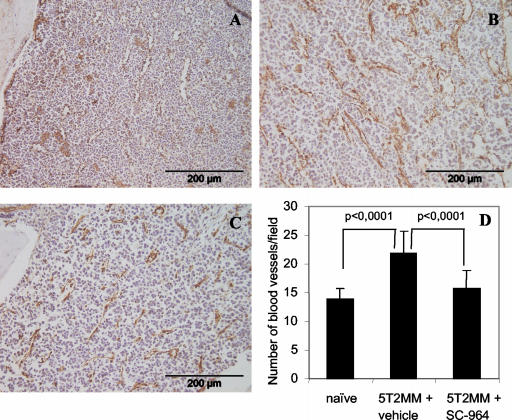
It has been demonstrated that angiogenesis is induced in MM both in human MM samples30 and in the 5TMM murine model.21 CD31 immunostaining of the BM from 5T2MM-diseased mice treated with vehicle (Figure 4B) demonstrated an increase in blood vessel density compared to staining of the BM from naïve mice (Figure 4A). This increase of the MVD was prevented in 5T2MM mice treated with SC-964 (Figure 4C). The number of blood vessels counted in 5T2MM-bearing mice was significantly increased compared to naïve mice (Figure 4D). There was a highly significant inhibition of 77% of new blood vessel formation in mice treated with SC-964 compared to mice treated with vehicle (Figure 4D) indicating that the MVD in the treated mice is almost back to the MVD counted in naïve mice. The direct role of MMPs in angiogenesis was further demonstrated in the rat aortic ring assay. Rat aortic rings cultured in conditioned medium of 5T2MM-diseased BM treated with vehicle (DMSO) exhibit a significant number of capillaries (Nv = 18.1 ± 0.59) with considerable branchings (Nb = 12.3 ± 7.42) (Figure 5A). In contrast, aortic rings cultured in conditioned medium supplied with SC-964 exhibit fewer capillaries (Nv = 7.2 ± 4.48) with less branchings (Nb = 0.8 ± 0.23) (Figure 5B). In addition, the maximal length of vessels was significantly reduced by MMP inhibitor treatment (0.3 ± 0.02 mm versus 0.8 ± 0.1 mm in control conditions). The differences were statistically significant with P < 0.02 for Nv and P < 0.006 for Nb and Lmax.
Figure 4.
The effect of MMP inhibition by SC-964 on angiogenesis. CD31 immunostaining of BM from a fore leg of a naïve mouse (A), a 5T2MM-diseased mouse treated with vehicle (B), and a 5T2MM-diseased mouse treated with SC-964 (C). The number of CD31-positive blood vessels was counted in the BM of naïve mice and 5T2MM-diseased mice treated with vehicle or SC-964 (D). Mean ± SD values of 18, 16, and 18 animals for, respectively, naïve, 5T2MM + vehicle, and 5T2MM + SC-964 are shown. Scale bars, 200 μm.
Figure 5.
Photomicrographs of rat aortic rings cultured in collagen gels in the presence of medium conditioned by 5T2MM-diseased BM with vehicle (0.6% DMSO) (A) or 6 μmol/L SC-964 (B). Photomicrographs from one experiment, representative of two, are shown. Nv, number of microvessels; Nb, total number of branching; Lmax, maximal microvessel length. Results represent the mean value ± SD of two independent experiments performed in triplicate. Scale bars, 1 mm.
Blocking of MMPs Inhibits the Development of Bone Disease
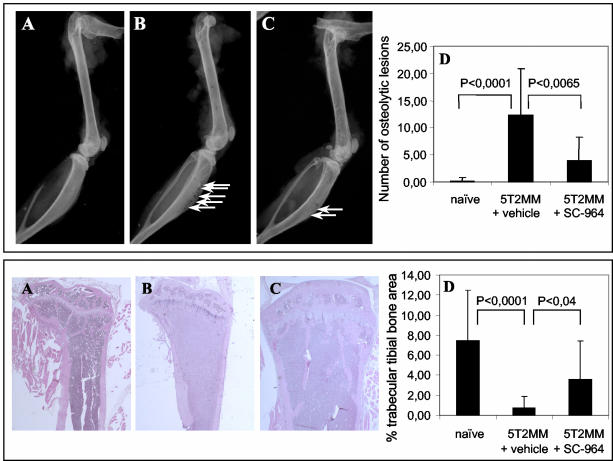
It has been previously demonstrated that injection of 5T2MM cells leads to the development of osteolytic bone disease.27 Several aspects in the development of bone disease were investigated. Radiographical analysis of 5T2MM-diseased mice treated with vehicle demonstrated a significantly increased number of osteolytic lesions in the tibia and femur (Figure 6, B and D, top) compared to naïve mice in which osteolytic lesions were absent (Figure 6, A and D, top). Treatment of mice bearing 5T2MM cells with SC-964 resulted in a significant decrease in the number of osteolytic lesions (Figure 6, C and D, top). Most of the osteolytic lesions were detectable in the tibia. The cancellous bone volume was measured in the proximal tibial metaphysis and the distal femoral metaphysis of naïve mice and 5T2MM-diseased mice treated with vehicle or SC-964. Injection of 5T2MM cells and treatment with vehicle resulted in an almost complete loss of cancellous bone in the tibial metaphysis (Figure 6B, bottom) compared to naïve mice in which the cancellous bone remained intact (Figure 6A, bottom). In 5T2MM-diseased mice treated with SC-964 the decrease in cancellous bone induced by the presence of 5T2MM cells was partially prevented (Figure 6C, bottom). The cancellous bone area was measured by image analysis. Results are given in Figure 6D (bottom). Similar results were obtained in the femoral metaphysis, but they were not statistically significant (results not shown).
Figure 6.
The effect of MMP inhibition on osteolytic bone disease. Top: Radiograph of femur and tibia from a naïve mouse (A), a 5T2MM-diseased mouse treated with vehicle (B), and a 5T2MM-diseased mouse treated with SC-964 (C). Arrows indicate the presence of osteolytic lesions. The number of osteolytic lesions was counted in the femur and tibia of naïve mice and 5T2MM-bearing mice treated with vehicle or SC-964 (D). Mean ± SD values of 12, 17, and 16 animals for, respectively, naïve, 5T2MM + vehicle, and 5T2MM + SC-964 are shown. Bottom: Section of the tibia from a naïve mouse (A), a 5T2MM-bearing mouse treated with vehicle (B), and a 5T2MM-bearing mouse treated with SC-964 (C). The trabecular bone area was measured in the tibia (D). Mean ± SD values of 18, 15, and 18 mice for, respectively, naïve, 5T2MM + vehicle, and 5T2MM + SC-964 are shown. Original magnifications, ×4.
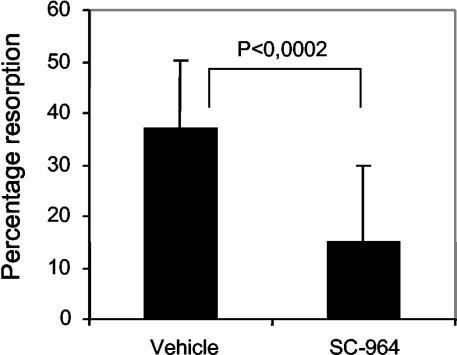
To investigate if MMP inhibition has an effect on osteoclast formation, osteoclasts were stained for TRAP and counted at the corticoendosteal bone surface. In naïve mice, few osteoclasts (2.49 ± 1.41, n = 10) were found on this surface. However, in mice injected with 5T2MM cells and treated with vehicle the number of TRAP-positive cells (6.17 ± 1.90, n = 8) was increased. In 5T2MM-bearing mice treated with SC-964, the number of osteoclasts (4.69 ± 2.62, n = 7) was decreased, but this was not statistically significant. The activity of osteoclasts can also be measured by the osteoclast resorption assay in vitro. The percentage of bone resorption in dentine slices treated with SC-964 (6 μmol/L) was relatively decreased by 60% compared to vehicle (0.6% DMSO) dentine slices (Figure 7).
Figure 7.
Percentage of bone resorption determined by counting pits on dentine slices incubated with DMSO or SC-964. Each condition contained four dentine slices. Pits were counted in five independent areas in each dentine slice. Results from one representative experiment of two are shown.
Discussion
MMPs are thought to be implicated in several processes in MM, but the exact role of the enzymes has not yet been identified. The 5T2MM mouse model is a good tool to study the role of MMPs in MM progression. SC-964 is a competitive substrate inhibitor that inhibits MMP-2, -3, -8, -9, and -13. In 5T2MM-diseased BM enriched for 5T2MM cells, mRNA expression of MMP-2, -8, -9, and -13 was detected, but no MMP-3. This is consistent with previous reports. Indeed, MMP-8, -9, and -13 have been demonstrated in human MM cell lines and patients9,31 and MMP-2 in BM stromal cells.9 Although MMP-2 mRNA was detected in 5T2MM-diseased BM, no MMP-2 protein was detected by gelatin zymography. Because the sensitivity of RT-PCR is higher than zymography, MMP-2 positivity in RT-PCR might be because of the production of MMP-2 by the contaminating stromal cells. Or the level of MMP-2 production is below the level of detection in the activity assay. MMP-8 and -13 can initiate the degradation of collagen types I, II, and III. The resulting cleavage products are denatured at body temperature to gelatin that can be further degraded by MMP-2 and -9. The gelatinases are also able to degrade collagen types IV and V. MMPs are secreted as inactive proenzymes and are activated extracellularly by proteolytic cleavage. The uPA/plasmin system is involved in the activation of proMMPs. Recent results demonstrated that uPA is expressed by 5T2MM cells,32 indicating that the MMPs in the BM can be activated. By inhibiting MMPs in the 5T2MM mouse model, we were able to determine which process may be regulated by MMPs in MM.
SC-964 treatment of mice injected with 5T2MM cells resulted in a decrease in tumor burden in the BM. This decrease was not because of a decreased homing of the 5T2MM cells to the BM. The in vitro proliferation of 5T2MM cells in co-culture with irradiated BM fibroblasts was slightly decreased in the presence of SC-964. The identity of the specific MMP involved in the regulation of tumor growth remains unknown. One possibility is that the release of growth factors from the extracellular matrix is inhibited by SC-964. Interleukin (IL)-6 and insulin-like growth factor-1 (IGF-1) are important growth factors for MM. Shedding of IL-6 receptor (IL-6R) and insulin-like growth factor binding protein-3 (IGFBP-3) is the result of proteolytic activity.8,33 The soluble IL-6R caused by shedding of the receptor from the cell membrane binds to IL-6 leading subsequently to an increased proliferation of MM cells.34 It is possible that shedding of IGFBPs increases the bioavailability of IGF-1 resulting in increased tumor growth. The inhibition of shedding of IL-6R and IGFBPs might thus result in a decline of tumor growth. These are the subjects of further studies.
It has recently been suggested that angiogenesis or neovascularization may play an important role in hematological malignancies.35 Angiogenesis, as measured by MVD, has been demonstrated in MM patients30 and in the 5TMM mouse model.21 Moreover, BM MVD also appears to be a prognostic factor in MM patients.36 In this study, treatment of 5T2MM-diseased mice with SC-964 resulted in an almost complete prevention of new blood vessel formation or angiogenesis. Rat aortic ring assays demonstrated a direct role of MMPs in neovascularization. Previous studies in other cancers already indicate that MMPs may play a role in angiogenesis.37–39 Degradation of the basement membrane, endothelial cell migration through the extracellular matrix followed by sprout elongation, lumen formation, and extracellular matrix remodeling require proteolytic activity. The influence of MMPs on angiogenesis can also be mediated by their effects on proangiogenic factors such as vascular endothelial growth factor.40 MMP-9 can trigger the angiogenic switch during carcinogenesis by releasing vascular endothelial growth factor from an extracellular reservoir.
One of the main characteristics of MM is the development of an osteolytic bone disease. In the present study, we demonstrated that inhibition of MMPs in 5T2MM-diseased mice resulted in a reduction in the number of osteolytic lesions. Treatment with SC-964 partially inhibited the decrease of cancellous bone volume induced by the presence of tumor cells. These results are consistent with data recently reported in a model of prostate cancer bone metastasis. Treatment with the broad-spectrum MMP inhibitor batimastat in a SCID-human model of prostate cancer metastasis prevented degradation of mineralized trabeculae and reduced the number of osteoclasts.41 There are a number of possible mechanisms that may contribute to the decrease in bone disease associated with MMP inhibition. First, MMPs are thought to participate in the recruitment of osteoclasts to sites of resorption.42 Second, MMPs themselves produced by MM cells or BM stromal cells participate in bone resorption.43,44 Third, MM cells produce factors responsible for stimulating osteoclastic bone resorption. The decrease in tumor load seen after MMP inhibition may therefore indirectly result in a decrease of bone disease. To investigate this, we examined the effect of SC-964 in in vitro osteoclast assays. The activity of osteoclasts was inhibited in the presence of SC-964. These results suggest that MMPs in MM invaded BM can influence the activity of the osteoclasts.
In conclusion, we could demonstrate that MMPs have a multifunctional role in the pathogenesis of MM. MMP inhibition not only resulted in reduction of tumor growth, but also had a significant effect on neovascularization and bone disease development. In vitro investigations confirmed that this inhibition was able to affect these processes directly. However, it is possible that indirect effects also occur. Treatment of MM-bearing mice with Fc-OPG or zoledronic acid resulted in a similar decrease of tumor load and osteolytic lesions.45,46 The reduction of tumor burden in the BM was not because of a direct effect of the two agents on MM cell proliferation. As already mentioned, MM cells produce osteoclast-activating factors. A decrease in tumor load may therefore result in a decrease of bone disease. On the other hand, the increased osteolytic activity in MM causes the release of several cytokines from the bone matrix, including IL-6, IGF, transforming growth factor-β, and basic fibroblast growth factor. These factors can enhance tumor growth and angiogenesis. In SC-964-treated animals the number of osteoclasts was decreased, but not significantly, suggesting the possibility that MMP activity is required for osteoclast formation and that this may indirectly affect tumor burden. Our group (unpublished results) and others have found a correlation between tumor load and angiogenesis.47 Whether a decrease in osteolytic bone disease has an influence on angiogenesis or the reverse needs to be explored further. To investigate if SC-964 might have clinical usefulness, a therapeutic setting where treatment starts from the onset of the disease (appearance of serum paraprotein), is necessary. Although MMP inhibitors were very effective in models of solid cancer, clinical trials were disappointing. However, it is now known that the functions of MMPs are complex. Recently, it has been demonstrated in a T-cell lymphoma model that an inhibitor with greater selectivity/specificity for MMP-9 in vitro showed greater efficacy against liver metastasis in vivo.48 In this study, a relatively broad-spectrum MMP inhibitor is used and it cannot be excluded that the inhibitor is inhibiting other metalloproteinases such as ADAMs. Additional studies are necessary to determine exactly which MMPs are involved in the different processes and at which stage of the disease to finally start clinical trials. It has been demonstrated that several MMPs are involved in the development of MM and that these enzymes might be interesting therapeutical targets.
Acknowledgments
We thank Frank Rylant, Gunther Vrolix, Liliane Moeneclaey, and Angelo Willems for their technical assistance; and Prof. F. Gorus (Department of Clinical Chemistry, Vrije Universiteit Brussel) for the performance of protein electrophoresis.
Footnotes
Address reprint requests to Dr. Karin Vanderkerken at Vrije Universiteit Brussel, Department HEIM, Laarbeeklaan 103, B-1090 Brussels, Belgium. E-mail: karin.vanderkerken@vub.ac.be.
Supported by the Onderzoeksraad-Vrije Universiteit Brussel, Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (clinical doctor grant to H.D.R.), Kom op tegen Kanker, Belgische Federatie tegen Kanker, Fortis, VIVAvzw (VIVA), European Commission (FP5 QLK3-CT02-02136 and FP6), Vlaamse Innovatie Soumenwerkingosverbander (VIS), and Leukemia Research Foundation (LRF) (to P.C.).
Karin Vanderkerken and Kewal Asosingh are postdoctoral fellows of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
References
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Noël A, Hajitou A, L’Hoir C, Maquoi E, Baramova E, Lewalle J, Remacle A, Kebers F, Brown P, Calberg-Bacq C, Foidart J. Inhibition of stromal matrix metalloproteases: effects on breast-tumor promotion by fibroblasts. Int J Cancer. 1998;76:267–273. doi: 10.1002/(sici)1097-0215(19980413)76:2<267::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakanishi I, Yamashita K, Hayakawa T, Okada Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci. 1993;104:991–999. doi: 10.1242/jcs.104.4.991. [DOI] [PubMed] [Google Scholar]
- Hua J, Muschel RJ. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996;56:5279–5284. [PubMed] [Google Scholar]
- Moses MA. The regulation of neovascularization by matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- O-Charoenrat P, Rhys-Evans P, Eccles S. A synthetic matrix metalloproteinase inhibitor prevents squamous carcinoma cell proliferation by interfering with epidermal growth factor receptor autocrine loops. Int J Cancer. 2002;100:527–533. doi: 10.1002/ijc.10531. [DOI] [PubMed] [Google Scholar]
- Manes S, Llorente M, Lacalle RA, Gomez-Mouton C, Kremer L, Mira E, Martinez -AC. The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem. 1999;274:6935–6945. doi: 10.1074/jbc.274.11.6935. [DOI] [PubMed] [Google Scholar]
- Barillé S, Akhoundi C, Collette M, Mellerin M, Rapp M, Harousseau J, Bataille R, Amiot M. Metalloproteinases in multiple myeloma: production of matrix metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction of MMP-1 by myeloma cells. Blood. 1997;90:1649–1655. [PubMed] [Google Scholar]
- Van Valckenborgh E, Bakkus M, Munaut C, Noël A, St Pierre Y, Asosingh K, Van Riet I, Van Camp B, Vanderkerken K. Upregulation of matrix metalloproteinase-9 in murine 5T33 multiple myeloma cells by interaction with bone marrow endothelial cells. Int J Cancer. 2002;101:512–518. doi: 10.1002/ijc.10642. [DOI] [PubMed] [Google Scholar]
- Vande Broek I, Asosingh K, Allegaert V, Leleu X, Facon T, Vanderkerken K, Van Camp B, Van Riet I. Bone marrow endothelial cells increase the invasiveness of human multiple myeloma cells through upregulation of MMP-9: evidence for a role of hepatocyte growth factor. Leukemia. 2004 doi: 10.1038/sj.leu.2403331. Mar 4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Barillé S, Bataille R, Rapp M, Harousseau J, Amiot M. Production of metalloproteinase-7 (matrilysin) by human myeloma cells and its potential involvement in metalloproteinase-2 activation. J Immunol. 1999;163:5723–5728. [PubMed] [Google Scholar]
- Kelly T, Borset M, Abe E, Gaddy-Kurten D, Sanderson R. Matrix metalloproteinases in multiple myeloma. Leuk Lymphoma. 2000;37:273–281. doi: 10.3109/10428190009089428. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Sounni N, Devy L, Grignet-Debrus C, Lewalle J, Li H, Deroanne CF, Lu H, Colige A, Nusgens BV, Frankenne F, Maron A, Yeh P, Perricaudet M, Chang Y, Soria C, Calberg-Bacq C, Foidart J, Noël A. Down-regulation of vascular endothelial growth factor by tissue inhibitor of metalloproteinase-2: effect on in vivo mammary tumor growth and angiogenesis. Cancer Res. 2001;61:3450–3457. [PubMed] [Google Scholar]
- Ahonen M, Baker AH, Kahari V. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310–2315. [PubMed] [Google Scholar]
- Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102:39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- McCarthy K, Maguire T, McGreal G, McDermott E, O’Higgins N, Duffy MJ. High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer. 1999;84:44–48. doi: 10.1002/(sici)1097-0215(19990219)84:1<44::aid-ijc9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Carron CP, Becker D, Duffin T, Goellner J, Knoerzer D, Mehta P, Munie G, Funckes-Shippy C, Swearingen C, Villamil C, Welsch D, Zhang K, Hollister B, Dexter D, De Crescenzo G, McWherter C. The highly selective MMP-1 sparing matrix metalloproteinase inhibitor SC-77964 provides broad-spectrum anti-tumor activity. Proc Am Assoc Cancer Res Annual Meeting. 2001;42:4975. (Abstract) [Google Scholar]
- Vanderkerken K, Goes E, De Raeve H, Radl J, Van Camp B. Follow-up of bone lesions in an experimental multiple myeloma mouse model: description of an in vivo technique using radiography dedicated for mammography. Br J Cancer. 1996;73:1463–1465. doi: 10.1038/bjc.1996.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkerken K, Asosingh K, Croucher P, Van Camp B. Multiple myeloma biology: lessons from the 5TMM models. Immunol Rev. 2003;194:196–206. doi: 10.1034/j.1600-065x.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Van Valckenborgh E, De Raeve H, Devy L, Blacher S, Munaut C, Noël A, Van Marck E, Van Riet I, Van Camp B, Vanderkerken K. Murine 5T multiple myeloma cells induce angiogenesis in vitro and in vivo. Br J Cancer. 2002;86:796–802. doi: 10.1038/sj.bjc.6600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radl J, De Glopper ED, Schuit HR, Zurcher C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J Immunol. 1979;122:609–613. [PubMed] [Google Scholar]
- Radl J, Croese JW, Zurcher C, Van den Enden-Vieveen MH, de Leeuw AM. Animal model of human disease. Multiple myeloma. Am J Pathol. 1988;132:593–597. [PMC free article] [PubMed] [Google Scholar]
- Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K. The 5TMM series: a useful in vivo mouse model of human multiple myeloma. Hematol J. 2000;1:351–356. doi: 10.1038/sj.thj.6200052. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K, De Raeve H, Goes E, Van Meirvenne S, Radl J, Van Riet I, Thielemans K, Van Camp B. Organ involvement and phenotypic adhesion profile of 5T2 and 5T33 myeloma cells in the C57BL/KaLwRij mouse. Br J Cancer. 1997;76:451–460. doi: 10.1038/bjc.1997.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkerken K, Asosingh K, Braet F, Van Riet I, Van Camp B. Insulin-like growth factor-1 acts as a chemoattractant factor for 5T2 multiple myeloma cells. Blood. 1999;93:235–241. [PubMed] [Google Scholar]
- Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJR, Holen I, Skerry TM, Dunstan CR, Russell GR, Van Camp B, Vanderkerken K. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K, De Greef C, Asosingh K, Arteta B, De Veerman M, Vande Broek I, Van Riet I, Kobayashi M, Smedsrod B, Van Camp B. Selective initial in vivo homing pattern of 5T2 multiple myeloma cells in the C57BL/KaLwRij mouse. Br J Cancer. 2000;82:953–959. doi: 10.1054/bjoc.1999.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher S, Devy L, Burbridge MF, Roland G, Tucker G, Noël A, Foidart J. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–142. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, Dammacco F. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Wahlgren J, Maisi P, Sorsa T, Sutinen M, Tervahartiala T, Pirilä E, Teronen O, Hietanen J, Tjäderhane L, Salo T. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J Pathol. 2001;194:217–224. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- Asosingh K, Menu E, Van Valckenborgh E, Vande Broek I, Van Riet I, Van Camp B, Vanderkerken K. Mechanisms involved in the differential bone marrow homing of CD45 subsets in 5T murine models of myeloma. Clin Exp Metastasis. 2002;19:583–591. doi: 10.1023/a:1020987830132. [DOI] [PubMed] [Google Scholar]
- Hargreaves PG, Wang F, Antcliff J, Murphy G, Lawry J, Russell RG, Croucher PI. Human myeloma cells shed the interleukin-6 receptor: inhibition by tissue inhibitor of metalloproteinase-3 and a hydroxamate-based metalloproteinase inhibitor. Br J Haematol. 1998;101:694–702. doi: 10.1046/j.1365-2141.1998.00754.x. [DOI] [PubMed] [Google Scholar]
- Thabard W, Barillé S, Collette M, Harousseau J, Rapp M, Bataille R, Amiot M. Myeloma cells release soluble interleukin-6Ralpha in relation to disease progression by two distinct mechanisms: alternative splicing and proteolytic cleavage. Clin Cancer Res. 1999;5:2693–2697. [PubMed] [Google Scholar]
- Mangi MH, Newland AC. Angiogenesis and angiogenic mediators in haematological malignancies. Br J Haematol. 2000;111:43–51. doi: 10.1046/j.1365-2141.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Sezer O, Niemoller K, Eucker J, Jakob C, Kaufmann O, Zavrski I, Dietel M, Possinger K. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol. 2002;79:574–577. doi: 10.1007/s002770000236. [DOI] [PubMed] [Google Scholar]
- Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- Naglich JG, Jure-Kunkel M, Gupta E, Fargnoli J, Henderson AJ, Lewin AC, Talbott R, Baxter A, Bird J, Savopoulos R, Wills R, Kramer RA, Trail PA. Inhibition of angiogenesis and metastasis in two murine models by the matrix metalloproteinase inhibitor, BMS-275291. Cancer Res. 2001;61:8480–8485. [PubMed] [Google Scholar]
- Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart J, Noël A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. EMBO J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R, Cher ML. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- Sato T, Foged NT, Delaisse JM. The migration of purified osteoclasts through collagen is inhibited by matrix metalloproteinase inhibitors. J Bone Miner Res. 1998;13:59–66. doi: 10.1359/jbmr.1998.13.1.59. [DOI] [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Beertsen W. Cysteine proteinases and matrix metalloproteinases play distinct roles in the subosteoclastic resorption zone. J Bone Miner Res. 1998;13:1420–1430. doi: 10.1359/jbmr.1998.13.9.1420. [DOI] [PubMed] [Google Scholar]
- Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL. Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem. 1997;272:22053–22058. doi: 10.1074/jbc.272.35.22053. [DOI] [PubMed] [Google Scholar]
- Croucher PI, De Raeve H, Perry MJ, Hijzen A, Shipman CM, Lippitt J, Green J, Van Marck E, Van Camp B, Vanderkerken K. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K, De Leenheer E, Shipman C, Asosingh K, Willems A, Van Camp B, Croucher P. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–289. [PubMed] [Google Scholar]
- Xu JL, Lai R, Kinoshita T, Nakashima N, Nagaska T. Proliferation, apoptosis, and intratumoral vascularity in multiple myeloma: correlation with the clinical stage and cytological grade. J Clin Pathol. 2002;55:530–534. doi: 10.1136/jcp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt M, Kopitz C, Pennington C, Watson KL, Krell HW, Bode W, Gansbacher B, Khokha R, Edwards DR, Krüger A. Increase in gelatinase-specificity of matrix metalloproteinase inhibitors correlates with antimetastatic efficacy in a T-cell lymphoma model. Cancer Res. 2002;62:5543–5550. [PubMed] [Google Scholar]