Abstract
The Lyme disease spirochete, Borrelia burgdorferi, is an extracellular microbe that causes persistent infection despite the development of strong immune responses against the bacterium. B. burgdorferi expresses several ligand-binding lipoproteins, including the decorin-binding proteins (Dbps) A and B, which may mediate attachment to decorin, a major component of the host extracellular matrix during murine infection. We show that B. burgdorferi was better protected in the joints and skin, two tissues with a higher decorin expression, than in the urinary bladder and heart, two tissues with a lower decorin expression, during chronic infection of wild-type mice. Targeted disruption of decorin alone completely abolished the protective niche in chronically infected decorin-deficient mice but did not affect the spirochete burden during early infection. The nature of protection appeared to be specific because the spirochetes with higher outer surface protein C expression were not protected while the protective niche seemed to favor the spirochetes with a higher dbpA expression during chronic infection. These data suggest that spirochetal DbpA may interact with host decorin during infection and such interactions could be a mechanism that B. burgdorferi uses to evade humoral immunity and establish chronic infection.
Borrelia burgdorferi, the Lyme disease spirochete, causes persistent mammalian infection despite the development of vigorous immune responses against the pathogen.1,2 This bacterium expresses lipoproteins to form an antigenic layer that protects itself from, and directly interacts with, the environment.3 Lipoproteins may stimulate innate responses via Toll-like receptors 1 and 2, enhancing adaptive immune responses to B. burgdorferi.4,5 Antibodies to many lipoproteins such as outer surface proteins (Osp) A, B, and C and decorin-binding protein (Dbp) A can effectively protect the mammalian host from an initial B. burgdorferi infection,6–9 but none of them are able to clear an established infection. Little is known about how this extracellular bacterium survives in the extremely hostile environment of chronic mammalian infection after specific immune responses have developed.
A broad array of spirochetal surface lipoproteins have been shown in vitro to have interactions with mammalian host antigens. These include some of OspE-related lipoproteins (Erps) and BBA68 that acquire complement inhibitor factor H and/or factor H-like protein 1,10–12 DbpA and DbpB,13–15 and the fibronectin-binding protein BBK32.16,17 Decorin and fibronectin are both major components of the host extracellular matrix (ECM).18 In addition, at least two integral outer membrane proteins P66 and Bgp (Borrelia glycosaminoglycan-binding protein) have been identified as spirochetal adhesins that mediate interactions of B. burgdorferi with the ECM and host cells.19–23 Spirochetes in tissue specimens taken from infected mice and patients with Lyme disease are often associated with collagenous connective tissues and vessel walls.24–26 These tissues are rich in ECM components. B. burgdorferi could reside in the ECM during a persistent infection. The interactions of the spirochetes with the ECM, mediated by Dbps, BBK32, and others, may therefore play critical roles in the persistence of B. burgdorferi in tissues.
It has been speculated for decades that microbial pathogens may acquire host antigens to avoid immune clearance. DbpA is the best-characterized ligand-binding lipoprotein of B. burgdorferi. A recent study shows that a decorin deficiency reduces the susceptibility of laboratory mice to Lyme arthritis.27 We used DbpA and decorin as a model system to explore the hypothesis that the interactions between a bacterial antigen and a host component may facilitate immune evasion.
Materials and Methods
Spirochete and Mouse Strains
B. burgdorferi B31 clone 5A1128 (a gift from Steven Norris, University of Texas, Houston, TX) was cultivated in Barbour-Stoenner-Kelly H complete medium at 33°C (Sigma Chemical Co., St. Louis, MO). BALB/c wild-type mice (Dcn+/+) and severe combined immunodeficient (SCID) mice on a BALB/c background were purchased from the Jackson Laboratories (Bar Harbor, ME). Decorin-deficient (Dcn−/−) mice on a BALB/c background were generated as previously described.27,29 All mice were 4 to 8 weeks old when they were infected.
Mouse Inoculation with Cultured and Host-Adapted Spirochetes
Mice were given one single intradermal injection of 105 cultured spirochetes or inoculated with host-adapted spirochetes through a tissue transplant. Mice that were infected with cultured B. burgdorferi were sacrificed 2 months after infection. Urinary bladders, hearts, joints, and dorsal skins (not from the inoculation site) were harvested and immediately frozen in liquid nitrogen. Frozen samples were stored at −80°C until DNA and RNA were isolated.
To prepare host-adapted spirochetes, donor mice were infected with cultured spirochetes for 3 months. Ear punches were taken and used to quantify spirochete burdens by quantitative polymerase chain reaction (qPCR) as described below. Donor ears were cut into small pieces. Each piece that that was estimated to contain ∼100 spirochetes was implanted into the dorsal skin of recipient mice as described previously.30 The donor and recipient mouse strains were identical. Recipient mice were sacrificed either 2 weeks or 2 months after infection. Urinary bladders, hearts, joints, and skins were collected and stored as described above.
Anti-Borrelia Serum Preparation and Passive Immunization
To prepare anti-Borrelia sera, BALB/c mice were infected with cultured B31 5A11 spirochetes as described above. Blood was drawn between 2 and 4 months after infection and sera were isolated, pooled, and stored at −20°C. Ten SCID mice were infected with cultured spirochetes for 2 months as described above and each subcutaneously received six doses of 100-μl anti-Borrelia sera at intervals of 2 days. All animals were sacrificed 3 days after the last passive immunization. Hearts, joints, and skins were collected and stored as described above.
RNA and DNA Preparation
Frozen bladder, heart, joint, and skin samples were transferred in liquid nitrogen and ground thoroughly with a mortar and pestle. An appropriate amount of ground tissue was transferred in a 500-μl polypropylene PCR tube for DNA preparation using the DNeasy mini kit (Qiagen Inc., Valencia, CA). RNA was isolated from the remaining tissue using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA). To ensure that there was no DNA contamination, RNA preparations were first digested in solution with RNase-free DNase I (Life Technologies, Inc., Gaithersburg, MD) at 37°C for 2 hours; and then loaded to the RNeasy mini columns and further treated with RNase-free DNase I (Qiagen) for an additional 20 minutes at room temperature. Doubly digested samples were repurified and analyzed for potential DNA contamination by PCR amplification of the flaB gene.
cDNA Preparation
The DNA-free RNA preparation was first annealed with the reverse oligonucleotide primers of flaB, dbpA, ospC, decorin, and actin genes (Table 1) at 65°C, 60°C, 55°C, 50°C, and 45°C each for 1 minute, in the presence of reverse transcription buffer (Invitrogen). dNTPs and SuperScript II RNase H− reverse transcriptase (Invitrogen) were added and reverse transcription was conducted at 42°C for 1 hour and then inactivated at 95°C for 5 minutes following the manufacturer’s instructions.
Table 1.
qPCR Primers and Probes
| Gene | Primer | Probe | |
|---|---|---|---|
| flaB | Forward | 5′-GAGTTTCTGGTAAGATTAATGCTC-3′ | 5′-AGAGGTTTGTCACAAGCTTCTAGAAATACTTCAAAGGC-3′ |
| Reverse | 5′-CATTTAAATTCCCTTCTGTTGTCTGA-3′ | ||
| dbpA | Forward | 5′-CTTAAACTAACTATACTTGTTAAC-3′ | 5′-TTATATCATGTGGACTAACAGGAGCAAC-3′ |
| Reverse | 5′-AATGTCTTTAGCGCTTCGTTC-3′ | ||
| ospC | Forward | 5′-TACGGATTCTAATGCGGTTTTAC-3′ | 5′-TGAAGCGTTGCTGTCATCTATAGATGAAATTGCTGCT-3′ |
| Reverse | 5′-GTGATTATTTTCGGTATCCAAACCA-3′ | ||
| actin* | Forward | 5′-CCATGTACCCAGGCATTGC-3′ | 5′-TGCAGAAGGAGATCACAGCCCTAGCACC-3′ |
| Reverse | 5′-CCAGACTGAGTACTTGCGTTC-3′ | ||
| actin† | Forward | 5′-CATCATGAAGTGTGACGTTGAC-3′ | 5′-GTATGCCAATACAGTGCTGTCTGGTGGTACCAC-3′ |
| Reverse | 5′-GCATCCTGTCAGCAATGCC-3′ | ||
| decorin | Forward | 5′-TGTCATCTTCGAGTGGTGCA-3′ | 5′-CACCCGACACAACCTTGCTAGACCTGC-3′ |
| Reverse | 5′-GAGGTTTGAATGCCTCTGGA-3′ |
Used for actin cDNA quantification.
Used for actin genomic DNA quantification.
qPCR DNA Concentration Standards
flab, dbpA, and ospC DNA concentration standards were prepared from cultured B. burgdorferi. B31 5A11 spirochetes were grown to stationary phase in BSK-H complete medium, counted in a Petroff-Hausser Counter (Hausser Scientific Partnership, Horsham, PA) and harvested by centrifugation at 13,000 rpm for 10 minutes. Pellets were digested with proteinase K (Qiagen) at 55°C for 2 hours, inactivated at 95°C for 10 minutes, and diluted to 100 to 105 spirochete DNA copies/μl.
To generate an actin DNA concentration standard for cDNA and DNA quantification, primers (forward, 5′-TGAGCGGTTCCGGTGTCC-3′; reverse, 5′-CAGTGAGGCCAGAATGGA-3′) were designed to amplify a 292-bp internal fragment of the actin gene by PCR using murine DNA as a template. To prepare a decorin DNA concentration standard for cDNA quantification, mouse cDNA was prepared as described above and PCR amplified to generate a 205-bp internal fragment of decorin cDNA using the primers (forward, 5′-GGACAAAGTGCCCTGGGA-3′; reverse, 5′-GTCCTTCAGGTTCTTGAAGGC-3′). Taq polymerase was purchased from Roche Diagnostics Co. (Indianapolis, IN). The PCR conditions used were: 95°C for 5 minutes; 95°C for 40 seconds, 50°C for 1 minute, 72°C for 40 seconds, 50 cycles; 72°C for 10 minutes. PCR products were purified using the Quick PCR Product Purification kit (Qiagen). Purity was examined by agarose gel electrophoresis. DNA concentrations were determined by measuring the optical density at 260-nm wavelength, converted to copy number, and diluted at 102 to 107 DNA copies/μl.
qPCR
qPCR analyses were performed using the iCycler (Bio-Rad Laboratories, Hercules, CA). The Platinum TaqDNA Polymerase High Fidelity kit was purchased from Invitrogen. The sequences of primers and internal probes of flaB, dbpA, ospC, decorin, and actin genes were listed in Table 1. TaqMan TAMRA probes were ordered from Applied Biosystems (Foster City, CA). Amplification was performed in a 50-μl final volume reaction mix in individual wells of a 96-well iCycler iQ PCR plate (Bio-Rad). Twelve wells of each plate were assigned for DNA standards at six different concentrations in duplicate. Each cDNA or DNA sample was amplified in duplicate for flaB, dbpA, ospC, decorin, or actin genes. A PCR program with the following parameters was used: 95°C for 5 minutes; 95°C for 30 seconds, 60°C for 1 minute, 50 cycles. The mean cDNA copy numbers for flaB, dbpA, decorin, and actin transcripts of each cDNA pool and mean DNA copy numbers for flaB and actin genes of each DNA sample were automatically calculated from duplicate wells using the iCycler software. Tissue spirochete burdens were calculated as flaB DNA copy numbers per 106 actin DNA copies. Mouse decorin expression levels were presented as decorin mRNA copy numbers per 103 actin mRNA transcripts. Spirochetal dbpA expression levels were expressed as dbpA mRNA copy numbers per 103 flaB mRNA transcripts.
Statistical Analysis
Data were analyzed using Microsoft Excel (Redmond, WA) software. A two-tailed Student’s t-test was used to analyze qPCR and quantitative reverse transcriptase-PCR data. A P value <0.05 was considered to be a significant difference.
Results
B. burgdorferi Is Better Protected Against Immune Clearance in the Joints and Skin than the Bladder and Heart
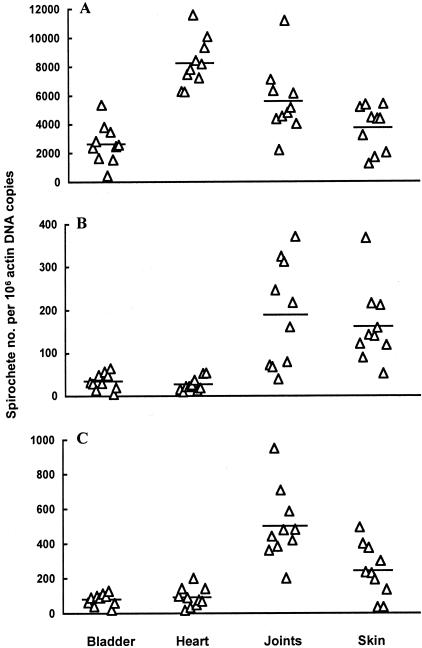
To define a protective niche for B. burgdorferi to resist immune clearance, the tissue spirochete burden was first investigated in infected SCID mice. Ten SCID mice were infected with cultured B. burgdorferi for 2 months and sacrificed. DNA samples were prepared from the urinary bladder, heart, joints, and skin and quantified for the tissue spirochete burden. These tissues were investigated for different reasons: the bladder has been shown to be a consistent source of B. burgdorferi and the skin is the most commonly examined biopsy site because it represents the tissue of initial entry. Finally, both the heart and joints are major sites of inflammation in the murine model of Lyme disease. The highest spirochete burden was detected in the heart, which was 48%, 123%, and 210% higher than the joints (P = 0.01), skin (P = 6.9 × 10−6), and bladder (P = 1.7 × 10−7), respectively (Figure 1A). The second highest spirochete burden was found in the joints, which was 34% and 52% higher than in the skin (P = 0.05) and bladder (P = 0.004), respectively. There was no significant difference in the spirochetal burden between the skin and bladder tissues (P = 0.12).
Figure 1.
B. burgdorferi is better protected against immune clearance in the joints and skin than the bladder and heart. Twenty SCID and 10 wild-type mice were infected with cultured B. burgdorferi. Ten of the SCID mice (A) and all of the wild-type mice (B) were sacrificed 2 months later. The rest of the SCID mice (C) were passively immunized with anti-Borrelia sera. DNA samples were prepared from bladder, heart, joint, and skin tissues and quantified for flaB and actin DNA copy numbers by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
We next examined the spirochete burden in wild-type mice infected in a similar manner. The tissue spirochete burden analysis revealed that the joint and skin tissues provided the better protection for B. burgdorferi to resist immune clearance (Figure 1B). The spirochete burden in the joint tissue was 5.4 and 7.0 times higher than those of heart (P = 5.9 × 10−4) and bladder tissues (P = 9.5 × 10−4), respectively; and the skin bacterial burden was 6.0 and 4.6 times higher than those of the heart (P = 1.6 × 10−4) and bladder tissues (P = 3.1 × 10−4). There were no significant differences in the tissue spirochete burden between the heart and bladder tissues (P = 0.34). The better protection in the joint and skin tissues was further demonstrated by a passive immunization study. Transferred antibodies more effectively reduced the tissue spirochete burden in the bladder and heart than the joints and skin (Figure 1C). The spirochete burden in the joint was 5.4 and 6.3 times higher than those of the heart (P = 1.1 × 10−5) and bladder tissues (P = 5.4 × 10−6), respectively. The bacterial burden in the skin tissue was 2.6 and 3.0 times higher than those of the heart (P = 8.7 × 10−3) and bladder tissues (P = 3.9 × 10−3). There was no significant difference in the tissue spirochetal burden between the heart and bladder tissues (P = 0.57).
Better Protection Correlates with Higher Tissue Decorin Expression
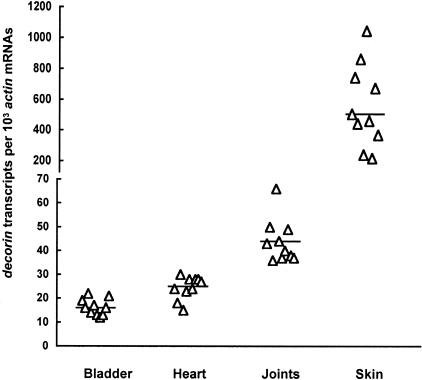
It is well documented that some of the tissues with the highest decorin expression are the skin, cartilage, and tendon.31,32 To establish a correlation between decorin levels and Borrelia persistence, we analyzed relative decorin expression in the bladder, heart, joint, and skin tissues. Total RNA was isolated and converted to cDNA and quantified for actin and decorin mRNA copy numbers. Decorin transcription in the joint tissue was 163% and 68% higher than bladder (P = 5.2 × 10−6) and heart tissues (P = 5.7 × 10−4), respectively (Figure 2). The expression levels in the skin were higher than those of all of the three tissues. These data are fully consistent with a large body of published evidence indicating high levels of decorin expression in the skin.29 B. burgdorferi was more sensitive to immune clearance in both bladder and heart tissues than the joint and skin (Figure 1, B and C), and the decorin expression levels in these two former tissues were significantly lower, suggesting that better protection of B. burgdorferi against immune clearance may require a threshold level of decorin expression.
Figure 2.
Tissue differential decorin expression. RNA samples were prepared from the bladder, heart, joints, and skin of the wild-type mice that had been used to generate data for Figure 1B, converted to cDNA, and analyzed by qPCR for actin and decorin mRNA copy numbers. The data are expressed as decorin mRNA copy numbers per 103 actin mRNA copies.
Decorin Is the Key Protective Niche Component
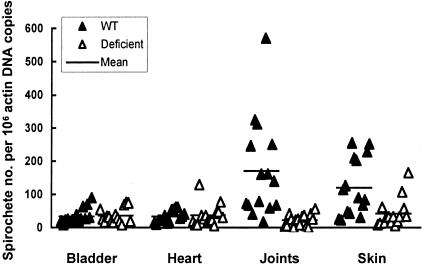
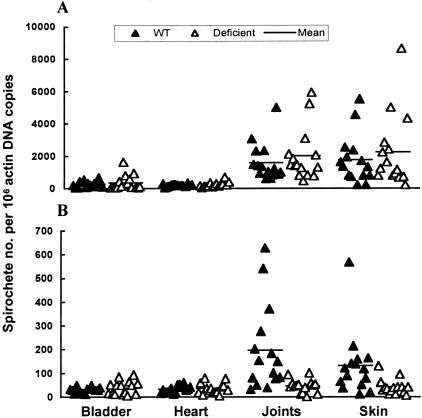
To demonstrate that decorin is the key component in establishing a protective niche, Dcn−/− mice were used to confirm this hypothesis. Dcn+/+ and Dcn−/− mice were infected with cultured B. burgdorferi for 2 months and sacrificed after the anti-Borrelia immune response was fully developed. The bacterial burdens in the bladder, heart, joints, and skin were analyzed by qPCR. As shown in Figure 3, significant differences in the spirochete burdens between Dcn+/+ and Dcn−/− mice were noted only in the joints and skin (P = 0.0006 and 0.004, respectively), the two tissues with the highest decorin expression but not in the bladder or heart tissues (P = 0.78 and 0.80). The average spirochete burdens in the joints and skin of Dcn+/+ mice were 7.8 and 2.9 times higher than those in the same tissues in Dcn−/− mice. In contrast, the spirochete burdens in all of the four tissues of Dcn−/− mice and in the bladder and heart tissues of Dcn+/+ mice were similar (P = 0.10 to 0.64). These data indicate that a decorin deficiency completely diminished the protective niche in the joint and skin tissues of Dcn−/− mice, further defining a key role for decorin in the formation of a protective niche against immune clearance of B. burgdorferi during chronic infection.
Figure 3.
Targeted disruption of decorin abolishes the protective niche for B. burgdorferi. Fifteen Dcn+/+ (WT) and 15 Dcn−/− mice (deficient) were infected with cultured B. burgdorferi and sacrificed 2 months later. DNA samples were prepared from bladder, heart, joint, and skin tissues and quantified for flaB and actin DNA copy numbers by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
The Protective Niche Appears to Favor Spirochetes with Higher dbpA Expression
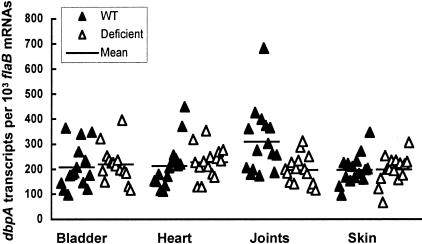
We next investigated whether decorin deficiency affected the gene expression of B. burgdorferi. dbpA mRNA transcripts were examined in the bladder, heart, joint, and skin tissues from both Dcn+/+ and Dcn−/− mice at 2 months after infection, a time point when the immune response against the spirochete had fully developed. No significant differences in dbpA expression were found in the bladder (P = 0.69), heart (P = 0.61), or skin (P = 0.69) between Dcn+/+ and Dcn−/− mice (Figure 4). However, higher levels of dbpA expression were observed in the joints of Dcn+/+ mice compared to Dcn−/− mice (P = 0.005). The dbpA expression level in the joint tissue of Dcn+/+ mice was 68% higher than the same tissue in Dcn−/− mice (Figure 4). This correlation between elevated dbpA expression in tissues containing elevated decorin expression eg, joints, indicated that a threshold level of decorin might be required to protect the spirochetes in this tissue, presumably against anti-Borrelia antibodies. Alternatively, the reduced dbpA expression observed in the joints of Dcn−/− mice could be a consequence related to the lack of interaction between DbpA and decorin because this interaction alone might influence dbpA expression. To rule out this possibility, dbpA expression was also examined in the joints of mice that had been infected just for 2 weeks. No significant differences in dbpA expression were noted between Dcn+/+ and Dcn−/− mice (data not shown), indicating that the presence of decorin did not affect dbpA expression before the maturation of immune responses. Taken together, these data suggest that the interactions between the DbpAs and decorin may give an advantage to DbpA-expressing spirochetes by preventing immune-mediated clearance of B. burgdorferi from high decorin-expressing tissues during chronic infection.
Figure 4.
Spirochetes with higher dbpA expression are better protected in the joints of wild-type mice. RNA samples were prepared from the bladder, heart, joints, and skin of the mice that had been used to generate data for Figure 3, converted to cDNA, and analyzed by qPCR for flaB and dbpA mRNA copy numbers. The data are expressed as dbpA mRNA copy numbers per 103 flaB mRNA copies.
The Protective Niche Is Not Apparent without a Matured Immune Response
After mammalian host infection, B. burgdorferi first replicates at the inoculation site and then rapidly disseminates and colonizes remote tissues. Infection also induces specific immune responses that effectively diminish tissue spirochete burdens. Our data demonstrated that deletion of decorin alone significantly reduced the spirochete burdens in chronically infected mice (Figure 3). This could be a function of impaired colonization by B. burgdorferi relating to the lack of host decorin and independent of immune pressure or the presence of decorin provided the bacteria with a protective niche against immune clearance, either by preventing Borrelia-specific antibodies from recognizing their targets or by making the bacteria unrecognizable to innate immune mechanisms. To distinguish between these two mechanisms of action, we examined tissue spirochete burdens before the anti-B. burgdorferi response was fully developed.
Thirty Dcn−/− and 30 Dcn+/+ mice were infected with host-adapted spirochetes from Borrelia-infected donor mice. Half of the mice were sacrificed at 2 weeks after inoculation. The spirochete burdens in the bladder, heart, joints, and skin were quantified by qPCR and are presented in Figure 5A. No significant differences in the spirochete burdens between decorin-deficient and control mice were detected in the bladder (P = 0.52), heart (P = 0.45), joints (P = 0.44), or skin (P = 0.50). These data indicate that the influence of decorin deficiency on the spirochete burden is insignificant before the maturation of the anti-B. burgdorferi antibody response.
Figure 5.
Decorin deficiency does not affect early infection. Thirty Dcn+/+ (WT) and 30 Dcn−/− mice (deficient) were infected with host-adapted B. burgdorferi. Half of them were sacrificed 2 weeks later (A) and the rest were euthanized 2 months after infection (B). DNA samples were prepared from the bladder, heart, joint, and skin tissues and examined for the tissue spirochetal burden by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
The remaining mice were allowed to fully develop an immune response against the spirochetes and sacrificed 2 months after infection. Quantification of spirochetes in various tissues confirmed the role of decorin in the protection of B. burgdorferi against immune clearance. The differences in the tissue spirochete burdens in the joints (P = 0.003) and skin (P = 0.02) between Dcn−/− and Dcn+/+ mice were 4.3 and 3.1 times, respectively. In contrast, bladder (P = 0.48) and heart tissues (P = 0.73) had similar spirochete numbers regardless of decorin genotype (Figure 5B), a result consistent with the data obtained from mice infected with cultured spirochetes (Figure 3).
The Protective Niche Appears to be Specific
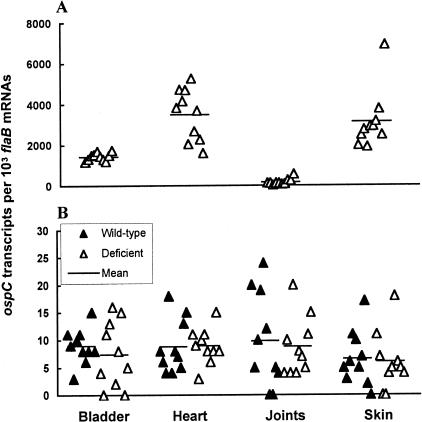
The crucial role for decorin in the establishment of the protective niche could result from two mechanisms. First, decorin, which has been shown to play a role in collagen fibril formation and ECM integrity, could nonspecifically affect the transportation of immunoglobulin molecules into the tissues. Second, decorin may bind to DbpA in such a manner as to specifically interfere with the interactions of anti-Borrelia antibody. To test the hypothesis, we examined whether the protective niche could protect ospC-expressing spirochetes from clearance. SCID, Dcn−/−, and Dcn+/+ mice were infected intradermally and sacrificed 2 months after infection. RNA samples were prepared from the bladder, heart, joint, and skin specimens, converted to cDNA and quantified for ospC and flaB mRNA copy numbers. In the absence of immune selection pressure, B. burgdorferi exhibited differential ospC expression in the four tissues (Figure 6A). B. burgdorferi more actively transcribed ospC in the heart and skin than the bladder and joints. The immune response elicited during the infection in both Dcn−/− and Dcn+/+ mice effectively reduced ospC expression to similar levels (P = 0.5 to 0.96) in all of the four tissues regardless of the fact that the joint and skin tissues of Dcn+/+ mice showed higher levels of decorin expression (Figure 6B). Thus, the presence of decorin had no impact on survival of ospC-expressing spirochetes, suggesting that the protective niche may be specific for dbpA-expressing spirochetes.
Figure 6.
The protective niche does not protect ospC-expressing spirochetes. RNA samples were prepared from the bladder, heart, joints, and skin of the SCID mice (A) that had been used to generate data for Figure 1A, and the wild-type and Dcn−/− mice (B) that had been used to generate data for Figure 5, converted to cDNA, and analyzed by qPCR for flaB and ospC mRNA copy numbers. The data are expressed as dbpA mRNA copy numbers per 103 flaB mRNA copies.
Discussion
This study has clearly shown the role of decorin in the protection of B. burgdorferi against immune clearance. In infected SCID mice, the heart tissue showed the second lowest decorin expression but harbored the highest spirochete number of all four tissues, whereas the skin tissue most abundantly expressed decorin but had similar spirochete burdens as the bladder tissue that had the lowest decorin expression, indicating that decorin has no role in spirochete colonization in the tissue when the immune response is absent. The immune response generated in wild-type mice more effectively reduced the spirochete burdens in both the bladder and heart, the two tissues with the lowest decorin expression, compared to the joints and skin, the two tissues with the highest decorin expression. The importance of decorin in the establishment of the protective niche is confirmed by the data obtained from Dcn−/− mice. Targeted disruption of decorin completely abolished the protective niche of the joints and skin, resulting in similar spirochete burdens in all of the four tissues. In contrast, a decorin deficiency did not affect the tissue spirochete burdens before the immune response had been fully developed. Our study has further shown that the protection may be specific for dbpA-expressing spirochetes because immune pressure significantly reduced dbpA expression in the joints of infected Dcn−/− mice compared to wild-type mice but effectively eliminated ospC-expressing spirochetes in all of the tissues of both Dcn−/− and Dcn+/+ mice. Thus, the protective capacity conferred by decorin is most likely because of specific interactions between host decorin and spirochetal DbpA.
B. burgdorferi infection of SCID mice results in constantly higher tissue spirochete burdens. There have been no reports indicating that innate immune components such as macrophages or neutrophils can directly kill spirochetes in vivo, suggesting that the high tissue spirochete burdens found in infected SCID mice may be a function of the spirochete but not the host immune system. Although nothing is known about how the tissue spirochete burdens are controlled in the absence of adaptive immune pressure, our study suggests that the decorin expression levels are not a contributing factor influencing the tissue spirochete burdens in chronically infected SCID mice as well as in acutely infected immunocompetent mice. The specific immune response showed the influence of the decorin expression level on the tissue spirochete burden. During chronic infection, the tissue spirochete burdens of Dcn+/+ mice were differentially affected depending on the tissue decorin expression level, while all of the tissues had similar spirochete burdens in Dcn−/− mice. These data support the crucial role of decorin in the formation of the protective niche for B. burgdorferi to resist immune clearance.
Decorin, a pleotropic molecule that is involved in maintaining the structural integrity of the ECM may also affect the efficacy of antibodies, in this case to Borrelia, by affecting the ECM’s integrity as a function of its expression levels in various tissues.29 To rule out this possibility, we examined whether the protection conferred by decorin is specific for dbpA-expressing spirochetes. We measured the ospC expression levels as an indicator of the number of ospC-expressing spirochetes in tissues. The immune response effectively eliminated ospC-expressing spirochetes in all tissues of both in Dcn+/+ and Dcn−/− mice. In contrast, the presence of decorin more effectively protected spirochetes with higher dbpA expression in the joint tissue of Dcn+/+ mice compared to Dcn−/− mice. Thus, this selective protection is most likely because of the existence of interactions of DbpA with decorin and such interactions probably selectively reduce the efficacy of specific DbpA antibody. Unlike OspC, whose expression may not be required for in vivo spirochetal survival, DbpA may be a crucial antigen facilitating persistent B. burgdorferi infection. This may be the reason why dbpA expression levels were similar in the joints of Dcn−/− mice and the bladder, heart, and skin of both Dcn+/+ and Dcn−/− mice. The DbpA levels found in these tissues may represent the minimum expression threshold required for spirochetal survival during chronic infection.
The repertoire of immune response to spirochetal antigens increases during B. burgdorferi infection and a significant bactericidal response can be detected as early as a week after infection.33 OspC is one of the spirochetal antigens that can induce an early strong humoral response.34,35 Although OspC antibodies select against OspC-producing spirochetes, these antibodies only effectively reduce the spirochete burdens in the bladder and heart (Liang and Fikrig, unpublished data). This is consistent with our observation that the spirochete burdens were lower in the bladder and heart than the joints and skin of both Dcn+/+ and Dcn−/− mice during early infection of immunocompetent mice although the heart tissue contained the highest spirochete burden in infected SCID mice. The lower spirochete burden in the bladder and heart tissues most likely resulted from the early strong anti-OspC immune response. In contrast, the anti-DbpA humoral response gradually develops within the first 2 months of infection.36,37 This may be a reason why significant differences in the tissue spirochete burdens of decorin-rich tissues could not be noted between Dcn+/+ and Dcn−/− mice during early infection.
Previous data suggest that humoral and cellular immune responses are indistinguishable between Dcn+/+ and Dcn−/− mice.27 The lower spirochete burden in the joints and skin of Dcn−/− mice compared to these of Dcn+/+ mice appeared not to result from a stronger immune response developing in the decorin-deficient mice. To determine whether Dcn−/− mice generated a stronger immune response against B. burgdorferi, antisera prepared from Dcn+/+ and Dcn−/− mice that had been infected with B. burgdorferi for 2 months were passively transferred to infected SCID mice. Tissue spirochete quantification showed that the antisera from Dcn+/+ mice reduced the tissue spirochete burden of SCID mice as effectively as that of Dcn−/− mice (Liang and Fikrig, unpublished data).
Although the membranous architecture of B. burgdorferi is not clear, lipoproteins are believed to shield the outer membrane of the bacterium.3 When major lipoproteins such as OspA, OspB, and OspC are expressed on cultured spirochetes, integral outer membrane proteins such as P66 and Bgp, and even other surface lipoproteins such as DbpA/B may be less accessible to their ligands.38 The cultivation of B. burgdorferi in dialysis membrane chambers implanted into rat peritoneal cavities alters spirochetal protein expression profiles, including the down-regulation of OspA.39 These changes indeed result in an enhanced binding in vitro to glycosaminoglycan and host cells.40 B. burgdorferi undergoes a series of modifications, the majority of which occur on the bacterial surface, during its transmission between the tick vector and the mammalian host. For instance, ospA expression is down-regulated by 105 times after B. burgdorferi migrates from the tick vector to the murine host (Liang and Fikrig, unpublished data). Infection triggers a strong humoral immune response in particular to lipoprotein antigens. Immune pressure may select against spirochetes that express specific lipoproteins.35,41 A typical example of a 400-fold decrease in ospC expression levels is associated with the development of immune responses generated against this antigen during infection. The elimination of these lipoproteins from the spirochete surface would allow adhesion molecules more accessible to their ligands. The enhanced interactions between the adhesins and their ligands would give a survival advantage to these spirochetes during chronic infection.
DbpA is one of several known ligand-binding lipoproteins of B. burgdorferi. The deletion of its ligand decorin alone resulted in 7.8- and 2.9-fold reductions in the spirochetal burdens in the joint and skin tissues of chronically infected Dcn−/− mice compared to Dcn+/+ mice. In contrast, decorin deficiency did not affect early infection. B. burgdorferi persistently expresses numbers of lipoproteins during chronic murine infection after immune responses against the pathogen have fully been developed.41 Most of them have unknown functions. Some of these lipoproteins may have ligand-binding properties and thus participate in immune evasion. In addition to lipoproteins, integral outer membrane proteins of B. burgdorferi may also be involved in the process. For instance, integral outer membrane protein P66 has been shown to recognize host β3-chain integrins in vitro.20,21 Although P66 is shielded by lipoproteins such as OspA and OspC on cultured spirochetes, it may become accessible to its ligand in a chronically infected host after some of the lipoproteins such as OspA and OspC are down-regulated as a function of either immune selection pressure or other factors.35,41–43 Therefore, binding to host components and coating itself with host antigens could be an extremely powerful immune evasion mechanism resulting in persistent Lyme borreliosis.
Acknowledgments
We thank Steven Norris (University of Texas, Houston, TX) for providing clonal isolate B31 5A11 and Debbie Beck for assistance with the murine studies.
Footnotes
Address reprint requests to Erol Fikrig, Section of Rheumatology, Department of Internal Medicine, Yale University School of Medicine, S525A, 300 Cedar St., New Haven, CT 06520-8031. E-mail: erol.fikrig@yale.edu.
Supported in part by the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR49405; National Institute of Allergy and Infectious Disease grants AI55959, AI32947, and AI49200) and the Centers for Disease Control (grant CCU618387).
E.F. is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
References
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- Seiler KP, Weis JJ. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- Haake DA. Spirochaetal lipoproteins and pathogenesis. Microbiology. 2000;146:1491–1504. doi: 10.1099/00221287-146-7-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Gilmore RD, Jr, Kappel KJ, Dolan MC, Burkot TR, Johnson BJ. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Hodzic E, Stevenson B, Barthold SW. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJ, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70:491–497. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, Brade V, Zipfel PF, Wallich R. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. 2004;279:2421–2429. doi: 10.1074/jbc.M308343200. [DOI] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Brown EL, Guo BP, O’Neal P, Hook M. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem. 1999;274:26272–26278. doi: 10.1074/jbc.274.37.26272. [DOI] [PubMed] [Google Scholar]
- Pikas DS, Brown EL, Gurusiddappa S, Lee LY, Xu Y, Hook M. Decorin-binding sites in the adhesin DbpA from Borrelia burgdorferi: a synthetic peptide approach. J Biol Chem. 2003;278:30920–30926. doi: 10.1074/jbc.M303979200. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, III, Flavell RA. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- Defoe G, Coburn J. Delineation of Borrelia burgdorferi p66 sequences required for integrin alpha(IIb)beta(3) recognition. Infect Immun. 2001;69:3455–3459. doi: 10.1128/IAI.69.5.3455-3459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen N, Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- Parveen N, Robbins D, Leong JM. Strain variation in glycosaminoglycan recognition influences cell-type-specific binding by Lyme disease spirochetes. Infect Immun. 1999;67:1743–1749. doi: 10.1128/iai.67.4.1743-1749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- Nocton JJ, Steere AC. Lyme disease. Adv Intern Med. 1995;40:69–117. [PubMed] [Google Scholar]
- Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook M. Resistance to Lyme disease in decorin-deficient mice. J Clin Invest. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Fikrig E, Bockenstedt LK, Persing DH. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HU, Johnson TL, Pal S, Tang LH, Rosenberg L, Neame PJ. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-Sepharose chromatography. J Biol Chem. 1989;264:2876–2884. [PubMed] [Google Scholar]
- Vogel KG, Evanko SP. Proteoglycans of fetal bovine tendon. J Biol Chem. 1987;262:13607–13613. [PubMed] [Google Scholar]
- Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003;71:4608–4613. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung BP, McHugh GL, Leong JM, Steere AC. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med. 2002;195:415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Yang X, Wikel SK, Schoeler GB, Caimano MJ, Radolf JD, Norgard MV. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun. 2000;68:4759–4764. doi: 10.1128/iai.68.8.4759-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen N, Caimano M, Radolf JD, Leong JM. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol Microbiol. 2003;47:1433–1444. doi: 10.1046/j.1365-2958.2003.03388.x. [DOI] [PubMed] [Google Scholar]
- Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]