Abstract
Ultraviolet (UV) irradiation from the sun reduces production of type I procollagen (COLI), the major structural protein in human skin. This reduction is a key feature of the pathophysiology of premature skin aging (photoaging). Photoaging is the most common form of skin damage and is associated with skin carcinoma. TGF-β/Smad pathway is the major regulator of type I procollagen synthesis in human skin. We have previously reported that UV irradiation impairs transforming growth factor-β (TGF-β)/Smad signaling in mink lung epithelial cells. We have investigated the mechanism of UV irradiation impairment of the TGF-β/Smad pathway and the impact of this impairment on type I procollagen production in human skin fibroblasts, the major collagen-producing cells in skin. We report here that UV irradiation impairs TGF-β/Smad pathway in human skin by down-regulation of TGF-β type II receptor (TβRII). This loss of TβRII occurs within 8 hours after UV irradiation and precedes down-regulation of type I procollagen expression in human skin in vivo. In human skin fibroblasts, UV-induced TβRII down-regulation is mediated by transcriptional repression and results in 90% reduction of specific, cell-surface binding of TGF-β. This loss of TβRII prevents downstream activation of Smad2/3 by TGF-β, thereby reducing expression of type I procollagen. Preventing loss of TβRII by overexpression protects against UV inhibition of type I procollagen gene expression in human skin fibroblasts. UV-induced down-regulation of TβRII, with attendant reduction of type I procollagen production, is a critical molecular mechanism in the pathophysiology of photoaging.
Ultraviolet (UV) irradiation from the sun is harmful to human skin. UV-induced skin damage includes sunburn, immunosuppression, cancer, and premature skin aging (photoaging).1,2 Thinning of the ozone layer, which allows greater amounts of UV irradiation to strike the surface of the earth, has fueled increased awareness of the potential health risks of solar UV irradiation. Growing awareness of the deleterious consequences of solar UV irradiation has been heightened by recognition of increased incidence of skin cancer, the most common form of human cancer.3 Although skin cancer is relatively common, its prevalence falls far short of that of photoaging, the most common form of skin damage caused by sun exposure. Photodamage is a complex condition that alters many aspects of skin, including epithelium, pigment, vasculature, and connective tissue.2 Damage to skin connective tissues is responsible for the characteristic aged appearance of photodamaged skin.4–6 The most abundant structural protein in skin connective tissue is type I collagen, which is responsible for conferring strength and resiliency. Type I collagen is synthesized primarily by fibroblasts residing within skin connective tissue (dermis). Type I collagen is synthesized as a soluble precursor, type I procollagen, which is secreted from fibroblasts and proteolytically processed to form insoluble collagen fibers.7 Fibroblasts organize and maintain these fibers in aligned three-dimensional arrays. Collagen in skin has a long half-life, estimated to be greater than 1 year.8 Disorganization, fragmentation, and dispersion of collagen bundles are prominent features of photodamaged human skin.5,9 Loss of the structural integrity of the collagenous extracellular matrix is believed to be primarily responsible for the wrinkled appearance of photodamaged skin.10
We have reported that UV irradiation disrupts the skin collagen matrix by two interdependent pathways: stimulating collagen degradation and inhibiting procollagen production.9,11 Molecular mechanisms that result in collagen degradation in photoaged skin are relatively well understood, namely UV irradiation activates MAP kinase signaling, which in turn induces transcription factor AP-1, which stimulates expression of matrix metalloproteinases (MMP), collagenase-1 (MMP-1), stromelysin-1 (MMP-3), and gelatinase B (MMP-9). These MMPs act together to breakdown interstitial collagen. However, the molecular mechanisms responsible for UV reduction of procollagen synthesis in photoaged skin remain primarily unknown. The objective of the studies reported here was to shed light on these mechanisms.
Transforming growth factor-β (TGF-β) is a ubiquitous, multifunctional cytokine that plays an important role in regulating procollagen synthesis.12–15 TGF-β initiates its cellular actions by binding to specific cell-surface receptor complexes typically composed of TGF-β type I (TβRI) and TGF-β type II (TβRII) receptors. Binding of TGF-β to TβRII activates the intrinsic serine/threonine kinase activity of TβRI, which phosphorylates transcription factors Smad2 and Smad3. Phosphorylated Smad2 and Smad3 combine with Smad4, and translocate into the nucleus, where they function to regulate transcription of specific genes that possess TGF-β response elements in their promoters.15 Type I collagen is one of many TGF-β-regulated genes. A wealth of evidence indicates that TGF-β plays a central role in controlling production of extracellular matrix proteins, and is critical for connective tissue regeneration during wound healing.16,17 Interference with TGF-β, Smad3, or Smad4 expression in skin fibroblasts, results in substantial reduction of type I procollagen gene expression, suggesting autocrine production of TGF-β is primarily responsible for type I procollagen synthesis.18–22 Additionally, overexpression of TGF-β1 in transgenic mice results in accumulation of type I collagen in skin connective tissue and other organs.23 Taken together, these data indicate that TGF-β is a critical regulator of type I procollagen synthesis.
UV irradiation is known to modulate a variety of cell-surface growth factor and cytokine receptors, signal transduction cascades, and transcription factors.24 We have previously reported that UV irradiation impairs TGF-β/Smad pathway in a well-characterized model cell system (mink lung epithelial cells) used to study TGF-β signaling mechanisms.25 This observation prompted us to investigate the potential connector between UV inhibition of TGFβ/Smad signaling and UV reduction of type I procollagen production in adult human skin fibroblasts. Our data demonstrate that UV irradiation impairs TGF-β/Smad pathway through transcriptional down-regulation of TβRII, and that this impairment is primarily responsible for reduced procollagen synthesis in human skin fibroblasts.
Materials and Methods
Procurement of Human Tissue Samples and UV Irradiation
Punch biopsies were obtained from healthy adult human volunteers as previously described.5,24 Sun-protected buttock skin of human volunteers was irradiated with 2 minimum erythema dose solar-simulated UV (SPEC 450 W xenon arc solar simulator). Minimal erythema dose for each patient was determined 24 hours after UV irradiation. UV-irradiated and nonirradiated skin samples were obtained from each patient by punch biopsy, as described previously.26 All procedures involving human subjects were approved by the University of Michigan Institutional Review Board and all patients provided written informed consent.
Cell Culture and UV Irradiation
Human skin dermal fibroblasts were cultured from keratome biopsies of healthy adult human skin as described previously.26 Cells used for study were between passages three and six. For UV irradiation, subconfluent cells were washed once with phosphate-buffered saline (PBS) and irradiated with UV (30 mJ/cm2) using an Ultralite Panelite (Davvlin, Bryan, OH) lamp containing six FS24T12 UVB-HO bulbs. A Kodacel filter was used to eliminate wavelengths below 290 nm (UVC). This UV source emits primarily UVB (70%, 290 to 320 nm) with some UVA2 (30%, 320 to 340 nm). The irradiation intensity was monitored with an IL400A phototherapy radiometer and a SED240/UVB/W photodetector (International Light, Newbury, MA). Cell viability before harvesting cells for measurements was determined by inspection trypsin blue exclusion. In all experiments, cell viability was greater that 95%.
RNA Isolation and Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from human skin punch biopsies and cultured human skin fibroblasts using the Micro RNA isolation kit (Stratagene, La Jolla, CA). The levels of mRNA expression were quantified by real-time RT-PCR as previously described.27 Briefly, total RNA (100 ng) was reverse-transcribed using the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA), and real-time RT-PCR was performed on a 7700 Sequence Detector (Applied Biosystems) using one-tenth of the reverse transcription reaction and TaqMan Universal PCR Master Mix reagents (Applied Biosystems). PCR primers and probes were purchased from Applied Biosystems custom oligonucleotide synthesis service. Primers and FAM-labeled probes for TβRI and TβRII real-time RT-PCR were as follows: TβRI sense primer, 5′-GTGTGCTTCGTCTGCATCTCA-3′; TβRI anti-sense primer, 5′-GCACTCGATGGTGAATGACAGT-3′; TβRI probe, 5′-CATGTTGATGGTCTATATCTGCCACAACCG-3. TβRII sense primer 5′-CTGTGT-GACTTTGGGCTTTCC-3′; TβRII anti-sense primer, 5′-TCCCACCTGCCCACTGTT-3′; TβRII probe, 5′-ACCCTACTCTGTCTGTGGATGACCTGGC-3′; type I procollagen sense primer, 5′-AGGACAAGAGGCATGTCTGGTT-3′; type I procollagen anti-sense primer, 5′-TTGCAGTGGTAGGTGATGTTCTG-3′; type I procollagen probe, 5′-TCCTGCGCCTGATGTCCACCG-3′. Primers and VIC-labeled probe for 36B4 were described previously.27 Multiplex RT-PCR reactions contained primers and probes for TβRI or TβRII, type I (α1) procollagen, and 36B4. TβRI, TβRII, type I procollagen, and 36B4 mRNA levels were quantified based on standard curves. For comparison among treatment groups, TβRI and TβRII levels were quantified based on the standard curves and normalized to the housekeeping gene 36B4 levels as an internal control.
In Situ Hybridization
Plasmid DNA containing TβRII cDNA27 was linearized with ApaI and HindIII for sense and anti-sense probes, respectively. Digoxigenin-labeled sense and anti-sense riboprobes for human TβRII were synthesized using T7 and SP6 ribonucleic polymerase, respectively. Frozen skin sections (5 μm) were mounted, fixed, treated, and hybridized as previously described.5 Hybridization signals were detected immunohistochemically by alkaline phosphatase-conjugated anti-digoxigenin antibody.
Laser Capture Microdissection (LCM)-Coupled Quantitative Real-Time RT-PCR
LCM-coupled quantitative real-time RT-PCR was performed as previously described.27 Briefly, human skin punch biopsies were embedded in OCT, sectioned, and stained with hematoxylin. Approximately 200 fibroblasts identified by histology and immunohistology [positive for type I (α1) procollagen and vimentin, and negative for CD45],27 were captured using LCM.28 Total RNA was extracted using a Micro RNA isolation kit (Stratagene) and quantitative real-time RT-PCR was performed as described above.
Western Analysis and Immunoprecipitation
Preparation of membrane proteins, Western analysis, and immunoprecipitation were performed as described previously.25
Whole Cell Binding of [125I]TGF-β1
Determination of [125I]TGF-β1 (Perkin Elmer Life and Analytical Science Products, Inc., Boston, MA) binding to intact human skin fibroblasts was performed as described previously.25 Briefly, subconfluent cells were sham or UV irradiated with UV as described above. At the indicated times after UV exposure, cells were washed once with Krebs-Ringer-Hepes (KRH)/bovine serum albumin binding buffer (50 mmol/L Hepes, pH 7.5, 128 mmol/L NaCl, 1.3 mmol/L CaCl2, 5 mmol/L KCl, 0.5% bovine serum albumin), and then incubated in KRH/bovine serum albumin for 30 minutes at 37°C. Cells were washed with 0.1% glacial acetic acid for 5 minutes at room temperature, and then incubated with 0.2 nmol/L [125I]TGF-β1 for 3 to 4 hours at 4°C. Nonspecific binding of [125I]TGF-β1 was determined by addition of a 100-fold excess of unlabeled TGF-β1. Cells were washed, harvested by scraping, and radioactivity levels were measured using a gamma counter.
Immunofluorescence Confocal Laser Microscopy
Immunofluorescence confocal laser microscopy was performed as described previously.25 Briefly, human skin fibroblasts (1 × 104) were seeded on eight-well chamber slides and sham or UV (30 mJ/cm2) irradiated for the indicated times, before addition of TGF-β1 (5 ng/ml). Cells were fixed with 0.5% Nonidet P-40, blocked with 2% bovine serum albumin, and incubated overnight with anti-phospho-Smad2 (Upstate Biotechnology, Lake Placid, NY) or anti-Smad3 (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C. Fluorescein isothiocyanate-labeled secondary antibodies were then added, fluorescein isothiocyanate-conjugated anti-rabbit IgG for phospho-Smad2 or anti-goat IgG for Smad3 (1:50 dilution). The fluorescence-stained cells were observed and photographed with a Bio-Rad MRC 1000 confocal microscope (Bio-Rad, Richmond, CA) through a ×60 objective.
Northern Analysis
Northern analysis was performed as described previously.25 Briefly, total RNA (20 μg) was resolved by 1.2% agarose electrophoresis, transferred to nylon membranes, and hybridized with TβRII cDNA probes27 labeled with [32P]dCTP by random priming. Each blot was stripped and rehybridized with the housekeeping gene 36B4 cDNA probes as internal control to monitor the sample load in each lane. The intensities of each band were quantified by STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and normalized to the 36B4 as an internal control.
Metabolic Labeling of Cells
Human skin fibroblasts at 80% confluence were preincubated in methionine/cysteine-free minimal essential medium (Sigma Chemical, St. Louis, MO) for 2 hours. Cells were then either sham or UV irradiated (30mJ/cm2). [35S]Methionine/cysteine (100 μCi/ml, PRO-MIX; Amersham Pharmacia Biotech) was immediately add to the cells for the indicated times. Whole cell extracts were prepared, and [35S]methionine/cysteine incorporation into total protein was determined, as described previously.25 Equal amounts of total [35S]-labeled protein (∼200 μg) from sham- and UV-irradiated cells, were incubated with an anti-TβRII antibody (2 μg, Santa Cruz Biotechnology) overnight at 4°C. Twenty μl of protein G/A agarose beads were added and samples were rotated for 4 hours at 4°C. Samples were washed three times with RIPA buffer and then subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels then were dried, visualized, and quantified by STORM PhosphorImager (Molecular Dynamics).
Transient Transfection and Luciferase Assays
Plasmid DNA (1 μg) containing the β-galactosidase gene (pCMVβ; Clontech Laboratories, Inc., Palo Alto, CA), used as an internal control for transfection efficiency, was transiently co-transfected with various reporter genes and expression vectors: TGF-β/luciferase reporter gene29 (SBEX4), containing four repetitions of the GTCTAGAC Smad3/4 binding motif (provided by Dr. Bert Vogelstein of the Johns Hopkins Oncology Center, Baltimore, MD), TβRII promoter (−1640/+62)/luciferase constructs30 (provided by Dr. Seong-Jin Kim of the National Cancer Institute, Bethesda, MD), type I(α2) procollagen reporter gene promoter (provided by Dr. Maria Trojanowska of the Medical University of South Carolina, Charleston, SC),31 and TβRII cDNA expression vector.25 All plasmids except TβRII expression vector were transfected into cells using Fugene 6 (Roche Molecular, Indianapolis, IN) according to the manufacturer’s protocol. TβRII expression vector was transiently transfected into cells using the human dermal fibroblasts nucleofector kit (Amaxa Biosystems, Koln, Germany) according to the manufacturer’s protocols. After 24 to 48 hours of transfection, cells were sham or UV irradiated as described above and treated with vehicle or TGF-β1 (5 ng/ml) for 16 hours. Forty-eight hours after transfection, cells were washed once with PBS and harvested in lysis buffer (PharMingen International, San Diego, CA). Luciferase activity was measured using an enhanced luciferase assay kit (PharMingen International) according to the manufacturer’s protocol. CAT assays were performed as described previously.32 Aliquots containing identical β-galactosidase activity were used for each luciferase assay and CAT assay.
Statistical Analysis
Comparisons among treatment groups were made with the paired t-test (two groups) or the repeated measures of analysis of variance (more than two groups). Multiple pair-wise comparisons were made with the Tukey studentized range test. All P values are two-tailed and considered significant when <0.05.
Results
UV Irradiation Represses Type I Procollagen Expression
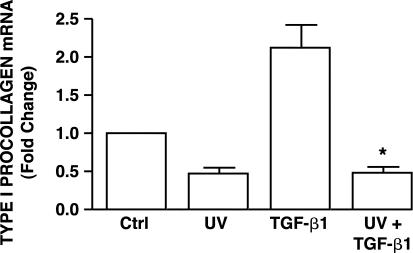
UV irradiation reduces type I procollagen production by fibroblasts in human skin in vivo.11 We used cultured adult human skin fibroblasts as a model to investigate the mechanisms of this reduction. We first examined the effects of UV irradiation on basal and TGF-β1-stimulated type I procollagen gene expression. UV irradiation reduced basal (no exogenous TGF-β) type I procollagen mRNA level 50% (Figure 1). Addition of TGF-β1 increased type I procollagen mRNA twofold, relative to vehicle-treated fibroblasts (Figure 1). This stimulation by TGF-β1 was completely abolished by exposure of fibroblasts to UV irradiation 8 hours before addition of TGF-β1 (Figure 1). The relatively low UV dose used in these experiments does not cause detectable morphological changes or reduced cell viability. These data indicate that UV irradiation inhibits both basal and TGF-β1-stimulated type I procollagen mRNA expression in cultured human skin fibroblasts, as was observed in human skin in vivo.11 UV irradiation reduces TGF-β-induction of Smad-dependent gene transcription.25
Figure 1.
UV irradiation inhibits TGF-β1-induced type I procollagen gene expression in cultured human skin fibroblasts. Cells were sham or UV irradiated (30 mJ/cm2). Vehicle or TGF-β1 (5 ng/ml) was added 8 hours after irradiation, and cells were harvested 16 hours later. Type I procollagen mRNA levels were determined by real-time RT-PCR and were normalized to mRNA levels of the housekeeping gene 36B4. Data are presented as fold change in type I procollagen levels relative to nontreated control (Ctrl) cells, and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared with TGF-β1 treatment.
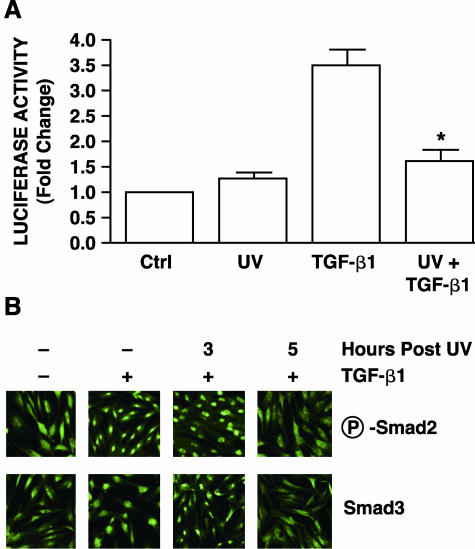
Having established that UV irradiation inhibits type I procollagen gene expression in human fibroblasts, we next investigated whether UV irradiation impairs the TGF-β/Smad pathway. To address this possibility, we investigated the effect of UV on TGF-β1-induced gene transcription, using TGF-β/Smad-regulated luciferase reporter gene. This reporter gene contains four repeats of the GTCTAGAC Smad3/4 binding element upstream of a SV40 minimal promoter.29 TGF-β1 activated the reporter construct more than threefold relative to levels in vehicle-treated human skin fibroblasts (Figure 2A). UV irradiation alone did not alter basal reporter activity. However, pretreatment of fibroblasts with UV irradiation 8 hours before addition of TGF-β1 repressed TGF-β1-induced reporter gene activation 90% (Figure 2A). These data indicate that UV irradiation impairs TGF-β-regulated, Smad-dependent gene expression in human skin fibroblasts.
Figure 2.
UV irradiation inhibits TGF-β/Smad signaling pathway in cultured human skin fibroblasts. A: UV irradiation inhibits TGF-β/Smad-regulated reporter gene in human skin fibroblasts. Cells were co-transiently transfected with TGF-β/Smad-regulated luciferase reporter constructs (SBEX4) and β-galactosidase expression vector (used as internal control). Twenty-four hours after transfection, cells were sham or UV irradiated (30 mJ/cm2), and then 8 hours later, cells were treated with vehicle or TGF-β1 (5 ng/ml) for 16 hours. Aliquots containing identical β-galactosidase activity were used for each luciferase assay. Data are presented as fold change in luciferase activity relative to activity in nontreated control (Ctrl) cells and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared with TGF-β1. B: UV irradiation inhibits TGF-β-induced Smad2 and Smad3 nuclear translocation in human skin fibroblasts. Cells were sham (−) or UV irradiated (30 mJ/cm2), and then treated at the indicated times after UV irradiation with vehicle (−) or TGF-β1 (+, 5 ng/ml) for 1 hour. Phosphorylated Smad2 (P-Smad2) and Smad3 nuclear translocation were determined by immunofluorescence confocal microscopy as described in Materials and Methods. Results are representative of three experiments.
Smad2/3-mediated gene transcription is dependent on translocation of Smad proteins into the nucleus. Therefore, we next examined the effect of UV irradiation on Smad2 and Smad3 nuclear translocation using immunofluorescence confocal laser microscopy. Smad2 and Smad3 were predominantly localized in the cytoplasm of untreated fibroblasts (Figure 2B) or fibroblasts treated with UV alone (data not shown). Addition of TGF-β1 resulted in substantial nuclear translocation of both phopspho-Smad2 and Smad3 within 1 hour of treatment (Figure 2B). Exposure of fibroblasts to UV irradiation 3 hours before addition of TGF-β1 had no effect on Smad 2/3 nuclear translocation. However, exposure to UV irradiation 5 hours before addition of TGF-β1 resulted in near complete inhibition of TGF-β1-induced Smad2 and Smad3 nuclear translocation (Figure 2B). As expected, UV inhibition of Smad3 nuclear translocation was accompanied by near complete loss of TGF-β-induced DNA binding to a Smad binding element (SBE) probe in electrophoretic mobility shift assays (data not shown).
UV Irradiation Reduces Type II TGF-β Receptor Expression
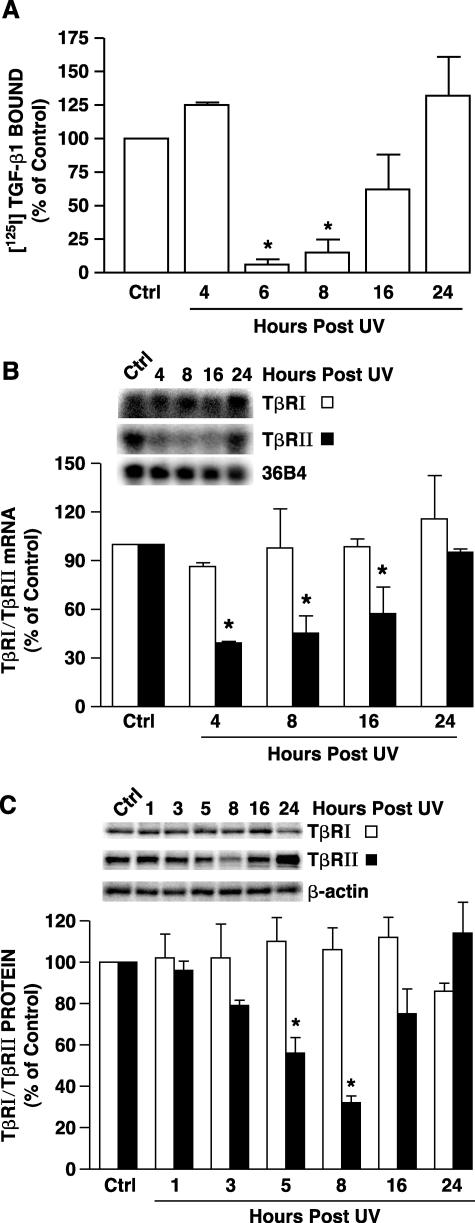
Taken together, the data presented above demonstrate that UV irradiation inhibits with TGF-β-induced signal transduction in human skin fibroblasts. This interference could occur because of physical and/or functional loss of TGF-β receptors. To investigate this possibility we determined the effect of UV irradiation on binding of TGF-β to its cell-surface receptors. Binding of [125I]TGF-β1 to human skin fibroblasts was determined 4 to 24 hours after exposure to UV irradiation. TGF-β1 binding was reduced by greater than 90% within 6 hours after UV irradiation (Figure 3A). TGF-β binding remained reduced for at least 8 hours, and gradually returned to its initial preirradiation level between 16 and 24 hours after UV irradiation (Figure 3A).
Figure 3.
UV irradiation reduces TβRII mRNA and protein in cultured human skin fibroblasts. A: UV irradiation inhibits TGF-β1 receptor binding in human skin fibroblasts. Cells were sham or UV irradiated (30 mJ/cm2). At the indicated times after UV specific binding of [125I]TGF-β1 to intact cells was determined as described in Materials and Methods. Data are presented as percentage of [125I]TGF-β1 binding relative to nonirradiated control (Ctrl) cells and are expressed as mean ± SEM, n = 4. *, P < 0.05, compared with control. B: UV irradiation reduces TβRII mRNA levels in human skin fibroblasts. Cells were sham or UV irradiated (30 mJ/cm2), total RNA was prepared at the indicated times after UV, and TβRI, TβRII, and 36B4 mRNA levels were determined by Northern analysis. Inset shows representative Northern blot. Data are presented as percentage of TβRI and TβRII levels relative to nonirradiated control (Ctrl) cells and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared with control. C: UV irradiation reduces TβRII protein levels in human skin fibroblasts. Cells were sham or UV irradiated (30 mJ/cm2), membrane fractions were prepared at the indicated time after UV, and TβRI and TβRII protein levels in membrane fractions were quantified by Western analysis. Inset shows representative Western blot for TβRI, TβRII, and β-actin control. Data are presented as percentage of TβRI and TβRII levels relative to nonirradiated control cells (Ctrl) and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared with control.
We next determined the effect of UV irradiation on TGF-β receptor mRNA and protein levels. TβRII mRNA levels were significantly decreased (60%) within 4 hours after UV, and remained decreased for at least 16 hours (Figure 3B). TβRII mRNA recovered to its initial level 24 hours after UV irradiation. In contrast, UV irradiation did not alter TβRI mRNA levels (Figure 3B). TβRII protein expression was also significantly reduced within 5 hours after UV followed by recovered 16 to 24 hours after UV (Figure 3C). In contrast, neither TβRI mRNA (Figure 3B) nor protein (Figure 3C) expression was affected by UV irradiation. These data indicate that UV irradiation specifically reduces TβRII.
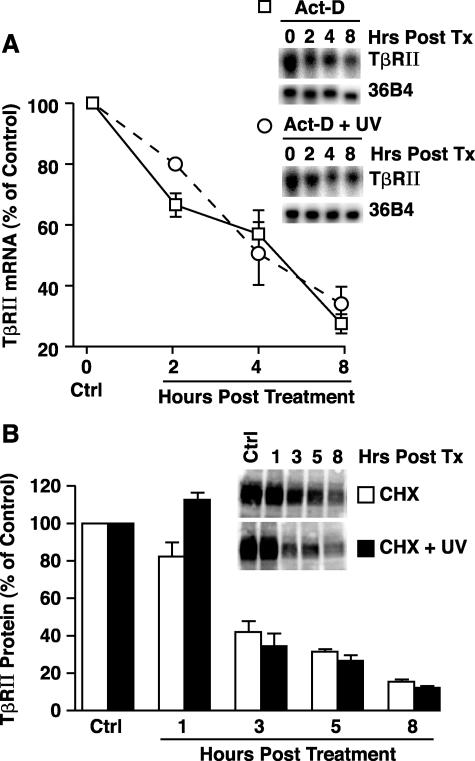
UV Irradiation Does Not Alter TβRII mRNA and Protein Stability
Reduced TβRII expression could result from increased degradation and/or reduced synthesis. To examine these possibilities we determined the effect of UV irradiation on TβRII mRNA and protein stability. Nonirradiated or UV-irradiated skin fibroblasts were treated with actinomycin D (10 μg/ml) or cycloheximide (10 μg/ml) to prevent de novo mRNA or protein synthesis, respectively, and the half-lives of TβRII mRNA and protein were determined. The half-life of TβRII mRNA was similar (∼4 hours), in both nonirradiated and UV-irradiated cells (Figure 4A). Similarly, the half-life of TβRII protein (∼3 hours) did not differ between nonirradiated and UV-irradiated fibroblasts (Figure 4B). These data demonstrate that UV irradiation does not accelerate degradation of either TβRII mRNA or TβRII protein.
Figure 4.
UV irradiation does not alter TβRII mRNA or protein stability. A: Fibroblasts were incubated with transcription inhibitor actinomycin D (Act-D, 10 μg/ml), either alone or in combination with UV irradiation (30mJ/cm2). Total RNA was isolated at the indicated times and subjected to Northern analysis. Data are presented as percentage of TβRII mRNA levels relative to vehicle-treated control cells (Ctrl), and are expressed as means + SEM, n = 3. B: Cells were incubated with translation inhibitor cycloheximide (CHX, 10 μg/ml), either alone or in combination with UV irradiation (30mJ/cm2). Whole cell extract was isolated at the indicated times and subjected to Western analysis. Data are presented as percentage of TβRII protein levels relative to vehicle-treated control cells (Ctrl) and are expressed as mean + SEM, n = 3.
UV Irradiation Inhibits TβRII Protein Synthesis and Transcription
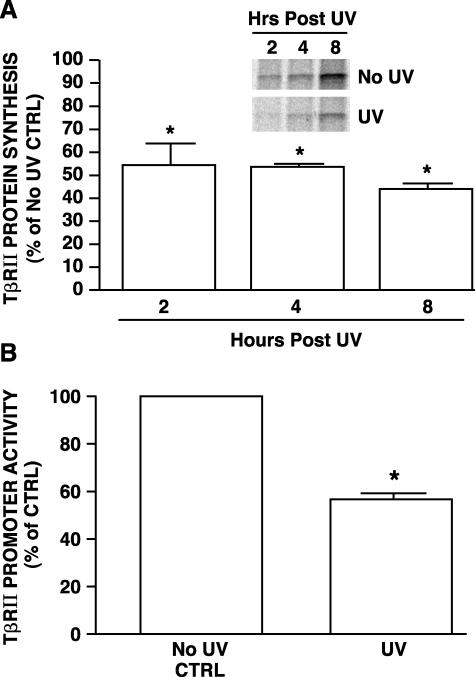
Given that UV irradiation does not accelerate breakdown of either TβRII mRNA or protein, the observed UV reduction of TβRII mRNA and protein must result from reduced synthesis. The ability of UV irradiation to inhibit TβRII protein synthesis was confirmed by comparison of de novo TβRII protein synthesis in sham- and UV-irradiated human skin fibroblasts. Immediately after sham or UV irradiation, [35S]methionine/cysteine was added to culture media, and the kinetics of incorporation of label into immunoprecipitated TβRII was determined. Synthesis of TβRII protein was significantly lower in UV-irradiated human skin fibroblasts, between 2 to 8 hours after UV irradiation, compared to nonirradiated fibroblasts (Figure 5A). This inhibition of TβRII protein synthesis was specific because [35S]methionine/cysteine incorporation into total protein was similar in sham- and UV-irradiated fibroblasts.
Figure 5.
UV irradiation inhibits TβRII protein synthesis and promoter transcription in human skin fibroblasts. A: UV irradiation inhibits TβRII protein synthesis in human skin fibroblasts. Cells were sham or UV irradiated and then immediately incubated with [35S]methionine/cysteine for the indicated times. [35S]-Labeled TβRII protein was immunoprecipitated and subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The bands represent [35S]-labeled TβRII protein synthesized during the indicated times. Bars are means ± SEM band intensities of TβRII protein levels in UV-irradiated cells, as a percentage in sham-irradiated cells. TβRII protein levels in sham-irradiated cells were assigned a value of 100% at each time point, n = 3. *, P < 0.05, compared with control. B: UV irradiation inhibits transcription of the TβRII promoter reporter in human skin fibroblasts. TβRII promoter/luciferase reporter (−1640/+62) was transiently co-transfected with β-galactosidase expression vector into the human skin fibroblasts. Twenty-four hours after transfection, cells were sham or UV irradiated (30 mJ/cm2). TβRII promoter activity was determined 8 hours after irradiation by luciferase assay. Aliquots containing identical β-galactosidase activity were used for each luciferase assay. Data are presented as percentage of control of promoter activity relative to activity in nonirradiated control cells (Ctrl) and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared with control.
We used a human TβRII promoter (−1640 to + 62)/luciferase reporter gene to assess the effect of UV irradiation on TβRII transcription. In sham-irradiated skin fibroblasts, the TβRII promoter construct was actively transcribed (Figure 5B). UV irradiation reduced reporter gene activity by nearly 50%, confirming the ability of UV irradiation to inhibit TβRII gene transcription.
Overexpression of TβRII Overcomes UV Inhibition of Type I Procollagen Gene Expression
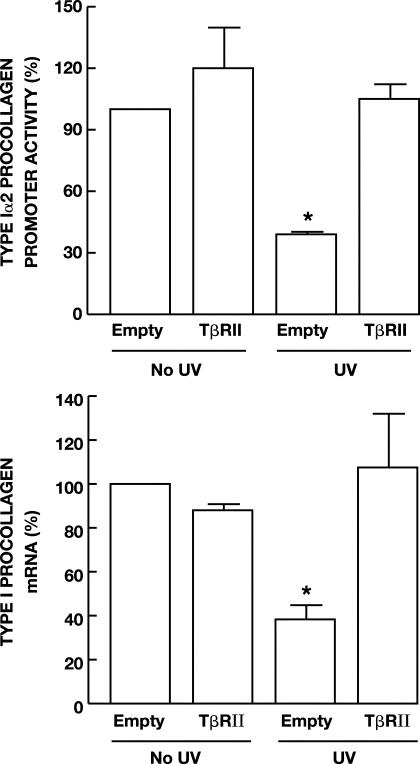
The above data demonstrate that UV irradiation inhibits production of TβRII and that this reduction impairs TGF-β responsiveness in human skin fibroblasts. Prevention of UV reduction of TβRII should counteract UV-induced loss of TGF-β responsiveness and thereby maintain TGF-β-dependent type I procollagen gene expression. To test this prediction, we determined the effect of overexpression of TβRII on UV inhibition of TGF-β-induced type I(α2) procollagen transcription. Type I(α2) procollagen promoter activity was increased 2.1-fold by TGF-β1 (data not shown). Pretreatment of cells with UV repressed TGF-β1-induced type I(α2) procollagen promoter activity 60% (Figure 6A). Overexpression of TβRII did not significantly alter promoter activity. In contrast, overexpression of TβRII completely prevented UV inhibition of type I(α2) procollagen promoter. Overexpression of TβRII also prevented UV irradiation inhibition of type I procollagen mRNA expression. UV irradiation reduced endogenous type I(α1) procollagen mRNA 62%, and this reduction was completely abrogated by overexpression of TβRII (Figure 6B). These data demonstrate that down-regulation of TβRII by UV irradiation is responsible for UV inhibition of type I procollagen gene expression in human skin fibroblasts.
Figure 6.
Overexpression of TβRII prevents UV inhibition of type I procollagen gene expression in human skin fibroblasts. A: Cells were transiently co-transfected with type I(α2) procollagen promoter CAT-reporter construct, β-galactosidase expression vector, and where indicated, empty or TβRII expression vector. Twenty-four hours after transfection, cells were sham or UV irradiated (30 mJ/cm2) and then 8 hours later treated with TGF-β1 (5 ng/ml) for 16 hours. Aliquots containing identical β-galactosidase activity were used for each procollagen promoter reporter activity assay. Data are presented as percentage of control in reporter CAT activity relative to empty vector control cells, and are expressed as mean ± SEM, n = 3. *, P < 0.05, compared to control. B: Human fibroblasts were transiently transfected with TβRII expression vector or empty vector (pCDNA 3.1). Forty-eight hours after transfection, cells were sham or UV irradiated (30 mJ/cm2). Sixteen hours after UV irradiation, type I(α1) procollagen and 36B4 (internal control) mRNA levels were quantified by real-time RT-PCR. Data are presented as percentage of type I(α1) procollagen mRNA levels (normalized to 36B4 mRNA levels) relative to nonirradiated empty vector control cells and are expressed as mean ± SEM, n = 4. *, P < 0.05, compared to control.
Solar-Simulated UV Irradiation Reduces TβRII mRNA Expression in Human Skin in Vivo
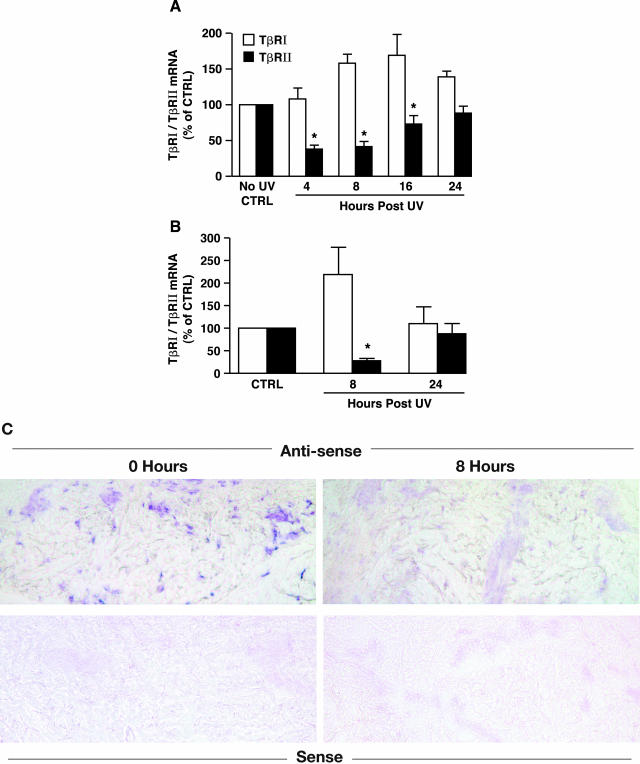
Finally, we investigated the effect of solar-simulated UV irradiation on TGF-β receptor mRNA expression in human skin in vivo. Sun-protected human skin was exposed to solar-simulated UV (2 minimum erythema dose) and full-thickness skin samples were dissected to separate epidermis and connective tissue (dermis).27 Total RNA was prepared from dermal fractions and TβRI and TβRII transcripts were quantified by real-time RT-PCR. TβRII mRNA was significantly reduced (60%) within 8 hours after UV and recovered to near its initial level by 24 hours after UV (Figure 7A). In contrast, TβRI mRNA expression was slightly increased 16 hours after UV irradiation (Figure 7A). The dermal fraction obtained by dissection of full-thickness skin inevitably contains some epidermal contamination as evidenced by detectable levels of epidermal keratin 14.27 To confirm that UV irradiation caused reduced expression of TβRII, but not TβRI, in dermal fibroblasts, we used LCM to obtain fibroblasts from frozen sections of skin. Total RNA was prepared from the captured fibroblasts and TβRI and TβRII expression were quantified by real-time RT-PCR. The level of TβRII mRNA in dermal fibroblasts was reduced 70% at 8 hours after solar-simulated UV, whereas TβRI mRNA expression was not significantly altered (Figure 7B). We next determined cellular localization of TβRII mRNA in human skin connective tissue in vivo by in situ hybridization. In nonirradiated skin, TβRII mRNA was expressed in cells throughout the dermis (Figure 7C). The level of TβRII mRNA was substantially reduced 8 hours after solar-simulated UV (Figure 7C). Hybridization of untreated and UV-exposed skin sections with sense TβRII probe yielded no detectable signals indicating that hybridization of the anti-sense probe was specific (Figure 7C).
Figure 7.
Solar-simulated UV irradiation reduces TβRII mRNA expression in human skin in vivo. A: Solar-simulated UV irradiation reduces TβRII mRNA in skin connective tissue. Sun-protected human skin was exposed to solar-simulated UV (2 minimum erythema dose). At the indicated times, full-thickness human skin samples were obtained and snap-frozen. Epidermis was removed from dermis by dissection with a scalpel, under a dissecting microscope. Total RNA was prepared from dermis. TβRI (open bars) and TβRII (filled bars) mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA levels of the housekeeping gene 36B4. Data are presented as percentage of TβRI and TβRII levels relative to nonirradiated control skin and are expressed as mean ± SEM, n = 6. *, P < 0.05, compared to control. B: Solar-simulated UV irradiation reduces TβRII mRNA in human skin fibroblasts in vivo. Fibroblasts in nonirradiated and solar-simulated UV-irradiated skin were captured by LCM as described in Materials and Methods. TβRI (open bars) and TβRII (filled bars) mRNA levels were quantified by real-time RT-PCR and normalized to mRNA levels of the housekeeping gene 36B4. Data are presented as percentage of TβRI and TβRII mRNA levels relative to nonirradiated control skin (Ctrl), and are expressed as mean ± SEM, n = 3 to 4. *, P < 0.05, compared to control. C: Dermal cellular localization of TβRII mRNA after solar-simulated UV irradiation in human skin in vivo. Skin samples were obtained at the indicated times after solar-simulated UV irradiation, and TβRII mRNA expression was determined by anti-sense riboprobe in situ hybridization. Sense riboprobe yielded minimal background signal. Results are representative of six patients.
Discussion
TGF-β is one of the major regulators of procollagen production in human skin. TGF-β acts through its cell surface receptors to activate transcription factors Smad 2/3, which regulate TGF-β target gene expression. We have previously reported that UV irradiation inhibits type I procollagen production in human skin in vivo.11 We have also previously reported that UV irradiation impairs the TGF-β/SMAD pathway in mink lung epithelial cell line, which is extensively used to investigate mechanisms of TGF-β/Smad signaling.25 These findings led us to investigate the mechanisms of UV irradiation inhibition of TGF-β/Smad pathway in human skin fibroblasts and the connection between this inhibition and the observed loss of type I procollagen in UV-exposed human skin. Our results demonstrate that UV irradiation impairs the TGF-β/Smad pathway by transcriptional inhibition of TβRII, in human skin fibroblasts. This reduction of TβRII expression causes reduced TGF-β responsiveness and thereby down-regulation of TGF-β target genes, including type I procollagen.
The significance of our study is that it elucidates the primary mechanism by which UV irradiation reduces type I procollagen production in human skin fibroblasts. Reduced type I procollagen production is a prominent feature of the pathophysiology of sun-exposed human skin. Because type I collagen is the major structural protein in the skin, reduced type I procollagen production is a critical factor in the aged appearance of sun-exposed skin. Therefore, elucidation of the molecular mechanism by which UV irradiation reduces type I procollagen production is an important contribution to our understanding of the pathophysiology of sun-induced premature skin aging.
UV irradiation of human skin in vivo and cultured human skin fibroblasts inhibits TGF-β/Smad signaling at its initial step by down-regulating TβRII expression. We previously observed that UV irradiation reduced TβRII expression in mink lung epithelial cells,25 suggesting that TβRII down-regulation is a fundamental response to UV irradiation, which is not restricted to specific cell types. In skin fibroblasts, UV irradiation inhibited both TβRII promoter transcription and protein synthesis. The kinetics of UV reduction of mRNA and protein levels were similar and therefore it is likely that reduced protein synthesis resulted, at least in part, from reduced mRNA levels. However, the possibility of direct UV inhibition of TβRII protein synthesis, independent of reduced mRNA levels, cannot be ruled out.
In contrast to TβRII, neither TβRI mRNA nor protein expression were reduced by UV irradiation. Discordant regulation of TβRI and TβRII by platelet-derived growth factor has also been reported in dermal fibroblasts. Platelet-derived growth factor up-regulates TβRII, whereas TβRI expression is unaffected.33 TβRI and TβRII receptors are also differentially expressed in a mouse model of wound healing in which both receptors are increased, but with distinct kinetics and different expression levels.34 TβRI and TβRII gene promoter sequences are dissimilar.30,35,36 The 5′-flanking region of the TβRI gene promoter is extremely GC-rich and contains multiple Sp1 sites, which are essential for basal and maximal promoter activity.35,36 The TβRII promoter also contains Sp1 regulatory elements; however, in addition it contains additional regulatory elements, including CRE/ATF, CCAAT box, EGR-1 binding site, and two overlapping ets sites, which are not present in the TβRI promoter. These differences in cis-regulator elements likely contribute to differential regulation of TβRI and TβRII gene expression.
Although regulation of type I procollagen expression is complex, and not fully understood, accumulated evidence indicates that transcriptional regulation plays a major role in controlling its production.37,38 Transcription of type I(α2) procollagen gene expression is directly regulated by TGF-β via a Smad3 binding element in its promoter.39 Inhibition of endogenous Smad3 or Smad4 expression results in abrogation of type I(α2) procollagen promoter activity and type I procollagen protein synthesis.19
We have previously reported that UV irradiation reduces TβRII mRNA expression in human skin in vivo.40 These data were obtained from the full thickness skin, and therefore represented alterations in the epidermis as well as the dermis. Although down-regulation of TβRII in the epidermal compartment is likely of importance in the overall response of skin to UV irradiation, it is not directly germane to regulation of procollagen production, which occurs in the dermis. To determine the expression level of TβRII in dermal fibroblasts, we used LCM coupled with quantitative real-time RT-PCR. This technology allowed us to select skin fibroblasts from the heterogeneous mixture of cell types in human skin. LCM coupled with quantitative real-time RT-PCR results revealed that the level of TβRII mRNA in dermal fibroblasts was reduced 70% by solar-simulated UV.
Our data indicate that UV inhibition of TβRII gene expression is the primary mechanism by which UV irradiation reduces type I procollagen expression in UV-irradiated skin because overexpression of TβRII completely overcomes UV inhibition of TGF-β-induced type I (α2) procollagen promoter activity and endogenous Type I (α1) procollagen mRNA expression in human skin fibroblasts. The TβRII promoter contains both positive and negative regulatory elements in addition to a core element that is required for basal expression. Choi and colleagues41 have identified a novel ets-related transcription (ERT) factor that plays a major role in the transcriptional regulation of the TβRII gene. Overexpression of ERT increased endogenous TβRII mRNA and protein expression, resulting in increased TGF-β responsiveness and growth inhibition of cancer cells.42 Conversely, early growth response factor-1 (Egr-1) can negatively regulate expression of TβRII.43 Egr-1 interacts directly with ERT binding region of the TβRII proximal promoter to repress TβRII promoter activity. Interestingly, we find that Egr-1 is rapidly and strongly induced on UV irradiation in human skin in vivo and in cultured human skin fibroblasts (unpublished data), raising the possibility that elevated Egr-1 may down-regulate TβRII expression. Whether ERT and Egr-1 transcription factors are involved in UV down-regulation of TβRII gene transcription remains to be determined.
In summary, our data demonstrate that UV irradiation impairs the TGF-β/Smad pathway, as a result of down-regulation of TβRII, and this impairment reduces procollagen synthesis in UV-irradiated human skin. Understanding the molecular basis for UV reduction of TβRII provides new insights into molecular mechanisms of photoaging and may facilitate identification of novel approaches for its prevention.
Acknowledgments
We thank Suzan Rehbine for procurement of tissue specimens, Yu-Chen Hu and Jingcheng Wang for technical assistance, Laura VanGoor for the preparation of graphic material, Ted Hamilton for statistical analysis, and Diane Fiolek for administrative assistance.
Footnotes
Address reprint requests to Gary J. Fisher, Ph.D., Department of Dermatology, University of Michigan Medical School, 1150 W. Medical Center Dr., Medical Science I, Room 6447, Ann Arbor, Michigan 48109-0609. E-mail: dianemch@umich.edu.
Supported by a grant from the National Institutes of Health (AG19364-02 to G.J.F.).
References
- Gilchrest BA, Yaar M. Ageing and photoageing of the skin: observations and the cellular and molecular level. Br J Dermatol. 1992;127:25–30. doi: 10.1111/j.1365-2133.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- Kaminer MS. Photodamage: magnitude of the problem. Gilchrest BA, editor. Cambridge: Blackwell Science,; Photodamage. 1995:pp 1–11. [Google Scholar]
- Brash D, Rudolph J, Simon J, Lin A, McKenna G, Baden H, Halperin A, Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R, Gartstein V, Kligman A, Montagna W, Allendorf R, Ridder G. Age, sunlight, and facial skin: a histologic and quantitative study. J Am Acad Dermatol. 1991;25:751–760. doi: 10.1016/s0190-9622(08)80964-4. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Smith J, Davidson E, Sams W, Clark R. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962;39:347–350. doi: 10.1038/jid.1962.122. [DOI] [PubMed] [Google Scholar]
- Uitto J. Biology of dermal cells and extracellular matrix. Fitzpatrick T, Eisen A, Wolff K, Freedberg I, Austen K, editors. New York: McGraw-Hill,; Dermatology in General Medicine. 1993:pp 299–314. [Google Scholar]
- Verzijl N, DeGroot J, Thorpe S, Bank R, Shaw M, Lyons T, Bijlsma W, Lafeber F, Baynes J, TeKoppele J. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, Kang S, Voorhees J. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:661–668. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-β signalling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-β Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E, Heldin CH, ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. EMBO J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Haukipuro K, Meikko J, Risteli L, Kairaluoma M, Risteli J. Synthesis of type I collagen in healing wounds in humans. Ann Surg. 1991;213:75–80. doi: 10.1097/00000658-199101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- Chen S-J, Yuan W, Lo S, Trojanowska M, Varga J. Interaction of Smad3 with a proximal Smad-binding element of the human α2(I) procollagen gene promoter required for transcriptional activation of TGF-β. J Cell Physiology. 2000;183:381–392. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148–155. doi: 10.1038/ki.1996.297. [DOI] [PubMed] [Google Scholar]
- Roberts A, Heine U, Flanders K, Sporn M. Transforming growth factor-β. Major role in regulation of extracellular matrix. Ann NY Acad Sci. 1990;580:225–232. doi: 10.1111/j.1749-6632.1990.tb17931.x. [DOI] [PubMed] [Google Scholar]
- Slack J, Liska D, Bornstein P. Regulation of expression of the type I collagen genes. Am J Med Genet. 1993;45:1940–1951. doi: 10.1002/ajmg.1320450203. [DOI] [PubMed] [Google Scholar]
- Mozes M, Bottinger E, Jacot T, Kopp J. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-β) isoforms in TGF-β transgenic mice. J Am Soc Nephrol. 1999;10:271–280. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Invest Dermatol. 1998;3:61–68. [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Esmann J, Griffiths CEM, Voorhees JJ. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M. Immuno-LCM: laser-capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–66. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad 3 and Smad 4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Bae H, Geiser A, Kim D, Chung M, Burmester J, Sporn M, Roberts A, Kim S. Characterization of the promoter region of the human transforming growth factor β type II receptor gene. J Biol Chem. 1995;270:29460–29468. doi: 10.1074/jbc.270.49.29460. [DOI] [PubMed] [Google Scholar]
- Ihn H, LeRoy E, Trojanowska M. Oncostatin M stimulates transcription of the human α2(I) collagen gene via the Sp1/Sp3-binding site. J Biol Chem. 1997;272:24666–24672. doi: 10.1074/jbc.272.39.24666. [DOI] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Cloning and characterization of the human protein kinase c-η promoter. J Biol Chem. 1999;274:28566–28574. doi: 10.1074/jbc.274.40.28566. [DOI] [PubMed] [Google Scholar]
- Czuwara-Ladykowska J, Gore E, Shegogue D, Smith E, Trojanowska M. Differential regulation of transforming growth factor-β receptors type I and II by platelet-derived growth factor in human dermal fibroblasts. Br J Dermatol. 2001;145:569–575. doi: 10.1046/j.1365-2133.2001.04443.x. [DOI] [PubMed] [Google Scholar]
- Frank S, Madlener M, Werner S. Transforming growth factors β1, β2, and β3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem. 1996;271:10188–10193. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- Bloom B, Humphries D, Kuang P, Fine A, Goldstein R. Structure and expression of the promoter for the R4/ALK5 human type I transforming growth factor-β receptor: regulation by TGF-β. Biochim Biophys Acta. 1996;1312:243–248. doi: 10.1016/0167-4889(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Ji C, Casinghino S, McCarthy T, Centrella M. Multiple and essential Sp1 binding sites in the promoter for transforming growth factor-β type I receptor. J Biol Chem. 1997;272:21260–21267. doi: 10.1074/jbc.272.34.21260. [DOI] [PubMed] [Google Scholar]
- Jimenez SA, Varga J, Olsen A, Li L, Diaz A, Herhal J, Koch J. Functional analog of human α1(I) procollagen gene promoter: differential activity in collagen producing and nonproducing cells and response to transforming growth factor β1. J Biol Chem. 1994;269:12684–12691. [PubMed] [Google Scholar]
- Inagaki Y, Truter S, Tanaka S, Di Liberto M, Ramirez F. Overlapping pathways mediate the opposing actions of tumor necrosis factor-α and transforming growth factor-β on α2(I) collagen gene transcription. J Biol Chem. 1995;270:3353–3358. doi: 10.1074/jbc.270.7.3353. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Yuan WH, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- Choi SG, Yi Y, Kim YS, Kato M, Chang J, Chung HW, Hahm KB, Yang HK, Rhee HH, Bang YJ, Kim SJ. A novel ets-related transcription factor, ERT/ESX/ESE-1, regulates expression of the transforming growth factor-β type II receptor. J Biol Chem. 1998;273:110–117. doi: 10.1074/jbc.273.1.110. [DOI] [PubMed] [Google Scholar]
- Chang J, Lee C, Hahm K, Youngsuk Y, Choi S-G, Kim S-J. Over-expression of ERT(ESX/ESE-1/ELF3), an ets-related transcription factor, induces endogenous TGF-β type II receptor expression and restores the TGF-β signaling pathway in Hs578t human breast cancer cells. Oncogene. 2000;19:151–154. doi: 10.1038/sj.onc.1203252. [DOI] [PubMed] [Google Scholar]
- Du B, Fu C, Kent K, Bush H, Jr, Schulick A, Kreiger K, Collins T, McCaffrey T. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-β type II receptor. J Biol Chem. 2000;275:39039–39047. doi: 10.1074/jbc.M005159200. [DOI] [PubMed] [Google Scholar]