Abstract
Wnt-1-induced secreted protein 1 (WISP-1) is a member of the CCN (connective tissue growth factor, Cyr61, NOV) family of growth factors. Experimental evidence suggests that CCN family members are involved in skeletogenesis and bone healing. To investigate the role of WISP-1 in osteogenic processes, we characterized its tissue and cellular expression and evaluated its activity in osteoblastic and chondrocytic cell culture models. During embryonic development, WISP-1 expression was restricted to osteoblasts and to osteoblastic progenitor cells of the perichondral mesenchyme. In vitro, we showed that WISP-1 expression in differentiating osteoblasts promotes BMP-2-induced osteoblastic differentiation. Using in situ and cell binding analysis, we demonstrated WISP-1 interaction with perichondral mesenchyme and undifferentiated chondrocytes. We evaluated the effect of WISP-1 on chondrocytes by generating stably transfected mouse chondrocytic cell lines. In these cells, WISP-1 increased proliferation and saturation density but repressed chondrocytic differentiation. Because of the similarity between skeletogenesis and bone healing, we also analyzed WISP-1 spatiotemporal expression in a fracture repair model. We found that WISP-1 expression recapitulates the pattern observed during skeletal development. Our data demonstrate that WISP-1 is an osteogenic potentiating factor promoting mesenchymal cell proliferation and osteoblastic differentiation while repressing chondrocytic differentiation. Therefore, we propose that WISP-1 plays an important regulatory role during bone development and fracture repair.
Wnt-1-induced secreted protein 1 (WISP-1) is a member of the CCN family of growth factors, which also includes connective tissue growth factor (CTGF), cysteine-rich 61 (Cyr61), nephroblastoma overexpressed (NOV), WISP-2, and WISP-3.1–4 WISP-1 is a target of the Wnt-1/Frizzled pathway and its expression is regulated by β-catenin.3,5 It is overexpressed by mesenchymal cells of the peritumoral stroma of several types of cancers and constitutes a putative paracrine effector of tumorigenesis.5–8 When expressed in normal fibroblasts, WISP-1 acts in an autocrine manner to accelerate cell growth, increase saturation density, induce morphological transformation, and promote tumorigenesis.5 WISP-1 activity and availability is modulated by its interaction with decorin and biglycan, two extracellular matrix-associated proteoglycans.9 Although WISP-1 involvement in tumor progression has gathered a lot of attention, its function in normal biological processes remains to be clarified.
Several genes involved in cancer progression have emerged as encoding critical elements of pathways involved in embryonic development.10 Among them, the Wnt/β-catenin signaling transduction pathway is known both for its central role in the etiology of numerous types of cancers and for its regulatory function during skeletogenesis.11–13 The Wnt signaling pathway acts on cell fate determination by modulating the expression of key players in a hierarchy of regulatory genes.14 In addition, the function of several Wnt target genes is consistent with control of cellular functions implicated in tumorigenesis and embryonic development.5,6,15 The Wnt pathway affects growth, patterning, and morphogenesis of skeletal elements by modulating chondrocyte and osteoblast differentiation.16–19
During vertebrate embryogenesis, most skeletal elements are first formed by cartilaginous templates that are progressively replaced by bone in a process called endochondral ossification.20–23 This process begins with the proliferation and condensation of committed osteochondroprogenitor mesenchymal cells into aggregates. Cells at the center of these aggregates differentiate into chondrocytes and initiate the synthesis of cartilage. Spindle-shaped cells surrounding the cartilage templates align longitudinally to form the perichondrium that separates the chondrocytes from the adjacent tissue. The chondrocytes at the distal ends of the templates continue to proliferate whereas the cells in the central region of the cartilage elements exit the cell cycle and become hypertrophic. Differentiation into hypertrophic chondrocytes is accompanied by the differentiation of the mesenchymal cells of the perichondrium into osteoblasts. Osteoblasts are responsible for the deposition of bone matrix forming the bone collar surrounding the hypertrophic region of the cartilage. The invasion of hypertrophic cartilage by blood vessels and osteogenic cells results in the replacement of the cartilage by bone. Alternately, in some skeletal elements, especially the flat bones of the skull, the osteochondroprogenitor cells bypass the cartilaginous template formation and directly differentiate into osteoblasts. This process is called intramembranous ossification. The Wnt/β-catenin pathway constitutes one of the essential molecular mechanisms regulating several aspects of bone development including chondrocyte and osteoblast differentiation and joint formation.16–19 Because WISP-1 is a Wnt/β-catenin-signaling pathway target gene, it could play an important regulatory role during vertebrate skeletal development.
In this study we describe WISP-1 spatiotemporal expression during skeletogenesis and show its association with mesenchymal and osteoblastic cells of bones developing via endochondral and intramembranous ossification processes. Using cell culture models, we further demonstrate that WISP-1 expression is associated with osteoblastic differentiation. In addition, we present evidence that WISP-1 interacts with chondrocytic cells and increases their proliferation and saturation density and prevents their differentiation. Finally using a bone fracture model, we show that WISP-1 expression during bone healing recapitulates the pattern observed during development. Taken together, the data presented here suggest an important role for WISP-1 in the osteochondroprogenitor’s maturation process during skeletogenesis and fracture repair.
Materials and Methods
Materials
Fatty acid ultra-free bovine serum albumin fraction V and the complete ethylenediaminetetraacetic acid-free protease inhibitor cocktail tablets were from Roche Molecular Biochemicals (Indianapolis, IN). The biotinylated horse anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Fluorescein isothiocyanate-conjugated streptavidin and Hoechst 33342 were from Molecular Probes (Eugene, OR). The Renaissance TSA indirect amplification kit was bought from NEN Life Science Products (Boston, MA). Vectashield mounting media was obtained from Vector (Burlingame, CA) and the Tissue-Tek OCT compound was from Miles (Elkhart, IN). Collagenase type 2, bovine insulin, transferrin, and sodium selenite were purchased from Sigma (St. Louis, MO). Recombinant human BMP-2 was purchased from R&D Systems (Minneapolis, MN) and recombinant human GDF-5 from Antigenix America Inc. (Huntington, NY). Recombinant WISP-1-Fc fusion protein and WISP-1 monoclonal antibody were generated as previously described.9
In Situ Hybridization
Localization of gene expression was executed as described previously24 using 33P-labeled sense and anti-sense riboprobes transcribed from a 740-bp polymerase chain reaction product corresponding to nucleotides 440 to 1180 of mouse WISP-1 (NM_018865).
Immunofluorescence
Sections (10 μm) of OCT-embedded rat E18 embryos were washed with phosphate-buffered saline (PBS) and the nonspecific binding sites were blocked for 20 minutes in PBS/3% bovine serum albumin containing 1.5% normal horse serum. Avidin and biotin binding sites were blocked with the avidin/biotin blocking kit from Vector and the slides were incubated with 1 μg/ml of mouse monoclonal anti-WISP-1 antibody (clone 9C10) in PBS/3% bovine serum albumin containing 1.5% normal horse serum for 1 hour, washed, and fixed in PBS/4% paraformaldehyde for 10 minutes. The sections were washed and incubated for 30 minutes with 1:200 biotinylated horse anti-mouse IgG in HBS-C/3% bovine serum albumin. The slides were washed, fixed, and the signal amplified using the TSA indirect amplification kit according to the manufacturer’s instructions. The slides were incubated for 30 minutes with streptavidin-conjugated fluorescein isothiocyanate (1:1000). The sections were washed, mounted in Vectashield mounting media containing 1 μg/ml of Hoechst 33342, and visualized under a Nikon Eclipse 800 fluorescent microscope.
In Situ Ligand Binding
Binding of WISP-1-Fc to rat embryo sections was evaluated using the in situ ligand-binding procedure previously described.9,25 No signal was detected when WISP-1-Fc was omitted or the anti-human IgG antibody replaced by an irrelevant antibody (anti-gp 120). The binding pattern described for WISP-1-Fc was unique and different from the binding pattern observed for a control protein (human IgG).
Primary Porcine Chondrocyte Isolation
Primary chondrocytes were isolated using a protocol previously described.26 Briefly, the metacarpo-phalangeal joint of 4- to 6-month-old female pigs was aseptically opened, and articular cartilage was dissected free of the underlying bone. The cartilage was pooled, minced, washed, and digested overnight at 37°C with collagenase. The digest was filtered through a 50-μm sieve and the cells were washed, seeded at 25,000 cell/cm2 in Ham-F12 containing 10% fetal bovine serum (FBS) and 4 μg/ml gentamicin, and maintained at 37°C under 5% CO2. Cells were fed every 3 days and reseeded every 5 days. After 11 days in culture, 50 to 60% of the primary chondrocytes had lost their chondrocytic character and reverted to a mesenchymal phenotype characterized by a spindloid bipolar shape and a switch from collagen 2 to collagen 1 expression.
Cell Binding
Binding of WISP-1-Fc to dedifferentiated porcine primary chondrocytes was executed as previously described.9 No signal was detected when WISP-1-Fc was omitted or the anti-human IgG antibody replaced by an irrelevant antibody (anti-gp 120).
Cell Culture
Normal human dermal fibroblasts and normal human lung fibroblasts were purchased from Cambrex (Walkersville, MD). The C57MG mouse mammary epithelial cell line was provided by Diane Pennica (Genentech, South San Francisco, CA). NIH/3T3 mouse fibroblasts, MC3T3-E1 clone 14 mouse calvaria preosteoblasts, and the mouse C2C12 skeletal muscle myoblasts were purchased from American Type Culture Collection (Manassas, VA). ST2 mouse bone marrow stromal cells and the ATDC5 mouse embryonal carcinoma-derived chondrogenic cell line were purchased from Riken (Tsukuba, Japan).
MC3T3-E1 cells were maintained in a mixture (1:1) of DME and Ham’s F-12 (DME/F12) medium supplemented with 10% FBS until they reached confluency. Osteoblastic differentiation was induced as previously described.27 Briefly, cells were grown to confluency in α-modified Eagle’s medium containing 10% FBS and treated with 50 μg/ml of ascorbic acid. The inorganic phosphate concentration was raised to 3 mmol/L and the cells were treated an additional 2 days. ST2 cells were maintained in RPMI 1640 containing 10% FBS and C2C12 cells in DME/F12 medium supplemented with 15% FBS. To induce osteoblastic differentiation, cells were grown to confluency and treated with 300 ng/ml of BMP-2.16,28
ATDC5 cells were maintained in DME/F12 medium supplemented with 5% FBS and 10 μg/ml bovine insulin, 10 μg/ml human transferrin, and 30 nmol/L sodium selenite. ATDC5 cells expressing a high level of WISP-1 (ATDC5/WISP-1H) or a lower level of WISP-1 (ATDC5/WISP-1L) were generated by co-transfecting human WISP-1 in a pRK vector with pSVi puromycin plasmid using Fugene6 according to the manufacturer’s instructions (Roche Diagnostics). After 48 hours, cells were selected in media containing 2 μg/ml of puromycin. After 2 weeks, clones were isolated and WISP-1 expression was evaluated by immunofluorescence. Control cell lines were generated using the same procedure after the transfection of the empty pRK vector. Chondrocytic differentiation was induced by treating ATDC5 cells with BMP-2 or GDF-5 as previously described.29 We measured ATDC5 cell proliferation by seeding 104 cells in 10-cm2 Petri dishes in culture media supplemented with 0.5% FBS. At indicated time points, we counted the viable cells using a hemacytometer after trypsinization.
Immunoprecipitation and Western Blot Analysis
Stably transfected ATDC5 cells (2 × 106) were cultured overnight in 4 ml of 1:1 Ham’s F-12:Dulbecco’s modified Eagle’s medium. A specific monoclonal antibody9 was used to immunoprecipitate WISP-1 from culture media and lysates using a previously described protocol.30 The immunoprecipitate was electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA) and electrotransferred to a polyvinylidene difluoride membrane (Bio-Rad). WISP-1 was immunodetected with a biotinylated monoclonal antibody and visualized with the West Femto chemiluminescent substrate (Pierce, Rockford, IL). An equivalent of 0.5 × 106 cells/lane and 0.2 × 106 cells/lane were analyzed for supernatant and cell lysate, respectively.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction Analysis
Total RNA was extracted from cells using the RNeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Specific primers and fluorogenic probes were used to amplify and quantitate gene expression (sequences available on request).31 The gene-specific signals were normalized to the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene. Triplicate sets of data were averaged for each condition. All TaqMan reverse transcriptase-polymerase chain reaction reagents were purchased from Applied Biosystems (Foster City, CA).
Alkaline Phosphatase Assay
Cells were washed twice with PBS and lysed in 20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, and 1% Triton X-100 for 5 minutes on ice. Twenty μl of the lysate were added to 80 μl of Attophos substrate (Roche) and incubated for 5 minutes at room temperature. The fluorescence was measured (excitation, 420 nm; emission, 560 nm) and the alkaline phosphatase activity was determined by comparison to a standard curve of enzymatic product. Cell lysates were analyzed for protein content using the micro-BCA Assay kit (Pierce) and alkaline phosphatase activity was normalized for total protein concentration.
Mouse Femoral Fracture Healing Model
A midshaft, fixed femur fracture was created in anesthetized 6- to 8-week-old male C57BL6 mice (Charles River Laboratories, Wilmington, MA) following a previously described procedure.32 All animal experimentation was conducted in accordance with national guidelines.
Results
Tissue Distribution of WISP-1
We performed in situ hybridization to elucidate the spatiotemporal profile of WISP-1 mRNA expression during embryonic skeletogenesis. At E10.5, before ossification begins, WISP-1 was weakly expressed in circumscribing precartilaginous condensations of developing endochondral bones (data not shown). As skeletal development progresses, WISP-1 expression increased in the mesenchymal cell layer surrounding the cartilage anlagen and subsequently in osteoblasts lining bones undergoing endochondral ossification (Figure 1, A to D). As early as E12.5, WISP-1 mRNA was expressed in regions of mesenchymal condensations comprising flat bones of the skull destined to undergo intramembranous ossification (Figure 1A). At this stage, some expression was also found in the myocardium and subcutaneous mesenchyme (data not shown). At E15.5, WISP-1 expression was high in osteoblasts and associated periosteal cells of vertebrae, ribs, and along the diaphysis forming the cortex of the long bone after ossification has begun (Figure 1, B and C). Notably, at E15.5, WISP-1 expression was more prominent at sites of intramembranous ossification (Figure 1D). The signal was predominantly in osteoblasts and periosteal cells of the developing calvarium and maxilla. WISP-1 was low or undetectable in chondrocytes and other cells surrounding osteogenic cells.
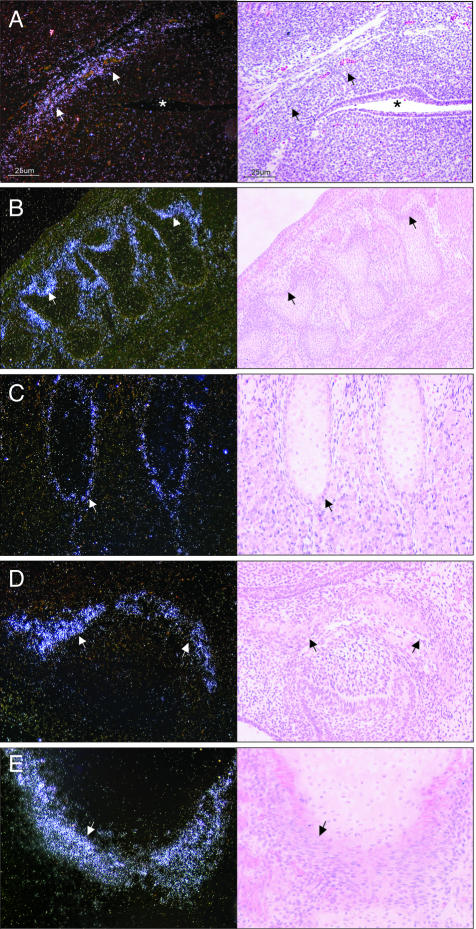
Figure 1.
In situ hybridization of WISP-1 expression during mouse development. Left: Dark-field images; right: corresponding bright-field images. A: Base of the skull dorsal of the oropharynx (*) at E12.5. At E15.5, WISP-1 is expressed in osteoblasts and mesenchymal cells adjacent to bones undergoing endochondral ossification (B, vertebras; C, ribs) and intramembranous ossification (D, ossification within palatal shelf of maxilla). WISP-1 expression was similarly distributed in human embryo lower limb (E, lateral border of head of tibia). Original magnifications: ×100 (A, D); ×40 (B); ×200 (C, E).
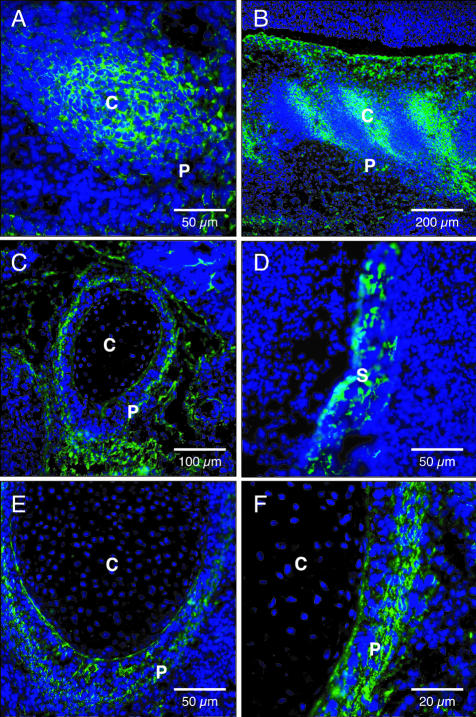
The presence of WISP-1 protein at sites of developing bone was assessed by immunofluorescence in E18 rat embryos. We observed an intense fluorescent staining pattern that closely matched the in situ hybridization expression profile (Figure 2). WISP-1 protein was found in osteoblasts at all sites of endochondral and intramembranous ossification. The staining was intense in osteoblasts lining the developing calvaria, mandible, clavicle, vertebrae, and ribs. No staining was observed in the perichondrium or chondroblasts.
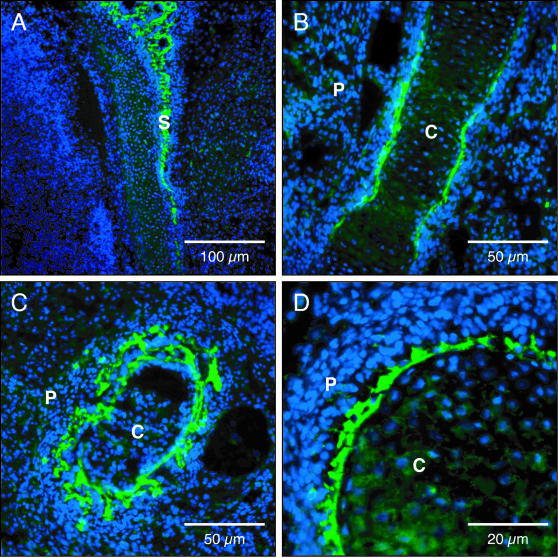
Figure 2.
Immunofluorescent localization of WISP-1 in rat embryo E18. Differentiating osteoblasts lining the calvaria (A), femur (B), and ribs (C, D). S, skull; P, periosteum; C, cartilage primordium. Original magnifications: ×100 (A); ×200 (B, C); ×400 (D).
WISP-1 Is Expressed by Differentiating Osteoblasts
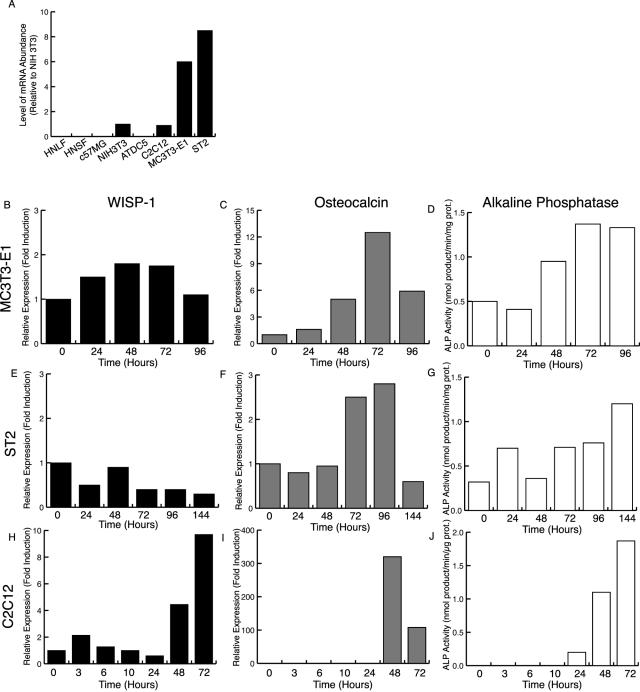
WISP-1 expression was measured in various cell types (Figure 3A). No WISP-1 expression was detected in primary human normal lung and skin fibroblasts, C57MG mammary epithelial cells, and ATDC5 chondrogenic cells. A comparable level of WISP-1 expression was found in NIH3T3 fibroblasts and C2C12 myoblasts. On the other hand, the level of WISP-1 expression was sixfold to ninefold higher in MC3T3-E1 calvaria preosteoblasts and ST2 osteoblastic bone marrow stromal cells when compared to NIH3T3 fibroblasts.
Figure 3.
WISP-1 is induced in differentiating osteoblasts. A: WISP-1 expression in different cell types. WISP-1 (B, E, H) and osteocalcin expression (C, F, I) and alkaline phosphatase activity (D, G, J) in MC3T3-E1 cells after ascorbic acid treatment (B–D), in ST2 cells after BMP-2 treatment (E–G), and in C2C12 cells after BMP-2 treatment (H–J).
We monitored WISP-1 expression during osteoblast differentiation using the MC3T3-E1 and ST2 osteogenic cell lines.16,27 When placed in differentiating medium, these cells progressively adopted an osteoblast phenotype as demonstrated by their increase in osteocalcin expression and alkaline phosphatase activity (Figure 3). In these cells, the level of WISP-1 expression did not change during the osteoblastic differentiation and remained elevated at all times. Because WISP-1 is expressed in preosteoblastic cells, it could represent an early event that precedes the commitment of MC3T3-E1 and ST2 cells to the osteoblastic lineage. To test this possibility, we measured WISP-1 expression in an osteoblastic transdifferentiation model using the C2C12 myoblasts.28 WISP-1 expression rapidly increased on BMP-2 induction of the osteogenic transdifferentiation (Figure 3H). WISP-1 expression was also induced by the pro-osteoblastic factors BMP-3, BMP-4, and BMP-6 but not by IGF-1, a pro-myoblastic factor for C2C12 cells (data not shown).33–35 These results suggest that WISP-1 is predominantly expressed by cells of the osteoblastic lineage and that its induction occurs early during the acquisition of this phenotype.
WISP-1 Promotes Osteoblast Differentiation
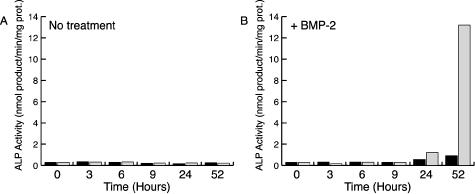
Because WISP-1 is induced during osteoblastic differentiation we evaluated its participation in this process. Although WISP-1 overexpression was not sufficient to trigger C2C12 cell osteoblastic differentiation, it greatly potentiated BMP-2 pro-osteoblastic activity (Figure 4). When treated with BMP-2, WISP-1-transfected cells demonstrated a 13- to 14-fold increase in alkaline phosphatase activity compared to cells transfected with a vector control (Figure 4). WISP-1 potentiation of pro-osteoblastic factors could promote lineage determination by facilitating the osteoblastic differentiation of progenitor cells.
Figure 4.
WISP-1 promotes BMP-2-induced osteoblastic differentiation. C2C12 cells were transiently transfected with an empty vector (black bars) or WISP-1 expression construct (gray bars). Forty-eight hours after transfection, the culture media was replaced by media containing 5% FBS (A) or media containing 5% FBS and 300 ng/ml of BMP-2 (B) and alkaline phosphatase activity was measured at the indicated time.
WISP-1 Binds to the Perichondrium
To better understand the role of WISP-1 in skeletal development we analyzed its in situ binding to sagittal sections of rat embryo. At embryonic stage E14, WISP-1 interacted with the perichondrial mesenchyme and the condensing prechondroblastic cells of cartilage primordium (Figure 5). At stage E18, WISP-1 bound only to mesenchymal cells of the perichondrium. No fluorescence was associated with chondroblasts or chondrocytes. No signal was detected with an unrelated antibody or when WISP-1 was omitted or replaced by a control protein.
Figure 5.
In situ WISP-1 binding in rat embryo. At E14, WISP-1 binding revealed an intense fluorescent signal associated with costal (A) and vertebral (B) condensed mesenchymal cells. At E18, WISP-1 bound to osteoblasts and perichondral mesenchyme of developing bones; mesenchyme surrounding cartilage primordium of rib (C), calvaria (D), mesenchyme surrounding cartilage primordium of distal part of radius (E, F). P, perichondrium; C, cartilage primordium; S, skull. Original magnifications: ×200 (A, D, E); ×40 (B); ×100 (C); ×400 (F).
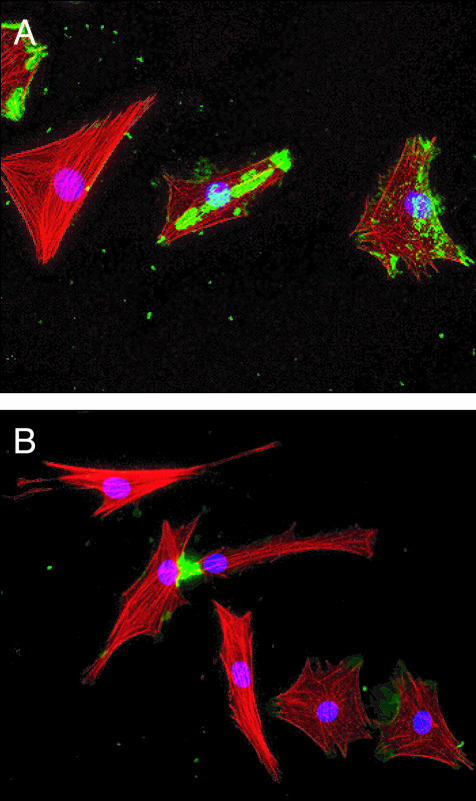
The interaction of WISP-1 with mesenchymal cells was evaluated using primary porcine chondrocytes that had adopted a mesenchymal phenotype after 11 days in culture. WISP-1 binding revealed an irregular pattern associated with patches and points of focal adhesion (Figure 6A). We observed intense fluorescent staining at points of contact between adjacent cells (Figure 6B). WISP-1 interaction with mesenchymal cells could be involved in cell-cell communication.
Figure 6.
WISP-1 binding to dedifferentiated chondrocytes. A: The binding of WISP-1 (green) to dedifferentiated primary porcine chondrocytes showed an irregular pattern associated with patches and point of focal adhesion. B: Intense staining was found at the point of contact of adjacent cells. Red, actin filament staining; blue, nuclear staining. Original magnifications, ×200.
WISP-1 Acts on Chondrocytic Cells
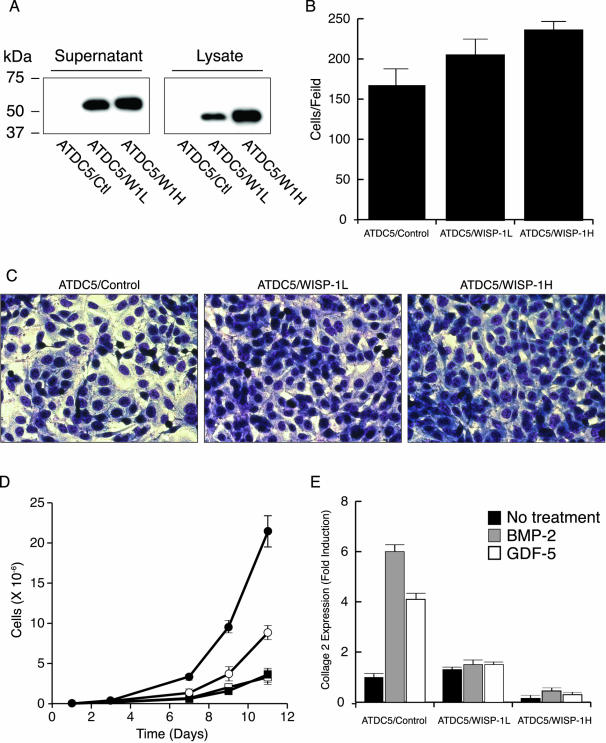
The WISP-1 binding pattern suggested that the protein might act on differentiating chondrocytes. We investigated this possibility by generating ATDC5 chondrogenic cell lines stably transfected with WISP-1. A cell line expressing a high level of WISP-1 (ATDC5/WISP-1H), a cell line expressing a low level of WISP-1 (ATDC5/WISP-1L), and a cell line transfected with an empty vector (ATDC5/control) were analyzed. Compared to ATDC5/WISP-1L cells, ATDC5/WISP-1H cells had a WISP-1 mRNA level 2.0 ± 0.7-fold higher (data not shown) and a protein level twofold higher (Figure 7A). When grown to confluency the WISP-1-expressing cell lines demonstrated a significant increase in density compared to the control cell line (Figure 7C). The saturation density of ATDC5/WISP-1H cell line increased by 1.4 ± 0.1-fold (t = 0.0075) and the ATDC5/WISP-1L by 1.2 ± 0.1-fold (t = 0.45) compared to the ATDC5/control cell line (Figure 7B). No significant differences were found between the density of the ATDC5/control cell line and the parental cell line at confluency (data not shown). The WISP-1 transfectants also demonstrated an increased proliferation compared to the ATDC5/control and the parental cell line. After 11 days, the ATDC5/WISP-1H and the ATDC5/WISP-1L cell population increased by 6- and 2.5-fold, respectively, compared to the ATDC5/control cell line (Figure 7D). The growth rate of the ATDC5/control cell line and the parental cell line were identical.
Figure 7.
WISP-1 represses chondrogenic differentiation of ATDC5 cells. A: Western blot of WISP-1 produced by the ATDC5/control, ATDC5/WISP-1L, and ATDC5/WISP-1H cell lines. Saturation density (B) and photomicrograph (C) of ATDC5 cell lines grown to confluency. D: Proliferation of ATDC5 (open squares), ATDC5/control (filled squares), ATDC5/WISP-1L (open circles), and ATDC5/WISP-1H cells (filled circles). E: Relative expression of collagen 2 in ATDC5/control, ATDC5/WISP-1L, and ATDC5/WISP-1H cells before (black bars) and after inducing chondrocytic differentiation by BMP-2 (gray bars) or GDF-5 (white bars).
We assessed the differentiation state of the ATDC5 cell lines by evaluating their collagen 2 expression level. Before chondrocytic differentiation was induced, the level of collagen 2 expression was comparable in ATDC5/control and ATDC5/WISP-1L cells but was 10-fold lower in the ATDC5/WISP-1H cells compared to the control cell line (Figure 7E). The induction of chondrocytic differentiation by BMP-2 or GDF-5, significantly increased collagen 2 expression in ATDC5/control cells. On the other hand, collagen 2 induction was greatly diminished in ATDC5/WISP-1L cells and nearly abolished in ATDC5/WISP-1H cells. These results indicate that WISP-1 increases prechondrogenic cell proliferation and saturation density and it prevents the progression of these cells along the chondrocytic lineage.
WISP-1 Expression Is Induced During Bone Fracture Repair
Because signals regulating embryonic bone formation are recapitulated during fracture repair, we evaluated WISP-1 temporal expression in a mouse model of bone fracture healing.36 WISP-1 signal was prominent at days 5 through 14 after fracture and gradually decreased until day 21 (Figure 8).
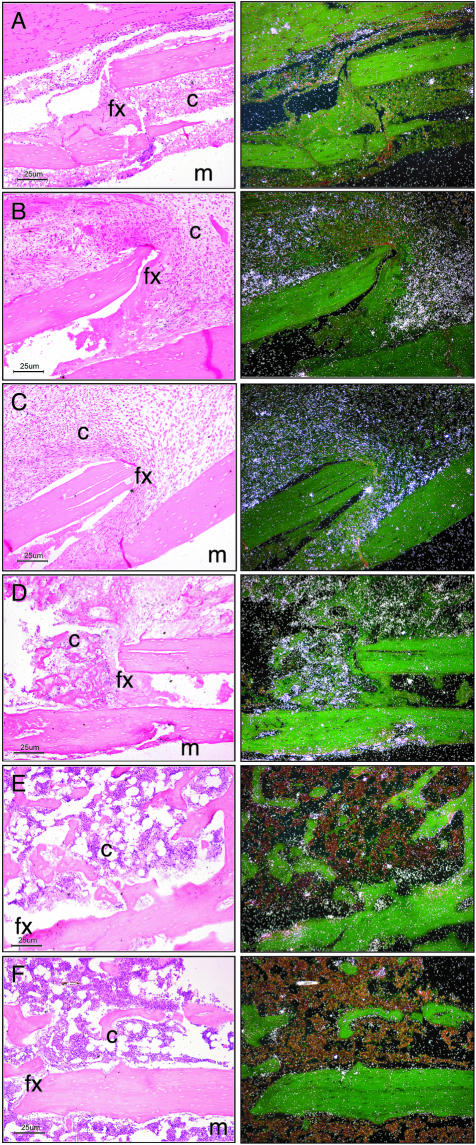
Figure 8.
In situ hybridization of WISP-1 expression during fracture repair. Left: Bright-field images; right: corresponding dark-field images. Photomicrographs showing the localization of WISP-1 expression at days 3 (A), 5 (B), 7 (C), 14 (D), 21 (E), and 28 (F) after fracture. Each image is oriented with the medullary cavity (m) at the bottom right; the cortex, fracture (fx), and callus (c) occupy the majority of the photomicrograph. Original magnifications, ×100.
At day 5 after fracture, WISP-1 was found in mesenchymal cells within the provisional callus formed along the periosteal surface. Weak expression was also observed in osteoblastic cells lining the periosteum adjacent to the fracture site. At day 7, the osteoblasts along the islands of woven bone within the provisional callus were expressing WISP-1. At day 14 after fracture, WISP-1 expression was strongest over osteoblasts aggregated along bone spicules bridging islands of woven bone within the hard callus. By day 21, WISP-1 signal was absent from the remodeled bony callus. WISP-1 temporal expression pattern implies a role in early fracture repair that would mirror its function during bone development.
Discussion
Skeletogenesis involves the commitment of mesenchymal progenitor cells to chondrogenic and osteogenic lineages and their terminal differentiation in chondrocytes or osteoblasts.20,21 Factors involved in the differentiation process are present in the committed progenitor cells of the appropriate lineage before terminal differentiation has taken place. During mouse development, WISP-1 expression was initiated at day 10.5 in pluripotent mesenchymal cells surrounding the cartilaginous skeletal templates. WISP-1 expression progressively increased during the mesenchymal condensation of the developing skull and appendicular skeleton and reached a maximum in newly differentiated osteoblasts. By day 15.5, WISP-1 was located in all osteoblasts regardless of their future mode of ossification. Although WISP-1 is expressed early during development, it was not found in mesenchymal cell aggregates that will later differentiate into chondrocytes through the endochondral process. WISP-1 expression was restricted to mesenchymal cells and cells of the osteoblastic lineage at sites of endochondral and intramembranous ossification. Using the C2C12 myoblast cell line, we further confirmed that WISP-1 expression gradually increased in cells induced to transdifferentiate along the osteoblastic lineage. Although WISP-1 overexpression did not modify the phenotype of these cells, it potentiated their BMP-2-promoted osteoblastic differentiation. Therefore, in lineage-specific progenitor cells, early WISP-1 induction by pro-osteoblastic factors could promote their progression along the osteoblastic pathway.
We performed in situ ligand binding and identified the potential site of WISP-1 action as the perichondral mesenchyme of developing bones. We confirmed WISP-1 interaction with mesenchymal cells using cultured dedifferentiated primary chondrocytes. WISP-1 is a glycosaminoglycan binding protein that interacts with cell surface and extracellular matrix-associated proteoglycan of mesenchymal cells.9 The staining pattern of WISP-1 binding at the surface of dedifferentiated chondrocytes differs from the pattern previously observed with human skin fibroblasts.9 Because WISP-1-binding pattern differences were previously reported for normal rat kidney fibroblasts and human skin fibroblasts, the cellular origin, the species, and phenotype could be responsible for these variations. WISP-1 specifically interacts with decorin and biglycan, two small leucine-rich secreted dermatan sulfate proteoglycans abundantly found in bone and cartilage.9,37–43 Decorin and biglycan are attached to the extracellular matrix through their interaction with collagen I, collagen II, collagen XIV, and fibronectin.44–47 The importance of decorin and biglycan in osteogenesis has been confirmed in knockout mouse models and human diseases.48–50 Biglycan and decorin distribution at sites of bone formation is consistent with WISP-1 in situ binding pattern.38–40 Consequently, WISP-1 is most likely bound to the surface of mesenchymal cells of the perichondrium through its interaction with these small leucine-rich proteoglycans.
The prominent structural similarities to extracellular components suggest that CCN proteins, including WISP-1, resemble the functionally diverse matricellular proteins, which are also characterized by a mosaic of matrix protein domains.51–54 Matricellular proteins have the ability to interact with multiple cell-surface receptors, cytokines, growth factors, proteases, and structural proteins.52,55–58 CCN protein interaction with multiple components of the extracellular matrix and the proteoglycan would therefore limit their diffusion. Consistent with this notion, WISP-1, CTGF, CYR61, and NOV were shown to remain associated with the cell surface after secretion.7,59–61 In vivo, WISP-1 is likely to be associated with the extracellular matrix-attached decorin and biglycan in the vicinity of secreting mesenchymal cells of the osteoblastic lineage. This specific interaction would modulate WISP-1 diffusion range, availability, and activity.
The importance of intercellular communication mediated by extracellular matrix proteins during limb development has been demonstrated.62 The concept of a growth factor and cytokine depot has been suggested for the proteoglycans.43 Consequently, WISP-1 tethered to the extracellular matrix could act in a paracrine manner on neighboring mesenchymal cells committed to the chondrogenic lineage.
To test this hypothesis we generated chondrocytic cell lines stably transfected with WISP-1. In these cell lines, WISP-1 increased proliferation, saturation density, and promoted the expression of genes associated with undifferentiated mesenchymal (vimentin, fibronectin; data not shown) cells while repressing genes linked to chondrocyte differentiation. In addition, it attenuated the induction of chondrocytic differentiation by added exogenous growth factors. Taken together, these results suggest that WISP-1 is a negative regulator of chondrocyte differentiation.
Chondrocyte proliferation, commitment, and differentiation depends on their local environment, autocrine, and paracrine regulation.63 Wnt genes were shown to be important paracrine regulators of chondrocyte and osteoblast differentiation during vertebrate skeletal development. Wnt-1, Wnt-5a, Wnt-7a, and Wnt-14 negatively regulate chondrogenesis whereas Wnt-4 and Wnt-8 promote chondrocyte maturation.17–19,64 Wnt signaling also promotes osteoblast differentiation and regulates bone accrual during development.65 Wnt regulatory activity requires the integrity of its pathway, suggesting that Wnt/β-catenin target genes are involved in the osteoblastic and chondrocytic differentiation of mesenchymal progenitor cells.16,17 Because WISP-1 is a Wnt/β-catenin downstream gene, it could constitute an effector of the Wnt regulatory cascade acting during skeletogenesis.3,5 The parallels found between the activity of WISP-1 and several Wnt genes on chondrocytes would support this hypothesis.19,66
During endochondral ossification, proliferation and condensation of mesenchymal cells are stopped by their differentiation into hypertrophic chondrocytes. The appropriate size and shape of the bones depends on a balance between proliferation and differentiation of mesenchymal cells forming the cartilage anlagens.67 In vitro, WISP-1 positively regulates osteoblastic differentiation while repressing chondrocytic differentiation. Because WISP-1 a secreted protein expressed by osteoblastic cells at sites of endochondral ossification during development, it could act in a paracrine manner to prevent premature completion of chondrocytic differentiation and ensure adequate morphogenesis of the skeletal structure. Alternatively, WISP-1 expressed at an early stage during osteoblastic differentiation could act through an autocrine mechanism and contribute to phenotype definition by promoting the completion of osteoblastic differentiation and preventing precursor cells from reverting to a chondrocytic lineage.
Several pathways regulating embryonic skeletal development are reactivated during bone healing.36 Moreover, the activation of the Wnt/β-catenin signaling pathway during bone healing was recently demonstrated.68 For these reasons, we analyzed WISP-1 expression patterns during fracture repair. Bone healing proceeds through three distinct phases, namely inflammation, reparation, and remodeling.69,70 The first phase begins with the activation of the inflammatory cell response and the recruitment and proliferation of mesenchymal stem cells surrounding the fracture site. During the reparation phase, endochondral and intramembranous bone synthesis takes place. Mesenchymal cells of the subperiostal bone differentiate into chondrocytes to form the fibrocartilaginous soft callus. Chondrocytes of the soft callus that progressively differentiate into hypertrophic chondrocytes are invaded by blood vessels and osteogenic cells and are ultimately replaced by bone. Also, the periosteal mesenchymal cells adjacent to the injured bone directly differentiate into osteoblasts and start the production of bone matrix to form the hard callus. The formation of primary bone is followed by extensive remodeling until the damaged skeletal element regains original shape and size. During the bone healing process, WISP-1 expression recapitulated the pattern observed during embryonic development.
Soon after bone fracture, WISP-1 was expressed in mesenchymal cells surrounding the site of injury. WISP-1 could prevent premature chondrocytic differentiation and promote growth and accumulation of mesenchymal cells at the fracture site. Similarly, CTGF, a closely related CCN family member, was suggested to participate in fibroblast recruitment, proliferation, and stimulation of extracellular matrix protein synthesis during fracture repair.71 Other factors including BMP-4, were also shown to recruit mesenchymal progenitor cells during the inflammation stage.72 Alternatively, WISP-1 could participate in mesenchymal stem cell recruitment by modulating BMP-4 activity. Vascular endothelial growth factor and noggin were shown to play such a function by, respectively, potentiating and antagonizing BMP-4 activity during bone repair.73,74 Because CTGF, was already shown to interact with BMP-4 and transforming growth factor-β and modulate their activity a similar role could be played by WISP-1.75
During the reparation stage, WISP-1 expression was limited to the osteoblasts lining the periosteum and the islands of woven bone within the provisional callus. Because bone matrix is formed at this stage, it is possible that WISP-1 plays a role in this process. By 3 weeks after fracture, the bones were reunited by hard callus and bone remodeling is taking place. No WISP-1 expression could be detected at 21 days after fracture indicating that WISP-1 is not likely implicated in the bone remodeling process.
Other members of the CCN family were found to have functions related to skeletogenesis and bone homeostasis. Cyr61 is expressed in chondrocytes of the developing limbs, ribs, vertebrae, and craniofacial elements where it promotes chondrogenic differentiation.76,77 During embryogenesis, CTGF expression is associated with condensed connective tissue and osteoblasts around bone and cartilage. It promotes chondrocyte and osteoblast proliferation and differentiation. It is also involved in bone mineralization.78,79 NOV expression is found in, chondrocytes, osteoclasts, and osteoblasts and may play a role in sustaining the growth of osteoblast-like cells.80 WISP-2 expression is localized to osteoblasts and chondrocytes where it is thought to play a role in bone turnover.81 WISP-3 mutations are responsible for progressive pseudorheumatoid dysplasia and its association with postnatal growth regulation and cartilage homeostasis has been proposed.82 Accumulating evidence for a link between the CCN family and skeletogenesis supports the involvement of WISP-1 in this process.
During bone development, the various CCN family members show either overlapping or exclusive expression patterns and reported activities for individual members are either similar or opposing. In addition, several types of receptors including integrins,83–85 low-density lipoprotein-related protein,86 and Notch87 were reported for this family. The absence of consensus suggests that a complex regulatory mechanism involving all members of this family of proteins could take place during embryogenesis to modulate the osteogenic process. We believe that WISP-1 plays an important role in this regulatory mechanism.
In conclusion, the data presented in this study demonstrate that WISP-1 is a osteogenic potentiating factor promoting mesenchymal cell proliferation while repressing chondrocytic differentiation. WISP-1 expression patterns and activity suggest an important osteogenic regulatory function of this protein during bone development and fracture repair.
Acknowledgments
We thank Kurt Schroeder and Nancy Chiang for WISP-1 monoclonal antibodies, Jessica Foster for the WISP-1-Fc protein, and Aparna Draksharapu for technical assistance.
Footnotes
Address reprint requests to Luc Desnoyers, Ph.D., Department of Molecular Oncology (M/S 42), Genentech Inc., 1 DNA Way, South San Francisco, CA 94080. E-mail: desnoyer@gene.com.
References
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Saeki H, Kinoshita J, Ohga T, Shimada M, Maehara Y. A novel variant of WISP1 lacking a von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene. 2001;20:5525–5532. doi: 10.1038/sj.onc.1204723. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Wnt genes and vertebrate development. Curr Opin Genet Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–318. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers L, Simonette RA, Vandlen RL, Fendly BM. Novel non-isotopic method for the localization of receptors in tissue sections. J Histochem Cytochem. 2001;49:1509–1518. doi: 10.1177/002215540104901204. [DOI] [PubMed] [Google Scholar]
- Cai LYJ, Starovasnik MA, Hogue DA, Hillan KJ, Mort JS, Filvaroff EH. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001;16:10–21. doi: 10.1006/cyto.2001.0939. [DOI] [PubMed] [Google Scholar]
- Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3–E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res. 1999;250:351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]
- Tice DA, Szeto W, Soloviev I, Rubinfeld B, Fong SE, Dugger DL, Winer J, Williams PM, Wieand D, Smith V, Schwall RH, Pennica D, Polakis P. Synergistic induction of tumor antigens by Wnt-1 signaling and retinoic acid revealed by gene expression profiling. J Biol Chem. 2002;277:14329–14335. doi: 10.1074/jbc.M200334200. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Street JBM, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasincic DJ, Calera MR, Farmer SR, Pilch PF. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol Cell Biol. 1996;16:5964–5973. doi: 10.1128/mcb.16.11.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Kaihara S, Bessho K, Okubo Y, Sonobe J, Komatsu Y, Miura M, Miyatake S, Nakao K, Iizuka T. Over expression of bone morphogenetic protein-3b (BMP-3b) using an adenoviral vector promote the osteoblastic differentiation in C2C12 cells and augment the bone formation induced by bone morphogenetic protein-2 (BMP-2) in rats. Life Sci. 2003;72:1683–1693. doi: 10.1016/s0024-3205(02)02477-3. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- Wilda M, Bachner D, Just W, Geerkens C, Kraus P, Vogel W, Hameister H. A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. J Bone Miner Res. 2000;15:2187–2196. doi: 10.1359/jbmr.2000.15.11.2187. [DOI] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, Kopp JB, Termine JD, Robey PG. The use of synthetic peptide antibodies and in situ hybridisation for investigating expression and localization of small proteoglycans of developing bone (biglycan and decorin). Cohn D, Glorieux FM, Martin TJ, editors. Amsterdam: Elsevier,; Calcium Regulating Hormones and Bone Metabolism. 1990:pp 201–206. [Google Scholar]
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Fisher LW. Biglycan and decorin in intact developing tissues: the in situ approach to their role in development, morphogenesis and tissue organization. Scott JE, editor. London: Portland Press,; Dermatan Sulfate ProtoglycansChemistry, Biology, Chemical Pathology. 1993:pp 193–205. [Google Scholar]
- Fisher LW, Termine JD, Dejter SW, Jr, Whitson SW, Yanagishita M, Kimura JH, Hascall VC, Kleinman HK, Hassell JR, Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983;258:6588–6594. [PubMed] [Google Scholar]
- Rosenberg LC, Choi HU, Tang LH, Johnson TL, Pal S, Webber C, Reiner A, Poole AR. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985;260:6304–6313. [PubMed] [Google Scholar]
- Iozzo RV, editor. New York: Marcel Dekker, Inc.,; ProteoglycansStructure, Biology and Molecular Interactions. 2000:pp 1–4. [Google Scholar]
- Kresse H, Liszio C, Schonherr E, Fisher LW. Critical role of glutamate in a central leucine-rich repeat of decorin for interaction with type I collagen. J Biol Chem. 1997;272:18404–18410. doi: 10.1074/jbc.272.29.18404. [DOI] [PubMed] [Google Scholar]
- Font B, Aubert-Foucher E, Goldschmidt D, Eichenberger D, van der Rest M. Binding of collagen XIV with the dermatan sulfate side chain of decorin. J Biol Chem. 1993;268:25015–25018. [PubMed] [Google Scholar]
- Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Winnemoller M, Schmidt G, Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol. 1991;54:10–17. [PubMed] [Google Scholar]
- Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis. 2002;5:153–165. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. Matrix Biol. 2000;19:555–556. doi: 10.1016/s0945-053x(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000;19:557–568. doi: 10.1016/s0945-053x(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Perbal B, Martinerie C, Sainson R, Werner M, He B, Roizman B. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci USA. 1999;96:869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- Steffen CL, Ball-Mirth DK, Harding PA, Bhattacharyya N, Pillai S, Brigstock DR. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors. 1998;15:199–213. doi: 10.3109/08977199809002117. [DOI] [PubMed] [Google Scholar]
- Lonai P. Epithelial mesenchymal interactions, the ECM and limb development. J Anat. 2003;202:43–50. doi: 10.1046/j.1469-7580.2003.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto R, Campanile G, Cancedda R, Dozin B. Modulation of commitment, proliferation, and differentiation of chondrogenic cells in defined culture medium. Endocrinology. 1997;138:4966–4976. doi: 10.1210/endo.138.11.5522. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251:142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. Correlation of expression of Wnt-1 in developing limbs with abnormalities in growth and skeletal patterning. Nature. 1993;362:546–549. doi: 10.1038/362546a0. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- Sandberg MM, Aro HT, Vuorio EI. Gene expression during bone repair. Clin Orthop. 1993:292–312. [PubMed] [Google Scholar]
- Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200:165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Nakata E, Nakanishi T, Kawai A, Asaumi K, Yamaai T, Asano M, Nishida T, Mitani S, Inoue H, Takigawa M. Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24) during fracture healing. Bone. 2002;31:441–447. doi: 10.1016/s8756-3282(02)00846-3. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- Takayama T, Miyairi T, Miwa T, Nagata N, Yoshimura Y. Application of the warm heart surgery to the open heart surgery in children. Nippon Kyobu Geka Gakkai Zasshi. 1994;42:874–878. [PubMed] [Google Scholar]
- Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TP, Lau LF. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 1992;3:645–654. [PubMed] [Google Scholar]
- Wong M, Kireeva ML, Kolesnikova TV, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev Biol. 1997;192:492–508. doi: 10.1006/dbio.1997.8766. [DOI] [PubMed] [Google Scholar]
- Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312:175–188. doi: 10.1007/s00441-003-0712-6. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Manara MC, Perbal B, Benini S, Strammiello R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami J, Yeger H, Picci P, Scotlandi K. The expression of ccn3(nov) gene in musculoskeletal tumors. Am J Pathol. 2002;160:849–859. doi: 10.1016/S0002-9440(10)64908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hand AT, Connor JR, Dodds RA, Ryan PJ, Trill JJ, Fisher SM, Nuttall ME, Lipshutz DB, Zou C, Hwang SM, Votta BJ, James IE, Rieman DJ, Gowen M, Lee JC. Identification and cloning of a connective tissue growth factor-like cDNA from human osteoblasts encoding a novel regulator of osteoblast functions. J Biol Chem. 1999;274:17123–17131. doi: 10.1074/jbc.274.24.17123. [DOI] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV, Rezai-Delui H, Legius E, Le Merrer M, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23:94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin {alpha}6{beta}1 binding site in the angiogenic inducer CCN1 (CYR61). J Biol Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, III, Carmichael DF. The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem. 2001;276:40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]