Abstract
We reported that high-molecular weight kininogen is proangiogenic by releasing bradykinin and that a monoclonal antibody to high-molecular weight kininogen, C11C1, blocked its binding to endothelial cells. We now test if this antibody can prevent arthritis and systemic inflammation in a Lewis rat model. We studied 32 animals for 16 days. Group I (negative control) received saline intraperitoneally. Group II (disease-treated) received peptidoglycan-polysaccharide simultaneously with C11C1. Group III (disease-untreated) received peptidoglycan-polysaccharide simultaneously with isotype-matched mouse IgG. Group IV (disease-free-treated) and group V (disease-free isotype-treated) received saline and C11C1 or mouse IgG. Analysis of joint diameter changes showed a decrease in the C11C1 disease-treated group compared to the disease-untreated group. The hind paw inflammatory score showed a decrease in the intensity and extent of inflammation between the disease-untreated and the C11C1 disease-treated group. Prekallikrein, high-molecular weight kininogen, factor XI, and factor XII were decreased in the disease-untreated group compared to the C11C1 disease-treated group. T-kininogen was increased in the disease-untreated group when compared with the C11C1 disease-treated group. Disease-free groups IV and V did not show any sign of inflammation at any time. This study shows that monoclonal antibody C11C1 attenuates plasma kallikrein-kinin system activation, local and systemic inflammation, indicating therapeutic potential in reactive arthritis.
Systemic inflammatory response syndrome is accompanied by activation of the plasma kallikrein-kinin system (KKS) and decrease of its component plasma proteins.1–3 Similar changes have been documented in clinical sepsis and hereditary angioedema.4–6
The KKS plays an important role in the pathophysiology of inflammatory events involved in cellular injury, including fibrinolysis, kinin formation, complement activation, cytokine secretion, and release of proteases. The KKS in humans and rats consist of four major proteins: factor XII (FXII), factor XI (FXI), prekallikrein (PK), and high-molecular weight kininogen (HK). HK complexed with PK binds to the endothelial surface where prolylcarboxypeptidase and/or activated FXII converts PK to kallikrein.7 The process is further amplified by kallikrein cleavage of FXII to form FXIIa.8
Kallikrein, in the presence of HK, stimulates neutrophil chemotaxis,9 aggregation, and oxygen consumption,10 and induces neutrophil elastase release.11 Kallikrein also primes neutrophils for superoxide production.12 Activated FXII stimulates neutrophil aggregation13 and interleukin-1 expression in monocytes.14 HK is a precursor of bradykinin (BK) and functions as a co-factor in KKS activation. Plasma kallikrein cleaves human and rat HK in a two-step pattern. The first and second cleavage yields a heavy chain (64 kd) joined by a single disulfide bond to a light chain (56 kd) and release of BK. The third cleavage occurs later and proteolyzes the 56-kd light chain to a 45-kd light chain and this two-chain molecule has been termed HKa. BK stimulates intestinal inflammation,15 mediates intestinal secretion,16 releases prostaglandins17,18 and nitric oxide, enhances microvascular flow and permeability,18 and thus is proinflammatory. After the cleavage of BK from HK, the resulting active co-factor, HKa, undergoes dramatic conformational changes as detected by electron microscopy.19 As a result of domain rearrangement, HKa acquires the ability to bind to anionic-charged surfaces20,21 and cell receptors.22,23
Direct involvement of the KKS in the pathogenesis of experimental acute arthritis24 and acute and chronic enterocolitis25,26 has been documented by previous studies from our laboratory. Our laboratory recently discovered a genetic difference in kininogen structure between resistant Buffalo and Fischer F344 inbred rats and the susceptible Lewis rat, that results in accelerated cleavage of HK in the latter.27 Genetically susceptible Lewis rats develop an acute arthropathy followed by chronic, progressive, destructive arthritis, anemia, and granulomatous hepatitis after a single intraperitoneal injection of the streptococcal cell wall components, peptidoglycan-polysaccharide (PG-PS) polymers.28–30 The KKS is an important contributor to the pathogenesis of this experimental model.24–26 Plasma PK levels declined in both the acute and chronic phases.25,31 HK, whose decline parallels BK release, also decreased throughout time in parallel with the inflammation. In fact, HK concentrations were inversely related to the degree of joint inflammation.31 Plasma T-kininogen is an acute-phase protein unique to rats and is another indicator of the inflammatory response in these animals.32,33
Seronegative inflammatory arthropathies are a group of chronic systemic inflammatory autoimmune disorders that affect 1% of the adult population.34 These diseases are thought to occur as an abnormal immunological response to an unidentified antigen(s) that may be bacterial in origin. The characteristic features include a persistent inflammatory synovitis involving peripheral joints.35 A symmetric distribution is the hallmark of the disease. Although the precise relationship to human disease is not known, the clinical, pathological, radiological, and immunological features of streptococcal cell wall-induced arthritis36,37 resemble that seen in human reactive arthritis. Furthermore, the disease severity remits and relapses in rats30 similar to the course of disease progression in patients.
The objective of this study is to examine the effects of a monoclonal antibody (mAb) targeted to HK on KKS and the local and systemic inflammation in a rodent model of reactive arthritis in the Lewis rat. We found that mAb C11C1 prevented the arthritis (joint swelling) throughout the entire course of the disease, decreased the intensity and extension of inflammation, attenuated KKS activation, and decreased systemic inflammation.
Materials and Methods
PG-PS Preparation
Purified, sterile PG-PS polymers from the cell walls of group A, type 3, strain D58 streptococcus pyogenes were purchased from Lee Laboratories (Grayson, GA) and prepared as previously described.38 The cell wall fragments were sonicated immediately before injection to disperse aggregates.
Purification of mAb C11C1
C11C1 is a murine mAb directed to an epitope on the unique 46-kd light chain of human HK previously identified in our laboratory.39 The epitope was mapped to human domain 5 of HK (H441-K502) by proteolytic digestion, immunoaffinity chromatography, and N-terminal sequencing.20 C11C1 inhibits the in vitro coagulation activity of human HK.39 This mAb reacts poorly with kininogens in plasma using the Western blot technique but binds using surface plasmon resonance.40 mAb C11C1 (IgG1κ) was produced in tissue culture supernatant. The IgG fraction was isolated by protein A affinity chromatography (Pro-Chem Inc., Acton, MA) according to the manufacturer’s recommendations. The antibody was a single band of 160 kd on nonreduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis and two bands of 55 and 25 kd in the presence of reducing conditions. The IgG fraction was dialyzed against 0.01 mol/L HEPES and 0.15 mol/L NaCl, pH 7.4, for 20 hours at 4°C. The levels of endotoxin present in purified C11C1 were determined using a chromogenic Limulus amebocyte lysate test (QCL-100; Bio-Whittaker, Walkersville, MD). The amount of endotoxin present was below the lowest detection levels (0.02 ng/ml). 1 EU/ml equals 0.1 ng/ml of lipopolysaccharide.
Mouse IgG
Purified murine IgG, (MOPC-21, isotyped-matched to mAb C11C1, IgG1) was purchased from Sigma Chemical Co. (St. Louis, MO). MOPC-21 was used as a control for C11C1 and given in the same concentration and at the same intervals as the mAb. MOPC-21 was diluted in the same buffer as C11C1 (0.01 mol/L HEPES, 0.15 mol/L NaCl, pH 7.4).
Experimental Protocol and Treatment
A total of 32 female-specific pathogen-free Lewis rats (Charles Rivers Laboratories, Raleigh, NC), weighing 140 to 170 g, were used. All animals were housed in the Temple University Health Science Center Animal Care Facility and had free access to food and water. The protocol was approved by the Institutional Animal Care and Use Committee before implementation and conformed to National Institutes of Health guidelines. Animals were randomly separated into five different groups and studied for a period of 16 days. Group I (negative control, n = 6) received sterile 0.15 mol/L NaCl intraperitoneally. Group II (disease-treated, n = 8) received PG-PS at a single dose of 15 μg of rhamnose/g mean body weight, administered as an intraperitoneal injection on day 0 of the study, followed by intraperitoneal injections of mAb C11C1 at a dose of 0.32 mg/rat (∼2 mg/kg body weight) every other day, starting on day 0. Group III (disease-untreated group, n = 6) received a single dose of PG-PS as the previous group and intraperitoneal injections of murine IgG MOPC-21 at a dose of 0.32 mg/rat, every other day starting on day 0. Group IV (disease-free-treated, n = 6) received sterile 0.15 mol/L NaCl intraperitoneally and injections of mAb C11C1 at a dose of 0.32 mg/rat, every other day, starting on day 0. Group V (disease-free isotype-treated, n = 6) received sterile 0.15 mol/L NaCl intraperitoneally and injections of MOPC-21 at a dose of 0.32 mg/rat, every other day, starting on day 0.
Evaluation of the Arthritis
After the injection of PG-PS, rats were weighed and examined every day during the first week, and then every other day during the entire time course of the protocol. The severity of arthritis was assessed using ankle joint diameters with digital caliper (Ultra-Call Mark III, F.V. Fowler Co., Inc., Newton, MA). Body weight and the mean of triplicate measurements of each hind paw were recorded every day during the first week and then every other day during the entire course of the protocol. The joint diameter is reported as change in joint diameter in mm from the baseline at 0 days.
Rats were sacrificed with carbon dioxide narcosis on day 16. Cardiac puncture was performed and blood was collected in a final concentration of 0.38% sodium citrate. Plasma was isolated from whole blood by double centrifugation (at 3000 rpm for 10 minutes and then at 10,000 rpm for 10 minutes) in polypropylene tubes at 23°C and aliquots of the supernatant were stored at −70°C until assay. The internal organs and hind paws were removed for gross and microscopic examination.
Plasma KKS Functional Assays
PK function levels were determined by a microtiter, amidolytic assay by using a chromogenic substrate, S-2302 (Pro-Phe-Arg-p-nitroanilide; Chromogenix, Moindal, Sweden) as described in our laboratory.41 FXI was measured as previously described by our published method.42 HK coagulant activity was evaluated by our modification43,44 of an activated partial thromboplastin time test assay.45 FXII coagulant activity was performed by a similar method using specifically FXII-deficient plasma (George King Bio-Medical, Overland Park, KS).
Determination of T-Kininogen in Rat Plasma
Plasma T-kininogen was measured by sandwich enzyme-linked immunoabsorbent assay, as described previously.32
Histopathology
On necropsy day, liver, spleen, kidneys, and hind paws were harvested and fixed in buffered formalin (Fisher Lab., NJ). The organs were weighed after fixation and the paws were decalcified in formic acid (Fisher Scientific, Pittsburgh, PA). The tissue was embedded in paraffin and the sections stained with hematoxylin and eosin (Fisher Scientific) for microscopic examination. To determine the hind paw inflammatory score, grading was performed in paraffin sections of 4 to 6 μm using a modification of the scoring system used by Rooney and colleagues46 and Koizumi and colleagues.47 Briefly, eight histopathological features characteristic to rheumatoid arthritis were included: synovium hyperplasia, presence of inflammatory infiltrates within the synovium, neovascularity within the synovium (more than three vessels per high-power field), subsynovial fibrosis, intra-articular exudates, percentage of intra-articular space occupied by pannus, cartilage erosion, and presence of subchondral inflammation. Each feature was rated 0 to 3 summing to a maximum score of 24 points. Pictures taken are shown at ×200.
Immunohistochemistry
Sections from each rat were stained with goat polyclonal anti-mouse IgG antibody to assess possible mAb C11C1 deposition in paws, liver, spleen, and kidney. The avidin-biotin immunoperoxidase complex was used (Vector Laboratories, Burlingame, CA). Mouse tissue was used as positive control.
Statistical Analysis
The dependent joint diameter was analyzed as a continuous variable for all analyses. Means, SE, and number of observations were tabulated for each group and time point. The experiment used a repeated measures design with each animal evaluated at 12 time points. Statistical analysis of any other data were performed by one-way analysis of variance, Student’s t-test, and Mann-Whitney rank sum test as indicated in the figure legends.
Results
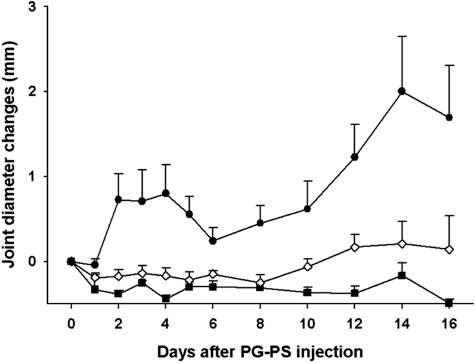
Joint Diameter Changes (Figure 1)
Figure 1.
Joint diameter changes. Comparison of the daily joint diameter changes between the disease-untreated group (filled circle; group III, PG-PS + MOPC-21), the disease-treated group (open diamond; group II, PG-PS + C11C1), and the negative control group (filled square; group I, saline + saline). Values are recorded as mean ± SEM (n = 6 to 8).
Groups I (saline-saline; negative control), IV (C11C1-treated, disease-free), and V (MOPC-21-treated, disease-free) did not develop any arthritis during the course of the study. In group III (disease-untreated) there was a biphasic increase in the joint diameter. The acute phase started on day 2 and peaked on day 4 (0.8 ± 0.3 mm). Joint swelling improved by day 6, with recurrence (chronic phase) on day 8 and peaking on day 14 (2.0 ± 0.6 mm). As expected, on necropsy (day 16) the changes in joint diameter were also significantly increased (1.7 ± 0.6 mm). Group II (C11C1, disease-treated) did not develop any significant paw swelling during the acute phase (days 2 to 4). Although there were mild changes occurring during the chronic phase (days 12 to 16) compared to group I (saline-saline; negative control), differences between group II (C11C1, disease-treated) and group I (saline-saline, negative control) were not statistically significant at any time during the course of the study. Comparing the differences between group II (C11C1, disease-treated) and group III (disease-untreated), there was significant improvement in group II (C11C1, disease-treated) in the acute phase (day 4, P = 0.001), in the chronic phase (day 14, P = 0.004), and on termination (day 16, P = <0.02). These data indicate that mAb C11C1 prevented the joint swelling (arthritis) throughout the acute phase of the disease and greatly ameliorated the chronic phase in this animal model of arthritis.
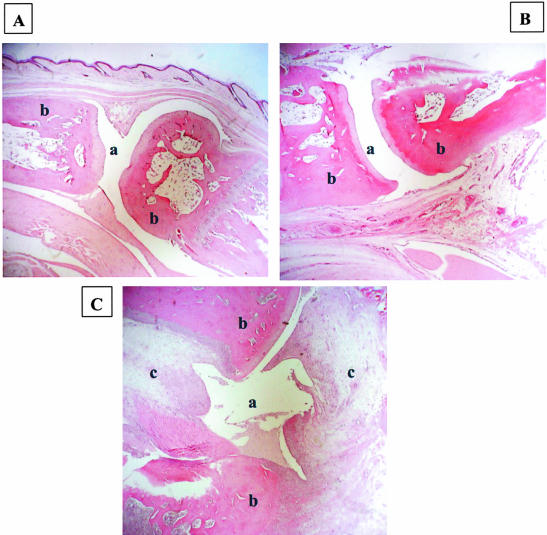
Hind Paw Histology Assessment (Figure 2)
Figure 2.
Hind paw histology. A: Negative control (group I, saline + saline). B: Disease-treated (group II, PG-PS + C11C1). C: Disease-untreated (group III, PG-PS + MOPC-21). a, joint space; b, bone; c, pannus within joint space. Original magnifications, ×200.
Groups I (saline-saline, negative control), IV (C11C1-treated, disease-free), and V (MOPC-21-treated, disease-free) did not show any inflammatory changes. A representative section from group I (negative control) is shown in Figure 2A. Group II (C11C1, disease-treated) (Figure 2B) showed sparse chronic inflammatory infiltrates with minimal thickening of the synovial lining. Group III (disease-untreated) (Figure 2C) showed diffuse inflammatory infiltrates with severe synovial thickening, pannus formation, cartilage destruction, and bone erosion. When comparing group II (C11C1, disease-treated) with group III (disease-untreated), there is marked attenuation of the joint inflammatory process in the animals treated with mAb C11C1.
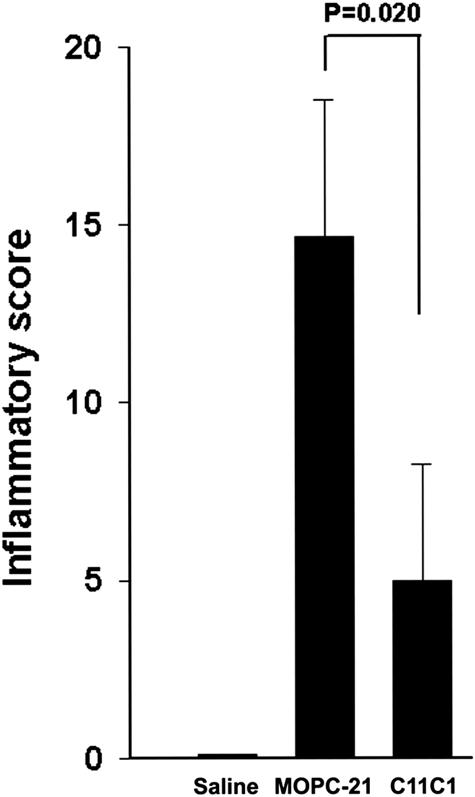
Hind Paw Inflammatory Score (Figure 3)
Figure 3.
Hind paw inflammatory score. Comparison of the disease-untreated group (group III, PG-PS + MOPC-21), the disease-treated group (group II, PG-PS + C11C1), and the negative control group (group I, saline + saline). Results are plotted as mean ± SEM (n = 6 to 8). Because of the skewed results, we applied the Mann-Whitney rank sum test for statistical analysis.
Groups I (saline-saline, negative control), IV (C11C1-treated, disease-free), and V (MOPC-21-treated, disease-free) had an inflammatory score of zero (no inflammation present). Group II (C11C1, disease-treated) had a skewed inflammatory score with a mean value of 5.0 ± 3.3 whereas group III (disease-untreated) had a skewed inflammatory score with a mean value of 14.7 ± 3.9 (Mann-Whitney rank sum test, P = 0.020). In summary, treatment with mAb C11C1 significantly decreased the intensity and extension of the joint inflammation.
Histopathology of Liver, Spleen, and Kidneys (Results Not Shown)
Microscopic examinations of sections from liver, spleen, and kidneys failed to demonstrate any changes in the disease-treated (group II, C11C1) or disease-free-treated groups (groups I, IV, and V). The disease-untreated (group III, MOPC-21) showed multiple granulomata in liver and spleen sections. Kidney sections showed moderate diffuse lymphocytic infiltrates. These results indicate that C11C1 treatment decreased the intensity and extension of systemic inflammation induced by PG-PS injection.
Immunohistochemistry (Results Not Shown)
There was no detectable mouse IgG deposition in any of the tissues studied (hind paws, liver, spleen, and kidney) in all groups, indicating that the changes were not because of antibody-antigen complexes.
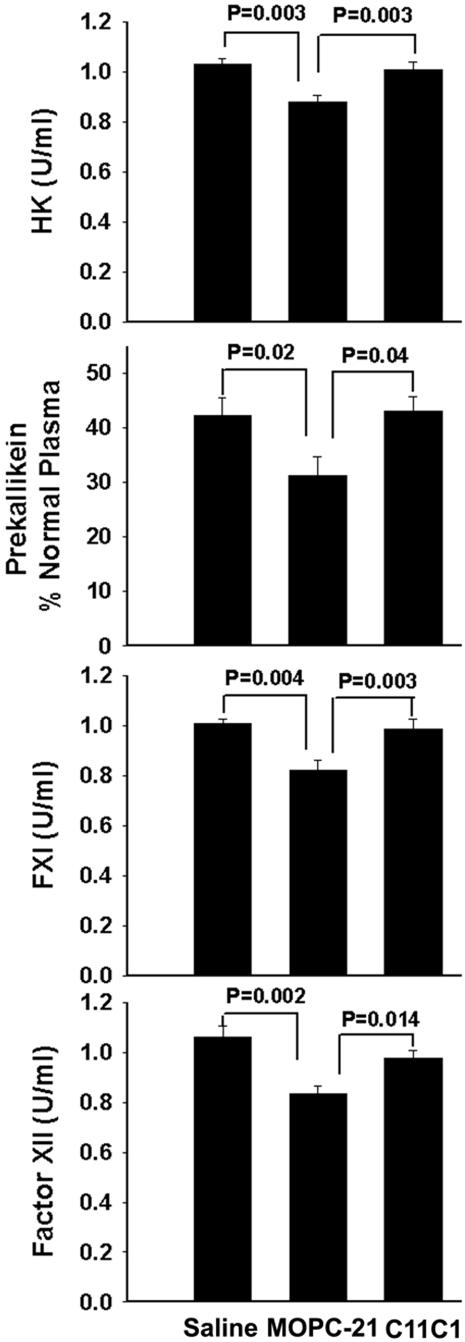
KKS Functional Assays (Figure 4)
Figure 4.
KKS functional assays. Determination of PK, HK, FXI, and FXII in the negative control group (group I, saline + saline), in the disease-treated group (group II, PG-PS + C11C1), and in the disease-untreated group (group III, PG-PS + MOPC-21). Only the disease-untreated group (group III) was significantly lower than the other two groups. Student’s t-test was applied for statistical analysis.
Evaluation of PK, HK, FXI, and FXII showed that the four proteins were not significantly different among the negative control (group I, saline), the disease-treated group (group II, C11C1), and disease-free groups (groups IV and V). PK, HK, FXI, and FXII were significantly decreased (P < 0.02) in the disease-untreated group (group III, MOPC-21) when compared with the disease-treated group (group II, C11C1) (Figure 4). This finding demonstrates that mAb C11C1 attenuates KKS activation.
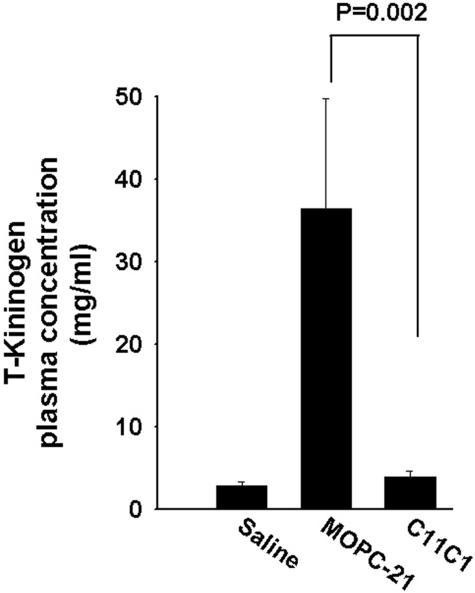
Plasma T-Kininogen Antigenic Levels (Figure 5)
Figure 5.
Plasma T-kininogen antigenic levels. Comparison of negative control, disease-treated (group II, PG-PS + C11C1), and disease-untreated (group III, PG-PS + MOPC-21) groups are shown. Only the disease-untreated group (group III) was significantly increased over the other two groups. Student-Newman-Keuls test was applied for statistical analysis.
The T-kininogen (rat acute phase reactant) was not significantly different among the negative control group (group I, saline), the disease-free groups (groups IV and V) and the disease-treated group (group II, C11C1) (P > 0.90). T-kininogen was significantly increased in the disease-untreated group (group III, MOPC-21) when compared with the disease-treated group (group II, C11C1) or the normal control (group I, saline) (P < 0.002). Systemic inflammation as measured by T-kininogen was substantially reversed in the disease-treated group.
Discussion
The results of the current study of experimental inflammatory arthritis in Lewis rats provide evidence that a mAb to HK modulates the inflammatory changes observed in this experimental model of reactive arthritis as evidenced by the significant decrease in joint diameter changes in the disease-treated group (group II, PG-PS + mAb C11C1) compared with the disease-untreated group using a sham antibody (group III, PG-PS + MOPC-21). Histological examination of the joints of the disease-untreated group demonstrated synovial thickening with diffuse inflammatory infiltrates within the synovial lining and pannus formation occupying the joint space in contrast to the disease-treated group that showed only minimal thickening of the synovial lining. The hind paw inflammatory score in the disease-untreated group was markedly elevated whereas the disease-treated group had minimal inflammation. Assays of PK, HK, FXI, and FXII showed that the four proteins were significantly decreased in the disease-untreated group, indicating KKS activation when compared with the disease-treated group. This finding demonstrates that mAb C11C1 attenuates KKS activation. Plasma T-kininogen concentrations were significantly increased in the disease-untreated group, indicating that systemic inflammation was substantially attenuated in the group treated with mAb C11C1.
Previous work in our laboratory has indicated that the KKS is activated in the Lewis rat model of arthritis and systemic inflammation after a single intraperitoneal injection of PG-APS.31 More recently, our work has led to the discovery of a genetic difference in kininogen structure between resistant Buffalo and Fischer F344 inbred rats and the susceptible Lewis rat, resulting in accelerated cleavage of HK in the latter.27 HKa then leads to increased production of inflammatory cytokines by mononuclear leukocytes.48 Many cytokines, including interleukin-1, interleukin-6, tumor necrosis factor-α, interferon-α, and interferon-β are involved in the initiation of synovitis in rheumatoid arthritis. In addition, the acute phase response involves activation of complement and subsequent neutrophil chemotaxis.49,50 These neutrophils, in turn, are found in the rheumatoid synovium and in synovial effusions.
Our laboratory has demonstrated that HK/HKa binds specifically, saturably, and reversibly to neutrophils.51 Further studies indicated that both the heavy chain (domain 3) and the light chain (domain 5) of HK were involved in the binding to neutrophil Mac-1 (αmβ2).52 Two specific mAbs, 2B5 to HK heavy chain domains 2 and 3, and C11C1 to HK light chain domain 5, inhibited by 99% and 93%, respectively, the binding of 125I-HK (8.3 nmol/L) to neutrophils.52 More recently, we demonstrated that two short sequences within domain 3 and a single amino acid sequence within domain 5 form part of all of the HK binding sites to neutrophils.53
We have previously demonstrated that a specific kallikrein inhibitor (P 8720) attenuated acute inflammatory changes and prevented arthritis and systemic complications in this PG-PS model.33 Furthermore, we have shown that B2R antagonists attenuate the inflammatory changes in the same animal model.54
The epitope of mAb C11C1 is mapped to H441-K502 of the HKa light chain.20 We recently reported that HK is proangiogenic by releasing BK and that mAb C11C1 blocks the binding of HK to endothelial cells.40,55 Because HK cleavage occurs by kallikrein on the endothelial cell surface,8 the mode of action of mAb C11C1 is by inhibiting the binding of HK to the surface. This action would decrease the cleavage of HK, the formation of HKa, and the proteolytic release of BK. Decreasing both of these mediators would inhibit the local inflammatory process in the joints and the systemic inflammation as shown in this study.
In summary, our results indicate that C11C1, a mAb to domain 5 of HK, attenuates KKS activation and local and systemic inflammation in the Lewis rat model of PG-PS-induced arthritis and systemic inflammation. HK may be an appropriate target for further drug discovery in the therapy of chronic inflammatory arthropathies.
Acknowledgments
We thank Virginia Sheaffer for editorial and administrative assistance with the text and graphics.
Footnotes
Address reprint requests to Robert W. Colman, The Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 North Broad St., Room 300 OMS, Philadelphia, PA 19140. E-mail: colmanr@temple.edu.
Supported by the National Institutes of Health (grants R01 CA-83121 and T 32 HL-07777) and the Pennsylvania Department of Health.
R.G.E. and A.U. contributed equally to this article.
References
- DeLa Cadena RA, Suffredini AF, Page JD, Pixley RA, Kaufman N, Parrillo JE, Colman RW. Activation of the kallikrein-kinin system after endotoxin administration to normal human volunteers. Blood. 1993;81:3313–3317. [PubMed] [Google Scholar]
- DeLa Cadena RA, Majluf-Cruz A, Stadnicki A, Tropea M, Reda D, Agosti JM, Colman RW, Suffredini AF. Recombinant tumor necrosis factor receptor P75 fusion protein (TNRF:Fc) alters endotoxin-induced activation of the kinin, fibrinolytic, and coagulation systems in normal humans. Thromb Haemost. 1998;80:114–118. [PubMed] [Google Scholar]
- Pixley RA, Zellis S, Bankes P, DeLa Cadena RA, Page JD, Scott CF, Kappelmayer J, Wyshock EG, Kelly JJ, Colman RW. Prognostic value of assessing contact system activation and factor V in systemic inflammatory response syndrome. Crit Care Med. 1995;23:41–51. doi: 10.1097/00003246-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Mason JW, Kleeberg U, Dolan P, Colman RW. Plasma kallikrein and Hageman factor in Gram-negative bacteremia. Ann Intern Med. 1970;73:545–551. doi: 10.7326/0003-4819-73-4-545. [DOI] [PubMed] [Google Scholar]
- Schapira M, Silver LD, Scott CF, Schmaier AH, Prograis LJ, Curd JG, Colman RW. Prekallikrein activation and high molecular weight kininogen consumption in hereditary angioedema. N Engl J Med. 1983;308:1050–1053. doi: 10.1056/NEJM198305053081802. [DOI] [PubMed] [Google Scholar]
- Kaufman N, Page JD, Pixley RA, Schein R, Schmaier AH, Colman RW. Alpha 2-macroglobulin-kallikrein complexes detect contact system activation in hereditary angioedema and human sepsis. Blood. 1991;77:2660–2667. [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- Rojkjaer R, Hasan AA, Motta G, Schousboe I, Schmaier AH. Factor XII does not initiate prekallikrein activation on endothelial cells. Thromb Haemost. 1998;80:74–81. [PubMed] [Google Scholar]
- Goetzl EJ, Austen KF. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974;53:591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M, Despland E, Scott CF, Boxer LA, Colman RW. Purified human plasma kallikrein aggregates human blood neutrophils. J Clin Invest. 1982;69:1199–1202. doi: 10.1172/JCI110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel YT, Kucich U, James HL, Scott CF, Schapira M, Zimmerman M, Cohen AB, Colman RW. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983;72:1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W, Huber I, Bouma BN, Lammle B. Purified human plasma kallikrein does not stimulate but primes neutrophils for superoxide production. Thromb Haemost. 1989;62:1121–1125. [PubMed] [Google Scholar]
- Wachtfogel YT, Pixley RA, Kucich U, Abrams W, Weinbaum G, Schapira M, Colman RW. Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood. 1986;67:1731–1737. [PubMed] [Google Scholar]
- Toossi Z, Sedor JR, Mettler MA, Everson B, Young T, Ratnoff OD. Induction of expression of monocyte interleukin 1 by Hageman factor (factor XII). Proc Natl Acad Sci USA. 1992;89:11969–11972. doi: 10.1073/pnas.89.24.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Gaginella TS, Kachur JF. Kinins as mediators of intestinal secretion. Am J Physiol. 1989;256:G1–G15. doi: 10.1152/ajpgi.1989.256.1.G1. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- Warren JB, Loi RK. Captopril increases skin microvascular blood flow secondary to bradykinin, nitric oxide, and prostaglandins. EMBO J. 1995;9:411–418. doi: 10.1096/fasebj.9.5.7896012. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Nagaswami C, Woodhead JL, DeLa Cadena RA, Page JD, Colman RW. The shape of high molecular weight kininogen: organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J Biol Chem. 1994;269:10100–10106. [PubMed] [Google Scholar]
- DeLa Cadena RA, Colman RW. The sequence HGLGHGHEQQHGLGHGH in the light chain of high molecular weight kininogen serves as a primary structural feature for zinc-dependent binding to an anionic surface. Prot Sci. 1992;1:151–160. doi: 10.1002/pro.5560010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CF, Silver LD, Schapira M, Colman RW. Cleavage of human high molecular weight kininogen markedly enhances its coagulant activity. Evidence that this molecule exists as a procofactor. J Clin Invest. 1984;73:954–962. doi: 10.1172/JCI111319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP, DeLa Cadena RA, Colman RW. Deletion mutagenesis of high molecular weight kininogen light chain: identification of two anionic surface binding subdomains. J Biol Chem. 1993;268:2486–2492. [PubMed] [Google Scholar]
- Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2+3 of the urokinase receptor. J Clin Invest. 1997;100:1481–1487. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais C, Jr, Couture R, Drapeau G, Colman RW, Adam AA. Involvement of endogenous kinins in the pathogenesis of peptidoglycan-induced arthritis in the Lewis rat. Arthritis Rheum. 1997;40:1327–1333. doi: 10.1002/1529-0131(199707)40:7<1327::AID-ART18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Sartor RB, Janardham R, Majluf-Cruz A, Kettner C, Adam AA, Colman RW. Specific inhibition of plasma kallikrein modulates chronic granulomatous intestinal and systemic inflammation in genetically susceptible rats. EMBO J. 1998;12:325–333. doi: 10.1096/fasebj.12.3.325. [DOI] [PubMed] [Google Scholar]
- Isordia-Salas I, Pixley RA, Li F, Sainz I, Sartor RB, Adam A, Colman RW. Kininogen deficiency modulates chronic intestinal inflammation in genetically susceptible rats. Am J Physiol. 2002;283:G180–G186. doi: 10.1152/ajpgi.00514.2001. [DOI] [PubMed] [Google Scholar]
- Isordia-Salas I, Pixley RA, Parekh H, Kunapuli SP, Li F, Stadnicki A, Lin Y, Sartor RB, Colman RW. The mutation Ser511Asn leads to N-glycosylation and increases the cleavage of high molecular weight kininogen in rats genetically susceptible to inflammation. Blood. 2003;102:2835–2842. doi: 10.1182/blood-2003-02-0661. [DOI] [PubMed] [Google Scholar]
- Sartor RB, Anderle SK, Rifai N, Goo DA, Cromartie WJ, Schwab JH. Protracted anemia associated with chronic, relapsing systemic inflammation induced by arthropathic peptidoglycan-polysaccharide polymers in rats. Infect Immun. 1989;57:1177–1185. doi: 10.1128/iai.57.4.1177-1185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris JD, Allen JB, Schwab JH. In vivo changes in complement induced with peptidoglycan-polysaccharide polymers from streptococcal cell walls. Infect Immun. 1982;35:377–380. doi: 10.1128/iai.35.1.377-380.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie WJ. Arthritis in rats after systemic injection of streptococcal cell or cell walls. J Exp Med. 1977;146:1585–1601. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLa Cadena RA, Laskin KJ, Pixley RA, Sartor RB, Schwab JH, Back N, Bedi GS, Fisher RS, Colman RW. Role of kallikrein-kinin system in pathogenesis of bacterial cell wall-induced inflammation. Am J Physiol. 1991;260:G213–G219. doi: 10.1152/ajpgi.1991.260.2.G213. [DOI] [PubMed] [Google Scholar]
- Adam A, Damas J, Calay G, Renard C, Remacle Volon G, Bourdon V. Quantification of rat T-kininogen using immunological methods. Application to inflammatory processes. Biochem Pharmacol. 1989;38:1569–1575. doi: 10.1016/0006-2952(89)90303-1. [DOI] [PubMed] [Google Scholar]
- DeLa Cadena RA, Stadnicki A, Uknis AB, Sartor RB, Kettner CA, Adam A, Colman RW. Inhibition of plasma kallikrein prevents peptidoglycan-induced arthritis in the Lewis rat. EMBO J. 1995;9:446–452. doi: 10.1096/fasebj.9.5.7896018. [DOI] [PubMed] [Google Scholar]
- Kvien TK, Glennas A, Melby K, Granfors K, Andrup O, Karstensen B, Thoen JE. Reactive arthritis: incidence, triggering agents and clinical presentation. J Rheumatol. 1994;21:115–122. [PubMed] [Google Scholar]
- Arnett FC. Seronegative spondylarthropathies. Bull Rheum Dis. 1987;37:1–12. [PubMed] [Google Scholar]
- Clark RL, Cuttino JT, Jr, Anderle SK, Cromartie WJ, Schwab JH. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979;22:25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Wilder RL. Streptococcal cell wall-induced polyarthritis in rats. Greenwald RA, Diamond HS, editors. Boca Raton: CRC Press,; Handbook of Animal Models for the Rheumatic Diseases. 1988:pp 33–40. [Google Scholar]
- Stimpson SA, Schwab JH. Pharmacological Methods in the Control of Inflammation. Chang JY, Lewis AJ, editors. New York: Alan R. Liss,; 1989:pp 381–394. [Google Scholar]
- Schmaier AH, Schutsky D, Farber A, Silver LD, Bradford HN, Colman RW. Determination of the bifunctional properties of high molecular weight kininogen by studies with monoclonal antibodies directed to each of its chains. J Biol Chem. 1987;262:1405–1411. [PubMed] [Google Scholar]
- Colman RW, Pixley RA, Sainz I, Song JS, Isordia-Salas I, Muhamed SN, Powell JA, Jr, Mousa SA. Inhibition of angiogenesis by antibody blocking the action of proangiogenic high-molecular-weight kininogen. J Thromb Haemost. 2003;1:164–170. doi: 10.1046/j.1538-7836.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- DeLa Cadena RA, Scott CF, Colman RW. Evaluation of a microassay for human plasma prekallikrein. J Lab Clin Med. 1987;109:601–607. [PubMed] [Google Scholar]
- Scott CF, Colman RW. A simple and accurate microplate assay for the determination of factor XI in plasma. J Lab Clin Med. 1988;111:708–714. [PubMed] [Google Scholar]
- Colman RW, Bagdasarian A, Talamo RC, Scott CF, Seavey M, Guimaraes JA, Pierce JV, Kaplan AP. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975;56:1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnicki A, DeLa Cadena RA, Sartor RB, Bender D, Kettner CA, Rath HC, Adam A, Colman RW. Selective plasma kallikrein inhibitor attenuates acute intestinal inflammation in Lewis rat. Dig Dis Sci. 1996;41:912–920. doi: 10.1007/BF02091530. [DOI] [PubMed] [Google Scholar]
- Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin: a simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- Rooney M, Condell D, Quinlan W, Daly L, Whelan A, Feighery C, Bresnihan B. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988;31:956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Koizumi F, Matsuno H, Wakaki K, Ishii Y, Kurashige Y, Nakamura H. Synovitis in rheumatoid arthritis: scoring of characteristic histopathological features. Pathol Int. 1999;49:298–304. doi: 10.1046/j.1440-1827.1999.00863.x. [DOI] [PubMed] [Google Scholar]
- Khan MM, Colman RW. Cleaved high molecular weight kininogen stimulates the secretion of interleukin 1-beta and monocyte chemotactic protein-1 and induces tissue factor in human blood mononuclear cells. Blood. 2003;102:793a. (Abstract) [Google Scholar]
- Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis Rheum. 2001;44:997–1002. doi: 10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gustafson EJ, Schmaier AH, Wachtfogel YT, Kaufman N, Kucich U, Colman RW. Human neutrophils contain and bind high molecular weight kininogen. J Clin Invest. 1989;84:28–35. doi: 10.1172/JCI114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel YT, DeLa Cadena RA, Kunapuli SP, Rick L, Miller M, Schultze RL, Altieri DC, Edgington TS, Colman RW. High molecular weight kininogen binds to Mac-1 on neutrophils by its heavy chain (domain 3) and its light chain (domain 5). J Biol Chem. 1994;269:19307–19312. [PubMed] [Google Scholar]
- Khan MMH, Kunapuli SP, Lin YZ, Majluf-Cruz A, DeLa Cadena RA, Cooper SL, Colman RW. Three noncontiguous peptides comprise binding sites on high molecular weight kininogen to neutrophils. Am J Physiol. 1998;275:H145–H150. doi: 10.1152/ajpheart.1998.275.1.H145. [DOI] [PubMed] [Google Scholar]
- Uknis AB, DeLa Cadena RA, Janardham R, Sartor RB, Whalley ET, Colman RW. Bradykinin receptor antagonists type 2 attenuate the inflammatory changes in peptidoglycan-induced acute arthritis in the Lewis rat. Inflamm Res. 2001;50:149–155. doi: 10.1007/s000110050739. [DOI] [PubMed] [Google Scholar]
- Song JS, Sainz IM, Cosenza SC, Isordia-Salos I, Bio A, Bradford HN, Guo YL, Pixley RA, Reddy EP, Colman RW: Inhibition of tumor angiogenesis in vivo by monoclonal antibody targeted to domain 5 of high molecular weight kininogen. Blood, e-published on line, May 25, 2004 [DOI] [PubMed] [Google Scholar]