Abstract
Transgenic mice mimicking certain features of Alzheimer’s disease (AD)-pathology, namely amyloid plaques and neurofibrillary tangles, have been developed in an effort to better understand the mechanism leading to the formation of these characteristic cerebral lesions. More recently, these animal models have been widely used to investigate emergent therapies aimed at the reduction of the cerebral amyloid load. Several studies have shown that immunotherapy targeting the amyloid peptide (Aβ) is efficacious at clearing the amyloid plaques or preventing their formation, and at reducing the memory/behavior impairment observed in these animals. In AD, different types of plaques likely have different pathogenic significance, and further characterization of plaque pathology in the PDAPP transgenic mice would enhance the evaluation of potential therapeutics. In the present study, a morphological classification of amyloid plaques present in the brains of PDAPP mice was established by using Thioflavin-S staining. Neuritic dystrophy associated with amyloid plaques was also investigated. Finally, the efficacy of passive immunization with anti-Aβ antibodies on the clearance of Thio-S positive amyloid plaques was studied. Our results show that distinct morphological types of plaques are differentially cleared depending upon the isotype of the antibody.
The definitive diagnosis of Alzheimer’s disease is based on the neuropathological examination of the brain and the concomitant observation of several pathological features. Along with neuronal and synaptic loss, the primary lesions are neurofibrillary tangles and parenchymal amyloid plaques.1 Amyloid plaques have been extensively characterized in Alzheimer’s disease or aged human brain, and several classifications, based on plaque morphology, have been established. Briefly, these classifications discriminate diffuse plaques, described as amorphous deposits with blurred borders, from the spherically shaped immature plaques, associated with few dystrophic neurites, and from the mature plaques, with a central dense core of amyloid surrounded by numerous dystrophic neurites.2,3 Amyloid plaques showing such a degeneration of neuronal processes in their vicinity, the so-called neuritic plaques, might be of clinical relevance.3,4 Thus, refining the characterization of neuritic degeneration and their association with plaques in human and animal models will potentially benefit the evaluation of therapeutic approaches to prevent degeneration of these neuronal processes.
In the last decade, several strains of transgenic mice have been produced and characterized in an attempt to find a model mimicking the pathology observed in the brain of Alzheimer’s disease (AD) patients. Numerous studies have shown that mice overexpressing any of several mutated forms of the gene for the human amyloid precursor protein (hAPP) develop amyloid deposits in their brains, and have some memory deficits.5–8 Interestingly, recent studies comparing the amyloid peptides in the brains of transgenic mice and those of AD patients have reported different physical and chemical properties. Numerous posttranslational modifications have been shown to be responsible for the insolubility of amyloid plaques in AD. Such modifications are either absent or found at reduced levels in transgenic mice, leading to a greater solubility of the amyloid in the animals.9,10
Transgenic mice developing amyloid deposits have been very useful to investigate emergent therapies aiming at the reduction of the cerebral amyloid load. In the past few years, Aβ-based immunotherapy has been shown to be efficacious in reducing the amyloid burden and to ameliorate memory/behavior impairment in different APP transgenic mice.11–14 Different mechanisms, which are not mutually exclusive, have been proposed for explaining the amyloid-clearing effects of Aβ-based immunotherapy: Fc receptor-mediated phagocytosis of the plaques through activated microglia,12 capture of soluble Aβ by circulating anti-Aβ antibodies,15 or disruption of Aβ assemblies by anti-Aβ antibodies.16,17 Recently, it has been shown that clearance of amyloid plaques after intracranial administration of anti-Aβ antibodies might involve a two-step mechanism, without microglial activation and Fc-independent during the first phase, with microglial activation and likely Fc-dependent during a later phase.18
In the present study, plaques in a transgenic Alzheimer mouse model were first classified by distinct amyloid and neuritic pathology characteristics. The differential clearance of these plaques was examined after passive immunization with Aβ that varied in isotype but had similar N-terminal epitopes. Our results indicate that clearance of deposited amyloid was dependent on both plaque morphology and antibody isotype.
Materials and Methods
Anti-Aβ Antibody Treatment
Twelve- to thirteen-month-old heterozygous transgenic mice expressing mutated hAPP (PDAPP mice,6) were used. Passively immunized mice received weekly intraperitoneal injections of anti-Aβ monoclonal antibodies of different isotypes (IgG1, clone 10D5; IgG2a, clone 12B4; or IgG2b, clone 12A11) at a concentration of 10 mg/kg in phosphate-buffered saline (PBS) for 6 months. Antibodies were obtained as described previously and recognized the same N-terminal epitope of Aβ peptide, ie, amino acids 3 to 7. PBS-treated age-matched PDAPP mice served as controls.19 Mice were sacrificed at 18 to 19 months of age. All animals analyzed were a subset (n = 11 to 13 for each group) of a larger study described previously,19 and the median amyloid burden in the frontal cortex for this subset of animals was not significantly different when compared to values of the entire group.
Preparation of Mouse Brain Tissue
Mice were anesthetized by CO2 exposure, perfused with cold saline, and brain tissues were fixed in 4% buffered paraformaldehyde. Forty-μm coronal sections were cut with a Leica vibratome 2000 (Nussloch, Germany), cryoprotected, and stored at −20°C.
Thioflavin-S Staining and Immunostaining
Floating sections from immunized and nonimmunized PDAPP mice were washed in PBS and mounted on Superfrost Plus slides coated with Vectabond (Vector Laboratories, Burlingame, CA), before being processed for Thioflavin-S (Thio-S) staining as described by Schmidt and collaborators.20 Briefly, sections were postfixed in 10% formalin for 10 minutes, then washed in PBS. After incubation for 10 minutes in 0.25% potassium permanganate, sections were washed in PBS and incubated in 2% potassium metabisulfite and 1% oxalic acid until they appeared white. Sections were then washed in water and stained for 10 minutes with a solution of 0.015% Thio-S in 50% ethanol. Finally, sections were washed in 50% ethanol and in water, then dried, and dipped into Histo-Clear before being coverslipped with Permount. All chemicals were from Sigma (St. Louis, MO).
A double-staining protocol was used to compare 3D6-immunoreactivity to Thio-S staining of plaques. Monoclonal antibody 3D6 recognizes residues 1 to 5 of the Aβ peptide,12 and was used at a final concentration of 2 μg/ml in PBS. The immunostaining procedure was performed on floating sections before the Thio-S staining. Sections were washed in PBS, and then incubated for 30 minutes with 0.5% Triton X-100 and H2O2 3% in PBS. Nonspecific binding was prevented by incubating the sections in 10% normal goat serum (Vector Laboratories) in PBS for 1 hour, before the incubation with the primary antibody at 4°C overnight. Sections were washed in PBS, and the specific staining was revealed with a goat anti-mouse antibody labeled with Alexa 594 (Molecular Probes, Eugene, OR). Sections were then mounted on Superfrost Plus slides coated with Vectabond, dried overnight, and the Thio-S staining was performed as described above. Finally, the sections were coverslipped with Vectashield (Vector Laboratories), and sealed with nail polish.
Plaque-associated dystrophic neurites were visualized with SMI312 or with AT8, as previously described.21–23 The immunostaining procedure was performed on floating sections as described above, before the Thio-S staining. SMI312 is a cocktail of monoclonal IgG1 antibodies recognizing phosphorylated medium and high-molecular weight neurofilaments (Sternberger Monoclonals Inc., Lutherville, MD), and was used at a final dilution of 1/3000 in PBS. AT8 is a monoclonal antibody recognizing phosphorylation sites Ser202 and Ser205 of tau proteins,24 and was used at a final concentration of 0.4 μg/ml.
Confocal Image Analysis
For the characterization and quantification of the different types of plaques, three serial Thio-S-stained sections per animal were taken, and three fields were randomly chosen within the frontal cortex for each section (a total of nine fields per animal), each field covering an area of 2719 μm2. This brain region is one of the most severely affected by AD-type pathology in PDAPP mice, and is the region in which all previous analyses were performed.11,12,19 Each individual field was imaged with a ×40, NA 1.3 objective using a Bio-Rad MRC1024ES confocal acquisition system (Bio-Rad, Hercules, CA) attached to a Nikon Eclipse 800 microscope (Nikon USA, Melville, NY). Plaques were imaged at the level of their largest cross-section, and their size determined by using the Bio-Rad LaserSharp2000 processing software.
The same methodological approach was used to compare 3D6 immunostaining and Thio-S staining. However, only three fields per animal were analyzed in 10 18-month-old PDAPP mice used as references to compare 3D6 versus Thio-S staining. The area occupied by 3D6-positive plaques, 3D6-positive/Thio-S-negative (diffuse) plaques, and Thio-S-positive plaques was determined.
To characterize the dystrophic neurites and their relationship to amyloid plaques, sections were imaged with the confocal acquisition system described above. Stacks of images were acquired at ×40, with a zoom factor ranging from 1.5 to 2.0. This approach has been useful to avoid any misinterpretation on the nature of a given plaque based on a single level image analysis. Imaging of the immunostaining revealed with Alexa 594 was performed with a Texas Red filter (excitation at 568 nm), and Thio-S staining was imaged with a fluorescein isothiocyanate filter (excitation at 488 nm).
Statistical Analysis
To assess whether a significant reduction of the different types of plaques exists between the treatment groups and the control group, a statistical analysis was performed using a power transformation. The optimal transformation was 0.5 following the method of Box and Cox.25 The data were then analyzed using a one-way analysis of variance and Dunnett’s test.
Results
Morphological Characterization of Amyloid Plaques in PDAPP Mice
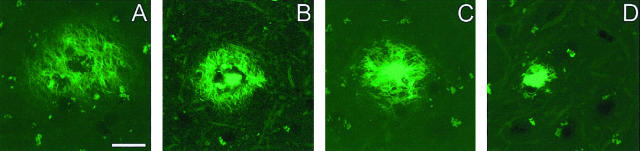
Amyloid plaques were classified into four different types based on their morphology after Thio-S staining. Thio-S-positive staining indicates that the amyloid in these plaques contains fibrillar material with a β-pleated sheet conformation. Type 1 plaques were aggregates of weakly Thio-S-reactive material with a reticular appearance, and were devoid of a central dense core (Figure 1A). They displayed an irregular shape, and their sizes were highly variable, with a cross-section generally ranging from 10 to 70 μm, although few plaques showed a larger diameter, up to 140 μm. Compact plaques were divided into types 2a, 2b, and 2c, and their common characteristic was a central dense core, strongly Thio-S-positive (Figure 1; B to D). Plaques type 2a exhibited a central core surrounded by a corona of fibrillar material (Figure 1B). A second type of compact plaque, plaque 2b, was characterized by fibrillar material radiating from the central dense core (Figure 1C). The size of the compact plaques 2a and 2b was relatively consistent with an average cross-section of 35 μm. Type 2b plaques sometimes occurred in clusters. Plaques type 2c, also called “burned-out” plaques by analogy with human classifications, were strongly Thio-S-positive without any surrounding weakly Thio-S-positive reticular material (Figure 1D). They were, on average, smaller than the other types of compact plaques (17 ± 4.7 μm). Considering that the size of the plaques type 2 (2a, 2b, or 2c) was relatively homogenous, they were quantified by determining their total number in the nine microscopic fields analyzed for each animal. Compact deposits type 2c displaying a cross-section smaller than 5 μm were not taken into account to avoid any confusion with granules of autofluorescent lipofuscin exhibiting the same appearance. All of the different types of plaques were visualized only if the sections were stained according to the protocol described above (see Material and Methods), which includes a pretreatment with potassium permanganate and potassium meta-bisulfite/oxalic acid. Plaques type 1 with a reticular appearance could not be visualized with another widely used Thio-S staining protocol, using a 1% Thio-S aqueous solution (data not shown).
Figure 1.
Morphological classification of amyloid plaques in PDAPP mice. The classification of the amyloid plaques was established based on the morphology after Thio-S staining. A: Plaques type 1 are displayed as a mesh of stained fibrils. Compact plaques exhibit a central dense core surrounded either by a corona of fibril-like material (type 2a, B), or radiating material (type 2b, C). The most compact plaques appeared as strongly Thio-S-positive structures without any surrounding materials. They are called “burned-out” plaques, as referred to the human neuropathology classification (type 2c, D). Scale bar in (A) represents 20 μm for (A–C) and 15 μm for (D).
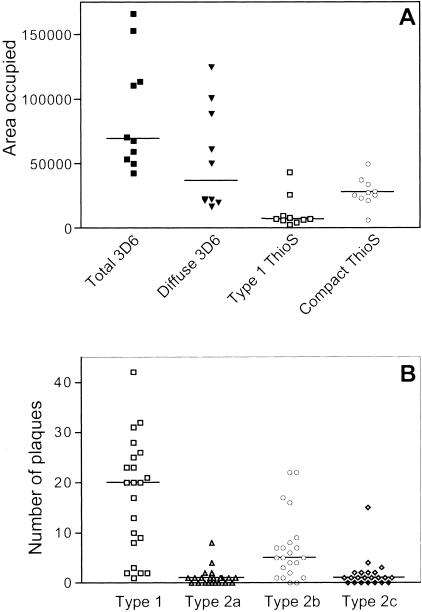
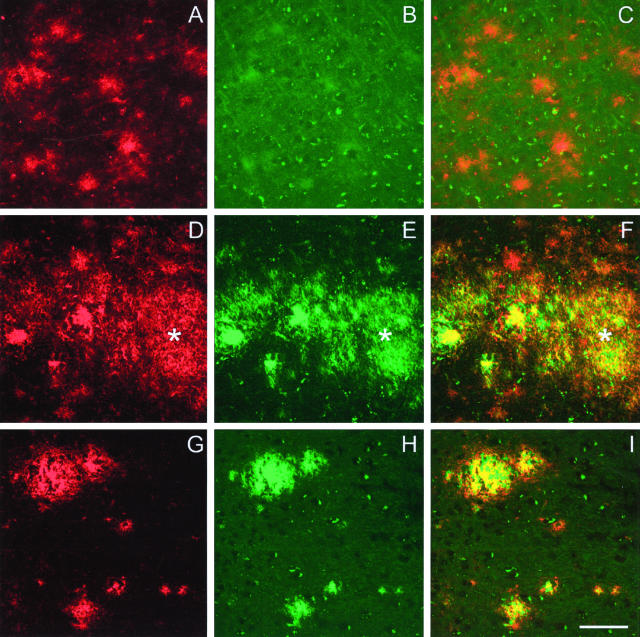
The double-staining protocol allowed us to compare Thio-S staining to 3D6 immunostaining, which was used in our previous studies.12,19 Quantification of the area covered by 3D6-positive compared to Thio-S-positive plaques was performed in 10 control PDAPP mice as described in Materials and Methods. As expected, immunostaining with 3D6 revealed more amyloid plaques than Thio-S staining (Figure 3A, total 3D6). Diffuse plaques, defined as 3D6 immunoreactive plaques with a reticular appearance, were mainly Thio-S-negative, even though some of them showed a very weak Thio-S hue (Figure 2; A to C). All Thio-S-positive plaques were also 3D6-positive. Plaques type 1 displayed a tight overlap between 3D6 immunoreactivity and Thio-S staining (Figure 2; D to F), and represented ∼20% of the total area covered by diffuse 3D6-positive plaques (Figure 3A; 7414 μm2 for type 1 Thio-S compared to 36641 μm2 for diffuse 3D6). The central core of compact type 2 plaques was consistently 3D6- and Thio-S-positive, whereas the periphery was usually 3D6 immunoreactive and Thio-S-negative (Figure 2, G to I).
Figure 3.
Different types of plaques in PDAPP mice. A: Thio-S-positive plaques represent a subset of the 3D6-immunoreactive plaques. Sections from 10 control animals were immunostained with 3D6 before being stained with Thio-S, and three fields per section were imaged by confocal microscopy. Each dot represents the median value of the area occupied for one animal. For each animal, total area occupied by all plaques 3D6-immunoreactive, area occupied only by diffuse plaques 3D6-immunoreactive, area occupied by type 1 plaque Thio-S-positive, and area occupied by compact plaque Thio-S-positive were determined with NIH Image 1.63. B: Relative proportion of the different types of plaques in control PDAPP mice. The number of plaques in control groups from two immunization studies were pooled together. Each dot represents the number of plaques for a given animal.
Figure 2.
Double-staining 3D6/Thio-S. Top: 3D6-immunoreactive diffuse plaques (A and C) are not detected by Thio-S staining (B). Middle: Plaques with a reticular appearance that are both 3D6- and Thio-S-positive are called plaques type 1 (asterisk, D to F) to differentiate them from the diffuse plaque 3D6-immunoreactive only. Bottom: Central dense core of compact plaques type 2b are immunolabeled with 3D6 (G and I) and stained by Thio-S (H and I). Periphery of the compact plaques type 2b are 3D6-immunoreactive (G and I), but mainly Thio-S-negative (I). Scale bar, 20 μm.
The relative distribution of the different types of Thio-S-positive plaques was determined by summing their respective number in 22 PDAPP control mice. Plaques type 1 and compact plaques type 2b were the most numerous, whereas type 2a and type 2c were rare, with a substantial number of animals not exhibiting any of these plaques (Figure 3B). Of a total of 593 plaques counted in the control animals, the relative percentage of the different types was as follows: 63.7% were type 1 plaques and 4.2%, 25.6%, and 6.4% were compact plaques type 2a, 2b, and 2c, respectively.
Neuritic Dystrophy Associated with Different Amyloid Plaque Types in PDAPP Mice
Double-labeling experiments, combining immunolabeling for phosphorylated neurofilaments and Thio-S staining, were performed to characterize the neuritic lesions associated with the different types of plaques. The denomination “dystrophic neurite” was applied to every neuritic process immunoreactive with SMI312 against phosphorylated neurofilaments, located in the vicinity of a plaque and displaying a distorted, swollen, or globular appearance. SMI312 was preferred over other antibodies because of its robust staining properties and because it is one of the most widely used for staining of dystrophic neurites in transgenic mice. In our previous reports, immunostaining with 8E5 against APP was used to visualize neuritic dystrophy.12,19 By using a double-labeling protocol 8E5 and SMI312, we verified that 8E5 labeling of dystrophic neurites always occurred together with SMI312 labeling around plaques (data not shown). Also, as previously reported, the immunolabeling of PDAPP brain sections with AT8, an antibody against hyperphosphorylated tau proteins, revealed short neuritic threads in the immediate vicinity of compact plaques.22 However, these thin dystrophic neurites were almost exclusively seen in the hippocampus (data not shown). Therefore, the following description addresses exclusively the dystrophic neurites immunolabeled with SMI312.
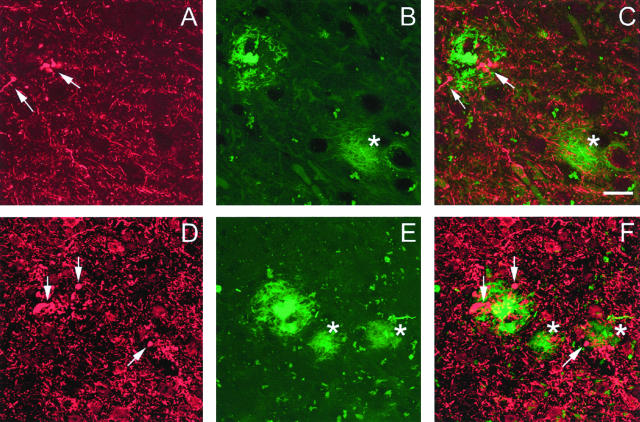
To visualize all dystrophic neurites associated with a single plaque, stacks of images with increments of 1 μm were performed from the top of the plaque to the bottom, with an additional margin of at least 2 μm on both sides of the plaque. Using this methodological approach, we showed that SMI312-positive neurites with a globular appearance were consistently associated with all types of compact plaques (Figure 4, C and F). The swollen morphology of these neurites was easily recognizable compared to the normal neurites, even when low magnification (×20) was used. In contrast, the association of the dystrophic neurites with plaques type 1 was less consistent, and plaques type 1 with or without dystrophic neurites were observed (Figure 4, C and F). It was determined in five 18-month-old control animals that 24% of plaques type 1 displayed associated dystrophic neurites.
Figure 4.
Neuritic lesions associated with plaques in PDAPP mice. Top: Swollen or distorted neurites immunoreactive with SMI 312 against phosphorylated neurofilaments (arrows in A and C) are associated with a compact plaque type 2a (B and C). A Thio-S-positive plaque type 1 in the same section (asterisk in B and C) was devoid of such associated lesion. Bottom: Clusters of dystrophic neurites (arrows in D and F) are localized in the immediate vicinity of a compact plaque type 2b (E and F). Very few neuritic lesions were associated with plaques type 1 (asterisks in E and F). The weaker SMI312 immunostaining observed in the top panel was because of the localization of the microscopic field, where a lower density of processes was observed. Scale bar in (C) represents 20 μm for (A–C) and 30 μm for (D–F).
Effects of Passive Immunization on Different Types of Amyloid Plaque
We analyzed the different plaques in the brains of PDAPP mice after immunization with Aβ antibodies of different isotypes [10D5 (IgG1), 12B4 (IgG2a), and 12A11 (IgG2b)]. As mentioned previously, the size of the type 1 plaques was highly variable. Therefore, the effect of the immunization was evaluated by measuring the percentage of the image area occupied by these plaques. A significant reduction of type 1 plaques compared to the control group was only observed after IgG2a (12B4) immunization (0 ± 0.20 versus 1.08 ± 0.46, P = 0.001; median ± SE; Figure 5A). In addition, a significant reduction of the number of compact plaques type 2b was also observed after IgG2a (12B4) immunization (3.0 ± 1.34, P = 0.007) compared to the control group (9.0 ± 2.12, Figure 5B). Finally, the effect of the passive immunization on the clearance of compact plaques type 2a or 2c was impossible to evaluate because of their very small number in the PDAPP mice (data not shown).
Figure 5.
Effect of passive immunization on removal of different types of plaques. Anti Aβ-antibodies of different isotypes have differential efficacy on the clearance of amyloid plaques. IgG2a antibody (12B4) was efficacious in significantly reducing both type 1 and compact plaques type 2b (P = 0.001 and 0.007, respectively). IgG1 (10D5) and IgG2b (12A11) antibodies had no significant effect on clearance of Thio-S-positive plaques, and were not significantly different from PBS-treated age-matched PDAPP control group.
Neuritic lesions without plaques were absent in sections from mice immunized with 12B4, as assessed by using a Thio-S/SMI312 double-labeling protocol (data not shown). This observation indicates that, in these mice, immunization with an IgG2a antibody also cleared the dystrophic neurites or prevented their formation.
Discussion
Different models of transgenic mice bearing one or several AD-related human genes have been developed and are widely used to investigate the pathological mechanisms leading to the formation of the characteristic cerebral lesions. The transgenic mice expressing a mutated human APP gene are of great interest for studies targeting the amyloid component of the pathological process because they display an accelerated AD-like phenotype.5–8 However, each individual line of these hAPP transgenic mice has its own characteristics in terms of the nature and density of amyloid plaques and other pathological features. Therefore, an accurate description and classification of the amyloid plaques is useful, especially if one wants to study the effect of therapeutic strategies on those plaques. With this goal, we established a morphological classification of amyloid plaques in PDAPP mice after Thio-S staining. The use of this method, rather than a classification based on immunostaining with anti-Aβ antibodies, was chosen to allow the visualization of all subsets of fibrillar amyloid plaques without regard for their chemical and immunological heterogeneity. Thus, we were able to differentiate four different types of Thio-S amyloid deposits.
The first category, named plaque type 1, included all plaques weakly stained by Thio-S, with a reticular appearance, and devoid of a central dense core. These plaques were morphologically similar to the diffuse plaques revealed by immunostaining with anti-Aβ antibodies. However, this original denomination based on immunostaining was not retained to avoid any confusion, because our classification was based solely on Thio-S staining. A second category of plaques was characterized by the presence of a central dense core, and included three different types. Compact plaques type 2a and type 2b exhibited a central dense core surrounded by weakly stained fibrillar material. The later was displayed either as a corona in plaques type 2a, or as a more homogeneous material radiating from the center in plaques type 2b. The compact plaques type 2a were very similar to the classical mature plaques described in AD.2,3 Finally, compact plaques type 2c had the appearance of the burned-out plaques described in human tissues,2,3 presenting only a dense compact core of Thio-S-positive material. Overall, the relative proportion of the different types of plaques is a feature of the PDAPP mice. Plaques type 1 are the most predominant in the frontal cortex of PDAPP mice. They account for ∼64% of the total number of plaques in the PDAPP mouse brain, whereas the compact plaques type 2b are the second most prominent, representing ∼25% of the total number. A different pattern has been described in the APP23 transgenic mice, with the compact plaque 2c being the most abundant and the compact 2b being rare.26 This difference emphasizes that each APP transgenic model is unique and displays its own pathological characteristics.
Neuritic dystrophy associated with senile plaques is a cardinal feature of AD brains.23,27 Dystrophic neurites containing paired-helical filaments are abundant in AD and their density correlates with clinical impairment.27 However, paired-helical filaments have never been observed in hAPP transgenic mice, even though dystrophic neurites immunoreactive for hyperphosphorylated tau but lacking the ultrastructure of the paired-helical filaments have been reported in the PDAPP and APP23 transgenic models.7,22,26 Dystrophic neurites immunoreactive for APP or neurofilament proteins have been extensively described in AD, and also in human normal aging and transgenic mice.22,23,28,29 We report here that Thio-S-positive compact plaques type 2 are always associated with dystrophic neurites in PDAPP mice, whereas plaques type 1 are less frequently associated with such neurites. However, the exact relationship between fibrillar Aβ and neuritic dystrophy and/or neuronal death is not yet fully understood. A recent report suggested, at least in cortical neuronal cultures, a direct link between the presence of fibrillar Aβ and the development of neuritic dystrophy and loss of synaptic integrity.30 Studies performed in AD or transgenic mice brains suggested that only the most compact and largest Thio-S-positive plaques exert potent local neurotoxic effect, leading to the formation of swollen axonal and dendritic processes,31 or leading to neuronal loss in their vicinity.32
In recent years, several studies have reported a beneficial effect of Aβ-based immunotherapy in transgenic mice models developing AD-like pathology. Thus, pathology can be prevented by immunization performed in young mice, and can also be reverted in older mice with pre-existing amyloid deposits.11,13,14,33 Interestingly, neuropathological examination of brains from a limited number of patients immunized with an aggregated form of Aβ42, ie, AN-1792, showed an apparent removal of diffuse and neuritic amyloid plaques in certain brain regions, as well as cellular Aβ immunoreactivity around the remaining plaques.34,35 Different mechanisms that are not exclusive could be responsible for the plaque clearance. Recently, Bard and collaborators19 have shown that antibodies directed against N-terminal epitopes of Aβ, directly administered or induced by active immunization with the corresponding peptides, were effective in reducing amyloid burden. Antibodies directed against C-terminal epitopes were inactive. Moreover, McLaurin and collaborators,36 showed that immunization with synthetic peptides encompassing the sequence 4 to 10 of Aβ was efficacious to clear amyloid plaques, but could also inhibit cytotoxicity and fibrillogenesis induced by amyloid. It has also been shown in our previous study that antibody isotype influences the degree of clearance, with IgG2a antibodies being more efficacious than IgG2b or IgG1 antibodies.19 However, the effects of immunization were evaluated in terms of reduction of the total amyloid burden, as revealed by immunostaining with anti-Aβ antibodies. The different types of plaques were not considered, although it is conceivable that in AD, the different types of plaques could have different pathogenic significance.2 In this context, there is a growing interest to analyze the effects of immunization on the different types of plaques separately.
Antibodies of different isotypes recognizing the same epitope, Aβ3–7 were compared for their efficacy in clearing different types of Thio-S-positive plaque, which represent only a subset of the 3D6-immunoreactive plaques. The only significant effect was achieved with an IgG2a antibody (12B4), which induced more than 90% reduction of type 1 and about 70% reduction of compact type 2b plaques. Immunization with IgG1 antibody (10D5), or IgG2b (12A11) had no effect on either type 1 or type 2 plaques. These results, as well as those indicating that dystrophic neurites were always associated with compact plaques and less frequently with type 1 plaques, should be considered in regard of our previous observations. In fact, immunization with IgG2a antibody (12B4), inducing a clearance of type 1 and compact type 2 plaques associated with dystrophic neurites, led to the reduction of the amyloid burden and also neuritic burden.19 On the other hand, immunization with IgG1 (10D5) or IgG2b (12A11) did not affect Thio-S-positive plaques, and this observation was in agreement with the absence of reduction of neuritic burden described in our previous report.19 Other studies have shown that intracranial injections of anti-Aβ IgG1 antibodies led, after 3 to 4 days, to clearance of compact Thio-S-positive plaques,18 and to normalization of neuritic alterations.37 One could explain these latter effects by the high and localized concentration of antibody obtained after intracranial injections. However, it might be difficult to compare these studies with those described by our group, in which antibodies are administered peripherally throughout a 6-month period of time and only limited amounts of antibodies get access to the brain through the blood-brain barrier.
Finally, and considering the paucity of vascular Aβ deposits in the PDAPP mouse, the present study did not address the question of the effect of passive immunization on such deposits. However, it might be of interest to investigate this issue because a recent study showed that immunization of APP23 transgenic mouse, characterized by prominent vascular Aβ deposition, with anti-Aβ IgG1 antibody resulted in a twofold increase in the rate of hemorrhages.38
Overall, the present study confirms that passive immunization with IgG2a antibodies directed against the N-terminal region of the Aβ peptide is efficacious to clear amyloid plaques in PDAPP mice. Experiments described in our previous study indicated that both antibody isotype and affinity for Fc receptors were key factors for the reduction of neuritic dystrophy in PDAPP mice.19 Here, we extend this observation by showing that IgG2a antibodies are efficacious in clearing fibrillar, Thio-S-positive plaques. The higher efficacy of IgG2a antibodies is consistent with their ability to best stimulate microglial and peripheral macrophage phagocytosis.19 Therefore, our observations support a crucial role for microglial Fc receptor-mediated phagocytosis in clearance of at least the fibrillar plaques. Alternatively, other mechanisms independent of the microglial Fc receptor18,39 might play a role in clearing diffuse, 3D6-immunoreactive, Thio-S-negative plaques and soluble Aβ moieties. Consistent with this hypothesis, Fc-knockout mice also showed reduction of plaque burden after Aβ immunotherapy.39
In conclusion, we report a morphological classification of the amyloid plaques in PDAPP mice, based on Thio-S staining, and compare it to the more commonly used immunostaining with anti-Aβ antibody. From the four different types of plaques described, type 1 plaques and compact plaques with a central dense core surrounded by fibrillar material (type 2b) represent remarkable features of the PDAPP mice. Two additional types of compact plaques, similar to the classical mature plaques (type 2a) and burned-out plaques (type 2c) in AD, are also present, albeit at very low number in these mice. We show that dystrophic neurites are always associated with the compact plaques, but not with most of the type 1 plaques. Passive immunization of PDAPP mice induce a reduction of the amyloid load because of plaques type 1, whereas the compact plaques are cleared to a greater extent by injecting the animals with anti-amyloid antibodies of the IgG2a isotype. The concomitant analysis of plaques, with and without effective therapy, will help to determine the type of plaque and underlying mechanisms that are most relevant to the pathophysiology of AD.
Note Added in Proof
Based on cloning and nucleotide sequencing performed recently for all antibodies used in the passive immunization protocol, it appears that the isotype of the antibody 12A11, described above as an IgG2b (as determined by an enzyme-linked immunosorbent assay isotyping method), is actually an IgG1. Therefore, an additional group of animals (n = 12) immunized with an IgG2b (group 3A3) has been analyzed, and the following data were obtained: the median value for the area occupied by plaque type1 is 1.55 ± 0.38 (compared to 1.08 ± 0.47 for the PBS group); the median value for the number of compact plaque type 2b is 11.5 ± 1.72 (compared to 9.0 ± 2.13 for the PBS group). Importantly, the overall conclusions of the present study remain unchanged, ie, IgG2a antibodies directed against the N-terminal of the Aβ peptide are the most efficacious in clearing fibrillar, Thio-S-positive plaques.
Acknowledgments
We thank Dr. Chuck Davis, Mrs. Bridget Schmitz, and Ms. Lily Chen for their helpful comments and assistance in the statistical analysis.
Footnotes
Address reprint requests to Manuel Buttini, Ph.D., 800 Gateway Blvd., South San Francisco, CA 94080. E-mail: manuel.buttini@elan.com.
References
- Mirra SS, Gearing M, Nash F. Neuropathologic assessment of Alzheimer’s disease. Neurology. 1997;49:S14–S16. doi: 10.1212/wnl.49.3_suppl_3.s14. [DOI] [PubMed] [Google Scholar]
- Delaère P, Duyckaerts C, He Y, Piette F, Hauw JJ. Subtypes and differential laminar distributions of beta A4 deposits in Alzheimer’s disease: relationship with the intellectual status of 26 cases. Acta Neuropathol. 1991;81:328–335. doi: 10.1007/BF00305876. [DOI] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Gomez-Isla T, Hyman BT. Abeta associated neuropil changes: correlation with neuronal loss and dementia. J Neuropathol Exp Neurol. 1998;57:1122–1130. doi: 10.1097/00005072-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah M, McConologue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Kokjohn TA, Beach TG, Sue LI, Brune D, Lopez JC, Kalback WM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Roher AE. Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem. 2001;276:12991–12998. doi: 10.1074/jbc.M007859200. [DOI] [PubMed] [Google Scholar]
- Kalback W, Watson MD, Kokjohn TA, Kuo YM, Weiss N, Luehrs DC, Lopez J, Brune D, Sisodia SS, Staufenbiel M, Emmerling M, Roher AE. APP transgenic mice Tg2576 accumulate Abeta peptides that are distinct from the chemically modified and insoluble peptides deposited in Alzheimer’s disease senile plaques. Biochemistry. 2002;41:922–928. doi: 10.1021/bi015685+. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St. George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Robinson KA, Lee VM, Trojanowski JQ. Chemical and immunological heterogeneity of fibrillar amyloid in plaques of Alzheimer’s disease and Down’s syndrome brains revealed by confocal microscopy. Am J Pathol. 1995;147:503–515. [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer’s disease. J Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Sisk A, Mallory M, Games D. Neurofibrillary pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. J Neuropathol Exp Neurol. 2001;60:357–368. doi: 10.1093/jnen/60.4.357. [DOI] [PubMed] [Google Scholar]
- Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J Royal Stat Soc. 1964;Series B:211–243. [Google Scholar]
- Danner S, Herzig MC, Staufenbiel M, Wiederhold KH. Spatial relation of amyloid plaque associated neurodegenerative and inflammatory processes in APP transgenic mice analyzed by confocal microscopy. Neurobiol Aging. 2002;23:S905. [Google Scholar]
- McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheimer’s disease. Ann Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, Alford M, DeTeresa R, Terry R. An antibody against phosphorylated neurofilaments identifies a subset of damaged association axons in Alzheimer’s disease. Am J Pathol. 1993;142:871–882. [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132:86–101. [PMC free article] [PubMed] [Google Scholar]
- Grace E, Rabiner C, Busciglio J. Characterization of neuronal dystrophy induced by fibrillar amyloid beta: implications for Alzheimer’s disease. Neuroscience. 2002;114:265–273. doi: 10.1016/s0306-4522(02)00241-5. [DOI] [PubMed] [Google Scholar]
- Brendza RP, O’Brien C, Simmons K, McKeel DW, Bales KR, Paul SM, Olney JW, Sanes JR, Holtzman DM. PDAPP; YFP double transgenic mice: a tool to study amyloid-beta associated changes in axonal, dendritic, and synaptic structures. J Comp Neurol. 2003;456:375–383. doi: 10.1002/cne.10536. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St. George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]