Abstract
In many different liver diseases, such as cirrhosis, degradation of the microcirculation, including obliteration of small portal or hepatic veins contributes to disease-associated portal hypertension. The present study demonstrates the importance of angiogenesis in the establishment of arteriovenous shunts and the accompanying changes to the venous bed. One aspect of angiogenesis involves the branching of new vessels from pre-existing ones, and the molecular mechanisms controlling it are complex and involve a coordinated effort between specific endothelial growth factors and their receptors, including the angiopoietins. We modulated the hepatic vasculature in mice by conditionally expressing angiopoietin-1 in hepatocytes. In mice exposed to angiopoietin-1 during development, arterial sprouting, enlarged arteries, marked loss of portal vein radicles, hepatic vein dilation, and suggestion of arteriovenous shunting were observed. Most importantly, these phenotypic changeswere completely reversed within 14 days of turning off transgene expression. Expression of excess angiopoietin-1 beginning in adulthood did not fully recapitulate the phenotype, but did result in enlarged vessels. Our findings suggest that controlling excessive angiogenesis during liver disease may promote the restoration of the portal vein circuit and aid in the resolution of disease-associated portal hypertension.
Many types of liver disease, including Budd-Chiari syndrome (BCS), hepatitis, cirrhosis, and focal nodular hyperplasia (FNH) are characterized by portal hypertension and liver failure. Histologically, they often involve fibrosis, nodular regeneration, and disturbed vascular architecture and it is these changes that account for the significant disturbance in the hepatic circulation.1–6 Historically these diseases have been widely regarded as irreversible, however several reports examining experimental animal models of fibrosis and human liver biopsies suggest that the fibrosis events related to some types of liver disease can be reversed.7–9 Nonetheless, the portal hypertension associated with liver disease appears irreversible, and most likely reflects the presence of arteriovenous (AV) shunts and aberrant veins.2,4–6 These uncontrolled angiogenic events suggest that controlling blood vessel development and plasticity may provide important insight into alternative treatment strategies targeting the vasculature, in addition to current strategies targeting the hepatocytes.
Angiogenesis refers to the expansion or remodeling of pre-existing blood vessels and is critical during normal embryonic vascular development and in the progression of several diseases.10 The molecular events governing angiogenesis are complex and involve multiple families of proteins and receptors, including the vascular endothelial growth factor family11 and the angiopoietin (Ang) family.10 Vascular endothelial growth factor is a potent angiogenic factor and in the liver, can contribute to the proliferation of sinusoidal endothelial cells (ECs) during liver regeneration12,13 and is increased after experimental induction of hepatic fibrosis and cirrhosis14,15 and in livers of patients suffering from cirrhosis,15,16 nodularregenerative hyperplasia (NRH), BCS, FNH, and other benign liver tumors.4,6,17 Ang-1, a member of the Ang family, has also been shown to play a role in vascular development and vascular-related disease processes.10 Ang-1 is the best characterized member of the Ang family and binds to Tie2, a receptor tyrosine kinase that is expressed on ECs lining blood vessels.18,19 In the vascular system, Ang-1 is involved in EC survival and migration, periendothelial cell recruitment, and tubule formation.10 In the liver, increased expression of Ang-1, Ang-2, and their receptor Tie2 have been observed in regenerating liver, after hepatectomy,20–22 in hepatocellular carcinoma,23–25 and in the damaged liver.26,27
The close interdependence between ECs, their growth factors, and the liver suggests that liver organogenesis may be dependent on angiogenic events.28 Similar requirements for ECs have been shown during development of the pancreas, heart, and kidney.29 After injury, it now also appears that the interaction between ECs and liver regeneration is critical,30 such that after hepatectomy, inhibition of angiogenic events decreases the rate of hepatic regeneration, and exposure to angiogenic stimuli accelerates hepatic regeneration. New evidence suggests the mechanism in which these angiogenic growth factors protect the liver is through EC-stimulated communication with neighboring parenchymal cells that then induces production of other growth and survival factors, which in turn protects the liver.31 Regardless of whether the effect is direct or indirect, it appears that the liver vasculature and the growth factors involved in its vascular development are closely interconnected with liver growth itself.
To examine more closely the role of Ang-1 in the development of the liver vasculature, we conditionally expressed Ang-1 in a hepatocyte-specific manner using a tetracycline-based binary conditional transgenic mouse system. Double-transgenic (DT) mice survived gestation but displayed a gross dilation of vessels on the subcapsular surface of the liver. Closer examination revealed loss of portal veins and signs of AV shunt formation, reminiscent of that observed in human liver disease. DT mice with the transgene turned off until adulthood, and then turned on for up to 14 weeks, showed only mild enlargement of surface vessels, suggesting the vascular effects elicited by Ang-1 occurred during development, and that the levels of excess Ang-1 produced in adults was not sufficient to elicit the same phenotype. Interestingly, the developmentally induced vascular phenotype in adults could be completely reversed as early as 2 weeks after turning the transgene off, providing important insight into the importance of angiogenesis in preventing regeneration of the portal venous bed and may explain the continued presence and mechanism of portal hypertension in individuals suffering from liver disease, even in the absence of fibrosis. Our findings may provide novel insight for providing treatment strategies, incorporating not only the targeting of fibrosis but also targeting the liver vasculature and uncontrolled angiogenic events.
Materials and Methods
Transgene Expression Analyses of Transgenic Mice
The pTET-Ang-1 IRES LacZ responder and the liver-enriched activator protein (LAP) driver lines have been described previously.32–34 Crosses were performed between pTET-Ang-1-IRES LacZ and the LAP tTA lines and offspring were genotyped by polymerase chain reaction (PCR) using DNA extracted from either tail or ear biopsies. DNA was prepared and PCR performed using primers as previously described.35 Littermates that inherited one or no transgenes served as experimental controls.
Transgene expression was determined using LacZ staining as previously described,18 reverse transcriptase (RT)-PCR analyses, and Western analyses (see below). Liver RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), DNase treated (Sigma-Aldrich, St. Louis, MO), and a two-step RT-PCR was done (CloneTech, Palo Alto, CA) all according to each manufacturer’s suggested protocol. Samples were run in duplicate with one set undergoing RT and a second set containing no RT to control for possible DNA contamination. PCR was completed on cDNA using primers against β-actin, mouse Ang-1, and human (transgene-specific) Ang-1 using the following primers: 3′β-actin-CTCTTTGATGTCACGCACGATTTC; 5′β-actin-primer-GTGGGCCGCTCTAGGCACCAA; 3′human Ang-1-primer-TGGGAAGGGAACGAGCCTATT; 5′human Ang-1-primer-AATCATCATAGTTGTGGAACGTAA;3′ mouse Ang-1-primer-GGGCCGGATCATCATGGTGGTGG; 5′ mouse Ang-1-primer-GCCTGGATTTCCAGAGGGGCTGG.
Western Analysis
Frozen pieces of liver tissue were homogenized in RIPA buffer and quantified using the BCA protein assay (Pierce, Rockford, IL). Equal loading of samples was confirmed by performing Western blotting on 30 μg of total protein from liver lysates as described previously,36 using a monoclonal antibody against β-actin (clone AC-15, Sigma) at a 1:5000 dilution. Densitometry was performed when necessary using a GS-800 densitometer (Bio-Rad, Hercules, CA) and Quantity One Software (Bio-Rad). Transgenic hAng-1 protein levels were determined by immunoprecipitating 1 to 2 mg of protein or 300 μl of Ang-1-Myc-His-conditioned media (as positive control; as described in Jones and colleagues37) with 1 μg of monoclonal anti-His antibody (R&D Systems, Minneapolis, MN) as described previously.38 Immunoprecipitations were followed by Western blotting with anti-His primary antibody at a 1:500 dilution and a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000, Stressgen, Victoria, BC, Canada). Blots were stripped and reprobed with a goat polyclonal anti-Ang-1 antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and a donkey anti-goat secondary (1:5000, Santa Cruz Biotechnology). Blots were reproduced at least three times.
Animal Paradigms
All animals received humane care according to the Canadian Council of Animal Care. Animals were kept on a standard light cycle and fed food and water ad libitum throughout the course of the experiments.
Animals Exposed to Transgenic Ang-1 During Development
Animals were sacrificed by cervical dislocation and the liver removed at 0 weeks (0 days old), 2 weeks (14 days old), between 4 and 12 weeks, and between 36 to 52 weeks of age (Table 1). To repress pTET Ang-1 IRES LacZ expression in adult mice exposed to the transgene during development, mice were given 100 μg/ml doxycycline (Dox) (Sigma) with 5% sucrose in their drinking water. Animals receiving Dox began the regime at 5 or 12 weeks of age, time points in which the liver phenotype was strongly visible (Table 1). Fresh Dox and sugar-supplemented water was provided at least two times per week for 2, 8, or 12 weeks (Table 1).
Table 1.
Number and Genotype of Animals Used and Summary of Phenotypic Observations
| Genotype/age | n | Enlarged arteries | Sprouting | Dilated hepatic veins | PV hypoplasia | Apoptosis/necrosis | Nodules |
|---|---|---|---|---|---|---|---|
| WT mice | |||||||
| P0 | 3 | 0* | 0 | 0 | 0 | 0 | 0 |
| 2 wks | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4–12 wks | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 36–52 wks | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 wks+ Dox (2 wks) | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 wks+ Dox (8 or 12 wks) | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| DT mice | |||||||
| P0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 wks | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4–12 wks | 8 | 5 (63) | 6 (75) | 7 (88) | 5 (63) | 3 (38) | 0 |
| 36–52 wks | 3 | 3 (100) | 2 (68) | 3 (100) | 2 (68) | 0 | 2 (68) |
| 12 wks+ Dox (2 wks) | 4 | 1 (25) | 1 (25) | 1 (25) | 1 (25) | 0 | 0 |
| 5 wks+ Dox (8 or 12 wks) | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
Data is presented as the number of animals with lesions graded as 2+ or greater by a pathologist (I.R.W.) as described previously.8 The number in parentheses represents the percentage (%). Wks, weeks of age or treatment.
Animals Exposed to Transgenic Ang-1 as Adults
Repression of Ang-1 transgene expression during development was achieved in a similar manner, such that Dox was administered to single transgenic (ST) mothers bred with ST males from the day of the first sign of the vaginal plug. The Dox treatment was maintained from conception until early adulthood, and was removed at the age of 6 weeks. Animals were then sacrificed at 0 days, 4 weeks, between 8 and 9 weeks, and between 12 and 14 weeks after Dox withdrawal.
Perfusion of Mice and Microcomputerized Axial Tomography (CT) Imaging
Control and wild-type littermates (n = 4 control; n = 4 DT), 8 to 12 weeks, were provided with deep anesthesia after an intraperitoneal injection of 6.5 mg/kg sodium pentobarbital. Littermates were perfused transcardially with phosphate-buffered saline containing heparin, followed by 4% paraformaldehyde, and then a solution containing either Microfil MV-122 (livers in Figure 2; Flow Tech Inc, Carver, MA) or 5% gelatin and 30 to 40% barium (livers in Figure 3), until the vasculature appeared to be filled. Animals were packed in wet ice for 15 minutes to allow for the hardening of gelatin, and the liver was removed and postfixed in 4% paraformaldehyde until processing for micro-CT analyses.
Figure 2.
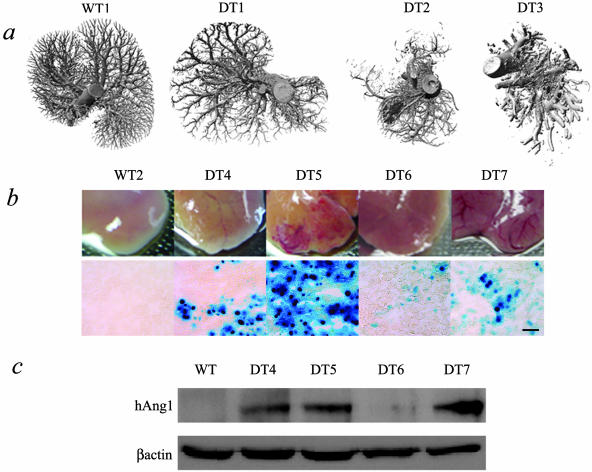
DT mice with more severe phenotypes express more transgenic Ang-1 protein. a: Micro-CT images demonstrate overall vascular phenotypes of WT and DT livers. Some DT livers (DT1) appeared remarkably normal whereas others showed a range of severity (DT2 to DT3). WT and ST mouse (ST shown) livers at the gross morphological level (middle row, b) display the characteristic smooth capsular surface with no visible veins, consistent with normal liver morphology whereas the DT (DT4 to DT7) livers show a range of phenotype consisting of small to very large increases in dilatation of veins under the capsular surface. The range of phenotype corresponds to levels of LacZ staining (bottom row of b) and levels of transgenic Ang-1 protein expressed in the livers of these mice (c), such that mice with severe phenotypes (DT5 and DT7) have more LacZ staining and express more transgenic Ang-1 protein. Scale bar, 100 μm.
Figure 3.
Ang-1 expression leads to alterations in hepatic vein architecture. ST and WT mouse livers (a) display the characteristic smooth capsular surface with no visible veins, consistent with normal liver morphology whereas DT livers (b) show a clear dilatation of veins under the capsular surface. Micro-CT images demonstrate even filling and the presence of portal and hepatic veins in WT and ST livers (c and e) and an obvious dilation of the hepatic veins and apparent reduction in the filling of the portal veins in DT livers (d and f). Interestingly, the dilated veins in the DT livers run parallel to the capsular surface (f) compared to the characteristic branching pattern displayed in the normal liver (e). At the microscopic level, WT and ST livers (g) stained with Masson Trichrome contain uniform portal and hepatic veins without any visible dilatation. h: DT livers present with a discernible dilatation of hepatic veins and an irregular distribution. a, c, e, and g are ST mice. Scale bar, 100 μm.
Three-dimensional CT data sets were acquired for each excised liver specimen at 35-μm isotropic resolution using an MS-8 micro-CT scanner (EVS Corp., London, Ontario, Canada). With the X-ray source at 80 kVp (mean energy of incident beam, 32 keV), these were acquired in 2.5 hours with 900 views and reconstructed using the Feldkamp algorithm39 for cone beam CT geometry. Renderings were produced by first segmenting the vessels from the surrounding tissue using seeded region growing,40 then extracting the vessel surface using marching cubes.
Morphological and Histological Analysis
Whole livers were dissected from the body cavity and immediately photographed with a digital camera. The livers were then dissected into lobes and either fixed overnight in Histochoice tissue fixative (Amresco, Solon, OH) or in 10% buffered formalin alone or in combination with a 1% solution of glutaraldehyde. Additional pieces of liver were frozen at −80°C. Blocks of fixed liver tissue were either embedded in paraffin, cut in 4-μm sections using a microtome, and stained with hematoxylin and eosin and Masson’s trichrome, or cryopreserved with 30% sucrose, cut in 10-μm pieces using a cryostat and subsequently used for immunohistochemistry.
Immunohistochemistry was performed using a goat polyclonal anti-Ang-2 antibody (1:150, Santa Cruz) on formalin-fixed tissue. Conditioned medium from a rat anti-Tek hybridoma (TEK4; a kind gift from Toshio Suda, Keio University41) was used undiluted at 50 μl per section, on Histochoice-fixed tissue. Antigens were detected through the use of a rabbit anti-goat secondary for Ang-2 and a goat anti-rabbit secondary for Tek (both 1:150, Vector Laboratories, Burlingame, CA), an avidin-biotin complex (Vectastain Elite ABC kit; Vector Laboratories), and a diaminobenzidine peroxidase substrate (Vector Laboratories). Slides were counterstained with methyl green (DAKO, Caspinteria, CA), dehydrated in acetone followed by successive xylene immersions, and mounted with Entellan (EM Science). Grading of liver phenotypes was completed by one gastroenterology pathologist specializing in liver pathology (I.R.W.) as described previously.2
Results
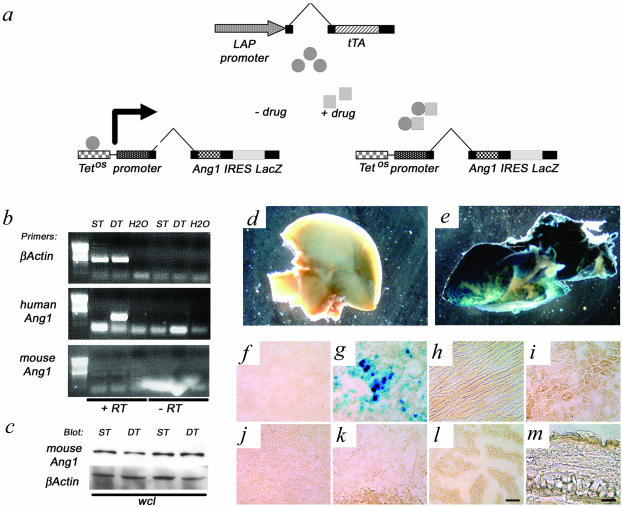
To gain insight into the role of the angiogenic growth factor Ang-1 in the development of liver vasculature, we expressed Ang-1 in a hepatocyte-specific manner from the onset of liver bud formation, using a Dox-based binary transgenic expression system (Figure 1a).32,35 DT mice survived gestation at the estimated Mendelian ratio and were similar in size and weight to their littermate controls. RT-PCR analyses for both human Ang-1 and mouse Ang-1 confirmed the expression of transgenic-derived human Ang-1 in DT and not in ST littermates (Figure 1b), and suggested that endogenous Ang-1 levels was not altered between DT and ST mice. No effects on endogenous levels of Ang-1 were confirmed at the protein level using an antibody that recognized mouse Ang-1 but not human Ang-1 (Figure 1; c and d). Cell-type specificity of transgenic Ang-1 was confined to hepatocytes in the liver beginning at embryonic day 9.5 (Figure 1g) and continued into adulthood, as evidenced by LacZ staining in DT mice and not in ST littermate controls (Figure 1; d to g). LacZ staining was confined in the DT mouse to the liver, as no staining was observed in the heart, kidney, spleen, lung, intestine, or skin (Figure 1; h to m). This expression was extinguished after exposure to Dox in the drinking water (see below).
Figure 1.
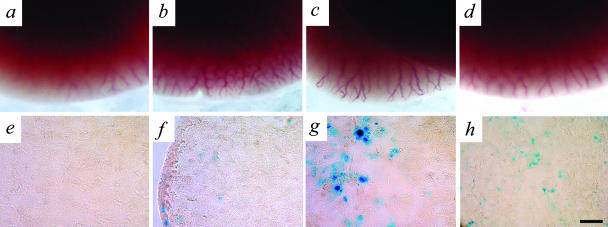
Generation and characterization of liver-specific Ang-overexpressing mice. a: Two independent lines of mice were used to induce the conditional expression of Ang-1 IRES LacZ. The tetracycline-responsive transactivator (tTA), expressed from the LAP promoter (driver transgene) associates with the tTA-binding site (TetOS) upstream of the human Ang-1 cDNA (responder transgene), which was bi-cis-tronically linked to the E. coli LacZ gene via an internal ribosomal entry site (IRES). In the absence of a tetracycline analog (−drug), Ang-1 is expressed and in the presence of this drug (+drug) Ang-1 expression in suppressed. b: RT-PCR expression analyses demonstrate the presence of human Ang-1 in the absence of Dox and that endogenous mouse Ang-1 levels do not dramatically change; +/− RT, presence or absence of reverse transcriptase, MMLV. c: Whole cell lysates were blotted with an antibody recognizing mouse Ang-1 and not human Ang-1, confirming that endogenous mouse Ang-1 protein levels do not change in the presence of the transgenic Ang-1. e and g: X-Gal staining in pieces and sections of double-transgenic (DT) adult liver showed strong expression of LacZ. d and f: No staining was observed in the single-transgenic (ST, d) or wild-type (WT, not shown) livers. LacZ expression in all DT mice was confined to the liver because no LacZ staining was observed in the heart (h), kidney (i), spleen (j), lung (k), intestine (l), or skin (m) of the ear. Scale bars: 100 μm (l); 50 μm (m).
A range of phenotype in the DT livers was observed with some DT livers having mild alterations in the capsular vasculature although still recognizable as transgenic; whereas others had grossly looking abnormal changes (Figure 2, a and b). This range in phenotype corresponded to levels of transgenic Ang-1 produced by each mouse (Figure 2; a to c). DT mice with severe phenotypes displayed increases in LacZ staining, in addition to increased levels of transgenic Ang-1. There was a remarkable correlation of Ang-1 transgene expression and the severity of the liver phenotype.
At the gross morphological level, DT mice showed a striking dilation of veins on the capsular surface of the liver when compared to control littermates (Figure 3, a and b), and micro-CT images of DT mice provided three-dimensional evidence for the irregularity of the distribution of the vessels, the marked reduction or pruning of the portal venous tree, and confirmed the gross observations of hepatic vein dilatation on the liver surface (Figure 2a and Figure 3, c to f). Histological examination of control mice and DT mice provided further verification of these macro-observations, such that control mice had normal livers containing large regular portal veins extending to the subcapsular region (Figure 3g) whereas the DT mice had notably dilated and irregularly shaped portal veins (Figure 3h). In addition to the venous dilation, a distinct reduction of portal vein branches was observed (Figure 3; c to h) and was accompanied by numerous irregular arterial branches unaccompanied by portal veins (see below).
Control mice had normal livers in which the portal veins were large and regular and no unaccompanied arteries were ever witnessed in these livers (Figure 4, a and b). Closer examination of control and DT livers revealed a significant reduction of portal vein branches in the DT livers (Figure 2a; Figure 3, c to h; Figure 4, c and d). The larger portal tracts not containing portal veins appeared to have enlarged arteries and ducts (Figure 4c). In addition, the reduction in portal vein branches was often accompanied by numerous irregular arterial branches (Figure 4, e and f). These arterial sprouts closely approached the hepatic veins suggesting the possibility of AV shunts. These phenotypic changes were quantitatively and qualitatively similar at all ages sampled beginning as early as 4 weeks of age and extending up to 52 weeks (Table 1).
Figure 4.
Ang-1-induced angiogenic events lead to liver reorganization. a: Masson Trichrome-stained sections from WT and ST livers demonstrate the characteristic pattern of organization of the portal tracts in proximity to a hepatic vein. b: All portal tracts in the WT liver contained a portal vein, portal artery, and a duct. c: In contrast, DT livers had disorganized portal tracts such that a marked enlargement of arteries was observed with very small portal veins or in smaller portal tracts, a complete absence of portal vein association. d: Higher magnification further demonstrates the absence of portal veins in two independent portal tracts. e–f: Arterial sprouts were observed without any associated portal veins, and these arterial twigs were localized closely with hepatic veins, suggesting the occurrence of AV shunts. a, artery; d, duct; hv, hepatic vein; pv, portal vein; pt, portal tract. Scale bars: ∼65 μm (a, c, e); ∼33 μm (b, d, and f).
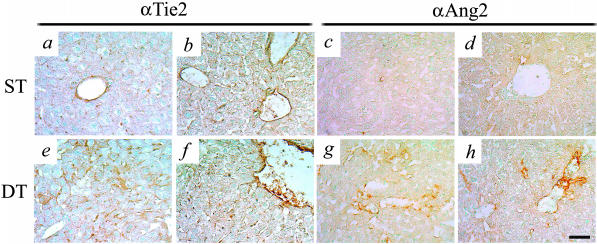
In vessels undergoing angiogenesis, Tie2 and Ang-2 protein levels were increased in ECs (Figure 5). Tie2 in ST, WT, and DT livers was seen in the ECs of hepatic veins and portal veins and arteries (Figure 5; a and b, e and f) and of particular interest was the observation of Tie2 immunoreactivity in the putative AV sprouts in the DT livers (Figure 5, e and f). In WT and ST mice, liver ECs showed very little or no basal expression of Ang-2 (Figure 5, c and d), whereas DT liver ECs showed increases in Ang-2 staining in remodeled hepatic veins and some sinusoidal endothelium (Figure 5, g and h). Both Tie2 and Ang-2 expression was increased in remodeled ECs, consistent with the suggested role of Ang-2.42 This role suggests that Ang-2 acts as a Tie2 antagonist leading to the destabilization of quiescent blood vessels, such that in the absence of concurrent expression of vascular endothelial growth factor, the ECs will die via apoptosis, whereas when expressed in the presence of vascular endothelial growth factor, the ECs will undergo mitosis, with the end result in both cases being vascular remodeling.43
Figure 5.
Ang-1-induced increases in Tie2 and Ang-2 expression in ECs. ECs in ST and WT mice hepatic veins and portal veins and arteries express the Ang receptor Tie2 (a and b), and very low basal levels of Ang-2 (c and d). e and f: Tie2 levels increase in DT mice undergoing angiogenic remodeling, and are seen in hepatic veins and AV sprouts. g and h: Significant increases in Ang-2 levels are observed in DT mice and appear to occur in vessels undergoing angiogenic remodeling. Scale bar, 50 μm.
To determine the age of onset of these phenotypic changes, we examined livers taken from postnatal day 0 and postnatal day 15 (2 weeks old) mice (Table 1). No differences in sinusoidal size were found in 0-day-old DT mice but at 2 weeks of age, sinusoidal dilation began appearing in the presence of normal peripheral portal veins. By 4 weeks of age, visible arterial sprouting was observed along with dilatation of hepatic veins and a marked reduction in portal veins (Table 1). No evidence of thrombosis or other degenerative changes was seen in these animals.
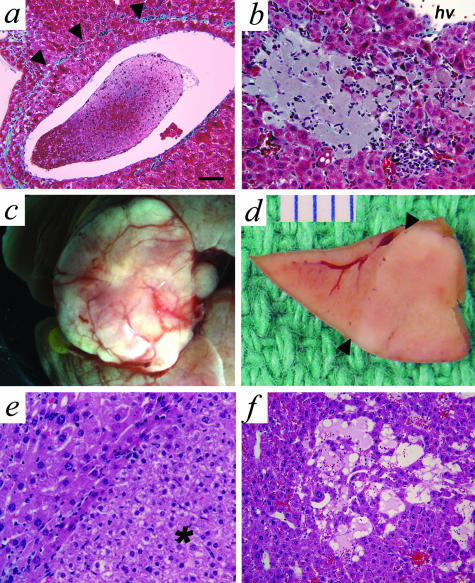
A minority of DT mice had more extreme vascular changes, including hepatic vein remodeling (Figure 6a) and focal necrosis (Figure 6b, Table 1). Moreover, large hepatocellular nodules (Figure 6; c to e) were observed in two of three animals between 36 to 52 weeks of age, representing either a hyperplastic or a neoplastic phenomenon, and these regions were often marked with significant dilatation of sinusoids also referred to as peliosis hepatis (Figure 6f).
Figure 6.
In severely affected DT mice, Ang-1-induced angiogenic events lead to hepatic vein remodeling, cell death, and nodular growths. a: Hepatic vein remodeling was seen as indicated by the presence of the original tunica media marked by a band of collagen (arrowheads). The tunica intima contains several layers of hepatocytes that encroach on the lumen. b: Small infarcts were observed in regions with absent portal veins. c: Some livers contained hyperplastic or neoplastic growths resembling FNH. d: In cross-section the tumor mass sits adjacent to normal-appearing liver tissue (arrowheads indicates the boundary) and is ∼1 cm in diameter. e: At the histological level, the margin of the tumor in e can be seen (arrowhead) and the tumor itself (*) is composed of normal-appearing hepatocytes without atypia. f: Within the region of the tumor a striking dilatation of the sinusoids, referred to as peliosis hepatis is seen. Scale bars: ∼65 μm (a); ∼33 μm (b, e, and f).
To determine whether the liver phenotype required the continued expression of Ang-1, groups of animals were given Dox to suppress Ang-1 expression for 2, 8, or 12 weeks beginning either at 5 weeks or 12 weeks of age (Table 1). The suppression of the transgenic Ang-1 was confirmed at the protein level (Figure 7a) in DT mice. All DT mice, with the exception of one (receiving Dox for 2 weeks), had completely resolved phenotypes. No arterial sprouts, loss of portal vein branches, or dilatation of hepatic veins were observed (Figure 7, b to d; Table 1) suggesting that reversal of the Ang-1-mediated phenotype could be resolved as quickly as 2 weeks. Because the portal veins were present in standard arrangement, the branching of portal tracts out to the periphery appeared to be normal and no irregular sprouts were seen except in the one animal (Figure 7, b to d). This silencing of Ang-1 expression was accompanied by a noticeable restoration toward wild-type morphology including a decrease in arterial sprouting and a remarkable development of the peripheral portal venous tree such that customary portal tracts were identified in a near classic arrangement in the peripheral tissue. Animals left untreated had severe phenotypic abnormalities. In mice treated with Dox for 2 weeks, we noted partial regression of the hepatocellular phenotype such that a micronodular pattern (Figure 7e) appeared in which the healthier hepatocytes appeared to develop in the regions of the hepatic veins.
Figure 7.
Ang-1-induced angiogenic events are reversible. a: Western blot analyses done on ST and DT mice before Dox exposure demonstrated the presence of transgenic Ang-1 in the DT mouse liver. After exposure to Dox (+Dox), transgenic Ang-1 production was suppressed. Three individual samples are shown (DT1 to DT3). b–d: Histological examination of DT mice having Ang-1 expression suppressed for 2 weeks with Dox returned the liver morphology back to normal with a uniform redistribution of veins. c and d: Histologically, normal portal tracts and normal hepatic veins without dilatation were observed in DT livers and the portal tracts contained veins, arteries, and ducts. e: Alternating regions of atrophic and nonatrophic hepatocytes were seen resembling the pattern of NRH, which may account for the mechanism of reversal. a, artery; d, duct; hv, hepatic vein; pv, portal vein; pt, portal tract. Scale bars: ∼65 μm (b and d); ∼33 μm (c).
To assess whether the liver phenotype formed as a result of excess Ang-1 during development, or whether we could recapitulate the phenotype by expressing Ang-1 in adulthood only, groups of animals were given Dox to suppress Ang-1 expression beginning at the time of conception until 6 weeks of age. Dox was removed and animals were evaluated 0 days (Figure 8, a and e), 4 weeks (Figure 8, b and f), 8 weeks (Figure 8, c and g), and 14 weeks (Figure 8, d and h) later. Mice exposed to Dox showed no transgene expression evidenced by lack of LacZ staining and after 4 weeks of Dox withdrawal, DT livers showed small alterations in the capsular surface vasculature at the lateral edges of the liver (Figure 8b), with low levels of LacZ staining. These findings were confirmed at 8 and 14 weeks after Dox removal (Figure 8; c and d, g and h), in which only moderate increases in vessel size were observed. Transgenic Ang-1 expression (indicated by LacZ staining), albeit present, did not ever reach the levels achieved during development (Figure 1, e and g) suggesting that perhaps if the developmental levels of Ang-1 could be attained the phenotype might be recapitulated in its entirety.
Figure 8.
Suppressing Ang-1 production during development and subsequent exposure to excess Ang-1 in adulthood leads to moderate levels of vascular remodeling. a and e: Mice exposed to Dox throughout gestation and into adulthood show no production of Ang-1 (as indicated by LacZ staining) and have completely normal looking livers. Transgenic Ang-1 production returns (as indicated by LacZ staining) 4 weeks (b, f), 8 weeks (c, g), and 14 weeks (d, h) after the removal of Dox exposure. Mild to moderate changes in the vasculature are observed at the edges of the liver as evidenced by moderate increases in vessel visibility and vessel size. No gross changes in vessel reorganization in the subcapsular space were seen in any of the DT livers.
Discussion
Here, we conditionally expressed Ang-1 in a hepatocyte-specific manner to determine the role of Ang-1 in liver development. DT mice lived through gestation but displayed a gross dilation of vessels on the subcapsular surface of the liver. Closer examination revealed a loss of portal veins and signs of AV shunt formation similar to that observed in human liver disease. In more severe cases, focal growths, peliosis, areas of focal necrosis, and hepatic vein remodeling were found. Most interesting was the finding that these phenotypes were completely reversed 2 weeks after extinguishing transgene expression, and may occur via a mechanism of nodular regeneration. In contrast to these developmental results, we were unable to recapitulate the phenotype in mice exposed to excess Ang-1 beginning in adulthood. This may simply reflect the lower transgenic Ang-1 levels we obtained after 9 weeks of transgene suppression.
In mice exposed to Ang-1 throughout development, we observed dilation of hepatic veins, loss of portal veins, and the formation of AV shunts (evidenced by Tie2-positive immunoreactivity) in the Ang-1-expressing mice. The presence of dilated hepatic veins and portal vein hypoplasia may reflect either the loss of directionality because of expression of the Ang-1 in hepatocytes or perhaps the high ambient blood pressure in the sinusoidal bed leading to lack of development of the portal veins. This latter theory is supported by our developmental observations that no differences in sinusoidal size were observed in the 0-day-old DT mice and that by 2 weeks of age sinusoidal dilation begins appearing in the presence of normal peripheral portal veins, however by 4 weeks of age, visible arterial sprouting accompanied by dilatation of hepatic veins and a marked reduction in portal veins was seen. These shunts appear to be a result of Ang-1-induced sprouting angiogenesis confirming reports of others that showed Ang-1-mediated sprouting in vitro44–46 and in vivo.47 These findings suggest that the dilatation of hepatic veins and sinusoids appears to be secondary to increased arterial flow. Consistent with this interpretation is that during this period of postnatal development, the liver is maturing with rapid elongation of the portal tracts (including the portal arteries) therefore the portal vein hypoplasia observed most likely represents the failure of these veins to develop. This theory is further supported in that no evidence of thrombosis or other degenerative changes were observed in these animals. Additional evidence supporting this theory is that in adult DT mice in which transgene expression was suppressed until development and maturation had occurred, no portal vein hypoplasia was observed, rather mild to moderate vascular dilation, confirming previous reports of increased vascularization and vessel diameter after exposure to Ang-1 in the skin and heart.34,47,48 Notwithstanding these arguments, as the Ang-1 transgene levels were significantly less than Ang-1 levels obtained during development, we cannot rule out a dose-response effect, such that if the Ang-1 protein levels were higher in the adult, sprouting could occur, leading to portal vein stasis, thrombosis, and hepatic vein dilation.
In the more severely affected DT mice exposed to Ang-1 during development, focal necrosis, large hepatocellular nodules, venous remodeling, and peliosis hepatis were all observed. Unique to this model is its similarity to the human condition of FNH which is accompanied with longstanding augmentation of angiogenesis, and recently has been found to correlate with increased Ang-1 expression.27 It has been demonstrated that some FNH lesions are associated with hepatic vein obstruction, and this event may trigger a secondary hormonal response that augments the regenerative capacity of the hepatocytes in the affected regions.49–51 Both animals with the large nodules had evidence of previous obliteration of medium and large hepatic veins in the region of the tumors, suggesting that this may be an early lesion rather than a secondary event.
To assess whether the observed developmental phenotypes were Ang-1-dependent and could be reversed, we silenced transgene expression by exposing the animals to Dox in their water as reported previously.35,41 The finding of normal portal veins and absence of arterial sprouts after only 2 weeks of suppression of Ang-1 is consistent with the view that inhibition of an ongoing chronic angiogenic stimulus may have allowed the veins to grow and, indeed, catch up with the development of other portal tract elements. We interpret this to be a result of a partial reduction in the arterial flow near the portal tracts and nearly complete reduction of pressure in the more peripheral regions near the hepatic veins. Of particular interest in mice treated with Dox for 2 weeks was the noted partial regression of the hepatocellular phenotype. We observed a micronodular pattern in which the healthier hepatocytes appeared to develop in the regions of the hepatic veins, whereas this restitution had not yet occurred in the periportal regions suggesting the return of the hepatocytes to their normal phenotype may be related to the character of the blood flow or pressure in the sinusoids. There is striking similarity of this nodular pattern to conditions in the human liver of NRH52 and nodularity adjacent to infarcts of Zahn53 where angiogenesis is known to occur.54 The finding of large nodules in two of our older DT animals and a pattern of NRH after withdrawal of Ang-1 expression in two animals suggest that this model may be useful for the study of these various nodular lesions of the human liver. However, it must be noted that the severity of the phenotype of these mice was assumed to not include the hepatocellular nodules and focal necrosis phenotypes, as these were observed only in older mice, and we began Dox treatment at 5 weeks of age. Thus our conclusions regarding reversibility should be limited to include reversal of the enlarged arteries, arterial sprouting, dilation of hepatic veins, and portal vein hypoplasia.
Angiogenesis occurs during the development of cirrhosis, NRH, FNH, Budd-Chiari syndrome, hepatitis, and hepatocellular carcinoma. The present model has many features that may aid in the elucidation of possible mechanisms involved in the development and regression of the angiogenic component of human cirrhosis and other chronic hepatic lesions. Human liver diseases are accompanied by marked obliteration of the peripheral portal and hepatic veins with secondary sprouting of the arterioles.1,4,8,17 In fact, in human cirrhosis the fibrous septa may disappear throughout time while the disease is inactive nonetheless, the portal hypertension often remains, along with histological evidence of unaccompanied arteries in the parenchyma as seen in this animal model. This situation suggests that the limiting step in the regression of cirrhosis is not removal of collagen but rather the regeneration of the portal venous circuit. It offers the possibility that the secondary angiogenesis may be preventing the restitution of the portal vein circuit and offers the hope that the control of excessive angiogenesis in the cirrhotic liver may aid the regression of cirrhosis and portal hypertension.
Our developmental model provides a liver phenotype reminiscent of the human diseases, whereas our adult onset has yet to be able to reproduce the same effects. This work, albeit not an exact animal model of liver disease, does provide important insight into the importance of angiogenesis in preventing regeneration of the portal venous bed and may explain the continued presence and mechanism of portal hypertension in individuals with otherwise regressed cirrhosis. Our findings may provide additional information that may aid in providing treatment strategies, incorporating the targeting of fibrotic events or hepatocyte toxicity, in addition to targeting the liver vasculature and uncontrolled angiogenesis.
Acknowledgments
We thank Maribelle Cruz for her assistance with animal husbandry and Janet Koprivnikar for her help with the micro-CT imaging.
Footnotes
Address reprint requests to Daniel J. Dumont, Sunnybrook and Women’s College Research Institute, 2075 Bayview Ave., Research Building, S-227, Toronto, Ontario, Canada, M4N 3M5. E-mail: dan.dumont@sw.ca.
Supported by the Canadian Institute for Health Research (to N.L.W., J.G.S., R.M.H.), the Heart and Stroke Foundation of Canada (studentship to C.S.), and the National Cancer Institute of Canada (to D.J.D.).
D.J.D. is a Canadian Institute for Health Research Scientist and R.M.H. holds a Canada Research Chair in Imaging.
Current address of N.L.W.: Department of Anatomy, Case Western Reserve University, Cleveland, OH 44106.
References
- Huet PM, Villeneuve JP, Pomier-Layrargues G, Marleau D. Hepatic circulation in cirrhosis. Clin Gastroenterol. 1985;14:155–168. [PubMed] [Google Scholar]
- Wanless IR. Epithelioid hemangioendothelioma, multiple focal nodular hyperplasias, and cavernous hemangiomas of the liver. Arch Pathol Lab Med. 2000;124:1105–1107. doi: 10.5858/2000-124-1105-EHMFNH. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- Wanless IR. Benign liver tumors. Clin Liver Dis. 2002;6:513–526. doi: 10.1016/s1089-3261(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488–496. doi: 10.1002/hep.510270224. [DOI] [PubMed] [Google Scholar]
- DeLeve LD. Vascular liver diseases. Curr Gastroenterol Rep. 2003;5:63–70. doi: 10.1007/s11894-003-0011-0. [DOI] [PubMed] [Google Scholar]
- Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443–446. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13:19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- Mochida S, Ishikawa K, Inao M, Shibuya M, Fujiwara K. Increased expressions of vascular endothelial growth factor and its receptors, flt-1 and KDR/flk-1, in regenerating rat liver. Biochem Biophys Res Commun. 1996;226:176–179. doi: 10.1006/bbrc.1996.1329. [DOI] [PubMed] [Google Scholar]
- Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30:911–915. doi: 10.1016/s0168-8278(99)80147-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, Ikeda H, Ohno A, Shibuya M, Fujiwara K. Expressions of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun. 1999;254:587–593. doi: 10.1006/bbrc.1998.9984. [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999;155:1065–1073. doi: 10.1016/S0002-9440(10)65209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol. 2001;16:1319–1328. doi: 10.1046/j.1440-1746.2001.02576.x. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135–1148. doi: 10.1053/jhep.2001.29624. [DOI] [PubMed] [Google Scholar]
- Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Kraizer Y, Mawasi N, Seagal J, Paizi M, Assy N, Spira G. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287:209–215. doi: 10.1006/bbrc.2001.5548. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Yamashita Yi Y, Ohga T, Shirabe K, Shimada M, Wands JR. Tie2 vascular endothelial receptor expression and function in hepatocellular carcinoma. Hepatology. 2002;35:861–867. doi: 10.1053/jhep.2002.32535. [DOI] [PubMed] [Google Scholar]
- Dhar DK, Naora H, Yamanoi A, Ono T, Kohno H, Otani H, Nagasue N. Requisite role of VEGF receptors in angiogenesis of hepatocellular carcinoma: a comparison with angiopoietin/Tie pathway. Anticancer Res. 2002;22:379–386. [PubMed] [Google Scholar]
- Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103:341–345. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Ohtsuru A, Futakuchi M, Kawashita Y, Nagayama Y, Fukuda E, Namba H, Shirai T, Kanematsu T, Yamashita S. Distinctive gene expression of receptor-type tyrosine kinase families during rat hepatocarcinogenesis. Int J Mol Med. 2002;9:473–480. [PubMed] [Google Scholar]
- Paradis V, Biáeche I, Dargáere D, Laurendeau I, Nectoux J, Degott C, Belghiti J, Vidaud M, Bedossa P. A quantitative gene expression study suggests a role for angiopoietins in focal nodular hyperplasia. Gastroenterology. 2003;124:651–659. doi: 10.1053/gast.2003.50104. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Bahary N, Zon LI. Development. Endothelium—chicken soup for the endoderm. Science. 2001;294:530–531. doi: 10.1126/science.1066282. [DOI] [PubMed] [Google Scholar]
- Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, Efstathiou JA, Holmgren L, Adamis AP, Rupnick M, Folkman J, O’Reilly MS. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237:530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lèubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, Goelman G, Keshet E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, Van Slyke P, Sturk C, Cruz M, Dumont DJ. Angiopoietin 1 expression levels in the myocardium direct coronary vessel development. Dev Dyn. 2004;229:500–509. doi: 10.1002/dvdy.10479. [DOI] [PubMed] [Google Scholar]
- Sarao R, Dumont DJ. Conditional transgene expression in endothelial cells. Transgenic Res. 1998;7:421–427. doi: 10.1023/a:1008837410485. [DOI] [PubMed] [Google Scholar]
- Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- Jones N, Dumont DJ. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–1108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- Feldkamp LA, Davis LA, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1:612–619. [Google Scholar]
- Adams RBL. Seeded region growing. IEEE Trans Pattern Analysis Mach Intell. 1994;16:641–647. [Google Scholar]
- Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–445. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- Zhu WH, MacIntyre A, Nicosia RF. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am J Pathol. 2002;161:823–830. doi: 10.1016/S0002-9440(10)64242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Rangheard AS, Vilgrain V, Audet P, O’Toole D, Vullierme MP, Valla D, Belghiti J, Menu Y. Focal nodular hyperplasia inducing hepatic vein obstruction. Am J Roentgenol. 2002;179:759–762. doi: 10.2214/ajr.179.3.1790759. [DOI] [PubMed] [Google Scholar]
- Arriv̄ae L, Dahan H, Tubiana JM. Hepatic vein obstruction in a case of focal nodular hyperplasia. Am J Roentgenol. 1999;173:857. doi: 10.2214/ajr.173.3.10470963. [DOI] [PubMed] [Google Scholar]
- Haber M, Reuben A, Burrell M, Oliverio P, Salem RR, West AB. Multiple focal nodular hyperplasia of the liver associated with hemihypertrophy and vascular malformations. Gastroenterology. 1995;108:1256–1262. doi: 10.1016/0016-5085(95)90228-7. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Lentz JS, Roberts EA. Partial nodular transformation of liver in an adult with persistent ductus venosus. Review with hypothesis on pathogenesis. Arch Pathol Lab Med. 1985;109:427–432. [PubMed] [Google Scholar]
- Shimamatsu K, Wanless IR. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343–350. doi: 10.1002/hep.510260214. [DOI] [PubMed] [Google Scholar]
- Wanless IR. Physioanatomic considerations. Schiff ER, Maddrey WC, editors. Philadelphia: Lippincott-Raven,; Schiff’s Disease of the Liver. 2002:pp 3–37. [Google Scholar]