Abstract
Chemokines and cytokines play a critical role in HIV infection, serving both to modulate virus replication and to recruit target cells to the site of infection. Platelet-derived growth factor (PDGF), a mitogen and chemoattractant for a wide variety of cells, is secreted by macrophages. Since macrophages are the target cells for lentiviral infection in the brain and PDGF is a known inducer of macrophage chemoattractant protein-1 (MCP)-1, a potent chemokine closely associated with HIV encephalitis, we investigated the association of PDGF-B chain (PDGF-B) with encephalitis in macaques caused by simian human immunodeficiency virus (SHIV), a chimera of HIV and SIV. Northern blot analysis confirmed elevated expression of PDGF-B chain mRNA in the brains from encephalitic macaques. Validation of these in vivo studies was confirmed in rhesus macrophage cultures infected with SHIVKU2 in which we demonstrated heightened expression of PDGF-B chain mRNA. Nuclear run-off analysis established transcriptional up-regulation of PDGF-B chain in virus-inoculated macrophage cultures. Reciprocally, addition of exogenous PDGF enhanced virus replication and MCP-1 expression in these cells. Inhibition of virus replication by tyrosine kinase inhibitor, STI-571, and by PDGF-B antisense oligonucleotides confirmed the specificity of the PDGF effect. Relevance of these findings was confirmed by analysis of archival brain tissue from SHIV encephalitic and non-encephalitic macaques for PDGF-B chain expression. PDGF-B chain protein expression was observed in the virus-infected cells in microglial nodules in the brains of SHIV-encephalitic macaques.
HIV-associated dementia (HAD) is a clinical disorder characterized by progressive cognitive, motor, and behavioral abnormalities1,2 caused by HIV-1 infection. Prominent neurological disease occurs in 15 to 20%3 of infected individuals and is associated with a marked depletion of CD4+T lymphocytes.4–6 HIV-1 encephalitis (HIVE), a common pathological manifestation of HAD includes infiltration of macrophages into the brain where they become productively infected with the virus.7,8 This is accompanied by considerable cytokine9 and chemokine10–12 dysregulation in the brain that often culminates into the unique pathological features that characterize this syndrome. Characteristic pathological changes of HIVE include perivascular and parenchymal accumulations of mononuclear cells, formation of microglial nodules and multinucleated giant cells, activation and proliferation of astrocytes, and neuronal dysfunction and loss.13,14 In the central nervous system (CNS), the majority of the cells that support productive viral replication are monocyte-derived macrophages and microglia.11,14 HIV invades the CNS early after infection where it later gives rise to cognitive, motor, and behavioral manifestations. A possible explanation of the emergence of productive virus replication in HIV-dementia in the brain is the increased monocytic infiltration across the blood-brain barrier. Although the mechanisms leading to entry of monocytes in the brain still remain unclear, it is speculated that secretory products, including CC chemokines, released by HIV-1-infected cells could be potentially important for recruitment of monocytes from blood to the brain. One such CC chemokine, macrophage chemoattractant protein (MCP)-1, has been shown to be the most potent among a variety of other major chemokines closely associated with AIDS dementia.15
Macaques infected with neuropathogenic strains of SIV12,16,17 and SHIV12,18,19 develop many of the pathological and behavioral changes observed in HIVE. These non-human primate models have served as excellent working models to explore the role of various cytokines and chemokines in enhancement of virus replication. Our recent findings aimed at highlighting gene expression profiles that accompany encephalitis demonstrated an up-regulation of platelet-derived growth factor-B chain (PDGF-B).20 This growth factor has hitherto not been recognized for having a role in the pathogenesis of HIV dementia. Four known PDGF ligands (A-D) have been described that can exert their effect via specific cell membrane receptors designated α and β.12,18,21,22 PDGF is a known mitogen and chemoattractant for a number of cell types both in vitro and in vivo and can activate early transcription of a number of otherwise quiescent genes, several of which encode potent cytokines and other proto-oncogenes.23 In addition to its role in various pathophysiological conditions24–32. PDGF is a known inducer of MCP-1,33 a chemokine that plays a pivotal role in HIVE.15,34 We therefore hypothesized that regulation of PDGF expression is critical for the development of the syndrome. In this study we therefore sought to explore the association of PDGF-B chain in virus replication in macaque macrophage cultures and in macaques with SHIV-E.
Materials and Methods
Viruses
We obtained SHIV-4 DNA encoding the env, tat, rev, and vpu genes of HIV-1 HXBc2 on a background of SIVmac239,35 from Dr. Joseph Sodroski, Harvard University. Viral DNA was transfected into CEMx174 cells to produce a virus that was used to initiate sequential passages in macaques. Virus isolated from cerebrospinal fluid obtained 8 weeks post-inoculation from pig-tailed macaque PNb in passage four was amplified in a culture of peripheral blood mononuclear cells (PBMC) from a normal macaque and subsequently designated as SHIVkU-136. A further passage of this virus in rhesus macaques gave rise to SHIVkU-237, the virus used in our study.
Macrophage Cultures
PBMCs from rhesus macaques were obtained by Ficoll-Hypaque (Sigma, St. Louis, MO) gradient centrifugation and suspended at a concentration of 2 × 106 cells/ml in macrophage differentiation medium consisting of RPMI medium supplemented with 20% heat-inactivated human serum, 5 U/ml of M-CSF (PeproTech, Inc, Rocky Hill, NJ), 100 U/ml of GM-CSF (PeproTech), and 5% heat-inactivated rhesus monkey serum. Six-well dishes (Costar, Cambridge, MA) were seeded with 3 ml of medium containing 6 to 10 × 106 cells/per well and incubated overnight at 37°C. Cultures were then rinsed to remove non-adherent cells, re-fed with macrophage differentiation medium, and maintained for 7 days to allow adherent monocytes to differentiate into mature macrophages. Cells in our cultures were exclusively macrophages as greater than 98% of them expressed the CD14 monocyte/macrophage-specific cell surface marker (data not shown). Experiments involving effects of infection on PDGF expression were performed in 6-well plates. The cells were inoculated with cell-free SHIVkU-2, at a multiplicity of 0.1 for 24 hours at 37°C. Cultures were then rinsed three times with RPMI medium and replenished with macrophage medium. All experiments involving treatment of cells with exogenous PDGF-BB protein were conducted under serum-free conditions since serum induces PDGF. Studies were performed on macrophage cultures derived from at least three separate animals and each experiment was performed in triplicate.
RT-PCR Analysis of PDGF mRNA in the Normal Rhesus Macrophage Cultures
Total RNA from macrophage cultures was extracted with Trizol (GIBCO BRL, Carlsbad, CA) and subjected to RT-PCR analysis using macaque-specific PDGF primers (Table 1). For RT reactions, one μg of total RNA from each sample was used in the Titan One-Tube RT-PCR System (Boehringer-Mannheim, Indianapolis, IN). The presence of any DNA contamination in the RNA preparations was tested by duplicate reactions, and subjecting one of them to 99°C for 2 minutes followed by 3 minutes at 95°C, to inactivate the RT activity. When present, DNA was removed with DNase I (Life Technologies, Gaithersburg, MD), followed by extraction. The reactions were carried out in a Perkin-Elmer DNA Thermal Cycler 480 with a temperature profile of 42°C for 30 minutes, 1 cycle; 94°C for 5 minutes, 1 cycle; 94°C for 30 seconds, 55°C for 30 seconds, 68°C for 45 seconds, 10 cycles; 94°C for 30 seconds, 55°C for 30 seconds, 68°C for 45 seconds, 5 second extension/cycle, 30 cycles; and 68°C for 6 minutes. PCR products were resolved by electrophoresis in 2% agarose gels (SeaKem MR; FMC BioProducts, Rockland, ME) in 0.04 mol/L Tris-acetate (pH 8.5)-0.001 mol/L EDTA containing 0.05 mg of ethidium bromide per ml. The UV fluorescence of cDNA bands was measured with a Kodak Imaging System (Eastman Kodak Company, Rochester, NY).
Table 1.
Primers for Semi-Quantitative RT-PCR Analyses of Platelet-Derived Growth Factor
| Primer | Position | Size of the PCR product (bp) | |
|---|---|---|---|
| PDGF-A | Forward 5′-TTT TCT GCC ATG CCT AAG TGT G-3′ | 1986–2007 | 494 |
| chain | Reverse 5′-GTG GAA AGT CAT TCA TCA CAG GG-3′ | 2460–2480 | |
| PDGF-B | Forward 5′-GCA CAC GCA TGA CAA GAC GGC-3′ | 1663–1683 | 461 |
| chain | Reverse 5′-AGG CAG GCT ATG CTG AGA GGT CC-3′ | 2102–2124 | |
| PDGFRA | Forward 5′-CCT GTA ACC TTA CAC AAC AGT GAG G-3′ | 611–635 | 432 |
| Reverse 5′-TTT CTT TGA CCT CCC TGG TAG C-3′ | 1022–1043 | ||
| PDGFRB | Forward 5′-ATG TCT ACA GAC TCC AGG TGT C-3′ | 1089–1110 | 443 |
| Reverse 5′-CTT TGA ACC ACA GGA CAG TGG-3′ | 1512–1532 |
GenBank accession numbers: PDGF-A chain - S 62078; PDGF-B chain - X 02811; PDGFRA - M21574; PDGFRB - BCO32224.
Determination of c-Fos Induction
Rhesus macrophages grown in chamber slides were rendered quiescent by serum starving for 48 hours and treated for 30 minutes with PDGF-BB (100 ng/ml). Cells were washed with PBS, fixed in Zn-formalin for 20 minutes, and then incubated overnight at room temperature with a rabbit polyclonal serum against c-Fos protein (Oncogene Research Products, San Diego, CA). Biotinylated goat anti-rabbit IgG (Dako Laboratory), peroxidase-conjugated streptavidin (Dako Laboratory), and NovaRed substrate (Vector Laboratories, Burlingame, CA), were used to visualize the reaction, which yields a reddish reaction product.
Slot Blot Analysis of PDGF-B Chain Following Inoculation with UV-Inactivated SHIV
RNA from rhesus macrophages was extracted by Trizol (Life Technologies) and the integrity of the RNA was confirmed by fractionation on 1.2% (wt:vol) agarose-formaldehyde gels and staining the ribosomal bands with ethidium bromide. For slot blot analysis, 0.6 to 5.0 μg aliquots of RNA were applied in a final 200 μl solution of 6.15 mol/L formaldehyde and 10X SSC (1.5 mol/L sodium chloride and 0.15 mol/L sodium citrate, pH 7.0) onto a Hybond-N+ nylon membrane (Amersham, Arlington Heights, IL). Five μg of ribosomal RNA was loaded onto the slot blot and used as a negative control. Total RNA from UV-inactivated SHIV-inoculated rhesus macrophages and control rhesus macrophages was denatured in a loading buffer containing 2.2 mol/L formaldehyde for 5 minutes at 65°C and electrophoresed in 1% (wt:vol) agarose gels containing 0.66 mol/L formaldehyde. The RNAs were transferred to nylon membrane using the capillary transfer technique. After transfer, the blots were air dried and fixed in an UV crosslinker (Stratagene, La Jolla, CA). The probe was labeled with deoxycytdine 5′-[α-32P] triphosphate by a random-primed labeling system (Amersham), with specific activity of 3.0 × 109 counts/min/μg DNA. Pre-hybridization (2 hours) and hybridization (overnight) conditions have been described earlier.38
Nuclear Run-Off Assay
Nuclear run-off transcript reactions were performed with isolated nuclei and [α-32P] UTP as described.39 Briefly, the nuclei from uninoculated and SHIVkU-2-inoculated rhesus macrophages were incubated with 10 μl of [α-32P] UTP (760 Ci/mmol, 10mCi/ml) for 30 minutes at 30°C. Labeled RNA was extracted by the phenol-chloroform and hybridization was carried out with equal numbers of labeled RNA counts on filters prepared by slot blotting of 10 μg of linearized plasmid probes. The filters were hybridized in plastic vials at 65°C for 48 hours, washed and exposed to x-ray film for detection of signal.
Effect of PDGF-BB on Virus Replication
Serum-starved rhesus macrophage cultures were inoculated with SHIVkU-2 for 4 hours at 37°C, following which the inoculated cells were maintained in the presence or absence of 100 ng/ml of recombinant PDGF-BB protein (R&D Systems). The medium was replenished every third day with fresh PDGF-BB. Supernatant fluids were collected at regular intervals for determination of SIV core antigen concentration by p27 ELISA (Coulter, FL, USA) as described by the manufacturer. Studies were performed on macrophage cultures from at least three separate animals and each experiment was performed in triplicate.
Synthetic Oligodeoxynucleotides
Purified oligodeoxynucleotides (ODNs) were synthesized using a 394 DNA/RNA synthesizer from Applied Biosystems (Foster City, CA). The 25-mer ODNs were targeted against the translation initiation site of PDGF-B chain. The antisense (AS) PDGF-B ODN sequence was 5′ TAC-AGC-AAA-TAC-CAT-ATT-AAA-CCC-T 3′, that for sense (S) PDGF-B cDNA was 5′ ATG-TCG-TTT-ATG-GTA-TAA-TTT-GGG-A 3′, and the scrambled (SC) PDGF-B ODN was 5′ TGC-AAT-TGA-GTA-TAA-TTT-GGG-TTT-C 3′. The rhesus macrophages cultures were infected as described above, following which the cultures were treated with ODNs (80 μmol/L/L) in the presence or absence of 100 ng/ml of recombinant PDGF-BB protein (R&D Systems). The medium was replenished every third day with fresh ODNs and PDGF-BB. To rule out non-specific effects of PDGF-B ODN, parallel experiments were carried out with an unrelated cytokine such as IL-8. Supernatant fluids were collected at regular intervals for determination of SIV core antigen concentration by p27 ELISA.
Inhibition of Virus Replication by Tyrosine Kinase Inhibitor, STI-571
Virus-infected rhesus macrophage cultures were incubated with or without (10 μmol/L) STI-571, an inhibitor of PDGF receptor tyrosine kinase (obtained from Novartis, Basel, Switzerland) for 3 hours and then stimulated with PDGF-BB (100 ng/ml). Supernatant fluids were collected at regular intervals for detection of virus antigen by ELISA.
MCP-1 Induction by PDGF-BB
To examine whether exogenous PDGF-BB could induce MCP-1 expression in rhesus macrophage cultures, both uninfected and infected cultures were maintained in serum-free medium and treated with exogenous PDGF-BB (100 ng/ml). Sequentially collected supernatant fluids were examined for MCP-1 protein using ELISA (R&D Systems). The ELISA detected concentrations as low as 10 pg/ml. Protein measurements were determined by comparison to a standard curve, which was run in duplicate with each assay.
Studies on Archival Brain Tissue
Five rhesus macaque monkeys previously used to define cytokine/chemokine gene expression profiles in the brain were used in this study. The five animals were infected with SHIV89.6P and all developed AIDS-defining illnesses. All five had also developed virus infection in the brain but only three of these animals developed CNS lesions and SHIV-E as demonstrated by histopathology of nine different regions of the brain.20 These three animals also had CNS-associated diverse opportunistic infections in the brain. The other two macaques died with AIDS but had no CNS lesions. Details of viral inoculation, disease course, processing of tissue samples, and histological analysis of the tissues have been described earlier.20 Prominent neuropathological changes were present in basal ganglia, motor cortex, and brain stem regions in the encephalitic animals.
Immunohistochemistry
Paraffin sections were used for immunohistochemical analysis. To detect the presence of PDGF-B chain protein or viral antigen, paraffin sections were treated with either a monoclonal antibody, PGF-007, directed against PDGF-B chain (obtained as a gift from Mochida Co, Japan) or FA2 antibody against SIV P27 (AIDS Reagent Center, National Institutes of Health, Bethesda, MD), followed by treatment with biotinylated goat anti-mouse IgG (Dako Laboratory), peroxidase-conjugated streptavidin (Dako Laboratory), and NovaRed substrate (Vector Laboratories), which yields a reddish reaction product.
Northern Blot Analysis of PDGF mRNA in the Brains of SHIV-Infected Rhesus Macaques with and without SHIV-E
Archival brain tissues form SHIV-infected macaques with and without SHIV-E were used in this study. RNA was isolated by homogenizing weighed portions of brain tissues in Trizol (Life Technologies) followed by precipitation with isopropanol. Briefly, total RNA (20 μg) from basal ganglion regions of the brains from SHIV-infected macaques with and without SHIV-E was denatured in a loading buffer containing 2.2 mol/L of formaldehyde for 5 minutes at 65°C and electrophoresed in 1% (w:v) agarose gels containing 0.66 mol/L of formaldehyde. The RNAs were transferred to nylon membrane using the capillary transfer technique. After transfer the blots were air dried and fixed in an UV crosslinker (Stratagene). To obtain PDGF-B chain riboprobe, macaque-specific PDGF-B chain sequences were amplified by PCR from a human PDGF-B chain cDNA (GenBank No. X02811) and subsequently cloned in a pGEM-T easy vector. The probe was labeled with deoxycytidine 5′-[α-32P] triphosphate by a random-primed labeling system (Amersham), with a specific activity of 3.0 × 109 counts/min/μg DNA. Prehybridization (2 hours) and hybridization (overnight) conditions were performed as described.38
Statistical Analysis
Two-factor analyses of variance with interaction were run to assess the effect of parameter and time on p27 and MCP-1 levels. When a significant interaction term was found (P ≤ 0.05), then pair-wise comparisons were performed across parameter at given time points and across time points within a specific parameter to determine exactly where significant differences occurred. For all pair-wise comparisons P ≤ 0.01 was deemed to be significant. Pair-wise comparisons at varying time points across parameters were not of interest and were not performed. Since we have destructive sampling in these experiments at each time point, time was considered a factor and not a repeated measurement.
Results
Expression of PDGF and Its Receptors in Rhesus Macrophage Cultures
In our previous studies, we had examined brains from five SHIV-infected macaques that succumbed to AIDS. Three of the five animals had developed CNS lesions and had lentiviral encephalitis associated with opportunistic infections. The two other animals died with AIDS but had no CNS lesions.20 Microarray analysis for cytokine and chemokine genes in the brains from the two groups of macaques demonstrated a up-regulation of PDGF-B chain in the brains of macaques with SHIV-E compared to brains of infected macaques without encephalitis.20 To understand whether macrophages, the primary target cells for virus production in the brain, expressed PDGF and its receptors, we assessed the expression of PDGF ligands, A & B chains, and their cognate receptors, α & β, by RT-PCR. As shown in Figure 1 A, these cells expressed RNA for PDGF-B chain and β receptor, however there was negligible detection of PDGF-A chain or its α receptor. Since microarray analysis of brains of macaques with and without SHIV-E revealed up-regulation of only the PDGF-B chain20 in this report, we focused only on the expression of the PDGF-B chain.
Figure 1.
A: Endogenous level of PDGF-A & B chains and PDGF receptors, α & β in rhesus macrophages. RNA from macrophage cultures was subjected to RT-PCR using macaque-specific PDGF primers. Amplified products were electrophoresed on 2% agarose gel. B: Nuclear staining of c-fos protein in serum-starved macrophages with (right) and without (left) PDGF-BB treatment. Rhesus macrophages rendered quiescent by serum starvation for 48 hours followed by stimulation with PDGF-BB (100 ng/ml) stained intensely with antibody directed toward c-fos protein. A reddish reaction product (NovaRed substrate) developed approximately 30 minutes after treatment, in and around the cell nuclei. Control cells which were not stimulated with PDGF-BB did not develop any color.
Cultured Rhesus Macrophages Have Functional PDGF β Receptors
Serum-starved macrophage cultures stimulated with PDGF-BB protein were stained intensely with an antibody directed toward c-Fos protein, whereas unstimulated did not (Figure 1B). Staining appeared approximately 30 minutes post-PDGF-BB treatment and was localized in the cell nuclei.
SHIVkU-2 Inoculation Enhanced PDGF-B Chain Expression in Rhesus Macrophage Cultures
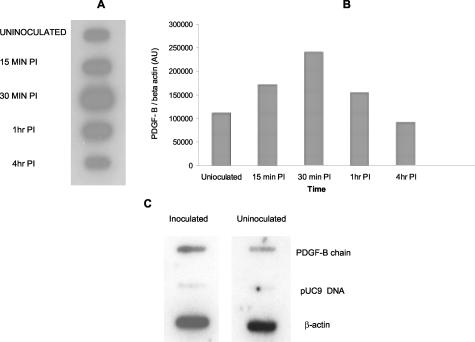
To determine whether our earlier in vivo microarray analysis20 of PDGF-B chain up-regulation in the brains of macaques with SHIV-E could be reproduced in cell culture, we inquired whether infection of macrophages with SHIVkU-2, could lead to an altered expression of PDGF-B chain RNA. Following inoculation with SHIVkU-2, rhesus macrophage cultures were harvested at various times post-inoculation and the RNA was assessed for expression of PDGF-B chain by slot blot analysis using 32P-labeled macaque-specific PDGF-B ribroprobe. As shown in Figure 2A, there was an increase in PDGF-B chain RNA at 30 minutes post-inoculation following which the levels returned to baseline values by 4 hours. A similar kinetic response was observed following inoculation of the cultures with UV-inactivated virus (data not shown). This indicated that transient virus-cell interaction was sufficient for induction of PDGF-B chain RNA, and that this process did not require infection or virus replication.
Figure 2.
A: Slot blot analysis of PDGF-B chain mRNA following treatment with UV-inactivated SHIV. Macrophages cultured in serum-free medium that were either uninoculated or treated with UV-inactivated SHIVkU- 2 were processed at varying times post-inoculation for RNA extraction. RNA was then spotted onto a slot blot and hybridized with [α-32 P] PDGF-B chain riboprobe. The autoradiogram is representative of two separate experiments. B: Laser densitometric scanning of the autoradiogram from uninoculated and inoculated macrophages. C: Nuclear run-off assay was performed with nuclei isolated from either uninfected or SHIVkU-2-treated macaque macrophages. Isolated nuclei were labeled with [α-32P] UTP and hybridized to specific cDNAS. PDGF-B chain intensity was normalized to that of β-actin. The figure is representative of two sets of experiments.
To determine whether the increase in PDGF-B chain expression following viral inoculation was mediated at the level of transcription, we performed nuclear run-off assays. The number of radioactive transcripts for PDGF-B chain synthesized in nuclear run-off assays by nuclei from uninfected and SHIVkU-2-inoculated macrophages (30 minutes post-inoculation) was assessed by hybridization of the transcripts to immobilized cDNAs for PDGF-B chain and β actin followed by autoradiography (Figure 2C). An increased rate of transcription for PDGF-B chain (1.6-fold) was noted in SHIVkU-2-inoculated macrophages as compared to uninoculated cells.
PDGF Enhances Virus Replication in Macrophage Cultures
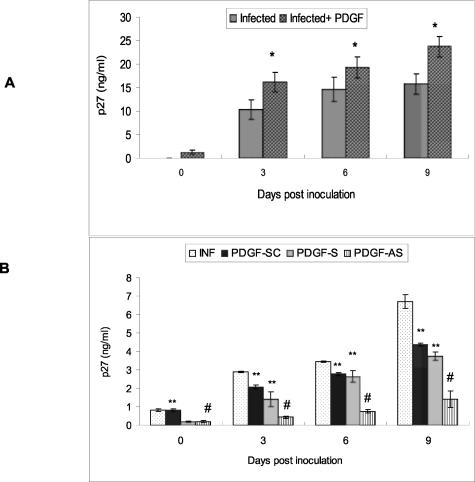
Since rhesus macrophage cultures express PDGF-B chain and its receptor, we inquired whether this factor had a role in regulating virus replication in these cells. SHIVkU-2-infected macrophage cultures were treated with 100 ng/ml of PDGF-BB in serum-free medium and the supernatant fluids collected periodically for assay of viral p27 protein by ELISA. As shown in Figure 3A, addition of PDGF led to increased viral p27 antigen production in the culture supernatants. To investigate whether the effect of PDGF-BB in modulating virus replication was specific, we tested the effect of antisense, sense, and scrambled PDGF ODNs on virus replication. As shown in Figure 3B, the inhibition of virus replication by PDGF-antisense ODN was highly significant (P < 0.0001) compared with that caused by sense and scrambled PDGF ODNs. This effect was specific for PDGF-BB since an unrelated cytokine, IL-8, had no effect on virus replication (data not shown).
Figure 3.
A: Effect of exogenous PDGF-BB on SHIVkU-2 virus replication. Supernatants of SHIV-infected rhesus macrophage cultures treated or untreated with recombinant PDGF-BB (100 ng/ml) were analyzed for p27 ELISA. B: Effect of PDGF-antisense ODNs on viral replication. p27 levels in supernatants of SHIV-infected rhesus macrophage cultures treated with PDGF-antisense, sense, and scrambled ODNs. All data represent the mean ± SEM for three values at each time point. The R2 value for the two-factor analysis of variance with interaction was 0.9606 (A) and 0 0.9863 (B), respectively. *, P < 0.01 infection versus infection plus PDGF; #, P < 0.0001 infection versus infection plus PDGF-antisense; **, P < 0.0001 PDGF-scrambled and sense versus PDGF-antisense.
STI-571, a Tyrosine Kinase Inhibitor, Inhibits PDGF-BB-Mediated Increase in SHIVkU-2 Replication in Macrophages
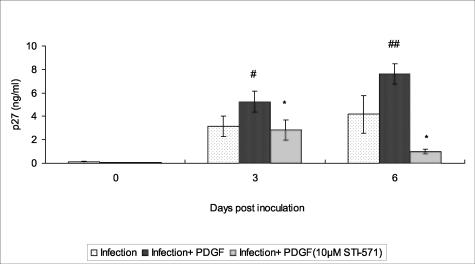
To further examine the specificity of PDGF-BB in enhancing virus replication, the effect of STI-571 (a selective inhibitor of PDGF receptor tyrosine kinase) on viral replication was monitored in rhesus macrophage cultures. As shown in Figure 4, SHIVkU-2-infected macrophages, when stimulated with PDGF-BB, showed enhanced virus replication with respect to time as compared to cells treated with virus alone. Treatment of virus-infected cells with the inhibitor, STI-571, followed by PDGF-BB treatment resulted in significant inhibition of PDGF-mediated increase in virus replication. These results clearly demonstrated that a PDGF-BB-mediated increase in viral replication occurs via activation and binding of PDGF to the PDGF-β receptor tyrosine kinase.
Figure 4.
Effect of STI-571 on viral p27 in PDGF-BB-treated SHIV-infected cultures. Supernatant fluids from SHIV-infected macrophage cultures treated with PDGF-BB or PDGF-BB plus STI-571 were monitored for viral p27 by ELISA. All data represent the mean ± SEM for three values at each time point. The R2 value for the two-factor analysis of variance with interaction was 0.9371. #, P < 0.005; ##, P < 0.0001, infection versus infection plus PDGF; *, P < 0.001 infection with PDGF versus infection with PDGF plus STI-571.
PDGF Induces MCP-1 in Rhesus Macrophages
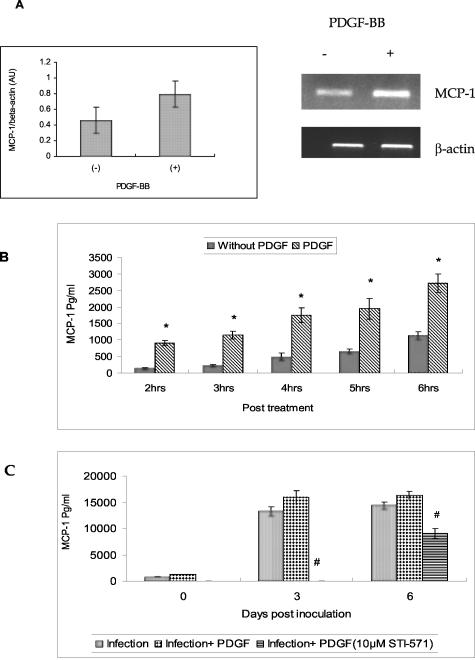
In addition to its role as a mitogen and chemoattractant,40,41 PDGF is also known to induce MCP-142 a potent chemokine whose levels are elevated in AIDS-dementia and whose function is thought to be the recruitment of monocytes from the blood to the brain.15,34 Since PDGF-B chain and MCP-1 were both up-regulated in SHIV-E,20,43 it was of interest to assess whether treatment of rhesus macrophages with PDGF-BB would also lead to induction of MCP-1 in rhesus macrophage cultures. In these studies serum-starved rhesus macrophage cultures were maintained in the presence or absence of PDGF-BB following which cells were harvested for MCP-1 RNA and the supernatant fluids collected for assay of MCP-1 protein by ELISA. As shown in Figure 5, treatment of macrophage cultures with exogenous PDGF-BB for 12 hours enhanced the expression of MCP-1 RNA (Figure 5A). This increase was also evident at the protein level (Figure 5B) in cells treated with PDGF-BB.
Figure 5.
A: Induction of MCP-1 mRNA following PDGF treatment. Laser densitometric scanning of MCP-1 RNA in macrophage cultures treated with and without exogenous PDGF-BB. RNA from cultures with and without the treatment was subjected to RT-PCR analysis using macaque-specific MCP-1 primers. B: Induction of MCP-1 protein following PDGF-BB stimulation. MCP-1 protein levels as measured by ELISA, in the culture supernatants of rhesus macrophage cultures that were treated with or without recombinant PDGF- BB at various time intervals. C: Inhibition of MCP-1 by STI-571. MCP-1 levels in the culture supernatants of SHIV-infected macrophage cultures that were treated either with recombinant PDGF-BB or PDGF-BB plus STI-571. All data represent the mean ± SEM for three values at each time point. The R2 value for the two-factor analysis of variance with interaction was 0.9695 (B) and 0.9993 (C), respectively. *, P < 0.0001 PDGF treated versus without PDGF treatment; #, P <0.0001 infection with PDGF versus infection with PDGF plus STI-571.
Since SHIV infection is also known to enhance MCP-1 expression,43 we also wanted to explore the effect of PDGF-BB and its inhibitor STI-571 on MCP-1 induction in infected macrophage cultures. MCP-1 protein expression was estimated, by ELISA, from the supernatant fluids collected sequentially on different days post-infection. As shown in Figure 5C, and similar to our previously published observations,43 SHIV infection was accompanied by enhanced MCP-1 protein expression in these cells even after 6 days post-infection. Treatment of infected macrophage cultures with PDGF-BB did not cause a significant increase in MCP-1 expression. Tyrosine kinase inhibitor, on the other hand, inhibited MCP-1 expression to levels lower than those cultures with infection alone indicating the role of PDGF in virus-mediated MCP-1 up-regulation.
Specificity of the PDGF-BB response in induction of MCP-1 was further confirmed by treatment of macrophage cultures with PDGF-B ODN and monitoring the inhibition of MCP-1 expression. Exposure of cells to antisense PDGF-B ODN resulted in almost 50% inhibition of MCP-1 protein production (data not shown). In contrast, the sense and scrambled PDGF-ODNs exhibited no such effect.
PDGF-B Chain Is Expressed in Encephalitic Brains of Rhesus Macaques Infected with CXCR4-Using Viruses
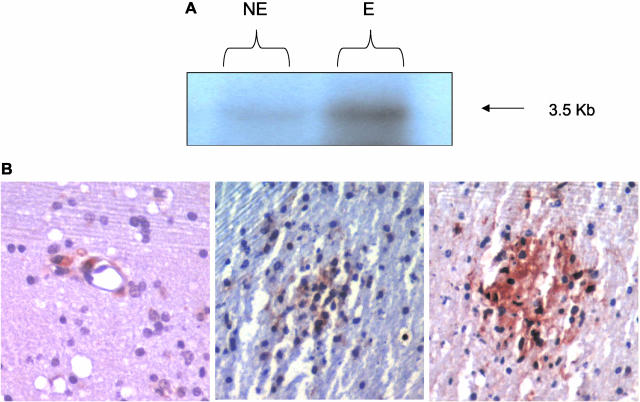
Induction of PDGF-B chain RNA following inoculation with SHIV prompted us to explore the possible implication of this phenomenon in vivo. Brain tissue from SHIV-infected macaques with and without encephalitis was assessed for expression of PDGF-B chain by Northern analysis. Equal amounts of RNA from brain samples from the two groups of animals were subjected Northern blotting using the labeled PDGF-B chain riboprobe. As shown in Figure 6A, the abundance of the 3.5-kb transcript of PDGF-B chain RNA was higher in the brains of encephalitic animals as compared to non-encephalitic animals. The localization of PDGF-B chain in the brains of encephalitic animals was further confirmed by immunohistochemical analysis. Brain sections from encephalitic animals demonstrated PDGF-B chain protein both in the perivascular macrophages and in the microglial nodules (Figure 6, left and middle panels). However, not all of the cells in the nodule stained positively for PDGF- B chain protein. On the other hand, the majority of the cells in these nodules comprised of virus-infected cells (Figure 6, right panel).
Figure 6.
A: Northern blot analysis of PDGF-B chain mRNA in the basal ganglion region of the brains of SHIV-infected macaques with (E) and without (NE) SHIV-E. The autoradiogram is representative of two separate experiments. B: Immunohistochemistry showing localization of PDGF-B protein in the perviascular macrophages (left) and in the microglial nodule (middle) in the basal ganglia region of the brains of macaques with SHIV-encephalitis. Immunohistochemistry for viral protein (right) in a serial section of the same brain region of the macaque showing viral-positive cells in the microglial nodule. Positive cells are indicated by a reddish reaction product. Original magnification, ×100 (right, middle, and left).
Discussion
Overexpression of the classical PDGFs, PDGF-A and PDGF-B, have been linked to several diseases, including cancer, fibrotic lung diseases, and atherosclerosis.24–28,44 Although two other novel PDGF ligands, PDGF-C and PDGF-D, have recently been discovered21,22 it has not yet been established whether these are linked to disease processes. PDGFs regulate a number of physiological and pathophysiological processes in many cell types via two tyrosine kinase receptors, PDGF α and β. While PDGF has been implicated in the pathogenesis of HIV-associated pulmonary hypertension45,46 and in AIDS-associated Kaposi’s sarcoma,47,48 its role in lentiviral encephalopathy has never been investigated. The impetus for the current study stems from the observation that in addition to its role in various disease phenotypes, PDGF-B is also known to induce the expression of MCP-1, a potent chemokine that is selectively accumulated in the brains and CSF of patients with AIDS dementia.15,34 Perhaps, the major role of MCP-1 in AIDS dementia is its ability to initiate the transmigration of monocytes across the blood-brain barrier.
Our preliminary findings on microarray analysis of brains of macaques with SHIV-E revealed an enhancement of PDGF-B chain RNA in infected macaques compared to animals without encephalitis.20 It was therefore of interest to first examine the endogenous levels of PDGF-A and B chains and their cognate receptors in cultured macrophages, the major cellular target for virus replication in the CNS.49 We demonstrated that rhesus macrophages express detectable levels of PDGF-B chain and β receptor RNA while the levels of the corresponding PDGF-A chain and α receptor were negligible. This suggested that the functional isoform in these cells is very likely PDGF-BB that could be functioning via the PDGF-β receptor.
Having determined that rhesus monocyte-derived macrophages express PDGF-B chain and its β receptor, it was of interest to explore whether virus infection could modulate changes in expression of these factors. Since PDGF is an early gene, we monitored its expression soon after virus attachment and observed that PDGF-B RNA was transiently up-regulated 30 minutes post-inoculation. Virus replication was not required for this induction since UV-inactivated virus had a similar effect. This suggested that the binding of the virus to its receptor on the macrophages transduced expression of PDGF gene expression. The mechanism of regulation of PDGF-B induction on virus binding occurred at the transcriptional levels as determined by nuclear run-off assays performed in infected versus uninfected cells.
Since PDGF is secreted by a variety of cells, it is possible that PDGF secreted by neighboring cells could affect virus replication. To simulate this effect, we tested whether treatment of SHIV-infected macrophage cultures with exogenous PDGF-BB could result in modulation of virus replication. We demonstrated that exogenous PDGF-BB enhanced virus replication in rhesus macrophage cultures. This effect was specific for PDGF as antisense PDGF-B ODN abolished this increase in virus replication. Furthermore, sense and scrambled PDGF-B ODNs exerted no inhibitory effect. Further corroboration of these findings was also demonstrated in experiments involving the tyrosine kinase inhibitor STI-571.44,50–53 Since PDGF exerts its effect via the tyrosine kinase receptors, blocking of the enzyme activity by STI-571 further confirmed the specificity of PDGF response.
In addition to its diverse functions in various pathophysiological states, PDGF is also known to stimulate expression of the immediate early gene set within target cells. A well-characterized example of a fast immediate early gene, such as c-fos, reaches peak levels within 30 minutes and returns to baseline within 2 hours.54,55
In contrast to the rapid but transient response exhibited by c-fos, slow immediate-early genes like c-myc and MCP-1 display a 60- to 90-minute lag time for initiation.56,57 While induction of MCP-1 by PDGF has been demonstrated in a variety of cell systems,58,59 its induction in monocyte-derived macrophages, the target cells for HIV replication in the CNS, has hithereto not been reported. Since virus infection leads to an increase in PDGF in the macrophages as shown above and because virus infection also leads to increased MCP-1 expression,43 it was essential to determine whether exogenous PDGF could, by itself, induce MCP-1 expression in rhesus macrophage cultures, irrespective of infection. Similar to other reports on induction of MCP-1, we found increased expression of both MCP-1 RNA and protein in macrophages treated with the growth factor. This effect was specific for PDGF-B since antisense PDGF-B ODN inhibited this induction. Corroborating the cell culture results, we found expression of PDGF-B chain RNA and protein were also up-regulated in the brains of macaques with SHIV encephalitis. Decreased expression was observed in infected macaques without encephalitis. Since the encephalitic lesions in the brains of macaques primarily comprise of cells of the macrophage lineage, it is speculated that virus-infected macrophages up-regulate PDGF-B chain and this in turn, induces MCP-1 that recruits more cells into the CNS. It was interesting that intense PDGF-B chain staining was observed in perivascular macrophages. In the microglial nodules that are comprised primarily of virus-infected cells, not all cells stained positively for PDGF-B chain. Furthermore, not all of the microglial nodules in the brain sections of encephalitic animals stained positively for PDGF-B chain protein. One possible explanation for this could be that macrophages exist in varying phenotypic states in the brain, and PDGF-B chain expression could be associated with macrophages with specific cell phenotype. It is also likely that the transient expression of PDGF could be responsible for this sporadic expression in only certain nodules.
Acknowledgments
We thank Dr. M. Sasahara, Toyama Medical and Pharmaceutical University, Japan for his invaluable advice, Novartis, Basel, Switzerland for providing us with STI-571, and Mingzhao Huang and Fenglan Jia for their technical help.
Footnotes
Address reprint requests to Shilpa J. Buch, Ph.D., Department of Microbiology, Immunology, and Molecular Genetics, 5000 Wahl Hall East, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS 66160. E-mail: sbuch@kumc.edu.
Supported by grants MH-62969–01, AI-29382, NS-32203, RR-16443, P51RR000165, and MH068212 from the National Institutes of Health, the KUMC Biomedical Research Training Program and the Swedish Research Council.
References
- Price RW, Sidtis JJ, Brew BJ. AIDS dementia complex and HIV-1 infection: a view from the clinic. Brain Pathol. 1991;1:155–162. doi: 10.1111/j.1750-3639.1991.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston: dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP. Dementia in AIDS patients: incidence and risk factors: Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Rosenberg ZF, Fauci AS. Immunopathology and pathogenesis of human immunodeficiency virus infection. Pediatr Infect Dis J. 1991;10:230–238. doi: 10.1097/00006454-199103000-00012. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Graziosi C, Fauci AS. The immunopathogenesis of human-immunodeficiency-virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Michaels J, Price RW, Rosenblum MK. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection, and fusion. Acta Neuropathol (Berl) 1988;76:373–379. doi: 10.1007/BF00686974. [DOI] [PubMed] [Google Scholar]
- Poli G, Vicenzi E, Ghezzi S, Lazzarin A. Cytokines in the acquired immunodeficiency syndrome and other infectious diseases. Int J Clin Lab Res. 1995;25:128–134. doi: 10.1007/BF02592553. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Dandekar S, Gardner MB. Neurobiology of simian and feline immunodeficiency virus infections. Brain Pathol. 1991;1:201–212. doi: 10.1111/j.1750-3639.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Sharer LR, Baskin GB, Cho ES, Murphey-Corb M, Blumberg BM, Epstein LG. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23(Suppl):S108–S112. doi: 10.1002/ana.410230727. [DOI] [PubMed] [Google Scholar]
- Smyth RJ, Yi Y, Singh A, Collman RG. Determinants of entry cofactor utilization and tropism in a dualtropic human immunodeficiency virus type 1 primary isolate. J Virol. 1998;72:4478–4484. doi: 10.1128/jvi.72.5.4478-4484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger KK, Dykhuizen M, Mitchen JL, Hinds PW, Preuninger BL, Wallace M, Thomson J, Montefiori DC, Lu Y, Pauza CD. CD4+-T-cell and CD20+-B-cell changes predict rapid disease progression after simian-human immunodeficiency virus infection in macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Potula R, Pinson D, Adany I, Li Z, Day J, Buch E, Segebrecht J, Villinger F, Liu Z, Huang M, Narayan O, Buch S. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. J Med Primatol. 2003;32:229–239. doi: 10.1034/j.1600-0684.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF α-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF β-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Deuel TF. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor: its potential roles in wound healing, atherosclerosis, neoplasia, and growth and development. Ciba Found Symp. 1985;116:98–112. doi: 10.1002/9780470720974.ch7. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW. Platelet-derived growth factor: its role in health and disease. Adv Exp Med Biol. 1988;234:9–21. doi: 10.1007/978-1-4757-1980-2_2. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70:III77–III82. [PubMed] [Google Scholar]
- Antoniades HN, Bravo MA, Avila RE, Galanopoulos T, Neville-Golden J, Maxwell M, Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud JM, Allam M, Martinet N, Pech M, Plenat F, Martinet Y. Presence of platelet-derived growth factor in normal and fibrotic lung is specifically associated with interstitial macrophages, while both interstitial macrophages and alveolar epithelial cells express the c-sis proto-oncogene. Am J Respir Cell Mol Biol. 1991;5:531–538. doi: 10.1165/ajrcmb/5.6.531. [DOI] [PubMed] [Google Scholar]
- Vignaud JM, Marie B, Klein N, Plenat F, Pech M, Borrelly J, Martinet N, Duprez A, Martinet Y. The role of platelet-derived growth factor production by tumor-associated macrophages in tumor stroma formation in lung cancer. Cancer Res. 1994;54:5455–5463. [PubMed] [Google Scholar]
- Brody AR. Control of lung fibroblast proliferation by macrophage-derived platelet-derived growth factor. Ann NY Acad Sci. 1994;725:193–199. doi: 10.1111/j.1749-6632.1994.tb39801.x. [DOI] [PubMed] [Google Scholar]
- Marinelli WA, Polunovsky VA, Harmon KR, Bitterman PB. Role of platelet-derived growth factor in pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:503–504. doi: 10.1165/ajrcmb/5.6.503. [DOI] [PubMed] [Google Scholar]
- Goppelt-Struebe M, Stroebel M. Synergistic induction of monocyte chemoattractant protein-1 (MCP-1) by platelet-derived growth factor and interleukin-1. FEBS Lett. 1995;374:375–378. doi: 10.1016/0014-5793(95)01155-8. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Li Z, Wang C, Jia F, Foresman L, Adany I, Pinson DM, Stephens EB, Narayan O. Chimeric SHIV that causes CD4+ T cell loss and AIDS in rhesus macaques. J Med Primatol. 1998;27:59–64. doi: 10.1111/j.1600-0684.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Covert J, Splitter G. Detection of cytokine transcriptional profiles from bovine peripheral blood mononuclear cells and CD4+ lymphocytes by reverse transcriptase polymerase chain reaction. Vet Immunol Immunopathol. 1995;49:39–50. doi: 10.1016/0165-2427(95)05451-b. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1:555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor and its role in health and disease. Philos Trans R Soc Lond B Biol Sci. 1990;327:155–169. doi: 10.1098/rstb.1990.0051. [DOI] [PubMed] [Google Scholar]
- Cochran BH, Reffel AC, Stiles CD. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Hicks A, Potula R, Sui YJ, Villinger F, Pinson D, Adany I, Li Z, Long C, Cheney P, Marcario J, Novembre F, Mueller N, Kumar A, Major E, Narayan O, Buch S. Neuropathogenesis of lentiviral infection in macaques: roles of CXCR4 and CCR5 viruses and interleukin-4 in enhancing monocyte chemoattractant protein-1 production in macrophages. Am J Pathol. 2002;161:813–822. doi: 10.1016/S0002-9440(10)64241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell-surface receptors in signal-transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Pellicelli AM, Palmieri F, Cicalini S, Petrosillo N. Pathogenesis of HIV-related pulmonary hypertension. Ann NY Acad Sci. 2001;946:82–94. doi: 10.1111/j.1749-6632.2001.tb03904.x. [DOI] [PubMed] [Google Scholar]
- Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, Emilie D. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV-seropositive and HIV-seronegative patients. Eur Respir J. 1998;11:554–559. [PubMed] [Google Scholar]
- Pistritto G, Ventura L, Mores N, Lacal PM, D’Onofrio C. Regulation of PDGF-B and PDGF receptor expression in the pathogenesis of Kaposi’s sarcoma in AIDS. Antibiot Chemother. 1994;46:73–87. doi: 10.1159/000423635. [DOI] [PubMed] [Google Scholar]
- Sturzl M, Roth WK, Brockmeyer NH, Zietz C, Speiser B, Hofschneider PH. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci USA. 1992;89:7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc Natl Acad Sci USA. 2001;279:658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom T, Shimizu A, O’Brien KP, Pietras K, Dal Cin P, Buchdunger E, Dumanski JP, Ostman A, Heldin CH. Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res. 2001;61:5778–5783. [PubMed] [Google Scholar]
- Tomasson MH, Williams IR, Hasserjian R, Udomsakdi C, McGrath SM, Schwaller J, Druker B, Gilliland DG. TEL/PDGFβR induces hematologic malignancies in mice that respond to a specific tyrosine kinase inhibitor. Blood. 1999;93:1707–1714. [PubMed] [Google Scholar]
- Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Gilliland DG, Druker BJ. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90:4947–4952. [PubMed] [Google Scholar]
- McGary EC, Weber K, Mills L, Doucet M, Lewis V, Lev DC, Fidler IJ, Bar-Eli M. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin Cancer Res. 2002;8:3584–3591. [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Kelly K, Siebenlist U. Mitogenic activation of normal T cells leads to increased initiation of transcription in the c-myc locus. J Biol Chem. 1988;263:4828–4831. [PubMed] [Google Scholar]
- Hall DJ, Alberta JA, Stiles CD. Labile repressors are involved in the transcriptional control of PDGF-responsive genes. Oncogene Res. 1989;4:177–184. [PubMed] [Google Scholar]
- Dean M, Levine RA, Ran W, Kindy MS, Sonenshein GE, Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986;261:9161–9166. [PubMed] [Google Scholar]
- Poon M, Hsu WC, Bogadanov VY, Taubman MB. Secretion of monocyte chemotactic activity by cultured rat aortic smooth muscle cells in response to PDGF is due predominantly to the induction of JE/MCP-1. Am J Pathol. 1996;149:307–317. [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Kim S, Ohta K, Nakao T, Miyazaki H, Nakatani T, Iwao H. Differential contribution of three mitogen-activated protein kinases to PDGF-BB-induced mesangial cell proliferation and gene expression. J Am Soc Nephrol. 2003;14:584–592. doi: 10.1097/01.asn.0000050415.97942.2f. [DOI] [PubMed] [Google Scholar]