Abstract
Aeromonas hydrophila, an uncommon human pathogen, can cause invasive infections in immunocompromised individuals. As the fluoroquinolones have been shown to be active in vitro against mesophilic aeromonads and clinical experience with the use of fluoroquinolones to treat aeromonads infections is limited, the antimicrobial activities of five selected drugs (ciprofloxacin, gatifloxacin, levofloxacin, lomefloxacin, and moxifloxacin) against A. hydrophila were studied in vitro and in mice. The MICs of the fluoroquinolones (except lomefloxacin), cefotaxime, and minocycline for 90% of 64 clinical isolates of A. hydrophila tested by the agar dilution method were ≤1 μg/ml. With a clinical cefotaxime-resistant strain, Ah 2743, in an in vitro time-kill study, at an inoculum of 7 × 105 CFU/ml incubated with fluoroquinolones, cefotaxime, or minocycline at concentrations equal to twice the MICs, the inhibitory effect lasted for less than 6 h and regrowth occurred thereafter. In an animal model with female BALB/c mice intraperitoneally infected with an inoculum of 1.1 × 107 CFU of Ah 2743, more mice in the ciprofloxacin-treated group survived (72.2%) than in the cefotaxime-, minocycline-, or cefotaxime-minocycline-treated group (P < 0.00001, log rank test). However, there were similar fatality rates, ranging from 71.4 to 87.5%, among mice treated with any of five fluoroquinolones. With a larger inoculum, 4.9 × 107 CFU, mice in the ciprofloxacin-treated group survived longer than those in the minocycline-, cefotaxime-, or cefotaxime-minocycline-treated group (30 h versus 18, 12, and 12 h, respectively [P < 0.002, log rank test]). However, in mice infected with cefotaxime-susceptible Ah 2556, ciprofloxacin was as effective as cefotaxime-minocycline. Thus, our results suggest that ciprofloxacin is at least as effective as cefotaxime-minocycline against murine A. hydrophila infections, which warrants clinical studies to delineate its role in human infections.
The genus Aeromonas is gram negative, oxidase positive, and a member of the family Vibrionaceae (1). Its separation from the genera Vibrio and Plesiomonas depends on resistance to the vibriostatic compound O/129, no growth in 6% sodium chloride, and absence of ornithine decarboxylase (except in A. veronii biovar veronii) (11). Aeromonads are ubiquitous in nature and can cause diseases in fish, reptiles, amphibians, and humans. Common clinical presentations are acute gastrointestinal illness, soft-tissue infections, and sepsis (12). Most invasive infections occur in patients with malignancies or liver cirrhosis (13) and are caused by A. hydrophila. The case fatality rate among patients with Aeromonas bacteremia ranges from 27.5 to 46% (5-7, 16).
In the literature, Aeromonas species isolates have been reported to be susceptible in vitro to a variety of antimicrobial agents, including expanded- or broad-spectrum cephalosporins, aminoglycosides, chloramphenicol, tetracycline, trimethoprim-sulfamethoxazole (18, 20), aztreonam, and fluoroquinolones (14). It has been demonstrated that cefotaxime and minocycline act synergistically against a cefotaxime-susceptible A. hydrophila isolate in vitro and in BALB/c mice with intraperitoneal infections (17). However, there is increasing resistance to broad-spectrum cephalosporins in clinical Aeromonas isolates in Taiwan (14). The optimal therapy for invasive infections caused by cefotaxime-resistant A. hydrophila is unknown.
The fluoroquinolones have been demonstrated to be as effective as cefotaxime-minocycline in vitro and in vivo at inhibiting Vibrio vulnificus (25), another marine pathogen causing a clinical presentation similar to that of A. hydrophila in cirrhotic patients in Taiwan (3). Clinical experience with ciprofloxacin in the treatment of human Aeromonas infections was very limited, and in the English literature, there were only several case reports describing successful treatment of Aeromonas gastrointestinal (19, 21, 24) and bloodstream (23) infections by ciprofloxacin. Comparative information about the antimicrobial activities of various fluoroquinolones or of fluoroquinolones and cefotaxime-minocycline were not available. Thus, the antibacterial activities of seven antimicrobial agents, including five fluoroquinolones, against A. hydrophila were evaluated both in vitro and in vivo and compared with that of cefotaxime-minocycline in the present study.
MATERIALS AND METHODS
Bacterial isolates.
Clinical isolates of A. hydrophila were collected from two medical centers in the Tainan area of Taiwan, i.e., the Chi Mei Foundation Medical Center and the National Cheng Kung University Hospital. In 2001, 64 strains were originally isolated from blood (25 strains), wounds (21 strains), bile (5 strains), pus (5 strains), sputum (3 strains), ascites (3 strains), and other body sites (2 strains). All isolates were identified as A. hydrophila by conventional biochemical methods as described previously (1). The organisms were stored at −70°C in Protect Bacterial Preservers (Technical Service Consultants Limited, Lancashire, England) before being cultured on Luria-Bertani agar (Difco Laboratories, Detroit, Mich.).
MIC and minimal bactericidal concentration (MBC) determinations.
The MICs of the following antimicrobial agents were determined by the agar dilution method as previously described (22): cefotaxime (Hoechst AG, Frankfurt, Germany), minocycline (American Cyanamid Co., Pearl River, N.Y.), moxifloxacin (Bayer AG, Frankfurt, Germany), gatifloxacin (Bristol-Myers Squibb, Humacao, Australia), levofloxacin (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan), ciprofloxacin (Bayer AG, Frankfurt, Germany), and lomefloxacin (Shionogi Pharmaceutical Co., Ltd., Osaka, Japan). All of the drugs except minocycline were incorporated into agar in serial twofold concentrations ranging from 0.00375 to 256 μg/ml, and the minocycline concentrations ranged from 0.03 to 128 μg/ml. The fluoroquinolone powder was dissolved in a 0.05 M NaOH solution, minocycline was dissolved in a 0.1 M NaOH solution, and cefotaxime was dissolved in sterile water, and then all were diluted with sterile water to the required test concentration. Final inocula of approximately 104 CFU per spot of inoculum were applied to the plates and incubated at 37°C for 24 h. Escherichia coli ATCC 25922 was used in each run as a control.
MICs and MBCs were determined by the broth macrodilution method. Briefly, serial doubling dilutions of antimicrobial agents were made in Mueller-Hinton broth. The final bacterial suspension in the mixture was adjusted to 5 × 105 CFU/ml, and the exact colony count was measured by plating 10-fold serially diluted specimens of 100-μl aliquots on drug-free nutrient agar (Difco Laboratories). The mixtures of bacteria and drugs were incubated at 37°C for 24 h. The MIC was defined as the lowest antibiotic concentration preventing visible growth in broth. Subsequently, 100 μl of the bacterial suspensions with no visible growth after 24 h of incubation was subcultured into drug-free nutrient agar at 37°C for 24 h. The MBC was defined as the lowest concentration of a drug yielding a colony count that was <0.1% of the initial inoculum.
Inhibitory effects of antimicrobial agents against A. hydrophila in time-kill studies.
Two A. hydrophila isolates, Ah 2743 and Ah 2556, originally isolated from the bloodstreams of two patients at the National Cheng Kung University Hospital, were selected for the time-kill studies. The former is resistant to cefotaxime (MIC, 128 μg/ml), and the latter is susceptible to cefotaxime (MIC, 0.03 μg/ml). Bacterial concentrations were diluted to approximate 5 × 105 CFU/ml in 25 ml of fresh Mueller-Hinton broth in a 125-ml conical glass flask. At minocycline or ciprofloxacin concentrations equal to four or more times the MIC, there was sustained in vitro inhibitory activity against Ah 2743 for at least 48 h, which was absent at lower drug concentrations. Therefore, the drug concentrations used in the following time-kill studies were adjusted to twice the MIC of each antimicrobial agent. Each flask was incubated at 37°C. Bacterial counts were examined at predetermined time points, i.e., 0, 2, 4, 6, 8, 12, 24, 30, 36, and 48 h, and measured by enumerating the colonies in 10-fold serially diluted specimens of 100-μl aliquots plated on nutrient agar (Difco Laboratories). The lower limit of detection was set at 10 colonies (100 CFU/ml). All experiments were performed at least twice for confirmation of the results.
In vivo efficacy of antimicrobial therapy in experimental mouse infection.
The marketed parenteral forms of cefotaxime, minocycline, and ciprofloxacin used in the in vivo experiments were provided by Hoechst Taiwan Co., Ltd.; Lederle Parenterals, Inc., Puerto Rico; and Bayer AG, respectively. Parenteral forms of moxifloxacin, levofloxacin, gatifloxacin, and lomefloxacin were not available in Taiwan, so their standard powders were diluted to the desired concentration. For the following three mouse experiments, solutions of antimicrobial agents were prepared on the morning of the day when each experiment was conducted, diluted in sterile 0.85% saline, and delivered intraperitoneally in a volume of 0.1 ml in sterile disposable plastic syringes. Antimicrobial agents were given at 2 h after the animal was infected.
Female inbred BALB/c mice (Animal Center, National Science Council, Taipei, Taiwan) weighing 20 g (5 to 6 weeks old), on average, were used throughout the study. A bacterial suspension in a volume of 0.1 ml was delivered subcutaneously into the right thigh of each mouse. The numbers of surviving mice were recorded at 6-h intervals after the initial treatment until 120 h. The experimental procedures and drug preparations were the same in all three mouse experiments, unless described specifically.
Experiment 1 was done to compare the therapeutic efficacies of ciprofloxacin, minocycline, cefotaxime, and cefotaxime-minocycline in mice infected with Ah 2743. In addition to the inoculum of 1 × 107 CFU, a larger inoculum of 5 × 107 CFU was used. There were five experiment groups: control (no antimicrobial agent was given), cefotaxime, minocycline, ciprofloxacin, and cefotaxime-minocycline. There were 9 or 10 mice in each group. The drug dosages given herein were as previously described (12, 17). Cefotaxime was given at 150 mg/kg of body weight every 6 h, and a loading dose of minocycline of 20 mg/kg of body weight was followed by a maintenance dose of 10 mg/kg every 12 h. A loading dose of ciprofloxacin of 16 mg/kg of body weight was followed by a maintenance dose of 8 mg/kg every 12 h. Antimicrobial agents were given for a total of 48 h. Control animals received 0.1 ml of sterile 0.85% saline.
The design of experiment 2 was intended to compare the efficacies of different fluoroquinolones for the treatment of mice infected with Ah 2743 at an anticipated inoculum of 107 CFU. There were six experiment groups with seven or eight mice in each group, including five groups treated with fluoroquinolones and a saline-treated control group. For moxifloxacin, levofloxacin, or gatifloxacin, a loading dose of 16 mg/kg of body weight was followed by a maintenance dose of 8 mg/kg every 24 h. For lomefloxacin, a loading dose of 8 mg/kg of body weight was followed by a maintenance dose of 4 mg/kg every 12 h. For ciprofloxacin, the doses were given as in experiment 1. Antimicrobial agents were given for a total of 48 h.
Previously, it has been demonstrated that among mice infected with cefotaxime-susceptible A. hydrophila, combination therapy with cefotaxime and minocycline results in a higher survival rate than does monotherapy with cefotaxime or minocycline (17). In experiment 3, the efficacy of ciprofloxacin in the treatment of mice infected with cefotaxime-susceptible Ah 2556 was compared with that of cefotaxime and minocycline in combination. There were three experiment groups, including one saline-treated group, with eight mice in each group. The doses and administration intervals of the three drugs were the same as those in experiment 1. All of our animal experiments were done in compliance with all of the relevant national guidelines of the Republic of China and with the Chi Mei Foundation Medical Center Animal Use Policy.
RESULTS
MIC and MBCs.
The results of antimicrobial susceptibility testing of 64 A. hydrophila isolates are shown in Table 1. The MICs of cefotaxime, minocycline, and all of the fluoroquinolones except lomefloxacin for 90% of the strains tested (MIC90s) were ≤1 μg/ml. The MIC of cefotaxime for Ah2743, used in in vitro time-kill and in vivo animal experiments, was 128 μg/ml, and that for Ah2556 in animal experiments was 0.03 μg/ml. By the broth macrodilution method, the MBC/MIC ratio of minocycline for Ah 2743 was 2 (2/1 μg/ml) and that of ciprofloxacin was 4 (0.06/0.015 μg/ml). For Ah2556, the MBC/MIC ratio of cefotaxime was 4 (4/1 μg/ml).
TABLE 1.
Susceptibilities of 64 clinical isolates of A. hydrophila to seven antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| For 50% of isolates | For 90% of isolates | Range | Ah 2556 | Ah 2743 | |
| Cefotaxime | 0.06 | 1 | 0.03-256 | 0.03 | 128 |
| Minocycline | 1 | 1 | 0.12-4 | 0.5 | 0.5 |
| Ciprofloxacin | 0.0075 | 0.5 | 0.00375-1 | 0.0075 | 0.0075 |
| Levofloxacin | 0.0075 | 0.25 | 0.00375-0.5 | 0.015 | 0.0075 |
| Gatifloxacin | 0.03 | 0.5 | 0.0075-1 | 0.03 | 0.03 |
| Moxifloxacin | 0.06 | 1 | 0.015-2 | 0.06 | 0.06 |
| Lomefloxacin | 0.06 | 2 | 0.03-4 | 0.06 | 0.06 |
Time-kill studies.
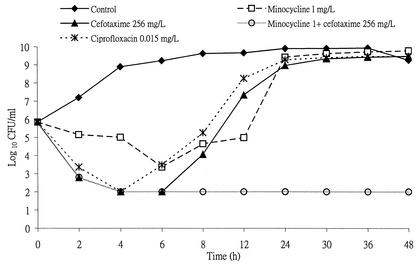
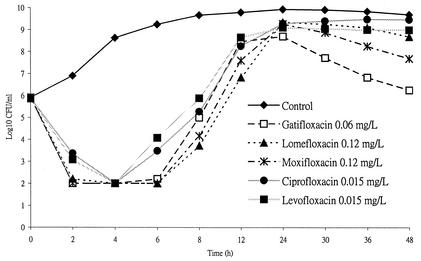
When Ah 2743 at an initial inoculum of 7 × 105 CFU/ml was incubated with cefotaxime, minocycline, or both in combination at a concentration equal to twice the MIC, bacterial growth was inhibited temporarily for 8 h, at most, in the case of minocycline, and A. hydrophila later regrew (Fig. 1). However, the combination of cefotaxime and minocycline demonstrated an enhanced effect on Ah 2743. Similar growth curves were noted when ciprofloxacin, levofloxacin, lomefloxacin, moxifloxacin, or gatifloxacin at a concentration equal to twice the MIC was incubated with Ah 2743 (Fig. 2). Each point plotted in Fig. 1 and 2 stands for the mean value of the results of duplicate experiments.
FIG. 1.
Time-kill curves of Ah 2743 at an initial inoculum of 7 × 105 CFU/ml after incubation with various drugs at a concentration equal to twice the MIC. L, liter.
FIG. 2.
Time-kill curves of Ah 2743 at an initial inoculum of 5.9 × 105 CFU/ml after incubation with fluoroquinolones at a concentration equal to twice the MIC. L, liter.
In vivo mouse study.
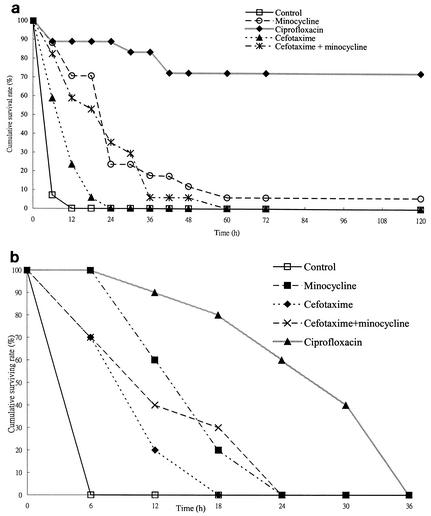
In experiment 1, with an inoculum of 1.1 × 107 CFU of Ah 2743, all mice in the control group died within 12 h (Fig. 3a). No mice treated with cefotaxime or cefotaxime plus minocycline and only one mouse treated with minocycline survived at 120 h. Thus, of four antimicrobial agent-treated groups, the survival rates at the end point were 72.2% in the ciprofloxacin-treated group, which is significantly higher than those in the other groups (P < 0.00001, log rank test). The other antimicrobial agent-treated groups had longer median survival times (12 h for the cefotaxime-treated group, 24 h for the minocycline-treated and cefotaxime-minocycline-treated groups) than the saline-treated control group (6 h; P < 0.003, log rank test). The survival time of the minocycline-treated or cefotaxime-minocycline-treated group was significantly longer than that of the cefotaxime-treated group (P < 0.003, log rank test). With a larger inoculum of 4.9 × 107 CFU, all of the mice, regardless of the antimicrobial regimen, died within 36 h (Fig. 3b). However, with regard to the median survival time of four antimicrobial agent-treated groups, mice in the ciprofloxacin-treated group survived significantly longer (30 h) than those in the other groups (6 h in the control group, 12 h in both the cefotaxime-treated and cefotaxime-minocycline-treated groups, and 18 h in the minocycline-treated group; P < 0.002, log rank test). Regardless of the inoculum size, the survival time of mice treated with minocycline was longer than that of mice treated with cefotaxime (P = 0.001 with an inoculum of 1.1 × 107 CFU; P < 0.03 with an inoculum of 4.9 × 107 CFU; log rank test).
FIG. 3.
Kaplan-Meier survival curves of five groups of mice infected with two inocula of Ah 2743, a cefotaxime-resistant bacteremic isolate, and treated with different antibiotics. (a) Inoculum of 1.1 × 107 CFU. (b) Inoculum of 4.9 × 107 CFU. The mice in all of the experimental groups, regardless of antimicrobial therapy, died within 36 h.
In experiment 2, with an inoculum of 9.6 × 106 CFU of Ah 2743, survival rates at 120 h among the mice treated with fluoroquinolones (five [71.4%] of seven in the ciprofloxacin-treated group, six [85.7%] of seven in the levofloxacin-treated group, seven [87.5%] of eight in the gatifloxacin-treated group, six [75%] of eight in the lomefloxacin-treated group, and seven [87.5%] of eight in the moxifloxacin-treated group) were significantly higher than that of the saline-treated control group (zero of seven; P < 0.01, log rank test) but not significantly different from each other (P > 0.05, log rank test).
With an inoculum of 1.2 × 107 CFU of Ah 2556 in experiment 3, all of the mice in the control group died within 24 h and all of the mice treated with ciprofloxacin or cefotaxime combined with minocycline survived until 120 h.
DISCUSSION
Many classes of antimicrobial agents, such as chloramphenicol, tetracycline, and trimethoprim-sulfamethoxazole (18, 20), aminoglycosides, broad-spectrum cephalosporins, imipenem, and meropenem (4), have been reported to be active against aeromonads. Until now, there was no well-designed, prospective, and randomized clinical trial to clearly delineate the optimal therapy for invasive Aeromonas infections. The rationale for the selection of optimal antimicrobial therapy can only be based on anecdotal case reports, in vitro susceptibility data, or in vivo animal experiments.
However, the list of rational antimicrobial therapies for Aeromonas infections is shortening as our knowledge of the mechanisms of β-lactam resistance is increasing. The finding of a coexisting chromosome-encoded cephalosporinase and carbapenemase (10, 26) in clinical isolates raises concerns about the emergence of resistant mutants during antimicrobial therapy (15). The emergence of cefotaxime resistance has been observed in 2 (3.4%) of 58 episodes of monomicrobial Aeromonas bacteremia treated with a cephalosporin (16). In Taiwan, only 50 and 60% of 234 clinical Aeromonas strains were susceptible to tetracycline and co-trimoxazole, respectively (14), which further decrease the potential choices for the treatment of Aeromonas infections.
Several studies have indicated that the excellent in vitro activity of fluoroquinolones against Aeromonas species (2, 18), as our study demonstrated that the fluoroquinolone MIC90s ranged from 0.25 μg/ml (levofloxacin) to 2 μg/ml (lomefloxacin). Information on the clinical use of fluoroquinolones for the treatment of Aeromonas infections in the English literature was mainly found in cases reports (8, 9, 19, 23, 24). As for invasive infections, there was only a clinical study comparing the relative efficacies of different antimicrobial regimens among cases of Aeromonas sepsis. In a case series retrospectively reviewing 104 episodes of monomicrobial Aeromonas bacteremia in southern Taiwan (16), the 72-h survival rate (7 [87.5%] of 8) of patients empirically treated with ciprofloxacin or ofloxacin was similar to that (23 [88.5%] of 26) of patients treated with a β-lactam agent in combination with an aminoglycoside and that (20 [60.1%] of 33) of those treated with a β-lactam agent. This clinical observation suggests the therapeutic potential of ciprofloxacin or ofloxacin monotherapy for Aeromonas sepsis.
In this study, using time-killing methods, there was no significant difference among the inhibitory activities of five fluoroquinolones against A. hydrophila. However, at a fluoroquinolone concentration equal to twice the MIC, regrowth of Ah 2743 occurred within 8 h of coincubation and the growth curve was different from that of V. vulnificus (VV 5823), against which the fluoroquinolones exhibited sustained inhibitory activity lasting for at least 48 h (25). The MIC for the surviving isolate after 24 h of incubation with ciprofloxacin was 0.03 μg/ml, which is four times that for the initial isolate. This suggests that, at such a drug concentration, there is only inhibitory activity for Ah 2743. The difference between the time-kill curves of Ah 2743 and VV 5823 is probably related to the fact that the former is more tolerant to ciprofloxacin (MBC/MIC ratio, 4) than is the latter (MBC/MIC ratio, ≤2).
In in vivo studies with mice infected with A. hydrophila, the therapeutic efficacy of ciprofloxacin, measured by the survival rate at 120 h or the median survival time, was greater than that of cefotaxime, minocycline, or both in combination. Corresponding to our previous report (17), in the present study, the therapeutic efficacy of minocycline was superior to that of cefotaxime, regardless of the inoculum size. The enhanced inhibitory activity of cefotaxime-minocycline in combination was demonstrated in the present time-kill study (Fig. 1), as it has been demonstrated against a cefotaxime-susceptible A. hydrophila isolate (17). However, the therapeutic benefit of cefotaxime-minocycline was absent in mice infected with a cefotaxime-resistant isolate, Ah2743, when they were treated by the use of the indicated doses of cefotaxime and minocycline. With similar doses of both antimicrobial agents, which will be adequate to exhibit synergistic activity against a cefotaxime-susceptible isolate in infected tissue, the therapeutic superiority of combination therapy will be apparent (17). Overall, our results suggest that ciprofloxacin is the most effective of the four regimens investigated against cefotaxime-resistant A. hydrophila. As for cefotaxime-susceptible strains, ciprofloxacin is as effective as cefotaxime-minocycline in the treatment of infected mice.
Our clinical experiences in the treatment of Aeromonas bacteremia, along with the in vitro and in vivo animal studies, indicate that ciprofloxacin is at least as effective as the combination of cefotaxime and minocycline and could be an option for the treatment of invasive Aeromonas infections.
Acknowledgments
This work was partially supported by grants from the National Science Council (NSC-91-2314-B-006-050, NSC-91-2314-B-384-002), the Center for Disease Control (DOH-91-DC-1015), the Department of Health, and the Chi Mei Medical Center (CMFHR 9023), Taiwan.
REFERENCES
- 1.Altweg M. 1999. Aeromonas and Plesiomonas, p. 507-516. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 2.Burgos, A., G. Quindos, R. Martinez, P. Rojo, and R. Cisterna. 1990. In vitro susceptibility of Aeromonas caviae, Aeromonas hydrophila and Aeromonas sobria to fifteen antibacterial agents. Eur. J. Clin. Microbiol. Infect. Dis 9:413-417. [DOI] [PubMed] [Google Scholar]
- 3.Chuang, Y. C., C. Y. Yuan, C. Y. Liu, C. K. Lan, and A. H. M. Huang. 1992. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 15:271-276. [DOI] [PubMed] [Google Scholar]
- 4.Clark, R. B. 1992. Antibiotic susceptibilities of the Vibrionaceae to meropenem and other antimicrobial agents. Diagn. Microbiol. Infect. Dis. 15:453-455. [DOI] [PubMed] [Google Scholar]
- 5.Dryden, M., and R. Munro. 1989. Aeromonas septicemia: relationship of species and clinical features. Pathology 21:111-114. [DOI] [PubMed] [Google Scholar]
- 6.Duthie, R., T. W. Ling, A. F. B. Cheng, and G. L. French. 1995. Aeromonas septicemia in Hong Kong: species distribution and associated disease. J. Infect. 30:241-244. [DOI] [PubMed] [Google Scholar]
- 7.Funada, H., and T. Matsuda. 1997. Aeromonas bacteremia in patients with hematologic diseases. Int. Med. 36:171-174. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Barca, E., C. Ardanuy, J. Carratala, A. Sanchez, A. Fernandez-Sevilla, and A. Granena. 1997. Fatal myofascial necrosis due to imipenem-resistant Aeromonas hydrophila. Scand. J. Infect. Dis. 29:91-92. [DOI] [PubMed] [Google Scholar]
- 9.Grobusch, M. P., K. Gobels, and D. Teichmann. 2001. Cellulitis and septicemia caused by Aeromonas hydrophila acquired at home. Infection 29:109-110. [DOI] [PubMed] [Google Scholar]
- 10.Hayes, M. V., C. J. Thomson, and S. G. B. Amyes. 1996. The “hidden” carbapenemase of Aeromonas hydrophila. J. Antimicrob. Chemother. 37:33-44. [DOI] [PubMed] [Google Scholar]
- 11.Hickman-Brenner, F. W., K. L. MacDonald, A. G. Steigerwalt, G. R. Fanning, D. J. Brenner, and J. J. Farmer III. 1987. Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J. Clin. Microbiol. 25:900-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, B. L., and M. H. Wilcox. 1995. Aeromonas infections and their treatment. J Antimicrob. Chemother. 35:453-461. [DOI] [PubMed] [Google Scholar]
- 13.Ko, W. C., and Y. C. Chuang. 1995. Aeromonas bacteremia: review of 59 episodes. Clin. Infect. Dis. 20:1298-1304. [DOI] [PubMed] [Google Scholar]
- 14.Ko, W. C., K. W. Yu, C. Y. Liu, C. T. Huang, H. S. Leu, and Y. C. Chuang. 1996. Increasing antibiotic resistance in clinical isolates of Aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 40:1260-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko, W. C., H. M. Wu, T. C. Chsng, J. J. Yan, and J. J. Wu. 1998. Inducible beta-lactam resistance in Aeromonas hydrophila: therapeutic challenge for antimicrobial therapy. J. Clin. Microbiol. 36:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko, W. C., H. C. Lee, Y. C. Chuang, C. C. Liu, and J. J. Wu. 2000. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteremia. J. Infect. 40:267-273. [DOI] [PubMed] [Google Scholar]
- 17.Ko, W. C., H. C. Lee, Y. C. Chuang, S. H. Ten, C. Y. Su, and J. J. Wu. 2001. In vitro and in vivo combinations of cefotaxime and minocycline against Aeromonas hydrophila. Antimicrob. Agents Chemother. 45: 1281-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler, J. M., and L. R. Ashdown. 1993. In vitro susceptibilities of tropical strains of Aeromonas species from Queensland, Australia, to 22 antimicrobial agents. Antimicrob. Agents Chemother. 37:905-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao, W. C., and M. S. Cappell. 1989. Treatment with ciprofloxacin of Aeromonas hydrophila associated colitis in a male with antibodies to the human immunodeficiency virus. J. Clin. Gastroenterol. 11:552-554. [DOI] [PubMed] [Google Scholar]
- 20.Motyl, M. R., G. McKinley, J. M. Janda. 1985. In vitro susceptibilities of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae to 22 antimicrobial agents. Antimicrob. Agents Chemother. 28:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathwani, D., R. B. Laing, G. Harvey, and C. C. Smith. 1991. Treatment of symptomatic enteric Aeromonas hydrophila infection with ciprofloxacin. Scand. J. Infect. Dis. 23:653-654. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS. 2000. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically; approved standard—fifth edition, M7-A5. NCCLS, Villanova, Pa.
- 23.Orlando, R., L. Mastrullo, D. De Blasi, M. L. Boffa, G. Zorzato, and E. Miraglia. 2001. Aeromonas sobria sepsis in a neutropenic patient. Haematologica 86:E11.. [PubMed] [Google Scholar]
- 24.Rautelin, H., M. L. Hanninen, A. Sivonen, U. Turunen, and V. Valtonen. 1995. Chronic diarrhea due to a single strain of Aeromonas caviae. Eur. J. Clin. Microbiol. Infect. Dis. 14:51-53. [DOI] [PubMed] [Google Scholar]
- 25.Tang, H. J., M. C. Chang, W. C. Ko, K. Y. Huang, C. L. Lee, and Y. C. Chuang. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 46:3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh, T. R., D. J. Payne, A. P. MacGowan, and P. M. Bennett. 1995. A clinical isolate of Aeromonas sobria with three chromosomally mediated inducible β-lactamases: a cephalosporinase, a penicillinase and a third enzyme, displaying carbapenemase activity. J. Antimicrob. Chemother. 35:271-279. [DOI] [PubMed] [Google Scholar]