Abstract
Human monocytotropic ehrlichiosis caused by Ehrlichia chaffeensis was reported in 1987. An animal model to study acute fatal ehrlichiosis in mice that has been developed closely resembles the fatal form of human monocytotropic ehrlichiosis. However, animal models for persistent infection in the genus Ehrlichia in immunocompetent mice have not been characterized. We report the histopathological progression of Ehrlichia muris infection in immunocompetent mice (AKR and C57BL/6 strains) correlated with their antibody response determined by indirect immunofluorescence and Western immunoblotting, and the distribution and quantity of the ehrlichial load by immunohistochemistry, polymerase chain reaction (PCR), and real-time PCR in lungs, liver, and spleen. Mild to moderate correlation was observed between histopathological grading in these organs and relative ehrlichial loads. The highest ehrlichial loads were present between days 4 and 14 after infection. E. muris was detected in tissues examined up to 150 days after infection by real-time PCR. Analysis of the serological response revealed several immunodominant antigens, including 200-, 180-, 100-, 73/75-, 45-, and 28-kd proteins. In conclusion, we have provided for the first time a complete histopathological, serological, immunohistochemical, and quantitative analysis of an animal model for the study of persistent ehrlichial infection.
Ehrlichiae are obligate intracellular bacteria that reside in a cytoplasmic vacuole and have evolved in close association with a mammal reservoir and a tick vector.1–6 The classification of ehrlichial organisms has undergone reorganization based on available genetic sequences.7
Five species of Ehrlichia are known to be human or veterinary pathogens: Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia canis, Ehrlichia ruminantium, and Ehrlichia muris. Ehrlichiae cause a persistent infection in their natural host, and in some accidental hosts they cause severe toxic shock-like illness [E. chaffeensis in humans and Ixodes ovatus ehrlichia (IOE) in experimentally inoculated mice].8,9 To date, it is not known what proportion of humans develop a chronic, persistent infection.
An animal model that mimics acute disease caused by E. chaffeensis in humans has been described that uses an unnamed Ehrlichia species isolated from I. ovatus ticks (IOE) in Japan.8–10 Other animals available for the study of ehrlichial infections include canines (E. canis or E. chaffeensis); deer (E. chaffeensis); and sheep, goats, and cattle (E. ruminantium). However, laboratory studies with these animals are difficult, and species-specific immunological reagents are not as widely available as for mice. In addition, research with E. ruminantium in this country is restricted by the United States Department of Agriculture.
In 1983, an infectious agent designated strain AS145 was isolated from the spleen of a wild mouse in Japan (Eothenomys kageus).11 In 1993, it was characterized as a member of the genus Ehrlichia based on morphological and antigenic criteria.11 In 1995, the organism was named E. muris based on 16S rRNA (rrs) base sequences, serological, morphological, and biological characteristics.12 Subsequently, E. muris has been isolated from Hemaphysalis flava ticks in the same areas where E. muris had originally been isolated.13 E. muris is most closely related to E. chaffeensis and causes nonlethal infection in laboratory mice.14 The disease consists of a short course of illness characterized by ruffled fur, lethargy, anorexia, splenomegaly, and lymphadenopathy (case-fatality rate, ∼0.3%). E. muris antibodies have been detected in focal areas in metropolitan Tokyo with seropositivity rates of up to 63% in Apodemus speciosus and A. argenteus. In humans, a serosurvey in Tokyo revealed presence of anti-E. muris antibodies in 20 of 1803 serum samples (1% seropositivity rate). The geographic distribution, animal reservoirs, vectors, and human exposure to E. muris are primarily unknown.13
In 1996, Kawahara and colleagues,14 reported impaired antigen-specific responses and enhanced polyclonal stimulation in mice infected with E. muris. Furthermore, it was documented that E. muris could be reisolated from infected mice up to 400 days after infection. However, no detailed histological studies have been performed to elucidate the histopathology of E. muris infection in mice.
In this study, we report detailed histopathological, immunohistochemical, serological, and polymerase chain reaction (PCR) analysis of the kinetics of the pathology, bacterial load, and humoral immune response in two strains of mice, namely C57BL/6 and AKR, infected with E. muris.
Materials and Methods
Ehrlichia
AKR and C57BL/6 mice were infected intraperitoneally with spleen homogenates of E. muris derived from the same mice strains. The animals were infected and the spleen harvested at day 9 after infection. Spleens were homogenized in sucrose-phosphate-glutamate buffer (21.8 mmol/L sucrose, 7.2 mmol/L K2HPO4, 3.1 mmol/L KH2PO4, 4.9 mmol/L l-glutamic acid, pH 7) 10% w/v, and 1 ml of the spleen suspension [10−2.5 tissue culture ID50 (TCID50)] was inoculated intraperitoneally into the experimental animals.
Infection of Mice
Thirty-six AKR and 38 C57BL/6 mice were used for the study. Four mice from each strain were sacrificed at each time point after infection (4, 9, 14, 20, and 30 days), and two mice from each strain were sacrificed at 60, 90, 120, and 150 days for C57BL/6 mice and similarly up to 120 days for AKR mice, and a complete necropsy was performed on each of the animals. Two animals from each strain were used as controls until day 30. These animals were inoculated with noninfected, homogenized spleen.
Histology and Immunohistochemistry
The liver, spleen, bone marrow, kidneys, testis, skeletal muscle, brain, and lungs were fixed in 10% neutral-buffered formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. Paraffin-embedded sections of the bone marrow were also stained by the periodic acid-Schiff method after diastase digestion.
Histopathological Grading
Grading of lesions in the liver, spleen, and lungs was performed blindly and independently of conventional PCR and real-time PCR. For lesions in the liver, the following parameters were used for grading: apoptotic hepatocytes, lobular lymphohistiocytic infiltrates (LHI), and perivascular (portal triads) LHI. Each parameter was scored based on a four-tier system (0 to 3) as follows: apoptotic hepatocytes: 0, 0 to 2 apoptotic hepatocytes per 50 high-power fields (HPFs); 1, 3 to 9 per 50 HPFs; 2, 10 to 15 per 50 HPFs; and 3, >15 per 50 HPFs; lobular LHI: 0, occasional LHI in hepatic lobules; 1, 10 to 40% of lobules involved; 2, 40 to 70%; and 3, >70%; perivascular LHI: 0, occasional LHI; 1, 10 to 40% of portal triads involved and perivascular cuffing up to three cells in thickness; 2, 40 to 70% of triads involved and perivascular cuffing four to seven cells in thickness; and 3, >70% of triads involved and perivascular cuffing more than seven cells in thickness. Each variable was scored independently. A total score was obtained from each animal by adding all parameters and obtaining the mean. Therefore, histopathological scores ranged from 0 to 9.
For lesions in the spleen, the parameters used for histopathological grading were apoptotic bodies and immunoblastic proliferation in lymphoid follicles (to analyze the immune response to E. muris), and percentage of white pulp in spleen sections, which includes the infiltration of the marginal zone by macrophages, some of which were infected with E. muris. Each variable was scored independently from 0 to 3. All scores were added and the mean score was obtained from each animal. Total scores ranged from 0 to 9. Apoptotic bodies in lymphoid follicles: 0, 0 to 5 apoptotic bodies/lymphoid follicle; 1, 6 to 30; 2, 31 to 50; 3, >50. Immunoblastic proliferation in lymphoid follicles: 0, 0 to 5 immunoblasts/lymphoid follicle; 1, 6 to 20; 2, 21 to 50; 3, >50. Percentage of white pulp in spleen sections: 0, <40%; 1, 40 to 60%; 2, 60 to 70%; 3, >70%.
The parameters for evaluation of the lungs included interstitial, peribronchial, and perivascular LHI, and margination of mononuclear inflammatory cells in the pulmonary vessels. Each parameter was scored independently from 0 to 3 and the scores were then added and their means obtained. Total scores ranged from 0 to 12. Interstitial LHI: this parameter was evaluated by measuring thickness of alveolar septa at the periphery of the lung. A total of 50 septa were evaluated and mean thickness was obtained (0, 1 to 3 μm; 1, 4 to 6 μm; 2, 7 to 12 μm; 3, >12 μm). Perivascular and peribronchial LHI: this parameter was evaluated by measuring the maximum width of LHI around bronchi and blood vessels. An average width of all LHI was then obtained and expressed as μm (0, occasional LHI; 1, 1 to 10 μm, patchy; 2, 11 to 30 μm, diffuse; 3, >30 μm, diffuse). Margination of peripheral blood mononuclear cells in pulmonary vessels: this parameter was evaluated by estimating the percentage of lumen occupied by mononuclear cells (0, no margination; 1, 0 to 30%; 2, 30 to 60%; 3, >60%). Apoptotic cells in the spleen and liver were identified based on morphological appearance on H&E stains, that is cells with condensed hypereosinophilic cytoplasm and chromatin condensation with nuclear shrinkage with or without nuclear fragmentation. The bone marrow was evaluated based on cellularity and myeloid:erythroid (M:E) ratio. No grading was developed for the bone marrow because of the subtlety of the histological changes under light microscopy.
Immunohistochemical Detection of E. muris in Tissues
Tissue sections of liver, spleen, bone marrow, and lungs from all of the animals were stained immunohistochemically as follows: the sections were deparaffinized by heating the glass slides at 70°C for 20 minutes, followed by immersion in three xylene baths for 5 minutes each. The slides were then rehydrated by immersion in a series of alcohol baths ranging from 100 to 80% for 3 minutes each. The slides were then washed in distilled water for 2 to 3 minutes. Endogenous peroxidase was blocked with a solution containing phosphate-buffered saline (PBS) with 3% H2O2 and 0.03% sodium azide for 10 minutes. Sections were then microwaved in 10 mmol/L sodium citrate buffer (pH 6.0) at 100°C for 10 minutes and transferred to PBS containing 0.01% Tween 20. Avidin/biotin blocking solution (Vector Laboratories, Burlingame, CA) was then applied for 15 minutes to the tissue sections, which were then washed in PBS-Tween 20. Glass slides were then placed in a solution containing 0.03% casein and PBS-Tween 20 for 30 minutes to block nonspecific antibody binding sites. The slides were then incubated with canine anti-E. chaffeensis polyclonal antibody (1:1000 dilution and kindly provided by Dr. Xue-jie Yu, University of Texas Medical Branch, Galveston, TX) for 45 minutes at 37°C. Negative controls were incubated with normal canine serum. The slides were then washed twice in PBS-Tween 20 for 10 minutes each. A biotinylated goat anti-dog IgG was then added at 1:800 dilution (Vector Laboratories) for 30 minutes at 37°C. Two washes in PBS-Tween 20 were performed for 10 minutes each. Streptavidin-horseradish peroxidase (DAKO, Carpinteria, CA) was then added for 20 minutes followed by addition of diaminobenzidine (DAKO) for 10 minutes. Slides were then washed with water and counterstained with hematoxylin.
Antibody Responses
Serum samples from infected and control mice were analyzed by indirect immunofluorescence assay using E. muris as an antigen. Antigen slides were prepared as follows: monolayers of P388D1 cells were grown in 150-cm2 flasks in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY) containing 5% fetal bovine serum (Hyclone, Logan, UT). Infection of the monolayer was monitored by Diff Quik (Imeb, Inc., Chicago, IL) staining until 80% of the cells were infected. The cells were harvested and antigen slides were prepared. Serum samples were serially diluted in twofold increments and 10 μl were applied to each slide well and incubated for 30 minutes at 37°C in a humidified chamber. The antigen slides were then washed three times in PBS, pH 7.4, and then incubated with 10 μl of fluorescein isothiocyanate-labeled anti-mouse IgG (Kirkegaard & Perry, Gaithersburg, MD) at a 1:100 dilution. The slides were then washed three times in PBS, pH 7.4, counterstained with Evans blue and examined under a Nikon Labphoto fluorescent microscope. Serological end-point titers were expressed as the reciprocal of the highest dilution at which specific fluorescence was detected.
Gel Electrophoresis and Protein Blotting
Purified E. muris (AS145 strain) was subjected to sodium dodecyl-sulfate polyacrylamide gel electrophoresis in 3-N-morpholinopropanesulfonic acid running buffer (NuPAGE; Invitrogen, Carlsbad, CA) under reducing conditions using 4 to 12% gradient Bis-Tris acrylamide gels with a two-dimensional well (NuPAGE, Invitrogen). An antioxidant (Invitrogen) was added to the upper chamber buffer during electrophoresis and to the transfer buffer during protein blotting to prevent partial protein renaturation as a result of disulfide bond reoxidation. Antigen was heated at 70°C for 10 minutes in lauryl dodecyl sulfate sample buffer (NuPAGE, Invitrogen) containing a reducing agent, dithiothreitol (Invitrogen), before loading. A protein standard was included for molecular mass determination (Precision Protein Standards, broad range, prestained; Bio-Rad, Hercules, CA). Proteins were transferred to supported nitrocellulose (BA85; Schliecher & Schuell, Keene, NH) using a semidry protein blotting unit (Bio-Rad) with 2× transfer buffer (NuPAGE, Invitrogen) at 15 V for 30 minutes.15
Western Immunoblotting
Membranes were incubated with blocking buffer [Tris-buffered saline (pH 8.0)/3% nonfat milk] for 1 hour, and damp blots were placed in a slotted blotting apparatus (Multiscreen, Bio-Rad). Primary antibodies were diluted (1:100) in the blocking buffer, and 450 μl were placed in each channel for 1 hour at room temperature and slowly rocked. Membranes were removed from the apparatus, washed with buffer (Tris-buffered saline and 0.05% Tween 20), and incubated for 1 hour with affinity-purified alkaline phosphatase-labeled goat anti-mouse IgG (H & L) (Kirkegaard & Perry Laboratories).
Detection of Ehrlichial DNA by PCR and Real-Time PCR
PCR Detection of E. muris in Tissues
Primers for the 16S rRNA subunit (rrs) gene specific for E. muris were used for this purpose. For the amplification of this gene fragment, a 50-μl reaction mixture containing 25 μl of PCR Master Mix (Roche, Indianapolis, IN), 1 μl (0.4 μmol/L final concentration) of each primer and 1 μl of DNA template diluted in 23 μl of water were used. The PCR Master Mix contained 1.25 U Taq polymerase, 0.2 mmol/L each of dATP, dCTP, dGTP, and dTTP, 10 mmol/L Tris-HCl, 50 mmol/L KCl, and 1.5 mmol/L MgCl2. The primers used for the amplification of E. muris DNA were ECB (5′-CGTATTACCGCGGCTGCTGGCT-3′) and ECC (5′-AGAACGAACGCTGGCGGCAAGCC-3′). All specimens for PCR testing were manipulated in an AirClean 600 Workstation (AirClean Systems, Raleigh, NC) with aspiration of the PCR mixture with cotton-filled tips. The PCR mixtures were placed in a GeneAmp PCR System 9700 thermocycler (PE Applied Biosystems, Foster City, CA) and amplified for 35 cycles: preheating at 94°C for 2 minutes, denaturing at 94°C for 45 seconds, annealing at 65°C for 45 seconds, and extension at 72°C for 60 seconds. Detection of amplified products was performed by ethidium bromide staining.
Real-Time PCR Quantification of E. muris DNA in Tissues
The target gene selected for amplification of E. muris DNA was the Ehrlichia-specific dsb gene, which encodes a disulfide bond-forming protein (GenBank accession no. AY236484). DNA was first extracted from infected mouse tissues by using the DNeasy tissue kit (Qiagen, Valencia, CA). E. muris DNA was amplified by using forward primer dsb 251FL (5′-GTAGCCTTCTTTGACTATTCCTGTGGCTAC-3′) and reverse primer dsb 251FL327RU (5′-AATGACGCCTCACCGAGTAT-3′) in a reaction mixture as follows: 12.5 μl of IQ SYBR Green supermix (Bio-Rad), 0.25 μl of forward primer, 0.25 μl of reverse primer (0.25 μmol/L final concentration), 1 μl of template, and 11 μl of distilled water. The cycling program consisted of cycles with the following temperatures: preheating, 95°C for 2 minutes; denaturing, 95°C for 15 seconds; annealing, 60°C for 30 seconds; and extension, 72°C for 20 seconds. All reactions were performed in an iCycler iQ real-time PCR detection system (Bio-Rad). The eukaryotic housekeeping gene GAPDH was amplified from the same DNA extracts and under the same experimental conditions in parallel by using forward primer mG3PDHgv-f(5′-GTCATCCCAGAGCTGAAC-3′) and reverse primer huG3PDHgv-r(5′-GTCATCATATTTGGCAGGTTTT-3′). The relative amounts of ehrlichial DNA were estimated by using threshold cycles for both the dsb gene and the GAPDH gene in each sample. Differences between their threshold cycles (ΔCt) was obtained followed by subtracting this difference from the reference sample (ΔΔCt), which for these experiments was the sample with the lowest amount of ehrlichial DNA. Finally, 2 was raised to the ΔΔCt power to obtain the relative amount of ehrlichial DNA in each sample when compared to the reference sample. Each sample was run in triplicate and the mean cycles were calculated. For final quantification of ehrlichial loads in each organ the relative amounts were averaged from all four animals in each experimental group up until day 30. Ehrlichial loads were averaged between two animals for days 60, 90, 120, and 150 (C57BL/6 mice) after infection.
Statistical analysis was performed with SigmaStat, version 2 (SPSS Inc., Chicago, IL) Differences of ehrlichial loads and histopathological scores between mouse strains were analyzed by t-test or Mann-Whitney rank sum test where indicated. Correlation between ehrlichial loads and histopathological scores was analyzed by linear regression analysis.
Results
Course of Disease
AKR mice developed ruffled fur, anorexia, and inactivity at approximately days 7 to 10 after which they recovered fully. C57BL/6 mice did not develop signs of illness when compared to AKR mice. No fatalities were documented during the entire duration of the study.
Histopathological Observations
Table 2 summarizes the histological score in both AKR and C57BL/6 mice until day 30 after infection. Briefly, the histopathology in both mouse strains was similar with significant differences in histological scores in the lungs at days 4, 14, and 30 after infection. A detailed histopathological description follows (Figure 1).
Table 2.
Summary of Real-Time PCR Results and Histological Grading in AKR and C57BL/6 Mice Infected with E. muris
| Day after infection | Spleen
|
Liver
|
Lungs
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative copies mean (SEM)*
|
Histological score† mean (SEM)
|
Relative copies mean (SEM)
|
Histological score mean (SEM)
|
Relative copies mean (SEM)
|
Histological score mean (SEM)
|
|||||||||||||
| AKR | C57BL/6 | P‡ | AKR | C57BL/6 | P | AKR | C57BL/6 | P | AKR | C57BL/6 | P | AKR | C57BL/6 | P | AKR | C57BL/6 | P | |
| 4 | 366,279 | 18,334 | 0.03 | 2.8 | 2.5 | 0.51 | 24,971 | 12,498 | 0.2 | 1.3 | 1.5 | 0.5 | 45,356 | 359 | 0.03 | 3.5 | 1.0 | 0.03 |
| (77,547) | (6528) | (0.2) | (0.3) | (6274) | (4670) | (0.3) | (0.3) | (9728) | (155) | (0.5) | (0) | |||||||
| 9 | 429,432 | 195,596 | 0.2 | 7.8 | 7.8 | 1.0 | 435,282 | 786,421 | 0.2 | 5.3 | 4.5 | 0.3 | 74,792 | 9243 | 0.001 | 7.5 | 8.3 | 0.4 |
| (154,436) | (42,503) | (0.5) | (0.3) | (169,258) | (145,103) | (0.6) | (0.3) | (7616) | (3918) | (0.6) | (0.5) | |||||||
| 14 | 247,291 | 12,289 | 0.01 | 9.0 | 7.3 | 0.21 | 188,037 | 24,422 | 0.003 | 7.5 | 6.3 | 0.07 | 82,150 | 803 | 0.03 | 7.8 | 9.8 | 0.05 |
| (37,167) | (634) | (1.1) | (0.6) | (33,387) | (5022) | (0.3) | (0.5) | (21,464) | (207) | (0.6) | (0.5) | |||||||
| 20 | 16,109 | 7535 | 0.26 | 7.0 | 7.0 | 1.0 | 4805 | 10,570 | 0.5 | 2.8 | 2.8 | 1.0 | 36,113 | 1097 | 0.004 | 6.0 | 5.8 | 0.81 |
| (6447) | (2511) | (0.6) | (0.4) | (1506) | (7891) | (0.5) | (0.3) | (7852) | (598) | (0.6) | (0.8) | |||||||
| 30 | 3649 | 991 | 0.2 | 5.8 | 5.3 | 0.4 | 3213 | 3297 | 0.9 | 2.5 | 3.0 | 0.5 | 4977 | 239 | 0.03 | 3.5 | 7.3 | 0.003 |
| (1799) | (268) | (0.5) | (0.3) | (2507) | (1955) | (0.3) | (0.7) | (1292) | (44) | (0.6) | (0.5) | |||||||
Units are expressed as relative amounts of ehrlichial DNA compared to the lowest amount of ehrlichial DNA present in the organs analyzed during the experiment.
Spleen, 0 to 9; liver, 0 to 9; lungs, 0 to 12. SEM, standard error of the mean.
P values calculated by t-test or Mann-Whitney rank sum test where applicable.
Figure 1.
A: Liver at 9 days after infection. LHI in the hepatic lobule and Kupffer cell hyperplasia (H&E). B: Liver at 30 days after infection. Prominent perivascular infiltrates with well-formed granulomas (arrows). Kupffer cell hyperplasia (H&E). C: Liver, day 14 after infection. Ehrlichial morulae in sinusoidal mononuclear cells (horseradish peroxidase/diaminobenzidine). D: Lung, day 9 after infection. Focal LHI (arrow) in the lung interstitium (H&E). E: Lung, day 20 after infection. Interstitial, perivascular (arrow), and peribronchial (arrow) LHI (H&E). F: Lung, day 14 after infection. Ehrlichial morulae in mononuclear cells infiltrating the lung interstitium (horseradish peroxidase/diaminobenzidine). G: Section of spleen at day 4 after infection. Infiltration of red pulp and marginal zone by macrophages (H&E). H: Spleen section at day 14 after infection. Markedly irregular lymphoid follicles and macrophage infiltration of red pulp (H&E). I: Spleen at day 10 after infection. Ehrlichial morulae in macrophages infiltrating red pulp (horseradish peroxidase/diaminobenzidine). Original magnifications: ×200 (A, B, D); ×1000 (C, F, I); ×100 (E, G, H).
Day 4
The spleen showed mild increases of white pulp and mildly increased numbers of immunoblasts and apoptotic bodies in lymphoid follicles. Increased quantities of macrophages were seen infiltrating the marginal zone and the red pulp. The liver had evidence of lobular LHI present in 10 to 40% of hepatic lobules and occasional perivascular LHI in portal triads. The lungs revealed mild thickening of alveolar septa by LHI and margination of mononuclear cells in pulmonary vessels. Bone marrow revealed 80% cellularity, an M:E ratio of 4 to 5:1 and normal amounts of megakaryocytes, which remained so throughout the study.
Days 9 and 14
The spleen had markedly irregular lymphoid follicles with moderate to severe expansion of the white pulp. Large numbers of apoptotic cells and immunoblasts were observed in the lymphoid follicles. Macrophages in the red pulp and marginal zone were prominent. Lobular and perivascular LHI were seen in the liver involving from 40 to >70% of hepatic lobules. Perivascular LHI ranged from two to more than six cells in thickness. Apoptotic hepatocytes ranged from 3 to more than 15 per 50 HPF. Hyperplasia of Kupffer cells and focal erythrophagocytosis were observed. The lungs revealed moderate to large collections of macrophages marginating within the pulmonary vasculature and perivascular cuffs of LHI. The interstitium also showed widening by LHI. Peribronchial LHI were mild to moderate and more prominent in C57BL/6 mice. The bone marrow cellularity increased to 90 to 100%, and the M:E ratio was 10:1. The expansion of the myeloid lineage was mostly because of macrophages, although increased numbers of neutrophils were also observed.
Day 20
At this time point, the lesions in the spleen were similar to the lesions described at days 9 and 14 after infection. The lesions in the lungs and liver started regressing at this time point, and presence of poorly formed granulomas was demonstrated in the liver and less so in the lungs. The M:E ratio in the bone marrow was 7:1 and its cellularity was 90 to 100%.
Day 30
Regression of the lesions continued in the liver and lungs with presence of well-defined granulomas in the liver (Figure 1B). The lesions in the lungs were more severe in C57BL/6 than AKR mice at this time point. The splenic lesions started their regression at this time point. The bone marrow showed 90% cellularity and the same M:E ratio as day 20 after infection.
Days 60, 90, 120, and 150 after Infection
The histological lesions regressed dramatically in all organs. At these time points, the lesions consisted of mild LHI in lungs and liver and mildly increased numbers of immunoblasts and apoptotic bodies in lymphoid follicles, which were slightly increased in size. Histological scores in both mouse strains ranged from 1.0 to 3.5 in all organs and ehrlichial burdens ranged from 1 to 177 relative copies of ehrlichial DNA (Table 4).
Table 4.
Correlation between Relative Number of Ehrlichial DNA Copies and Histological Grading in AKR and C57BL/6 Mice Infected with E. muris
| Mouse strain | Organ | r value |
|---|---|---|
| AKR | Spleen | 0.109 |
| Liver | 0.658 | |
| Lungs | 0.835 | |
| C57BL/6 | Spleen | 0.438 |
| Liver | 0.256 | |
| Lungs | 0.331 |
Immunolocalization of E. muris with Polyclonal Anti-E. muris Antibody
Organisms were detected by immunohistochemistry as early as day 4 and until day 30 after infection. No organisms were detected after day 30 of infection. More ehrlichial morulae were observed on days 9, 14, and 20 after infection. In the spleen, morulae were mostly limited to macrophages in the marginal zones and red pulp. Ehrlichial morulae in the lungs were present in macrophages in the interstitium and in the mononuclear cells marginated to the vessel wall. Fewer morulae were detected in the perivascular cuffs. In the liver, ehrlichial organisms were present in the mononuclear cells present in the lobules, sinusoids, and in the lumens of the portal vessels. Occasional morulae were observed in hepatocytes. In all organs the morphology of the morulae ranged from single, large, membrane-bound structures occupying most of the cytoplasm to small multiple intracytoplasmic inclusions (Figure 1, C, F, and I).
Antibody Responses Detected by Immunofluorescence Assay and Western Immunoblotting
End-point titers of ≥1:80 were considered positive, based on results derived from sera from healthy uninfected mice. AKR mice developed an end-point titer ranging from <1:80 to 1:80 at day 9 after infection, and the titer rose to 1:2560 to 1:5120 at day 30, remaining at high titers for the entire duration of the experiment (120 days).
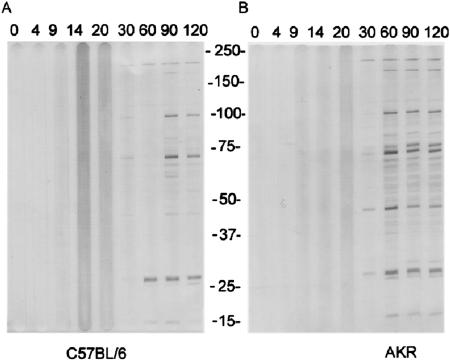
In C57BL/6 mice end-point titers by immunofluorescence assay rose less sharply on days 14 and 20 but otherwise were similar to those in AKR mice (Table 1). Antibodies against E. muris were detected by Western immunoblotting at day 30 after infection in both mice strains. The immunodominant bands representing antibodies against the 200-, 180-, 100-, 73/75-, 45-, and 28-kd range were identified at this time point. Reactivity against the 100-, 75/73-, 45-, and 28-kd bands became stronger at days 60, 90, and 120 days after infection. An additional weak band in the 16-kd range was also observed at day 60 after infection and thereafter (Figure 2).
Table 1.
Summary of Indirect Immunofluorescent Assay, Western Immunoblotting, and PCR Results of E. muris Animal Model
| Day after infection | IFA titers
|
Western immunoblot
|
PCR*
|
Real-time PCR
|
IHC
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | |
| 4 | − | − | − | − | S, L, Lu | S | + | + | + | + |
| 9 | 1:80 | 1:80 | − | − | S, L, Lu | S, L, Lu | + | + | + | + |
| 14 | 1:640 | 1:80 | − | − | S, L, Lu | S, L, Lu | + | + | + | + |
| 20 | 1:640 | 1:160 | − | − | S, L, Lu | S, L, Lu | + | + | + | + |
| 30 | 1:5120 | 1:2560 | + | + | S, L, Lu | S, L, Lu | + | + | + | + |
| 60 | 1:10240 | 1:5120 | + | + | S, L, Lu | L | + | + | − | − |
| 90 | 1:10240 | 1:5120 | + | + | S, L | − | + | + | − | − |
| 120 | 1:5120 | 1:10240 | + | + | − | − | + | + | − | − |
| 150 | ND | 1:10240 | ND | + | ND | − | ND | + | ND | − |
S, Spleen; L, liver; Lu, lung; ND, not done.
Figure 2.
Western immunoblots demonstrating the kinetics of antibody responses (IgG) to E. muris whole-cell lysates in pooled sera from two strains of mice (C57BL/6, A; AKR, B) experimentally infected intraperitoneally with live E. muris. Serum samples were collected at time points up to 120 days after inoculation as indicated above each corresponding lane. Molecular mass is shown in kilodaltons (15 to 250 kd).
Detection and Quantitation of E. muris in Tissues by PCR and Real-Time PCR
Ehrlichial organisms were detected by conventional PCR from day 4 after infection until day 90 after infection in AKR mice and until day 60 after infection in C57BL/6 mice (Table 1). Analysis by real-time PCR revealed the presence of ehrlichial DNA in liver, lungs, and spleen from day 4 after infection and through the end of the study (day 150 after infection in C57BL/6 mice and day 120 after infection in AKR mice; Table 3). The highest ehrlichial loads during the entire study were detected in the spleen and liver on days 4 to 14 after infection in both mouse strains. Statistically significant differences (Table 2) between the two strains were seen in the spleen at days 4 and 14, after infection but no significant differences were noted in their histopathological scores. Liver loads were significantly higher in C57BL/6 mice at day 14 after infection, and similar in both mouse strains at other time points. No significant differences were observed in liver histological scores between the two strains. Ehrlichial loads in the lungs were significantly higher in AKR mice when compared to C57BL/6 mice at all time points. Lung histological scores were also significantly different at days 4, 14, and 30, but paradoxically lung scores were higher in C57BL/6 mice at days 14 and 30 when the ehrlichial loads were lower than in AKR mice. Linear regression analysis between ehrlichial loads and histopathological scores at time points between 4 and 30 days revealed correlation coefficients ranging from 0.1 to 0.8 (Table 4).
Table 3.
Summary of Real-Time Results and Histological Grading in AKR and C57BL/6 Mice Infected with E. muris, Days 60 to 150 after Infection
| Day after infection | Spleen
|
Liver
|
Lungs
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative copies*
|
Histological score†
|
Relative copies
|
Histological score
|
Relative copies
|
Histological score
|
|||||||
| AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | AKR | C57BL/6 | |
| 60 | 80 | 176 | 2.0 | 2.5 | 14 | 128 | 1.5 | 1.5 | 177 | 28 | 1.0 | 1.0 |
| 90 | 9 | 27 | 2.5 | 3.5 | 1 | 45 | 1.75 | 1.0 | 2 | 27 | 1.5 | 1.5 |
| 120 | 1 | 2.7 | 2.5 | 2.0 | 2 | 62 | 1.0 | 0.75 | 1 | 1 | 1.0 | 1.5 |
| 150 | ND | 1 | ND | 2.5 | ND | 1 | ND | 0.5 | ND | 2 | ND | 1.5 |
ND, Not done.
Units are expressed as relative amounts of ehrlichial DNA compared to the lowest amount of ehrlichial DNA present in the organs analyzed during the experiment. The value represents the average of two animals used at each time point. Therefore, SEM is not provided.
Spleen, 0 to 9; liver, 0 to 9; lungs, 0 to 12. The value represents the average of two animals used at each time point.
Discussion
The first case of human monocytotropic ehrlichiosis caused by E. chaffeensis was described 17 years ago. In 2000, Sotomayor and colleagues8 and Okada and colleagues9 reported a mouse model of fatal human monocytotropic ehrlichiosis using an agent closely related to E. chaffeensis, namely IOE. This animal model has been useful for studying acute, fatal ehrlichiosis because it greatly resembles the human histopathology present in human monocytotropic ehrlichiosis. Murine models using E. chaffeensis and E. ruminantium have been reported.16–19 E. chaffeensis causes a subclinical disease in mice with no described histopathological lesions, and the study of E. ruminantium is prohibited in this country by the United States Department of Agriculture. E. muris causes a systemic infection in immunocompetent mice in association with clinical signs and in addition establishes a persistent, lifelong infection.14 These features make this model excellent for the investigation of pathogenesis and mechanisms of immunity in persistent ehrlichial infections.
In this study we have described the histopathological progression of acute E. muris infection and the persistent subclinical infection that followed in two different strains of mice for up to 150 days. Histopathological analysis in this study revealed a common theme in ehrlichial infections caused by the E. chaffeensis genogroup: infection of the mononuclear phagocytic cells with development of histopathological lesions in the liver, lungs, spleen, and bone marrow similar to the lesions described in humans and other animal models of ehrlichial and anaplasma infections.20–26 The degree of histopathological involvement in the spleen correlated well with the description of marked gross splenomegaly at day 15 after infection by Kawahara and colleagues14 This observation is most likely because of the dramatic increase in size of splenic follicles and the infiltration of the marginal zone and red pulp by macrophages. The clinical course in the animals correlated well with the histopathological grading of the organs involved: after day 20 of infection, the mice were clinically healthy. By day 30 after infection, histopathological regression of all lesions was dramatic. In addition, the appearance of activated macrophages in the liver (and less so in the lungs) at day 20 after infection correlated well with a drop of ehrlichial burden after day 20 of infection in all organs. Well-formed granulomas were documented in the liver at day 30 after infection, a time at which the ehrlichial burden was dramatically decreased when compared to day 14 after infection. Mild histopathological lesions could still be seen in some organs on day 150 after infection, and low levels of ehrlichial DNA were detected in all organs examined by real-time PCR.
The spectrum of severity in immunocompetent animals infected with organisms of the E. chaffeensis genogroup ranges from highly lethal infection as in the IOE mouse model, sheep infected with E. ruminantium, and cases of human monocytotropic ehrlichiosis resembling toxic shock syndrome or Rocky Mountain spotted fever, to chronic, persistent infection as in E. canis infections in dogs, E. chaffeensis in white-tailed deer, E. ruminantium in wild African ruminants, and E. muris infections in mice.5,8,9,14,27–40 The severity of pathology in the IOE model and the immunopathological response present in those animals seem to explain the lethality of the infection.41 One of the main differences between IOE and E. muris infection histologically is the absence of hepatic necrosis in the latter. The changes in the spleen are very similar to the ones described in the IOE animal model, namely follicular hyperplasia, infiltration of the marginal zone and red pulp by mononuclear cells, and lymphoid activation, although the degree of splenomegaly in IOE-infected mice is not as severe as in E. muris-infected animals, perhaps because of the demise of the animals early in the disease process. Likewise, granuloma formation was not identified in the fatal IOE model. Also of note is the absence of bone marrow necrosis in the E. muris animal model, a finding that was prominent in the IOE infection. Further studies are needed to better delineate the immune response in the E. muris mouse model.
The host immune response has been proposed as responsible for the pathological lesions seen in ehrlichioses microscopically.22–24 One of the findings that support this hypothesis is the paucity of ehrlichial organisms demonstrated by immunohistochemistry in pathological lesions. This study seems to confirm previously published data obtained with experiments performed with Anaplasma phagocytophilum.42 In such experiments, histopathological lesions were graded in liver, and bacterial burden was evaluated in blood. Even though our experimental protocol was different (direct quantification of ehrlichial burden in tissues), a rather weak correlation was observed between histopathological grading and ehrlichial burden quantified by real-time PCR.
The presence of persistently high titers of antibodies in the sera of infected mice suggests continuous stimulation of the immune system by E. muris. Kawahara and colleagues13,14 reported low end-point titers of anti-E. muris antibodies in experimentally infected mice and antibody titers as high as 1:2560 in wild rodents. The latter results are more in agreement with our data, which demonstrated high end-point antibody titers after day 30 of infection. The different sensitivity between Western immunoblotting and immunofluorescence assay in detecting anti-E. muris antibodies at early time points (up to 20 days after infection) suggests that the antibodies that developed early in the disease process are probably directed against conformational epitopes that were denatured during Western blot analysis.
The majority of immunoreactive E. muris proteins recognized by C57BL/6 and AKR mice appeared to correspond to the major immunoreactive proteins recently identified in E. canis based on molecular mass.15 Immunoreactive proteins larger than 100 kd have been recently identified in E. chaffeensis and E. canis.15,43,44 The two large immunoreactive proteins (180 and 200 kd) identified in this study may be orthologs of the glycoproteins identified in E. canis and E. chaffeensis, which exhibit similar molecular masses and are strongly immunoreactive. Two major immunoreactive proteins identified in E. canis that were weakly reactive or not reactive in E. muris included the 19-kd and 37-kd proteins, respectively. Conformational epitopes could explain the difference in reactivity of these proteins among different Ehrlichia spp. as differences in reactivity of 19-kd orthologs (MAP2) in E. canis and E. chaffeensis by Western blot appear to be attributed to conformationally dependent epitopes.45
Kinetics of the antibody response to the immunoreactive proteins of E. muris indicated that the 28-kd antigen was recognized early in the immune response. In this study, mice were inoculated intraperitoneally, and the early response to the 28-kd antigen is consistent with studies with E. canis that reported an early response to the 28-kd antigen in dogs inoculated intravenously.46 In contrast, dogs experimentally infected with E. canis by intradermal or subcutaneous routes responded to the 28-kd antigen later in the immune response.15 These findings appear to suggest that the route of inoculation can alter the kinetics of the antibody response to specific antigens.
The differences between routine (gel-based ethidium bromide staining) PCR detection of ehrlichial DNA and real-time PCR are impressive. Real-time PCR is by far a much more sensitive technique for detection of low levels of ehrlichial organisms in this particular animal model. In addition, analysis of relative burdens of ehrlichial DNA by organ revealed multiple noteworthy findings. First of all, there was a weak correlation between ehrlichial burden and histopathological scores. Secondly, the highest infectious titer described by Kawahara and colleagues in the spleen occurred at day 10 after infection, which is in agreement with our data that show the highest ehrlichial burden in the spleen at day 9 after infection.11 Third, the heaviest ehrlichial load was present in the liver and spleen, followed by the lungs in both strains of mice.
In summary, this report provides a complete histopathological, immunohistochemical, and quantitative microbial analysis of an animal model for the study of persistent ehrlichial infections in mice. Detailed investigation of the immune response and development of candidate vaccines will be facilitated by the availability of this model.
Acknowledgments
We thank Kelly Cassity and Susan Butler for expert secretarial assistance in the preparation of this manuscript.
Footnotes
Address reprint requests to David H. Walker, M.D., Professor and Chairman, Department of Pathology, Executive Director, Center for Biodefense and Emerging Infectious Diseases, University of Texas Medical Branch, 301 University Blvd., Keiller Bldg., Galveston, TX 77555-0609. E-mail: dwalker@utmb.edu.
Supported by a grant from the National Institute of Allergy and Infectious Diseases (AI 31431).
References
- Walker DH. Diagnosing human ehrlichioses. ASM News. 2000;66:287–291. [Google Scholar]
- Olano JP, Masters E, Cullman L, Hogrefe W, Yu XJ, Walker DH. Human monocytotropic ehrlichiosis (HME): epidemiological, clinical and laboratory diagnosis of a newly emergent infection in the United States. Raoult D, Brouqui P, editors. Paris: Elsevier,; Rickettsiae and Rickettsial Diseases at the Turn of the Third Millenium. 1999:pp 262–268. [Google Scholar]
- Rikihisa Y. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1999;1:367–376. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- Olano JP, Walker DH. Human ehrlichioses. Med Clin North Am. 2002;86:375–392. doi: 10.1016/s0025-7125(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Infect. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Sotomayor EA, Popov VL, Feng HM, Walker DH, Olano JP. Animal model of fatal human monocytotropic ehrlichiosis. Am J Pathol. 2001;158:757–769. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Tajima T, Kawahara M, Rikihisa Y. Ehrlichial proliferation and acute hepatocellular necrosis in immunocompetent mice experimentally infected with the HF strain of Ehrlichia, closely related to Ehrlichia chaffeensis. J Comp Pathol. 2001;124:165–171. doi: 10.1053/jcpa.2000.0447. [DOI] [PubMed] [Google Scholar]
- Shibata S, Kawahara M, Rikihisa Y, Fujita H, Watanabe Y, Suto C, Ito T. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Suto C, Rikihisa Y, Yamamoto S, Tsuboi Y. Characterization of ehrlichial organisms isolated from a wild mouse. J Clin Microbiol. 1993;31:89–96. doi: 10.1128/jcm.31.1.89-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott J, Fuerst PA, Kawahara M, Suto C. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Ito T, Suto C, Shibata S, Rikihisa Y, Hata K, Hirai K. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Suto C, Shibata S, Futohashi M, Rikihisa Y. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol Immunol. 1996;40:575–581. doi: 10.1111/j.1348-0421.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- McBride JW, Corstvet RE, Gaunt SD, Boudreaux C, Guedry T, Walker DH. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect Immun. 2003;71:2516–2524. doi: 10.1128/IAI.71.5.2516-2524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta RR, Wilkerson MJ, Cheng C, Rokey AM, Chapes SK. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect Immun. 2002;70:380–388. doi: 10.1128/IAI.70.1.380-388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow GM, Yager E, Shilo K, Collins DN, Chu FK. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect Immun. 1998;66:3892–3899. doi: 10.1128/iai.66.8.3892-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, III, Dawson JE. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet Microbiol. 1996;52:103–112. doi: 10.1016/0378-1135(96)00064-8. [DOI] [PubMed] [Google Scholar]
- Byrom B, Obwolo M, Barbet AF, Mahan SM. A polarized Th1 type immune response to Cowdria ruminantium infection is detected in immune DBA/2 mice. J Parasitol. 2000;86:983–992. doi: 10.1645/0022-3395(2000)086[0983:APTTIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Dawson JE, Walker DH. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993;24:391–396. doi: 10.1016/0046-8177(93)90087-w. [DOI] [PubMed] [Google Scholar]
- Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- Lepidi H, Bunnell JE, Martin ME, Madigan JE, Stuen S, Dumler JS. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am J Trop Med Hyg. 2000;62:29–37. doi: 10.4269/ajtmh.2000.62.29. [DOI] [PubMed] [Google Scholar]
- Bunnell JE, Trigiani ER, Srinivas SR, Dumler JS. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J Infect Dis. 1999;180:546–550. doi: 10.1086/314902. [DOI] [PubMed] [Google Scholar]
- Martin ME, Bunnell JE, Dumler JS. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J Infect Dis. 2000;181:374–378. doi: 10.1086/315206. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Brouqui P, Aronson J, Taylor JP, Walker DH. Identification of Ehrlichia in human tissue. N Engl J Med. 1991;325:1109–1110. doi: 10.1056/NEJM199110103251517. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Sutker WL, Walker DH. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- Mahan SM, Smith GE, Kumbula D, Burridge MJ, Barbet AF. Reduction in mortality from heartwater in cattle, sheep and goats exposed to field challenge using an inactivated vaccine. Vet Parasitol. 2001;97:295–308. doi: 10.1016/s0304-4017(01)00437-x. [DOI] [PubMed] [Google Scholar]
- Safdar N, Love RB, Maki DG. Severe Ehrlichia chaffeensis infection in a lung transplant recipient: a review of ehrlichiosis in the immunocompromised patient. Emerg Infect Dis. 2002;8:320–323. doi: 10.3201/eid0803.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TR, Edwards KM, Standaert SM. Severe ehrlichiosis in an adolescent taking trimethoprim-sulfamethoxazole. Pediatr Infect Dis J. 2000;19:170–172. doi: 10.1097/00006454-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Martin GS, Christman BW, Standaert SM. Rapidly fatal infection with Ehrlichia chaffeensis. N Engl J Med. 1999;341:763–764. doi: 10.1056/NEJM199909023411014. [DOI] [PubMed] [Google Scholar]
- Carpenter CF, Gandhi TK, Kong LK, Corey GR, Chen SM, Walker DH, Dumler JS, Breitschwerdt E, Hegarty B, Sexton DJ. The incidence of ehrlichial and rickettsial infection in patients with unexplained fever and recent history of tick bite in central North Carolina. J Infect Dis. 1999;180:900–903. doi: 10.1086/314954. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Folk SM, Shore GM, Machado LJ, Huycke MM, Slater LN, Liddell AM, Buller RS, Storch GA, Monson TP, Rimland D, Sumner JW, Singleton J, Bloch KC, Tang YW, Standaert SM, Childs JE. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586–1594. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- Neer TM, Breitschwerdt EB, Greene RT, Lappin MR. Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. J Vet Intern Med. 2002;16:309–315. doi: 10.1892/0891-6640(2002)016<0309:csoedo>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17:74–80. doi: 10.1016/s1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- Frank JR, Breitschwerdt EB. A retrospective study of ehrlichiosis in 62 dogs from North Carolina and Virginia. J Vet Intern Med. 1999;13:194–201. doi: 10.1892/0891-6640(1999)013<0194:arsoei>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WR, Lockhart JM, Stallknecht DE, Howerth EW, Dawson JE, Rechav Y. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J Wildl Dis. 2001;37:538–546. doi: 10.7589/0090-3558-37.3.538. [DOI] [PubMed] [Google Scholar]
- Little SE, Howerth EW. Ehrlichia chaffeensis in archived tissues of a white-tailed deer. J Wildl Dis. 1999;35:596–599. doi: 10.7589/0090-3558-35.3.596. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, Lockhart JM, Nettles VF, Olson JG, Childs JE. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: ixodidae). J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Ismail N, Soong L, McBride JR, Valbuena G, Olano JP, Feng HM, Walker DH. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172:1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- Martin ME, Caspersen K, Dumler JS. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am J Pathol. 2001;158:1881–1888. doi: 10.1016/s0002-9440(10)64145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Crocquet-Valdes P, Walker DH. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–154. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- Yu XJ, McBride JW, Diaz CM, Walker DH. Molecular cloning and characterization of the 120-kilodalton protein gene of Ehrlichia canis and application of the recombinant 120-kilodalton protein for serodiagnosis of canine ehrlichiosis. J Clin Microbiol. 2000;38:369–374. doi: 10.1128/jcm.38.1.369-374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleman AR, Barbet AF, Bowie MV, Sorenson HL, Wong SJ, Belanger M. Expression of a gene encoding the major antigenic protein 2 homolog of Ehrlichia chaffeensis and potential application for serodiagnosis. J Clin Microbiol. 2000;38:3705–3709. doi: 10.1128/jcm.38.10.3705-3709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]