Abstract
Mutations in the NPHS1 gene encoding nephrin lead to congenital nephrotic syndrome of the Finnish type. Nephrin is a key component of the glomerular slit diaphragms between epithelial foot processes, but its role in the pathogenesis of this disease is poorly understood. To further clarify the molecular mechanisms involved we investigated the interactions between nephrin and other components of the foot processes and filtration slits, especially adherens junction proteins, and searched for novel nephrin interacting proteins. Using co-immunoprecipitation and pull-down assays we show here that nephrin forms a multiprotein complex with cadherins and p120 catenin and with three scaffolding proteins, ZO-1, CD2AP, and CASK, in kidney glomeruli and when expressed in Madin-Darby canine kidney cells. CASK was identified as a novel binding partner of nephrin by mass spectrometry and was localized to podocytes in the glomerulus. CASK is a scaffolding protein that participates in maintenance of polarized epithelial cell architecture by linking membrane proteins and signaling molecules to the actin cytoskeleton. Our results support a model whereby the glomerular slit diaphragms are composed of cell adhesion molecules of the immunoglobulin and cadherin superfamilies that are connected to each other and to the actin cytoskeleton and signaling networks via the cytoplasmic scaffolding proteins CASK, CD2AP, and ZO-1.
A key finding in understanding the molecular mechanisms involved in the pathogenesis of proteinuria was the identification of the NPHS1 gene, which is mutated in the congenital nephrotic syndrome of the Finnish type.1 NPHS1 encodes for nephrin,1 a member of the immunoglobulin (Ig) superfamily of cell adhesion molecules,2 which is localized in the glomerular slit diaphragm and has been suggested to form the framework of this specialized cell adhesion structure.3–5
Slit diaphragms are important components of the glomerular filtration barrier. They bridge the urinary spaces between the foot processes of the glomerular epithelium and serve to attach foot processes to one another. They are considered to represent specialized cell junctions because they are derived from typical junctional complexes during glomerular development,6 and in nephrotic animals the slit diaphragms are replaced by tight junctions.7,8 In addition to nephrin several other novel proteins, Neph19,10 and podocin,11 have been localized to the slit diaphragm along with several proteins characteristic of tight and adherens junctions including ZO-1 and cadherins. ZO-1 is an actin-associated protein and is concentrated on the cytoplasmic side of tight junctions in polarized epithelial cells.12,13 In the glomerulus it is concentrated on the cytoplasmic side of the slit diaphragm14,15 and has been suggested to function as a scaffolding protein that organizes signaling complexes at the slit diaphragm.16 P-cadherin,17,18 a classical cadherin, and FAT,19 a cadherin superfamily member of unknown function, have also been localized to the slit diaphragm. Cadherins are key components of adherens junctions that mediate Ca2+-dependent, homotypic cell adhesion.20,21 Their presence in the slit diaphragm suggests that the slit diaphragm may have some of the properties of a modified adherens junction.17
Although these components of adherens and tight junctions have been reported to be present in the slit diaphragm region, it is not known if they simply co-localize with nephrin or whether they bind to nephrin. In this study we set out to determine whether nephrin can interact with cadherins and ZO-1 and to identify additional nephrin binding partners. To facilitate our studies we generated a Madin-Darby canine kidney (MDCK) cell line stably expressing nephrin. MDCK cells are well-characterized polarized epithelial cells with tight and adherens junctions, and they endogenously express several components of the glomerular slit diaphragms including ZO-1 and cadherins. We obtained evidence based on immunoprecipitation and pull-down assays performed on both glomerular lysates and lysates of MDCK cells expressing nephrin that nephrin forms a multiprotein complex with cadherins, p120 catenin, ZO-1, CD2AP, and CASK. CASK (calcium/calmodulin-dependent serine protein kinase) is a multidomain scaffolding protein that belongs to the membrane-associated guanylate kinase (MAGUK) family and participates in the regulation of the polarized epithelial cell phenotype.22,23 This complex most likely plays an important role in establishing the structural integrity and functional properties of the glomerular slit diaphragms and filtration slits.
Materials and Methods
Materials
Chemical reagents were from Sigma (St. Louis, MO) or Fisher Biotech (Tustin, CA) and detergents from Sigma or Calbiochem (San Diego, CA). Restriction endonucleases were from New England Biolabs (Beverly, MA). Cell culture plastics were obtained from Corning (Corning, NY) and Kodak Biomax MR film from Fisher Biotech.
Construction of Vectors
To produce a retroviral vector carrying nephrin, full-length rat nephrin cDNA24 was subcloned into SalI/ClaI sites in the pLNCX2 vector (BD Biosciences Clontech, Palo Alto, CA). To produce glutathione S-transferase (GST)-nephrin tail, the cytoplasmic domain of rat nephrin (amino acids 1083 to 1234) was subcloned into EcoRI/XhoI sites in pGEX-4T-1 vector (Amersham Biosciences, Buckinghamshire, UK). The GST-CD2AP 3.SH3 domain fusion protein construct was produced as described.25
Cell Culture, Retroviral Infection, and Selection of Cells Stably Overexpressing Nephrin
MDCK I and human embryonic kidney 293 (HEK 293) cells were maintained in a humidified atmosphere containing 95% air-5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium, high glucose (Gibco Invitrogen, Grand Island, NY), supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/L glutamine. For retroviral packaging, HEK 293 cells were transiently co-transfected with full-length rat nephrin cDNA in pLNCX2 and the packaging vector, pKAT 2 (obtained from Dr. Mark Kamps, University of California San Diego, La Jolla, CA), or pLNCX2 and pKAT 2 alone as a control, using calcium phosphate. Medium containing the virus was collected 24 to 48 hours after transfection, filtered through a 0.45-μm filter (Millipore, Bedford, MA) and stored at −80°C.
MDCK cells were plated on a 10-cm dish the day before infection to obtain 25% confluent cultures at the time of infection. One ml of viral stock in 3 ml of culture medium with 8 μg/ml of polybrene (hexadimethrine bromide; Sigma) was added to the cells. After 4 hours, 6 ml of culture medium were added, and 24 hours after infection the virus-containing medium was removed and replaced by culture medium supplemented with 1 mg/ml of G418 (Gibco Invitrogen) for 10 days to select for cells stably expressing nephrin.
Antibodies
Six nephrin antibodies were used: anti-nephrin 5-1-6 IgG, a mouse monoclonal antibody (mAb) against the extracellular domain of nephrin26 (obtained from Dr. Fujio Shimizu, Niigata University, Niigata, Japan); mouse mAb anti-nephrin 043 raised against the extracellular domain of rat nephrin (amino acids 749 to 1030); and four rabbit polyclonal antibodies, including protein A-purified (Amersham Biosciences) anti-nephrin 1109, raised against human nephrin,27 anti-nephrin 029, raised against the cytoplasmic tail of rat nephrin,27 anti-nephrin 1054, raised against the cytoplasmic tail of human nephrin,24 and anti-nephrin (LH) raised against the cytoplasmic tail of mouse nephrin3 (obtained from Dr. Lawrence Holzman, University of Michigan, Ann Arbor, MI). Rabbit anti-CD2AP R209 was raised against amino acids 331 to 637 of mouse CD2AP, and anti-CD2AP 1774 was raised against mouse CD2AP as described.28 Affinity-purified rabbit anti-pan-cadherin IgG against the C-terminus of N-cadherin, anti-ZO-1, and anti-CASK IgG and rat mAb anti-P-cadherin IgG were from Zymed (South San Francisco, CA). Rabbit anti-P-cadherin serum, raised against the cytoplasmic tail of mouse P-cadherin,29 was obtained from Dr. Raghu Kalluri (Harvard Medical School, Boston, MA). Anti-p120 catenin and anti-E-cadherin (rr1) mAbs were from BD Transduction Laboratories (BD Biosciences, San Jose, CA) and the Developmental Studies Hybridoma Bank (Iowa City, IA), respectively. Anti-protein disulfide isomerase mAb was from StressGen (Victoria, BC, Canada). Horseradish peroxidase-conjugated goat anti-rabbit, anti-mouse, and anti-rat IgG were purchased from Biodesign International (Saco, ME), Bio-Rad Laboratories (Hercules, CA), and Zymed, respectively, and rabbit IgG TrueBlot from eBioscience (San Diego, CA). Highly cross-absorbed Alexa 488 goat anti-mouse and Alexa 568 goat anti-rabbit IgG were from Molecular Probes (Eugene, OR). Anti-rabbit (10 nm) and anti-mouse (5 nm) IgG gold conjugates were purchased from Amersham Biosciences.
Preparation of Cell Lysates
MDCK-nephrin or MDCK-mock cells on 100-mm dishes were washed twice with ice-cold phosphate-buffered saline (PBS) and scraped from the dish with a cell lifter into 1 ml of ice-cold lysis buffer [0.5% Nonidet P-40, 10 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA) with or without 0.25% Triton X-100 (TX-100)] supplemented with 1× Complete, EDTA-free proteinase inhibitor cocktail (Roche, Mannheim, Germany), 50 mmol/L sodium fluoride (to inhibit Ser/Thr phosphatases), and 1 mmol/L sodium vanadate (to inhibit tyrosine phosphatases). Cell lysates were incubated at 4°C for 10 minutes with rotation, and detergent insoluble material was removed by centrifugation (10,000 × g for 2 minutes).
Preparation of Membrane and Cytosolic Fractions of MDCK-Nephrin Cells and Immunoblotting
MDCK-nephrin cells were homogenized and fractions prepared by centrifugation of a postnuclear supernatant (100,000 × g at 4°C for 1 hour) as previously described.30 Fractions were reconstituted to an equal volume containing a final concentration of 1× Laemmli sample buffer and boiled. Equal volumes of the fractions were separated by 7.5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore). After blocking with 5% nonfat milk in Tris-buffered saline supplemented with 0.1% Tween-20 (TBS-Tween), membranes were incubated with primary antibodies diluted in 1% nonfat milk in TBS-Tween at room temperature (1 hour) followed by horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG or with rabbit IgG TrueBlot (1 hour). Detection was by enhanced chemiluminescence (SuperSignal; Pierce Chemical Co., Rockford, IL).
Preparation of Glomerular Lysates
Glomerular fractions (containing >95% glomeruli) were isolated from kidney cortices of male Sprague-Dawley rats (150 to 200 g; Harlan Sprague Dawley, Indianapolis, IN) by graded sieving as previously described.31 Glomerular lysates were prepared by incubation in 1% Nonidet P-40, 20 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.5, 150 mmol/L NaCl, 100 mmol/L potassium iodide (KI)31 (for pull-downs), or in 1% TX-100, 0.5% Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.6, 1 mmol/L EDTA, 1 mmol/L EGTA (for immunoprecipitation) supplemented with 1× Complete, 50 mmol/L sodium fluoride, and 1 mmol/L sodium vanadate at 4°C for 30 minutes, and detergent insoluble material was removed by centrifugation (10,000 × g for 2 minutes).
Deglycosylation and Dephosphorylation
Removal of N-linked oligosaccharides was performed with an Enzymatic deglycosylation kit (Glyco, Novato, CA). Briefly, 10 μg of cell or glomerular lysates were treated with SDS and β-mercaptoethanol, followed by incubation with 2.5 mU of N-glycanase at 37°C for 4 hours. For dephosphorylation, 10 μg of cell or glomerular lysates were incubated with 18 U alkaline phosphatase (Roche) in 50 mmol/L Tris-HCl, pH 9, 1 mmol/L MgCl2, 0.1 mmol/L ZnCl2, 1 mmol/L spermidine, at 30°C for 8 hours. Proteins were separated by SDS-PAGE for immunoblotting.
Indirect Immunofluorescence
MDCK cells were seeded on polycarbonate Transwell filters (Costar, Cambridge, MA) at 1 × 105 cells/cm2 and allowed to grow for 2 to 4 days. Monolayers were fixed in 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer, pH 7.4, for 30 minutes, permeabilized with 0.15% TX-100 in PBS for 10 minutes, and incubated with primary antibodies overnight at 4°C. For double labeling cells were incubated simultaneously with two antibodies followed by detection with Alexa 488 goat anti-mouse and Alexa 568 goat anti-rabbit IgG in 5% fetal calf serum in PBS for 2 hours at room temperature. Samples were mounted in 75% glycerol in PBS containing 1 mg/ml of paraphenylinediamine. Images were collected with an MRC-1000 confocal imaging system (Bio-Rad Laboratories) and processed using Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Kidneys were either rapidly frozen in liquid nitrogen or perfusion fixed with PFA.31 Cryostat sections were cut from unfixed kidney tissue, fixed with acetone, and stained with anti-P-cadherin as previously described.29 For preparation of semithin sections, PFA-fixed kidneys were cryoprotected and frozen in liquid nitrogen.32 Semithin cryosections (0.5 to 0.7 μm) were cut with a Leica Ultracut UCT microtome equipped with an FCS cryoattachment at −100°C and stained as above. Samples were examined either by confocal microscopy or with a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, NY). Images were collected with a ORCA-ER camera (Hamamatsu, Bridgewater, NJ) using Scion image version 1.59 (Scion Corp., Frederick, MA) and processed using Adobe Photoshop 5.5.
Immunoelectron Microscopy
Cells were fixed in 4% PFA for 45 minutes followed by 15 minutes in 8% PFA, cryoprotected in sucrose, frozen in liquid nitrogen, and ultrathin cryosections (80 nm) were cut and processed as described.32 Sections were incubated with anti-nephrin 1109 and anti-E-cadherin IgG overnight at 4°C, followed by 5- or 10-nm gold-conjugated goat anti-rabbit or anti-mouse IgG for 2 hours at room temperature. Sections were adsorption stained31 and examined in a JEOL 1200 EX-II electron microscope.
Fluorescence-Activated Cell Sorting (FACS) of Cells Showing Different Levels of Nephrin Expression
MDCK-nephrin cells were labeled in suspension with anti-nephrin 5-1-6 IgG for 15 minutes at 4°C followed by Alexa 488 goat anti-mouse IgG. Cells were sorted by FACS33 to produce cell populations and pure subclones with different levels of nephrin expression. Sorting was performed with FACS Vantage SE, equipped with the CloneCyt Plus System and Quick Width Pulse Processing Plus (Becton Dickinson Immunocytometry Systems, San Jose, CA) in the University of California San Diego Cancer Center Flow Cytometry Shared Resource. Laser output power (488 nm) was set to 60 mW and green fluorescence was detected with the standard 530/30-nm filter.
Co-Immunoprecipitation Assays
MDCK-nephrin cell and glomerular lysates were precleared with protein A-Sepharose at 4°C for 1 hour and incubated with anti-nephrin or preimmune IgG (5 μg) or anti-CD2AP serum (5 μl) for 16 to 20 hours at 4°C. Immune complexes were bound to protein A-Sepharose beads at 4°C for 1 hour, washed three times with lysis buffer, and boiled in Laemmli sample buffer for immunoblotting.
Pull-Down Assays
GST and GST-nephrin tail and GST-CD2AP 3.SH3 domain fusion proteins were expressed in Escherichia coli BL21(DE3) (Stratagene, La Jolla, CA) and purified on glutathione-Sepharose beads (Amersham Biosciences). MDCK-nephrin cell and glomerular lysates were precleared with glutathione-Sepharose beads at 4°C for 1 hour, followed by incubation at 4°C for 4 hours or overnight with GST-nephrin tail, GST-CD2AP 3.SH3 domain, or GST alone (20 μg each) immobilized on beads. Beads were washed extensively with lysis buffer and boiled in Laemmli sample buffer. Proteins were separated by SDS-PAGE for immunoblotting.
Sucrose Velocity Gradient Centrifugation
Velocity gradient centrifugation was performed as described.34 MDCK-nephrin cells were lysed as described above and insoluble material was removed by centrifugation (15,000 × g for 15 minutes at 4°C). Cell lysates were applied on top of a 5 to 25% continuous sucrose gradient on a 750-μl, 60% sucrose cushion. Sucrose solutions were prepared in lysis buffer containing 0.05% Nonidet P-40 and 0.025% TX-100. After centrifugation for 15 hours at 4°C at 40,000 rpm in a SW 41 rotor, the fractions were collected from the top, and the sucrose concentration of each fraction was calculated from the refractive index. Samples of each fraction were immunoblotted with nephrin, pan-cadherin, and CD2AP antibodies, and their sedimentation coefficients were assessed using protein molecular weight standards (Sigma) with known S values.
Measurement of Transepithelial Electrical Resistance (TER)
Cells were seeded on polycarbonate Transwell filters at 1 × 105 cells/cm2. TER was measured with a Millicell-ERS (electrical resistance system; Millipore) and normalized to the area of the filter according to the manufacturer’s instructions.
Mass Spectrometry Analysis
Pull-down assays with GST-nephrin tail and GST alone were performed on glomerular lysates as described above. Bound proteins were separated by SDS-PAGE and stained with GelCode Blue (Pierce Chemical Co.). The ∼110-kd band obtained in the GST-nephrin tail pull-down was excised from the gel and processed for mass spectrometry.35 Briefly, the excised band was washed with water and destained with acetonitrile and 100 mmol/L NH4HCO3, followed by drying in a speed vac. The sample was reduced and alkylated by treatment with 10 mmol/L dithiothreitol and 55 mmol/L iodoacetamide, followed by washes with acetonitrile and 100 mmol/L NH4HCO3, and drying in a speed vac. In-gel trypsin digestion was performed with 12.5 ng/μl of trypsin (Promega, Madison, WI) in 50 mmol/L NH4HCO3 and 5 mmol/L CaCl2 on an ice bath for 45 minutes, followed by incubation at 37°C overnight in buffer alone. The digested peptides were extracted with 25 mmol/L NH4HCO3 followed by 5% formic acid and acetonitrile. Mass spectrometry was performed at the Scripps Center for Mass Spectrometry, and the peptides obtained by NanoLC-MS/MS analysis were identified by searching the Mascot/NCBInr database.
Results
Nephrin Is Expressed as a 180-kd Transmembrane Protein in MDCK-Nephrin Cells
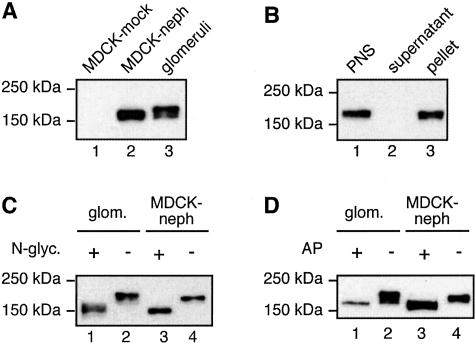
To establish a cell culture model for investigating the interactions of nephrin we prepared MDCK cells stably expressing nephrin (MDCK-nephrin cells) using a retrovirus expression system. In these cells, nephrin appeared as an ∼180-kd band that migrated slightly faster than endogenous nephrin in glomeruli (Figure 1A). Immunoblotting analysis of membrane (100,000 × g pellet) and cytosolic (100,000 × g supernatant) fractions prepared from MDCK-nephrin cells (Figure 1B) indicated that nephrin is associated exclusively with membranes.
Figure 1.
Immunoblot analysis of nephrin expressed in MDCK cells. A: In MDCK cells stably expressing nephrin (lane 2), nephrin appears as an ∼180-kd band that migrates slightly faster than endogenous nephrin in glomerular lysates (lane 3). Nephrin is not detected in MDCK cells infected with vector alone (lane 1). MDCK cells were retrovirally infected with a vector carrying nephrin and selected with G418 to obtain cells stably expressing nephrin. MDCK-nephrin cell and glomerular lysates were prepared as described in Materials and Methods and 10 μg of the lysates were separated by 7.5% SDS-PAGE followed by immunoblotting with anti-nephrin 043 IgG. B: Nephrin is detected in membrane fractions when expressed in MDCK cells. MDCK-nephrin cells were lysed with hypotonic buffer followed by fractionation of the postnuclear supernatant (PNS, lane 1) into cytosolic (supernatant, lane 2) and membrane (pellet, lane 3) fractions. Equal volumes of the fractions were analyzed by immunoblotting as in A. C: Nephrin is N-glycosylated when expressed in MDCK cells. N-glycanase induces a shift in the mobility of nephrin in both glomeruli (lanes 1 and 2) and MDCK-nephrin cells (lanes 3 and 4). Ten μg of MDCK-nephrin cell and glomerular lysates were incubated with 2.5 mU of N-glycanase at 37°C for 4 hours and immunoblotted as in A. D: Nephrin is phosphorylated when expressed in MDCK cells. Alkaline phosphatase induces a shift in the mobility of nephrin in both glomeruli (lanes 1 and 2) and MDCK-nephrin cells (lanes 3 and 4). Ten μg of MDCK-nephrin cell and glomerular lysates were incubated with 18 U of alkaline phosphatase at 30°C for 8 hours and immunoblotted as above.
Nephrin Is N-Glycosylated and Phosphorylated in MDCK-Nephrin Cells
Nephrin contains multiple putative N-glycosylation sites1 and is N-glycosylated in glomeruli.3 Nephrin is similarly modified when expressed in MDCK cells, because treatment of cell lysates with N-glycanase (removes N-linked oligosaccharides) resulted in a shift in the electrophoretic mobility of nephrin from ∼180 to 185 to ∼150 to 160 kd (Figure 1C).
Rat nephrin has also been shown to be tyrosine phosphorylated in glomeruli.36 To determine whether rat nephrin is phosphorylated when expressed in MDCK cells, we digested cell lysates with alkaline phosphatase. This treatment resulted in a shift in the electrophoretic mobility of nephrin from ∼180 to 185 to ∼160 to 170 kd (Figure 1D). These results indicate that when rat nephrin is expressed in MDCK cells its posttranslational modifications are similar to those in podocytes.
Sorting and Cloning of MDCK-Nephrin Cells by FACS
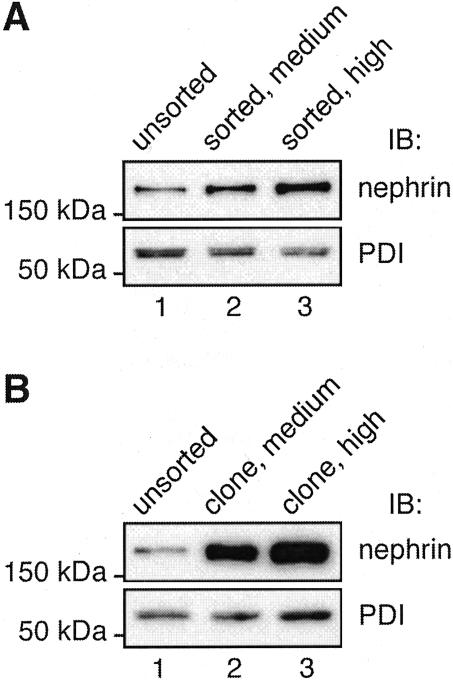
Next we examined the levels of expression of nephrin in MDCK-nephrin cells by indirect immunofluorescence on cell monolayers followed by confocal laser-scanning microscopy. Considerable variation was seen in nephrin expression among the cell population with some cells showing hardly any signal and others showing very high levels (data not shown). To obtain cells with more uniform expression levels of nephrin we FACS sorted the cells after labeling nephrin at the cell surface with anti-nephrin 5-1-6. Bulk cell populations were obtained by collecting cells showing medium or high levels of fluorescence, representing ∼13% and ∼7.4% of the total cell population, respectively. The level of nephrin expression was increased (two to three times) in mixed cell populations sorted for medium and high levels of nephrin compared to unsorted cells (Figure 2A). We also clonally expanded individual cells to obtain subclones in which the level of nephrin expression (Figure 2B) was dramatically increased (>10 times).
Figure 2.
Nephrin expression in FACS MDCK-nephrin cells. A: MDCK-nephrin cell populations selected for medium (lane 2) or high (lane 3) nephrin express more nephrin than unsorted MDCK-nephrin cells (lane 1). B: Clones of MDCK-nephrin cells selected for medium (lane 2) or high (lane 3) nephrin express much higher levels of nephrin (>10-fold) than unsorted cells (lane 1). Ten μg of cell lysates were separated by 10% SDS-PAGE and immunoblotted with anti-nephrin 043 IgG. Anti-protein disulfide isomerase (PDI) IgG (bottom) was used as a control for loading.
Nephrin Is Present on Both Apical and Lateral Domains of the Cell Membrane in MDCK Cells
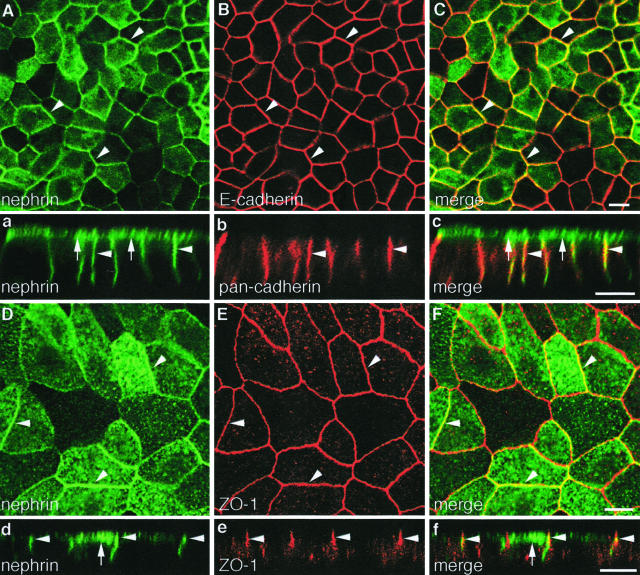
The sorted cell populations and clones also showed higher and more uniform nephrin expression by immunofluorescence (Figure 3, Aa and Dd). Analysis of confocal sections of cell monolayers showed that nephrin is localized on both apical (Figure 3, a and d) and lateral domains of the cell membrane and is distributed along the latter beyond the apical junctional complex (Figure 3, a and d). Staining of the apical cell surface was confirmed by incubating nonfixed, nonpermeabilized cell monolayers with an antibody, anti-nephrin 5-1-6, that binds to the extracellular domain of nephrin. Thus the distribution of nephrin in MDCK cells resembles that in the glomerulus where nephrin has been localized in both the junctional or slit diaphragm region and at the apical domain of podocytes.3–5
Figure 3.
Nephrin partially co-localizes with cadherins and ZO-1 in MDCK-nephrin cells. Double-immunofluorescence labeling of MDCK-nephrin cell monolayers for nephrin and E-cadherin (A–C), nephrin and pan-cadherin (a–c), and nephrin and ZO-1 (Dd–Ff). Nephrin is localized on both the apical (arrows) and lateral (arrowheads) plasma membrane domains as seen by confocal microscopy (Aa and Dd). Nephrin and E-cadherin partially co-localize along the lateral plasma membrane (arrowheads), as seen in horizontal sections (A–C). Often linear staining for both nephrin and cadherins is seen along the entire lateral cell membrane in vertical sections (a–c). Nephrin partially co-localizes with ZO-1 (arrowheads) as seen in both horizontal sections at the level of the tight junctions (D–F) and vertical sections (d–f). Cells cultured on filters were fixed with PFA, permeabilized, and double labeled with anti-nephrin 1109 and anti-E-cadherin IgG, or anti-nephrin 5-1-6 and either anti-pan-cadherin or anti-ZO-1 IgG, followed by confocal microscopy. Scale bars, 10 μm.
Nephrin Partially Co-Localizes with Junctional Proteins in MDCK-Nephrin Cells
To find out if nephrin co-localizes with junctional proteins, we performed double labeling for components of adherens junctions (cadherins and p120 catenin) and tight junctions (ZO-1). Confocal analysis of horizontal (Figure 3, A to C) and vertical (Figure 3, a to c) sections of cells selected for high levels of nephrin showed that endogenous cadherins and nephrin partially co-localize along the lateral cell membrane. This is in accord with previous studies showing that cadherin expression extends along the entire lateral cell membrane in MDCK cells and is not restricted to adherens junctions in the junctional complex.37 No staining for either nephrin or cadherins was observed on the basal cell membrane. Similar results were obtained with p120 catenin (not shown), a cytoplasmic protein that associates with cadherins and regulates cell adhesion.38 We also examined the distribution of nephrin and ZO-1 and found that these proteins partially overlapped at the level of the tight junctions (Figure 3, D to F and d to f). Nephrin incorporation into the junctional region did not cause any obvious morphological changes in the structure of the junctions or changes in the expression pattern of adherens and tight junction proteins in MDCK cells.
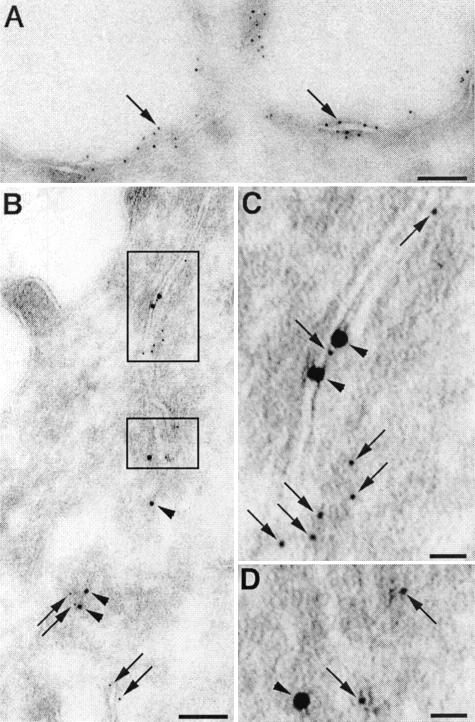
We also performed double-immunogold labeling for nephrin and E-cadherin on ultrathin cryosections of MDCK-nephrin cells. In keeping with the immunofluorescence results, nephrin was seen to be distributed along both the apical (Figure 4A) and lateral (Figure 4B) plasma membrane domains. Gold particles for both nephrin and E-cadherin were concentrated along adherens junctions in the apical junctional complex (Figure 4, B and C) as well as below it (Figure 4D). As already mentioned, in podocytes nephrin was found both at the slit diaphragm and along the apical plasma membrane.3–5
Figure 4.
Immunogold labeling of nephrin and E-cadherin in MDCK-nephrin cells. A: Nephrin (5 nm gold, arrows) is detected on the apical plasma membrane. B: Double labeling of nephrin (5 nm gold, arrows) and E-cadherin (10 nm gold, arrowheads) in cell-cell junctions in the region of the apical junctional complex (boxed areas). C: Higher magnification of the top boxed region in B showing co-localization of nephrin (arrows, small gold) and E-cadherin (arrowheads, large gold) in the junctional complex close to the apical domain. D: Higher magnification of the bottom boxed region in B showing nephrin and E-cadherin in cell-cell junctions along the lateral plasma membrane below the apical junctional complex. Ultrathin cryosections were incubated with anti-nephrin 1109 and anti-E-cadherin IgG followed by goat anti-rabbit (5 nm) gold or anti-mouse (10 nm) gold IgG conjugates. Scale bars: 100 nm (A, B); 20 nm (C, D).
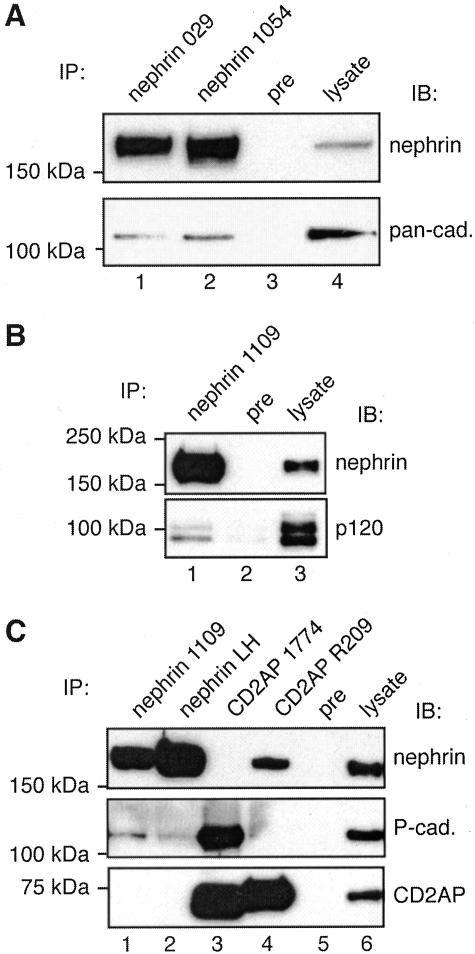
Nephrin Forms a Multiprotein Complex with P-Cadherin, p120 Catenin, ZO-1, and CD2AP in MDCK-Nephrin Cells
The localization of nephrin to slit diaphragms in the glomerulus and cell junctions in MDCK cells raises the question of whether nephrin actually associates with junctional proteins or simply co-distributes with them. To gain information on this point, we performed immunoprecipitation on MDCK-nephrin cell lysates with four different nephrin antibodies followed by immunoblotting with a pan-cadherin antibody (recognizes N-, E-, P-, and R-cadherins), an antibody that specifically recognizes P-cadherin (the predominant cadherin expressed in podocytes17), and antibodies against ZO-1 and α-, β-, and p120 catenin. We detected both cadherins (Figure 5A) including P-cadherin (Figure 5C) and p120 catenin (Figure 5B) in nephrin immunoprecipitates, indicating that nephrin, cadherins and p120 catenin are in the same protein complex. We also performed immunoprecipitation assays with two different CD2AP antibodies and detected cadherins (Figure 6A), including P-cadherin (Figure 5C), and nephrin (Figure 5C) in the immunoprecipitates, indicating that CD2AP is also a component of the nephrin-cadherin complex. CD2AP has previously been shown to associate with nephrin and has been suggested to link nephrin to the actin cytoskeleton.25,39,40 To validate the existence of these multiprotein complexes we also used an alternative approach and performed pull-down assays with GST-nephrin tail on MDCK-nephrin cell lysates. We found that ZO-1 as well as p120 catenin and CD2AP bound to GST-nephrin tail but not to GST alone (Figure 6B). We were unable to detect α- or β-catenin in either the immunoprecipitates or pull-downs. Thus, using four different nephrin antibodies and two different CD2AP antibodies for immunoprecipitation we found that nephrin associates with adherens junction proteins as well as its previously characterized interaction partner CD2AP when expressed in MDCK cells. Results of pull-down assays with GST-nephrin tail make it clear that the interactions occur through the cytoplasmic tail of nephrin, but our results do not provide information on whether the binding is direct or indirect.
Figure 5.
Nephrin, cadherins, p120 catenin, and CD2AP co-precipitate in MDCK-nephrin cells. A and B: Cadherins (lanes 1 and 2 in A) and p120 catenin (lane 1 in B) co-immunoprecipitate with nephrin. No cadherins or p120 catenin can be detected in precipitates obtained with preimmune serum (lane 3 in A, lane 2 in B). MDCK-nephrin cell lysate (100 μg, lane 4 in A; 10 μg, lane 3 in B) is included as control. MDCK-nephrin cells were lysed in 0.5% Nonidet P-40, 10 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1 mmol/L EDTA with proteinase inhibitors. Immunoprecipitations were performed on ∼2 mg cell lysate with anti-nephrin 029, 1054 (lanes 1 and 2 in A), and 1109 (lane 1 in B), or preimmune IgG as described in Materials and Methods. Precipitated proteins were separated by 7.5% SDS-PAGE and immunoblotted with anti-pan-cadherin, anti-p120 catenin, or anti-nephrin 043 IgG. C: P-cadherin co-immunoprecipitates with nephrin (lanes 1 and 2, middle) and CD2AP (lane 3, middle). Nephrin can also be immunoprecipitated with anti-CD2AP (lane 4, top). No P-cadherin or nephrin can be detected in precipitates obtained with preimmune serum (lane 5). MDCK-nephrin cell lysate (5 μg in top and middle panels; 25 μg in bottom panel, lane 6) is included as control. Immunoprecipitations were performed as described above with the indicated antibodies to nephrin and CD2AP, and the precipitated proteins were analyzed by immunoblotting with anti-nephrin 043, anti-P-cadherin, or anti-CD2AP 1774.
Figure 6.
Association between nephrin, CD2AP, and junctional proteins. A: Cadherins co-immunoprecipitate with CD2AP (lane 1). No cadherins can be detected in precipitates obtained with preimmune serum (lane 2). MDCK-nephrin cell lysate (upper panel, 25 μg; lower panel, 100 μg, lane 3) is included as control. Immunoprecipitations were performed as described in Figure 5 with anti-CD2AP 1774 or preimmune IgG, and the precipitated proteins were analyzed by immunoblotting with anti-pan-cadherin or anti-CD2AP 1774. B: Pull-down assays on MDCK cell lysates. p120 catenin, ZO-1, and CD2AP are pulled down with GST-nephrin tail (lane 1) but not with GST alone (lane 2). Two μg of MDCK-nephrin cell lysate is included as control in the immunoblotting with p120 catenin and CD2AP and 25 μg with ZO-1 (lane 3). MDCK-nephrin cells were lysed as in Figure 5. Twenty μg of nephrin cytoplasmic domain fused to GST (nephrin-GST) or GST alone (GST) were incubated with ∼1 mg of cell lysate for 4 hours and immunoblotted with anti-p120 catenin, anti-ZO-1, or anti-CD2AP. TX-100 (0.25%) was added in CD2AP pull-downs.
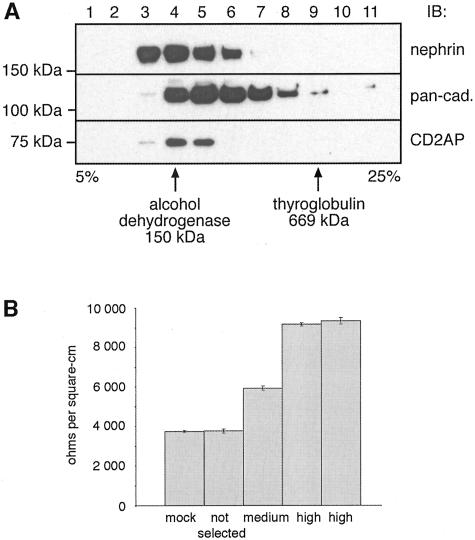
Nephrin Co-Sediments with Cadherins and CD2AP
Co-sedimentation represents another assay commonly used to analyze if proteins are present in the same complex.41 When we subjected detergent-solubilized, MDCK-nephrin cell lysates to velocity gradient centrifugation on sucrose gradients we found that nephrin and cadherins co-sedimented and were broadly distributed across the gradient. Moreover, nephrin, cadherins, and CD2AP co-sedimented in fractions 4 and 5 (Figure 7A) in a region of the gradient heavier than expected of monomers. These results are in keeping with the immunoprecipitation and pull-down data indicating the existence of these proteins in a co-sedimentable multiprotein complex.
Figure 7.
A: Nephrin, cadherins, and CD2AP co-sediment by sucrose velocity gradient centrifugation. MDCK-nephrin cells were lysed, loaded on top of a 5 to 25% continuous sucrose gradient and centrifuged as described in Materials and Methods. One-ml fractions were collected and a 40-μl aliquot of each fraction was separated by SDS-PAGE followed by immunoblotting with anti-nephrin 043, anti-pan-cadherin, and anti-CD2AP 1774 IgG. The locations of protein standards (alcohol dehydrogenase, 150 kd; and thyroglobulin, 669 kd) is indicated. Nephrin and cadherins have a broad distribution in the gradient with cadherins found in fractions 4 to 9 and nephrin in fractions 3 to 6. CD2AP is detected in fractions 4 and 5. B: Expression of nephrin increases the TER of MDCK cell monolayers. In MDCK cell clones expressing medium and high levels of nephrin TER is increased 1.6- and 2.5-fold (average: 5950, 9170, and 9350 ohms/cm2, respectively) compared to control MDCK cells (mock; average, 3740 ohms/cm2). TER values of a mixed population (not selected) of MDCK-nephrin cells are similar (average, 3760 ohms/cm2) to those of control MDCK cells. Cells were cultured on filters and TER was measured with a Millipore ERS electrical resistance system. Values are mean ± SD of four individual cell culture inserts of one representative experiment. The same result was obtained in three separate experiments.
Nephrin Expression Increases TER in MDCK Cells
Nephrin belongs to the Ig superfamily of cell adhesion receptors. Its incorporation into junctions of MDCK cells might be expected to affect the properties of these junctions. To find out if this is the case we measured TER of the monolayers after they reached confluency. MDCK-nephrin clones expressing medium and high levels of nephrin showed TER values that were 1.6-fold and 2.5-fold higher (average, 5950 and 9260 ohms/cm2) than TER values of MDCK-mock cells (average, 3740 ohms/cm2; Figure 7B). The TER of a mixed population of MDCK-nephrin cells (average, 3760 ohms/cm2) was similar to that of MDCK-mock cells. Thus when nephrin is incorporated into cell-cell junctions the tightness of the cell monolayers increases in parallel with the amount of nephrin expressed.
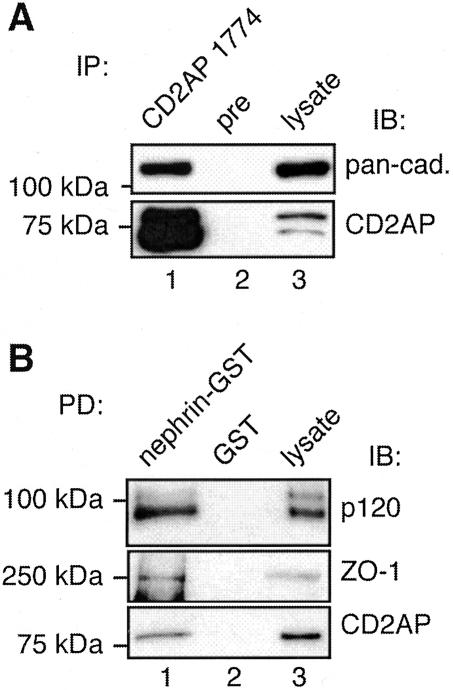
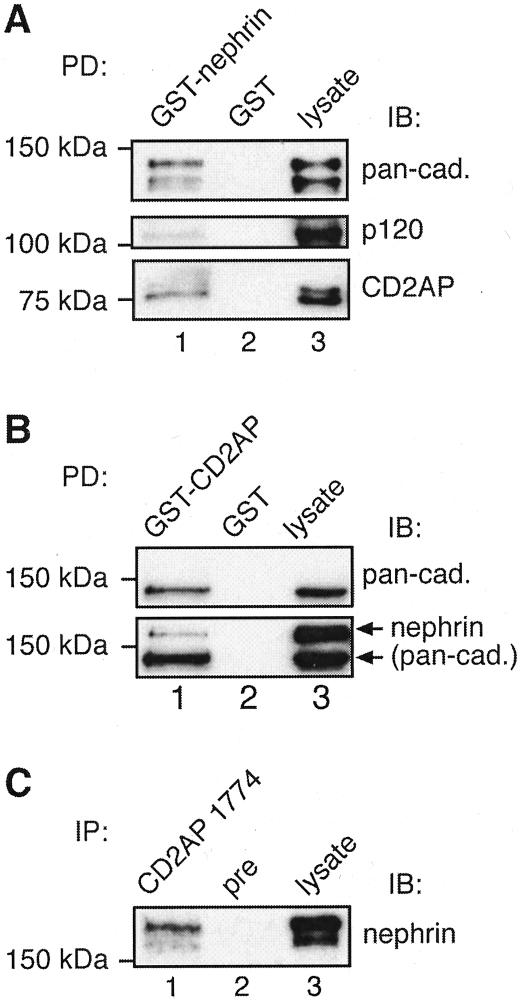
Nephrin Associates with CD2AP and Adherens Junction Proteins in Rat Glomeruli
Next we checked whether the association between adherens junction proteins, CD2AP and nephrin seen in MDCK cells expressing nephrin applies to endogenous proteins in glomeruli. Using pull-down assays with GST-nephrin tail on glomerular lysates, we found that cadherins, p120 catenin, and CD2AP bound to GST-nephrin tail but not to GST alone (Figure 8A). To further confirm the interactions between nephrin, cadherins, and CD2AP we performed pull-down assays on glomerular lysates with GST-CD2AP 3.SH3 domain that was previously shown to mediate the interaction between CD2AP and nephrin.25 We found that both nephrin and cadherins bound to GST-CD2AP (Figure 8B).
Figure 8.
Nephrin, cadherins, p120 catenin, and CD2AP form a multiprotein complex in glomeruli. A: Cadherins, p120 catenin, and CD2AP bind to GST-nephrin tail (lane 1) but not to GST alone (lane 2). Glomerular lysate (2 μg for pan-cadherin and p120 catenin and 5 μg for CD2AP) is included as a control (lane 3). Isolated glomeruli were lysed in 1% Nonidet P-40, 20 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 100 mmol/L KI with proteinase inhibitors. Four hundred μg of glomerular lysate was incubated with 20 μg of GST-nephrin tail or GST alone as described in Figure 6 and immunoblotted with anti-pan-cadherin, anti-p120 catenin, or anti-CD2AP 1774. B: Cadherins and nephrin bind to GST-CD2AP (lane 1) but not to GST alone (lane 2). Glomerular lysate (2 μg) is included as a control (lane 3). The same membrane was first immunoblotted with anti-pan-cadherin IgG (top), followed by immunoblotting with anti-nephrin 043 IgG (bottom). C: Nephrin co-immunoprecipitates with CD2AP from glomerular lysates (lane 1). No nephrin can be detected in precipitates obtained with preimmune serum (lane 2). Glomerular lysate (5 μg) is included as control (lane 3). Isolated glomeruli were lysed in 1% TX-100, 0.5% Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.6, 1 mmol/L EDTA, 1 mmol/L EGTA with proteinase inhibitors. Glomerular lysate (850 μg) was incubated overnight with anti-CD2AP R209 or preimmune IgG, and the precipitated proteins were immunoblotted with anti-nephrin 043.
Finally, we performed immunoprecipitation assays with anti-CD2AP and found that nephrin co-precipitates with CD2AP (Figure 8C). We were unable to work out solubilization conditions that maintained the interaction between nephrin and cadherins. The results of our pull-down and co-immunoprecipitation assays verify that nephrin, adherens junction proteins, and CD2AP are components of a multiprotein complex in glomeruli as well as in MDCK-nephrin cells.
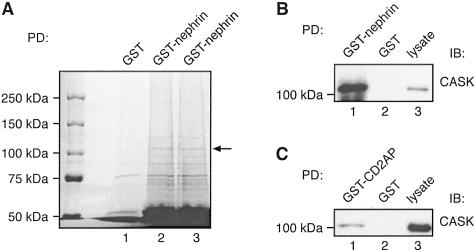
Nephrin Associates with CASK in Glomeruli
To identify additional components of the nephrin multiprotein complex in glomeruli we performed pull-down assays on glomerular lysates with GST-nephrin tail as above, separated the proteins that bound to the beads, and stained them with GelCode Blue. Eight bands were pulled down with GST-nephrin tail but not with GST alone. The strongest band, ∼110 kd (Figure 9A), was cut out, digested with trypsin, and the peptides obtained by NanoLC-MS/MS analysis were used to search the Mascot/NCBInr database. Nineteen peptides matched the rat CASK protein sequence. CASK is an adaptor protein whose N-terminus is highly homologous to Ca2+, calmodulin-dependent protein kinases (CaM kinases) and its C-terminus is very similar to the dlg-homologs DLG2, p55, PSD95/SAP90, and ZO-1/ZO-2.23 CASK has been shown to be present in many epithelial cells including MDCK cells.42–44 We performed immunoblotting with CASK antibodies on glomerular proteins that bound to GST-nephrin tail and confirmed the presence of CASK (Figure 9B). We also performed pull-down assays with GST-CD2AP on glomerular lysates and found that CASK binds to GST-CD2AP but not to GST alone (Figure 9C). Thus CASK is a component of the nephrin multiprotein complex in glomeruli. CASK was also detected in pull-downs with GST-nephrin tail in MDCK-nephrin cells, although the interaction was weaker than in glomeruli (not shown).
Figure 9.
A: Identification of CASK in GST-nephrin tail pull-downs by mass spectrometry. Twenty μg of GST-nephrin tail or GST alone were incubated with 400 μg of glomerular lysate as in Figure 6. Bound proteins were resolved by 6% SDS-PAGE and stained with GelCode Blue. A prominent ∼110-kd band (arrow) present in the GST-nephrin tail (lanes 2 and 3) but not GST (lane 1) pull-downs was excised, digested with trypsin, and identified by mass spectrometry as CASK. To upscale the reactions, six parallel pull-downs with GST-nephrin tail and three with GST alone were performed, and three parallel samples were combined per lane. The ∼110-kd bands observed in GST-nephrin tail pull-downs in lanes 2 and 3 were combined for analysis by mass spectrometry. B and C: The presence of CASK in GST-nephrin tail (B) and CD2AP (C) pull-downs was confirmed by immunoblotting. Twenty μg of GST-nephrin tail, GST-CD2AP, or GST alone were incubated with 400 μg of glomerular lysate as in Figure 8, and immunoblotted with anti-CASK IgG. CASK binds to GST-nephrin tail (lane 1 in B) and GST-CD2AP (lane 1 in C) but not to GST alone (lane 2). Glomerular lysate (10 μg) is included as control (lane 3).
Localization of P-Cadherin and CASK in Glomerular Epithelial Cells and in MDCK-Nephrin Cells
P-cadherin was previously shown to be expressed in podocytes and to be localized in the slit diaphragm region by immunoelectron microscopy.17,18 To confirm the presence of P-cadherin in podocytes, we performed immunofluorescence labeling of kidney cryostat sections with P-cadherin-specific antibodies and confirmed previous work that P-cadherin is localized in podocytes (Figure 10A).
Figure 10.
Localization of P-cadherin and CASK in mouse and rat glomeruli. A: P-cadherin localizes to the foot process layer of podocytes (arrowheads) in mouse glomeruli. Mouse kidney cryostat sections were fixed with acetone, stained with anti-P-cadherin IgG, and analyzed by confocal microscopy. B: CASK is found primarily in the cell bodies (arrows) and foot processes (arrowheads) of podocytes. C: Higher magnification of the marked region in B showing that CASK is distributed throughout the cell bodies of podocytes (arrows) and is especially concentrated in the foot processes (arrowheads). Rat kidneys were perfusion-fixed with PFA, processed for semithin cryosectioning, labeled with anti-CASK IgG, and examined by immunofluorescence. Scale bars, 10 μm.
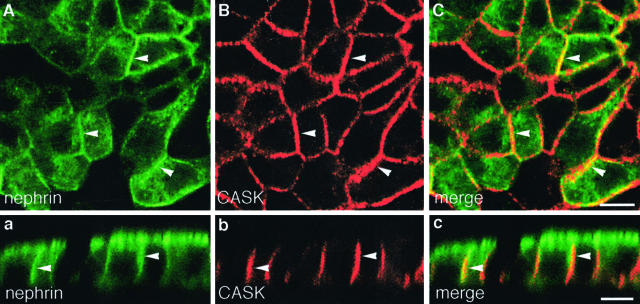
Immunofluorescence labeling of semithin cryosections prepared from rat kidney revealed that CASK is found primarily in podocytes (Figure 10, B and C). These findings confirm previous results indicating that CASK is expressed in the glomerulus43 and further establish that CASK is localized primarily in podocytes. In MDCK-nephrin cells, we found CASK to be expressed along the lateral cell membrane (Figure 11, B and b) as described previously.42,43 When cells were double labeled for nephrin and CASK, the two proteins partially co-localized (Figure 11, A to C and a to c). We conclude that CASK is expressed in podocytes and MDCK-nephrin cells in the appropriate location to interact with nephrin.
Figure 11.
Localization of CASK in MDCK-nephrin cells. A–C: In horizontal sections of MDCK-nephrin cell monolayers the distribution of nephrin and CASK partially overlaps on the lateral plasma membrane as shown by the yellow areas (arrowheads) in the merged image. a–c: In vertical sections the signal for CASK extends along the entire lateral cell membrane and coincides with that of nephrin (arrowheads in merged image). Cells cultured on filters were processed as in Figure 3 and labeled with anti-nephrin 5-1-6 and anti-CASK IgG. Scale bars, 10 μm.
Discussion
Two classes of cell adhesion molecules, those of the Ig superfamily, nephrin3–5 and Neph1,9,10 and those of the cadherin superfamily, P-cadherin17,18 and FAT,19 have previously been shown to be localized in part to the slit diaphragm region of podocytes. In this study we set out to determine whether nephrin interacts with adherens junction components and to identify additional binding partners of nephrin. To facilitate investigation of these problems we developed MDCK cell lines stably expressing nephrin. Here we demonstrate for the first time that nephrin forms a multiprotein complex with adherens junction (P-cadherin, p120 catenin) and tight junction (ZO-1) proteins as well as with the cytoplasmic adaptor proteins CASK and CD2AP in MDCK-nephrin cells and in rat glomeruli and that the interaction occurs through the cytoplasmic tail of nephrin. This conclusion is based on our findings that adherens junction proteins co-immunoprecipitate with nephrin in MDCK cells and bind to nephrin tail in pull-down assays on glomerular lysates.
Our results do not provide definitive information on whether the interaction between nephrin and cadherins is direct or is mediated via other proteins. The presence of the scaffolding proteins CASK, CD2AP, and ZO-1 in nephrin-cadherin complexes raises the possibility that one or more of these cytosolic adaptor proteins could link nephrin to adherens junction proteins. This seems likely because each of these proteins has been shown to connect members of the complex to the actin cytoskeleton: ZO-1 links the tight junction proteins claudins and occludin to the actin cytoskeleton,45 and it also binds α-, β-, and γ-catenins during early stages of junctional assembly.46 CD2AP binds actin47 and has been suggested to link nephrin to the actin cytoskeleton.39 CASK may also participate in linking nephrin to actin, as CASK has previously been shown to connect other membrane proteins, eg, syndecan-244 and neurexins,48 to the actin cytoskeleton.
Another novel finding in our work is the demonstration that CASK23 is present in podocytes and is part of the nephrin multiprotein complex. CASK, a member of the MAGUK family of scaffolding proteins, has previously been shown to be associated with several other specialized cell junctions, including synapses23 and tight junctions.49 It was originally identified as a binding partner of neurexins (neural cell adhesion molecules)23 and is believed to play a role in regulating neuronal and epithelial cell polarity.22 CASK forms a multiprotein complex with kainate receptors, cadherins and β- and p120 catenin in brain and was suggested to stabilize kainate receptors at the synaptic membrane during synapse formation and remodeling.50 In MDCK cells, CASK has been shown to participate in lateral targeting of proteins.42 Based on its function in other systems it is likely that in podocytes, CASK functions in a similar manner to stabilize nephrin-cadherin protein complexes, to organize specific signaling complexes, to participate in targeting of proteins, and to provide structural integrity to the slit diaphragm by linking the multiprotein complex to the actin cytoskeleton.
Tight junctions and adherens junctions are closely linked functionally in polarized epithelia. Adherens junctions are the first junctions formed between MDCK cell monolayers and are required for formation of tight junctions.51 There is also a close connection between adherens junctions and tight junctions in the glomerulus. Slit diaphragms arise from typical tight and adherens junctions between developing podocytes,6 and in the nephrotic syndrome induced by treatment with puromycin aminonucleoside (PAN) or protamine sulfate, slit diaphragms are lost and replaced by tight junctions.7,8 Our demonstration that nephrin forms a multiprotein complex with cadherins and p120 catenin in glomeruli suggests an interplay between cadherin-based Ca2+-dependent cell adhesion and Ig-mediated, Ca2+-independent cell adhesion in podocytes.
We were unable to detect ZO-1 in nephrin pull-downs performed on glomerular lysates; however, recent studies report association of ZO-1 with Neph1,10,16 another cell adhesion protein of the Ig superfamily expressed in podocytes that forms heterodimers with nephrin.9,10,52 It was suggested that the interaction between nephrin and Neph1 partially stabilizes the interaction between Neph1 and ZO-1.10 The question of how these two cell adhesion systems contribute to the regulation of slit diaphragms remains to be answered.
It is now recognized that signaling proteins are concentrated along sites of both cell-cell and cell-matrix attachment and that cell junctions are important signaling centers that influence a wide variety of cell functions.53 Cadherins mediate Ca2+-dependent homotypic cell-cell adhesion and regulate cell recognition, cell polarity, tissue morphogenesis, and tumor suppression, among others.21 Catenins interact with the cytoplasmic tail of cadherins and link them to the actin cytoskeleton and to signaling cascades.20 Both β- and p120 catenins play an important role in regulation of cell proliferation by associating with transcription factors.20,38
It is clear from nephrin and CD2AP knockout mice,39,54 nephrin TRAP mice,55 and human patients with congenital nephrotic syndrome of the Finnish type1 that nephrin and CD2AP expression are essential for normal glomerular development and function. Interestingly, P-cadherin knockout mice have no glomerular defects,56 suggesting either that P-cadherin is not essential or that other members of the cadherin superfamily substitute for P-cadherin in these mice. The pathways by which nephrin influences cell behavior have just begun to be investigated. Nephrin, together with podocin, stimulates the AP-1 transcription factor via activation of mitogen-activated protein kinases,57 associates with the p85 regulatory subunit of phosphoinositide 3-OH kinase (PI3K), stimulates PI3K-dependent AKT signaling in podocytes,58 and is phosphorylated by Fyn, a Src family kinase.36 The events that trigger these effects and the functional consequences remain to be worked out.
We have found MDCK cells to be a valuable model system to analyze the interactions between nephrin and adherens junction proteins. When expressed in MDCK cells, nephrin behaves as in glomeruli: it is localized to cell-cell contacts and the apical cell membrane, is N-glycosylated and phosphorylated, and interacts with the same binding partners as in glomeruli. Thus MDCK-nephrin cells provide a valuable alternative to working with isolated glomeruli or conditionally immortalized podocytes for investigating the interactions of nephrin. Immortalized podocytes have the limitation that nephrin expression is low59 or ceases on extended passaging.40 Furthermore, podocyte cell lines do not form continuous monolayers with distinct apical and basolateral domains. A limitation of both podocytes in culture and MDCK cells is that they do not make typical foot processes or slit diaphragms.
We were somewhat surprised to find that nephrin had an effect on the junctional properties of MDCK cells in that it increased the tightness of tight junctions as measured by the TER of cell monolayers. This is most likely explained by the fact that nephrin associates with actin,60 and changes in actin at adherens junctions are thought to regulate paracellular permeability.61 Previously we showed that expression of podocalyxin, another podocyte protein that associates with actin and maintains the filtration slits open in glomeruli, inhibits cell-cell adhesion and decreases TER in MDCK cells.62 Our finding that nephrin associates with ZO-1 in MDCK-nephrin cells may explain the effects of nephrin expression on the properties of tight junctions because ZO-1 localizes to the cytoplasmic surface of tight junctions and serves as the major scaffolding protein for tight junction strands.12,13
In conclusion, we show here that nephrin is a component of a large protein complex composed of cell adhesion receptors and cytosolic scaffolding proteins that connect the complex to the actin cytoskeleton and to signaling networks. The complex interactions between nephrin and adherens junction proteins mediated by the scaffolding proteins CASK, CD2AP, and ZO-1 are likely to be crucial for maintaining the structural integrity and signaling properties of the glomerular slit diaphragms.
Acknowledgments
We thank Dennis Young, University of California San Diego Cancer Center Flow Cytometry Shared Resource, for advice and assistance with the cell sorting.
Footnotes
Address reprint requests to Dr. Marilyn G. Farquhar, University of California San Diego, Department of Cellular and Molecular Medicine, 9500 Gilman Dr., La Jolla, CA 92093-0651. E-mail: mfarquhar@ucsd.edu.
Supported by the National Institutes of Health (grant DK 17724 to M.G.F.), the Academy of Finland (fellowship to S.L.), the Sigrid Jusélius Foundation (fellowship to S.L.), the University of Helsinki (to E.L. and H.H.), the Finnish Diabetes Foundation, and the Päivikki and Sakari Sohlberg Foundation (to H.H.).
References
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Lemmon V. Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr Opin Cell Biol. 2001;13:611–618. doi: 10.1016/s0955-0674(00)00259-3. [DOI] [PubMed] [Google Scholar]
- Holzman LB, John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cells. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Holthöfer H, Ahola H, Solin M-L, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D. Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol. 1999;155:1681–1687. doi: 10.1016/S0002-9440(10)65483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occludin junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978;39:90–100. [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis: evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976;34:43–59. [PubMed] [Google Scholar]
- Barletta G-M, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Simons M, Reiser J, Saleem MA, Faul C, Kritz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Van Itallie CM, Anderson J. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1992;89:7075–7079. doi: 10.1073/pnas.89.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, Sellin L, Walz G, Benzing T. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem. 2003;278:13417–13421. doi: 10.1074/jbc.C200678200. [DOI] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H. Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol. 2000;157:1905–1916. doi: 10.1016/S0002-9440(10)64829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Kihara I, Yamamoto T. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001;59:1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;51:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Caruana G. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int J Dev Biol. 2002;46:511–518. [PubMed] [Google Scholar]
- Hata Y, Butz S, Südhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola H, Wang S-X, Luimula P, Solin M-L, Holzman LB, Holthöfer H. Cloning and expression of the rat nephrin homolog. Am J Pathol. 1999;155:907–913. doi: 10.1016/S0002-9440(10)65190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmén T, Lehtonen S, Ora A, Kerjaschki D, Antignac C, Lehtonen E, Holthöfer H. Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J Am Soc Nephrol. 2002;13:1766–1772. doi: 10.1097/01.asn.0000019842.50870.41. [DOI] [PubMed] [Google Scholar]
- Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola H, Heikkilä E, Åström E, Inagaki M, Izawa I, Pavenstädt H, Kerjaschki D, Holthöfer H. A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol. 2003;14:1731–1737. doi: 10.1097/01.asn.0000075553.33781.9f. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Ora A, Olkkonen VM, Geng L, Zerial M, Somlo S, Lehtonen E. In vivo interaction of the adapter protein CD2-associated protein with the type 2 polycystic kidney disease protein, polycystin-2. J Biol Chem. 2000;275:32888–32893. doi: 10.1074/jbc.M006624200. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Grunkemeyer JA, Sudhakar A, Zeisberg M, Cosgrove D, Morello R, Lee B, Sugimoto H, Kalluri R. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem. 2002;277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–201. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–1598. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Farquhar MG. Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol. 1995;257:259–279. doi: 10.1016/s0076-6879(95)57031-4. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen Z, Yu JF, Young D, Bashey A, Ho AD, Law P. Correlation between IL-3 receptor expression and growth potential of human CD34+ hematopoietic cells from different tissues. Stem Cells. 1999;17:265–272. doi: 10.1002/stem.170265. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D, Dekan G, Aronson PS, Farquhar MG. Biosynthesis of the gp330/44-kDa Heymann nephritis antigenic complex: assembly takes place in the ER. Am J Physiol. 1993;264:F1011–F1020. doi: 10.1152/ajprenal.1993.264.6.F1011. [DOI] [PubMed] [Google Scholar]
- Pandey A, Andersen JS, Mann M. Use of mass spectrometry to study signaling pathways. Sci STKE. 2000;37:PL1. doi: 10.1126/stke.2000.37.pl1. [DOI] [PubMed] [Google Scholar]
- Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113:1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- Shih N-Y, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- Shih N-Y, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Fan S, Makarova O, Straight S, Margolis B. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol. 2002;22:1778–1791. doi: 10.1128/MCB.22.6.1778-1791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen O, Liu H, Wade JB, Merot J, Welling PA. Basolateral membrane expression of the Kir 2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex. Am J Physiol. 2002;282:C183–C195. doi: 10.1152/ajpcell.00249.2001. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Wood DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen S, Zhao F, Lehtonen E. CD2-associated protein directly interacts with the actin cytoskeleton. Am J Physiol. 2002;283:F734–F743. doi: 10.1152/ajprenal.00312.2001. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- Martínez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial Caco-2 cells. J Biol Chem. 2001;276:9291–9296. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mège R-M, Mulle C. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14:918–926. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- Brown E, Dejana E. Cell-cell and cell-matrix interactions—running, jumping, standing still. Curr Opin Cell Biol. 2003;15:505–508. [Google Scholar]
- Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Rantanen M, Palmén T, Pätäri A, Ahola H, Lehtonen S, Åström E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthöfer H. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol. 2002;13:1586–1594. doi: 10.1097/01.asn.0000016142.29721.22. [DOI] [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Köttgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276:41543–41546. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol. 2002;13:1385–1389. doi: 10.1097/01.asn.0000013297.11876.5b. [DOI] [PubMed] [Google Scholar]
- Yuan H, Takeuchi E, Salant D. Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am J Physiol. 2002;282:F585–F591. doi: 10.1152/ajprenal.00290.2001. [DOI] [PubMed] [Google Scholar]
- Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]