Abstract
The function of scavenger receptor class B, type I (SR-BI) in the liver as a high-density lipoprotein receptor that promotes the selective uptake of cholesteryl esters is well defined. Its role in macrophages, however, is primarily unknown, because it functions in the uptake of (modified) lipoproteins as well as the secretion of cholesterol to high-density lipoproteins. In this study, the biological role of SR-BI on bone marrow-derived cells, including macrophages, in lipid metabolism and atherosclerosis was assessed by selective disruption of SR-BI in bone marrow in two established models of atherosclerosis: low-density lipoprotein (LDL) receptor-deficient mice that develop extensive atherosclerosis on a Western-type diet and wild-type mice that develop fatty streak lesions when fed a high-cholesterol diet containing 0.5% cholate. The presence of SR-BI in bone marrow-derived cells in LDLr−/− mice decreased lesion development after 9 and 12 weeks of Western-type diet feeding, indicating that macrophage SR-BI protects against lesion development. At 6 weeks, no significant effect of SR-BI in bone marrow-derived cells on lesion development was observed. Interestingly, after only 4 weeks of Western-type diet feeding of transplanted LDLr−/− mice and in wild-type mice on a high-cholesterol/cholate diet, the presence of SR-BI in bone marrow-derived cells increased the development of small fatty streak lesions. It thus appears that, depending on the stage of atherosclerotic lesion development, SR-BI in bone marrow-derived cells is either pro-atherogenic or anti-atherogenic, indicating a unique dual role in the pathogenesis of atherosclerosis.
Macrophage-derived foam cells play an important role in all stages of atherosclerotic lesion development.1 Macrophage-derived foam cells are the predominant constituent of the fatty streak, the early atherosclerotic lesion. In advanced atherosclerotic lesions, foam cells are detected as clusters of cells surrounding a core of lipid and necrotic material, where they modulate the stability of the atherosclerotic lesion. Macrophage-derived foam cells develop as a result of excessive accumulation of lipoprotein-derived cholesterol.2 Because macrophages are incapable of limiting their uptake of modified lipoproteins via scavenger receptors, they heavily depend on cholesterol efflux mechanisms for maintaining cholesterol homeostasis within the cell.3–6 First, as proposed by Phillips and colleagues,7 driven by a concentration gradient, cholesterol can efflux from cells by passive diffusion to extracellular acceptor particles, such as high-density lipoprotein (HDL). In addition, cholesterol is effluxed to extracellular lipid-free apolipoprotein (apo) acceptors, such as apoAI, facilitated by the ATP-binding cassette transporter 1 (ABCA1).8,9 Furthermore, it has been demonstrated that the rate of cholesterol efflux from various cell types correlates with the expression of scavenger receptor class B, type I (SR-BI).10–13 SR-BI is an 82-kd glycosylated plasma membrane protein, capable of binding a wide array of native and modified lipoproteins.14–16 SR-BI is abundantly expressed in liver and steroidogenic tissues, where it mediates the selective uptake of cholesteryl esters from HDL.14,17,18 Several lines of evidence, however, suggest that it can also stimulate the bi-directional flux of free cholesterol between cells and extracellular lipoprotein particles.
SR-BI is expressed in lipid-laden macrophages in human and murine atherosclerotic lesions,10,19,20 indicating that SR-BI might play an important role locally in the arterial wall, in addition to its systemic role in determining serum HDL cholesterol levels by mediating hepatic uptake and biliary secretion of HDL cholesterol. Locally in the arterial wall, SR-BI expression by macrophages may protect against atherosclerosis by stimulating cholesterol efflux and preventing foam cell formation. On the other hand, its function in the uptake of both modified and native lipoproteins might enhance foam cell formation rendering macrophage SR-BI a proatherogenic factor.15,21
Macrophages, present in atherosclerotic lesions, primarily depend on infiltration from bone marrow-derived monocytes into the arterial wall. Therefore, in the present study, we investigated the effects of selective disruption of SR-BI in bone marrow-derived cells and thus macrophages on atherosclerotic lesion development in two established models of atherosclerosis: low-density lipoprotein (LDL) receptor-deficient mice that develop extensive atherosclerosis on a Western-type diet and wild-type (WT) mice that develop fatty streak lesions when fed a high-cholesterol diet containing 0.5% cholate. SR-BI on bone marrow-derived cells protected against the development of atherosclerosis in LDL receptor deficient (LDLr−/−) mice fed a Western-type diet for 9 and 12 weeks. While this article was under preparation, two articles appeared indicating that SR-BI in bone marrow-derived cells lowered atherosclerotic lesion development in LDLr−/−22 and apoE−/−23 mice. In addition, we now demonstrate that SR-BI in bone marrow-derived cells facilitates the development of small fatty streak lesions in LDLr−/− mice fed a Western-type diet for only 4 weeks and in WT mice fed a high-cholesterol/cholate diet. These data support a unique dual role for SR-BI on bone marrow-derived cells in atherosclerotic lesion development.
Materials and Methods
Mice
Class B, type I scavenger receptor (SR-BI)-deficient mice were kindly provided by Dr. M. Krieger (Massachusetts Institute of Technology, Cambridge, MA).24 Mice are on a mixed C57BL6/129 background. Heterozygous SR-BI-deficient mice were cross-bred to generate homozygous SR-BI−/− knockout progeny and SR-BI+/+ WT littermates. The presence of the targeted and WT SR-BI alleles was assessed by polymerase chain reaction (PCR) amplification of DNA extracted from tail biopsies (primers 5′-GAT-GGG-ACA-TGG-GAC-ACG-AAG-CCA-TTC-T-3′ and 5′-TCT-GTC-TCC-GTC-TCC-TTC-AGG-TCC-TGA-3′). Homozygous LDL receptor knockout25 (LDLr−/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) as mating pairs and bred at the Gorlaeus Laboratory, Leiden, The Netherlands. Mice were housed in sterilized filter-top cages and given unlimited access to food and water. Mice were maintained on sterilized regular chow, containing 4.3% (w/w) fat with no added cholesterol (RM3; Special Diet Services, Witham, UK), or were fed a semisynthetic Western-type diet containing 15% (w/w) fat and 0.25% (w/w) cholesterol (Diet W; Hope Farms, Woerden, The Netherlands) or a semisynthetic high-cholesterol/cholate diet containing 15% (w/w) fat, 1% (w/w) cholesterol, and 0.5% cholate (Diet N, Hope Farms).26 Drinking water was supplied with antibiotics (83 mg/L ciprofloxacin and 67 mg/L polymyxin B sulfate) and 6.5 g/L sucrose. Animal experiments were performed at the Gorlaeus Laboratories of the Leiden/Amsterdam Center for Drug Research in accordance with the National Laws. All experimental protocols were approved by the Ethics Committee for Animal Experiments of Leiden University.
Bone Marrow Transplantation
To induce bone marrow aplasia, female LDLr−/− and WT mice were exposed to a single dose of 9 Gy (0.19 Gy/min, 200 kV, 4 mA) total body irradiation, using an Andrex Smart 225 Röntgen source (YXLON Int., Copenhagen, Denmark) with a 6-mm aluminum filter, 1 day before transplantation. Bone marrow was isolated by flushing the femurs and tibias from female SR-BI−/− mice or female WT littermates with phosphate-buffered saline (PBS). Single-cell suspensions were prepared by passing the cells through a 30-μm nylon gauze. Irradiated recipients received 0.5 × 107 bone marrow cells by intravenous injection into the tail vein.
Assessment of Chimerism
The hematological chimerism of the transplanted mice was determined in genomic DNA from bone marrow by PCR. Two oligonucleotides were used for PCR amplification to detect both the WT and the null mutant SR-BI gene simultaneously, as described above.
Lipid Analyses
After an overnight fasting-period, ∼100 μl of blood was drawn from each individual mouse by tail bleeding. The concentrations of free cholesterol and cholesteryl esters in serum were determined using enzymatic colorimetric assays (Roche Diagnostics, Mannheim, Germany). The distribution of lipids over the different lipoproteins in serum was determined by fractionation of 30 μl of serum of each mouse using a Superose 6 column (3.2 × 30 mm, Smart-System; Pharmacia, Uppsala, Sweden). Total cholesterol content of the effluent was determined using enzymatic colorimetric assay (Roche Diagnostics). Erythrocyte cholesterol content was analyzed using enzymatic colorimetric assays after lipid extraction. Briefly, 30 μl of washed and packed erythrocytes were extracted by subsequent addition of 100 μl of methanol and 100 μl of chloroform. The extract supernatants were evaporated under nitrogen, redissolved in 30 μl of ethanol, and used for analysis.
Histological Analysis of the Aortic Root
To analyze the development of atherosclerosis at the aortic root, transplanted LDLr−/− mice were sacrificed after 4, 6, 9, and 12 weeks of feeding the Western-type diet, respectively. Transplanted WT mice were fed a high-cholesterol/cholate diet for 10 or 12 weeks before sacrifice. The arterial tree was perfused in situ with PBS (100 mmHg) for 20 minutes via a cannula in the left ventricular apex. The heart plus aortic root and descending aorta were excised and stored in 3.7% neutral-buffered formalin (Formal-fixx; Shandon Scientific Ltd., UK). The atherosclerotic lesion areas in oil red O-stained cryostat sections of the aortic root were quantified using the Leica image analysis system, consisting of a Leica DMRE microscope coupled to a video camera and Leica Qwin Imaging software (Leica Ltd., Cambridge, UK). Mean lesion area (in μm2) was calculated from 10 oil red O-stained sections, starting at the appearance of the tricuspid valves. For morphological analysis, sections were stained with Masson’s Trichrome Accustain according to manufacturer’s instructions (Sigma).
Macrophage Cholesterol Efflux Studies
Thioglycollate-elicited macrophages were isolated from SR-BI+/+ and SR-BI−/− mice 5 days after injection of 1 ml of Brewer’s thioglycollate medium (Difco, Detroit, MI). After washing, cells were seeded on 24-well plates at a density of 0.5 × 106 cells in 500 μl of Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% (w/v) bovine calf serum, 2 mmol/L l-glutamine, 100 μg/ml streptomycin, and 100 IU/ml penicillin. After 1 hour, nonadherent cells were removed by washing. After 2 days in culture, the cells were washed and incubated with 0.5 μCi/ml 3H-cholesterol (Amersham Biosciences, Buckinghamshire, UK) in DMEM/0.2% bovine serum albumin for 24 hours at 37°C to load the cells with cholesterol. In addition, an in vivo method was developed to load peritoneal macrophages with 3H-cholesterol, allowing the study of cholesterol efflux with minimal culture times. Briefly, SR-BI+/+ and SR-BI−/− mice, fed regular chow diet, were injected intraperitoneally with 1 ml of 3% Brewer’s thioglycollate medium (Difco). After 5 days, the elicited SR-BI+/+ and SR-BI−/− peritoneal macrophages were labeled in vivo by intraperitoneal injection of 3H-cholesterol. The injection sample was prepared by dissolving 6.25 μCi of 3H-cholesterol in 6.25 μl of ethanol and subsequent addition of 500 μl PBS at 37°C. At 3.5 hours after injection, peritoneal macrophages were harvested and seeded on 24-well plates at a density of 0.5 × 106 cells in 500 μl of DMEM/0.2% bovine serum albumin. After 1 hour, nonadherent cells were removed by washing.
Cholesterol efflux from macrophages, loaded with cholesterol in vitro or in vivo, was subsequently studied by incubation of the cells with DMEM/0.2% free fatty acid (FFA) free bovine serum albumin alone, or supplemented with either 10 μg/ml of apolipoprotein AI (Calbiochem) or 50 μg/ml of human HDL (isolated according to Redgrave and colleagues27). After a 24-hour efflux period, radioactivity in the cells and medium was determined by liquid scintillation counting. Cholesterol efflux is defined as (dpmmedium/dpmcell + dpmmedium) × 100%.
Macrophage Association Studies
β-Very low-density lipoprotein (VLDL) was isolated from rats that were fed a diet containing 2% cholesterol, 5% olive oil, and 0.5% cholate (Hope Farms) for 2 weeks. After overnight fasting, blood was collected from the abdominal aorta and β-VLDL was isolated using a discontinuous potassium bromide gradient, as described by Redgrave and colleagues.27 The fraction of d < 1.006 = g/ml was isolated and dialyzed against bovine serum albumin/1 mmol/L ethylenediaminetetraacetic acid. The β-VLDL consisted for 14.6 ± 2.1% of triacylglycerols, 15.8 ± 1.1% phospholipids, 49.4 ± 3.1% cholesteryl esters, 9.9 ± 1.0% free cholesterol, and 10.3 ± 0.7% protein and displayed β-mobility on agarose gels. The isolated β-VLDL was labeled with 125I at pH = 10.0 according to McFarlane,28 modified as described earlier.29 Human HDL was isolated by differential ultracentrifugation as described by Redgrave and colleagues27 and subsequently labeled with 3H-cholesterol oleate (Amersham) by exchange from donor particles as reported previously.30 Thioglycollate-elicited macrophages were isolated 5 days after injection of 1 ml of Brewer’s thioglycollate medium. After washing, cells were either directly used for association studies or seeded on 24-well plates as described above and used for association studies after 3 days in culture. Association of β-VLDL or HDL to the SR-BI+/+ and SR-BI−/− macrophages was determined by incubation with the indicated amounts of 125I-β-VLDL or 3H-CE HDL in DMEM/2% bovine serum albumin at 37°C. After 3 hours of incubation, the cells were washed and lysed in 0.1 mol/L NaOH, and cell protein content was determined according to Lowry and colleagues31 Finally, the cell-associated radioactivity was determined.
Statistical Analyses
Statistical analyses were performed using the unpaired Student’s t-test (Instat GraphPad software, San Diego, CA).
Results
Selective Disruption of SR-BI on Bone Marrow-Derived Cells in WT and LDL Receptor-Deficient Mice
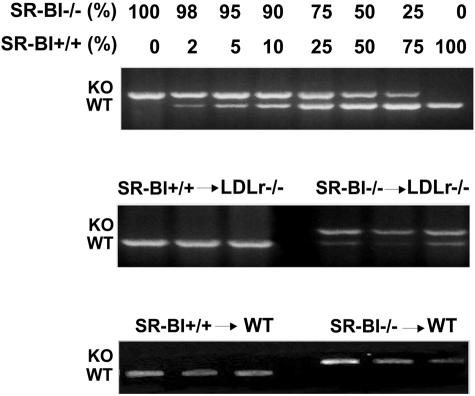
To assess the role of bone marrow-derived SR-BI in atherosclerotic lesion development, we used the technique of bone marrow transplantation to selectively disrupt SR-BI in hematopoietic cells. Bone marrow from previously generated SR-BI-deficient mice was transplanted into either WT or LDLr−/− mice, which represent established models for the development of atherosclerosis. Successful reconstitution of recipients with hematopoietic donor cells was established by PCR-assisted amplification using primers specific for the WT and the null mutant SR-BI gene (Figure 1). Genomic DNA isolated from the recipient mice, transplanted with bone marrow from SR-BI knockout mice contained a prominent band indicative of the disrupted allele, whereas only a faint WT band was visible. The control transplanted mice only displayed the WT band, indicating that the bone marrow transfer was successful.
Figure 1.
Verification of success of bone marrow transplantation. Verification of successful reconstitution with donor hematopoietic cells by PCR amplification of the WT and the null mutant SR-BI gene using genomic DNA isolated from bone marrow.
Effect of Disruption of SR-BI on Bone Marrow-Derived Cells in LDLr−/− Mice
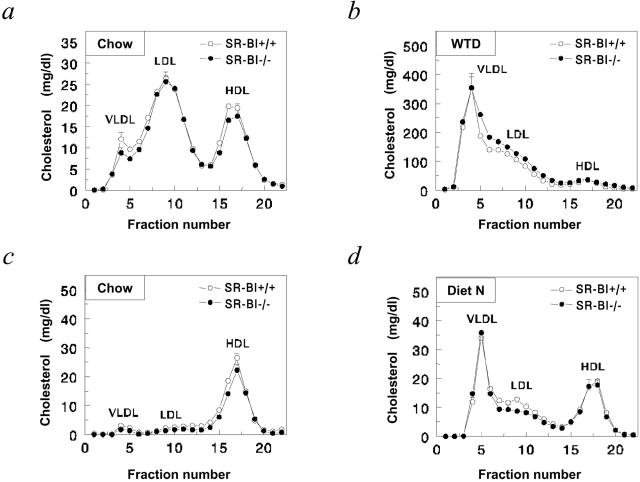
During the course of the experiment, the effects of disruption of SR-BI in bone marrow-derived cells in LDLr−/− mice on serum lipid levels were carefully monitored. On regular chow diet, the majority of the cholesterol in LDLr−/− mice is transported by LDL and HDL (Figure 2). Disruption of SR-BI in bone marrow-derived cells did not significantly affect serum cholesterol levels (Table 1) nor the distribution of cholesterol over the lipoproteins in the circulation. To induce atherosclerotic lesion development, the transplanted mice were fed a Western-type diet, containing 0.25% cholesterol and 15% fat, starting at 8 weeks after transplantation. On challenging the mice with the Western-type diet, serum cholesterol levels increased approximately sixfold in both groups of mice, primarily because of an increase in VLDL and LDL cholesterol (Table 1, Figure 2). Under these conditions, also no effect of SR-BI disruption in bone marrow-derived cells on serum cholesterol levels was observed. Furthermore, the cholesterol content of erythrocytes was comparable in both groups (0.59 ± 0.05 μg/μl in SR-BI+/+ → LDLr−/− mice versus 0.63 ± 0.06 μg/μl in SR-BI−/− → LDLr−/− chimeras).
Figure 2.
Effect of disruption of SR-BI in bone marrow-derived cells on serum cholesterol distribution in WT and LDL receptor-deficient (LDLr−/−) mice. Blood samples were drawn after an overnight fast at 8 weeks after transplant while feeding regular chow diet (chow) and at 16 weeks after bone marrow transplantation after 8 weeks of feeding a high-cholesterol/cholate (diet N) or Western-type diet (WTD). Sera from individual mice were loaded onto a Superose 6 column and fractions were collected. Fractions 3 to 7 represent VLDL; fraction 8 to 14, LDL; and fractions 15 to 19, HDL, respectively. The distribution of cholesterol over the different lipoproteins in LDLr−/− (a and b) or WT recipients (c and d) transplanted with SR-BI+/+ (open circles) or SR-BI−/− (filled circles) bone marrow is shown. Values represent the mean ± SEM of at least eight mice. No statistically significant differences were observed.
Table 1.
Effect of Disruption of SR-BI in Bone Marrow-Derived Cells on Serum Lipid Levels in LDLr−/− and Wild-Type Mice
| Mice | Time (weeks) | Diet | Free cholesterol (mg/dl) | Cholesteryl esters (mg/dl) | HDL cholesterol (mg/dl) |
|---|---|---|---|---|---|
| SR-BI+/+ → LDLr−/− | Baseline | Chow | 102 ± 6 | 347 ± 34 | ND |
| 8 | Chow | 113 ± 5 | 407 ± 24 | 205 ± 10 | |
| 16 | WTD | 468 ± 27 | 2659 ± 143 | 392 ± 45 | |
| SR-BI−/− → LDLr−/− | Baseline | Chow | 95 ± 5 | 388 ± 26 | ND |
| 8 | Chow | 105 ± 4 | 404 ± 19 | 184 ± 14 | |
| 16 | WTD | 541 ± 69 | 2690 ± 263 | 468 ± 51 | |
| SR-BI+/+ → WT | Baseline | Chow | 24 ± 2 | 116 ± 12 | ND |
| 8 | Chow | 33 ± 2 | 118 ± 8 | 78 ± 4 | |
| 16 | Diet N | 61 ± 5 | 295 ± 11 | 62 ± 7 | |
| SR-BI−/− → WT | Baseline | Chow | 24 ± 2 | 103 ± 5 | ND |
| 8 | Chow | 27 ± 2 | 109 ± 7 | 64 ± 6 | |
| 16 | Diet N | 46 ± 3 | 292 ± 14 | 58 ± 5 |
Serum lipids were measured in LDLr−/− and SR-BI+/+ wild-type (WT) mice before transplantation (baseline) and at 8 and 16 weeks after transplantation with SR-BI+/+ or SR-BI−/− bone marrow. At 8 weeks after transplantation, the regular chow diet was switched to a Western-type diet (WTD) or a high-cholesterol/cholate diet (diet N). Data represent mean ± SEM of at least eight mice. No significant differences between SR-BI+/+ → LDLr−/− versus SR-BI+/+ → LDLr−/− were noticed, while also the SR-BI+/+ → WT versus SR-BI+/+ → WT were not significantly different.
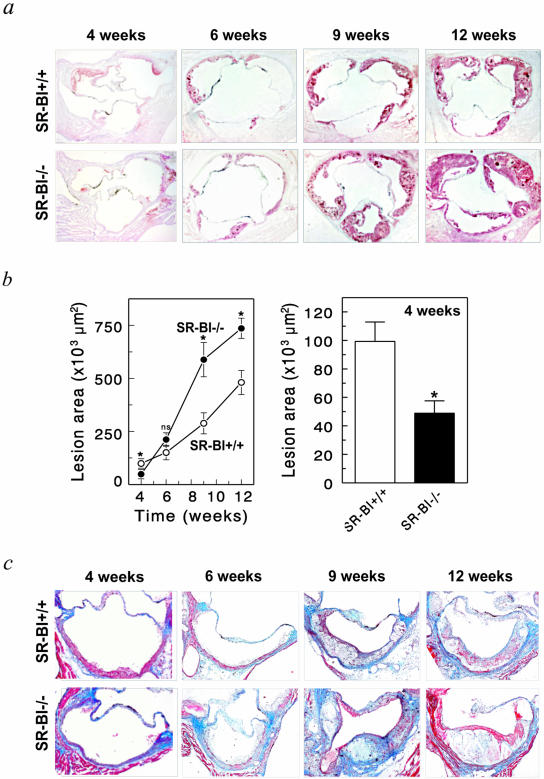
Atherosclerotic lesion development was analyzed in the aortic root of SR-BI+/+ → LDLr−/− mice and in SR-BI−/− → LDLr−/− chimeras after 4, 6, 9, and 12 weeks of Western-type diet feeding (Figure 3). After 4 and 6 weeks of diet feeding initial lesions are observed with isolated macrophage foam cells. After 9 weeks, lesions have progressed to intermediate lesions with multiple foam cell layers, small extracellular lipid pools, and a thin fibrous cap. After 12 weeks, advanced lesions are observed with a (extracellular) lipid core and fibrotic layer and frequently observed calcification, cholesterol clefts, and necrosis. Disruption of SR-BI in bone marrow-derived cells induced lesion development after 9 and 12 weeks on the Western-type diet. At 9 weeks, the mean atherosclerotic lesion area was increased twofold as a result of SR-BI deficiency in bone marrow-derived cells [588 ± 81 × 103 μm2 in SR-BI−/− → LDLr−/− mice (n = 10) versus 288 ± 49 × 103 μm2 in SR-BI+/+ → LDLr−/− mice (n = 18), P = 0.0034]. At 12 weeks, the mean atherosclerotic lesion area was increased 1.5-fold as a result of SR-BI deficiency in bone marrow-derived cells from 480 ± 57 × 103 μm2 in SR-BI+/+ → LDLr−/− mice (n = 12) to 736 ± 48 × 103 μm2 in SR-BI−/− → LDLr−/− mice (n = 23), P = 0.0055. Morphological analysis of sections stained with Masson’s Trichrome revealed that lesions of mice reconstituted with SR-BI−/− bone marrow appear more advanced with larger necrotic cores (Figure 3c). At 6 weeks, representing a time point with initial lesions, the effect of absence of SR-BI in bone marrow-derived cells on lesion development was only marginal and failed to reach statistical significance [211 ± 32 × 103 μm2 in SR-BI−/− → LDLr−/− mice (n = 6) versus 151 ± 34 × 103 μm2 in SR-BI+/+ → LDLr−/− mice (n = 11), P = 0.2618]. Interestingly, after only 4 weeks of Western-type diet feeding disruption of SR-BI in bone marrow-derived cells resulted in a twofold reduction in mean atherosclerotic lesion area [49 ± 9 × 103 μm2 in SR-BI−/− → LDLr−/− mice (n = 5) versus 99 ± 14 × 103 μm2 in SR-BI+/+ → LDLr−/− mice (n = 5), P = 0.0125]. Because SR-BI facilitated atherosclerosis in LDLr−/− mice after only 4 weeks of Western-type diet feeding, in contrast to the protective role at 9 and 12 weeks, this prompted us to study the effect in a second model of atherosclerosis with small fatty streak lesions: WT mice on a high-cholesterol/cholate diet.
Figure 3.
Disruption of SR-BI in bone marrow-derived cells induces atherosclerotic lesion development in LDLr−/− mice. Formation of atherosclerotic lesions was determined at the aortic root of SR-BI+/+ → LDLr−/−, and SR-BI−/− → LDLr−/− chimeras that were fed a Western-type diet (0.5% cholesterol, 15% fat) for 4, 6, 9, and 12 weeks, respectively. a and b: The mean lesion area was calculated from oil red O-stained cross-sections of the aortic root at the level of the tricuspid valves. Values represent the mean ± SEM of at least six mice. *, Statistically significant difference, P < 0.01, as compared to SR-BI+/+ → LDLr−/− mice. c: Morphological staining of atherosclerotic lesions in the aortic root with Masson’s Trichrome Accustain, which stains cytoplasm and muscle fibers red and collagen blue. Original magnifications, ×50.
Effect of Disruption of SR-BI in Bone Marrow-Derived Cells in WT Mice
On regular chow diet, the majority of the cholesterol in WT mice is transported by HDL (Figure 2). Disruption of SR-BI in bone marrow-derived cells did not significantly affect serum cholesterol levels (Table 1) or the distribution of cholesterol over the lipoproteins in the circulation (Figure 2). To induce atherosclerotic lesion development, the transplanted mice were fed a high-cholesterol diet, containing 1% cholesterol, 15% fat, and 0.5% cholate, starting at 8 weeks after transplantation. On challenging the mice with the high-cholesterol/cholate diet, serum cholesterol levels increased approximately threefold in both groups of mice, primarily because of an increase in VLDL and LDL cholesterol (Table 1, Figure 2).
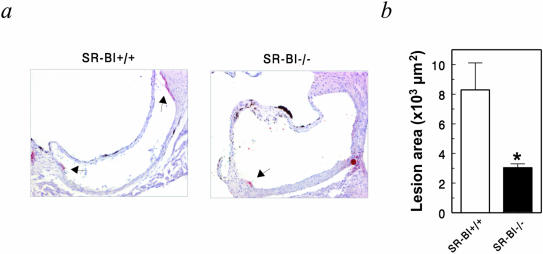
Atherosclerotic lesion development was analyzed in the aortic root of SR-BI+/+ → WT mice and in SR-BI−/− → WT chimeras after 10 weeks of high-cholesterol/cholate diet feeding (Figure 4). As observed in the LDLr−/− mice fed the Western-type diet for only 4 weeks, disruption of SR-BI in bone marrow-derived cells inhibited atherosclerotic lesion development in WT mice [8.3 ± 1.8 × 103 μm2 in SR-BI+/+ → WT mice (n = 9) versus 3.0 ± 0.6 × 103 μm2 in SR-BI−/− → WT mice (n = 7), P = 0.0256]. In a separate independent transplantation experiment also a threefold decrease in lesion size was observed in the absence of bone marrow-derived SR-BI after 12 weeks of the high-cholesterol/cholate diet feeding [56 ± 14 × 103 μm2 in SR-BI+/+ → WT mice (n = 6) versus 18 ± 4 × 103 μm2 in SR-BI−/− → WT mice (n = 6), P = 0.0237]. Thus, apparently also under these conditions the presence of SR-BI on bone marrow-derived cells facilitated lesion formation.
Figure 4.
Disruption of SR-BI in bone marrow-derived cells inhibits atherosclerotic lesion development in WT mice. a: Formation of atherosclerotic lesions was determined at the aortic root of SR-BI+/+ → WT and SR-BI−/− → WT chimeras that were fed a high-cholesterol/cholate diet (1% cholesterol, 15% fat, 0.5% cholate) for 10 weeks. b: The mean lesion area was calculated from oil red O-stained cross-sections of the aortic root at the level of the tricuspid valves. Values represent the mean ± SEM of at least seven mice. *, Statistically significant difference of P < 0.01, as compared to SR-BI+/+ → WT. Original magnifications, ×100.
Dual Role of Macrophage SR-BI in Cellular Cholesterol Homeostasis
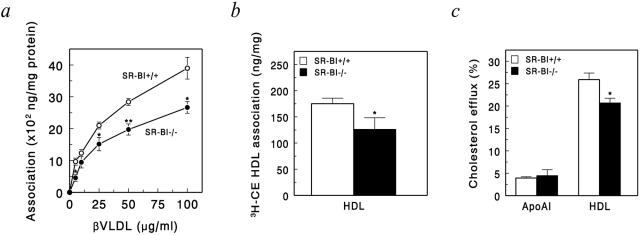
SR-BI is a multifunctional receptor capable of binding a wide array of native and modified lipoproteins. To investigate the relative importance of macrophage SR-BI in the uptake of atherogenic lipoproteins and efflux of cholesterol, we analyzed the effect of macrophage SR-BI deficiency on the association of β-VLDL and HDL, as well as the efflux of cholesterol to apoAI and HDL (Figure 5). Interestingly, deficiency for SR-BI resulted in a reduced association of the atherogenic lipoprotein β-VLDL to macrophages (Figure 5a). Surprisingly, no effect of SR-BI deficiency was observed on cholesterol efflux or association of HDL-CE using macrophages that had been cultured for 3 days (data not shown). However, using freshly isolated macrophages, we did observe a 28% reduction of HDL-CE association in the absence of macrophage SR-BI (Figure 5b). Therefore, to analyze the effect of macrophage SR-BI deficiency on cholesterol efflux under conditions with minimized cultures times, thioglycollate-elicited macrophages were loaded in vivo with 3H-cholesterol by intraperitoneal injection of 3H-cholesterol. After isolation of the macrophages, cholesterol efflux was subsequently studied in vitro. Interestingly, under these conditions macrophage SR-BI deficiency resulted in 20% reduction of cholesterol efflux to HDL, whereas efflux to apoAI was not affected (Figure 5c). Thus, depending on the culture conditions and the extracellular lipoproteins present, SR-BI might either mediate the uptake of cholesterol-rich lipoproteins or induce cholesterol efflux to HDL, indicating a dual role for macrophage SR-BI in cellular cholesterol homeostasis.
Figure 5.
Dual role for macrophage SR-BI in cellular cholesterol homeostasis. a: Association of 125I-βVLDL to SR-BI+/+ and SR-BI−/− peritoneal macrophages after 3 hours of incubation at 37°C at the indicated concentrations after 3 days in culture (n = 4). b: Association of 50 μg/ml of 3H-cholesteryl ester-labeled HDL to freshly isolated peritoneal macrophages after 3 hours of incubation at 37°C (n = 3). c: ApoAI (10 μg/ml) and HDL (50 μg/ml) induced cellular cholesterol efflux from in vivo 3H-cholesterol-labeled peritoneal macrophages isolated from SR-BI+/+ or SR-BI−/− mice, analyzed for 24 hours (n = 3). Values are means ± SEM of three or four individual mice. Statistically significant difference of *, P < 0.05 and **, P < 0.01 as compared to SR-BI+/+ macrophages.
Discussion
Several lines of evidence indicate an anti-atherogenic role for scavenger receptor BI (SR-BI) in atherogenesis. Huszar and colleagues32 showed that LDL receptor-deficient mice with an attenuated expression of SR-BI are more susceptible to atherosclerotic lesion development. Furthermore, disruption of SR-BI in WT33 as well as in LDLr−/− mice22 results in a highly increased susceptibility to atherosclerotic lesion development. When cross-bred onto the apolipoprotein E knockout (apoE−/−) background, SR-BI deficiency leads to severe cardiac dysfunction and premature death.34,35 Hepatic overexpression of SR-BI, on the other hand, protects against the development of atherosclerosis.36–38
This anti-atherogenic function of SR-BI can primarily be attributed to its role in the uptake of HDL cholesteryl esters by the liver. SR-BI, however, is also expressed in lipid-laden macrophages in atherosclerotic lesions,10,19,20 indicating that SR-BI might play an additional important role locally in the arterial wall. In this study we show that SR-BI in bone marrow-derived cells, including macrophages, directly affects atherosclerotic lesion development. Interestingly, SR-BI in bone marrow-derived cells lowered atherosclerotic lesion development in LDLr−/− mice after 9 and 12 weeks of Western-type diet feeding. At 6 weeks, no significant effect was observed, while after only 4 weeks of Western-type diet-feeding SR-BI in bone marrow-derived cells facilitated atherogenesis. This was confirmed in WT mice that only develop small fatty streak lesions on a high-cholesterol/cholate diet. While this article was under preparation, two articles appeared demonstrating that SR-BI in bone marrow-derived cells lowered atherosclerotic lesion development in LDLr−/−22 and apoE−/−23 mice. Our data in LDLr−/− mice at 9 and 12 weeks of Western-type diet feeding are in agreement with this interpretation. However, we currently show that SR-BI in bone marrow-derived cells has a dual role in atherosclerotic lesion development and that, depending on the stage of atheroscleotic lesion development, SR-BI in bone marrow-derived cells is either anti-atherogenic or pro-atherogenic.
SR-BI is a multifunctional receptor capable of binding a wide array of native and modified lipoproteins. Its primary function is promoting the selective uptake of cholesteryl esters from HDL.39 In addition to its role as a HDL receptor, SR-BI functions as a binding-site for atherogenic lipoproteins, including native LDL and modified LDL.14–16 Adenoviral overexpression of SR-BI in liver reduces serum VLDL and LDL cholesterol levels in C57BL/6 mice36,40 and reverses fibrate-induced hypercholesterolemia in apoE-deficient mice.41 Furthermore, SR-BI transgenics display reduced VLDL and LDL levels,37,42 whereas disruption of SR-BI in apoE−/− mice results in an increase in circulating VLDL and LDL levels.34 Thus, SR-BI may also play a role in the metabolism of apoB-containing lipoproteins in vivo. In this study we show that the association of β-VLDL, a highly atherogenic lipoprotein, to macrophages in the presence of SR-BI is increased. Previously, we have shown that in the absence of the LDL receptor, a class B scavenger receptor, most likely SR-BI, is the primary receptor for the association of β-VLDL to macrophages and contributes to selective uptake of cholesteryl esters from β-VLDL.43 This function as a receptor for the uptake of cholesteryl esters from atherogenic apoB-containing lipoproteins is expected to induce foam cell formation and thus facilitate atherosclerotic lesion development. Indeed, the presence of macrophage SR-BI does facilitate atherosclerotic lesion development in LDLr−/− mice fed a Western-type diet for only 4 weeks and in WT mice on a high-cholesterol/cholate diet.
In LDLr−/− mice fed Western-type diet for 9 and 12 weeks, however, a protective role in atherosclerotic lesion development was observed. De la Llera-Moya and colleagues10 were the first to demonstrate that overexpression of SR-BI in cultured cells increases the rate of cholesterol efflux from cells to HDL particles. Furthermore, the rate of cholesterol efflux directly correlates with the level of SR-BI expression on a variety of cultured cells. In agreement, we found that cholesterol efflux from in vivo cholesterol-loaded SR-BI-deficient macrophages to HDL is impaired, whereas no effect on efflux to apoAI could be demonstrated. This is consistent with the generally accepted model that lipid-free apoAI is the ligand for cholesterol efflux via ABCA1, whereas SR-BI effluxes cholesterol to fully lipidated HDL.44,45 The function of SR-BI as a mediator of cholesterol efflux could explain the observed increase in atherosclerosis in the absence of macrophage SR-BI as observed in LDLr−/− mice. Several lines of evidence support that SR-BI can mediate the bidirectional movement of free cholesterol (FC) between cells and lipoproteins down a FC concentration gradient.10–12,46 Depending on the direction of the FC concentration gradient, either net efflux or net influx of cholesterol will occur. This leads to a unique function of macrophage SR-BI, that it can facilitate initial lesion formation and inhibit more advanced lesion formation when foam cells are heavily loaded with free cholesterol.
In addition to SR-BI, macrophages express ABCA1 and apoE that play an important role in sterol efflux pathways.3–6 Previously, we have shown that macrophage ABCA1 deficiency47 as well as apoE deficiency48 induce atherosclerotic lesion development. Chen and colleagues49 showed that SR-BI inhibits ABCA1-mediated cholesterol efflux by facilitating the reuptake of cholesterol (but not phospholipid) effluxed to apoAI. Furthermore, enhanced SR-BI expression in macrophages inhibits apoE-mediated cholesterol efflux by accelerating the degradation of newly synthesized apoE.50 Depending on the stage of atherosclerotic lesion development, these pathways might potentially interact differently, thereby modulating the direction of net sterol flux in macrophages.
The pathogenesis of atherosclerosis involves a complicated sequence of events in which various cell types, including endothelial cells, smooth muscle cells, and macrophages interact.1 In addition to its function in bidirectional cholesterol transport, SR-BI has also been implicated in the delivery of anti-oxidants as α-tocopherol,51,52 endothelium- and nitric-oxide-dependent arterial relaxation,53,54 and the removal of apoptotic cells.55 Progression of atherosclerotic lesions is characterized by an ongoing chronic inflammatory reaction and extensive cellular necrosis and apoptosis. It is therefore possible that impaired phagocytic activity of lesion macrophages because of the absence of SR-BI may have led to impaired clearance of apoptotic material, thereby inducing more excessive inflammatory responses and more rapid progression of the atherosclerotic lesion in absence of bone marrow-derived SR-BI.
Finally, other bone marrow-derived cells expressing SR-BI, in addition to macrophages, might have contributed to the effects at different stages of atherosclerotic lesion development. SR-BI deficiency is associated with impaired erythrocyte maturation as a result of increased cellular cholesterol levels.56 Selective disruption of SR-BI in bone marrow-derived cells, however, did not affect the erythrocyte cholesterol content. This is in agreement with recent data from Covey and colleagues.22 Recently, it was also shown that human platelets express CLA-1, the human homologue of SR-BI and that the levels of CLA-1 expression correlated inversely with platelet aggregation.57 No evidence of thrombosis was found in atherosclerotic lesions of mice reconstituted with SR-BI-deficient bone marrow. It is thus unlikely that altered erythrocyte or platelet function might have contributed to atherosclerotic lesion development in mice reconstituted with SR-BI-deficient bone marrow.
In conclusion, we have demonstrated that SR-BI in bone marrow-derived cells, including macrophages, has a dual role in atherosclerotic lesion development. This will probably also be relevant for the pathogenesis of atherosclerosis in humans. CLA-1, the human homologue of murine SR-BI is strongly induced on differentiation from monocytes into macrophages.19,20 Furthermore, in human carotid atherosclerotic lesions, CLA-1-positive staining is observed in the subendothelial region and the lipid core, co-localizing with specific macrophage markers.19,20 Thus, high levels of CLA-1/SR-BI are present in both human and murine atherosclerotic lesions, suggesting also a possible dual role for this scavenger receptor in atherosclerotic lesion development in humans.
Footnotes
Address reprint requests to M. Van Eck, Division of Biopharmaceutics, Gorlaeus Laboratories, Einsteinweg 55, 2333 CC Leiden, The Netherlands. E-mail: m.eck@lacdr.leidenuniv.nl.
Supported by The Netherlands Heart Foundation (grant 2001T041 to M.V.E.) and an International HDL Research Award (to M.V.E.).
References
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1991;340:115–125. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Kruth HS. Macrophage foam cells and atherosclerosis. Front Biosci. 2001;6:D429–D455. doi: 10.2741/kruth. [DOI] [PubMed] [Google Scholar]
- Rothblat GH, De la Llera-Moya M, Atger V, Kellner-Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- Tall AR, Costet P, Wang N. Regulation of mechanisms of macrophage cholesterol efflux. J Clin Invest. 2002;110:899–904. doi: 10.1172/JCI16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Bortnick AE, Kellner-Weibel G, De La Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- Von Eckardstein A. Cholesterol efflux from macrophages and other cells. Cur Opin Lipidol. 1996;7:308–319. doi: 10.1097/00041433-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Phillips MC, Johnson WJ, Rothblat GH. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochem Biophys Acta. 1987;906:223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- De la Llera-Moya M, Rothblat GH, Connelly MA, Kellner-Weibel G, Sakr SW, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- Yancey PG, De la Llera-Moya M, Swarnaker S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem. 2000;275:36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- Gu X, Kozarsky K, Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J Biol Chem. 2000;275:29993–30001. doi: 10.1074/jbc.275.39.29993. [DOI] [PubMed] [Google Scholar]
- Acton S, Rigotti A, Landschultz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- Gu X, Lawrence R, Krieger M. Dissociation of the high density lipoprotein and low density lipoprotein binding activities of murine scavenger receptor class B type I (mSR-BI) using retrovirus library-based activity dissection. J Biol Chem. 2000;275:9120–9130. doi: 10.1074/jbc.275.13.9120. [DOI] [PubMed] [Google Scholar]
- Landschulz KT, Pathak RP, Rigotti A, Krieger M. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl H, Hyatt M, Hobbs HH. Transport of lipids from high and low density lipoproteins via scavenger receptor-BI. J Biol Chem. 1999;274:32692–32698. doi: 10.1074/jbc.274.46.32692. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- Stangl H, Cao G, Wyne KL, Hobbs HH. Scavenger receptor, class B, type I-dependent stimulation of cholesterol esterification by high density lipoproteins, low density lipoproteins, and nonlipoprotein cholesterol. J Biol Chem. 1998;273:31002–31008. doi: 10.1074/jbc.273.47.31002. [DOI] [PubMed] [Google Scholar]
- Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yancey PG, Su YR, Babaev VR, Zhang Y, Fazio S, Linton MF. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low-density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina PM, Verstuyft J, Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990;31:859–869. [PubMed] [Google Scholar]
- Redgrave TG, Roberts DCK, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- McFarlane AS. Efficient trace-labelling of proteins with iodine. Nature. 1958;182:53–57. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Nagelkerke JF, Barto KP, Van Berkel TJC. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer and parenchymal cells. J Biol Chem. 1983;258:12221–12227. [PubMed] [Google Scholar]
- Pieters MN, Schouten D, Bakkeren HF, Esbach B, Brouwer A, Knook DL, Van Berkel TJC. Selective uptake of cholesteryl esters from apolipoprotein-E-free high-density lipoproteins by rat parenchymal cells in vivo is efficiently coupled to bile acid synthesis. Biochem J. 1991;280:359–365. doi: 10.1042/bj2800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Huszar D, Lee Varban M, Rinninger F, Feeley R, Arai T, Fairchild-Huntress V, Donovan MJ, Tall AR. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler Thromb Vasc Biol. 2000;20:1068–1073. doi: 10.1161/01.atv.20.4.1068. [DOI] [PubMed] [Google Scholar]
- Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IST, Kruijt JK, Kuipers F, Van Berkel TJC. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Gong E, Royer L, Cooper PN, Francone OL, Rubin EM. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- Trigatti B, Rigotti A, Krieger M. The role of the high-density lipoprotein receptor SR-BI in cholesterol metabolism. Curr Opin Lipidol. 2000;11:123–131. doi: 10.1097/00041433-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–415. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- Fu T, Kozarsky KF, Borensztajn J. Overexpression of SR-BI by adenoviral vector reverses the fibrate-induced hypercholesterolemia of apolipoprotein E-deficient mice. J Biol Chem. 2003;278:52559–52563. doi: 10.1074/jbc.M310892200. [DOI] [PubMed] [Google Scholar]
- Wang N, Arai T, Ji Y, Rinninger F, Tall AR. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J Biol Chem. 1998;273:32920–32926. doi: 10.1074/jbc.273.49.32920. [DOI] [PubMed] [Google Scholar]
- Herijgers N, Van Eck M, Korporaal SJ, Hoogerbrugge PM, Van Berkel TJC. Relative importance of the LDL receptor and scavenger receptor class B in the beta-VLDL-induced uptake and accumulation of cholesteryl esters by peritoneal macrophages. J Lipid Res. 2000;41:1163–1171. [PubMed] [Google Scholar]
- Yancey PG, Kawashiri MA, Moore R, Glick JM, Williams DL, Connelly MA, Rader DJ, Rothblat GH. In vivo modulation of HDL phospholipid has opposing effects on SR-BI- and ABCA1-mediated cholesterol efflux. J Lipid Res. 2004;45:337–346. doi: 10.1194/jlr.M300231-JLR200. [DOI] [PubMed] [Google Scholar]
- Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- Kellner-Weibel G, de La Llera-Moya M, Connelly MA, Stoudt G, Christian AE, Haynes MP, Williams DL, Rothblat GH. Expression of scavenger receptor BI in COS-7 cells alters cholesterol content and distribution. Biochemistry. 2000;39:221–229. doi: 10.1021/bi991666c. [DOI] [PubMed] [Google Scholar]
- Van Eck M, Bos IST, Kaminski WE, Orso E, Rothe G, Twisk J, Bottcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung WP, Van Berkel TJC, Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci USA. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M, Herijgers N, Vidgeon-Hart M, Pearce NJ, Hoogerbrugge PM, Groot PH, Van Berkel TJC. Accelerated atherosclerosis in C57Bl/6 mice transplanted with ApoE-deficient bone marrow. Atherosclerosis. 2000;150:71–80. doi: 10.1016/s0021-9150(99)00372-x. [DOI] [PubMed] [Google Scholar]
- Chen W, Silver DL, Smith JD, Tall AR. Scavenger receptor-BI inhibits ATP-binding cassette transporter 1-mediated cholesterol efflux in macrophages. J Biol Chem. 2000;275:30794–30800. doi: 10.1074/jbc.M004552200. [DOI] [PubMed] [Google Scholar]
- Huang ZH, Mazzone T. ApoE-dependent sterol efflux from macrophages is modulated by scavenger receptor class B type I expression. J Lipid Res. 2002;43:375–382. [PubMed] [Google Scholar]
- Goti D, Hrzenjak A, Levak-Frank S, Frank S, Van der Westhuyzen DR, Malle E, Sattler W. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- Mardones P, Strobel P, Miranda S, Leighton F, Quinones V, Amigo L, Rozowski J, Krieger M, Rigotti A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr. 2002;132:443–449. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- Gong M, Wilson M, Kelly T, Su W, Dressman J, Kincer J, Matveev SV, Guo L, Guerin T, Li XA, Zhu W, Uittenbogaard A, Smart EJ. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J Clin Invest. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imachi H, Murao K, Hiramine C, Sayo Y, Sato M, Hosokawa H, Ishida T, Kodama T, Quehenberger O, Steinberg D, Takahara J. Human scavenger receptor B1 is involved in recognition of apoptotic thymocytes by thymic nurse cells. Lab Invest. 2000;80:263–270. doi: 10.1038/labinvest.3780029. [DOI] [PubMed] [Google Scholar]
- Holm TM, Braun A, Trigatti BL, Brugnara C, Sakamoto M, Krieger M, Andrews NC. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002;99:1817–1824. doi: 10.1182/blood.v99.5.1817. [DOI] [PubMed] [Google Scholar]
- Imachi H, Murao K, Cao W, Tada S, Taminato T, Wong NCW, Takahara J, Ishida T. Expression of human scavenger receptor B1 on and in human platelets. Arterioscler Thromb Vasc Biol. 2003;23:898–904. doi: 10.1161/01.ATV.0000067429.46333.7B. [DOI] [PubMed] [Google Scholar]