Abstract
Increased proteolytic activity of matrix metalloproteinases (MMPs) may promote articular destruction such as occurs in rheumatoid arthritis and osteoarthritis. Recently, we reported that synovial tissue and fluid obtained from patients with rheumatoid arthritis contained higher activity of macrophage elastase (MMP-12). To examine the hypothesis that MMP-12 may potentially enhance the progression of arthritis, we investigated the effects of overexpression of MMP-12 on inflammatory arthritis in transgenic rabbits that express the human MMP-12 transgene in the macrophage lineage. Inflammatory arthritis was produced by articular injection of carrageenan solution and the degree of inflammatory arthritis in transgenic rabbits was compared with that in control rabbits. We found that overexpression of MMP-12 in transgenic rabbits significantly enhanced the arthritic lesions, resulting in severe synovial thickening, pannus formation, and prominent macrophage infiltration at an early stage and a marked destruction of articular cartilage associated with loss of proteoglycan at a later stage. These results demonstrate that excessive MMP-12 expression exacerbates articular connective tissue and cartilage degradation and thus plays a critical role in the development of inflammatory joint disease.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that possess the ability to degrade all of the extracellular matrix components associated with tissue destruction, and are believed to play important roles in many physiological and pathological processes.1,2 Among MMPs, MMP-12, also known as macrophage elastase (HME), is the predominant MMP produced by macrophages.3,4 Accumulating evidence has shown that increased activity of MMP-12 secreted from inflammatory macrophages is associated with several destructive diseases, including atherosclerosis,5 aortic aneurysm formation,6 and emphysema.7 Although the major substrate for MMP-12 is elastin, MMP-12 is able to degrade a broad spectrum of substrates such as type IV collagen, fibronectin, laminin, vitronectin, proteoglycans (PGs), chondroitin sulfate, myelin basic protein, α-1-anti-trypsin, and plasminogen.8,9 Moreover, human and mouse MMP-12 undergo self-activation through autolytic processing, and recombinant rabbit MMP-12 has been shown to activate other MMPs such as MMP-2 and MMP-3.5 Importantly, MMP-12 expression of macrophages is highly regulated by inflammatory mediators such as GM-CSF, MCP-1,10,11 and CD40 ligands,12 suggesting that in many inflammatory processes, once MMP-12 is up-regulated, there is a cascade of activation of other MMPs that leads to extracellular matrix degradation.
Rheumatoid arthritis (RA) is a chronic disease characterized by articular tissue destruction and irreversible joint damage. The molecular mechanisms associated with rheumatoid tissue destruction have not been fully elucidated, but it seems that macrophage infiltration is directly involved in the severity of the articular destruction.13,14 Ample evidence has shown that in the RA synovial membrane, the number of macrophages rather than lymphocytes determines the outcome of joint destruction.15–17 A possible mechanism for this macrophage-mediated joint destruction has been proposed, and many proteolytic enzymes appear to be involved. It is generally believed that the joint destruction in RA is mediated by the concerted action of various proteinases, among which the MMPs appear to play a major role.18 During the past few decades, MMPs such as collagenase (MMP-1) and stromelysin (MMP-3) have been found to be elevated in the cartilage as well as in the synovial fluid of RA patients and have been considered the rate-limiting enzymes in the collagen degradation.18,19 More recently, MMP-9 and membrane-type MMPs have also been implicated in RA,20,21 suggesting that many kinds of MMPs may be associated with the destruction of cartilage and connective tissues.13 Because inflammatory macrophages can produce a large amount of MMPs, we postulated that it is likely that excess MMP-12 production by macrophages plays a crucial role in both the initiation and the progression of RA via a mechanism possibly similar to those responsible for atherosclerosis and aortic aneurysm formation.
Recently, our laboratory demonstrated that along with increased MMP-3 and MMP-9, there is increased MMP-12 in both synovial tissue and fluid from RA patients compared to those from patients with osteoarthritis.22 To examine the hypothesis that MMP-12 may act as an important mediator in the pathogenesis of RA, we used MMP-12 transgenic (Tg) rabbits that specifically overexpress human MMP-12 only in macrophages and investigated the effect of overexpressed MMP-12 on the development of experimentally induced inflammatory arthritis. To the best of our knowledge, the present study provides the first evidence showing that MMP-12 up-regulation affects the process of inflammatory arthritis. Our results not only shed fresh light on the mechanism of articular destruction but also have implications for the notion that inhibition of MMP-12 activity may be a potential therapeutic target for the treatment of joint destruction in RA.
Materials and Methods
Rabbits
Human MMP-12 Tg rabbits were produced in our laboratory as described recently.23 The human MMP-12 transgene was under the control of the human scavenger receptor-A enhancer/promoter, a macrophage-specific promoter,24 and therefore, the hMMP-12 transgene was expressed in the macrophage lineage of Tg rabbits.23 In this study, a founder rabbit (designated as F2) was mated with non-Tg rabbits to obtain hemizygous rabbits for the following study. A total of 18 female Tg and 18 littermates (4 to 5 months old) fed a chow diet were used for the evaluation of MMP-12 effects on the experimentally induced inflammatory arthritis. All animal experiments were performed with the approval of the Animal Research Committee of the University of Tsukuba, Tsukuba, Japan.
Induction of Experimental Arthritis by Carrageenan Injection
We adopted the experimentally induced arthritis model in rabbits. For this purpose, rabbits were anesthetized with sodium pentobarbital solution and the right hind knee joint was intra-articularly injected with 0.3 ml of sterile λ-carrageenan solution (1%) (Wako Chemicals, Osaka, Japan) as described previously.25,26 The left knee joint was injected with the same amount of sterile saline as a sham control. Rabbits were sacrificed at 7, 14, and 35 days after carrageenan injection and both knee joints were excised. The whole knee joints were cut sagittally into four segments. Synovial samples were collected from the joint capsule for Western blot and Northern blot analysis. The synovial samples and joints were fixed in 10% neutral buffered formalin and embedded in paraffin for histological observation. The joints were decalcified in 14% ethylenediaminetetraacetic acid before the preparation of the sections. Paraffin sections (3 μm thick) were stained with hematoxylin and eosin (H&E) to assess the degree of inflammation and synovial proliferation. For the evaluation of articular cartilage degradation, sections of the joints were also stained with safranin O and counterstained with fast green (see below) and Masson’s trichrome. In addition, three rabbits from each group were used for collecting the synovial fluid for Western blot and zymographic analyses.
Quantitative Assessment of Articular Synovitis and Cartilage Degradation
Synovitis was histologically evaluated by scoring the changes of the synovial membrane: 0, no change; 1, synovial lining cell hyperplasia; 2, villous formation (<500-μm projections); and 3, papillary proliferation (>500-μm projections) based on the grading method reported by Rooney and colleagues.27 Three sagittal sections from each joint (anterior and posterior synovium) were observed and the average total score and distribution of each score were calculated. Macrophage infiltration and MMP-12 expression in the synovial tissues were assessed using immunohistochemical staining (see below). The size of pannus formation within the articular space was determined using the MacSCOPE image analysis system. To evaluate the articular cartilage damage, the PG content of the articular cartilage of the noncalcified layer of both the lateral and the medial sides of the tibia and femoral condyles was measured according to the method of Mankin and colleagues28 and quantitated using the MacSCOPE image analysis system. Three sagittal sections per knee were measured, and the results were expressed as the mean percentage of safranin O staining area relative to the whole cartilage PG compared to the PG content of the sham-operated group, which was defined as 100%.
Immunohistochemistry
Immunohistochemical staining was performed using a labeled streptavidin biotin kit (Nichirei Co, Tokyo, Japan) according to manufacturer’s instructions. After blocking of endogenous peroxidase activity, the sections were blocked with 10% goat serum for 60 minutes at room temperature. In this study, we were specifically interested in investigating macrophage infiltration and MMP-12 expression in the lesions of arthritis. For this purpose, we used monoclonal antibodies (mAbs) against rabbit macrophages (RAM11, 1:200) from Dako Cytomation (Carpinteria, CA) and against the human MMP-12 catalytic domain (MAB917, 1:20) from R&D Systems (Minneapolis, MN). Immunostained slides for macrophage and MMP-12 intensity were quantitated using the MacSCOPE image analysis system.23 The percentage of the total area with positive color in each section was recorded and expressed as percent for macrophage distribution or MMP-12 expression of the whole joint cavity.
Northern Blot and Real-Time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) Analysis
Total RNA from various tissues and isolated peritoneal and alveolar macrophages23 was rapidly isolated using Trizol reagent (Invitrogen, Life Technologies, Inc., Carlsbad, CA). Northern blot analysis was performed using 32P-labeled human MMP-12 cDNA probe as described.10 The bands of MMP-12 detected on X-ray film were scanned using a GS-700 imaging densitometer and the results were expressed relative to the control β-actin signals in the same samples. The expression of the hMMP-12 in lung, liver, spleen, and bone marrow in Tg rabbits was confirmed using the Qiagen OneStep RT-PCR system.23 MMP-12 mRNA expression levels in peritoneal and alveolar macrophages were evaluated by real-time RT-PCR (DNA Engine Opticon; MJ Research, Tokyo, Japan) using DyNAmo SYBR Green qPCR kit (Finnzymes Bioworks, Inc., Espoo, Finland) according to the manufacturer’s instructions. The primers for hMMP-12 (forward, 5′-ACA CAT TTC GCC TCT CTG CT-3′; reverse, 5′-CCT TCA GCC AGA AGA ACC TG-3′; 191 bp; nucleotides,749 to 940) were used. Rabbit endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward, 5′-GCT GAA CGG GAA ACT CAC TG-3′; reverse, 5′-CCA GCA TCG AAG GTA GAG GA-3′, 266 bp) was used as an internal control.
Western Blot Analysis
To identify MMP-12 and MMP-3 proteins in the synovium and synovial fluid, 40 mg of frozen synovium in liquid nitrogen was homogenized in ice-cold suspension buffer (10 mmol/L Tris-HCl, pH 7.6, 100 mmol/L NaCl) supplemented with a proteinase inhibitor cocktail (Sigma, St. Louis, MO). The supernatant was collected and the protein content was measured using a Bio-Rad protein assay kit. Ten-μg aliquots of the crude proteins from synovium and synovial fluid were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for Western blotting and probed with an Ab against hMMP-1210 and mouse monoclonal Ab against hMMP-3 (Fuji Chemical, Toyama, Japan). The immunocomplexed proteins were identified by reaction with a horseradish peroxidase-conjugated goat antibody to rabbit IgG followed by enhanced chemiluminescent detection (Amersham, Piscataway, NJ). To quantitate the relative change of MMP-12 and MMP-3 levels, MMP-12 and MMP-3 bands were scanned using a GS-700 imaging densitometer and the results were expressed relative to the β-actin signals.
Zymography
Substrate gel zymography of the activity of MMPs of crude proteins from synovial fluid was performed using the method described previously.23
Quantitative Macrophage Migration Assay
To evaluate the effect of MMP-12 on macrophage migration ability, we performed a chemotaxis assay using Biocoat cell culture inserts coated with murine laminin (Becton Dickinson Labware, Bedford, MA). Alveolar macrophages (0.5 ml) (2.5 × 105 cells/ml) in 1640 medium (Invitrogen) were plated on the upper wells. The lower compartments were loaded with the same medium containing recombinant human monocyte chemotactic protein-1 (MCP-1) at 10 ng/ml (Pepro Tech, London, UK). After 48 hours of incubation (37°C, 5% CO2), the number of macrophages having penetrated the gels was determined by counting 10 high-power fields at random from each well.
Statistical Analyses
All values were expressed as mean ± SE and statistical significance was analyzed using Student’s t-test or the Mann-Whitney’s U-test for nonparametric analysis. Statistical significance was set at P < 0.05.
Results
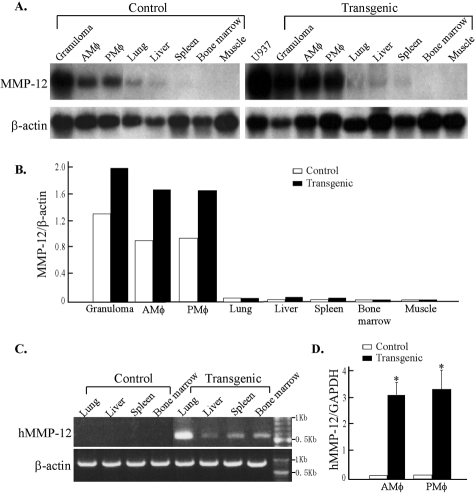
Northern blot analysis of eight different tissues showed that Tg rabbits had higher MMP-12 expression in macrophages isolated from the peritoneal cavity, lung, and carrageenan-induced subcutaneous granulomas than those from control rabbits (Figure 1, A and B). In the lung, liver, spleen, and bone marrow, which contain significant numbers of macrophage-lineage cells, weak expression of MMP-12 was detected after a longer exposure (data not shown) and the hMMP-12 expression was detected by RT-PCR (Figure 1C). Quantification of the MMP-12 expression levels in macrophages by real-time RT-PCR showed that there was significant increase of MMP-12 expression in both peritoneal and alveolar macrophages of Tg rabbits (Figure 1D).
Figure 1-4264.
Northern blotting analysis of MMP-12 mRNA tissue distribution in Tg and control rabbits. A: MMP-12 mRNA expression is increased in Tg rabbits compared to control rabbits. Rehybridization of the membrane with a human β-actin probe showed that similar amounts of RNA had been loaded in each lane. B: To quantitate the relative elevation of MMP-12 expression, the bands of MMP-12 and β-actin were scanned using a densitometry scanner, and the ratio of MMP-12 to β-actin was calculated. Total RNA isolated from human U937 cell-derived macrophages was used as a positive control.10C: Specific expression of hMMP-12 was demonstrated in the lung, liver, spleen, and bone marrow in Tg rabbits by RT-PCR. D: Increased MMP-12 expression levels in macrophages of Tg rabbits were shown by real-time RT-PCR analysis. N = 3 for each group; *, P < 0.01 versus control
Pathological Observations of Carrageenan-Induced Arthritis
Effect of Increased MMP-12 Expression on Synovitis
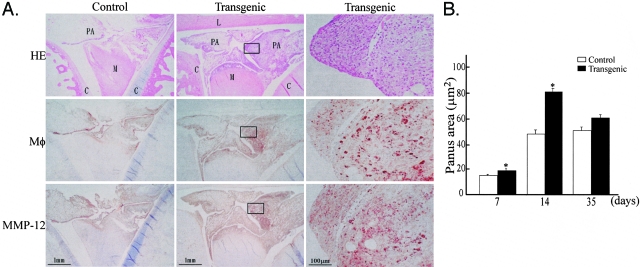
Inflammatory arthritis was investigated at 7, 14, and 35 days in an attempt to evaluate the effect of overexpressed MMP-12 on the lesions from various stages. Grossly, the treated joints in both Tg and control rabbits were slightly swollen for up to 3 days (data not shown). At 7 days, the synovium was significantly thickened because of a variable amount of synovial lining cell hyperplasia and extensive inflammatory infiltration consisting mainly of macrophages, with few lymphocytes and neutrophils (Figure 2A). A cluster of focal fibrin deposition on the synovial surface was observed occasionally (data not shown). The synovium of Tg rabbits contained more macrophages associated with increased MMP-12 immunoreactive proteins (Figure 2A). Synovial thickening reached a peak at 14 days but was constantly severe in Tg rabbits. Quantification of the synovitis score revealed that the synovitis was pronounced in Tg rabbits at all stages: 1.5-fold at 7 days, 1.46-fold at 14 days, and 1.24-fold high at 35 days over control rabbits (Figure 2B). The increased synovial thickness found in carrageenan-induced arthritis was not found in saline-injected sham joints of either Tg or control rabbits (Figure 2A).
Figure 2-4264.
Increased expression of human MMP-12 induced severe synovitis in Tg rabbits. A: right knee joints were intra-articularly injected with saline (left, control) and carrageenan (middle and right) as described in Materials and Methods. The synovial membrane from rabbits at 7 days was stained with either H&E (top), mAb against rabbit macrophages (middle), or MMP-12 (bottom). The synovium of Tg rabbits was markedly thickened compared to that of control rabbits. Note that many macrophages were accumulated in the thickened synovial membrane of Tg rabbits and were stained with Ab against MMP-12. B: Synovitis was evaluated by calculating average scores as described in Materials and Methods. Values are expressed as mean ± SE (n = 5 for each group). *, P < 0.01 versus control.
Effect of Increased MMP-12 Expression on Pannus Formation
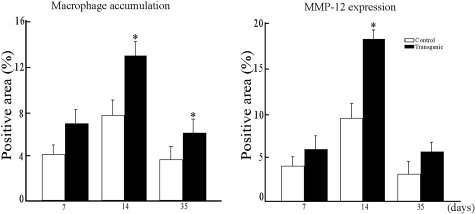
The second major pathological features examined were those of the pannus formation in the articular cavity, which became most evident at 14 days (Figure 3A). Compared to the lesions in control rabbits, the pannus lesions in Tg rabbits were significantly larger in size and contained more macrophages accompanied by increased levels of MMP-12 proteins. Quantification of the pannus lesions revealed that there was an increase of 1.27-fold at 7 days, 1.63-fold at 14 days, and 1.17-fold at 35 days in the lesion size in Tg rabbits compared to that in control rabbits (Figure 3B). Of note, the increased synovial thickening and pannus formation found in Tg rabbits were clearly correlated with both macrophage infiltration and MMP-12 staining intensity (Figure 4).
Figure 3-4264.
Increased expression of MMP-12 induced extensive pannus formation in Tg rabbits. A: joints from rabbits at 14 days after injection of carrageenan were prepared and stained with H&E (top), or with Ab against rabbit macrophages (middle), or MMP-12 (bottom). The details of the lesions in Tg rabbits are also shown at higher magnification (right). B: The size of the pannus formation from Tg rabbits was larger than that in control rabbits, and the pannus lesions in Tg rabbits contained more macrophage infiltration and MMP-12 immunoreactive proteins. The pannus formation reached the maximal extent at 14 days. Values are expressed as mean ± SE (n = 5 for each group). *, P < 0.01 versus control. M, meniscus; C, cartilage; L, ligament; PA, pannus.
Figure 4-4264.
Immunostaining intensity of macrophages (left) and MMP-12 (right). Quantitative analysis of macrophage accumulation and MMP-12 expression was performed using Macscope image analysis as described in Materials and Methods. Data are expressed as the average percentage of immunopositive area within the joint cavity (n = 4 for each group). *, P < 0.05 versus control.
Effect of Increased MMP-12 Expression on Cartilage Destruction
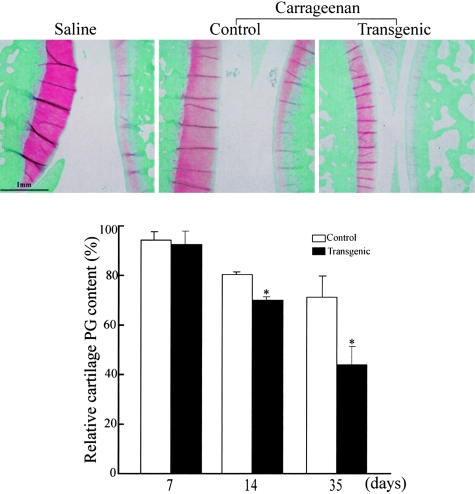
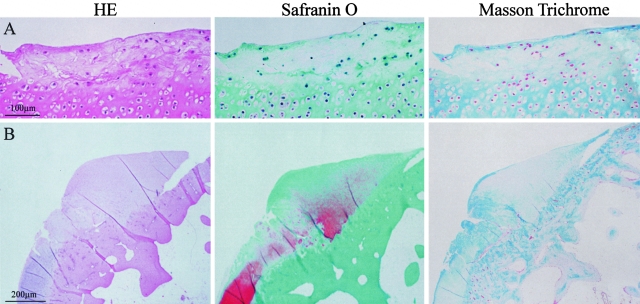
At 35 days, while the inflammatory components of both synovial thickening and pannus formation tended to regress, the lesions were characterized by cartilage destruction as shown by reduced intensity of safranin O staining (Figure 5). At this stage, we assessed the articular cartilage damage by comparing the cartilage PG depletion between the two groups. We found that PG contents as stained by safranin O were significantly reduced at 14 days and 35 days in Tg rabbits compared to control rabbits (Figure 5). Histological observation revealed that the cartilage destruction in Tg rabbits was so evident that the articular surface was rough, necrotic, or eroded accompanied by the reduction of PG and collagen (Figure 6).
Figure 5-4264.
Effect of increased MMP-12 expression on the articular destruction. Representative micrographs of joint sections from a sham control (left), carrageenan-injected control (middle), and carrageenan-injected Tg (right) rabbits at 35 days were stained with safranin O. The cartilage PG contents (stained by safranin O) at 14 and 35 days were markedly reduced in Tg rabbits compared to control rabbits (bottom). The PG content in the sham-operated saline-injected group was defined as 100%. Data are expressed as the average percentage of safranin O-stained area within the joint cavity (n = 7 for each group). *, P < 0.05 versus control.
Figure 6-4264.
Histological demonstration of marked cartilage destruction in Tg rabbits at 35 days. Micrographs of sections from the joints of carrageenan-injected Tg rabbits stained with H&E (left), safranin O (middle), and Masson’s trichrome (right) show that there was cartilage degeneration and necrosis (top) or erosion (bottom), associated with the loss of safranin O and Masson’s trichrome staining in the articular surface.
Increased MMP-12 Expression in Arthritis of Tg Rabbits
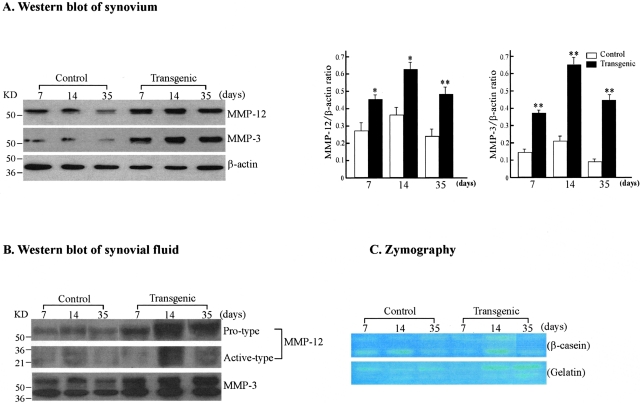
To further examine whether the increased arthritic lesions in Tg rabbit joints were correlated with MMP-12 overexpression, we investigated the MMP-12 in the synovial tissue and fluid by Northern blotting, Western blotting, and zymography. Northern blot analysis of the synovial tissue detected weak expression in both groups, but the expression of MMP-12 transcripts in Tg synovial tissue was higher than that in control rabbits (data not shown). In accordance with the findings of immunohistochemical staining, Western blot analysis confirmed that at all stages of the analyses, synovial tissue as well as synovial fluid from Tg rabbits consistently contained high levels of MMP-12 associated with higher levels of MMP-3, suggesting that MMP-12 may be involved in the activation of other MMPs (Figure 7A). Moreover, increased amounts of the active types of MMP-12 and MMP-3 were found in the synovial fluid of Tg rabbits (Figure 7B). Zymographic assays showed that increased MMP-12 proteins (especially at 14 days) were associated with β-casein digestion, indicating that these proteins were enzymatically functional (Figure 7C). Gelatinolytic activity of synovial fluid in Tg rabbits was also higher than that in control rabbits during the whole period of experiment.
Figure 7-4264.
Immunoblotting and zymographic analyses of MMP-12 and MMP-3 in the synovium and synovial fluid. A: Western blot analysis of MMP-12 and MMP-3 expression in the synovium. The synovial tissue collected from control and Tg arthritic rabbits at 7, 14, and 35 days was homogenized. Ten μg of the crude proteins from each sample were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions, and probed with Ab against either MMP-12 (top) or MMP-3 (middle). The same membrane was reprobed with mAb against β-actin to verify that equal amounts of proteins were loaded (bottom). The relative levels of MMP-12 and MMP-3 was quantitated by calculating the optical density (OD) of each signal intensity on the films using a densitometer (Bio-Rad) and normalized relative to the amount of β-actin protein (right). The analysis was performed in triplicate and values are expressed as the mean ± SE. *, P < 0.05 or **, P < 0.001 versus control. B: Western blot analysis of MMP-12 and MMP-3 expression in the synovial fluid from rabbits with inflammatory arthritis. Ten μg of proteins of the synovial fluid from control and Tg arthritic rabbits at 7, 14, and 35 days were analyzed as above. Note that synovial fluid from Tg rabbits contained more MMP-12 and MMP-3 proteins (both pro- and active type) than that from control rabbits. C: Zymographic analysis of MMP activity in the synovial fluid. To assess the enzymatic activity of MMPs, zymography with β-casein (top) and gelatin (bottom) as substrates was performed. The caseinolytic activity present at 54 and 45 kd in Tg rabbit synovial fluid was markedly increased compared to that in the control (top) at 14 days. The gelatinolytic activity in Tg rabbit synovial fluid tended to be stronger than that in controls at 14 and 35 days.
Macrophage Migration Assay
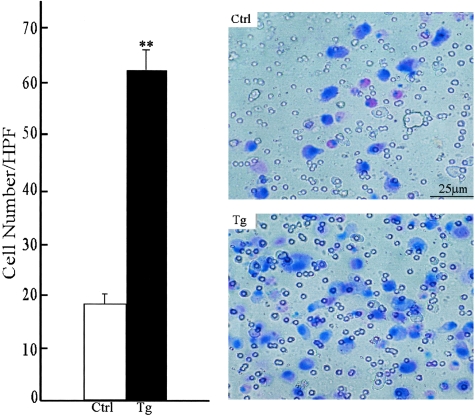
To determine whether the MMP-12 transgene affects the migration and chemotaxis properties of the macrophages, we compared their capacity to invade an immobilized extracellular matrix, laminin, in vitro. Without a chemoattractant, neither Tg nor control rabbit macrophages showed migratory activity (less than five cells in the whole well, data not shown). In response to the presence of a chemoattractant, MCP-1, the number of gel-invading macrophages from Tg rabbits was 3.26-fold greater than that from control rabbits (Figure 8).
Figure 8-4264.
Migration properties of transgenic and control alveolar macrophages. The chemotaxis assay was performed as described in Materials and Methods. Results are expressed as the average number of cells that penetrated the laminin gel after 48 hours of incubation at 37°C (left). The data are expressed as the mean ± SD (n = 5 for each group). **, P < 0.001 versus control. A representative micrograph of membrane with migrating macrophages stained by Diff-Quick was shown on the right.
Discussion
In a recent study, we demonstrated that synovial membrane and fluid from patients with RA contained higher MMP-12 activity than those from patients with osteoarthritis.22 Although these results indicate that MMP-12 may be associated with RA, it is not clear whether MMP-12 directly participates in the pathogenesis of RA. In this study, we examined the hypothesis that increased MMP-12 potentially enhances the progress of inflammatory arthritis. We used Tg rabbits that express a human MMP-12 transgene in a macrophage-specific pattern23 and used the carrageenan-induced inflammatory arthritis model.26,29 This arthritis model has several advantages: it is reproducible and is rapidly induced by a single injection of a small amount of carrageenan (carrageenan polysaccharide), which is relatively nontoxic to the animal as a whole29 and thus is considered an appropriate model for elucidating the relationship between the inflammatory response and the destruction of cartilage matrix.30 The lesions present in this experimentally induced arthritis model possess many features that mimic those of human RA, such as synovitis, pannus formation, and degradative changes in the articular cartilage matrix associated with loss of PG.31,32 Compared to the lesions in control rabbits, the lesions in Tg rabbits showed severe synovitis, pannus formation, and cartilage destruction, suggesting that increased MMP-12 is involved in the enhancement of the lesion formation.
Several mechanisms may be operative in the enhancement of inflammatory arthritis found in MMP-12 Tg rabbits. First, in the joint lesions of Tg rabbits, there was higher expression of MMP-12 protein derived from macrophages. Increased MMP-12 enzymatic activity may directly induce the degradation of connective tissue (mainly collagen and PG) and cartilage. Although the major substrates for MMP-12 are elastin, MMP-12 also has the ability to digest other extracellular matrix components such as type IV collagen, fibronectin, laminin, vitronectin, PGs, and chondroitin sulfate.8,9 Besides, MMP-12 can activate other MMPs such as MMP-2 and MMP-3, and consequently, exacerbate the proteolytic process.5 In this study, we also demonstrated that MMP-3 expression is increased in Tg rabbits (Figure 7). Therefore, it is likely that MMP-12 directly and/or indirectly (via concerted action with other MMPs) affects the processes of inflammatory arthritis. Second, there were more macrophages in the lesions of Tg rabbits than in those of control rabbits (Figure 4), which raises the possibility that increased MMP-12 from macrophages may result in the enhanced degradation of extracellular matrix surrounding the cells, thereby facilitating the migration of macrophages themselves toward chemoattractants. This notion is supported by the results of a chemotaxis assay showing that Tg rabbit macrophages had higher capacity of invading the extracellular matrix in vitro(Figure 8). A noteworthy finding in this study was that increased MMP-12 expression in the lesions was apparently associated with the increased number of macrophages and the lesion severity. In a separate study, we examined this issue using carrageenan-induced subcutaneous granuloma models in Tg rabbits and found that overexpression of MMP-12 significantly increased the size of subcutaneous granulomas.23
Finally, enhanced expression and production of MMP-12 may also be mediated by local cytokines and growth factors via paracrine and/or autocrine pathways in the synovial milieu. For example, GM-CSF and MCP-1 are known to be important activators of MMP-12,10,11 whereas PPAR-γ and transforming growth factor-β along with TIMPs are able to repress the expression of MMP-12.33,34 In this regard, one can state that up-regulation of these cytokines in arthritis may subsequently elevate the expression of MMP-12 because these cytokines are ubiquitously present in RA tissue. Nevertheless, direct contacts or interactions between T lymphocytes and macrophages, which are diffusely present in the RA synovial tissue, may also markedly induce MMP-12 expression. This assumption is supported by the demonstration that CD40/CD40 ligand signaling significantly augments the expression of MMPs.10,12 It is currently unknown, however, whether all these inflammatory processes are involved in the regulation of MMP-12 expression in vivo. In future studies, we need to investigate whether blocking MMP-12 activity will ameliorate the progression of RA and whether inhibition of MMP-12 can be used as a therapeutic strategy to treat RA. In this aspect, several MMP inhibitors have been developed and it will be interesting to test the efficacy of these inhibitors.35
In conclusion, the increased expression of MMP-12 in Tg rabbits dramatically exacerbated synovial hyperplasia, pannus formation, and the degeneration of articular cartilage in an experimentally induced inflammatory arthritis. These results provide evidence for a potential role of MMP-12 in the pathogenesis of RA and imply that the inhibition of MMP-12 may be potentially therapeutic for the treatment of inflammatory joint diseases.
Footnotes
Address reprint requests to Dr. Jianglin Fan, Cardiovascular Disease Laboratory, Department of Pathology, Institute of Basic Medical Sciences, University of Tsukuba, Tsukuba, 305-8575, Japan. E-mail: j-lfan@md.tsukuba.ac.jp.
Supported in part by grants-in-aid for scientific research from Mext (KAKENHI.13470046), the Mochida Memorial Foundation, the Uehara Memorial Foundation, and the Mitsubishi Pharma Research Foundation, and a grant from the Center for Tsukuba Advanced Research Alliance at the University of Tsukuba.
References
- Shapiro SD. Diverse roles of macrophage matrix metalloproteinases in tissue destruction and tumor growth. Thromb Haemost. 1999;82:846–849. [PubMed] [Google Scholar]
- Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- Werb Z, Gordon S. Elastase secretion by stimulated macrophages Characterization and regulation. J Exp Med. 1975;142:361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda MJ, Werb Z. Mouse macrophage elastase Purification and characterization as a metalloproteinase. Biochem J. 1981;193:589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol. 1998;153:109–119. doi: 10.1016/s0002-9440(10)65551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- Gronski TJ, Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189–12194. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Matsumoto S, Watanabe T. Induction and regulation of matrix metalloproteinase-12 by cytokines and CD40 signaling in monocyte/macrophages. Biochem Biophys Res Commun. 2000;269:808–815. doi: 10.1006/bbrc.2000.2368. [DOI] [PubMed] [Google Scholar]
- Wu L, Tanimoto A, Murata Y, Fan J, Sasaguri Y, Watanabe T. Induction of human matrix metalloproteinase-12 gene transcriptional activity by GM-CSF requires the AP-1 binding site in human U937 monocytic cells. Biochem Biophys Res Commun. 2001;285:300–307. doi: 10.1006/bbrc.2001.5161. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Sukhova GK, Atkinson E, Levesque E, Herman M, Graber P, Basset P, Libby P. Expression of stromelysin-3 in atherosclerotic lesions: regulation via CD40-CD40 ligand signaling in vitro and in vivo. J Exp Med. 1999;189:843–853. doi: 10.1084/jem.189.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol JA, Mix KS, Brinckerhoff CE, Vincenti MP, Clark IM. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull’s-eye or missing the mark? Arthritis Rheum. 2002;46:13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM, Mengshol JA, Mix KS, Vincenti MP, Clark IM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum. 1991;34:1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Clark IM, Brinckerhoff CE. Using inhibitors of metalloproteinases to treat arthritis Easier said than done? Arthritis Rheum. 1994;37:1115–1126. doi: 10.1002/art.1780370802. [DOI] [PubMed] [Google Scholar]
- Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996;39:1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, Billingham M, Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43:1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Liu M, Sun H, Wang X, Koike T, Mishima H, Ikeda K, Watanabe T, Ochiai N, Fan J: Increased expression of macrophage elastase (MMP-12) is associated with rheumatoid arthritis. Arthritis Rheum 2004, in press [DOI] [PubMed] [Google Scholar]
- Fan J, Wang X, Wu L, Matsumoto S, Liang J, Ichikawa T, Koike T, Sun H, Shikama H, Sasaguri Y, Watanabe T. Macrophage-specific overexpression of human matrix metalloproteinase-12 in transgenic rabbits. Transgenic Res. 2004;13:261–267. doi: 10.1023/b:trag.0000034717.70729.61. [DOI] [PubMed] [Google Scholar]
- Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci USA. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A, Gillard G. Carrageenan-induced arthritis I The effect of intraarticular carrageenin on the chemical composition of articular cartilage. Arthritis Rheum. 1976;19:769–776. doi: 10.1002/1529-0131(197607/08)19:4<769::aid-art1780190419>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Santer V, Srirantana A, Lowther DA. Carrageenin-induced arthritis: v A morphological study of the development of inflammation in acute arthritis. Semin Arthritis Rheum. 1983;13:160–167. doi: 10.1016/0049-0172(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Rooney M, Condell D, Quinlan W, Daly L, Whelan A, Feighery C, Bresnihan B. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988;31:956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Mankin H, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg. 1971;53:523–537. [PubMed] [Google Scholar]
- Lowther DA, Gillard GC. Carrageenin-induced arthritis I The effect of intraarticular carrageenin on the chemical composition of articular cartilage. Arthritis Rheum. 1976;19:769–776. doi: 10.1002/1529-0131(197607/08)19:4<769::aid-art1780190419>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Podworny NV, Kandel RA, Renlund RC, Grynpas MD. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999;26:1972–1982. [PubMed] [Google Scholar]
- Gillard GC, Lowther DA. Carrageenin-induced arthritis II Effect of intraarticular injection of carrageenin on the synthesis of proteoglycan in articular cartilage. Arthritis Rheum. 1976;19:918–922. doi: 10.1002/art.1780190513. [DOI] [PubMed] [Google Scholar]
- Senior RM, Campbell EJ, Landis JA, Cox FR, Kuhn C, Koren HS. Elastase of U-937 monocytelike cells Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982;69:384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARgamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma (PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg MW, Jain MK, Werner F, Sibinga NE, Wiesel P, Wang H, Topper JN, Perrella MA, Lee ME. Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J Biol Chem. 2000;275:25766–25773. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- Borkakoti N. Matrix metalloprotease inhibitors: design from structure. Biochem Soc Trans. 2004;32:17–20. doi: 10.1042/bst0320017. [DOI] [PubMed] [Google Scholar]